Introduction
Pancreatic cancer is a malignant disease, and the
mortality is almost equal to the incident rate due to its
aggressive progression and poor prognosis (1). Most pancreatic cancer-related deaths
are caused by extremely low diagnostic rate because of its
non-distinctive symptoms. Patients with early-stage disease have
been reported to have better outcomes, while the majority of
patients have no chance of operation once they are diagnosed with
terminal-stage, even with metastasis (2). Although, palliative chemotherapy and
local chemotherapy are the treatment options for those patients
with metastasis, the long-term survival rate is still low. After a
long period of searching, more explorations have focused on the
promising targeted drugs and the molecular basis of the cancer
(3), especially the successful
application of FOLFIRINOX protocol (4) and the use of nab-paclitaxel (5), hence, gene therapy is considered as
one of the most promising procedures for improving cancer
prognoses. Thus, unraveling the molecular mechanisms contributing
to pancreatic cancer initiation and progression is urgent.
Recent research has demonstrated the retinal
determination gene network (GDRN), a feed-forward transcriptional
subcircuit, plays an essential role in regulating cell
proliferation and differentiation during embryo development, as
well as correlated with cell migration directly (6). Human Dachshund homologue 1 (DACH1) has
been implicated as a component of the GDRN, which interacts with
other genes of Pax6, Six3, Six1/2, Eya. Noteworthy, the expression
of DACH1 gene is altered in different cancers, it was suppressed in
breast, prostate, and gastric cancer (7–9), but
overexpressed in ovarian cancer and myeloid leukemia (10,11).
However, our previous study detected the expression of DACH1 as
significantly upregulated in Capan-1 pancreatic cancer cells
compared with the normal counterpart, the immortalized epithelial
cell HPDE6-c7, but its role in pancreatic tumor genesis has not yet
been fully elucidated.
In our present study, we focused on the effect of
DACH1 in tumor cell proliferation and mobility. DACH1 expression
was determined both at the mRNA and protein levels via
semi-quantitative RT-PCR and western blotting, respectively.
Besides, the changes in proliferation and growth of Capan-1 cells
after knockdown of DACH1 gene were observed with colony formation
and CCK8 assays, while cell cycle and apoptosis were examined with
a flow cytometer. Cell migration and invasion abilities were then
detected by the chamber assay. Furthermore, the signaling proteins
involved in the tumor cell apoptosis and invasion were investigated
using western blotting.
Materials and methods
Cell lines culture and transfection
Four pancreatic cancer cell lines (PANC-1, BxPC-3,
AsPC-1 and Capan-1), the immortalized pancreatic ductal epithelial
cells (HPDE6-c7) and the bacterial liquid pGenesil-1 were obtained
from Chongqing Key Laboratory of Molecular Oncology and Epigenetics
(The First Affiliated Hospital of Chongqing Medical University,
Chongqing, China). The cells were maintained in high-glucose
Dulbecco's modified Eagle's medium (DMEM, Hyclone, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA,
USA) and antibiotics, including 100 U/ml penicillin and 100 mg/ml
streptomycin (Beyotime Biotechnology, Jiangsu, China). Cells were
incubated at 37°C in a humidified atmosphere with 5 %
CO2 and passaged 1:3 once 80% confluence was reached
approximately every 2–3 days. Cells were passaged twice, seeded
onto a six-well plate and cultured overnight. Then 250 μl
serum-free DMEM was separately mixed with 4 μg plasmids and
5 μl Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) for
5 min at room temperature, subsequently, the diluted plasmid DNA
was combined gently with diluted transfected agent Lipo for further
30 min. Then the mixtures without serum were added in each well for
cell starvation at 37° with 5% CO2 incubator, after 4–6
h starvation, the medium was changed with the complete medium
containing 10% FBS.
RNA isolation and semi-quantitative
RT-PCR analysis
Total RNA was separately isolated from cell lines by
using TRIzol reagent (Takara, Dalian, China), reverse transcribed
using Go Script Reverse Transcription System (Promega, Madison, WI,
USA), and then semi-quantitative RT-PCR was performed with Go
Taq® Flexi DNA polymerase (Promega). The temperature
profile used for PCR was 95°C initial denaturation for 2 min,
followed by 36 cycles of 95°C denaturation for 30 sec, 55°C primer
annealing for 30 sec and 72°C extension for 30 sec, ultimately with
a 3 min extension step at 72°C. β-actin was amplified as a positive
control with 23 cycles as well. The amplification products were
electrophoresed into a 2% agarose gel (Biowest, Barcelona, Spain),
then images were captured by Bio-Rad gel imaging instrument and
analyzed with Quantity One software. The primer sets for DACH1 gene
were: forward, 5′-TTGCATACGGTCTACACCAAG-3′; reverse,
5′-TGAGACCAGGGACAGAATGC-3′. β-actin primers: forward,
5′-CTCCATCCTGGCCTCGCTGT-3′; reverse,
5′-GCTGTCACCTTCACCGTTCC-3′.
Design and synthesis of shRNA
The Homo sapien DACH1 gene sequence (GenBank
Accession no. NM_080759.5) was identified in the NCBI gene bank.
According to the principles of siRNA design, three small
interfering RNA (siRNA) targeting human DACH1 (site at 1325th,
1543th and 1744th base) were designed by primer design software
Primer Premier 5 and Oligo 6, which has no sequences homologous
with any other gene. Moreover, a 21-base non-specific sequence was
designed as a negative control. Then, the short hairpin structure
was constructed with a stem-loop TTCG, connecting the DACH1-target
siRNA sequence and its reverse complementary sequence. By BLAST
with the sequences in the international nucleotide database, the
specificities of the three DACH1-target shRNAs were confirmed.
These four oligonucleotides were synthesized by Invitrogen
(Shanghai, China).
Construction of recombinant plasmid
The four groups of oligonucleotides were separately
dissolved in distilled water, making the final concentration at 50
μmol/l, and were then mixed with 5X annealing buffer for DNA
oligos according to the manual (Nobleryder, Beijing, China). The
mixture was incubated in 95°C for 2 min, and then dropped to 4°C by
gradient cooling every 90 sec in the polymerase chain reaction
(PCR) machine. The empty vector pGenesil-1 was extracted from
bacterial liquid by E.Z.N.A. Plasmid Mini kit I (Omega Bio-Tek,
Norcross, GA, USA) and double digested with BamHI and
HindIII enzyme (Takara) in a 37°C water bath for 6 h.
Afterward, E.Z.N.A Gel Extraction kit (Omega Bio-Tek) was used to
recycle the restriction fragment after endonuclease reaction.
Subsequently, T4 DNA ligase (Takara) was chosen to connect the
shRNA with the purified pGenesil-1 plasmid fragment at 16°C in the
metal bath overnight. The ligation products were transformed into
the competent Escherichia coli cells DH5α (Tiangen, Beijing,
China). Recombinant plasmids were extracted by E.Z.N.A. Plasmid
Mini kit I for analysis of enzyme identification and sequencing by
Sangon Biotech Co. (Shanghai, China). The correct plasmids were
named as pshRNA1-DACH1, pshRNA2-DACH1, pshRNA3-DACH1,
pGenesil-1-shRNA-negative control (pshRNA-NC).
Recombinant plasmid transfections and
identification of the most effective inhibitory rate group
Endotoxin-free plasmids were extracted using the
E.Z.N.A. Endo-Free Plasmid Mini kit I (Omega Bio-Tek). Pancreatic
cancer cell line Capan-1, which expresses high level of endogenous
DACH1, was transfected with the recombinant plasmids via
Lipofectamine 2000 according to the protocol mentioned above. Cells
were divided into six groups: pshRNA1/2/3-DACH1, pshRNA-NC,
pGenesil-1 and untreated cell. Forty-eight hours after
transfection, the expression of DACH1 was tested by
semi-quantitative RT-PCR and western blotting. The band intensity
values were then analyzed with Quantity One software to choose the
most effective interference plasmid. Besides, the expression of GFP
was observed under an inverted fluorescence microscope (Leica DM
IRB).
Western blot analysis
Total protein was isolated from cells from each
group by using RIPA lysis buffer and PMSF at a ratio of 100:1 and
the protein concentration was detected by BCA protein assay kit
(Beyotime Biotechnology). A total of 40 μg of protein lysate
for each sample was separated by using sodium dodecyl-sulfate
polyacrylamide gel electrophoresis (SDS-PAGE), followed by
electrophoretic transfer to PVDF membranes (Millipore, Billerica,
MA, USA) at a constant current of 250 mA and the membranes were
blocked with 5% nonfat milk for 1–2 h. Then membranes were
incubated with the first antibody at 4°C overnight, followed with
HRP-conjugated goat anti-rabbit/mouse IgG (Cell Signaling
Technology, Danvers, MA, USA). The proteins of interest were
detected using BeyoECL plus reagent (Beyotime Biotechnology). The
antibodies for western blot analyses were as follows: rabbit
anti-DACH1 (Proteintech, Wuhan, China), mouse anti-β-actin, rabbit
anti-Bcl-2, anti-caspase-3 and anti-PARP (Cell Signaling
Technology), rabbit anti-E-cadherin, mouse anti-N-cadherin and
rabbit anti-Vimentin (Abcam, Cambridge, MA, USA). The band
intensity values were analyzed with Quantity One software. Results
were recorded as fold changes compared to an internal reference
standard.
Cell proliferation assay
To assess the influence of the interference plasmid
transfections on cell growth rates, Capan-1 cells were seeded onto
96-well plates in triplicate at a density of 5000 cells/well and
cultured overnight. Cells were transfected with three groups of
plasmids respectively: pshRNA1-DACH1, which identified the highest
inhibitory rate, the negative control pshRNA-NC and the empty
vector pGenesil-1. At 24, 48, 72 h incubation after treated, 10
μl CCK8 solution was added to each well and wells containing
medium without cells were used as blank control. The plates were
incubated for further 2 h. Cell viability was measured with the
Cell Counting Kit8 (CCK8, Dojindo Molecular Technologies, Japan).
The optical density (OD) values were measured at 450 nm and data
are shown as means ± SD.
G418 concentration screening and colony
formation assay
Capan-1 cells were cultured in a 24-well plate with
a cell density of 1×104 each well. The screening
concentrations of G418 were 400, 500, 600, 700, 800, 900, 1000, and
1100 μg/ml, and three replicates were made for each concentration.
Then, a corresponding volume of G418 solution (Amresco, Solon, OH,
USA) was added to each well combined with complement medium and
cells, and changed with new culture medium every 2–3 days. After
selection for two weeks, the minimum lethal concentration of G418
to Capan-1 cells was regarded as the final selection
concentration.
Capan-1 cells were monolayer cultured in petri
dishes until adherence confluence of ~70%, then transfected with
pshRNA1-DACH1, pshRNA-NC and pGenesil-1 plasmid via Lipofectamine
2000. After 48 h, cells with different treatments were separately
collected, the suspension was inoculated into a new 6-well plate,
which was selected for 2 weeks using G418 with a mini selection
concentration of 800 μg/ml. Culture medium and G418 were
changed every 2–3 days. After 14 days of selection, cells were
fixed with 4% paraformaldehyde for 30 min, followed by 0.5% crystal
violet staining for further 30 min. Colonies with >50 cells were
counted.
Detection of cell migration by Transwell
assay
Cell mobility was evaluated by using a 24-well
Transwell chamber with an 8 μm pore-size PET membrane
(Corning Inc., Corning, NY, USA). Briefly, cells were divided into
three groups transfected with appropriate plasmids as mentioned
above. After 48 h transfection, cells were digested with
Trypsin/EDTA and collected from culture flasks. Capan-1 cells were
then suspended in serum-free medium at a density of
2.5×105 cells/ml and 200 μl cell suspension was
added into the upper well, and 700 μl of the complete medium
containing 10% FBS was placed into the lower chamber. The 24-well
plate was cultured at 37°C, in 5% CO2 incubator for 24
h. Cells that migrated from the upper chamber to the lower surface
of the PET membrane were fixed with 40 g/l paraformaldehyde for 30
min and then stained with 1 g/l crystal violet for another 30 min.
Finally, the Transwell chamber was washed with PBS buffer and five
random visual fields were counted to calculate the average number
under a light microscope (×200).
Transwell invasion assay
To detect the effect of knockdown of DACH1 on cell
invasion ability, 24-well Transwell chambers (8 μm
pore-size) were used. Matrigel (BD Biosciences, San Jose, CA, USA)
was mixed with cold DMEM without fetal bovine serum at a ratio of
1:7, which simulates the extracellular matrix structure. Then the
mixtures were loaded onto the upper chamber and incubated at 37°C
for 4 h. Capan-1 cells, transfected with the pshRNA-DACH1,
pshRNA-NC and vector plasmids, were collected and suspended in
serum-free medium. When the mixtures converted to solid state,
approximately 3×104 cells from each group were planted
into the upper compartment and allowed to penetrate to the lower
well containing complete medium with 10% FBS. After incubation for
36 h at 37°C humidified atmosphere with 5% CO2, cells on
the upper side of the filter were wiped off with cotton swabs,
subsequently, the invaded cells on the bottom of the PET membrane
were fixed with paraformaldehyde then stained with crystal violet
as above. The numbers of the invasion cell were counted at five
different areas.
Cell cycle and apoptosis by flow
cytometer
The pshRNA-DACH1, pshRNA-NC and vector plasmids were
separately transfected into Capan-1 cells as described above. For
cell cycle analysis, cells were treated for 48 h, later digested by
Trypsin without EDTA, suspended and washed twice by pre-cold PBS
buffer, then fixed in ice-cold 70% ethanol at 4°C overnight and
analyzed by flow cytometer in Academy of Life Science, Chongqing
Medical University. For assessing cell apoptosis, three groups of
cells were cultured for 48 h, digested with Trypsin/EDTA-free,
suspended in PBS buffer and flow cytometry analysis was immediately
performed. Each experiment was repeated three times.
Statistical analysis
Statistical analysis was performed using SPSS
version 18.0 software. Measurement data were presented as mean ±
SD. The expression difference of DACH1 between pancreatic cancer
cell lines and normal cells was calculated by the Student's t-test,
while comparison of three different treated groups was assessed
using one-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
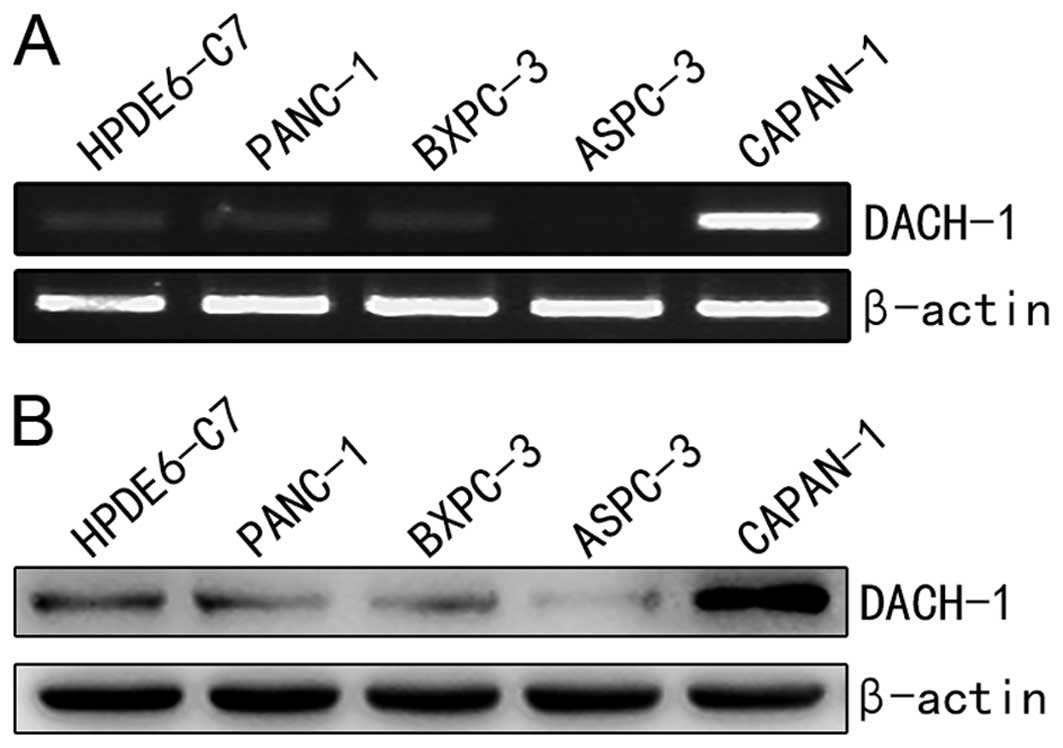
DACH1 is overexpressed in Capan-1 cells
compared to normal epithelial cells
DACH1 expression was altered in different cancers as
previously described (7,9,10,12).
To explore the expression difference of DACH1 between pancreatic
normal epithelial cell HPDE6-c7 cells and cancer cell lines
(PANC-1, BxPC-3, AsPC-1, Capan-1), semi-quantitative RT-PCR and
western blotting were performed. As shown in Fig. 1A, the mRNA level of DACH1 was
significantly higher in Capan-1 cell line, compared with that in
the immortalized epithelial cells. Moreover, DACH1 protein
expression was further confirmed abundant in Capan-1 cancer cells
(Fig. 1B), which correspond to the
RT-PCR analysis. These results indicated that DACH1 was upregulated
in Capan-1 pancreatic cancer cells, we assumed DACH1 might act as a
cancer-promoting gene in pancreas, which may play an opposite role
in tumor proliferation and in the growth of pancreatic cells as
compared to cancer cells originating from other organs such as
breast, prostate and stomach.
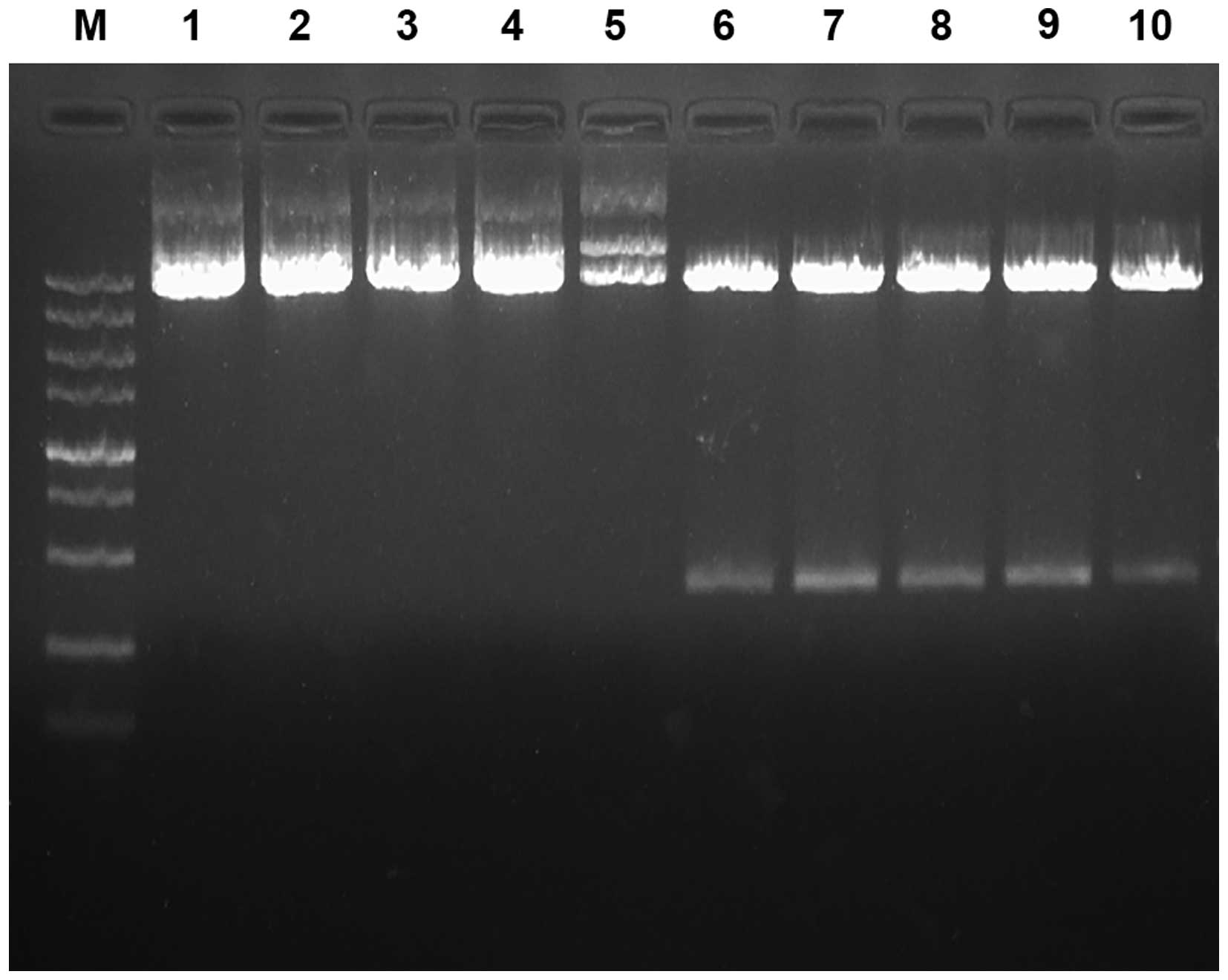
Identification of recombinant plasmid
construction
Three DACH1-shRNA were designed and synthesized
while a nonspecific shRNA as negative control (Table I), which were then inserted into
pGenesil-1 vector plasmid. The recombinant plasmids and pGenesil-1
were identified by using EcoRI single enzyme digestion and
EcoRI/HindIII double enzyme digestion. Our data
demonstrated that with restriction endonuclease digestion, the DNA
fragments of the four pshRNA-DACH1 were greater than pGenesil-1
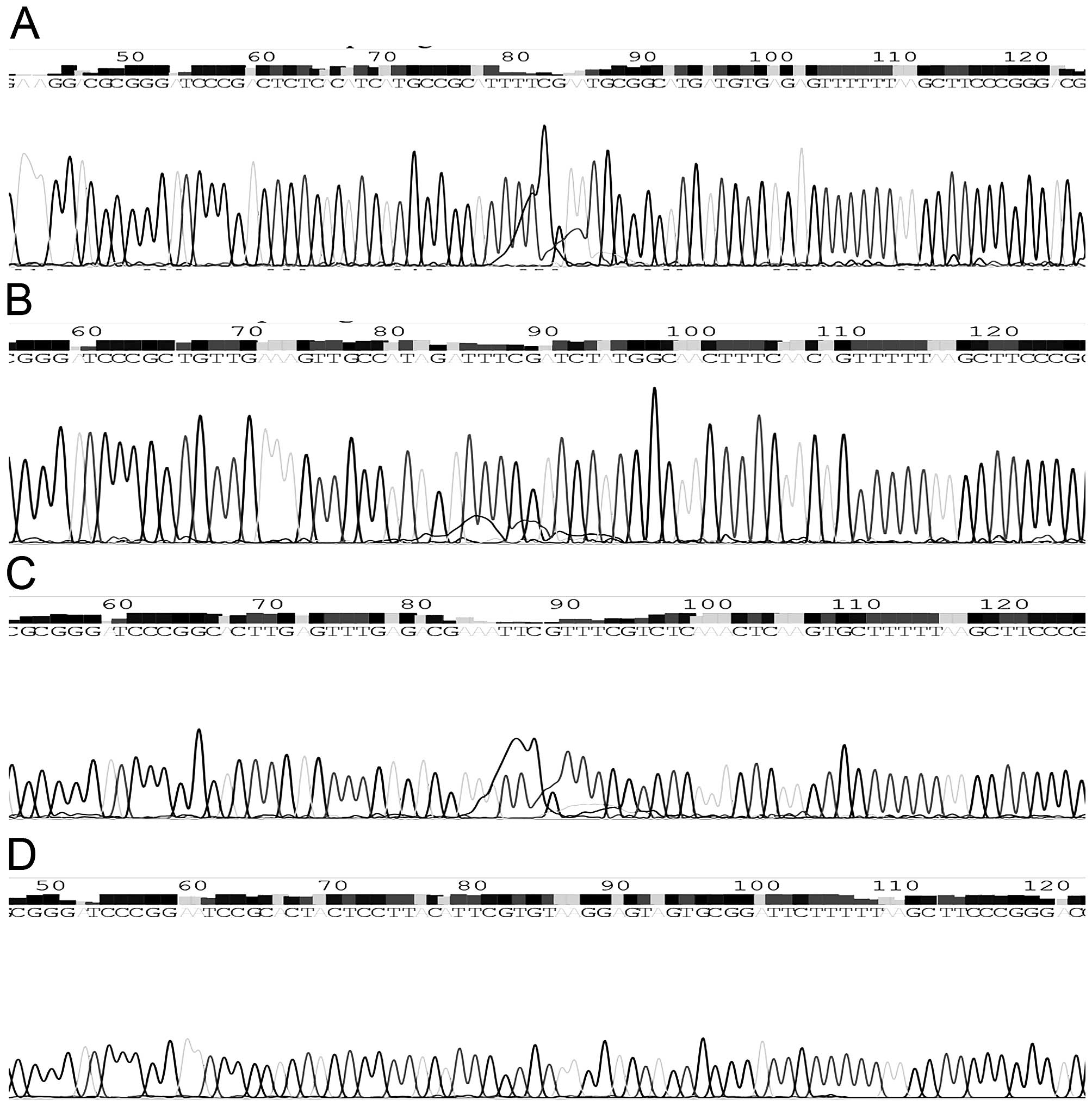
(Fig. 2). Simultaneously,
sequencing analysis showed that the inserted fragment completely
matched with the expected sequence (Fig. 3). All these results confirmed that
the interference plasmids were constructed successfully.
 | Table ISequences of the four shRNA targeting
DACH1. |
Table I
Sequences of the four shRNA targeting
DACH1.
| Target sequences | Nucleotide
sequences |
|---|
| 1.
ACTCTCACATCATGCCGCATT |
5′-GATCCCGACTCTCACATCATGCCGCATTTTCGAATGCGGCATGATGTGAGAGTTTTTTA-3′ |
|
5′-AGCTTAAAAAACTCTCACATCATGCCGCATTCGAAAATGCGGCATGATGTGAGAGTCGG-3′ |
| 2.
CTGTTGAAAGTTGCCATAGAT |
5′-GATCCCGCTGTTGAAAGTTGCCATAGATTTCGATCTATGGCAACTTTCAACAGTTTTTA-3′ |
|
5′-AGCTTAAAAACTGTTGAAAGTTGCCATAGATCGAAATCTATGGCAACTTTCAACAGCGG-3′ |
| 3.
GCACTTGAGTTTGAGACGAAA |
5′-GATCCCGGCACTTGAGTTTGAGACGAAATTCGTTTCGTCTCAAACTCAAGTGCTTTTTA-3′ |
|
5′-AGCTTAAAAAGCACTTGAGTTTGAGACGAAACGAATTTCGTCTCAAACTCAAGTGCCGG-3′ |
| 4.
GAATCCGCACTACTCCTTACA |
5′-GATCCCGGAATCCGCACTACTCCTTACATTCGTGTAAGGAGTAGTGCGGATTCTTTTTA-3′ |
| (Negative
control) |
5′-AGCTTAAAAAGAATCCGCACTACTCCTTACACGAATGTAAGGAGTAGTGCGGATTCCGG-3′ |
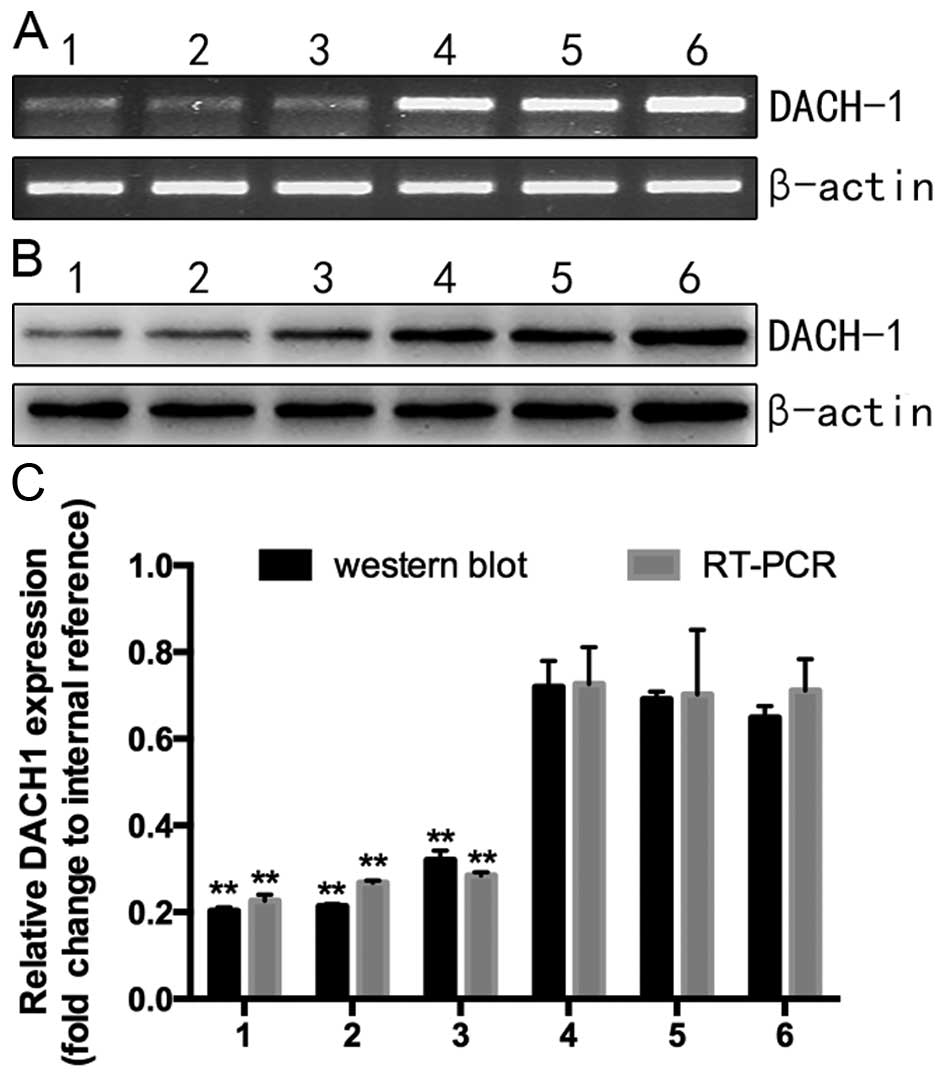
Knockdown efficiency of DACH1-shRNA1/2/3
sequences
To analyze the knockdown efficiency of the
interference plasmid targeting DACH1, samples were divided into six
groups with different treatments: three interference groups
(pshRNA1/2/3-DACH1), pshRNA-NC, the empty plasmid pGenesil-1 and
the untreated cell group. After 48 h transfection, the mRNA and
protein levels of treated and untreated groups were, respectively,
detected by RT-PCR and western blotting. As shown in Fig. 4A, the RNA expression of the six
groups was lowest in the pshRNA1-DACH1, which was consistent with
the protein level (Fig. 4B). Based
on these statistics, we chose pshRNA1-DACH1 as the most efficient
interference plasmid to complete the following experiment.
RNAi-mediated knockdown of DACH1 inhibits
the proliferation and growth of Capan-1 pancreatic cancer
cells
The effect of shRNA targeting DACH1 on cancer cell
proliferation and growth was then evaluated by using CCK8 assay and
colony formation test. As shown in Fig.
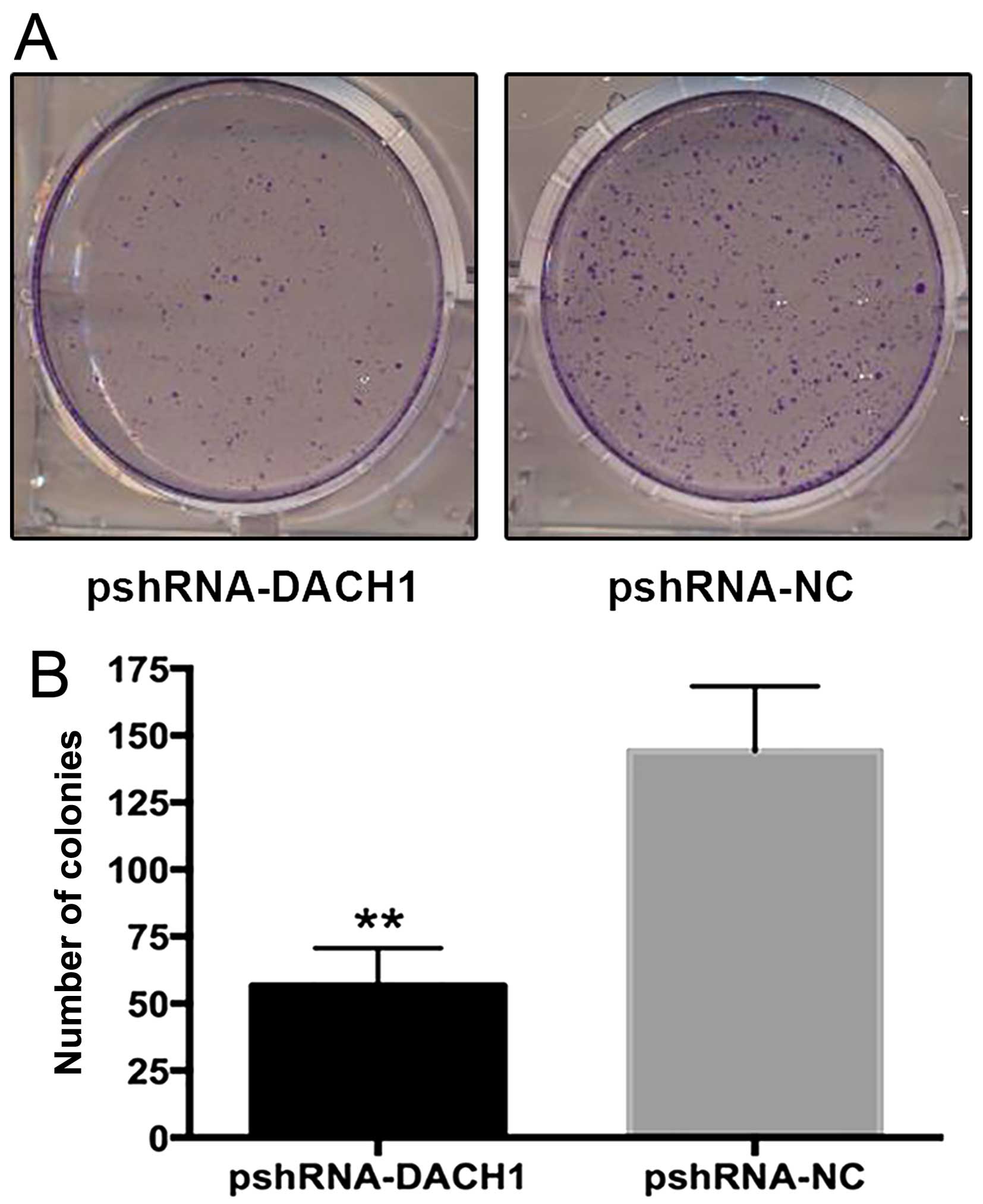
5, the survival colony number of the interference group
compared to the negative control was 56±14 versus 144±24
(P<0.01), indicating ~60% reduction in RNAi-mediated transfected
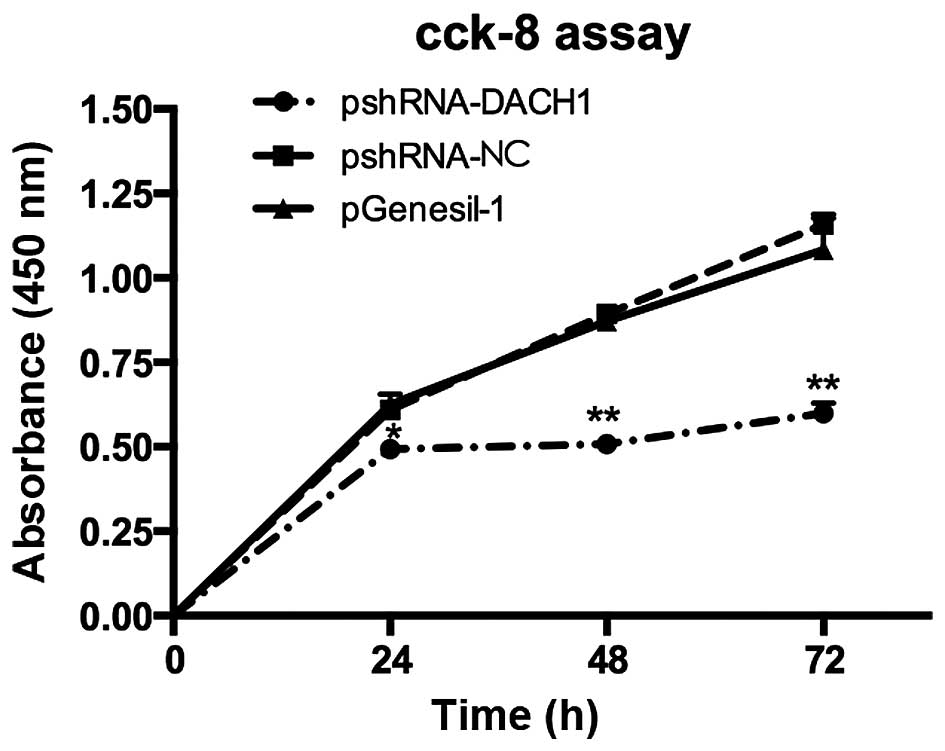
cells. To further confirm the cell viability, CCK-8 kit was used in
accordance with the instruction steps. The OD values were measured
with enzyme-link meters at 450 nm and recorded in Table II. The statistics showed that cell
proliferation rates were all significantly reduced after 24 h, 48
or 72 h transfection with pshRNA1-DACH1 in Capan-1 cells (Fig. 6). Therefore, the results
demonstrated that knockdown of DACH1 could inhibit the
proliferation and growth of Capan-1 cells.
 | Table IIEffect of recombinant plasmids on
cell proliferation.a |
Table II
Effect of recombinant plasmids on
cell proliferation.a
| Plasmids | 24 h | 48 h | 72 h |
|---|
| pshRNA-DACH1 | 0.49±0.013 | 0.51±0.020 | 0.60±0.029 |
| pshRNA-NC | 0.61±0.020 | 0.89±0.002 | 1.16±0.027 |
| pGenesil-1 | 0.63±0.029 | 0.87±0.029 | 1.08±0.094 |
Effect of downregulating DACH1 expression
on Capan-1 cancer cell cycle, apoptosis and the apoptotic
markers
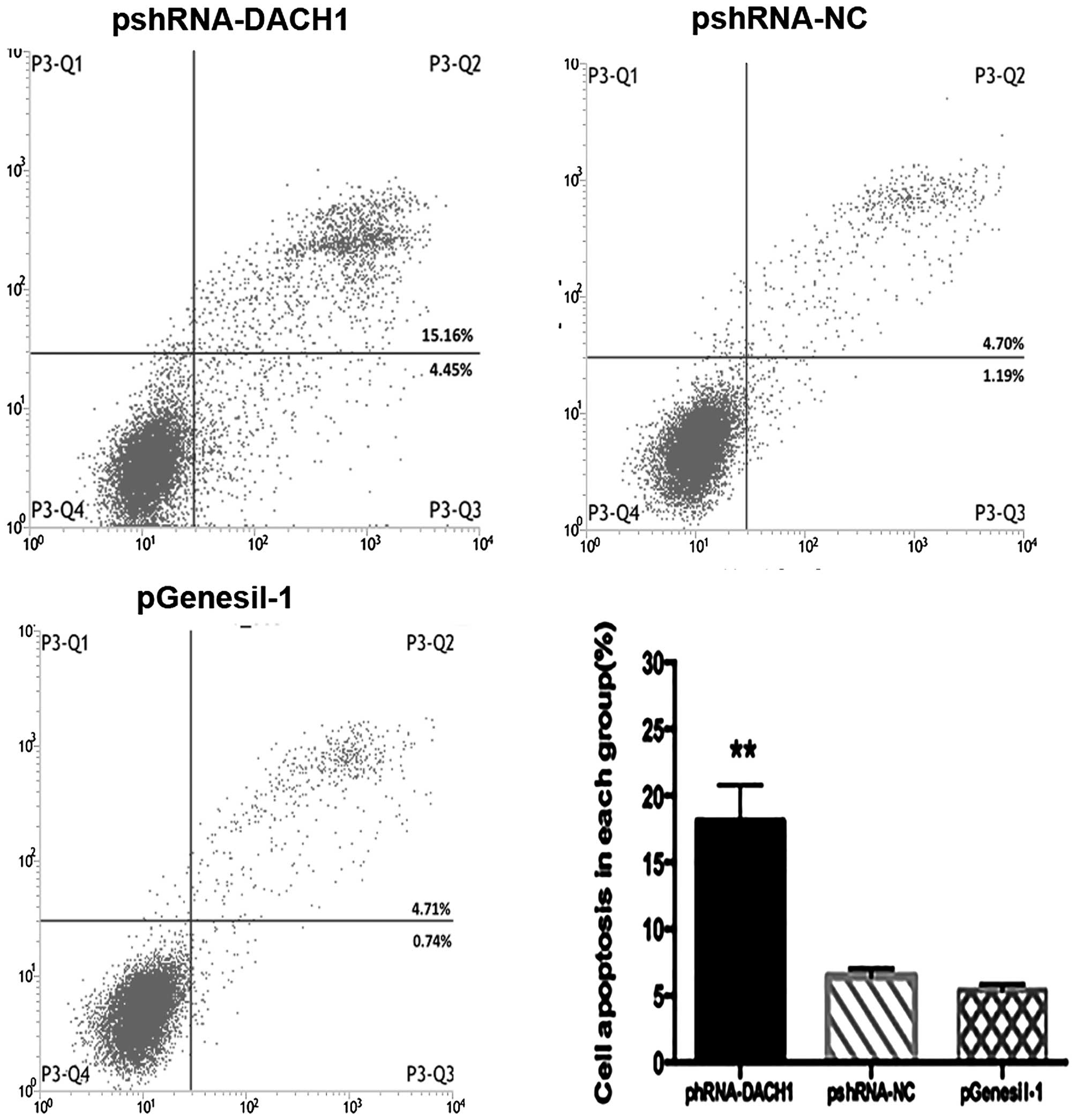
Cell cycle and apoptosis were analyzed by flow
cytometer with Annexin V-FITC/PI staining. Results of cell cycle
distribution among pshRNA-, control- and vector-transfected groups
have no significant difference (data not shown). However,
representative results of apoptosis in different treatment groups
are shown in Fig. 7, and revealed a
significant increase of apoptotic cells in the pshRNA-transfected
group in comparison with the control one. To further explore the
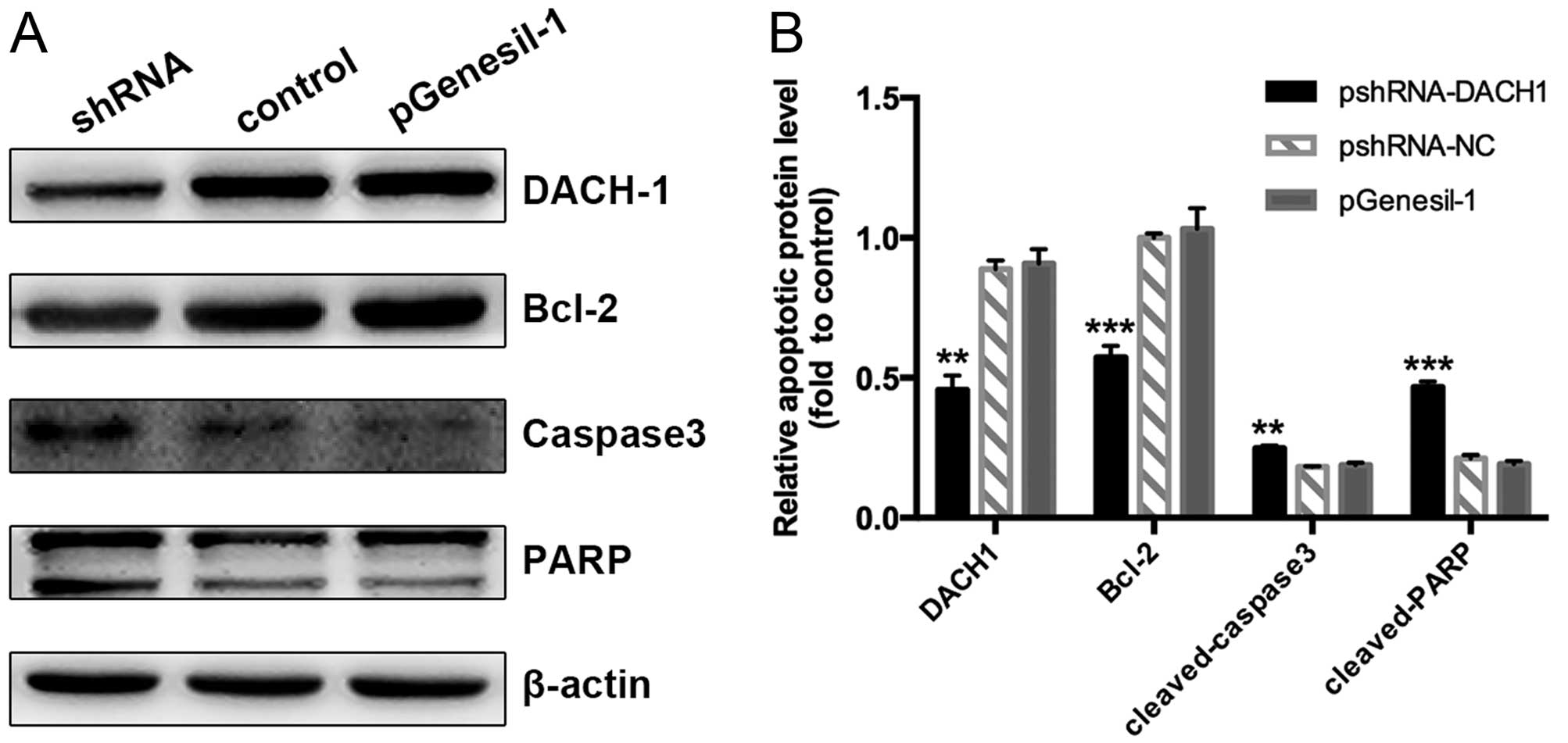
significance of DACH1 on cell apoptosis and the involved
mechanisms, we investigated the expression of apoptotic markers
using western blotting. Our data showed that downregulation of the
DACH1 activity inhibited Bcl2 accumulation in Capan-1 cancer cells,
inducing its downstream target genes the cleaved-caspase 3 and
cleaved poly (ADP-ribose) polymerase PARP expression, compared to
the control groups (Fig. 8A and B).
Taken together, these results indicated that knockdown of DACH1
expression could inhibit tumor cell proliferation and growth,
induced cell apoptosis, which may function as a tumor-promoting
gene in pancreatic cancer.
DACH1 modulates Capan-1 tumor cell
migration and invasion via EMT
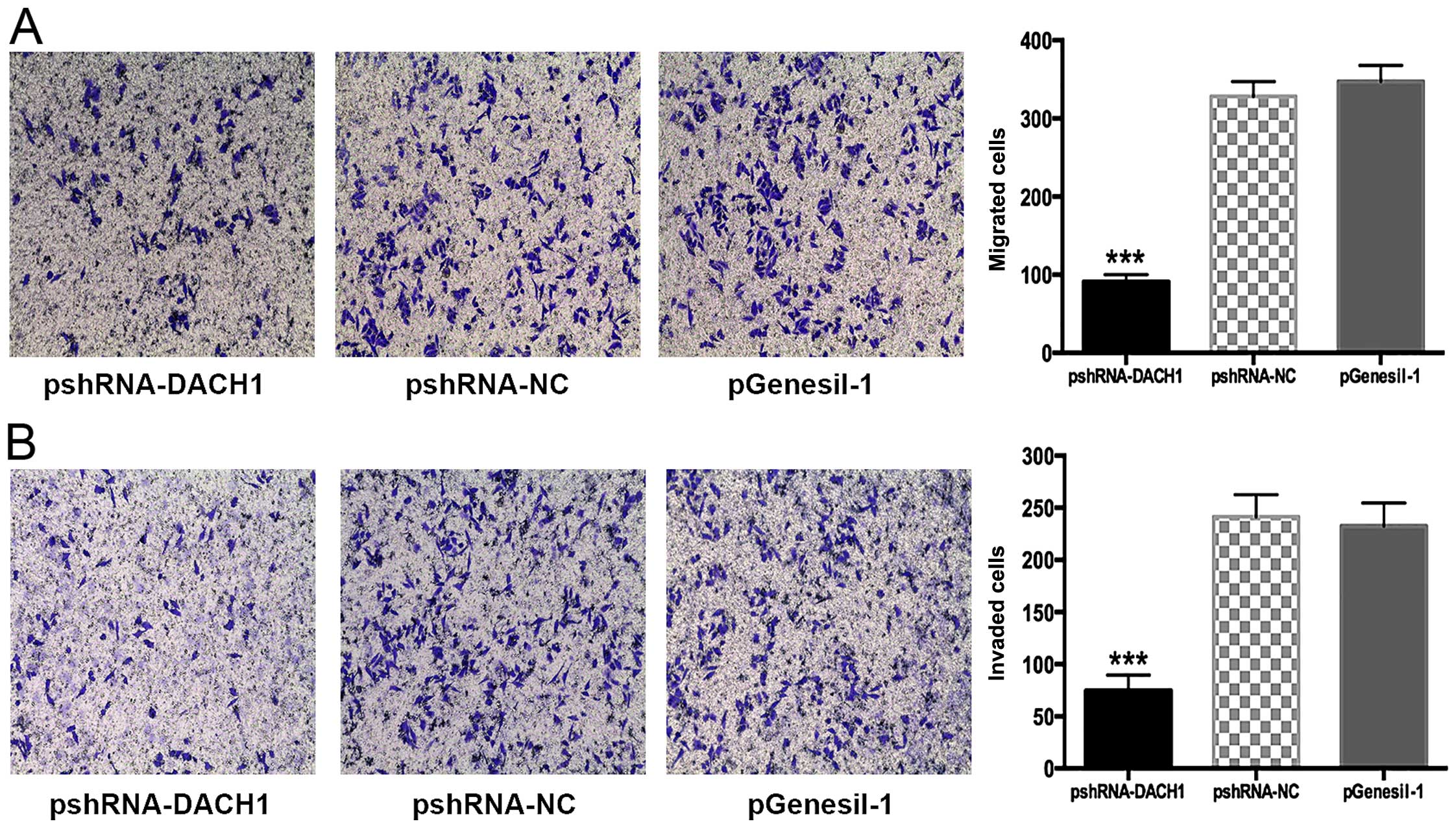
To determine whether DACH1 could influence tumor
cellular mobility, Transwell assay was performed. In Transwell
migration or invasion experiments, the invaded cell numbers were
significantly less in the pshRNA1-DACH1 interference cells than the
control cells (P<0.05) (Fig. 9A and
B). Our results demonstrated DACH1 indeed possess the ability
to promote metastasis in Capan-1 pancreatic cancer cells.
Tumor cell metastasis is a complex process with
multiple factors, which are the biological properties of malignant
carcinomas. Epithelial-mesenchymal transition (EMT) is known as a
dynamic cellular process and often occurs during cancer cell
migration and invasion (13). To
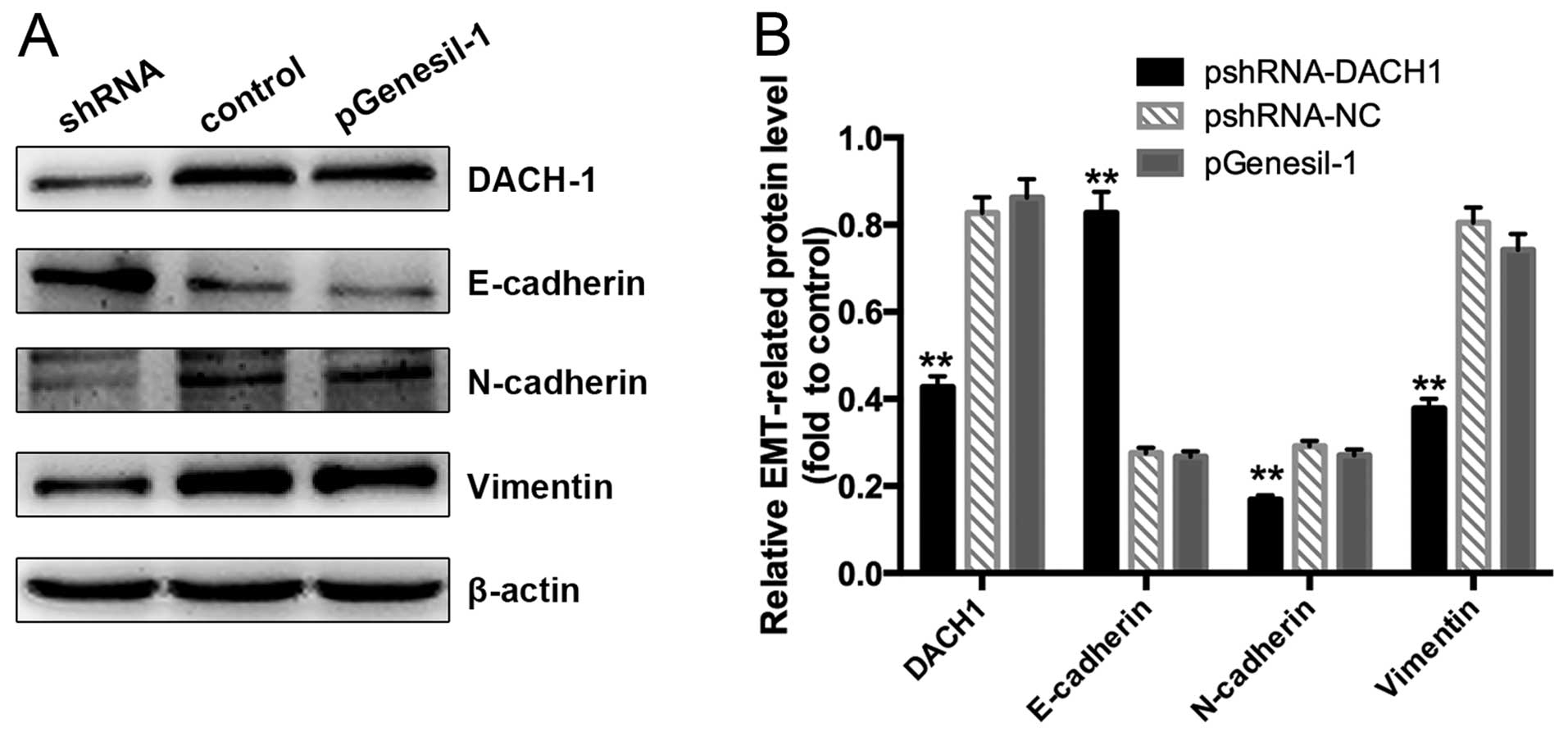
extend our analysis, we further examined whether downregulated
expression of DACH1 in Capan-1 cancer cells was able to trigger a
shift from mesenchyme to epithelia. As shown in Fig. 10A and B, a remarkable increase of
E-cadherin and reduction of N-cadherin and Vimentin expression were
detected in Capan-1 cells which were transfected with pshRNA-DACH1
plasmid. These findings suggested that knockdown the expression of
DACH1 in Capan-1 cells could result in preventing EMT.
Discussion
The incidence of pancreatic cancer has increased in
recent years, ranking the sixth in China, which would bring us a
huge burden of cancer-related death in the future (14,15).
To raise the survival rate and improve the therapeutic outcome, a
multidisciplinary approach must be used to more effectively manage
patients (3), including surgery,
novel drug combinations, and radiation therapy. Fortunately,
tremendous upswing in our understanding of the fundamental genetics
provide hope to advance targeted therapy for the neoplasms of the
pancreas. Currently, carbohydrate antigen (CA) 19-9 is the only
predictive biomarker recognized in general for pancreatic cancer
prognosis. However, in practical clinical application, it shows
poor to moderate sensitivity and its specificity is limited to
screenings of early pancreatic cancer (16).
The RDGN has been explored to integrate multiple
signaling pathways, and is pivotal for the development of many
organs such as eyes, muscle, ear, gonads and the central nervous
system (17). It comprises several
genes to govern tissue specification fate, mutation in any of these
may contribute to the failure of normal development. The dachshund
gene, a component of the RDGN, encodes conserved, nuclear proteins
which play a prominent role in controlling retinal cell fate
determination and leg development in Drosophila (18). The Dach/DACH, the mammalian
homologues of the dachshund gene, was respectively isolated from
mouse and human genes. It shared two highly conserved domains with
Drosophila-Dachbox-N and Dachbox-C (19), which are likely to be functional
domains. Dachbox-N is conserved with the N-terminal of Ski/Sno
proto-oncogenes, which is known as DACH Ski/Sno (DS) domain and
mediates DNA binding and transcriptional activation. Further
investigations have identified that the synergistic effect between
Eya and Dach among mouse homologues was not through a direct
interaction, but was mediated by the involvement of CREB binding
protein (CBP) (20). CBP is a
transcription mediator involved in histone acetyltransferase (HAT)
activity. However, the molecular mapping studies also found that
DACH1 was coprecipitated with histone deacetylase 3 (HDAC3)
(21). This suggests that the
altered expression and function of DACH1 in different cancers is
associated with its complex nature, such as it could either
interact with HAT or histone deacetylase requires
clarification.
In the present study, we analyzed the differential
expression of DACH1 among four cancer cell lines and the normal
counterparts at both mRNA and protein levels. The expression of
DACH1 was abundant in Capan-1 pancreatic cancer cell lines, but
downregulated in normal duct epithelial cell lines. Then, we
applied RNA interference technology to investigate its function and
related mechanisms in pancreatic cancer. As mentioned above, three
recombinant interference plasmids and the negative control one were
constructed successfully and transfected into Capan-1 cancer cells.
CCK8 assay and flow cytometry analysis demonstrated that
downregulation of DACH1 activity with shRNA indeed inhibits
pancreatic cancer cell proliferation by mostly inducing apoptosis,
but had no effect on cell cycling. Apoptosis is a programmed
cellular suicide mechanism and includes series of signal
transduction molecules. Defects in the control of apoptosis are
considered to be hallmarks of tumor genesis (22). The first discovery of a cell death
regulator was cloned from human B-cell lymphomas, namely bcl-2
gene, which acts as a pro-survival regulator (23). Previous research has declared that
deregulated expression of Bcl-2 and its related proteins may
protect cells from apoptotic stimuli, leading to caspase
activation, and subsequent tumor development and maintenance
(24). Our data have shown that the
decrease of Bcl-2 protein was consistent with downregulation of
DACH1, while the downstream genes cleaved-caspase 3 and PARP
expression were upregulated. These findings raise a possibility
that DACH1-mediated regulation of pancreatic cancer cell apoptosis
activates Bcl-2 signaling axis and results in caspase cascade
activation.
Additionally, we reported that knockdown of DACH1
suppressed Capan-1 cell migration and invasion through inhibiting
the EMT process. E-cadherin is recognized as a characteristic
molecule of the epithelial phenotype, which modulates the adherent
junctions to promote desmosome formation and ultimately regulates
cell morphogenesis (25). Loss of
E-cadherin expression is observed in many poorly differentiated
carcinomas, and parallels the expression of mesenchymal cell marker
N-cadherin, further indicating that EMT is correlated with the
progression of primary tumors to invasive carcinoma (26,27).
In the past decades, studies have found that a zinc-finger protein
belonging to Snail family controlled EMT and Snail1 acted as a
transcriptional repressor of the E-cadherin via binding to the
E-box (28,29). Recent research demonstrated that
DACH1 expression suppressed EMT in breast cancer by modulating
Snai1 (30). The regulation of
epithelial or mesechymal markers is vital in EMT process and DACH1
inhibits carcinoma metastasis by interacting with the EMT factor
Snail, thus, we explored whether DACH1 expression could regulate
migration and EMT in pancreatic cancer. Corresponding to our
speculation, our results demonstrated that knockdown of DACH1
activity repressed Capan-1 pancreatic cancer cell migration and
invasiveness in Tranwell assay, furthermore, downregulation of
DACH1 resulted in increased expression of the epithelial marker
E-cadherin, simultaneously upregulation the two mesenchymal markers
N-cadherin and Vimentin. All these results supported our hypothesis
that DACH1 expression plays an essential role in regulating cell
invasive ability via EMT in pancreatic cancer cells. The detailed
mechanism of the molecular interaction with DACH1 in regulating EMT
is worthy of further investigation.
Collectively, our study indicates that DACH1
expression was upregulated in Capan-1 pancreatic cancer cells, and
inhibition of its activity could remarkably repress cell
proliferation and invasion. This suggests that DACH1 may function
either as an oncogene or tumor suppressor gene in different tumor
types. However, whether there are other molecular mechanisms and
signaling pathways interacting with DACH1 in tumorigenesis in
vivo or in vitro should be verified in future
research.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang QH; Coordination Group of The
Committee on Pancreatic Cancer: Clinical analysis of 2340 cases of
pancreatic cancer. Zhonghua Yi Xue Za Zhi. 84:214–218. 2004.In
Chinese. PubMed/NCBI
|
|
3
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al Groupe Tumeurs Digestives of Unicancer;
PRODIGE Intergroup: FOLFIRINOX versus gemcitabine for metastatic
pancreatic cancer. N Engl J Med. 364:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martik ML and McClay DR: Deployment of a
retinal determination gene network drives directed cell migration
in the sea urchin embryo. eLife. 4:42015. View Article : Google Scholar
|
|
7
|
Wu K, Li A, Rao M, Liu M, Dailey V, Yang
Y, Di Vizio D, Wang C, Lisanti MP, Sauter G, et al: DACH1 is a cell
fate determination factor that inhibits cyclin D1 and breast tumor
growth. Mol Cell Biol. 26:7116–7129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu K, Katiyar S, Witkiewicz A, Li A, McCue
P, Song LN, Tian L, Jin M and Pestell RG: The cell fate
determination factor dachshund inhibits androgen receptor signaling
and prostate cancer cellular growth. Cancer Res. 69:3347–3355.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan W, Wu K, Herman JG, Brock MV, Zhou Y,
Lu Y, Zhang Z, Yang Y and Guo M: Epigenetic silencing of DACH1
induces the invasion and metastasis of gastric cancer by activating
TGF-β signalling. J Cell Mol Med. 18:2499–2511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sunde JS, Donninger H, Wu K, Johnson ME,
Pestell RG, Rose GS, Mok SC, Brady J, Bonome T and Birrer MJ:
Expression profiling identifies altered expression of genes that
contribute to the inhibition of transforming growth factor-beta
signaling in ovarian cancer. Cancer Res. 66:8404–8412. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JW, Kim HS, Kim S, Hwang J, Kim YH,
Lim GY, Sohn WJ, Yoon SR, Kim JY, Park TS, et al: DACH1 regulates
cell cycle progression of myeloid cells through the control of
cyclin D, Cdk 4/6 and p21Cip1. Biochem Biophys Res Commun.
420:91–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nan F, Lü Q, Zhou J, Cheng L, Popov VM,
Wei S, Kong B, Pestell RG, Lisanti MP, Jiang J, et al: Altered
expression of DACH1 and cyclin D1 in endometrial cancer. Cancer
Biol Ther. 8:1534–1539. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
16
|
Chen Y, Hao J, Ma W, Tang Y, Gao C and Hao
X: Improvement in treatment and outcome of pancreatic ductal
adenocarcinoma in north China. J Gastrointest Surg. 15:1026–1034.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Popov VM, Wu K, Zhou J, Powell MJ, Mardon
G, Wang C and Pestell RG: The Dachshund gene in development and
hormone-responsive tumorigenesis. Trends Endocrinol Metab.
21:41–49. 2010. View Article : Google Scholar :
|
|
18
|
Mardon G, Solomon NM and Rubin GM:
Dachshund encodes a nuclear protein required for normal eye and leg
development in Drosophila. Development. 120:3473–3486.
1994.PubMed/NCBI
|
|
19
|
Hammond KL, Hanson IM, Brown AG, Lettice
LA and Hill RE: Mammalian and Drosophila dachshund genes are
related to the Ski proto-oncogene and are expressed in eye and
limb. Mech Dev. 74:121–131. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ikeda K, Watanabe Y, Ohto H and Kawakami
K: Molecular interaction and synergistic activation of a promoter
by Six, Eya, and Dach proteins mediated through CREB binding
protein. Mol Cell Biol. 22:6759–6766. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu K, Yang Y, Wang C, Davoli MA, D'Amico
M, Li A, Cveklova K, Kozmik Z, Lisanti MP, Russell RG, et al: DACH1
inhibits transforming growth factor-beta signaling through binding
Smad4. J Biol Chem. 278:51673–51684. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsujimoto Y, Finger LR, Yunis J, Nowell PC
and Croce CM: Cloning of the chromosome breakpoint of neoplastic B
cells with the t(14;18) chromosome translocation. Science.
226:1097–1099. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kelly PN and Strasser A: The role of Bcl-2
and its pro-survival relatives in tumourigenesis and cancer
therapy. Cell Death Differ. 18:1414–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Batlle E and Wilkinson DG: Molecular
mechanisms of cell segregation and boundary formation in
development and tumorigenesis. Cold Spring Harb Perspect Biol.
4:a0082272012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moustakas A and Heldin CH: Signaling
networks guiding epithelial-mesenchymal transitions during
embryogenesis and cancer progression. Cancer Sci. 98:1512–1520.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Y and Zhou BP: Snail: More than EMT.
Cell Adhes Migr. 4:199–203. 2010. View Article : Google Scholar
|
|
29
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao F, Wang M, Li S, Bai X, Bi H, Liu Y,
Ao X, Jia Z and Wu H: DACH1 inhibits SNAI1-mediated
epithelial-mesenchymal transition and represses breast carcinoma
metastasis. Oncogenesis. 4:e1432015. View Article : Google Scholar : PubMed/NCBI
|