Introduction
The results of cytogenetic studies indicate that
telomeres are specific and complex structures localized at the end
of the chromosome, without which the genome integrity could not be
preserved. Telomeres are highly conserved among all eukaryotic
chromosomes in humans and other vertebrates and consist of simple
tandem repeats, such as AGGGTT (1–3).
Telomere shortening initiates cellular transformation through the
induction of chromosomal instability (4). Telomerase is a ribonucleoprotein
enzyme that enables the synthesis of telomeric DNA in eukaryotes.
The human telomerase consists of human telomerase RNA (hTR),
telomerase reverse transcriptase (hTERT) and the accessory proteins
dyskerin, NOP10, NHP2 and GAR1 (5).
Telomerase activity (TA) level is positively associated with cell
proliferation in most cancer cell types and renewal of tissues
(6,7). Telomerase maintains telomere length
(TL) homeostasis and allows cancer cells to escape the
antiproliferative obstacles caused by short telomeres (8).
Esophageal carcinoma is the seventh most common
cancer and the fifth leading cause of cancer-related deaths in
developing countries. Esophageal squamous cell carcinoma (ESCC) and
adenocarcinoma are the two main types of esophageal cancer. In the
highest-risk region, which extends from Northern Iran through the
Central Asian to North-Central China, 90% of the cases are ESCC,
contrasting to only 26% in the US (9). Currently, surgery, radiotherapy and
chemotherapy remain the three mainstays in the treatment of ESCC,
and therapy is best achieved by a multidisciplinary approach.
Radiotherapy has played a critical role in the management of
unresectable esophageal carcinoma (10). Nevertheless, intrinsic
radioresistance accounts for the high recurrence and poor 5-year
survival of ESCC patients (11). As
in many other types of cancer, esophageal cancer cells have a
reduced TL, and evidence has demonstrated that telomerase
contributes to telomere maintenance, DNA repair, and the survival
of esophageal cancer cells (12,13).
This indicates that telomerase could be a target for the induction
of enhanced sensitivity of esophageal cancer cells to
radiation.
The common medications used in highly active
antiretroviral therapy (HAART) for AIDS, zidovudine, abacavir and
lamivudine, are frequently used in anti-AIDS treatment. In
addition, the embedding of virus in DNA and telomere lengthening
are both courses of reverse transcription. Short telomeres induce
hypersensitivity to ionizing radiation in mammals (14,15).
Zhou et al reported that zidovudine exerts a
radiosensitization effect on human malignant glioma cells by
inhibiting the activity of telomerase (16). To date, there are few reported
experiments providing evidence that lamivudine and abacavir could
be radiosensitizers of cancer cell lines. However, zidovudine,
lamivudine and abacavir significantly inhibited TA in activated
peripheral blood mononuclear cells in vitro (17). In the present study, we hypothesized
that zidovudine, abacavir and lamivudine could all be used as
radiosensitizers for ESCC cells.
In the present study, we investigated the action of
zidovudine, abacavir and lamivudine in ESCC cell lines, either used
as a single therapeutic agent or in combination with radiation, and
determined clonogenic survival fraction, DNA damage, TA, TL and
cell apoptosis. Through this process, we attempted to ellucidate
the mechanism for achieving elevated sensitivity to radiation.
Materials and methods
Cell culture and reagents
Human ESCC cell line Eca109 [China Center for Type
Culture Collection (CCTCC)] and Eca9706 [American Type Culture
Collection (ATCC), Manassas, VA, USA] were cultured in RPMI-1640
medium supplemented with 10% calf serum (both from Gibco Life
Technologies, Grand Island, NY, USA), and 100 U/ml penicillin G and
streptomycin. Cells were incubated in a humidified atmosphere
containing 5% CO2 at 37°C. Zidovudine, abacavir, and
lamivudine are all reference material [National Institutes for Food
and Drug Control (NIFDC) China].
Experimental grouping
Experimental grouping was carried out as folows: i)
phosphate-buffered saline (PBS) control group, without the
application of drugs and radiation; ii) radiation group, cells
exposed to radiation; iii) drug groups, treated respectively with
zidovudine (0.02 mM), abacavir (0.03 mM) and lamivudine (0.025 mM)
for 48 h; clonal efficiency assay was used for concentration
screening of zidovudine, abacavir and lamivudine, and
IC20 concentrations were selected for our assays; iv)
radiation and drug combination groups, exposure to radiation after
administration of the respective drug doses for 48 h.
Clonal efficiency assay
Eca109 and Eca9706 cells were seeded in 6-well
plates at various cell densities for the different radiation doses
(0, 2, 4, 6 and 8 Gy). A medical linear accelerator (Varian Clinac
23 EX; USA) was used to irradiate the cells for determination of
clonogenic cell survival. Cells were incubated with or without
zidovudine, abacavir and lamivudine for 48 h in the 37°C incubator.
After being irradiated at an average dose rate of 100 cGy/min, the
cells were incubated for 12 days, fixed with ethanol and stained by
crystal violet. Clonogenic cells were defined as those containing
at least 50 cells. The survival curves were obtained with GraphPad
Prism 5 to establish the mean lethal dose (D0), quasi-threshold
dose (Dq) and survival fractions at 2 Gy (SF2). The
radiosensitivity was quantified by the sensitization enhancement
ratio (SER).
DNA damage measurement
DNA damage was measured using OxiSelect™ comet assay
kit (Cell Biolabs Inc., San Diego, CA, USA). We conducted alkaline
electrophoresis to detect any single-stranded and double-stranded
DNA breaks (SSBs and DSBs) according to the kit instructions. Cell
samples were harvested and washed with PBS. Cell suspension was
mixed with molten agarose before being transfered to the OxiSelect™
comet slide, and then 75 µl of this mixture was added to the
slides. The embedded cells were treated with lysis buffer at 4°C
for 1 h which was then replaced with pre-chilled alkaline buffer
and placed at 4°C for 30 min. Finally, the samples were
electrophoresed in a horizontal chamber to separate intact DNA from
the damaged fractions. Following electrophoresis, the samples were
dried, stained with Vista Green DNA Dye provided in the kit, and
visualized by epifluorescence microscopy. At least 30 cells/slide
were examined, and DNA damage was analyzed using the Comet Assay
Software Project (CASP). To quantify the DNA damage, the tail
moment was calculated as the product of the migration of the DNA as
well as the relative amount of DNA in the tail.
DNA isolation
DNA isolation from the cell samples was performed
with QIAamp® DNA Mini and Blood Mini kit (Qiagen,
Duesseldorf, Germany) following the manufacturer's recommendations.
Cells were harvested by cell scraper and washed with PBS. After
resuspension in PBS to a final volume of 200 µl, 20
µl of proteinase K was added to each sample. Then, 200
µl buffer AL was applied to the samples, which were
afterwards incubated at 56°C for 10 min. Aliquts of 200 µl
ethanol were added to the samples, and the mixtures were transfered
to QIAamp Mini spin columns (in 2-ml collection tubes).
Furthermore, these tubes were centrifuged at 6,000 × g for 1 min,
the columns were placed in clean 2-ml collection tubes, and 500
µl of buffer AW1 was added to the columns. Next, the
procedure was repeated. Finally, 500 µl buffer AW2 was
admixed to the columns, and centrifugation was performed at 20,000
× g for 3 min. The columns were put in clean 1.5-ml microcentrifuge
tubes and were washed with distilled water. DNA concentration was
determined using a spectrophotometer (DeNovix, Wilmington, DE, USA)
and DNA samples were prepared for TL assessment.
Quantitative real-time RT-PCR
analysis
One hour after exposure to radiation of 2 Gy, TL of
all the samples was assessed using SYBR-Green Real-Time PCR Master
Mix (Toyobo, Osaka, Japan) by Bio-Rad Single Color Real-Time PCR
system (Bio-Rad, Hercules, CA, USA). The synthesized primer
sequences (Sangon Biotech, Shanghai, China) were as follows: Telo,
5′-GGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTT TGGGTT-3′ (forward primer) and
Telo, 5′-GGCTTGCCTTACCCTTACCCTTACCCTTACCCT-3′ (reverse primer);
36B4, 5′-CAGCAAGTGGGAAGGTGTAATCC-3′ (forward primer) and
5′-CCCATTCTATCATCAACGGGTACAA-3′ (reverse primer). Relative TL was
calculated by the 2−ΔΔct method.
The detection of TA was carried out 1 h later
followed by a single dose of irradiation of 2 Gy via the
TRAPeze® kit RT Telomerase Detection kit (Merck
Millipore, Darmstadt, Germany), as recommended by the manufacturer.
PCR amplification was run in Bio-Rad Single Color Real-Time PCR
system. Standard curves obtained through the TSR8 quantitation
control were included in the kit providing a technique for
quantitation of TA relative to TSR8 product amplification. The
linear equation derived from the data curve fit was employed to
extrapolate arbitrary telomerase units relative to the TSR8
amplification using the experimental sample average Ct values.
Flow cytometric analysis of
apoptosis
Eca109 and Eca9706 cells were plated and exposed to
zidovudine, abacavir and lamivudine for 48 h and then were
irradiated with a single dose of 6 Gy. Cells were collected 20 h
after radiation, washed with cold 1X PBS, and apoptosis analysis
was performed with the Annexin V-FITC apoptosis detection kit and
then analyzed by flow cytometry (both from BD Biosciences, San
Jose, CA, USA).
Statistical analysis
All data are presented as means ± SD of three
independent experiments and were analyzed using GraphPad Prism 5
software. Statistical analyses were performed using ANOVA with post
hoc Tukey's test. Differences with P-value <0.05 were considered
significant.
Results
Zidovudine, abacavir and lamivudine
radiosensitize ESCC cells
To examine the effect of zidovudine, abacavir, and
lamivudine on radiosensitization, clonogenic cell survival assays
were performed in the Eca109 and Eca9706 cancer cells. The results
of the clonal efficiency assay showed that treatment with
zidovudine, abacavir and lamivudine substantially enhanced the
radiation response in the Eca109 and Eca9706 cells (Fig. 1). We obtained D0, Dq, SF2 and
SERD0 by fitting the survival curves into the single-hit
multi-target model y = 1 − [1 − e(−kx)]N. The
characteristics of the survival curves of Eca109 and Eca9706 cells
are presented in Tables I and
II. The values of SF2 were 0.809
and 0.850 for Eca109 and Eca9706 cells. After the treatment with
zidovudine, abacavir and lamivudine, SF2 values were 0.682, 0.678
and 0.695 in the the Eca109 cells and 0.815, 0.810 and 0.794 in the
Eca9706 cells, respectively (P<0.05). The SERD0 data
revealed that Eca109 cells were more sensitive to the action of
these three drugs than Eca9706 cancer cells. These results
indicated that zidovudine, abacavir and lamivudine may be used as
radiosensitizers to improve the anticancer effect of
radiotherapy.
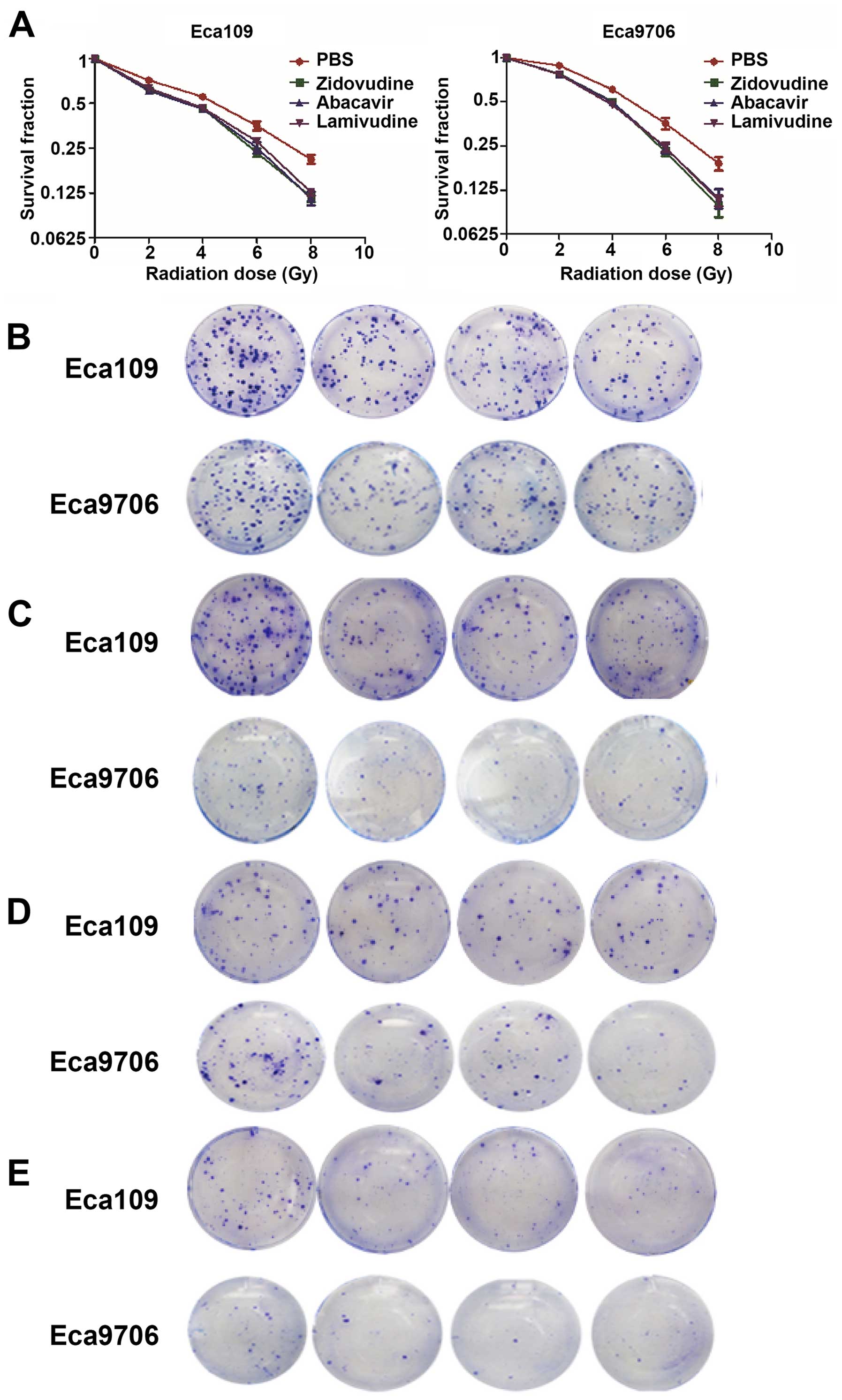 | Figure 1(A) Zidovudine, abacavir and
lamivudine radiosensitize ESCC cell lines. After treatment with
zidovudine, abacavir and lamivudine for 48 h, Eca109 and Eca9706
cells were irradiated at a single dose of 0, 2, 4, 6 or 8 Gy.
P<0.05 for each radiation dose in combination with drugs vs.
each radiation dose alone for both of the two cell lines. The
clonogenic ability of the Eca109 and Eca9706 cell lines irradiated
by a single dose of radiation was inhibited by zidovudine, abacavir
and lamivudine. Zidovudine, abacavir and lamivudine sensitized the
Eca109 and Eca9706 cancer cells to radiation, respectively, at the
doses of (B) 2, (C) 4, (D) 6 and (E) 8 Gy. |
 | Table IRadionsensitization effect of
zidovudine, abacavir and lamivudine on Eca109 cells. |
Table I
Radionsensitization effect of
zidovudine, abacavir and lamivudine on Eca109 cells.
| D0 | Dq | SF2 | SER |
|---|
| Radiation + PBS | 2.602±0.133 | 2.769±0.086 | 0.809±0.008 | |
| Radiation +
zidovudine | 2.168±0.085 | 1.945±0.088 | 0.682±0.010 | 1.200±0.013 |
| Radiation +
abacavir | 2.208±0.183 | 1.901±0.050 | 0.678±0.005 | 1.178±0.018 |
| Radiation +
lamivudine | 2.140±0.076 | 1.930±0.183 | 0.695±0.020 | 1.216±0.016 |
 | Table IIRadiosensitization effect of
zidovudine, abacavir and lamivudine on Eca9706 cells. |
Table II
Radiosensitization effect of
zidovudine, abacavir and lamivudine on Eca9706 cells.
| D0 | Dq | SF2 | SERD0 |
|---|
| Radiation + PBS | 3.582±0.133 | 2.882±0.086 | 0.850±0.008 | |
| Radiation +
zidovudine | 3.279±0.085 | 2.517±0.088 | 0.815±0.010 | 1.092±0.013 |
| Radiation +
abacavir | 3.374±0.183 | 2.441±0.050 | 0.810±0.005 | 1.063±0.018 |
| Radiation +
lamivudine | 3.194±0.076 | 2.325±0.183 | 0.794±0.020 | 1.121±0.016 |
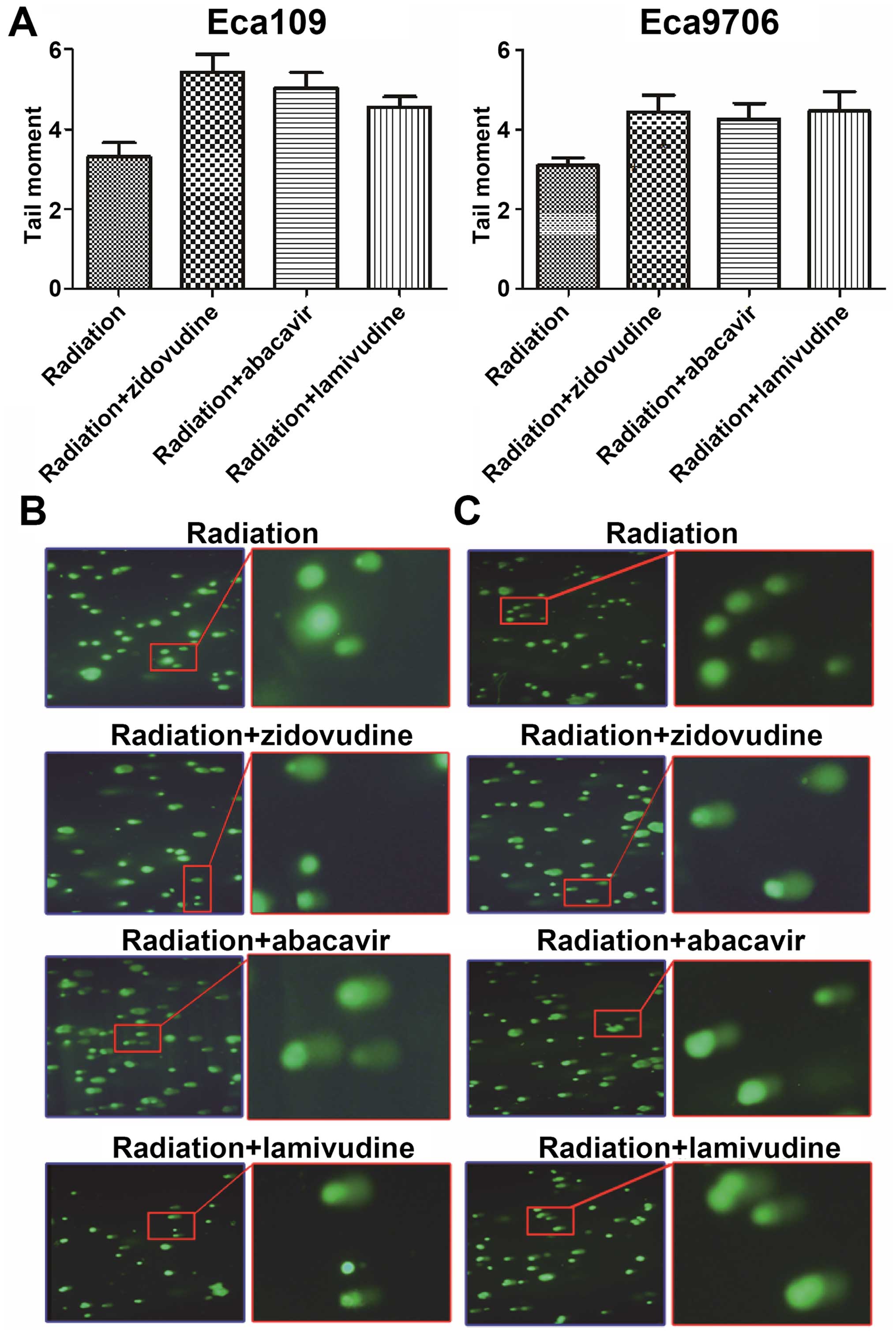
Increase in post-irradiation DNA damage
is induced by zidovudine, abacavir and lamivudine
The comet assay was used to measure DNA damage. The
values of tolomere maintainence (TM) were calculated by CASPLab
(Fig. 2). Higher values of TM were
obtained in the drug and radiation groups than these values in the
radiation-only group in both the Eca109 and Eca9706 cells
(P<0.05). However, there was no difference in the values of TM
following treatment with any two of the agents, zidovudine,
abacavir and lamivudine (P>0.05). This experiment demonstrated
that zidovudine, abacavir and lamivudine increased the DNA damage
of irradiated Eca109 and Eca9706 cancer cells.
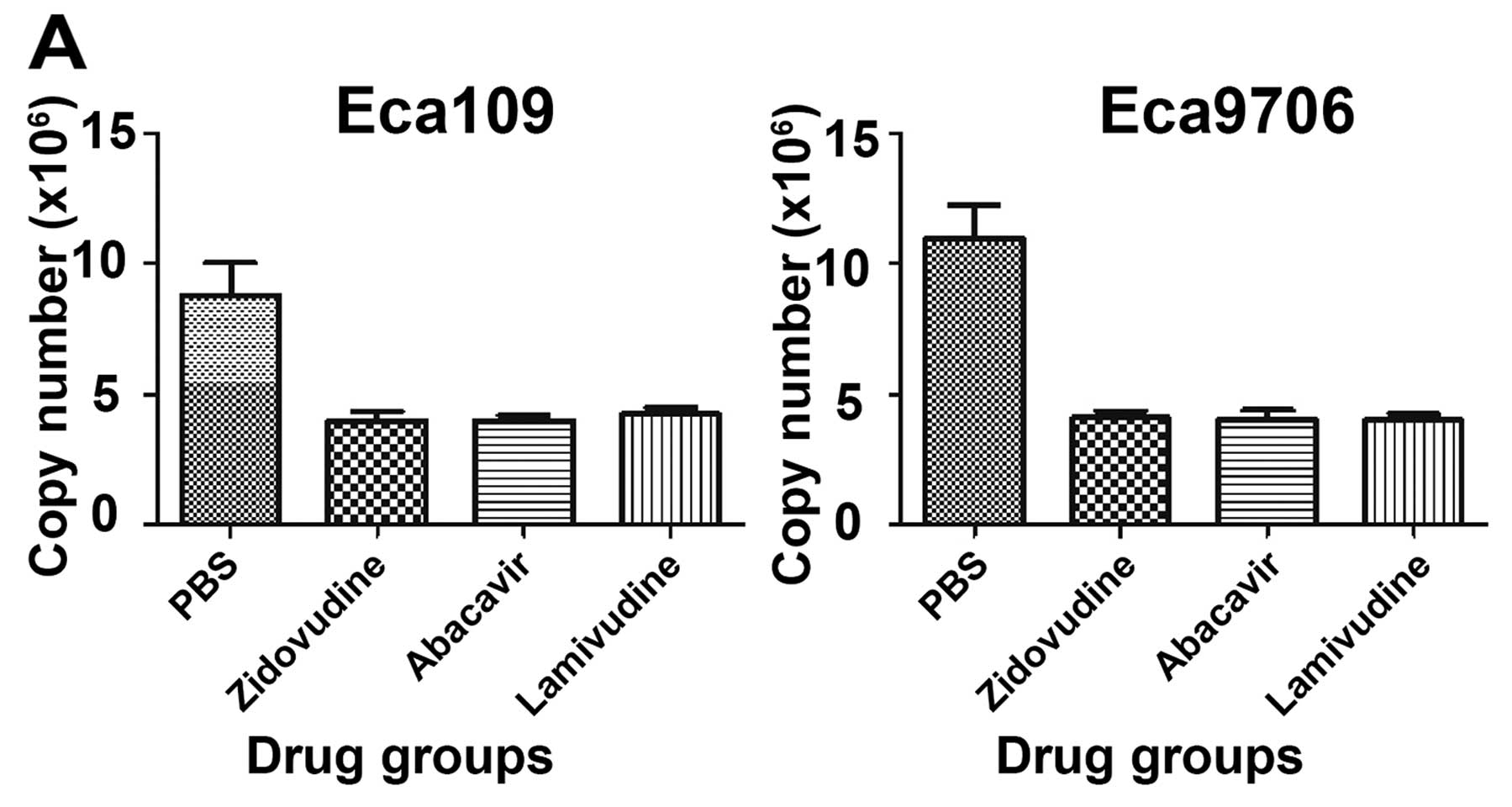
Inhibition of TA by zidovudine, abacavir
and lamivudine
TA from the cell extracts was evaluated by the
linear equation acquired from the curve fit using the amplification
data from different concentrations of TSR8. The copy number was
used to denote the TA of the experimental groups (Fig. 3). Compared to the PBS control group,
TA of the Eca109 and Eca9706 cells was upregulated by radiation but
was downregulated by zidovudine, abacavir and lamivudine. For the
drug groups, the ratios of TA inhibition were 54.8, 54.5 and 51.9%
in the Eca109 cells (P<0.05), and 62.5, 63.1 and 63.3% in the
Eca9706 cells (P<0.05). TA of the radiation-only group was
increased by 27.3 and 10.1% compared with that of the Eca109 and
Eca9706 cells in the PBS control group. The inhibition rates in the
drug and radiation groups were 55.8, 54.8 and 54.3% in Eca109 and
52.0, 54.7 and 53.3% in Eca9706 cancer cells (P<0.05). It was
obvious that TA of the Eca109 and Eca9706 cells was suppressed by
the action of zidovudine, abacavir and lamivudine regardless of
whether or not radiation was used.
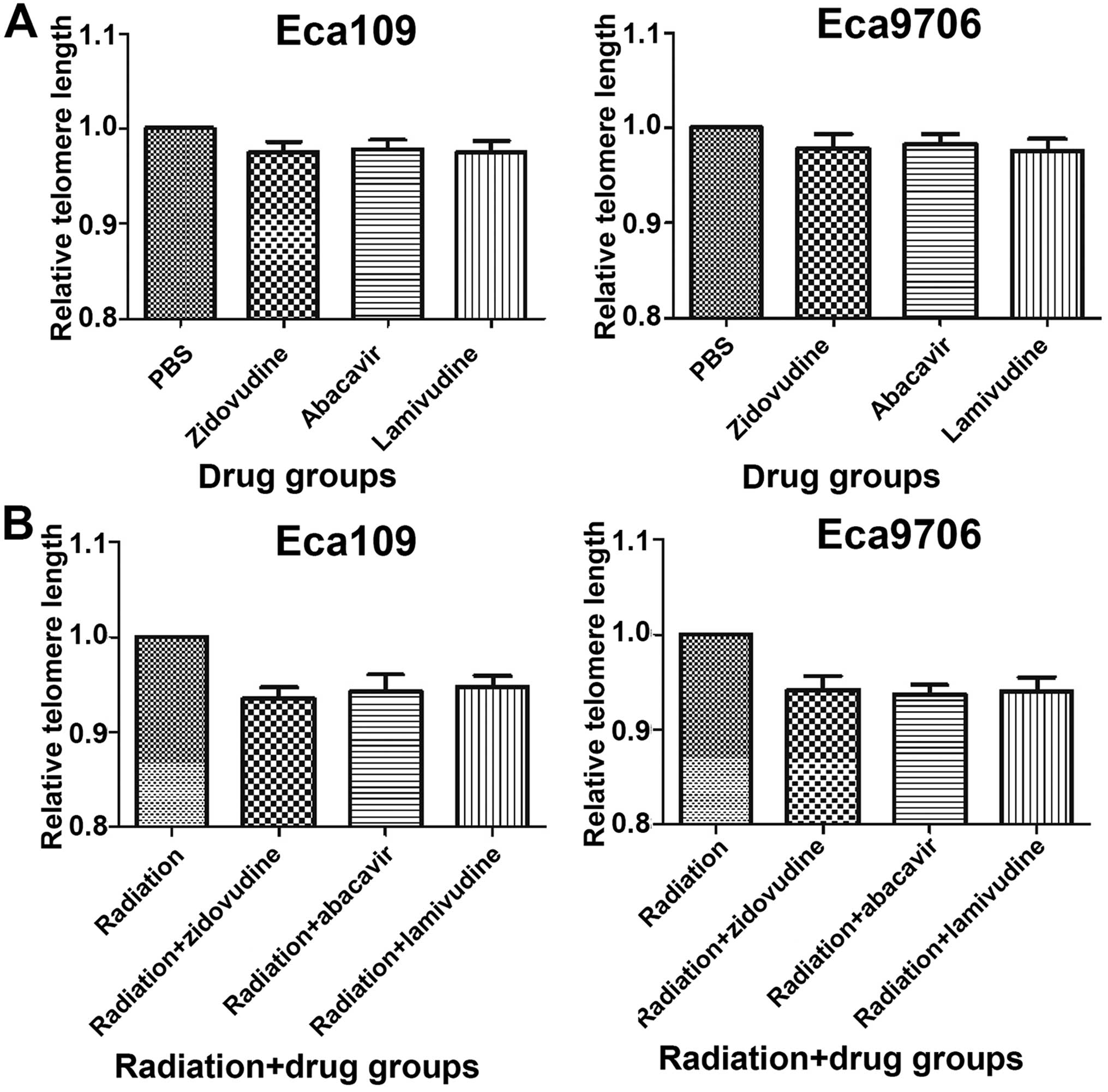
Shortening of TL is induced by the
administration of zidovudine, abacavir and lamivudine after
radiation
The relative TL was measured by real-time PCR
(Fig. 4). There was no difference
between the PBS and drug groups, which demonstrated that
zidovudine, abacavir and lamivudine did not inhibit telomere
lengthening of the Eca109 (P>0.05) and Eca9706 cells (P>0.05)
after the treatment with these drugs for 48 h. When combined with
radiation, the TL in the radiation group (P=0.224 in Eca109,
P=0.413 in Eca9706) and the radiation and drug groups (P<0.05
for the three drugs in Eca109 and Eca9706 cells) was shorter than
that in the PBS control group. Moreover, the radiation-only group
exhibited higher values of TL than did the radiation and drug
groups in the two cell lines, respectively (P<0.05). The results
indicated that radiation decreased TL length, and the application
of zidovudine, abacavir and lamivudine shortened TL
additionally.
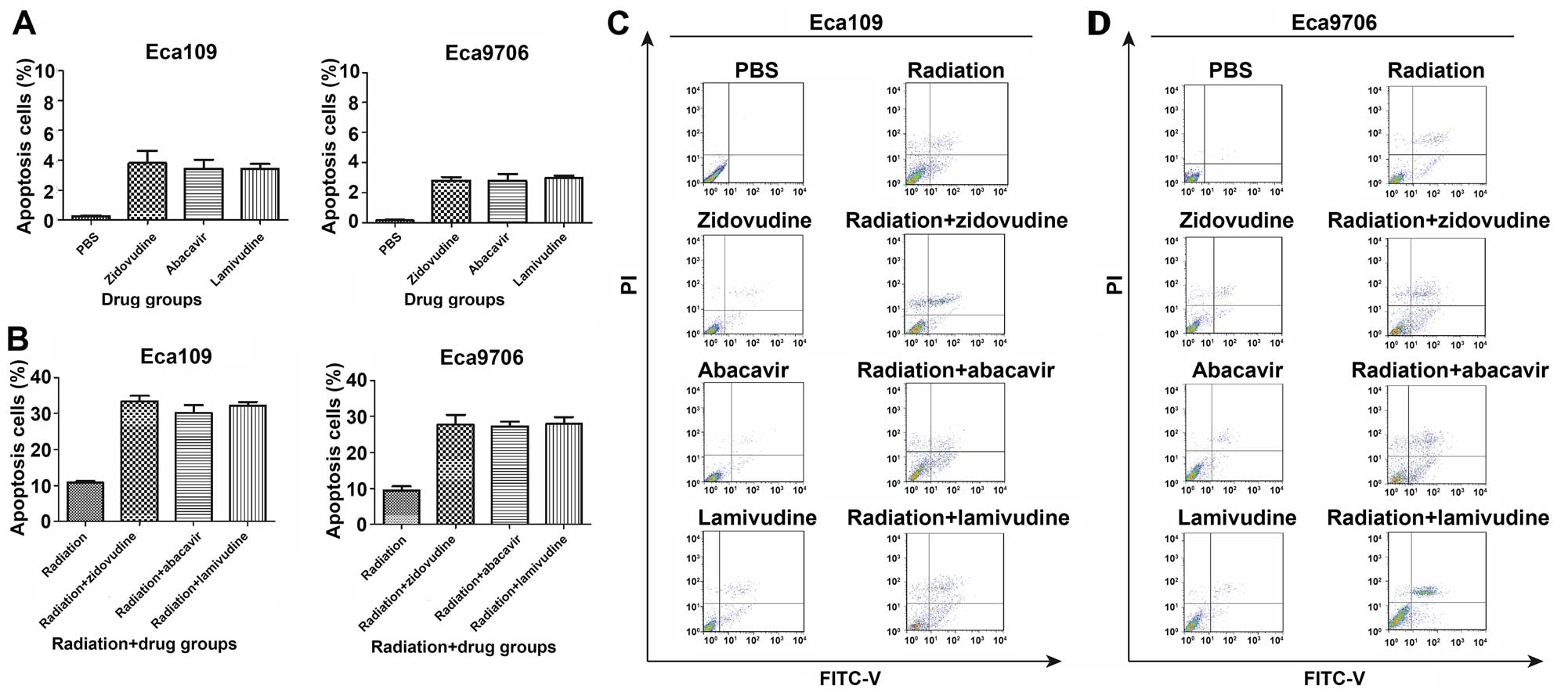
Zidovudine, abacavir and lamivudine
radiosensitize ESCC cells through induction of apoptosis
The apoptosis ratios were significantly higher in
the combination treatment groups than that in the radiation alone
or following the separate administration of zidovudine, abacavir
and lamivudine (Fig. 5). Radiation
induced cell apoptosis in 10.8% of Eca109 and in 9.5% of the
Eca9706 cells. The individual treatments with zidovudine, abacavir
and lamivudine in the Eca109 cells induced apoptosis rates of ~3.8,
3.4 and 3.4%, respectively, whereas these values for Eca9706 cells
were ~2.8, 2.7 and 3.0%. The combination of radiation and
zidovudine, abacavir and lamivudine caused an increase in the
apoptosis rates, and they reached values of 33.5, 30.2 and 32.2% in
the Eca109 cells, and 27.8, 27.2 and 27.9% in the Eca9706 cells,
correspondingly, which were significantly higher than those in the
radiation-only group (P<0.05). These results indicated that
zidovudine, abacavir and lamivudine sensitize Eca109 and Eca9706 to
radiation by stimulating cell apoptosis.
Discussion
Radiotherapy plays a critical role in the treatment
of esophageal cancer. Radioresistance has significantly decreased
the efficacy of radiotherapy for esophageal cancer therapy
(18). Our experimental results
indicated that the combined treatment with zidovudine, abacavir and
lamivudine significantly improved the efficacy of radiotherapy. We
first observed that in combination with radiation doses of 2, 4, 6
and 8 Gy, zidovudine, abacavir and lamivudine decreased the colony
forming efficiency of the Eca109 and Eca9706 cells. Consistent with
the results of the clonal efficiency assay, the comet assay
findings demonstrated that these drugs promoted DNA damage
(including SSBs and DSBs) of irradiated ESCC cells. Wong et
al reported that the radiosensitivity of the cells with
dysfunctional telomeres was related to chromosomal massive
fragmentation and aberrations (19). Moreover, irrespective of whether or
not radiation was applied, telomerase activity (TA) was
successfully inhibited by these drugs. Furthermore, the levels of
TA in the groups with only radiation and drug treatment were higher
than in those in the drug groups, confirming that the DNA damage
induced by radiation can increase TA, and that telomerase protects
DNA against double-stranded DNA (dsDNA)-damaging events (20,21).
However, in the absence of radiation, telomeres were not shortened
by the application of zidovudine, abacavir and lamivudine. This
result indicates that the inhibition of telomerase during a short
period of time (48 h) could not shorten telomeres significantly.
Notably, telomere length (TL) was reduced when radiation was used
in the treatments (combined with drugs or separately). This
phenomenon may be explained by the findings of a previous
investigation, in which the telomere was revealed as highly
sensitive to radiation (22–24).
Furthermore, apoptotic flow cytometric analysis demonstrated that
zidovudine, abacavir and lamivudine sensitized ESCC cells to
radiation by increasing apoptotic cell death. These results
suggested that these three drugs could sensitize ESCC cells to
radiotherapy via the possible mechanisms of increasing
radiation-induced DNA damage, deregulating TA, decreasing shortened
TL caused by radiation, and promoting the apoptosis of Eca109 and
Eca9706 cancer cells.
Currently, research on radiosensitizers has been
conducted through the mechanisms of hypoxia, topoisomerases,
micro-tubules and caspases. Numerous of these examinations have
performed well in pre-clinical trials, but information needs to be
provided on the safety and practicability of these approaches in
clinical trials (25). From another
point of view, DNA damage response is a determinant of cancer
outcome after radiotherapy (26),
and telomerase dysfunction in mice imparted a radiosensitivity
syndrome correlated with accelerated mortality (19). Consequently, a telomerase inhibitor
could act as a radiosensitizer for maximizing the benefits of
radiotherapy. It has been reported that curcumin could inhibit
telomerase, shorten telomeres in glioblastoma and medulloblastoma
cells, and enhance the radiation-induced inhibition of
neuroblastoma cell survival through suppression of TA (27,28).
Additionally, the telomerase inhibitor imetelstat was found to
exhibit similar effects on glioblastoma and esophageal cancer cells
(29,30). However, there is no sufficient
evidence to describe the adverse drug reactions and potency in
clinical trials.
New uses for an already approved drug could be a
strategy for shortening the drug approval period. The most frequent
drug-related adverse events of zidovudine were nausea, headache and
fatigue (31). The negative effects
of abacavir that have most often occurred are fatigue, nausea,
vomiting, abdominal pain, diarrhea, headache, rash and dyspepsia.
However, in a study, the most common treatment-limiting event was
hypersensitivity reaction (31). As
for lamivudine, increased serum transaminase activities,
neutropenia and hyperactivity were the most frequent side-effects
(31). All of these data were
collected in an anti-virus clinical environment. Whether the
adverse effects will be similar among cancer patients is uncertain.
In order to use more effectively zidovudine, abacavir and
lamivudine as radiosensitizers in clinical practice, we will carry
out further in vivo studies to investigate their
efficacy.
In conclusion, the present study demonstrated that
the combination of zidovudine, abacavir and lamivudine exhibited
pronounced effects on decreasing the colony formation ability and
stimulating DNA damage of ESCC cells through TL shortening, TA
reduction and promotion of apoptosis. Zidovudine, abacavir and
lamivudine may be valuable when used as radiosensitizers for cancer
radiotherapy.
Acknowledgments
The present study was performed through the support
of the National Natural Science Fund (approval no. 81572958).
References
|
1
|
Martínez P and Blasco MA: Replicating
through telomeres: A means to an end. Trends Biochem Sci.
40:504–515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blackburn EH: Structure and function of
telomeres. Nature. 350:569–573. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Londoño-Vallejo JA and Wellinger RJ:
Telomeres and telomerase dance to the rhythm of the cell cycle.
Trends Biochem Sci. 37:391–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Günes C and Rudolph KL: The role of
telomeres in stem cells and cancer. Cell. 152:390–393. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmidt JC and Cech TR: Human telomerase:
Biogenesis, trafficking, recruitment, and activation. Genes Dev.
29:1095–1105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shay JW and Bacchetti S: A survey of
telomerase activity in human cancer. Eur J Cancer. 33:787–791.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Artandi SE and DePinho RA: Telomeres and
telomerase in cancer. Carcinogenesis. 31:9–18. 2010. View Article : Google Scholar :
|
|
8
|
Xu L, Li S and Stohr BA: The role of
telomere biology in cancer. Annu Rev Pathol. 8:49–78. 2013.
View Article : Google Scholar
|
|
9
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang C, Chen X, Li L, Zhou Y, Wang C and
Hou S: The association between telomere length and cancer
prognosis: Evidence from a meta-analysis. PLoS One.
10:e01331742015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pal J, Gold JS, Munshi NC and Shammas MA:
Biology of telomeres: Importance in etiology of esophageal cancer
and as therapeutic target. Transl Res. 162:364–370. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goytisolo FA, Samper E, Martín-Caballero
J, Finnon P, Herrera E, Flores JM, Bouffler SD and Blasco MA: Short
telomeres result in organismal hypersensitivity to ionizing
radiation in mammals. J Exp Med. 192:1625–1636. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCaul JA, Gordon KE, Clark LJ and
Parkinson EK: Telomerase inhibition and the future management of
head-and-neck cancer. Lancet Oncol. 3:280–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou FX, Liao ZK, Dai J, Xiong J, Xie CH,
Luo ZG, Liu SQ and Zhou YF: Radiosensitization effect of zidovudine
on human malignant glioma cells. Biochem Biophys Res Commun.
354:351–356. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leeansyah E, Cameron PU, Solomon A,
Tennakoon S, Velayudham P, Gouillou M, Spelman T, Hearps A, Fairley
C, Smit V, et al: Inhibition of telomerase activity by human
immunodeficiency virus (HIV) nucleos(t)ide reverse transcriptase
inhibitors: A potential factor contributing to HIV-associated
accelerated aging. J Infect Dis. 207:1157–1165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shridhar R, Almhanna K, Meredith KL,
Biagioli MC, Chuong MD, Cruz A and Hoffe SE: Radiation therapy and
esophageal cancer. Cancer Control. 20:97–110. 2013.PubMed/NCBI
|
|
19
|
Wong KK, Chang S, Weiler SR, Ganesan S,
Chaudhuri J, Zhu C, Artandi SE, Rudolph KL, Gottlieb GJ, Chin L, et
al: Telomere dysfunction impairs DNA repair and enhances
sensitivity to ionizing radiation. Nat Genet. 26:85–88. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akiyama M, Ozaki K, Kawano T, Yamada O,
Kawauchi K, Ida H and Yamada H: Telomerase activation as a repair
response to radiation-induced DNA damage in Y79 retinoblastoma
cells. Cancer Lett. 340:82–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fleisig HB, Hukezalie KR, Thompson CA,
Au-Yeung TT, Ludlow AT, Zhao CR and Wong JM: Telomerase reverse
transcriptase expression protects transformed human cells against
DNA-damaging agents, and increases tolerance to chromosomal
instability. Oncogene. 35:218–227. 2016. View Article : Google Scholar
|
|
22
|
Zschenker O, Kulkarni A, Miller D,
Reynolds GE, Granger-Locatelli M, Pottier G, Sabatier L and Murnane
JP: Increased sensitivity of subtelomeric regions to DNA
double-strand breaks in a human cancer cell line. DNA Repair.
8:886–900. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bouffler SD, Blasco MA, Cox R and Smith
PJ: Telomeric sequences, radiation sensitivity and genomic
instability. Int J Radiat Biol. 77:995–1005. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mirjolet C, Boidot R, Saliques S,
Ghiringhelli F, Maingon P and Créhange G: The role of telomeres in
predicting individual radiosensitivity of patients with cancer in
the era of personalized radiotherapy. Cancer Treat Rev. 41:354–360.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Sun C, Mao A, Zhang X, Zhou X,
Wang Z and Zhang H: Radiosensitization to X-ray radiation by
telomerase inhibitor MST-312 in human hepatoma HepG2 cells. Life
Sci. 123:43–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goldstein M and Kastan MB: The DNA damage
response: Implications for tumor responses to radiation and
chemotherapy. Annu Rev Med. 66:129–143. 2015. View Article : Google Scholar
|
|
27
|
Khaw AK and Hande MP, Kalthur G and Hande
MP: Curcumin inhibits telomerase and induces telomere shortening
and apoptosis in brain tumour cells. J Cell Biochem. 114:1257–1270.
2013. View Article : Google Scholar
|
|
28
|
Aravindan N, Veeraraghavan J,
Madhusoodhanan R, Herman TS and Natarajan M: Curcumin regulates
low-linear energy transfer γ-radiation-induced NFκB-dependent
telomerase activity in human neuroblastoma cells. Int J Radiat
Oncol Biol Phys. 79:1206–1215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ferrandon S, Malleval C, El Hamdani B,
Battiston-Montagne P, Bolbos R, Langlois JB, Manas P, Gryaznov SM,
Alphonse G, Honnorat J, et al: Telomerase inhibition improves tumor
response to radiotherapy in a murine orthotopic model of human
glioblastoma. Mol Cancer. 14:1342015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu X, Smavadati S, Nordfjäll K, Karlsson
K, Qvarnström F, Simonsson M, Bergqvist M, Gryaznov S, Ekman S and
Paulsson-Karlsson Y: Telomerase antagonist imetelstat inhibits
esophageal cancer cell growth and increases radiation-induced DNA
breaks. Biochim Biophys Acta. 1823:2130–2135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aronson JK and Dukes MNG: Meyler's side
effects of drugs: The international encyclopedia of adverse drug
reactions and interactions. Elsevier; Amsterdam, Boston: 2006
|