Introduction
In the cancer microenvironment, complex interactions
between tumor cells and the surrounding tissues have important
roles in tumor growth and metastasis. In addition to their direct
biological activities mediating invasive processes by degrading the
extracellular matrix (ECM) via the production of cytokines and
proteolytic enzymes, cancer cells alter their adjacent stroma in
order to form a supportive cancer micro-environment (1). Fibroblasts within the tumor stroma,
known as carcinoma-associated fibroblasts (CAFs), were reported to
accelerate tumor progression and metastasis (2). CAFs express alpha-smooth muscle actin
(α-SMA) and produce ECM proteins such as fibronectin and collagen.
CAFs also produce a number of important factors that directly
promote growth in the adjacent epithelium.
Oral squamous cell carcinoma (OSCC) is one of the
leading causes of cancer-related death. Although OSCC accounts for
approximatelly 3% of all common cancers, the relative survival
rates of OSCC patients are poor because of high local metastasis
and recurrence rates, despite the development of various treatment
methods (3). Kawashiri et al
(4) reported that the presence of
CAFs in OSCC patients was correlated with the tumor stage,
metastasis and a poor prognosis. CAFs thus, appear to play a
critical role in many of the biological functions of the
cancer-stroma interplay at the tumor-host borderline, called the
invasive front, via cancer cell-cell adhesion, cancer cell-ECM
interactions, ECM degradation and the expression of cytokines.
Vascular endothelial growth factor (VEGF) is one of
the most important angiogenic factors. In OSCC patients, the
overexpression of VEGF has been identified as an independent
prognostic indicator for survival and recurrence (4). In VEGF gene expression, alternate
splice site selection in the terminal exon of mRNA yields two
families of VEGF isoforms: the pro-angiogenic family and the
anti-angiogenic family of VEGF. Proximal splice site selection in
exon 8 yields the pro-angiogenic VEGFxxx isoform (xxx is
the number of amino acids such as 121, 145, 165, 183, 189 and 206),
whereas distal splice site selection generates the anti-angiogenic
VEGFxxx isoform (5).
Among the pro-angiogenic isoforms, VEGF165 is known
to play critical roles in angiogenesis (6). VEGF165b has been identified as an
alternative isoform of VEGF165 via differential splice acceptor
site selection in the 3′-untranslated region (3′-utr) within exon
8. VEGF165b antagonizes VEGF165-induced endothelial cell
proliferation and competitively binds to vascular endothelial
growth factor receptor 2 (VEGFR2), resulting in the inhibition of
downstream signal transduction pathways (7–10).
However, it has been unknown whether VEGF165b affects OSCC stroma
cell biological activity, particularly in fibroblasts.
In the present study, we investigated whether
VEGF165b produced from OSCC cells affects the biological activity
of stromal cells and whether VEGF165b acts on specific molecular
targets associated with the biology of OSCC patients.
Materials and methods
Materials
For these in vitro experiments, recombinant
human VEGF165 (rVEGF165) and VEGF165b (rVEGF165b) were purchased
commercially from R&D Systems (Minneapolis, MN, USA) and
dissolved in sterile phosphate-buffered saline (PBS) containing at
least 0.1% bovine serum albumin (BSA) at a concentration of 100
μg/ml, and stored at −20°C prior to use.
Patients and cell lines
Seventy-one consecutive patients with OSCC (44 men,
27 women; median age, 70 years; range, 32–93 years) who underwent
surgical resection with curative intent between January 2006 and
December 2013 at the Showa University Dental Hospital were enrolled
in this study. Four OSCC cell lines (HSC2, 3, 4 and SAS) derived
from human oral squamous cell carcinoma were cultured at 37°C with
5% CO2 in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS). Normal human dermal
fibroblasts (NHDFs) derived from human dermals were purchased from
Lonza Biologics (Basel, Switzerland) and used between passages 2
and 10. These cells were also cultured at 37°C with 5%
CO2 in DMEM supplemented with 10% FBS.
Human umbilical vein endothelial cells (HUVECs)
derived from human umbilical cords were purchased from Lonza
Biologics and used between passages 6 and 10. These cells were
cultured in endothelial cell basal medium (EBM)-2 complete medium
(Lonza Biologics). Prior to their use in experiments, the HUVECs
were maintained in EBM-2 medium without hydrocortisone for ≥24 h.
The removal of hydrocortisone was necessary, since it inhibits
metalloproteinase production. Once passed and plated, the HUVECs
grew normally even in the absence of hydrocortisone. Hypoxia
experiments were performed for the indicated times in a humidified
multigas incubator (model BL-43MD; Bio-Labo, Tokyo, Japan)
calibrated to deliver 5% CO2, 2% O2 and 93%
N2 at 37°C.
Bead-based assays
Bio-Plex Pro™ assays with protein profiles using an
immunobead-based system were performed following the Bio-Rad
systems protocol (Bio-Rad Laboratories, Hercules, CA, USA).
Bio-Plex Pro™ Human Cytokine Standard Group I 24-Plex panels of
capture antibody-coated beads and labeled detection antibodies were
used with the cell lysate samples. Initially, the 96-well
filter-bottom plates were pre-wet with phosphoprotein wash buffer,
and 50 μl of the sample was added to each well in duplicate.
The plate was then incubated for 1 h and washed three times with
phosphoprotein wash buffer. Next, 50 μl of diluted biotin
antibody was added to each well and incubated for 30 min. The plate
was then washed, and 50 μl of diluted Streptavidin-PE (a
component of the Bio-Plex reagent kit) was added to each well and
incubated for 10 min. All incubations were performed at room
temperature on a shaker set at 300 rpm. Finally, the plate was
washed again with 100 μl of phosphoprotein wash buffer, and
125 μl of bead resuspension buffer was added. The median
relative fluorescence units were measured using a Bio-Plex Assay
Reader (Bio-Rad Laboratories).
RNA extraction and quantitative real-time
polymerase chain reaction (qPCR)
For the detection of Vegf165b mRNA on various
OSCC cell lines, total RNA was extracted from growing OSCC and NHDF
cells at the logarithmic phase in a 3.5-cm dish using
TRIzol® reagent (Life Technologies, Tokyo, Japan)
according to the manufacturer's directions.
For the detection of gelatinase mRNA,
subconfluent NHDF cells in a 3.5-cm dish were cultured for 24 h in
DMEM containing 10% FBS. The cells were replenished with fresh
medium without FBS and further cultured for 6 h. One of various
concentrations of rVEGF165 or rVEGF165b was then added, and the
culture was continued for 12 h. The complementary DNA (cDNA)
mixture was generated by reverse transcription using the iScrip™
cDNA Synthesis kit (Bio-rad Laboratories, Tokyo, Japan) and then
used as a template for the subsequent real-time PCR analysis using
iQ™ SYBR® Green Supermix (Bio-Rad Laboratories) and the
MyiQ™2 Two-Color Real-time PCR detection system (Bio-Rad
Laboratories). The primers used were
5′-tgtttgtacaagatccgcagacgtg-3′ and
5′-tcaccgcctcggcttgtcacatctgcaagtacgtt-3′ for vegf165 and
5′-tgtttgtacaagatccgcagacgtg-3′ and
5′-gttctgtatcagtctttcctggtgagagatctgca-3′ for vegf165b
(11). For vegf165, an
initial denaturation at 96°C for 5 min was followed by 30 cycles of
denaturation at 96°C for 30 sec, annealing at 55°C for 30 sec and
elongation at 72°C for 60 sec.
For vegf165b, an initial denaturation at 96°C
for 5 min was followed by 45 cycles of denaturation at 96°C for 30
sec, annealing at 55°C for 30 sec, and elongation at 72°C for 60
sec. The other primers were: for MMP-2,
5′-ccacgtgagaagccatggggcccc-3′ and
5′-gcagcctagccagcagtcggatttgatg-3′; for MMP-9,
5′-gcccgacccgagctgactc-3′ and 5′-ttcagggcgaggaccatagagg-3′; and for
18S ribosomal RNA, 5′-tcctgccagtagcatatgctg-3′ and
5′-agaggagcgagcgaccaaagg-3′. All results were normalized to 18S.
The threshold cycle (Ct) values for 18S and the genes of interest
were measured for each sample, and the relative transcript levels
were calculated by the ΔΔCT method.
Western blot analysis
A western blot analysis was carried out as
previously described (12). For the
detection of VEGF165b expression on the various OSCC cell lines,
total cellular proteins were harvested from growing OSCC and NHDF
cells at the logarithmic phase in a 3.5-cm dish.
For the detection of α-SMA and the determination of
the focal adhesion kinase (FAK) expression, subconfluent NHDF cells
in a 3.5-cm dish were cultured for 24 h in DMEM containing 10% FBS.
The cells were replenished with fresh medium without FBS and
further cultured for 6 h, and then the cells were exposed to one of
various concentrations of rVEGF165 or rVEGF165b and harvested after
12 h or at the indicated time for α-SMA expression or FAK
expression, respectively.
For the hypoxic experiments, subconfluent NHDFs and
the OSCC cell lines in a 3.5-cm dish were cultured for 24 h in 2 ml
of DMEM containing 10% FBS. The cells were replenished with 2 ml of
fresh medium and left under normoxic or hypoxic conditions for the
desired times. The primary antibodies human VEGF165 (catalog no.
AF-293-NA) and VEGF165b (cat. no. MAB3045) were obtained from
R&D Systems. Anti-actin antibodies, anti-FAK, anti-phospho-FAK
antibodies, and peroxide-conjugated secondary antibody were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Polyclonal mouse IgG anti-α-SMA antibody was obtained from Abcam
(Cambridge, MA, USA).
Cell proliferation assays
Cell proliferation assays were performed as
previously described (13). The
monolayer cell proliferation was measured using a
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
assay kit (roche Diagnostics, indianapolis, IN, USA) that measures
a purple formazan compound produced by viable cells. The cells
(5×103/well) were seeded with DMEM in 96-well plates
(Falcon Laboratories, McLean, VA, USA). After 24 h, the cells were
treated with various concentrations (1, 10, 20, 50 and 100 ng/ml)
of rVEGF165 or rVEGF165b and then further cultured for 3 days.
ECM adhesion assay
Adhesion assays were performed using the CytoSelect™
48-Well Cell Adhesion Assay (Cell Biolabs, Inc., San Diego, CA,
USA). NHDF cells (1×105) were seeded onto the wells in
serum-free DMEM containing 100 ng/ml of rVEGF165, rVEGF165b or both
isoforms and then incubated at 37°C for 6 h in a tissue culture
incubator prior to washing and staining. Gelatin zymography was
performed using a Gelatin Zymography kit (Cosmo Bio Co., Ltd.,
Tokyo, Japan).
Subconfluent NHDF cells in a 3.5-cm dish were
cultured for 24 h in 2 ml of DMEM containing 10% FBS. The cells
were replenished with 2 ml of fresh medium without FBS containing
one of various concentrations of rVEGF165, rVEGF165b or both
isoforms and further cultured for 18 h. Next, 2 ml of the
supernatant was harvested and then concentrated to 50 μl by
the Amicon Ultra-0.5 centrifugal filter device (Merck Millipore,
Billerica, MA, USA).
Immunohistochemical assay
For immunohistochemical staining, paraffin-embedded
tissues were cut at 4 μm. Slides were deparaffinized in
xylene for 5 min three times, in 100% ethanol for 5 min twice, in
90% ethanol for 5 min, in 80% ethanol for 5 min, in 70% ethanol for
5 min, in distilled water for 5 min, and finally in Tris-buffered
saline (TBS) for 5 min three times. After being deparaffinized and
rehydrated, the sections were heated in 10 mM sodium citrate buffer
for 20 min in an autoclave at 120°C. The sections were incubated in
3% H2O2-methanol for 10 min to inactivate
endogenous peroxidase. Non-specific binding was blocked with
Protein Block Serum-Free (Dako Japan, Tokyo, Japan) for 30 min at
room temperature.
The tissue sections were then incubated in a 1:50
dilution of monoclonal mouse IgG anti-human VEGF165b antibody
(R&D Systems) and with a ready-to-use monoclonal mouse antibody
IgG CD34 (Monosan, Uden, The Netherlands) placed on the sections
for 2 h in humidified boxes at room temperature. A 1:50 dilution of
antigen affinity-purified polyclonal goat IgG anti-human VEGF165
antibody (R&D Systems) and polyclonal mouse igG anti-α-SMA
actin antibody (Abcam) were placed on the sections in humidified
boxes and left at 4°C overnight. After being rinsed with TBS for 3
min three times, the sections were incubated with a biotinylated
secondary antibody, EnVision+ mouse/HRP (Dako) or polyclonal rabbit
anti-goat immunoglobulins/HRP (Dako) for 30 min at room
temperature. The sections were stained using the Liquid
DAB+ Substrate chromogen system (Dako). The slides were
mounted prior to observation under a conventional light microscope.
The clinicopathological findings were assessed based on the
international union Against Cancer TNM staging system (14). The histological mode of invasion was
classified according to the method of Anneroth et al
(15).
Statistical analyses
The statistical analyses for the qPCR and adhesion
assays were carried out using Student's t-test. The resulting data
are shown as the means ± standard deviations. P-values <0.05
were considered significant. We used Pearson's Chi-square test to
analyze the correlation between the clinicopathological features
and the expressions of VEGF165 and VEGF165b.
Results
The protein expression profiles of the
NHDFs and the OSCC lines
We used immunobead-based systems to compare the
expression protein profiles of a variety of cytokines in NHDFs and
four OSCC lines. A detailed comparison of the concentrations from
the cell layer led to the identification of interleukin (IL)-1β,
IL-8, basic fibroblast growth factor (bFGF), granulocyte-macrophage
colony-stimulating factor (GM-CSF), interferon γ-induced protein 10
(IP-10) and VEGF, which showed significant differences between the
NHDFs and the OSCC lines. Of these identified cytokines, the
expression of one angiogenic factor, VEGF, was markedly increased
on several OSCC lines compared to the NHDFs (table I). We therefore focused on this
potent angiogenic factor of interest as a clinicopathological
characteristic of OSCC.
 | Table IProtein expression profiles of the
NHDFs and the OSCC lines. |
Table I
Protein expression profiles of the
NHDFs and the OSCC lines.
| Gene name | Functional
class | Fibroblast | HSC2 | HSC3 | HSC4 |
|---|
| IL-1β | Cytokine | OOR< | 152 | 16.8 | 22.9 |
| IL-1ra | Cytokine | 19.4 | 36.9 | 72.4 | 32.6 |
| IL-2 | Cytokine | OOR< | 0.4 | OOR< | OOR< |
| IL-4 | Cytokine | 0.5 | 1.7 | 1.3 | 0.7 |
| IL-5 | Cytokine | OOR< | OOR< | OOR< | OOR< |
| IL-6 | Cytokine | 1.4 | 14.3 | 35.5 | 25.2 |
| IL-7 | Cytokine | 5.9 | 18.4 | 21.1 | 14.1 |
| IL-8 | Cytokine | 1.9 | 162.9 | 214.4 | 7.2 |
| IL-9 | Cytokine | OOR< | 3 | 4.3 | 2 |
| IL-10 | Cytokine | OOR< | 17.7 | 10.2 | 11.9 |
| IL-12 | Cytokine | 0.6 | 9.4 | 3.9 | 7.6 |
| IL-13 | Cytokine | 0.2 | 0.7 | 0.9 | 0.6 |
| IL-15 | Cytokine | OOR< | OOR< | OOR< | OOR< |
| IL-17 | Cytokine | OOR< | 0.6 | 2.4 | 0.8 |
| bFGF | Cytokine | 2777.3 | 945.1 | 2820 | 576.7 |
| G-CSF | Cytokine | 145.6 | 243.6 | 571 | 248.4 |
| GM-CSF | Cytokine | OOR< | 41.9 | 55.3 | 34.4 |
| IFN-γ | Cytokine | 9.5 | 20.3 | 17.8 | 9.8 |
| IP-10 | Chemokine | OOR< | 152 | 16.8 | 22.9 |
| MCP-1 | Chemokine | 0.9 | 1 | 81.2 | 0.4 |
| MIP-1 | Chemokine | 1 | 0.4 | 0.2 | 0.2 |
| RANTES | Chemokine
receptor | 2 | 1 | 0.4 | 0.2 |
| TNF-α | Chemokine | 44.8 | 116.5 | 95.7 | 53.8 |
| VEGF | Growth factor | OOR< | 278.2 | 78.9 | 194.3 |
The RNA and protein levels of both
VEGF165 and VEGF165b in the NHDFs and OSCC lines
It is well known that various isoforms of VEGF are
produced from a single gene by means of alternative splicing. Of
these isoforms, VEGF165b was isolated as the anti-angiogenic VEGF
splice variant (16). In the
present study, we first evaluated the mrNA and protein levels of
both VEGF165 and VEGF165b expressions in the NHDFs and the four
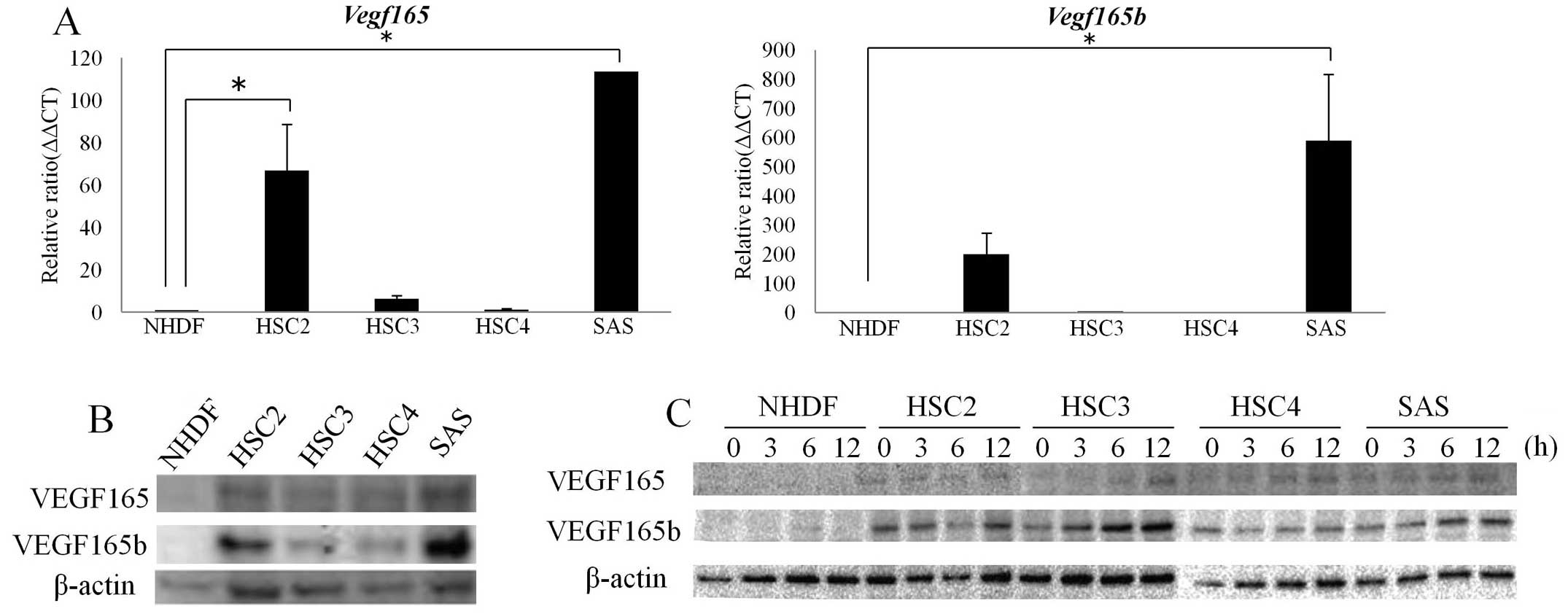
OSCC lines. As shown in Fig. 1A,
the NHDFs and the HSC3 and HSC4 cells faintly expressed
vegf165 mRNA, whereas the HSC2 and SAS cells more strongly
expressed vegf165 mrNA compared to the NHDFs and HSC3 and
HSC4 cells.
Notably, the vegf165b mRNA levels displayed a
pattern of expression similar to that observed for the
vegf165 mRNA levels in the NHDF, HSC2, HSC3, HSC4 and SAS
cells (Fig. 1A). At the protein
level, the HSC2 and SAS cells highly expressed both VEGF165 and
VEGF165b, whereas the NHDF, HSC3 and HSC4 cells expressed VEGF165
and VEGF165b only weakly, suggesting that the protein expression
patterns of both VEGF165 and VEGF165b are correlated with the
patterns observed for the mRNA levels.
Since hypoxia could be a contributing factor in the
stimulation of the switch of VEGF165b to VEGF165 during
angiogenesis (17), we proceeded to
determine whether or not the VEGF165b expression could be
upregulated in OSCC cells in response to hypoxic culture
conditions. As shown in Fig. 1C,
the hypoxia treatment of the OSCC lines along a 12-h time course
resulted in significant VEGF165b protein enhancement until 12 h of
hypoxic exposure in HSC2, HSC3 and SAS cells, whereas the VEGF165
protein level showed a faint but similar tendency to increase until
the 12-h time point of hypoxic exposure in all four OSCC lines.
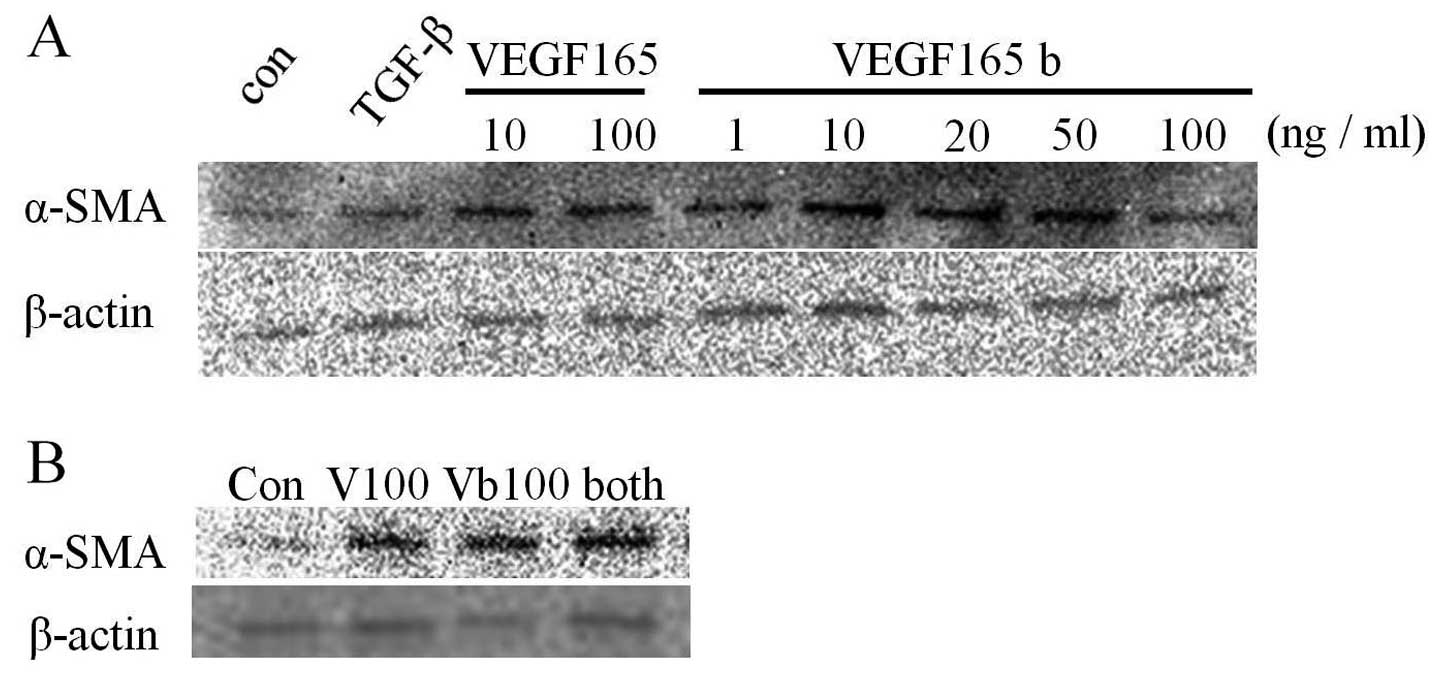
VEGF165b modulates the fibroblast
transdifferentiation to myofibroblasts
Transforming growth factor-beta (TGF-β) was
identified as the main factor in the promotion of the
transdifferentiation of fibroblast to myofibroblasts (18). Myofibroblasts are differentiated
fibroblasts that express α-SMA. Here we examined whether VEGF165b
regulates the transdifferentiation of fibroblasts into
myofibroblasts. As shown in Fig.
2A, not only VEGF165 but also VEGF165b induced α-SMA
expression, indicating that the normal fibroblasts
transdifferentiated into myofibroblasts, i.e., the so-called CAFs,
promoted by VEGF165 and VEGF165b secreted from the tumor cells.
Notably, the addition of both VEGF isoforms also induced α-SMA
expression despite the isoforms' competitive binding to the same
receptor (VEGFR2).
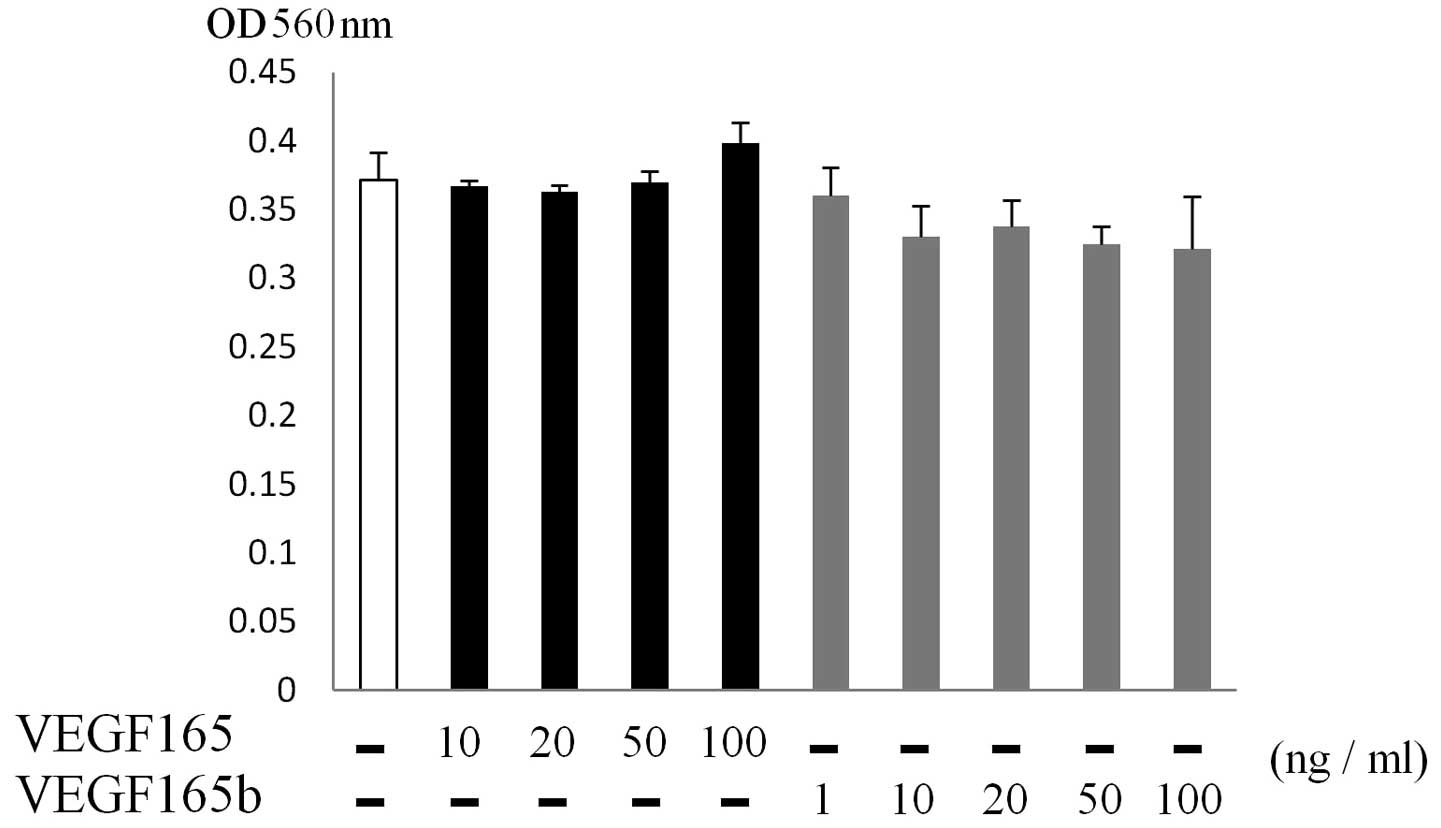
VEGF165b did not stimulate the
proliferation of fibroblasts
Since CAFs promote tumor invasion and migration
through paracrine stimulation (2),
we examined the ability of VEGF165b to induce the proliferation of
fibroblasts at various concentrations of VEGF165b (1–100 ng/ml). As
shown in Fig. 3, VEGF165 or
VEGF165b had no effect on the proliferation of fibroblasts at any
of the concentrations used. In the HUVECs, although VEGF165
stimulated the direct proliferation of HUVECs, no proliferation
activity of VEGF165b was observed even with the dose of 100 ng/ml
(data not shown).
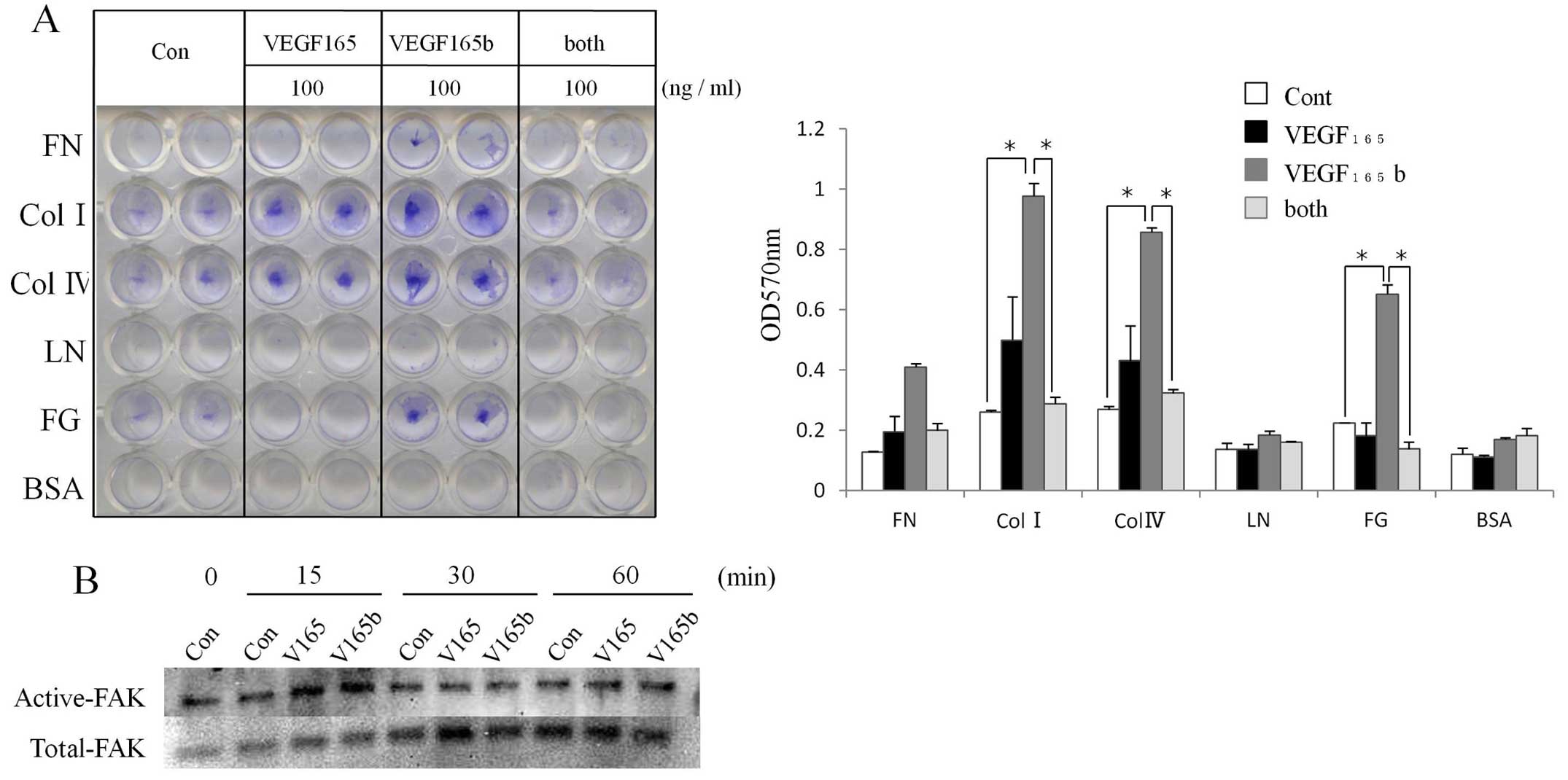
The effect of VEGF165b on the ECM
adhesion in fibroblasts
Since it is well known that CAFs produce ECM
proteins such as fibronectin and collagen, we examined the ECM
adhesion in fibroblasts incubated with 100 ng/ml of VEGF165,
VEGF165b, or both isoforms in a cell adhesion assay. As shown in
Fig. 4A, VEGF165b more strongly
increased adhesion to collagen II, collagen IV, and fibrinogen
compared to VEGF165. Interestingly, the addition of both isoforms
resulted in less adhesion than the use of either isoform alone.
It was reported that for the VEGF signal
transduction related to adhesion of ECM, the activation of FAK and
the protein paxillin was essential, leading to the recruitment of
actin-anchoring proteins to organize the focal adhesion plaque
(19). We therefore examined the
phosphorylation of FAK when VEGF165b was added to NHDFs, and the
results demonstrated that both VEGF165 and VEGF165b alone at 100
ng/ml induced the phosphorylation of FAK as early as at 15 min
(Fig. 4B).
The effect of VEGF165b on the proteolytic
activities in fibroblasts
Matrix metalloproteinases (MMPs) secreted from
cancer cells are critical for tumor invasion by the degradation of
the ECM (20), but it was recently
observed that MMPs produced from surrounding normal cells (such as
fibroblasts and endothelial cells) may be important in the tumor
microenvironment. We next examined the expression of MMPs and the
proteolytic activities in fibroblasts incubated with VEGF165 and
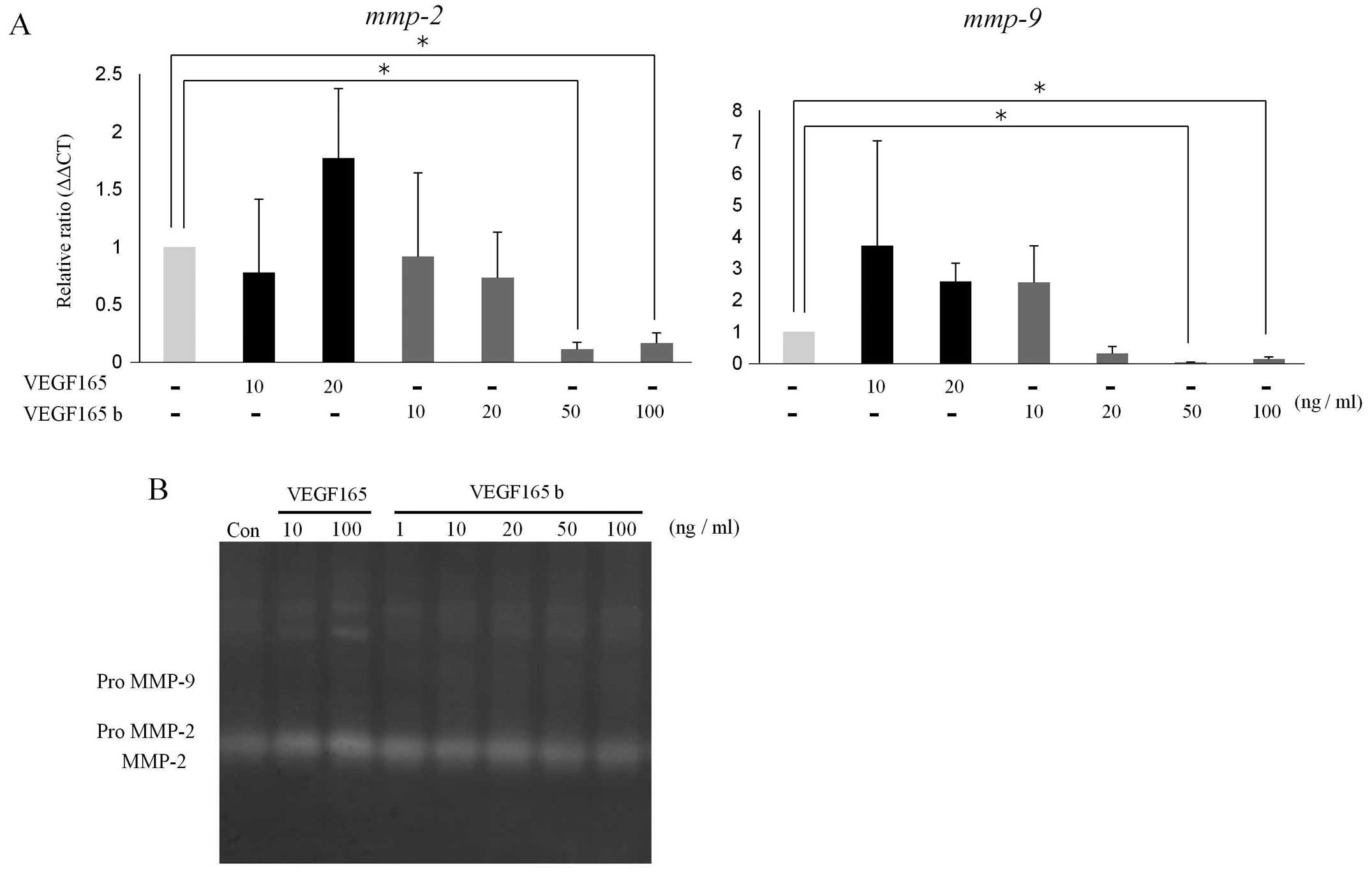
VEGF165b, using qPCR and gelatin zymography. As shown in Fig. 5A, VEGF165b did not affect the levels
of the gelatinase (mmp-2 and mmp-9) mrNA
significantly, but VEGF165 induced the expression of gelatinases.
Likewise, predominant proteolytic bands migrating at molecular
weights indicating MMP-2 species were increased with 100 ng/ml of
VEGF165, but VEGF165b incubation at the various concentrations did
not result in a significant increase in the proteolytic
activities.
The relationships between VEGF165b
expression and clinicopathological factors
We evaluated the correlations between the VEGF165b
expression and clinicopathological criteria (i.e., the T, N and M
categories, the mode of invasion, and the degree of
differentiation). As shown in Table
II, VEGF165b-positive staining was seen in almost all
categories regardless of the T or N category. Distant metastasis
related to the difference of VEGF isoforms was not evaluated,
because we observed only two cases of metastasis.
 | Table IICorrelations between the
clinicopathological features and the expressions of VEGF165 and
VEGF165b. |
Table II
Correlations between the
clinicopathological features and the expressions of VEGF165 and
VEGF165b.
| VEGF165 aberrant
expression
| VEGF165b aberrant
expression
|
|---|
| Positive
(n=28) | Negative
(n=43) | P-value | Positive
(n=69) | Negative (n=2) | P-value |
|---|
| Age (years) | | | | | | |
| ≥0 | 21 | 38 | 0.141738 | 55 | 2 | 0.477108 |
| <60 | 7 | 5 | | 14 | 0 | |
| Gender | | | | | | |
| Male | 20 | 24 | 0.185323 | 42 | 2 | 0.261113 |
| Female | 8 | 19 | | 27 | 0 | |
| T
classification | | | | | | |
| T1–T2 | 16 | 28 | 0.498811 | 43 | 1 | 0.723506 |
| T3–T4 | 12 | 15 | | 26 | 1 | |
| N
classification | | | | | | |
| ≥1 | 6 | 8 | 0.770075 | 14 | 0 | 0.477108 |
| <0 | 22 | 35 | | 55 | 2 | |
| Distant
metastasis | | | | | | |
| Yes | 0 | 2 | 0.0455 | 2 | 0 | 0.807048 |
| No | 2 | 0 | | 67 | 2 | |
|
YK-classification | | | | | | |
| 1/2/3 | 23 | 38 | 0.46087 | 59 | 2 | 0.56135 |
| 4C/4D | 5 | 5 | | 10 | 0 | |
|
Differentiation | | | | | | |
| Well/moderate | 24 | 40 | 0.312676 | 62 | 2 | 0.635188 |
| Poor | 4 | 3 | | 7 | 0 | |
We also observed that the VEGF165b-positive staining
in OSCC cells was not related to the mode of invasion or the degree
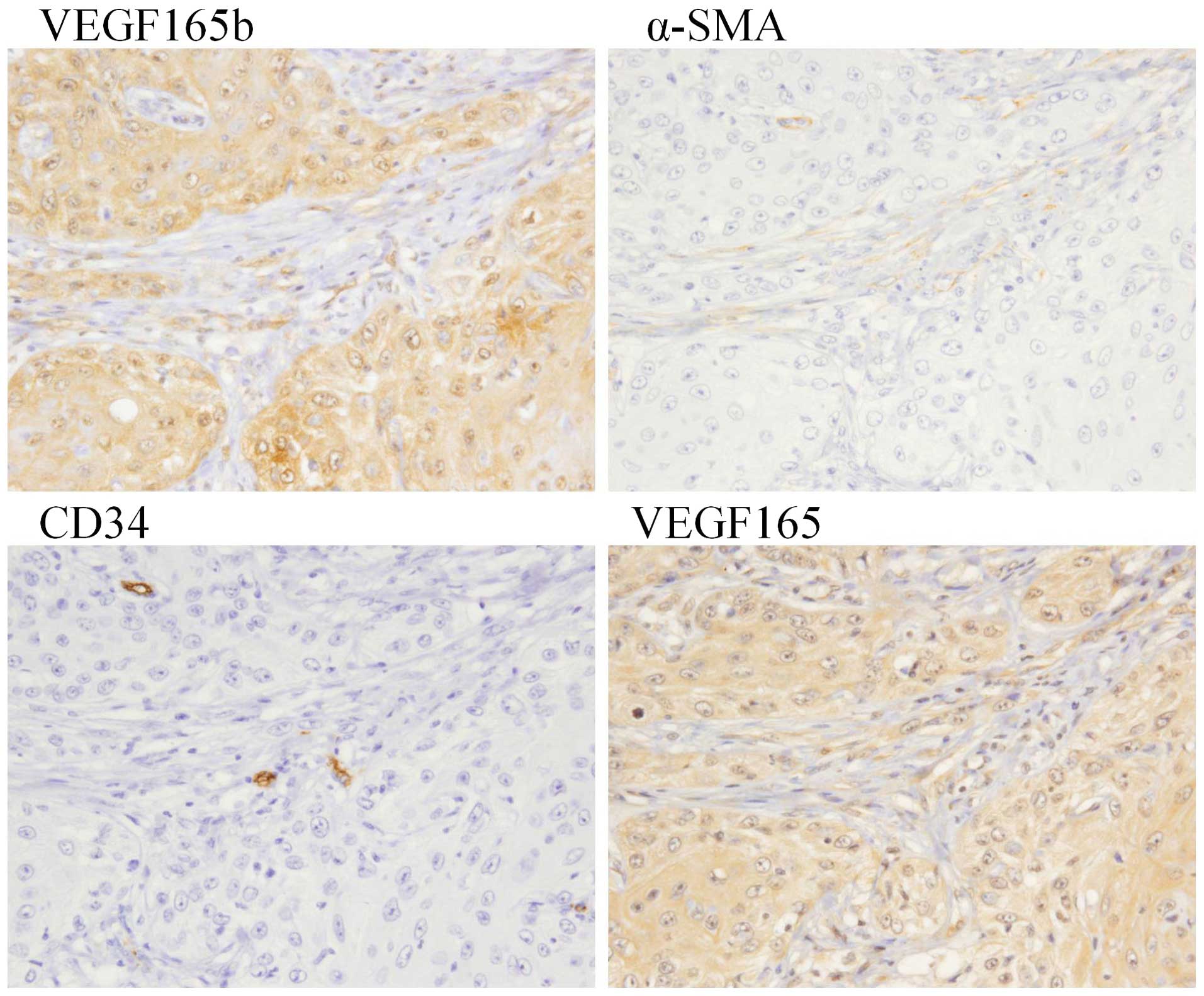
of differentiation. Fig. 6 provides
a representative histological pattern of both VEGF isoforms and the
α-SMA and CD34 expression observed in a patient with OSCC in the
tongue region. Strong expression of VEGF165 and VEGF165b in both
the nucleus and cytoplasm of tumor cells was observed. However,
there was a difference in the level of VEGF165b expression compared
to that of VEGF165 expression; i.e., an increasing tendency of
VEGF165b expression was observed in the boundary between tumor
cells and stroma cells that express α-SMA. No correlation was
observed between the VEGF165b expression or the VEGF165 expression
in tumor cells and the CD34 expression in endothelial cells.
Discussion
Cancer development and progression are controlled by
cellular interactions derived from a complex relationship between
stromal, epithelial and ECM components. The stromal
micro-environment surrounding cancer cells is known as 'reactive
stroma' that is characterized by modified ECM composition,
increased microvessel density, inflammatory cells, and fibroblasts
with an activated phenotype, termed CAFs (21,22).
These CAFs are thought to play a central role in the complex
process of tumor development and progression.
The VEGFxxxb family of isoforms is
generated by C-terminal distal splice site selection. VEGF165b was
identified as an alternative isoform which was an alternative
splice site in the terminal exon 8 of the vegf mRNA. The
precise mechanism of the biological action of VEGFxxxb
has not been fully elucidated, but it was reported that VEGF165b
binds to both VEGFR1 (23) and
VEGFR2 (5), competing with VEGF165,
and it initiated only weak signaling of the receptor to induce
tyrosine phosphorylation, leading to the inhibition of endothelial
cell proliferation, migration and vasodilation as well as in
vitro experimental angiogenesis and tumor growth (23,24).
In the present study, both the mRNA and protein
expression levels of VEGF165b in some of the OSCC lines were higher
than those in the NHDFs. We also observed that VEGF165b did not
promote the cell growth or invasive capabilities on the NHDFs. One
aspect of the molecular mechanisms that was elucidated is that
VEGF165b did not affect the level of gelatinase on NHDFs, whereas
it induced the cell adhesive capabilities to ECM through activated
FAK signaling pathways. In addition, VEGF165b modulates fibroblasts
transdifferentiation to myofibroblasts through α-SMA expression. We
also observed an increasing tendency of VEGF165b expression in the
boundary between cancer cells and stroma cells.
In light of our present findings, it is plausible
that the reactive stroma of OSCC indirectly acts in the process of
anti-angiogenesis via these VEGF165b-activated fibroblasts in a
paracrine manner, although OSCC cells themselves secrete an
anti-angiogenic factor, VEGF165b. On the other hand, Chen et
al (25) reported that VEGF165b
could inhibit the migration and invasion of cancer cells in an
autocrine-dependent manner through the inhibition of the expression
of VEGF165 as well as the competitive binding of VEGF165b to VEGFR.
Since the switching of the expression of VEGF from VEGF165 to
VEGF165b is related to a reduction in tumor growth rates (8,17) and
to microenvironmental conditions such as hypoxia (17), it is possible that OSCC cells
themselves enable a finely tuned modulation of the expression ratio
among VEGF isoforms under hypoxia, and these cells may promote
their own progression by controlling the surrounding stroma via
cellular interactions such as CAFs.
Kawashiri et al (4) reported that in the invasive front of
OSCC tissue, myofibroblasts increased gradually with the mode of
invasion and degree of differentiation. They also observed that
patients with α-SMA-positive staining had increased locoregional
lymph node metastasis and myofibroblast appearance, leading to a
prolonged disease-specific 5-year survival rate. Of note, our
present results confirmed that VEGF165b secreted from OSCC cell
lines may modulate the fibroblast transdifferentiation into
myofibroblasts through α-SMA expression. However, our data
(Table II) do not permit us to
conclude that OSCC tissue with VEGF165b-positive staining is
related to the mode of invasion or degree of differentiation. Since
VEGF165-positive staining was not seen most of our OSCC samples
despite the degree of invasion, VEGF165b-positive staining may be
more useful for the detection of malignant cells, especially in the
invasive front of 4C/4D tissue.
Our present findings showed that VEGF165b secreted
from OSCC cells may contribute to the process of anti-angiogenesis
by stopping the activity of MMPs and by activating cell adhesive
capabilities to ECM in CAFs surrounding tumor cells. Further
studies are necessary for the elucidation of the biological
activity of VEGF165b in OSCC cells.
Abbreviations:
|
bFGF
|
basic fibroblast growth factor
|
|
CAF
|
carcinoma-associated fibroblast
|
|
ECM
|
extracellular matrix
|
|
FAK
|
focal adhesion kinase
|
|
GM-CSF
|
granulocyte-macrophage
colony-stimulating factor
|
|
HUVEC
|
human umbilical vein endothelial
cell
|
|
IP-10
|
interferon gamma-induced protein
10
|
|
NHDF
|
normal human dermal fibroblast
|
|
OSCC
|
oral squamous cell carcinoma
|
|
qPCR
|
quantitative real-time polymerase
chain reaction
|
|
TGF-β
|
transforming growth factor-beta
|
|
VEGF
|
vascular endothelial growth factor
|
|
VEGFR2
|
vascular endothelial growth factor
receptor 2
|
|
α-SMA
|
alpha-smooth muscle actin
|
Acknowledgments
The present study was supported in part by
Grants-in-Aid for Scientific research (C) to S.K. and Y.M. from the
Japan Society for the Promotion of Science.
References
|
1
|
Rodrigues-Lisoni FC, Peitl P Jr, Vidotto
A, Polachini GM, Maniglia JV, Carmona-raphe J, Cunha BR, Henrique
T, Souza CF, Teixeira RA, et al Head and Neck Genome Project
GENCAPO: Genomics and proteomics approaches to the study of
cancer-stroma interactions. BMC Med Genomics. 3:142010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xing F, Saidou J and Watabe K: Cancer
associated fibroblasts (CAFs) in tumor microenvironment. Front
Biosci (Landmark Ed). 15:166–179. 2010. View Article : Google Scholar
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawashiri S, Tanaka A, Noguchi N, Hase T,
Nakaya H, Ohara T, Kato K and Yamamoto E: Significance of stromal
desmoplasia and myofibroblast appearance at the invasive front in
squamous cell carcinoma of the oral cavity. Head Neck.
31:1346–1353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Woolard J, Wang WY, Bevan HS, Qiu Y,
Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E,
Perrin R, et al: VEGF165b, an inhibitory vascular endothelial
growth factor splice variant: Mechanism of action, in vivo effect
on angiogenesis and endogenous protein expression. Cancer Res.
64:7822–7835. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soker S, Takashima S, Miao HQ, Neufeld G
and Klagsbrun M: Neuropilin-1 is expressed by endothelial and tumor
cells as an isoform-specific receptor for vascular endothelial
growth factor. Cell. 92:735–745. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ladomery MR, Harper SJ and Bates DO:
Alternative splicing in angiogenesis: The vascular endothelial
growth factor paradigm. Cancer Lett. 249:133–142. 2007. View Article : Google Scholar
|
|
8
|
Nowak DG, Amin EM, Rennel ES,
Hoareau-Aveilla C, Gammons M, Damodoran G, Hagiwara M, Harper SJ,
Woolard J, Ladomery MR, et al: Regulation of vascular endothelial
growth factor (VEGF) splicing from pro-angiogenic to
anti-angiogenic isoforms: A novel therapeutic strategy for
angiogenesis. J Biol Chem. 285:5532–5540. 2010. View Article : Google Scholar :
|
|
9
|
Rennel ES, Hamdollah-Zadeh MA, Wheatley
ER, Magnussen A, Schüler Y, Kelly SP, Finucane C, Ellison D,
Cebe-Suarez S, Ballmer-Hofer K, et al: Recombinant human VEGF165b
protein is an effective anti-cancer agent in mice. Eur J Cancer.
44:1883–1894. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Varey AH, Rennel ES, Qiu Y, Bevan HS,
Perrin RM, Raffy S, Dixon AR, Paraskeva C, Zaccheo O, Hassan AB, et
al: VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and
inhibits bevacizumab treatment in experimental colorectal
carcinoma: Balance of pro- and antiangiogenic VEGF-A isoforms has
implications for therapy. Br J Cancer. 98:1366–1379. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tayama M, Furuhata T, Inafuku Y, Okita K,
Nishidate T, Mizuguchi T, Kimura Y and Hirata K: Vascular
endothelial growth factor 165b expression in stromal cells and
colorectal cancer. World J Gastroenterol. 17:4867–4874. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsukamoto H, Kondo S, Mukudai Y, Nagumo T,
Yasuda A, Kurihara Y, Kamatani T and Shintani S: Evaluation of
anticancer activities of benzo[c]phenanthridine alkaloid
sanguinarine in oral squamous cell carcinoma cell line. Anticancer
Res. 31:2841–2846. 2011.PubMed/NCBI
|
|
13
|
Li C, Yazawa K, Kondo S, Mukudai Y, Sato
D, Kurihara Y, Kamatani T and Shintani S: The root bark of Paeonia
moutan is a potential anticancer agent in human oral squamous cell
carcinoma cells. Anticancer Res. 32:2625–2630. 2012.PubMed/NCBI
|
|
14
|
Sobin LH and Fleming ID: TNM
Classification of Malignant tumors, Fifth Edition. Union
International Contre le Cancer and the American Joint Committee on
Cancer. Cancer. 80:1803–1804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anneroth G, Batsakis J and Luna M: Review
of the literature and a recommended system of malignancy grading in
oral squamous cell carcinomas. Scand J Dent Res. 95:229–249.
1987.PubMed/NCBI
|
|
16
|
Perrin RM, Konopatskaya O, Qiu Y, Harper
S, Bates DO and Churchill AJ: Diabetic retinopathy is associated
with a switch in splicing from anti- to pro-angiogenic isoforms of
vascular endothelial growth factor. Diabetologia. 48:2422–2427.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rennel E, Waine E, Guan H, Schüler Y,
Leenders W, Woolard J, Sugiono M, Gillatt D, Kleinerman E, Bates D,
et al: The endogenous anti-angiogenic VEGF isoform, VEGF165b
inhibits human tumour growth in mice. Br J Cancer. 98:1250–1257.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tuxhorn JA, McAlhany SJ, Yang F, Dang TD
and Rowley DR: Inhibition of transforming growth factor-beta
activity decreases angiogenesis in a human prostate cancer-reactive
stroma xenograft model. Cancer Res. 62:6021–6025. 2002.PubMed/NCBI
|
|
19
|
Birukova AA, Cokic I, Moldobaeva N and
Birukov KG: Paxillin is involved in the differential regulation of
endothelial barrier by HGF and VEGF. Am J Respir Cell Mol Biol.
40:99–107. 2009. View Article : Google Scholar :
|
|
20
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Wever O and Mareel M: Role of tissue
stroma in cancer cell invasion. J Pathol. 200:429–447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Micke P and Ostman A: Tumour-stroma
interaction: Cancer-associated fibroblasts as novel targets in
anti-cancer therapy? Lung Cancer. 45(Suppl 2): S163–S175. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cébe Suarez S, Pieren M, Cariolato L, Arn
S, Hoffmann U, Bogucki A, Manlius C, Wood J and Ballmer-Hofer K: A
VEGF-A splice variant defective for heparan sulfate and
neuropilin-1 binding shows attenuated signaling through VEGFR-2.
Cell Mol Life Sci. 63:2067–2077. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bates DO, Cui TG, Doughty JM, Winkler M,
Sugiono M, Shields JD, Peat D, Gillatt D and Harper SJ: VEGF165b,
an inhibitory splice variant of vascular endothelial growth factor,
is downregulated in renal cell carcinoma. Cancer Res. 62:4123–4131.
2002.PubMed/NCBI
|
|
25
|
Chen J, Li Z, Zhang S, Zhang R, Dassarath
M and Wu G: Effects of exogenous VEGF(165)b on invasion and
migration of human lung adenocarcinoma A549 cells. J Huazhong Univ
Sci Technolog Med Sci. 31:619–624. 2011. View Article : Google Scholar : PubMed/NCBI
|