Introduction
Cholangiocarcinoma (CCA) is a severe tumor
originating from epithelial cells in the intrahepatic and
extrahepatic bile ducts and is associated with a poor prognosis
(1,2). Surgical resection is the predominant
treatment, however, the majority of patients are diagnosed too late
to resect or present with metastatic disease (3). Additionally, the lack of biomarkers
and effective non-surgical therapeutic modalities limit current
treatment options. To date, no second-line therapy has definitely
demonstrated improved long-term survival (4). Therefore, improving the understanding
of the molecular mechanisms of carcinogenesis is required to
develop novel therapies for the treatment of CCA.
The immunological response to cancer is an important
protective mechanism against cancer. However, the escape of tumors
from immune surveillance has been attributed to immune system
dysfunction, leading to tumor progression, metastasis and
recurrence. There are a number of strategies that enable tumor
cells to escape immune surveillance, including dysfunctional major
histocompatibility complex class I molecule, immunosuppressive
factors and aberrant expression of costimulatory molecules. While
the regulatory mechanisms by which costimulatory molecules regulate
the immune system have received research focus, the B7 family has
not been investigated in detail.
The costimulatory B7 family are cell-surface protein
ligands, providing stimulatory and inhibitory signals to regulate
the T cell response. The B7 family comprises seven members, B7.1
(CD80), B7.2 (CD86), B7-DC (CD273, PD-L2), B7-H1 (CD274, PD-L1),
B7-H2 (ICOS-L), B7-H3 (CD276) and B7-H4 (B7x, B7S1) (5). B7-H4 is a new member of the B7 family,
and is a type I-transmembrane protein and functions via a glycosyl
phosphate-dylinositol linkage, binding to a currently unidentified
receptor. B7-H4 is expressed at low levels in various peripheral
tissues, including lung, colon, liver, kidney, pancreas, small
bowel, breast and uterus (6,7).
Aberrant expression of B7-H4 has been observed in several tumor
types, including those of the breast, skin, lungs, colon, kidney,
brain and ovaries (8). B7-H4 has
been demonstrated to effect the negative regulation of T
cell-mediated immune responses by inhibiting T cell activation,
proliferation, cytokine production and cytotoxic activity (6). Furthermore, previous studies have
reported that the expression levels of B7-H4 are correlated with
clinicopathological parameters, and B7-H4 is currently considered
to be a prognostic marker in various tumors. However, the
expression levels of B7-H4 have not been investigated in different
tumor types, and its correlation with clinical outcomes remains
controversial (8). In particular,
the expression of B7-H4 in CCA and its clinical significance have
not been analyzed in detail. Further investigation of the
association between B7-H4 and CCA is required, and may provide
potential molecular targets for improved methods of detection and
treatment.
In the present study, the expression of B7-H4, and
its correlation with clinicopathological parameters in CAA were
investigated. The prognostic value of B7-H4 was evaluated using the
Kaplan-Meier estimator and Cox regression analysis. The present
study aimed to examine the tumor microenvironment by investigating
the association between B7-H4 protein and the density of various T
lymphocytes. Additionally, to understand the functional role of
B7-H4 in antitumor T cell responses, we carried out co-culture with
CD8+ T cytotoxic lymphocytes (CTLs) to identify its
impact on the suppression of CTL activity. Together, the results
suggest that B7-H4 may represent a novel prognostic predictor, in
addition to being a potential target for antitumor immunotherapy
for patients with CCA.
Materials and methods
CCA patients and clinical samples
Tissues were obtained from 137 patients who
underwent surgery at the Southwest Hospital (Chongqing, China)
between 2005 and 2011. Patients who underwent pre-operative
treatment, such as radiotherapy and/or chemotherapy, were excluded.
A total of 110 cancerous and 28 lymph node metastatic samples from
the patients were collected. A total of 19 chronic inflammatory
bile duct samples from patients with hepatolithiasis and 8 biliary
adenoma samples were also collected from Southwest Hospital. All
samples were obtained with informed consent from all patients,
according to the protocols approved by the Institutional Review
Board of the Southwest Hospital, Third Military Medical University.
The pathological reports were reviewed and the clinical features of
the 110 patients with CCAs are presented in Table I. Tumor-node-metastasis stages were
assigned according to the 6th Union for International Cancer
Control. Overall survival was calculated from the date of surgery
to the date of mortality or last contact. Recurrence-free survival
was computed from the date of surgery to the date of
recurrence.
 | Table ICorrelation between B7-H4 expression
and the CCA patient clinical features. |
Table I
Correlation between B7-H4 expression
and the CCA patient clinical features.
| Clinical
parameters | Cases | B7-H4 expression
| χ | P-valueb |
|---|
| Positive (%) | Negative (%) |
|---|
| Gender | | | | 1.163 | 0.184 |
| Men | 68 | 30 (44.1) | 38 (55.9) | | |
| Women | 42 | 24 (57.1) | 18 (42.9) | | |
| Age (years) | | | | 0.546 | 0.460 |
| ≤60 | 69 | 32 (46.4) | 37 (53.6) | | |
| >60 | 41 | 22 (53.7) | 19 (46.3) | | |
| Tumor location | | | | 0.896 | 0.334 |
| Intrahepatic | 18 | 7 (38.9) | 11 (61.1) | | |
| Extrahepatic | 92 | 47 (51.1) | 45 (48.9) | | |
| Tumor size
(cm) | | | | 0.115 | 0.734 |
| ≤5 | 91 | 44 (48.4) | 47 (51.6) | | |
| >5 | 19 | 10 (52.6) | 9 (47.4) | | |
| Tumor (T)
statusa | | | | 9.385 | 0.025c |
| T1 | 23 | 6 (26.1) | 17 (73.9) | | |
| T2 | 41 | 20 (48.8) | 21 (51.2) | | |
| T3 | 30 | 16 (53.3) | 14 (46.7) | | |
| T4 | 16 | 12 (75.0) | 4 (25.0) | | |
| Lymph node (N)
metastasis | | | | 4.464 | 0.035c |
| With | 42 | 26 (61.9) | 16 (38.1) | | |
| Without | 68 | 28 (41.2) | 40 (58.8) | | |
| Distant metastasis
(M) | | | | 3.434 | 0.064 |
| With | 19 | 13 (68.4) | 6 (31.6) | | |
| Without | 91 | 41 (45.1) | 50 (54.9) | | |
| UICC stagea | | | | 9.895 | 0.019c |
| I | 47 | 15 (31.9) | 32 (68.1) | | |
| II | 31 | 20 (64.5) | 11 (35.5) | | |
| III | 8 | 5 (62.5) | 3 (37.5) | | |
| IV | 24 | 14 (58.3) | 10 (41.7) | | |
Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded resected tissue
blocks were cut into 4-mm sections and mounted on charged glass
slides, deparaffinized and rehydrated in a graded series of
ethanol. Endogenous peroxidase activity was blocked with a solution
of 3% H2O2 in methanol for 30 min. Following
washing in phosphate-buffered saline (PBS), antigen retrieval was
performed in a citrate buffer (pH 6.0) at 120°C for 15 min. After
cooling and washing 3 times with PBS (pH 7.4) for 5 min each,
sections were incubated with the primary antibodies against B7-H4
(LLC.250473; dilution 1:200; Abbiotec, San Diego, CA, USA)
(9), CD4 (TA802240S; dilution
1:150), CD8 (TA802079S; dilution 1:200) (both from OriGene
Technologies, Inc., Rockville, MD, USA) in a humid chamber at 4°C
overnight. The sections were then washed with PBS and incubated
with a secondary polymeric peroxidase-labeled rabbit anti-mouse
antibody (Dako, Glostrup, Denmark) for 1 h at 37°C. Subsequently,
the nuclei were counterstained with hematoxylin and visualized with
3,3-diaminobenzidine tetrahydrochloride (Dako). A negative control
was performed using PBS instead of the primary antibodies under the
same conditions. The sections were dehydrated, cleared and
mounted.
Evaluation of IHC staining
Two independent investigators (G.F. and J.P.), who
were blinded to the patient clinicopathological data, analyzed the
IHC images. Expression of B7-H4 was analyzed in 10 different
high-power fields (HPFs). IHC for B7-H4 showed cytoplasmic and
membrane staining. The intensity (I) of staining was scored as
negative (0), weak (1), moderate
(2), or strong (3). The proportion (P) of B7-H4-positive
cells was defined as: 0, no staining; 1, <10% tumor cells with
staining; 2, 10–50% tumor cells with staining; 3, 50–80% tumor
cells with staining; 4, >80% tumor cells with staining. Samples
with IHC scores (P × I) ≤3 were considered as negative and with
scores >3 were defined as positive.
Additionally, the expression levels of
CD4/CD8-positive T lymphocytes in the tumor nest and tumor stroma
were separately determined according to IHC staining. The
evaluation was based on a review of 10 different HPFs that showed
the highest level of lymphocytic infiltrates for each case (low
<10%, high ≥10%).
CCA cell lines and transfection
Two human CCA cell lines (QBC939 and RBE), were
obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China) (10,11), and cultured in RPMI-1640 medium with
10% fetal bovine serum (FBS; GE Healthcare Life Sciences, Chalfont,
UK). All cell lines were incubated at 37°C in a humidified
atmosphere containing 5% CO2.
Cells were transfected with lentiviral vectors
encoding short hairpin RNA (Shanghai GenePharma Co., Ltd.,
Shanghai, China) targeting human B7-H4 for B7-H4 knockdown or a
scrambled shRNA-NC (Shanghai GenePharma Co., Ltd.) as the control.
The QBC939 and RBE cell lines were co-transfected with lentiviral
vectors. The recombinant vector was named GFP&Puro-B7-H4-shRNA,
according to the manufacturer's recommendations. For the QBC939 and
RBE cell lines, a multiplicity of infection of 10 was used to
achieve >90% transfection. The cells were cultured for 72 h
following transfection. Stably transfected QBC939 and RBE cells
were selected using puromycin. The efficiency of knockdown was
detected using western blotting.
Western blot analysis
Seventy-two hours after co-transfection with the
lentiviral vectors, cell extracts were prepared on ice using RIPA
lysis buffer plus a complete protease inhibitor cocktail (both from
Beyotime, China). Protein quantitation was measured by a BCA
protein assay kit (Thermo Scientific, Rockford, IL, USA) according
to the manufacturer's instructions. Ten micrograms of protein/lane
were separated on 8% acrylamide gels by sodium dodecyl sulfate
(SDS) gel electrophoresis and transferred to polyvinylidene
fluoride (PVDF) membranes. After blockage of non-specific binding
sites using 5% skim milk with Tris-buffered saline with Tween-20
(TBST) at 4°C overnight, the membranes were incubated for 2 h at
37°C with goat polyclonal affinity purified anti-human B7-H4
antibody (ab130151; dilution 1:1,000; Abcam, Cambridge, MA, USA). A
GAPDH antibody (10494-1-AP; dilution 1:1,000; Proteintech, China)
was used as an internal control. Following primary antibody
incubation, the membranes were washed in TBST and incubated with
horseradish peroxidase (HRP)-conjugated appropriate secondary
antibodies (Dako) for 1 h at 37°C. The membranes were washed with
TBST followed by visualization using enhanced chemiluminescence
(Millipore, Billerica, MA, USA) according to the manufacturer's
protocol.
Generation of CCA-specific CTLs
Peripheral blood mononuclear cells (PBMCs) were
isolated by density gradient centrifugation using Histopaque-1077
(Sigma-Aldrich, Munich, Germany) from 11 healthy donors. PBMCs were
seeded into 6-well culture plates containing 2 ml RPMI-1640 medium
and 10% FBS at a final concentration of 5–10×106
cells/well. Following 2 h of incubation, non-adherent cells were
removed by gentle washing with warm medium. The non-adherent cells
(effector lymphocytes) were cryopreserved in FBS supplemented with
10% dimethyl sulfoxide. The resultant adherent cells containing
dendritic cells (DCs) were cultured in medium supplemented with 500
U/ml recombinant human granulocyte-macrophage colony-stimulating
factor (GM-CSF) and 1,000 U/ml recombinant human interleukin 4
(IL-4) (both from PreproTech, Inc., Rocky Hill, NJ, USA) at 37°C in
5% CO2 (12). Every 2
days, one-half of the medium was replaced with fresh medium
containing a double concentration of GM-CSF and IL-4 as indicated
above. Following 5 days in culture, 10 ng/ml of recombinant human
tumor necrosis factor-α (TNF-α; PreproTech, Inc.) was added to the
medium to induce phenotypic and functional maturation of DCs
(12). CCA cells were induced to
apoptotic tumor cells (ATCs) with 100 µg/ml mitomycin for 24
h (Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China), and were presented by DCs to induce specific CTLs in
vitro. Subsequently, the isolated non-adherent effector
lymphocytes were co-cultured with the ATC-pulsed autologous DCs in
a 6-well plate in the presence of 10 ng/ml recombinant human
interleukin-7 (IL-7; PreproTech, Inc.). Half the medium was
replaced with complete medium supplemented with 30 IU/ml
recombinant human interleukin 2 (IL-2; PreproTech, Inc.) every 3
days. Following 7 days in culture, the lymphocytes were
re-stimulated with the ATC-pulsed autologous DCs in medium
containing 10 ng/ml IL-7 and 20 U/ml IL-2. On day 10, following the
fourth round of re-stimulation, the cells were harvested and tested
using CCA-specific CTL assay (13).
CD8+ T-mediated CTLs were purified by negative depletion
using a CD8+ T cell isolation kit (Miltenyi Biotec,
Bergisch Gladbach, Germany).
CTL cytotoxicity assay
CTL activity was evaluated using the CytoTox
96® non-radioactive cytotoxicity assay (Promega,
Madison, WI, USA) based on lactate dehydrogenase (LDH) release.
After washing, the target cells were counted and seeded into
96-well V-bottomed culture plates. Varying numbers of CTLs were
added to a final volume of 100 µl at the effector to target
(E/T) ratios of 2.5:1, 5:1, 10:1 and 20:1 and incubated for 4 h at
37°C. The supernatants were harvested and the assay plates were
incubated for 30 min at room temperature, protected from light. The
absorbance at 490 nm was recorded within 1 h after adding the stop
solution. The corrected values were used in the following formula
to compute percent cytotoxicity: Cytotoxicity = [(Experimental -
Effector Spontaneous - Target Spontaneous)/(Target Maximum - Target
Spontaneous)] × 100%.
Statistical analysis
Data were analyzed using SPSS software, version 19.0
(IBM SPSS, Armonk, NY, USA). Group comparisons of continuous data
were made using t-test on independent means. For categorical data,
Chi-square analysis or the Fisher's exact test was used. The
Kaplan-Meier estimator and Cox analysis were used for overall
survival and recurrence-free survival. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of B7-H4 as detected by IHC
analysis in CCA
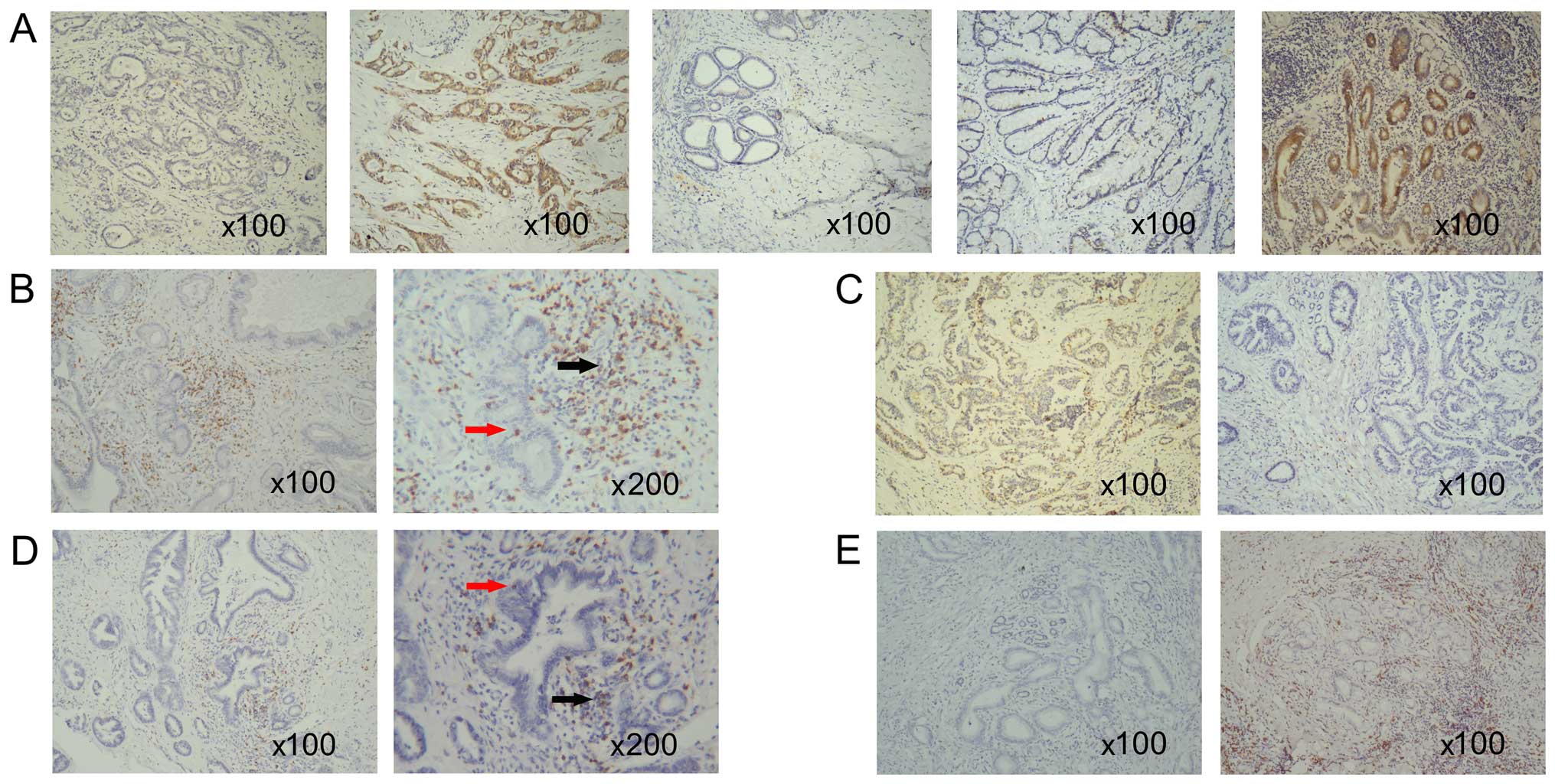
Representative IHC images of B7-H4 are presented in
Fig. 1. The expression of B7-H4
protein was detected in 54/110 (49.1%) cancerous tissues, 15/28
(53.6%) lymph node metastatic tissues and 4/19 (21.1%) chronic
inflammatory bile duct tissue samples (Table II). Notably, in the inflammatory
bile duct tissues, B7-H4 was predominantly expressed in the
infiltrating mononuclear cells rather than the epithelial cells of
the bile duct, which is in line with its role during inflammatory
reactions. In addition, 8 biliary adenoma samples (Fig. 1) stained negative for B7-H4. As
shown in Table II, positive
staining of B7-H4 was detected in cancerous and lymph node
metastatic samples, which was significantly greater compared with
the non-tumorous tissues. These data indicated that the high
expression of B7-H4 was specific to the CCA tissues.
 | Table IIDifferences in B7-H4 expression in
CCA and chronic inflammatory bile duct samples. |
Table II
Differences in B7-H4 expression in
CCA and chronic inflammatory bile duct samples.
| Sample | Cases | B7-H4 expression
| P-value |
|---|
| Positive (%) | Negative (%) |
|---|
| Cancerous | 110 | 54 (49.1) | 56 (50.9) | Control |
| Lymph node
metastatic | 28 | 15 (53.6) | 13 (46.4) | 0.672 |
| Chronic
inflammatory bile duct | 19 | 4 (21.1) | 15 (78.9) | 0.023a |
Expression of B7-H4 is significantly
associated with clinicopathological features including tumor
status, lymph node metastasis and International Union Against
Cancer (UICC) stage in CCA
As B7-H4 was highly expressed in the cancer tissues
of patients with CCA, its expression was investigated as to whether
it correlates with clinicopathological parameters in patients with
CCA. The clinicopathological features of the 110 cases of CCA were
grouped by positive or negative B7-H4 expression. As shown in
Table I, B7-H4 expression in CCA
tissues was significantly associated with tumor status (P=0.025),
lymph node metastasis (P=0.035) and UICC stage (P=0.019), however,
not with gender, age, tumor location and size. The data indicate
that the cases of CCA positive for B7-H4 exhibited more extensive
metastatic behavior.
Expression of B7-H4 indicates poorer
prognosis in patients with CCA
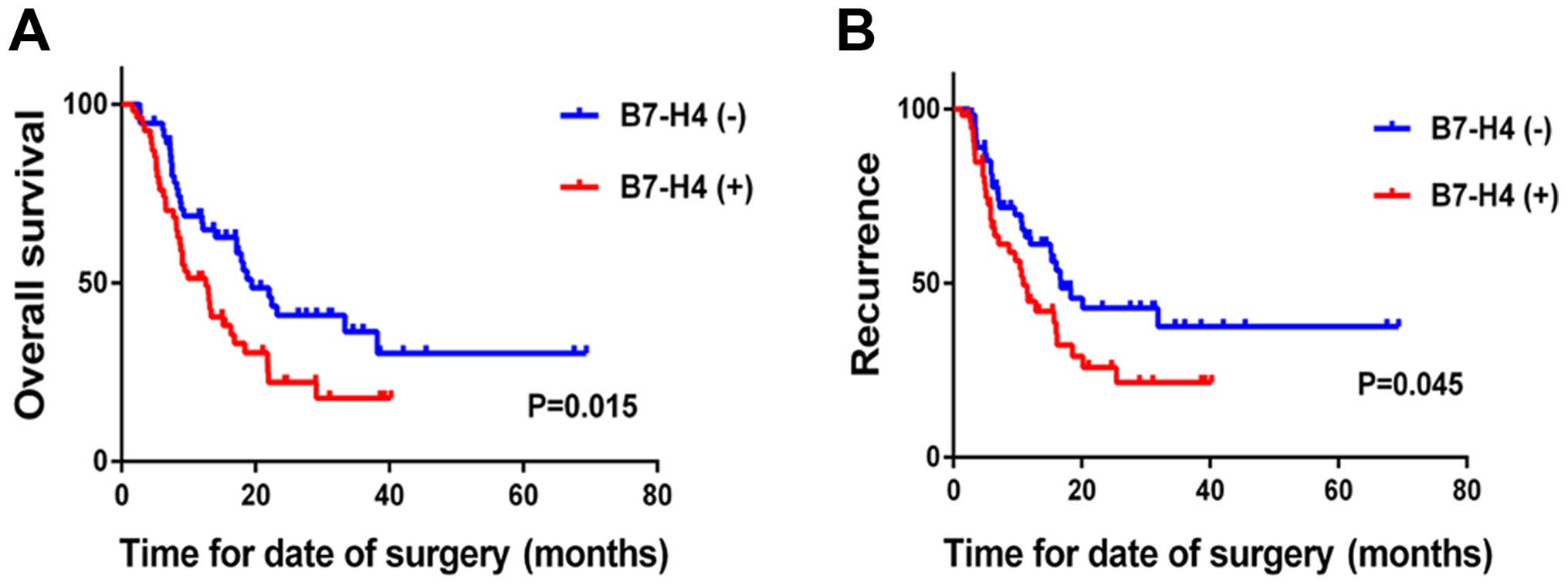
The results indicated that the expression of B7-H4
correlates with adverse pathological features, which are associated
with patient prognosis. First, the correlation of B7-H4 expression
with disease prognosis was analyzed. The results indicated that the
expression of B7-H4 is associated with a poorer outcome following
surgery. The median overall survival time of patients negative for
B7-H4 was 19.5 months, which is longer than the 12.6 months
observed in patients positive for the expression of B7-H4 (P=0.015;
Table III). In addition, the
median disease-free survival time of patients negative for B7-H4
expression was longer than that of patients with positive B7-H4
expression (16.7 vs. 10.9 months; P=0.046; Table III). Kaplan-Meier analysis
indicated that the expression of B7-H4 is associated with reduced
survival (log-rank P=0.015; Fig.
2A) and time to recurrence (log-rank P=0.046; Fig. 2B) in the 110 cases. To further
validate these observations, multivariate Cox analysis was used,
which showed that B7-H4 expression was an independent indicator of
poorer overall survival and early recurrence [hazard ratio
(HR)=1.786; 95% confidence interval (CI), 1.110–2.872; P=0.017; and
HR=2.062; 95% CI, 1.160–3.665; P=0.014; Table V]. Taken together, the data suggest
that aberrant expression of B7-H4 may be a risk factor for poorer
prognosis in patients with CCA following surgical resection.
 | Table IIIUnivariate analysis of various
clinicopathological parameters in relation to the survival of
patients with CCA. |
Table III
Univariate analysis of various
clinicopathological parameters in relation to the survival of
patients with CCA.
| Clinical
parameters | N (%) | Overall survival
| Disease-free
survival
|
|---|
| Median
(months) | Log-rank
(P-value) | Median
(months) | Log-rank
(P-value) |
|---|
| Gender |
| Male | 57 (62.0) | 13.4 | 0.914 | 15.3 | 0.855 |
| Female | 35 (38.0) | 14.0 | | 16.0 | |
| Age (years) |
| ≤60 | 58 (63.0) | 16.9 | 0.160 | 16.0 | 0.192 |
| >60 | 34 (37.0) | 12.6 | | 11.5 | |
| Tumor size
(cm) |
| ≤5 | 81 (88.0) | 17.1 | <0.001b | 17.2 | <0.001b |
| >5 | 11 (12.0) | 6.2 | | 5.8 | |
| Tumor (T)
statusa |
| T1 | 11 (12.0) | | <0.001b | | <0.001b |
| T2 | 39 (42.4) | 16.3 | | 15.2 | |
| T3 | 27 (29.3) | 8.5 | | 6.1 | |
| T4 | 15 (16.3) | 8.9 | | 9.6 | |
| Lymph node (N)
metastasis |
| With | 37 (40.2) | 8.4 | <0.001b | 6.9 | 0.03b |
| Without | 55 (59.8) | 21.9 | | 16.2 | |
| Distant metastasis
(M) |
| With | 17 (18.5) | 8.2 | <0.001b | 6.1 | <0.001b |
| Without | 55 (81.5) | 18.2 | | 16.0 | |
| UICC stagea |
| I | 39 (42.4) | 23.2 | <0.001b | 31.9 | <0.001b |
| II | 30 (32.6) | 8.4 | | 10.6 | |
| III | 6 (6.5) | 17.2 | | 15.7 | |
| IV | 17 (18.5) | 8.5 | | 6.4 | |
| B7-H4
expression |
| Positive | 47 (51.1) | 12.6 | 0.015b | 10.9 | 0.046b |
| Negative | 45 (48.9) | 19.5 | | 16.7 | |
 | Table VDifferences in Cox analysis for
overall survival and recurrence-free survival of CCAs after
surgical resection (n=110). |
Table V
Differences in Cox analysis for
overall survival and recurrence-free survival of CCAs after
surgical resection (n=110).
| Factors | Overall survival
| Recurrence-free
survival
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Expression of B7-H4
(+/−) | 1.786
(1.110–2.872) | 0.017a | 2.062
(1.160–3.665) | 0.014a |
| Gender
(male/female) | 1.049
(0.647–1.701) | 0.603 | 0.816
(0.480–1.387) | 0.452 |
| Age, years (≤60,
>60) | 0.728
(0.443–1.195) | 0.112 | 0.796
(0.472–1.343) | 0.392 |
| Location
(hilar/distal) | 1.451
(0.700–3.008) | 0.241 | 2.610
(1.025–6.650) | 0.044a |
| Histologic grade
(G1-2/G3) | 1.435
(0.841–2.447) | 0.204 | 1.598
(0.890–2.867) | 0.116 |
Expression of B7-H4 in tumor cells is
inversely associated with the density of CD8+ T cells in
the tumor stroma
Previous studies have shown that B7-H4 is able to
directly or indirectly modulate immune infiltrate cells, which are
comprised predominantly of CD4+ helper T cells and
CD8+ cytotoxic T cells (8,14–16).
The presence of tumor-infiltrating lymphocytes (TILs) within the
tumor stroma or nest is considered an indicator of the host immune
response to the tumor (17).
Therefore, whether the expression of B7-H4 correlates with
CD4+ and CD8+ TILs was investigated in the
110 CCA tissues. As shown in Table
IV, levels of B7-H4 expression in the tumor cells was inversely
correlated with the density of CD8+ T cells in the tumor
stroma (P=0.0004), however, was not correlated with the density of
CD8+ T cells in the tumor nest (P= 0.776). In addition,
there was no significant association between B7-H4 expression and
the CD4+ T cells in the tumor stroma or nest (P=0.567
and P=0.822, respectively). Therefore, these data provide further
evidence of the potential role of B7-H4 in the suppression of
cellular immune surveillance in patients with CAA, and in
particular tumor infiltrating CD8+ T cells.
 | Table IVCorrelation between B7-H4 expression
and the densities of TILs in the CCA tissue sections. |
Table IV
Correlation between B7-H4 expression
and the densities of TILs in the CCA tissue sections.
| B7-H4
expression | Cases | CD4+ T
cells
| CD8+ T
cells
|
|---|
In tumor nest
| In tumor stroma
| In tumor nest
| In tumor stroma
|
|---|
| Low | High | P-value | Low | High | P-value | Low | High | P-value | Low | High | P-value |
|---|
| Positive | 54 | 42 | 12 | 0.567 | 48 | 6 | 0.822 | 38 | 16 | 0.776 | 35 | 19 | 0.004a |
| Negative | 56 | 46 | 10 | | 49 | 7 | | 38 | 18 | | 21 | 35 | |
Knockdown of B7-H4 increases
CD8+ T-mediated cytotoxicity (CTL) in CCA cell
lines
Considering the above observations, which indicated
a negative correlation between B7-H4 expression and the density of
CD8+ T cells in the tumor stroma, the impact of B7-H4 on
CD8+ T cells was further investigated in vitro.
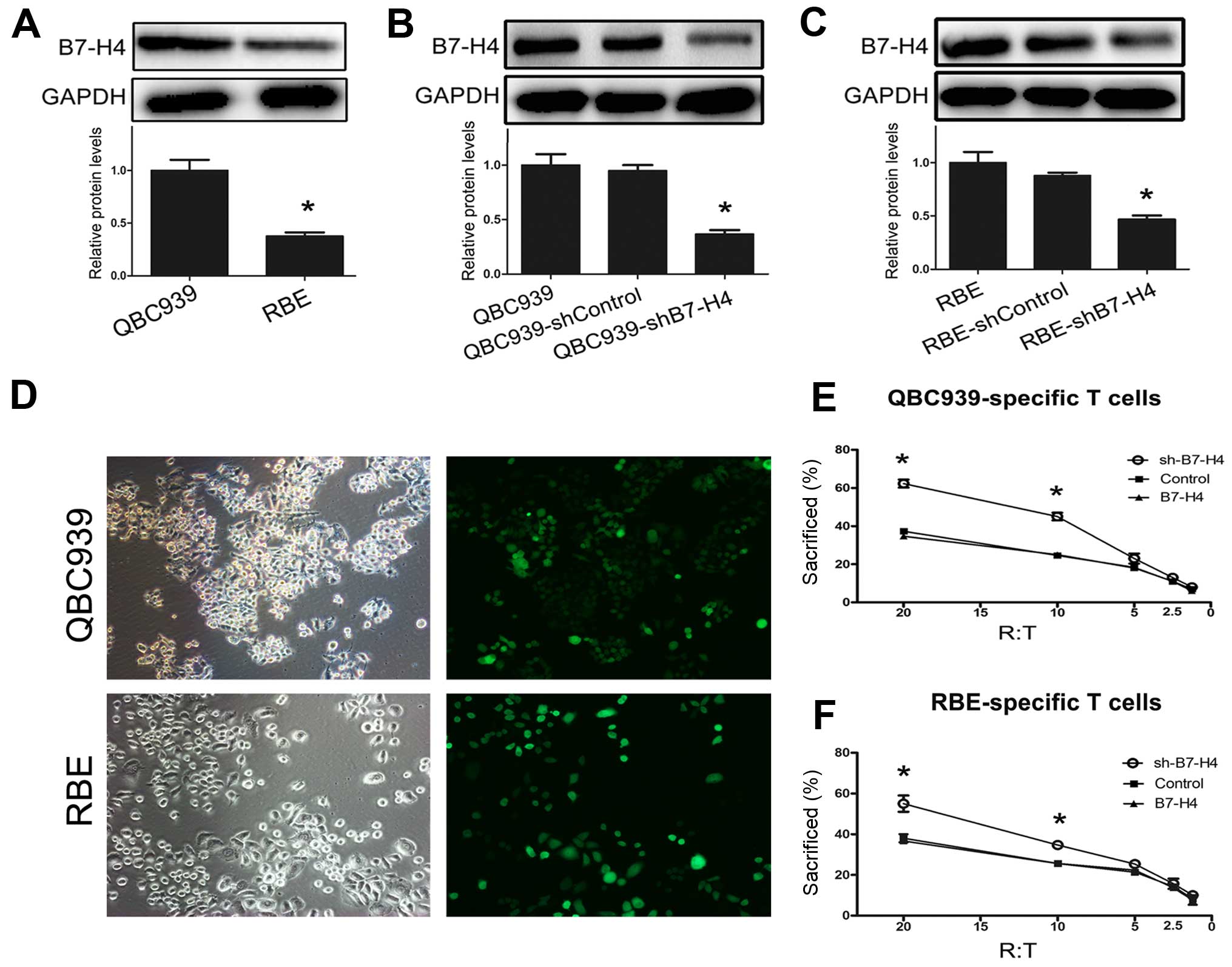
Western blot analysis showed that B7-H4 was expressed in QBC939 and
RBE cells (Fig. 3A). Notably, the
protein expression levels of B7-H4 were significantly greater in
QBC939 cells compared with RBE cells (Fig. 3A). Knockdown of B7-H4 was performed
in QBC939 and RBE cells using B7-H4-shRNA lentiviral transfection
(Fig. 3B and C). By culturing with
CD8+ cytotoxic T cells, the cytotoxicity of
CD8+ T cells was markedly improved by the knockdown of
B7-H4 in QBC939 and RBE cells (Fig. 3E
and F). These data indicate that knockdown of B7-H4 in tumors
increases CD8+ T cell-mediated cytotoxicity in
vitro.
Discussion
Epidemiological studies have demonstrated that the
incidence of CCA has been increasing in recent years (18). However, patients with CCA have a
poor prognosis, with a median survival of <24 months, due to
late diagnosis and the limited efficacy of non-surgical therapies
(19). B7-H4, as a negative
regulator of T cell responses, has been observed to be expressed in
a variety of human tumors. Numerous studies that focus on the
clinical significance of B7-H4 have been reported (20–23).
However, there are limited studies regarding the expression of
B7-H4 in CCA, and its functional relevance has not been reported in
detail. The present study, to the best of our knowledge, is the
first demonstration that the expression of B7-H4 is low in
noncancerous and high in CCA tissues and lymph node metastases.
These results are in accordance with previous studies in which the
expression of B7-H4 has been observed in gastric and lung cancer
tissues, with a positive rate of 44.9 and 40.7% (24,25).
Furthermore, ovarian, breast and esophageal squamous carcinoma have
demonstrated higher expression of B7-H4 in 93.5, 94.8 and 95.5% of
cases, respectively (2,20,26).
In addition, the present study observed that positive expression of
B7-H4 is associated with tumor status, lymph node metastasis and
tumor stage in CCA. These data are in accordance with the
association between the expression of B7-H4 and clinicopathological
factors associated with the prognosis of tumor patients reported in
previous studies (20–23). In contrast to the present study,
Tringler et al observed no significant association between
B7-H4 expression and grade, stage or other clinicopathological
features in breast cancer (2). This
discrepancy may be explained by the tumor heterogeneity between CCA
and breast cancer. Therefore, the present study demonstrates that
B7-H4 expression is associated with advanced CCA, and indicates a
more aggressive biological potential.
Due to the aforementioned results, the present study
further analyzed the association between B7-H4 and the prognosis of
patients with tumors. The results suggest that B7-H4 is an
independent factor in the prognosis of patients with CCA.
The presence of T-lymphocytes within the tumor
microenvironment is considered an important component of the
antitumor immune response and reflects the process of ̔cancer
immunoediting̓ in solid tumors (27). In the present study, the immune
responses against cancer cells were investigated using IHC of TILs
in CCA. TILs in the tumor microenvironment are predominantly
CD4+ and CD8+ T cells, which are considered
to be the effector cells in the Th2 and Th1 antitumor immune
responses, respectively. A subset of CD4+ TILs was
selected to examine the T-helper population, and a subset of
CD8+ TILs was selected to specifically examine the
cytotoxic T cell population (17,28).
The CD8+ TILs serve a vital role in the killing of tumor
cells. Previous studies have shown that increased B7-H4 expression
is involved in shaping the tumor microenvironment by modulating the
infiltration of CD3+ and CD8+ TILs in breast
cancer (14), however, the subtypes
of the T lymphocytes were not further analyzed. The association
between B7-H4 and CD4+, CD8+ TILs has not
been reported in CCA. The present study demonstrated that the
expression of B7-H4 is inversely correlated with the density of
CD8+, not with CD4+ TILs in the tumor stroma.
Notably, CD4+ and CD8+ TILs exhibit low
expression in the tumor nest regardless of the expression levels of
B7-H4. This phenomenon may be due to the immunosuppression of the
local tumor nest. These data suggest that B7-H4 may reduce the
total number of infiltrating lymphocytes in tumor stroma, in
particular, CD8+ TILs rather than CD4+ TILs,
by inhibiting their recruitment or survival in the tumor
microenvironment.
The present study indicated that B7-H4 acts as a
negative regulator of T cells, by inhibiting the infiltration of
the CD8+ TILs. However, the functionality of the immune
cells is of greater importance compared to the frequencies of
immune infiltrates (28,30). Therefore, the impact of
cancer-associated B7-H4 on CCA-specific CD8+ T
cytotoxicity was further explored in vitro. Sica et
al (6) reported that B7-H4
inhibits T cell proliferation and cytotoxicity against allogeneic
antigens in vitro. Additionally, it has been demonstrated in
lung cancer that blockade of B7-H4 using neutralizing monoclonal
antibodies promotes the apoptosis of T cells, and inhibits
CTL-mediated cytotoxicity (29). In
contrast to inhibiting the function of B7-H4 using B7-H4 Ig, the
present study knocked down the expression of B7-H4 using lentiviral
vectors encoding shRNA. This enables the direct observation of the
reduction of B7-H4 in tumor cells, and the screening for stable
tumor cell lines which express low levels of B7-H4. Following
co-culture with CCA cells transfected with lentiviral vectors,
CD8+ T cell-mediated cytotoxicity was measured.
Following the reduction in the expression of B7-H4, the
CD8+ T cell-mediated cytotoxicity was increased.
Therefore, the present study suggests that it may be possible to
increase CD8+ T cell-mediated cytotoxicity using
treatments to reduce or block B7-H4.
B7-H4 may contribute to the inhibition of
CD8+ T cell-mediated cytotoxicity, however, the
mechanism has not been investigated in detail. Previous studies
have suggested various possible explanations for the suppression of
cytotoxicity. B7-H4 has been reported to interfere with T cell
activation, at least in part, through signaling pathways downstream
of CD28, including protein kinase B, extracellular signal-regulated
kinase and c-Jun N-terminal kinase (30). As a cell-surface protein, B7-H4 is
extensively N-glycosylated, which appears to regulate surrounding T
cell function (31). Alternatively,
B7-H4 may partially contribute to the production of cytokines such
as TGF-β and IL-6 (16,32). These cytokines are able to induce
CD8+ CTLs to differentiate into non-cytotoxic
IL-17-producing cells (33). The
results of the present study provide evidence that B7-H4 may serve
an important role in shielding tumors from immune surveillance by
reducing the number and cytotoxic ability of CD8+
TILs.
In conclusion, the present study showed that B7-H4
is overexpressed in CCA and is associated with multiple aggressive
tumor features and poor prognosis. The aberrant expression of B7-H4
observed in CCA cells may significantly suppress the number and
cytotoxicity of CD8+ T cells in the tumor
microenvironment. However, the precise role of B7-H4 in T cell
regulation and the underlying mechanisms remain to be fully
elucidated. Further studies are required to explore the specific
role of B7-H4 in CCA. The present study indicates that B7-H4 may be
a promising new target for the diagnosis and treatment of patients
with CCA.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (nos. 81071729 and 8127237). We
thank Spandidos Publications for providing English Language editing
Service.
References
|
1
|
Lee BS, Cha BH, Park EC and Roh J: Risk
factors for perihilar cholangiocarcinoma: A hospital-based
case-control study. Liver Int. 35:1048–1053. 2015. View Article : Google Scholar
|
|
2
|
Tringler B, Zhuo S, Pilkington G, Torkko
KC, Singh M, Lucia MS, Heinz DE, Papkoff J and Shroyer KR: B7-h4 is
highly expressed in ductal and lobular breast cancer. Clin Cancer
Res. 11:1842–1848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mavros MN, Economopoulos KP, Alexiou VG
and Pawlik TM: Treatment and prognosis for patients with
intrahepatic cholangiocarcinoma: Systematic review and
meta-analysis. JAMA Surg. 149:565–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jarnagin WR and Shoup M: Surgical
management of cholangiocarcinoma. Semin Liver Dis. 24:189–199.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seliger B, Marincola FM, Ferrone S and
Abken H: The complex role of B7 molecules in tumor immunology.
Trends Mol Med. 14:550–559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sica GL, Choi IH, Zhu G, Tamada K, Wang
SD, Tamura H, Chapoval AI, Flies DB, Bajorath J and Chen L: B7-H4,
a molecule of the B7 family, negatively regulates T cell immunity.
Immunity. 18:849–861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prasad DVR, Richards S, Mai XM and Dong C:
B7S1, a novel B7 family member that negatively regulates T cell
activation. Immunity. 18:863–873. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng X, Li XD, Wu CP, Lu BF and Jiang JT:
Expression of costimulatory molecule B7-H4 in human malignant
tumors. Onkologie. 35:700–705. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Basta P, Galazka K, Mach P, Jozwicki W,
Walentowicz M and Wicherek L: The immunohistochemical analysis of
RCAS1, HLA-G, and B7H4-positive macrophages in partial and complete
hydatidiform mole in both applied therapeutic surgery and surgery
followed by chemotherapy. Am J Reprod Immunol. 65:164–172. 2011.
View Article : Google Scholar
|
|
10
|
Tian F, Li D, Chen J, Liu W, Cai L, Li J,
Jiang P, Liu Z, Zhao X, Guo F, et al: Aberrant expression of GATA
binding protein 6 correlates with poor prognosis and promotes
metastasis in cholangiocarcinoma. Eur J Cancer. 49:1771–1780. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He Q, Cai L, Shuai L, Li D, Wang C, Liu Y,
Li X, Li Z and Wang S: Ars2 is overexpressed in human
cholangiocarcinomas and its depletion increases PTEN and PDCD4 by
decreasing microRNA-21. Mol Carcinog. 52:286–296. 2013. View Article : Google Scholar
|
|
12
|
Li B, Wang Y, Chen J, Wu H and Chen W:
Identification of a new HLA-A*0201-restricted CD8+ T
cell epitope from hepatocellular carcinoma-associated antigen
HCA587. Clin Exp Immunol. 140:310–319. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao Y, Chen L, Wei W, Deng X, Ma L and Hao
S: Tumor cell-derived exosome-targeted dendritic cells stimulate
stronger CD8+ CTL responses and antitumor immunities.
Biochem Biophys Res Commun. 436:60–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mugler KC, Singh M, Tringler B, Torkko KC,
Liu W, Papkoff J and Shroyer KR: B7-h4 expression in a range of
breast pathology: Correlation with tumor T-cell infiltration. Appl
Immunohistochem Mol Morphol. 15:363–370. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smith JB, Stashwick C and Powell DJ Jr:
B7-H4 as a potential target for immunotherapy for gynecologic
cancers: A closer look. Gynecol Oncol. 134:181–189. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Wang T, Xu M, Xiao L, Luo Y, Huang
W, Zhang Y and Geng W: B7-H4 overexpression impairs the immune
response of T cells in human cervical carcinomas. Hum Immunol.
75:1203–1209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Drescher KM and Lynch HT: Tumor
infiltrating lymphocytes (TILs): Lessons learned in 30 years of
study. Clin Appl Immunol Rev. 5:149–166. 2005. View Article : Google Scholar
|
|
18
|
Plentz RR and Malek NP: Clinical
presentation, risk factors and staging systems of
cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 29:245–252.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nathan H, Pawlik TM, Wolfgang CL, Choti
MA, Cameron JL and Schulick RD: Trends in survival after surgery
for cholangiocarcinoma: A 30-year population-based SEER database
analysis. J Gastrointest Surg. 11:1488–1497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan
M, Shan BE, Lu BF and Zhang XG: B7-H4 expression associates with
cancer progression and predicts patient's survival in human
esophageal squamous cell carcinoma. Cancer Immunol Immunother.
60:1047–1055. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krambeck AE, Thompson RH, Dong H, Lohse
CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC and Kwon
ED: B7-H4 expression in renal cell carcinoma and tumor vasculature:
Associations with cancer progression and survival. Proc Natl Acad
Sci USA. 103:10391–10396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu J, Chu B-F, Yang Y-P, Zhang SL, Zhuang
M, Lu WJ and Liu YB: B7-H4 expression is associated with cancer
progression and predicts patient survival in human thyroid cancer.
Asian Pac J Cancer Prev. 14:3011–3015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu W, Shibata K, Koya Y, Kajiyama H,
Senga T, Yamashita M and Kikkawa F: B7-H4 overexpression correlates
with a poor prognosis for cervical cancer patients. Mol Clin Oncol.
2:219–225. 2014.PubMed/NCBI
|
|
24
|
Sun Y, Wang Y, Zhao J, Gu M, Giscombe R,
Lefvert AK and Wang X: B7-H3 and B7-H4 expression in non-small-cell
lung cancer. Lung Cancer. 53:143–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang J, Zhu Y, Wu C, Shen Y, Wei W, Chen
L, Zheng X, Sun J, Lu B and Zhang X: Tumor expression of B7-H4
predicts poor survival of patients suffering from gastric cancer.
Cancer Immunol Immunother. 59:1707–1714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tringler B, Liu W, Corral L, Torkko KC,
Enomoto T, Davidson S, Lucia MS, Heinz DE, Papkoff J and Shroyer
KR: B7-H4 overexpression in ovarian tumors. Gynecol Oncol.
100:44–52. 2006. View Article : Google Scholar
|
|
27
|
Dunn GP, Old LJ and Schreiber RD: The
immunobiology of cancer immunosurveillance and immunoediting.
Immunity. 21:137–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiou SH, Sheu BC, Chang WC, Huang SC and
Hong-Nerng H: Current concepts of tumor-infiltrating lymphocytes in
human malignancies. J Reprod Immunol. 67:35–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen C, Qu QX, Shen Y, Mu CY, Zhu YB,
Zhang XG and Huang JA: Induced expression of B7-H4 on the surface
of lung cancer cell by the tumor-associated macrophages: A
potential mechanism of immune escape. Cancer Lett. 317:99–105.
2012. View Article : Google Scholar
|
|
30
|
Wang X, Hao J, Metzger DL, Ao Z, Chen L,
Ou D, Verchere CB, Mui A and Warnock GL: B7-H4 treatment of T cells
inhibits ERK, JNK, p38, and AKT activation. PLoS One. 7:e282322012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Salceda S, Tang T, Kmet M, Munteanu A,
Ghosh M, Macina R, Liu W, Pilkington G and Papkoff J: The
immunomodulatory protein B7-H4 is overexpressed in breast and
ovarian cancers and promotes epithelial cell transformation. Exp
Cell Res. 306:128–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kryczek I, Wei S, Zhu G, Myers L, Mottram
P, Cheng P, Chen L, Coukos G and Zou W: Relationship between B7-H4,
regulatory T cells, and patient outcome in human ovarian carcinoma.
Cancer Res. 67:8900–8905. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh
CL, Yeh YC, Chou AH, Chang SR, Hsiao KN, Yu FW, et al: Induction of
a distinct CD8 Tnc17 subset by transforming growth factor-beta and
interleukin-6. J Leukoc Biol. 82:354–360. 2007. View Article : Google Scholar : PubMed/NCBI
|