Introduction
Prostate cancer (PCa) is one of the most common
malignancies in men (1). This tumor
is androgen-dependent and is treated using surgery, radiotherapy
and hormone therapy (2). However,
PCa frequently progresses into a hormone-independent, highly
aggressive and invasive disease (3)
presenting with multiple drug resistance. This neoplasia is also
the most common malignancy in which the chromosome 17q21 is
segregated (4–6) suggesting that genes located in the
immediate vicinity of 17q21 can be important in PCa
development/progression (5).
p75NTR is a 75-kDa glycoprotein receptor that belong to
the tumor necrosis factor (TNF) receptor superfamily and binds
mainly to nerve growth factor (NGF) and has structural and
sequential similarity to the TNF receptor (7). Notably, the human p75NTR
gene locus has been mapped distal to 17q21–q22 (6). The prostate gland is one of the most
abundant sources of NGF controlling cell proliferation and
apoptosis (8,9). However, p75NTR is
progressively lost during the progression of PCa (10–12).
Loss of expression of p75NTR protein is correlated with
increased Gleason's score of organ-confined pathological prostate
tissues (12–14), and is completely absent in prostate
tumor cells derived from metastases (11). The re-expression of
p75NTR was previously shown in PCa to retard cell cycle
progression by inducing accumulation of cells in the G1 phase with
a concomitant reduction in cells in the S phase thus inducing
apoptosis (15) and reverting
androgen-independent growth (16).
p75NTR can be upregulated by prolonged treatment with
NGF (17) or GnRH treatment
(18) indicating epigenetic
mechanisms of protein expression.
DNA hypermethylation plays an important role in the
downregulation of genes important for cell death in PCa.
Demethylating agents have been shown to prevent tumorigenesis and
delay androgen-independent disease (19,20) in
a TRAMP mouse model of PCa as well as to revert androgen
independence (19,21,22)
and chemoresistance in prostate tumor cells (23).
In the present study, we demonstrated that
azacitidine treatment induced a dose-dependent increase in
p75NTR expression. This was in agreement with a recent
report showing that azacitidine and estrogen treatment induced
p75NTR (24) in 22rv1
cells. Blocking NGF antibodies or specific p75NTR
inhibitors reverted this effect. Taken together, our results
suggest that the NGF network could be a candidate for future
pharmacological manipulation in PCa therapy.
Materials and methods
Reagents
All the materials for the tissue culture were
purchased from EuroClone S.p.A. (Milan, Italy). Antibodies: Bcl-2
(sc-509), Bax (N20) (sc-493), Bad (C20) (sc-943), p-Bad ser112
(sc-7998), Bcl-xL (H2) (sc-8392), TrkA (C20) (sc-20537), p-TrkA
(E6) (sc-8058), p75NTR (H92) (sc-5634), TRADD (A-5)
(sc46653), RIP (H207) (sc 7881), TRAF2 (D-3) (sc-136997), DR4 (C20)
(sc-6823) and DR5 (N19) (sc-7192) were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). DNMT1, DNMAT3a and DNMT3b
antibodies were purchased from BioCarta LLC (San Diego, CA, USA).
Plasticware was obtained from Nunc (Roskilde, Denmark). Azacitidine
(Vidaza®) was obtained from Celgene Corporation (Summit,
NJ, USA). The p38 MAPK inhibitor SB202190 and ibuprofen were
purchased from Sigma-Aldrich Italy (Milan, Italy). Ro 08-2750, a
selective p75NTR inhibitor, was purchased from Tocris
Bioscience (Bristol, UK).
Cell lines
Two aggressive PCa models (PC3 and 22rv1 cell lines)
were obtained from the American Tissue Culture Collection (ATCC;
Rockville, MD, USA) and DSMZ (Braunschweig, Germany), respectively,
and were grown as recommended.
Growth assays
Cells were seeded at a density of 2×104
cells/ml into 24-well plates. Cells were left to attach and grow in
5% FCS and Dulbecco's modified Eagle's medium (DMEM). Next, the
cells were cultured under appropriate experimental conditions.
Morphological controls were performed every day with an inverted
phase-contrast photomicroscope (Nikon Diaphot, Tokyo, Japan) before
cell trypsinization and counting by the NucleoCounter™ NC-100
automated cell counter system (Chemotec, Gydevang, Denmark). The
effect on cell proliferation was measured by taking the mean cell
number with respect to the controls over time for the different
treatment groups.
Cell cycle and apoptosis analysis
Adherent cells were trypsinized, pooled with the
culture supernatant containing the apoptotic cells already detached
from the dish and centrifuged. Cells (1×106) were washed
in phosphate-buffered saline (PBS) and fixed for 30 min by the
addition of 1 ml of 70% ethanol. Apoptosis and cell cycle analyses
were performed using the Tali Cell Cycle kit and the Tali apoptosis
kit, Annexin V-Alexa Fluor 488 and propidium iodide-based staining
(Life Technologies Italia, Monza, Italy). Apoptosis was further
confirmed by cytofluorimetric analysis following the manufacturer's
instructions. Stained cells were then measured on a Tali
Image-Based Cytometer. Caspase 8 and −9 activities were evaluated
using enzymatic assays from GenScript (Piscataway, NJ, USA).
Western blot analysis
Cells (1×106) were washed with cold PBS
and immediately lysed with 100 μl of lysis buffer (50 mM
HEPES, pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton x-100, 1 mM
EDTA, 1 mM EGTA, 50 mM NaF, 1 Mm sodium orthovanadate, 30 mM
p-nitrophenyl phosphate, 10 mM sodium pyrophosphate, 1 mM
phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin and 10
μg/ml leupeptin). Cell lysates were electrophoresed in 7%
SDS-PAGE, and separated proteins were transferred to a
nitrocellulose membrane and probed with the appropriate antibodies
as indicated.
In vivo treatments
Male CD1 nude mice (Charles River, Milan, Italy)
were maintained under the guidelines established by our institution
(university of L'Aquila, Medical School and Science and Technology
School Board Regulations, complying with the Italian government
regulation no. 116, January 27, 1992, for the use of laboratory
animals). All mice received s.c. flank injections of
1×106 PC3 and 22v1 cells. Tumor growth was assessed by a
bi-weekly measurement of tumor diameters with a Vernier calliper
(length × width). Tumor weight was calculated as previously
indicated (22). Treatments were
started when tumor volumes reached ~80 mm3 (day 0) and
were stopped after 28 days. Mice were grouped as follows: group 1,
10 mice received intraperitoneal (i.p.) injections of 100 l PBS for
a consecutive 7 days; group 2: 10 mice received i.p. injections of
100 l of azacitidine (Aza-CR) (0.8 mg/kg) for a consecutive 7 days.
Tumors were fixed in paraformaldehyde for hystochemical and
immunohystochemical analyses. Indirect immunoperoxidase staining of
tumor xenografts was performed on paraffin-embedded 4-μm
tissue sections. Briefly, the sections were incubated with the
primary antibodies overnight at 4°C. Next avidin-biotin
assays were carried out using the Vectastain Elite kit (Vector
Laboratories, Burlingame, CA, USA). Mayer's hematoxylin was used as
a nuclear counterstain.
Statistical analysis
Continuous data are presented as the mean ± standard
deviation (SD), and were compared using an umpired Student's
t-test.
Results
Azacitidine restores the expression of
p75NTR in PC3 and 22rv1 cell models
In the present study, we demonstrated that the
antiproliferative and pro-apoptotic effects induced by azacitidine
at non-toxic concentrations (ranging between 0.1 and 0.5 μM)
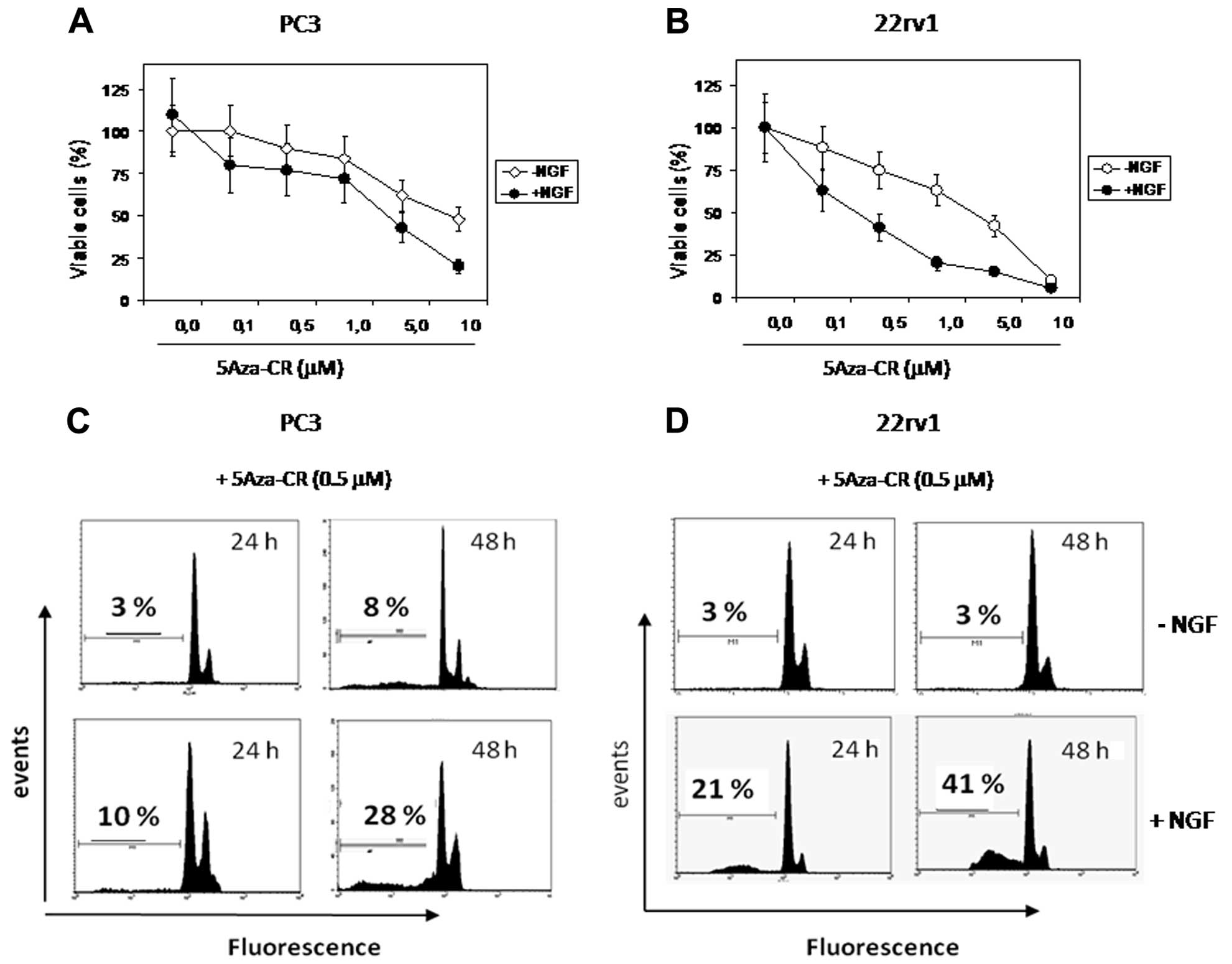
were enhanced in the presence of 10 ng/ml NGF. We observed that
NGF-induced growth inhibition was significantly higher when
compared to data observed following treatment with the
demethylating agent alone both in the PC3 (Fig. 1A) and 22rv1 cells (Fig. 1B). The addition of NGF triggered a
significant and early apoptosis in both cell lines (Fig. 1C and D). Next, we analyzed the
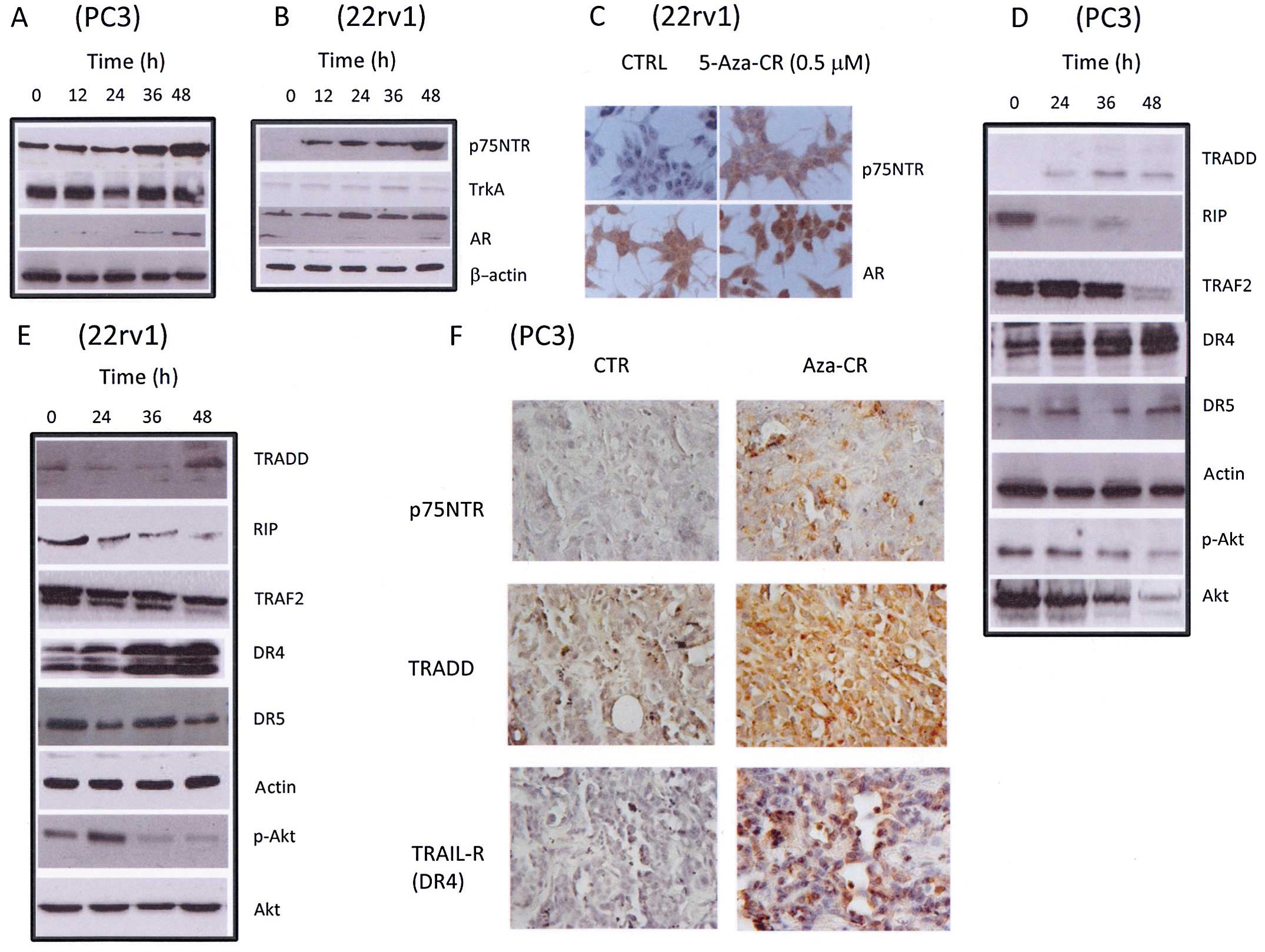
molecular arrangement induced by treatments using western blotting.
We observed that expression of the high affinity p75NTR,
but not expression of the low affinity receptor (TrkA) was
increased after in vitro administration of azacitidine in a
time-dependent manner in the PC3 and 22rv1 cells (Fig. 2A and B). As a positive control we
used the androgen receptor (AR) which was widely demonstrated to be
induced after azacitidine treatment (19,25).
Western blot analyses were confirmed by immunocytochemical analyses
(Fig. 2C) in the aggressive 22rv1
cell line. The same tumor cell models were examined for expression
levels of downstream components proximal to p75NTR
(TRADD, RIP, DR4, DR5 and TRAF2) in both the PC3 (Fig. 2D) and 22rv1 cells (Fig. 2E). Previously, we demonstrated that
an intraperitoneal administration of azacitidine
(Vidaza®, 0.8 mg/kg for 7 consecutive days) in nude male
mice subcutaneously inoculated with 22rv1 and PC3 cells was able to
reduce tumor growth both in the PC3 and 22rv1 xenograft models
(22). In the present study, we
demonstrated that reduction in the tumor mass after Aza-CR
treatment was associated with p75NTR re-expression in
vivo (Fig. 2F). Cell death was
dependent on activation of the caspase 9 pathway which we also
verified in vivo by analyzing FasL and TRAIL protein
expression in the control and Aza-CR-treated tumors.
Azacitidine induces
p75NTR-mediated cell death
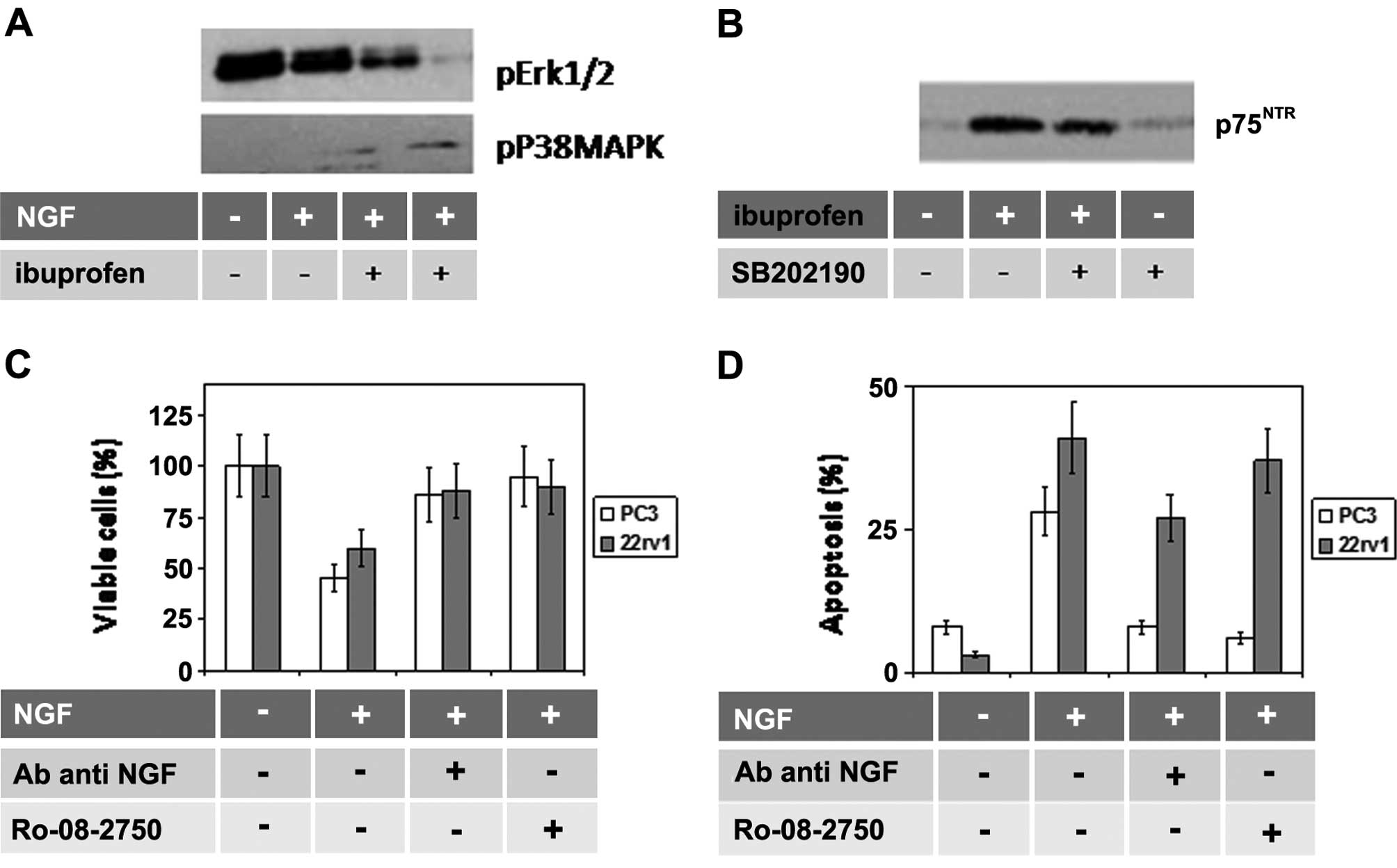
It has been previously demonstrated that p38 MAPK
activation, associated with ibuprofen treatment, is related to
induction of p75NTR in PCa cells (26). Therefore, considering that the
antitumor effects of azacitidine are associated with increased p38
MAPK activity necessary for the induction of cell cycle arrest in
the G2 phase (27,28) and apoptosis (29), we verified whether the p38 MAPK
inhibitor, SB202190, was able to prevent the induction of
p75NTR using as a positive control, treatment with
ibuprofen (1 μM). In agreement with the literature data, we
demonstrated that: i) NGF reduced ERK activation, but not p38 MAPK
(Fig. 3A), and ii) ibuprofen
induced p75NTR (Fig. 3B)
whereas p38 MAPK inhibition was able to reduce p75NTR
expression (Fig. 3B). Similar
results were obtained using P38 MAPK siRNA (data not shown)
indicating that p75NTR expression was under p38
MAPK-dependent epigenetic control. Thus, we analyzed the role of
p75NTR in the azacitidine-induced cell death. For this
aim we used a blocking antibody for p75NTR as well as
the non-peptide antagonist of NGF, Ro 08-2750 [able to bind to the
NGF dimer and inhibit selectively p75NTR at
submicromolar concentrations whereas both p75NTR and
TrkA receptors were blocked at >5 μM (29)]. Both anti-NGF agents significantly
increased the cell viability (Fig.
3C) and apoptosis (Fig. 3D)
induced by co-treatment with azacitidine and NGF. Conversely the
p75NTR induction by Aza-CR treatment was partial after
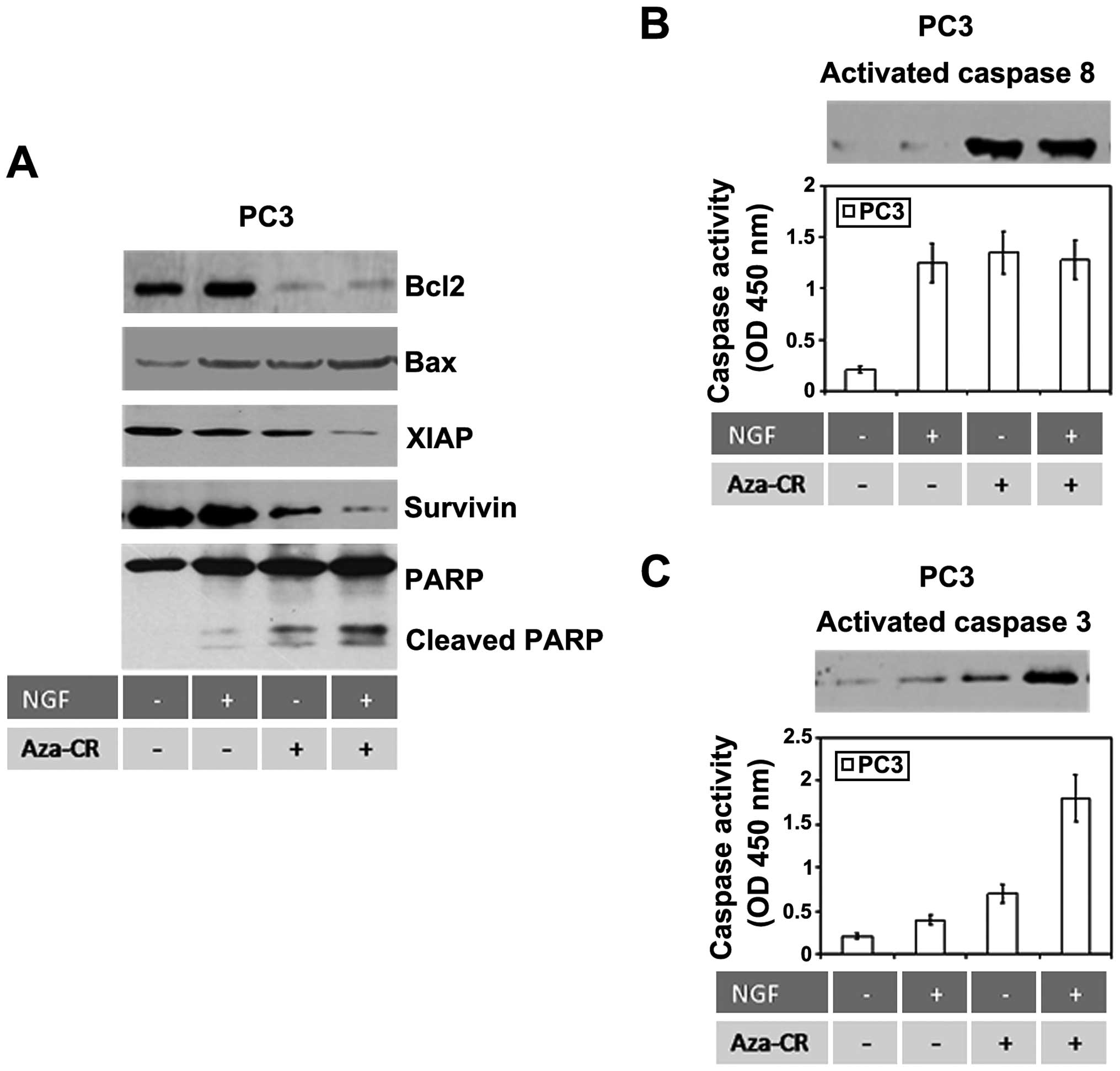
SB202190 (data not shown). Increased p75NTR observed
after azacitidine treatment was related to increased expression of
Bax and PARP cleavage as well as to reduced levels of Bcl2, XIAP
and survivin (Fig. 4A) with caspase
8-dependent caspase 3 activation (Fig.
4B and C) in agreement with our in vivo effects
(30).
Discussion
The low-affinity neurotrophin receptor
p75NTR, a member of the tumor necrosis factor (TNF)
receptor superfamily, is expressed in prostate gland (6–11) and
has been implicated in promoting cell apoptosis and death through a
conserved intracellular death domain (31–34).
In the present study, we demonstrated that azacitidine was able to
restore the expression of p75NTR in
p75NTR-negative and aggressive prostate cancer (PCa)
cell lines. This was dependent on increased p38 MAPK activation and
the inhibition of DNMT actvitiy (35,36),
and this was in agreement with a recent study in which estrogen and
azacitidine modulated the increased expression of p75NTR
and apoptosis in 22rv1 cells (24).
Previously, it has been shown that treatment with nonsteroidal
anti-inflammatory drugs induces p75NTR expression
leading to p75NTR-mediated decreased survival through a
p38 MAPK-mediated mechanism (26–28).
p38 MAPK could be a complementary mechanism by which prostate tumor
cells re-express p75NTR. The re-expression of
p75NTR tumor suppressor activity is associated with
reduced tumor cell growth and activation of caspase 9-dependent
caspase 3 activation. Since the re-expression of p75NTR
does not alter levels of TrkA, these changes in cell cycle
progression can be directly attributed to the changes in levels of
the p75NTR protein. These effects were increased in the
presence of NGF and indicate a TRAIL-mediated crosstalk with TRAILR
(DR4) (37,38) or FAS:FASL signaling (39,40).
In addition, cell cycle arrest, differentiation and apoptosis after
p75NTR activation could involve further intracellular
signals. It has been demonstrated that anti-inflammatory compounds
modulate DNMT expression/activity (41,42)
via phospholipase A2, able for example to induce cell arrest and
differentiation in rat NK cells (43), and PPARγ activation (43) but not PPARβ, involved in
differentiation of neuronal cells (44,45).
In the absence of a ligand, p75NTR-dependent cell cycle
arrest was accompanied by a rank-order increase in the apoptosis of
PCa cells. NGF increased the apoptosis, via mitochondrial
activation of a caspase cascade, induced by p75NTR
re-expression. Taken together, it seems clear that
p75NTR expression selectively alters specific cell cycle
regulatory molecules that retard progression. In conclusion, the
present study showed that p75NTR retards cell cycle
progression and induces NGF-mediated apoptosis and suggests that
the NGF network could be a candidate for future pharmacological
manipulation for aggressive PCa.
References
|
1
|
Malvezzi M, Bertuccio P, Levi F, La
Vecchia C and Negri E: European cancer mortality predictions for
the year 2014. Ann Oncol. 25:1650–1656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thoreson GR, Gayed BA, Chung PH and Raj
GV: Emerging therapies in castration resistant prostate cancer. Can
J Urol. 21(Supp 1): S98–S105. 2014.
|
|
3
|
Matsumoto K, Hagiwara M, Tanaka N,
Hayakawa N, Ishida M, Ninomiya A, Nakajima Y and Nakamura S:
Survival following primary androgen deprivation therapy for
localized intermediate- or high-risk prostate cancer: Comparison
with the life expectancy of the age-matched normal population. Med
Oncol. 31:9792014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rettig WJ, Thomson TM, Spengler BA,
Biedler JL and Old LJ: Assignment of human nerve growth factor
receptor gene to chromosome 17 and regulation of receptor
expression in somatic cell hybrids. Somat Cell Mol Genet.
12:441–447. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haiman CA, Chen GK, Blot WJ, Strom SS,
Berndt SI, Kittles RA, Rybicki BA, Isaacs WB, Ingles SA, Stanford
JL, et al: Genome-wide association study of prostate cancer in men
of African ancestry identifies a susceptibility locus at 17q21. Nat
Genet. 43:570–573. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watson SK, Woolcock BW, Fee JN, Bainbridge
TC, Webber D, Kinahan TJ, Lam WL and Vielkind JR: Minimum altered
regions in early prostate cancer progression identified by high
resolution whole genome tiling path BAC array comparative
hybridization. Prostate. 69:961–975. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnson D, Lanahan A, Buck CR, Sehgal A,
Morgan C, Mercer E, Bothwell M and Chao M: Expression and structure
of the human NGF receptor. Cell. 47:545–554. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sigala S, Bodei S, Missale C, Zani D,
Simeone C, Cunico SC and Spano PF: Gene expression profile of
prostate cancer cell lines: Effect of nerve growth factor
treatment. Mol Cell Endocrinol. 284:11–20. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rende M, Rambotti MG, Stabile AM, Pistilli
A, Montagnoli C, Chiarelli MT and Mearini E: Novel localization of
low affinity NGF receptor (p75) in the stroma of prostate cancer
and possible implication in neoplastic invasion: An
immunohistochemical and ultracytochemical study. Prostate.
70:555–561. 2010.
|
|
10
|
Arrighi N, Bodei S, Zani D, Simeone C,
Cunico SC, Missale C, Spano P and Sigala S: Nerve growth factor
signaling in prostate health and disease. Growth Factors.
28:191–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Festuccia C, Gravina GL, Muzi P, Pomante
R, Ventura L, Ricevuto E, Vicentini C and Bologna M: In vitro and
in vivo effects of bicalutamide on the expression of TrkA and P75
neurotrophin receptors in prostate carcinoma. Prostate.
67:1255–1264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bassili M, Birman E, Schor NF and Saragovi
HU: Differential roles of Trk and p75 neurotrophin receptors in
tumorigenesis and chemoresistance ex vivo and in vivo. Cancer
Chemother Pharmacol. 65:1047–1056. 2010. View Article : Google Scholar
|
|
13
|
Perez M, Regan T, Pflug B, Lynch J and
Djakiew D: Loss of low-affinity nerve growth factor receptor during
malignant transformation of the human prostate. Prostate.
30:274–279. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fromont G, Godet J, Pires C, Yacoub M,
Dore B and Irani J: Biological significance of perineural invasion
(PNI) in prostate cancer. Prostate. 72:542–548. 2012. View Article : Google Scholar
|
|
15
|
Khwaja F, Tabassum A, Allen J and Djakiew
D: The p75NTR tumor suppressor induces cell cycle arrest
facilitating caspase mediated apoptosis in prostate tumor cells.
Biochem Biophys Res Commun. 341:1184–1192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Molloy NH, Read DE and Gorman AM: Nerve
growth factor in cancer cell death and survival. Cancers.
3:510–530. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sigala S, Tognazzi N, Rizzetti MC, Faraoni
I, Missale C, Bonmassar E and Spano P: Nerve growth factor induces
the re-expression of functional androgen receptors and
p75NGFR in the androgen-insensitive prostate cancer cell
line DU145. Eur J Endocrinol. 147:407–415. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sánchez C, Clementi M, Benitez D,
Contreras H, Huidobro C and Castellón E: Effect of GnRH analogs on
the expression of TrkA and p75 neurotrophin receptors in primary
cell cultures from human prostate adenocarcinoma. Prostate.
65:195–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gravina GL, Marampon F, Piccolella M,
Motta M, Ventura L, Pomante R, Popov VM, Zani BM, Pestell RG,
Tombolini V, et al: Hormonal therapy promotes hormone-resistant
phenotype by increasing DNMT activity and expression in prostate
cancer models. Endocrinology. 152:4550–4561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gravina GL, Marampon F, Di Staso M,
Bonfili P, Vitturini A, Jannini EA, Pestell RG, Tombolini V and
Festuccia C: 5-Azacitidine restores and amplifies the bicalutamide
response on preclinical models of androgen receptor expressing or
deficient prostate tumors. Prostate. 70:1166–1178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCabe MT, Low JA, Daignault S, Imperiale
MJ, Wojno KJ and Day ML: Inhibition of DNA methyltransferase
activity prevents tumorigenesis in a mouse model of prostate
cancer. Cancer Res. 66:385–392. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zorn CS, Wojno KJ, McCabe MT, Kuefer R,
Gschwend JE and Day ML: 5-aza-2′-deoxycytidine delays
androgen-independent disease and improves survival in the
transgenic adenocarcinoma of the mouse prostate mouse model of
prostate cancer. Clin Cancer Res. 13:2136–2143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Festuccia C, Gravina GL, D'Alessandro AM,
Muzi P, Millimaggi D, Dolo V, Ricevuto E, Vicentini C and Bologna
M: Azacitidine improves antitumor effects of docetaxel and
cisplatin in aggressive prostate cancer models. Endocr Relat
Cancer. 16:401–413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu JD, Yang K, Mao QQ, Kong DB, Zheng XY
and Xie LP: Estrogen in combination with 5-azacitidine up-regulates
p75NTR expression and induces apoptosis in 22Rv1 prostate cancer
cells. Mol Med Rep. 2:831–836. 2009.PubMed/NCBI
|
|
25
|
Gravina GL, Festuccia C, Millimaggi D,
Dolo V, Tombolini V, de Vito M, Vicentini C and Bologna M: Chronic
azacitidine treatment results in differentiating effects,
sensitizes against bicalutamide in androgen-independent prostate
cancer cells. Prostate. 68:793–801. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khwaja F, Allen J, Lynch J, Andrews P and
Djakiew D: Ibuprofen inhibits survival of bladder cancer cells by
induced expression of the p75NTR tumor suppressor
protein. Cancer Res. 64:6207–6213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uzgare AR, Kaplan PJ and Greenberg NM:
Differential expression and/or activation of P38 MAPK, erk1/2, and
jnk during the initiation and progression of prostate cancer.
Prostate. 55:128–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cho SD, Li G, Hu H, Jiang C, Kang KS, Lee
YS, Kim SH and Lu J: Involvement of c-Jun N-terminal kinase in G2/M
arrest and caspase-mediated apoptosis induced by sulforaphane in
DU145 prostate cancer cells. Nutr Cancer. 52:213–224. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eibl JK, Strasser BC and Ross GM:
Identification of novel pyrazoloquinazolinecarboxilate analogues to
inhibit nerve growth factor in vitro. Eur J Pharmacol. 708:30–37.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Festuccia C, Gravina GL, D'Alessandro AM,
Millimaggi D, Di Rocco C, Dolo V, Ricevuto E, Vicentini C and
Bologna M: Downmodulation of dimethyl transferase activity enhances
tumor necrosis factor-related apoptosis-inducing ligand-induced
apoptosis in prostate cancer cells. Int J Oncol. 33:381–388.
2008.PubMed/NCBI
|
|
31
|
He W, Wang Q, Xu J, Xu X, Padilla MT, Ren
G, Gou X and Lin Y: Attenuation of TNFSF10/TRAIL-induced apoptosis
by an autophagic survival pathway involving TRAF2- and
RIPK1/RIP1-mediated MAPK8/JNK activation. Autophagy. 8:1811–1821.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Labsch S, Liu L, Bauer N, Zhang Y,
Aleksandrowicz E, Gladkich J, Schönsiegel F and Herr I:
Sulforaphane and TRAIL induce a synergistic elimination of advanced
prostate cancer stem-like cells. Int J oncol. 44:1470–1480.
2014.PubMed/NCBI
|
|
33
|
Szliszka E, Zydowicz G, Mizgala E and Krol
W: Artepillin C (3,5-diprenyl-4-hydroxycinnamic acid) sensitizes
LNCaP prostate cancer cells to TRAIL-induced apoptosis. Int J
Oncol. 41:818–828. 2012.PubMed/NCBI
|
|
34
|
Jung YH, Lim EJ, Heo J, Kwon TK and Kim
YH: Tunicamycin sensitizes human prostate cells to TRAIL-induced
apoptosis by upregulation of TRAIL receptors and downregulation of
cIAP2. Int J Oncol. 40:1941–1948. 2012.PubMed/NCBI
|
|
35
|
Gober MD, Smith CC, Ueda K, Toretsky JA
and Aurelian L: Forced expression of the H11 heat shock protein can
be regulated by DNA methylation and trigger apoptosis in human
cells. J Biol Chem. 278:37600–37609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Monks TJ, Xie R, Tikoo K and Lau SS:
Ros-induced histone modifications and their role in cell survival
and cell death. Drug Metab Rev. 38:755–767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang D, Lu J and Tindall DJ: Androgens
regulate TRAIL-induced cell death in prostate cancer cells via
multiple mechanisms. Cancer Lett. 335:136–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shankar S and Srivastava RK: Enhancement
of therapeutic potential of TRAIL by cancer chemotherapy and
irradiation: Mechanisms and clinical implications. Drug Resist
Updat. 7:139–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mitsiades N, Poulaki V, Tseleni-Balafouta
S, Koutras DA and Stamenkovic I: Thyroid carcinoma cells are
resistant to FAS-mediated apoptosis but sensitive to tumor necrosis
factor-related apoptosis-inducing ligand. Cancer Res. 60:4122–4129.
2000.PubMed/NCBI
|
|
40
|
Mimouni-Rongy M, White JH, Weinstein DE,
Desbarats J and Almazan G: Fas ligand acts as a counter-receptor in
Schwann cells and induces the secretion of bioactive nerve growth
factor. J Neuroimmunol. 230:17–25. 2011. View Article : Google Scholar
|
|
41
|
Yin L and Chung WO: Epigenetic regulation
of human β-defensin 2 and CC chemokine ligand 20 expression in
gingival epithelial cells in response to oral bacteria. Mucosal
Immunol. 4:409–419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Javierre BM and Richardson B: A new
epigenetic challenge: Systemic lupus erythematosus. Adv Exp Med
Biol. 711:117–136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cifone MG, Botti D, Festuccia C,
Napolitano T, del Grosso E, Cavallo G, Chessa MA and Santoni A:
Involvement of phospholipase A2 activation and arachidonic acid
metabolism in the cytotoxic functions of rat NK cells. Cell
Immunol. 148:247–258. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Benedetti E, Galzio R, Cinque B, Biordi L,
D'Amico MA, D'Angelo B, Laurenti G, Ricci A, Festuccia C, Cifone
MG, et al: Biomolecular characterization of human glioblastoma
cells in primary cultures: Differentiating and antiangiogenic
effects of natural and synthetic PPARgamma agonists. J Cell
Physiol. 217:93–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Di Loreto S, D'Angelo B, D'Amico MA,
Benedetti E, Cristiano L, Cinque B, Cifone MG, Cerù MP, Festuccia C
and Cimini A: PPARbeta agonists trigger neuronal differentiation in
the human neuroblastoma cell line SH-SY5Y. J Cell Physiol.
211:837–847. 2007. View Article : Google Scholar : PubMed/NCBI
|