Introduction
Malignant melanoma, a neoplasm of melanocytic
origin, is the most invasive and deadly form of skin cancer.
Although it comprises less than 5% of all dermatologic cancer
cases, melanoma is responsible for the great majority of skin
cancer-related deaths. During the past 10 years, the incidence and
annual mortality of melanoma has increased more rapidly than any
other type of cancer (1,2). Various environmental and genetic
factors including ultraviolet (UV) light and oncogenic BRAF
mutations, respectively, have been reported to affect the
development and progression of melanoma (3,4).
Treatment for melanoma mainly depends on the properties of the
tumor and the time of diagnosis. If recognized in earlier stages,
the tumor may be removed surgically. However, in the metastatic
stage, melanoma becomes highly resistant to conventional therapies
and nodal metastasis is associated with a poor prognosis for
patients with advanced melanoma. Despite the recent FDA approvals
of six drugs including three immunotherapies and three targeted
therapies for the treatment of melanoma, the poor clinical
prognosis and the lack of curative therapies for patients with
advanced melanoma necessitate the development of new successful
therapies (5,6).
Microphthalmia-associated transcription factor
(MITF), a master regulator of melanocyte development and
differentiation, is associated with melanoma development and
progression (7). MITF
transcriptionally activates a number of genes that play
pro-survival roles in melanoma such as cyclin dependent kinase 2
(CDK2), hypoxia-inducible factor 1α (HIF1α) and
c-MET (8–10). The transcription and
post-translational modifications of MITF gene are strongly
influenced by multiple upstream pathways, including c-Kit,
Wnt/β-catenin and α-melanocyte stimulating hormone (α-MSH)
(11–13). Activation of melanocortin 1 receptor
(MC1R) by binding of α-MSH induces cAMP production via activation
of adenylyl cyclase (AC) and phosphorylates cAMP response
element-binding protein (CREB). Phosphorylated CREB directly binds
to the MITF promoter region and stimulates MITF
transcription (14). Recent studies
have shown that α-MSH/cAMP pathway upregulates c-MET and HIF1α
expression, through MITF that binds and activates their promoters,
thereby contributing to melanoma progression (9,15). The
oncogenic potential of MITF suggests that MITF inhibition may be an
attractive therapeutic approach in malignant melanoma.
7,8-Dihydroxyflavone (7,8-DHF) is a monophenolic
flavone with diverse biological effects (Fig. 1). It has been found to act as a
small molecule agonist of tyrosine kinase receptor B (TrkB) that
has neurotrophic effects in various neurological diseases (16). 7,8-DHF also possesses potent
antioxidant activity independent of its actions on TrkB, and thus
protects against glutamate-induced excitotoxicity,
6-hydroxydopamine-induced dopaminergic neurotoxicity and oxidative
stress-induced genotoxicity (17–19).
In addition, the anticancer effects of 7,8-DHF in human monocytic
leukemia and oral squamous cancer cells have been reported
(20,21). However, its effect in melanoma has
not been explored. In the present study, first we assessed the
chemotherapeutic potential and underlying mechanisms of action of
7,8-DHF on malignant melanoma cells. Our results demonstrated that
7,8-DHF could efficiently suppress proliferation, survival,
migration, invasion, and differentiation of highly metastatic
melanoma cell line B16F10 via blocking α-MSH/cAMP/MITF pathway. In
addition, we suggest that 7,8-DHF might be applied in combination
therapy with resveratrol, a known therapeutic agent against
melanoma.
Materials and methods
Materials
7,8-Dihydroxyflavone (7,8-DHF) was purchased from
Tokyo Chemical Industry (Tokyo, Japan). Alpha-melanocyte
stimulating hormone (α-MSH), Matrigel®, and
Transwell® chamber systems were obtained from
Sigma-Aldrich (St. Louis, MO, USA), BD Biosciences (San Jose, CA,
USA) and Corning Costar (Acton, MA, USA), respectively.
L-3,4-dihydroxyphenylalanin (L-DOPA) was obtained from Acros
Organics (Geel, Belgium). Anti-MITF, anti-HIF-1α, and anti-VEGF
antibodies were purchased from Abcam (Cambridge, MA, USA), BD
Bioscience (Bedford, MA, USA) and Santa Cruz Biotechnology (Santa
Cruz, CA, USA), respectively. Anti-phospho-cMET, anti-c-MET,
anti-phospho-AKT, anti-AKT, anti-phospho-ERK1/2, anti-ERK1/2, and
anti-β-actin antibodies were obtained from Cell Signaling
Technology (Danvers, MA, USA).
Cell culture and cell growth assay
B16F10 melanoma cells were grown in Dulbecco's
modified Eagle's medium (Invitrogen, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (FBS; Invitrogen). Cells
were maintained at 37°C in a humidified 5% CO2
incubator. For cell growth assay, B16F10 cells were plated at
2×103 cells/well in 96-well culture plates (SPL Life
Sciences Co., Ltd., Gyeonggi, Korea). 7,8-DHF (0–100 μM) was
added to each well in the presence of α-MSH (10 nM) and the cells
were incubated for 72 h. Cell growth was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay.
Cell viability assay
Cell viability assay was performed using the trypan
blue exclusion method. B16F10 cells were seeded at
1.5×104 cells/well in 24-well culture plates (SPL Life
Sciences). 7,8-DHF (0–40 μM) was added to each well in the
presence of α-MSH (10 nM) and the cells were incubated for up to 72
h. After 72 h, the cells were stained with trypan blue and counted
using a hemocytometer.
Colony formation assay
B16F10 cells were seeded at 5×102
cells/well in 6-well culture plates (SPL Life Sciences). 7,8-DHF
was treated to each well in the presence of α-MSH (10 nM) and the
cells were incubated for 10 days until colonies were formed. Cells
were fixed with 4% formaldehyde, stained with 0.25% crystal violet
for 10 min and washed with double-distilled water.
Wound healing assay
The confluent monolayer B16F10 cells were scratched
using a tip and each well was washed with PBS to remove
non-adherent cells. The cells were treated with various
concentrations of 7,8-DHF in the presence of α-MSH (10 nM) and then
incubated for up to 48 h. The perimeter of the area with a central
cell-free zone was confirmed under an optical microscope (Olympus,
Center Valley, PA, USA).
Chemoinvasion assay
Cell invasion was assayed using a
Transwell® chamber system with polycarbonate filter
inserts with a pore size of 8.0 μm. The lower side of the
filter was coated with 10 μl gelatin (1 mg/ml) and the upper
side was coated with 10 μl Matrigel® (3 mg/ml).
B16F10 cells (1×105) were placed in the upper chamber of
the filter and 7,8-DHF was added to the lower chamber in the
presence of α-MSH (10 nM) and 0.5% BSA. The chamber was incubated
at 37°C for 24 h, and the cells were subsequently fixed with
methanol and stained with hematoxylin and eosin (H&E). The
total number of cells that invaded the lower chamber of the filter
was counted using an optical microscope (Olympus).
Measurement of melanin content
B16F10 cells (15×104 cells/well) were
plated in 12-well culture plates (SPL Life Sciences) and then
treated with various concentrations of 7,8-DHF in the presence or
absence of α-MSH (10 nM) for 72 h. The cells were then washed with
PBS and lysed in 150 μl of 1 M NaOH at 95°C. A total of 100
μl of the lysate was added in 96-well microplate and the
absorbance was measured at 405 nm using a microplate
spectrophotometer (Thermo Scientific Multiskan®
Spectrum).
Determination of cellular tyrosinase
activity
Tyrosinase activity was estimated by measuring the
rate of L-DOPA oxidation. B16F10 cells were plated in 12-well
culture plates at a density of 15×104 cells/well and
then treated with various concentrations of 7,8-DHF in the presence
or absence of α-MSH (10 nM) for 72 h. The cells were washed with
PBS and lysed in 50 mM phosphate buffer (pH 6.8) containing 1%
Triton X-100 and 0.1 mM phenylmethylsulfonyl fluoride. Cellular
lysates were centrifuged at 12,000 rpm for 20 min at 4°C. The
supernatant was collected and the protein content was determined by
the Bradford method. The cellular extract was incubated with L-DOPA
(1.25 mM) in 25 mM phosphate buffer (pH 6.8) and the absorbance at
475 nm was read until the reaction has finished.
Measurement of cAMP levels
B16F10 cells were treated with various
concentrations of 7,8-DHF in the presence or absence of α-MSH (10
nM) and incubated for 15 min. The cells were lysed in 0.1 M HCl,
and intracellular cAMP generation was determined using
enzyme-linked immunosorbent assay (ELISA) with a cAMP direct
immunoassay kit (Abcam, Cambridge, MA, USA) according to the
manufacturer's instructions. The cAMP levels were normalized to
total protein content.
Western blot analysis
Cell lysates were separated by 10% SDS-PAGE
electrophoresis and the separated proteins were transferred to
polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA)
using standard electroblotting procedures. The blots were blocked
and immunolabeled with primary antibodies against MITF, HIF-1α,
VEGF, phospho-cMET, c-MET, phospho-AKT, AKT, phospho-ERK1/2, ERK1/2
and β-actin overnight at 4°C. Immunolabeling was detected with an
enhanced chemiluminescence (ECL) kit (Bio-Rad Laboratories,
Hercules, CA, USA), according to the manufacturer's
instructions.
Statistical analysis
The results are expressed as the mean ± standard
error (SE). Student's t-test was used to determine statistical
significance between the control and the test groups. A P-value of
<0.05 was considered to indicate a statistically significant
result.
Results
Inhibition of cell growth and clonogenic
survival by 7,8-DHF in B16F10 cells
α-MSH has been reported to elicit a variety of
biological responses that bring about melanoma development as well
as melanocyte differentiation (22–24).
We thus assessed the anticancer activities of 7,8-DHF against
malignant melanoma in α-MSH-stimulated B16F10 cells. To determine
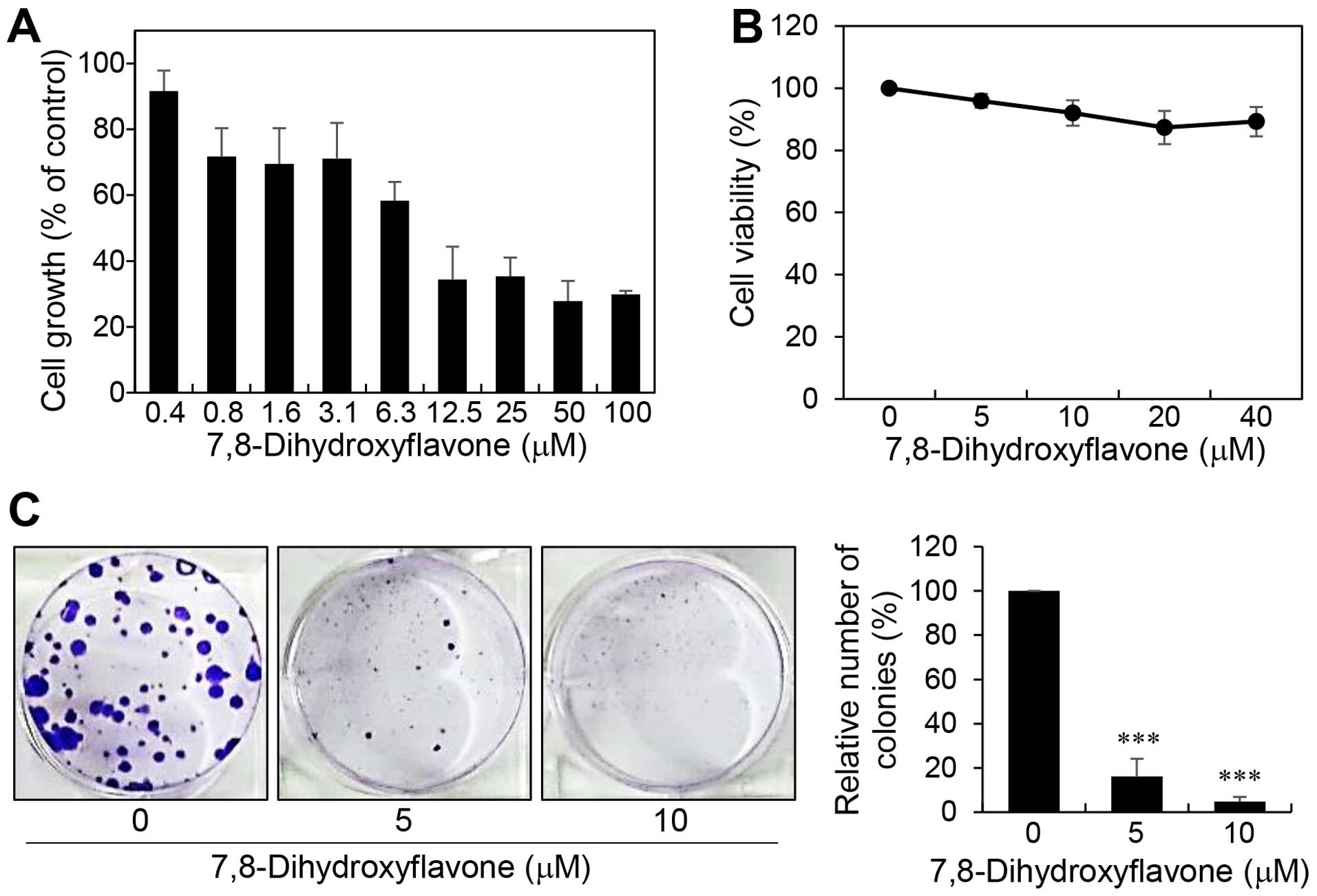
whether 7,8-DHF affects melanoma cell growth, B16F10 cells were
treated with various concentrations of 7,8-DHF for 72 h and the MTT
assay was performed. 7,8-DHF dose-dependently inhibited the
proliferation of B16F10 with an IC50 value of 9.04
μM (Fig. 2A). Notably, a
trypan blue viability test revealed that 7,8-DHF treatment did not
show cytotoxic effects up to 40 μM (Fig. 2B). These data indicate that the
inhibition of melanoma cell growth by 7,8-DHF resulted from a
cytostatic effect. To further evaluate the tumor suppressive effect
of 7,8-DHF, we carried out clonogenic assay, which is an in
vitro cell survival assay based on the ability of a single cell
to grow into a colony. Treatment of B16F10 cells with 7,8-DHF
resulted in a remarkable inhibition of the colony-forming ability
(Fig. 2C). Taken together, these
results suggest that 7,8-DHF may be considered a novel anticancer
agent with potent antiproliferative activity against melanoma
cells.
The inhibitory effects of 7,8-DHF on
B16F10 cell migration and invasion
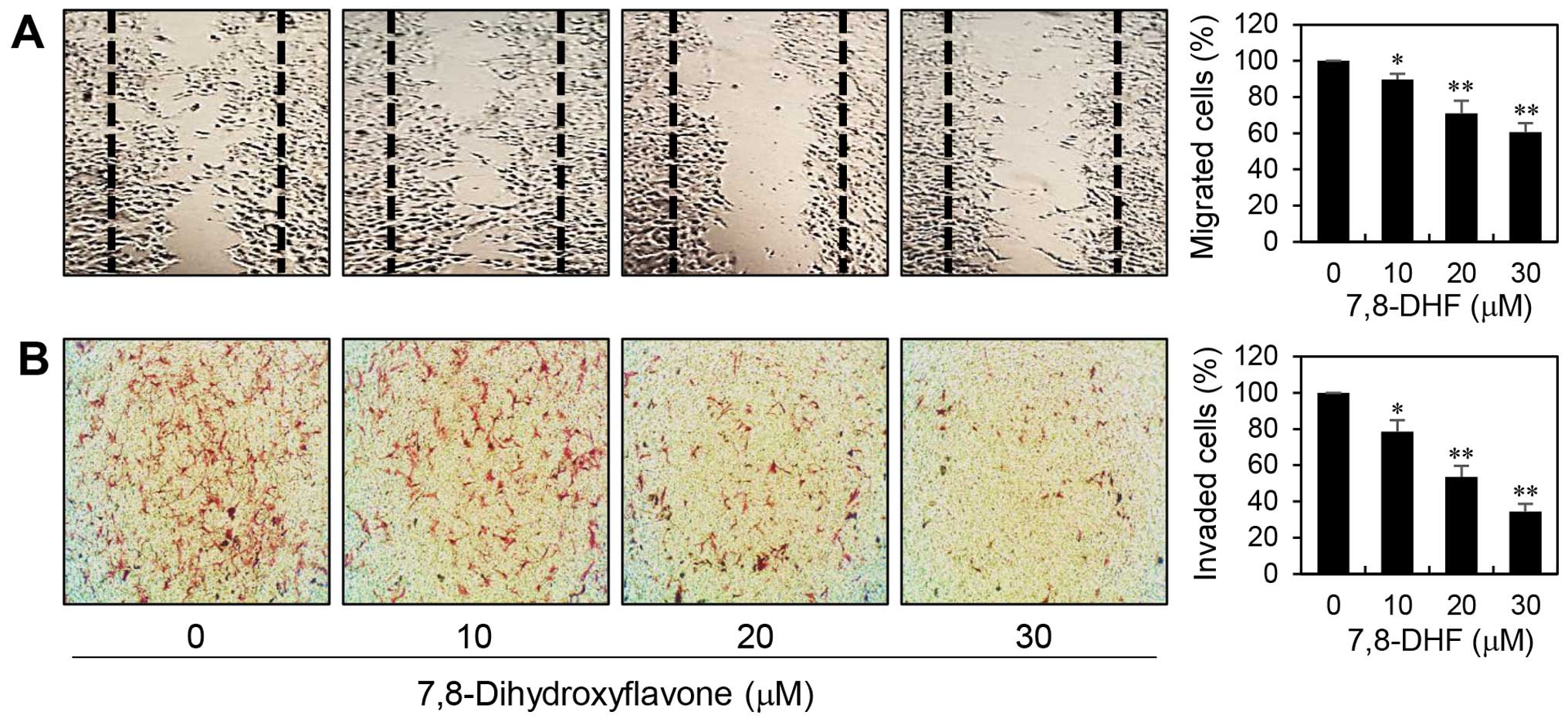
The most dangerous aspect of melanoma is its ability
to spread to other parts of the body. To assess the inhibitory
potential of 7,8-DHF on the metastatic ability of melanoma cells,
we first confirmed its effect on migration of α-MSH-stimulated
B16F10 cells using wound healing assay. As shown in Fig. 3A, relative to untreated control
cells, treatment with 7,8-DHF for 48 h reduced the migration
capacity of B16F10 cells in a dose-dependent manner. Next, the
effect of 7,8-DHF on invasive potential of α-MSH-treated B16F10
cells was investigated by employing the Matrigel matrix-coated
Transwell chamber assay. As shown in Fig. 3B, 7,8-DHF treatment significantly decreased
the invasiveness of B16F10 cells. These data demonstrate that
7,8-DHF has the chemotherapeutic potential to suppress melanoma
metastasis.
Antimelanogenic effect of 7,8-DHF in
B16F10 cells
Malignant melanocytes tend to exhibit upregulated
melanogenesis and defective melanosomes (25). Cellular tyrosinase activity is the
major factor that stimulates melanin synthesis and ultimately
induces melanogenesis (26). We
thus determined the cellular tyrosinase activity and melanin
content in order to investigate the effect of 7,8-DHF on
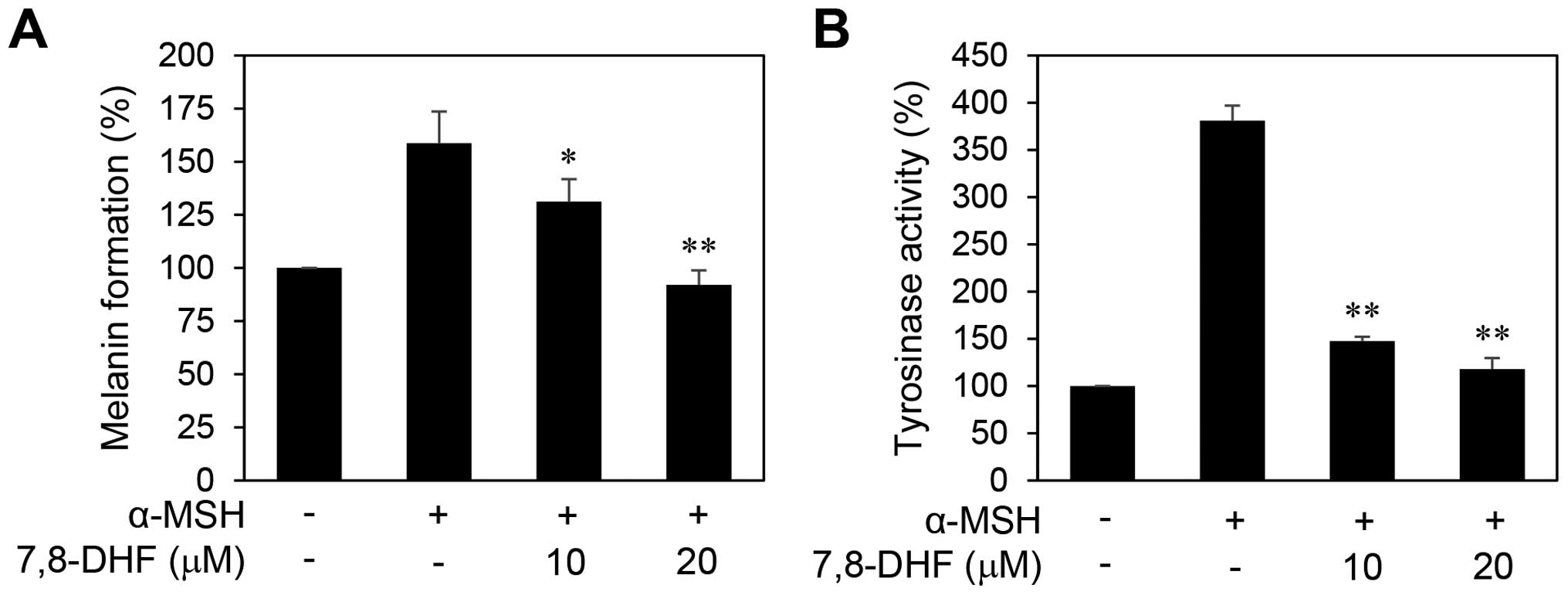
melanogenesis of B16F10 cells. The cells were stimulated by α-MSH
in the presence or absence of 7,8-DHF for 72 h. As shown in
Fig. 4, treatment with 7,8-DHF
clearly blocked the melanin formation and tyrosinase activity of
B16F10 cells induced by α-MSH, indicating that 7,8-DHF
downregulates the differentiation of melanoma cells associated with
melanogenesis.
Downregulation of MITF and its downstream
transcription targets by 7,8-DHF
MITF is not only a key player in melanocyte
development but is thought to also function as an oncogene in
melanoma (7). α-MSH can increase
intracellular cAMP levels and consequently activate MITF expression
(14). To elucidate the anticancer
mechanisms of 7,8-DHF against melanoma, we investigated the effect
of 7,8-DHF on α-MSH/cAMP/MITF signaling network in melanoma cells.
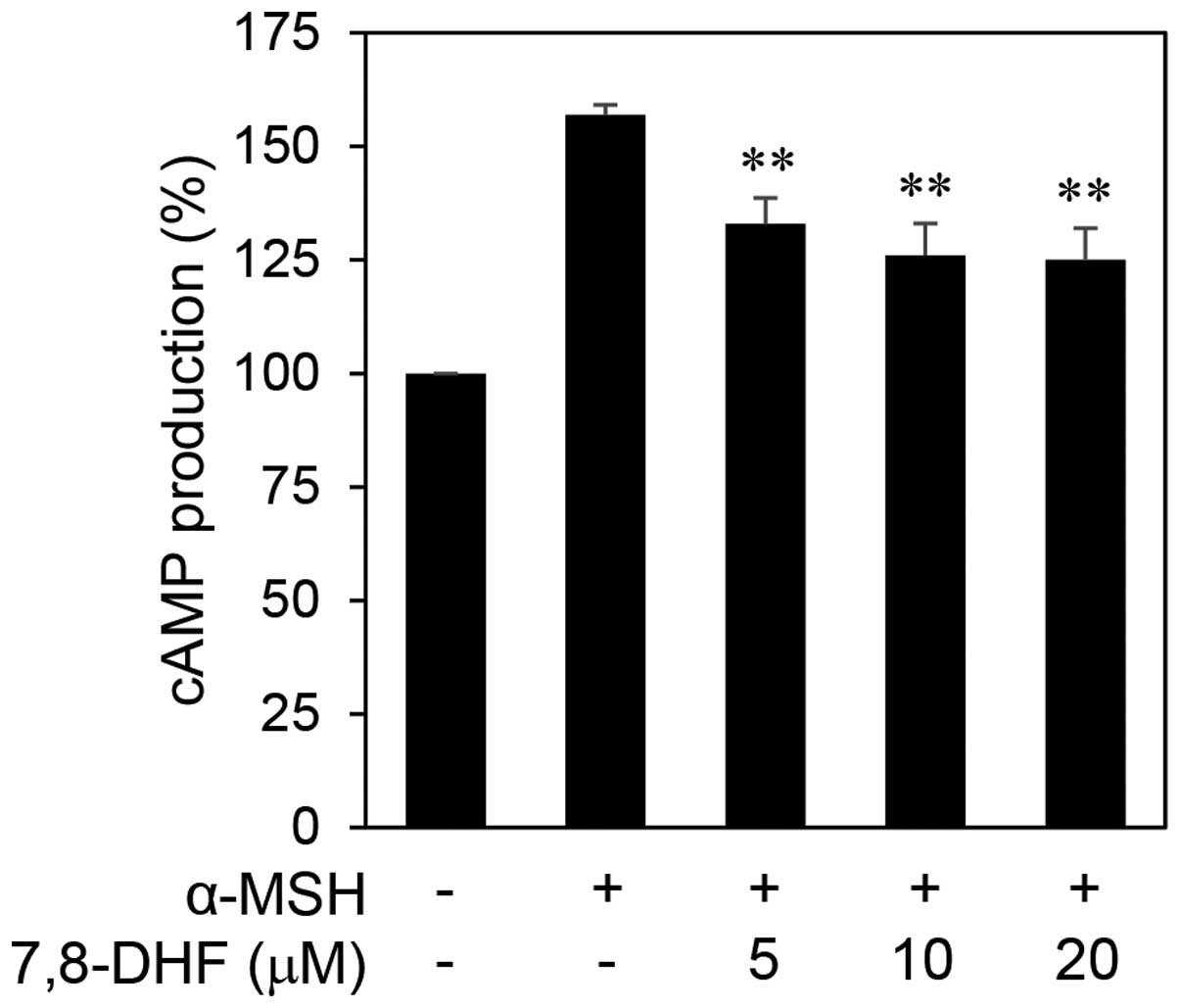
As shown in Fig. 5, treatment with
7,8-DHF significantly reduced the intracellular cAMP levels in
B16F10 cells stimulated by α-MSH. We next examined the effect of
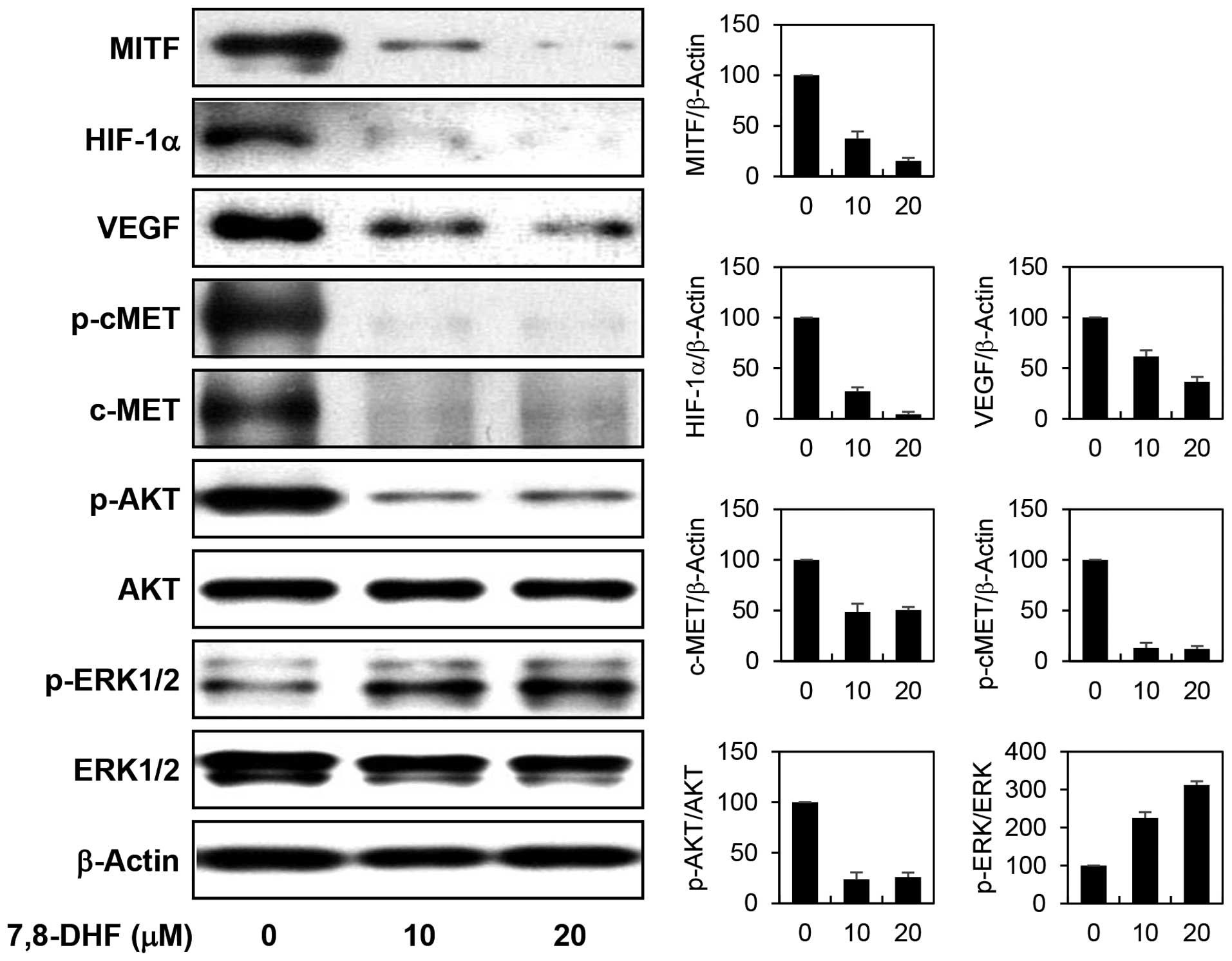
7,8-DHF on the expression of MITF and its downstream transcription
targets. 7,8-DHF markedly decreased the protein levels of MITF in
α-MSH-stimulated B16F10 cells (Fig.
6). In addition, the expression of HIF1α and c-MET, the major
transcriptional targets of MITF which have been implicated in
antiapoptotic and metastatic potential in melanoma, was prominently
downregulated by 7,8-DHF treatment. Furthermore, the inhibition of
HIF1α resulted in a remarkable reduction in expression of VEGF, its
transcriptional target that is required for tumor angiogenesis. In
addition, the diminished c-MET levels were also linked to the
blockade of c-MET signaling such as inhibiting phosphorylation of
c-MET and its downstream effector AKT. However, 7,8-DHF treatment
rather increased the phosphorylation of extracellular
signal-regulated kinases (ERK1/2), another c-MET downstream
effector. Recent studies have revealed that activation of ERK
induces phosphorylation and degradation of MITF and consequently
downregulates α-MSH-induced melanogenesis by inhibiting tyrosinase
activity and expression (27,28).
Accordingly, 7,8-DHF-induced activation of ERK1/2 may partly
account for the antimelanogenic effect of the compound. These
results collectively suggest that 7,8-DHF may exhibit the potential
anticancer activity against melanoma through the downregulation of
cAMP levels and subsequent suppression of the expression of MITF
and its key oncogenic target genes such as HIF1α and c-MET.
Enhanced anticancer effect of combination
treatment with 7,8-DHF and resveratrol
Resveratrol is a naturally-occurring compound that
possesses anticancer capabilities (29). A recent study demonstrated that
resveratrol inhibits MITF expression, viability, and invasiveness
activated by α-MSH in melanoma cells (30). To further exploit promising
combination therapies of 7,8-DHF, the anticancer effects of 7,8-DHF
and resveratrol either alone or in combination were examined in
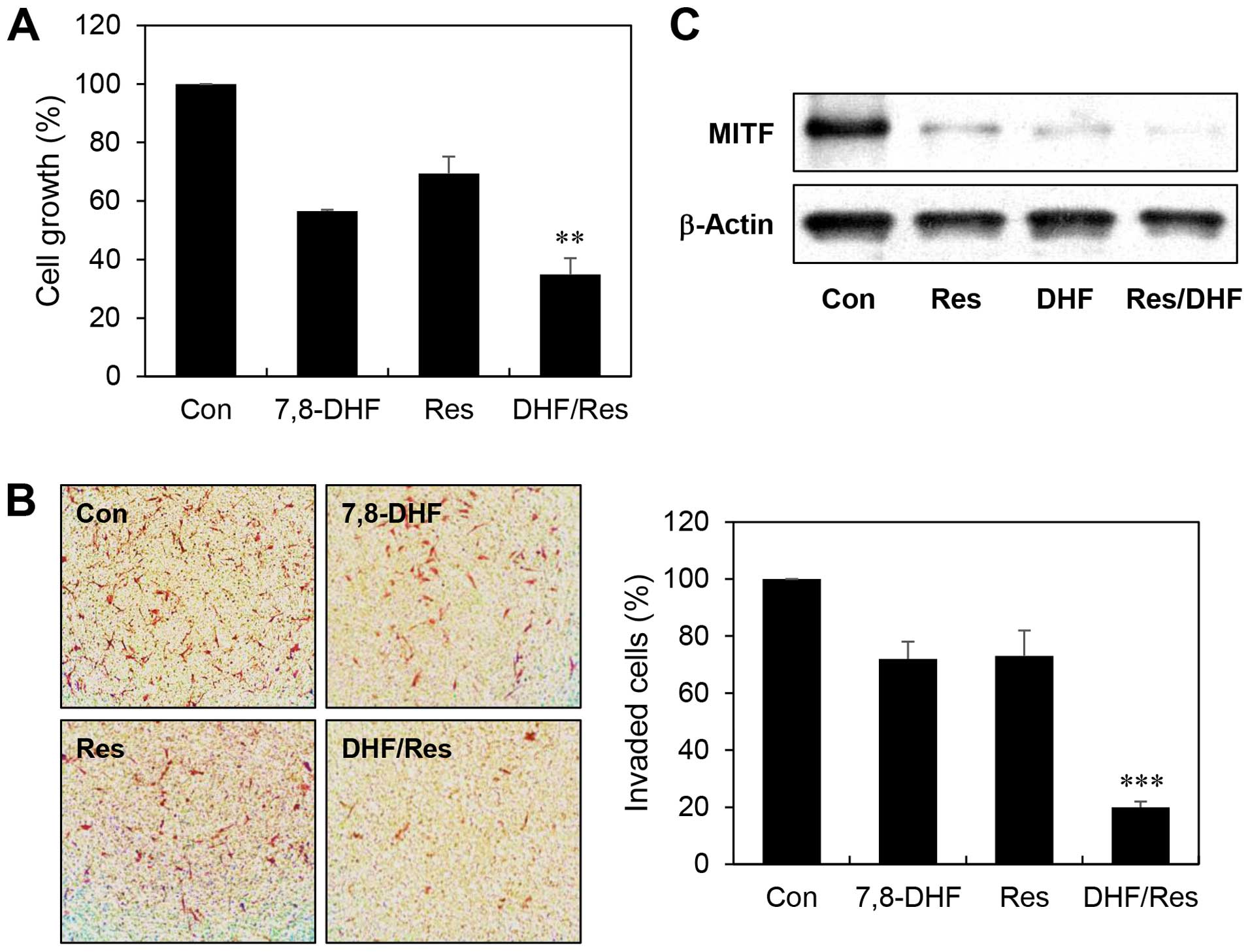
α-MSH-stimulated B16F10 cells. At the concentrations tested,
combined treatment with 7,8-DHF and resveratrol more efficiently
inhibited the melanoma cell growth compared with single agent
treatment (growth inhibition of 43, 31 and 65% with 7,8-DHF,
resveratrol, and the 7,8-DHF/resveratrol combination, respectively)
(Fig. 7A). Furthermore, combination
of the two compounds greatly increased suppression of the melanoma
cell invasion compared to either agent alone (invasion inhibition
of 28, 27 and 80% with 7,8-DHF, resveratrol, and the
7,8-DHF/resveratrol combination, respectively) (Fig. 7B). Notably, the combined treatment
resulted in an enhanced reduction in MITF expression levels
compared with inhibition by single agent treatment (Fig. 7C). These findings suggest that
7,8-DHF might be used to treat melanoma growth and metastasis in
combination with resveratrol.
Discussion
Malignant melanoma remains one of the cancers most
resistant to treatment, and the incidence and mortality rates are
increasing rapidly worldwide (1,2).
Through our continuing efforts to discover more effective and less
toxic anticancer agents for treatment of melanoma,
7,8-dihydroxyflavone (7,8-DHF), a monophenolic flavone, was newly
identified as a potential anticancer compound against melanoma.
Although the inhibitory effect of 7,8-DHF in some types of cancer,
like human monocytic leukemia and oral squamous carcinoma (20,21),
has been described previously, there is no such report on melanoma
so far. The key finding of the present study is that 7,8-DHF could
inhibit the growth, metastasis, and melanogenesis of melanoma cells
through downregulation of microphthalmia-associated transcription
factor (MITF) and its related oncogenic pathways.
MITF regulates multiple targets, which are involved
in various cellular processes such as cell cycle progression,
survival, motility, invasion and differentiation, in melanocytes
and melanoma cells (7). In human
metastatic melanoma, amplification of MITF was observed and
correlated with decreased overall patient survival (31). Ectopic MITF expression in
conjunction with the BRAF(V600E) mutant transformed primary human
melanocytes. In addition, disruption of MITF sensitized melanoma
cells to chemotherapeutic agents such as docetaxel and cisplatin.
Recent evidence has also shown that hypoxia-inducible factor 1α
(HIF1α) is a transcriptional target of MITF and the upregulation of
α-MSH/cAMP/MITF/HIF1 pathway contributes to survival,
neovascularization and metabolic adaptation of melanoma cells
(9). Moreover, overexpression of
c-MET, another target of MITF, has been associated with a poor
clinical outcome in human melanoma samples. c-MET expression was
increased by α-MSH/cAMP/MITF pathway, resulting in invasive growth
and antiapoptotic effect of melanoma cells (10). These findings demonstrate the
oncogenic potential of MITF, indicating that inhibition of MITF
could be an attractive therapeutic strategy to target malignant
melanoma. In the present study, we found that 7,8-DHF effectively
blocks α-MSH/cAMP/MITF pathway in melanoma cells. 7,8-DHF inhibited
cAMP production in α-MSH-stimulated B16F10 cells and consequently
suppressed not only MITF but also HIF1α and c-MET expression,
thereby inhibiting both expression of VEGF and phosphorylation of
c-MET and AKT, their related oncogenic networks. In addition, the
activation of ERK by 7,8-DHF is thought to cause the
antimelanogenic effect in B16F10 cells by mediating downregulation
of α-MSH/cAMP/MITF/tyrosinase pathway. These observations suggest
that 7,8-DHF could be an effective chemotherapeutic agent for
melanoma treatment through inhibition of MITF.
Previous studies have demonstrated that resveratrol,
a natural polyphenolic compound, has both antiproliferative and
proapoptotic effects in various cancer types including melanoma
(29,30). Resveratrol has been also found to
sensitize several types of malignancies to anticancer drugs such as
temozolomide and paclitaxel (32,33).
More recently, resveratrol enhanced the suppressive effect of
imatinib on α-MSH signaling, viability and invasiveness in melanoma
cells (30). In the present study,
we further evaluated the effect of 7,8-DHF in combination with
resveratrol on melanoma cell growth and invasion. Combination
treatment was more effective in inhibiting growth and invasion of
α-MSH-stimulated B16F10 cells compared to single agents.
Furthermore, combination of 7,8-DHF and resveratrol was more
powerful in reducing expression of MITF when compared to individual
agents. Based on our data, we suggest that the use of 7,8-DHF, as
single agent or in conjunction with known chemotherapeutic agents,
may be a promising strategy for the management of malignant
melanoma.
Acknowledgments
The present study was supported by the Sun Moon
University Research Grant of 2015.
References
|
1
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cummins DL, Cummins JM, Pantle H,
Silverman MA, Leonard AL and Chanmugam A: Cutaneous malignant
melanoma. Mayo Clin Proc. 81:500–507. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato M, Liu W, Akhand AA, Hossain K,
Takeda K, Takahashi M and Nakashima I: Ultraviolet radiation
induces both full activation of ret kinase and malignant
melanocytic tumor promotion in RFP-RET-transgenic mice. J Invest
Dermatol. 115:1157–1158. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Libra M, Malaponte G, Navolanic PM,
Gangemi P, Bevelacqua V, Proietti L, Bruni B, Stivala F, Mazzarino
MC, Travali S, et al: Analysis of BRAF mutation in primary and
metastatic melanoma. Cell Cycle. 4:1382–1384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hocker TL, Singh MK and Tsao H: Melanoma
genetics and therapeutic approaches in the 21st century: Moving
from the benchside to the bedside. J Invest Dermatol.
128:2575–2595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsao H, Chin L, Garraway LA and Fisher DE:
Melanoma: From mutations to medicine. Genes Dev. 26:1131–1155.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levy C, Khaled M and Fisher DE: MITF:
Master regulator of melanocyte development and melanoma oncogene.
Trends Mol Med. 12:406–414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du J, Widlund HR, Horstmann MA, Ramaswamy
S, Ross K, Huber WE, Nishimura EK, Golub TR and Fisher DE: Critical
role of CDK2 for melanoma growth linked to its melanocyte-specific
transcriptional regulation by MITF. Cancer Cell. 6:565–576. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buscà R, Berra E, Gaggioli C, Khaled M,
Bille K, Marchetti B, Thyss R, Fitsialos G, Larribère L, Bertolotto
C, et al: Hypoxia-inducible factor 1{alpha} is a new target of
microphthalmia-associated transcription factor (MITF) in melanoma
cells. J Cell Biol. 170:49–59. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McGill GG, Haq R, Nishimura EK and Fisher
DE: c-Met expression is regulated by Mitf in the melanocyte
lineage. J Biol Chem. 281:10365–10373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yasumoto K, Takeda K, Saito H, Watanabe K,
Takahashi K and Shibahara S: Microphthalmia-associated
transcription factor interacts with LEF-1, a mediator of Wnt
signaling. EMBO J. 21:2703–2714. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Price ER, Horstmann MA, Wells AG,
Weilbaecher KN, Takemoto CM, Landis MW and Fisher DE:
alpha-Melanocyte-stimulating hormone signaling regulates expression
of microphthalmia, a gene deficient in Waardenburg syndrome. J Biol
Chem. 273:33042–33047. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu M, Hemesath TJ, Takemoto CM, Horstmann
MA, Wells AG, Price ER, Fisher DZ and Fisher DE: c-Kit triggers
dual phosphorylations, which couple activation and degradation of
the essential melanocyte factor Mi. Genes Dev. 14:301–312.
2000.PubMed/NCBI
|
|
14
|
Bertolotto C, Abbe P, Hemesath TJ, Bille
K, Fisher DE, Ortonne JP and Ballotti R: Microphthalmia gene
product as a signal transducer in cAMP-induced differentiation of
melanocytes. J Cell Biol. 142:827–835. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beuret L, Flori E, Denoyelle C, Bille K,
Busca R, Picardo M, Bertolotto C and Ballotti R: Up-regulation of
MET expression by alpha-melanocyte-stimulating hormone and MITF
allows hepatocyte growth factor to protect melanocytes and melanoma
cells from apoptosis. J Biol Chem. 282:14140–14147. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jang SW, Liu X, Yepes M, Shepherd KR,
Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, et al: A
selective TrkB agonist with potent neurotrophic activities by
7,8-dihydroxyflavone. Proc Natl Acad Sci USA. 107:2687–2692. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen J, Chua KW, Chua CC, Yu H, Pei A,
Chua BH, Hamdy RC, Xu X and Liu CF: Antioxidant activity of
7,8-dihydroxyflavone provides neuroprotection against
glutamate-induced toxicity. Neurosci Lett. 499:181–185. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang R, Kang KA, Piao MJ, Ko DO, Wang ZH,
Chang WY, You HJ, Lee IK, Kim BJ, Kang SS, et al: Preventive effect
of 7,8-dihydroxyflavone against oxidative stress induced
genotoxicity. Biol Pharm Bull. 32:166–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han X, Zhu S, Wang B, Chen L, Li R, Yao W
and Qu Z: Antioxidant action of 7,8-dihydroxyflavone protects PC12
cells against 6-hydroxydopamine-induced cytotoxicity. Neurochem
Int. 64:18–23. 2014. View Article : Google Scholar
|
|
20
|
Lee RH, Shin JC, Kim KH, Choi YH, Chae JI
and Shim JH: Apoptotic effects of 7,8-dihydroxyflavone in human
oral squamous cancer cells through suppression of Sp1. Oncol Rep.
33:631–638. 2015.
|
|
21
|
Park HY, Kim GY, Hyun JW, Kim ND, Kim CG,
Kim WJ, Yoo YH and Choi YH: 7,8-dihydroxyflavone induces g1 arrest
of the cell cycle in U937 human monocytic leukemia cells via
induction of the Cdk inhibitor p27 and downregulation of pRB
phosphorylation. Oncol Rep. 28:353–357. 2012.PubMed/NCBI
|
|
22
|
Hunt G, Todd C, Cresswell JE and Thody AJ:
Alpha-melanocyte stimulating hormone and its analogue Nle4DPhe7
alpha-MSH affect morphology, tyrosinase activity and melanogenesis
in cultured human melanocytes. J Cell Sci. 107:205–211.
1994.PubMed/NCBI
|
|
23
|
Rusciano D, Lorenzoni P and Burger MM:
Regulation of c-met expression in B16 murine melanoma cells by
melanocyte stimulating hormone. J Cell Sci. 112:623–630.
1999.PubMed/NCBI
|
|
24
|
Poser I and Bosserhoff AK: Transcription
factors involved in development and progression of malignant
melanoma. Histol Histopathol. 19:173–188. 2004.PubMed/NCBI
|
|
25
|
Riley PA: Melanogenesis and melanoma.
Pigment Cell Res. 16:548–552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Costin GE and Hearing VJ: Human skin
pigmentation: Melanocytes modulate skin color in response to
stress. FASEB J. 21:976–994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JH, Jang JY, Park C, Kim BW, Choi YH
and Choi BT: Curcumin suppresses alpha-melanocyte stimulating
hormone-stimulated melanogenesis in B16F10 cells. Int J Mol Med.
26:101–106. 2010.PubMed/NCBI
|
|
28
|
Jin SH, Lee YY and Kang HY:
Methyl-beta-cyclodextrin, a specific cholesterol-binding agent,
inhibits melanogenesis in human melanocytes through activation of
ERK. Arch Dermatol Res. 300:451–454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gusman J, Malonne H and Atassi G: A
reappraisal of the potential chemopreventive and chemotherapeutic
properties of resveratrol. Carcinogenesis. 22:1111–1117. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen YJ, Chen YY, Lin YF, Hu HY and Liao
HF: Resveratrol inhibits alpha-melanocyte-stimulating hormone
signaling, viability, and invasiveness in melanoma cells. Evid
Based Complement Alternat Med. 2013:6321212013.PubMed/NCBI
|
|
31
|
Garraway LA, Widlund HR, Rubin MA, Getz G,
Berger AJ, Ramaswamy S, Beroukhim R, Milner DA, Granter SR, Du J,
et al: Integrative genomic analyses identify MITF as a lineage
survival oncogene amplified in malignant melanoma. Nature.
436:117–122. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Osmond GW, Augustine CK, Zipfel PA,
Padussis J and Tyler DS: Enhancing melanoma treatment with
resveratrol. J Surg Res. 172:109–115. 2012. View Article : Google Scholar
|
|
33
|
Fulda S and Debatin KM: Sensitization for
anticancer drug-induced apoptosis by the chemopreventive agent
resveratrol. Oncogene. 23:6702–6711. 2004. View Article : Google Scholar : PubMed/NCBI
|