Introduction
Gastric cancer (GC) is a life-threatening malignant
tumor in humans and the second most common cause of cancer-related
deaths worldwide (1). Despite the
extensive efforts to develop new therapeutic strategies for GC and
despite that the incidence rates of GC have noticeably decreased in
most countries in the world, it remains the most common cause of
cancer-related mortality worldwide, particularly in Eastern Asian
countries (2). In China, the
incidence of GC ranks second among all types of malignant tumors.
GC patients often present with an advanced stage at diagnosis and
may also have metastasis when initial symptoms occur. As such tumor
recurrence and metastasis impose great difficulty for the
prevention and treatment of GC.
Cancer stem cells (CSCs), firstly found in patients
with acute myeloid leukemia (3),
are a unique subpopulation of cancer cells that have similar
characteristics to normal stem cells and display unlimited
proliferation potential, self-renewal ability and capability to
generate heterogeneous lineages of cancer cells. Numerous studies
have demonstrated that CSCs also exist in solid tumors such as
breast and brain cancer, glioma and pancreatic cancer (4–8). CSCs
were suggested to play a key role in tumor initiation, invasion,
metastasis and drug resistance. Currently, CSCs have been proposed
as a therapeutic target in the treatment of cancers (9).
There is growing evidence suggesting the existence
of gastric cancer stem cells (GCSCs). GCSCs were firstly isolated
and identified from human GC cell lines using a defined cell
surface marker CD44 in 2009 (10).
This study confirmed that CD44+ GC cells exhibit cancer
stem cell properties such as self-renewal and high tumorigenicity.
GCSCs were successfully isolated using CD90 in a previous study
(11). In addition, the stem cell
markers CD44, CD133 and ALDH1 have been recommended for identifying
GCSCs (12,13). Self-renewal and lineage capacity are
characteristics of all stem cells. Recently, in order to acquire
CSCs, a variety of research methods have been developed based on
these features. Liu et al (14) obtained GCSCs from human GC cells by
cultivating cancer cells in stem-condition culture systems. This
method has been used as a representative method by which to obtain
GCSCs.
Forkhead box protein M1 (FoxM1), a transcription
factor, is an important member of the forkhead transcription
family. The International Society for Molecular and Cell Biology
and Biotechnology Protocols and Research (ISMCBBPR) recognized
Forkhead box protein M1 (FOXM1) as the 2010 Molecule of the Year
due to its growing potential as a target for cancer therapies. The
FoxM1 transcriptional factor is essential for cell cycle
progression and cell survival. Upregulation of FoxM1 has been found
in various types of cancers, suggesting that it may be involved in
the initiation of human carcinogenesis (15–17).
Accumulating evidence suggests that FoxM1 plays an essential role
in cancer development and progression by enhancing drug resistance
and cancer cell metastasis (18).
In addition, alterations in the FoxM1 signaling pathway are
reportedly associated with tumorigenesis (19,20).
It has been reported that overexpression of FoxM1 leads to
epithelial-mesenchymal transition (EMT) by the acquisition of EMT
phenotype and downregulation of FoxM1 leads to the inhibition of
EMT in GC cell lines (21).
Moreover, FoxM1 has been shown to be a key transcription factor in
regulating CSC characteristics in several studies (22,23).
Thus, FoxM1 may be a new molecular target for discovering tumor
therapeutic agents that target CSCs.
The transcription factor Twist is considered as one
of the major inducers of the EMT process, and plays a significant
role in tumor metastasis through various signal transcription
pathways, including Akt, Ras, signal transducer and activator of
transcription 3 (STAT3), mitogen-activated protein kinase (MAPK)
and Wnt signaling (24,25). Twist is encoded by the Twist1 gene
located on human chromosome 7p21 and belongs to the family of basic
helix-loop-helix (bHLH) transcription factors (26). Several studies have shown that
Twist1 plays an essential role in the regulation of CSC functions
and features. For example, overexpression of Twist can facilitate
the generation of a breast cancer stem cell phenotype (27). Activation of AKT and β-catenin
pathways induced by overexpression of Twist is crucial for the
maintenance of the characteristics of breast and cervical CSCs
(28).
Genistein, a natural isoflavone, was first isolated
from soy products. It has been demonstrated that genistein is a
potential chemopreventive agent that inhibits carcinogenesis by
mediating multiple regulatory pathways. Our previous studies
confirmed that a novel synthetic genistein derivative,
7-difluoromethoxyl-5,4′-di-n-octyl genistein (DFOG), inhibited the
growth of GC cells by suppressing FoxM1 (29) and halted the self-renewal of ovarian
cancer stem cell by activating Foxo3a (30). In the present study, we investigated
the effect of DFOG on gastric cancer stem-like cells (GCSLCs) and
its potential mechanism for the first time. The results confirmed
that DFOG can attenuate the characteristics of GCSLCs involving the
decreased expression level of FoxM1. We also evaluated the effects
of DFOG on EMT of GCSLCs. The results demonstrated that DFOG was
able to reverse the EMT phenotype in GCSLCs by suppressing the
expression of Twist1. The present study suggests that DFOG may be a
potential therapeutic drug for the treatment of GC by targeting
CSCs.
Materials and methods
Cell culture and reagents
Human GC SGC-7901 cells were purchased from the
China Center for Type Culture Collection (CCTCC; Wuhan, China) and
were maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 100 IU/ml
penicillin and 100 µg/ml streptomycin in a humidified
incubator containing 5% CO2 at 37°C. FBS, trypsin and
DMEM were purchased from HyClone (Thermo Scientific, Waltham, MA,
USA).
Sphere-forming and self-renewal
assay
Parental cells (PCs) were collected and washed to
remove serum, and were then suspended in serum-free stem cell
conditional medium containing DMEM/F12 (Gibco-Invitrogen, Carlsbad,
CA, USA) supplemented with 50X B27 (Invitrogen, Carlsbad, CA, USA),
20 ng/ml EGF, 20 ng/ml bFGF (both from eBioscience, Inc., San
Diego, CA, USA), 4 µg/ml insulin, 100 IU/ml penicillin G and
100 µg/ml streptomycin. After that, the cells were plated in
ultra-low adherence culture plates (6-wells) at a density of 10,000
cells/ml and maintained in a humidified incubator containing 5%
CO2 at 37°C. After 5 days of culture, the first
generation of sphere-forming cells (SFCs) was obtained after
trypsin-EDTA digestion. The first-generation SFCs were further
cultured and expanded at a density of 10,000 cells/well in
ultra-low adhesion 6-well culture plates to obtain the SFCs.
Scratch assays
The PCs and third-generation SFCs were seeded in
6-well plates at a density of 4×105/well in DMEM
supplemented with 10% FBS. When the cells grew to 85% confluency,
the wound was generated by scratching the surface of the plates in
the central region with a 200 µl pipette tip. Washing was
performed 2 times using phosphate-buffered saline (PBS) to remove
floating cells and debris. The cells were incubated for 48 h, and
were imaged at 0 and 48 h in the same location of the wound,
respectively. The numbers of cells in the scratch area were
counted, and the migratory rate of the cells was determined in
relation to the migratory rate of the PCs considered as the
standard migration rate (100%).
Transwell chamber invasion assay in
vitro
DMEM (1.0 ml) supplemented with 10% FBS as a
chemical inducer was added to the 24-well cell culture plate which
was then embedded in the Transwell chamber. A total of 10,000 PCs
or SFCs were plated in the top chamber of the Transwell coated with
Matrigel. After being cultured for 24 h, the cells that had not
invaded through the pores of the insert were cleared with a sterile
cotton swab and discarded. The cells that invaded to the lower
chamber were fixed with methanol, stained with crystal violet and
counted under an optical microscope with the migration rate of the
PCs or SFCs treated with 0.1% dimethyl sulfoxide (DMSO) considered
as the standard invasion rate (100%).
Western blot analysis
The cells were washed with PBS once, and lysed in 1
ml lysis enzyme buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.2
mM EDTA, 0.2% NP-40, 10% glycerol, 1 M β-Me, 1 µg/ml
Trasylol, 0.5 µg/ml leupeptin, 0.1 mM
Na3VO4, 0.5 mM 4-NPP, 0.5 mM NaF and protease
inhibitors]. The cells were scraped and collected after incubation
for 20 min at 4°C. The lysates was centrifuged at 13,200 rpm for 5
min at 4°C to prepare whole cell extracts. The Bradford assay
(Bio-Rad Laboratories, Hercules, CA, USA) was used to determine the
protein content. The proteins were separated and extracted using
10% SDS-polyacrylamide gel electrophoresis, and transferred to a
polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica,
MA, USA). The membranes were detected using mouse antibodies
against CD133 (Santa Cruz Biotechnology, Santa Cruz, CA, USA),
CD44, ALDH1 (both from Cell Signaling Technology, Danvers, MA,
USA), N-cadherin (Upstate Biotechnology, Inc., Lake Placid, NY,
USA), E-cadherin (BD Transduction), FoxM1 (Santa Cruz
Biotechnology), Twist1 (Cell Signaling Technology) and β-actin
(Sigma, St. Louis, MO, USA), respectively.
Statistical analysis
Data are presented as the mean ± SE (mean ± SD) and
were analyzed by SPSS 17.0 statistical software. Multiple
comparisons were performed by one-way ANOVA and pair-wise
comparison was conducted by the LSD t-test method. The Dunnett's
method was used for unequal variances. P<0.05 as considered to
indicate a statistically significant result.
Results
Synthesis and identification of DFOG
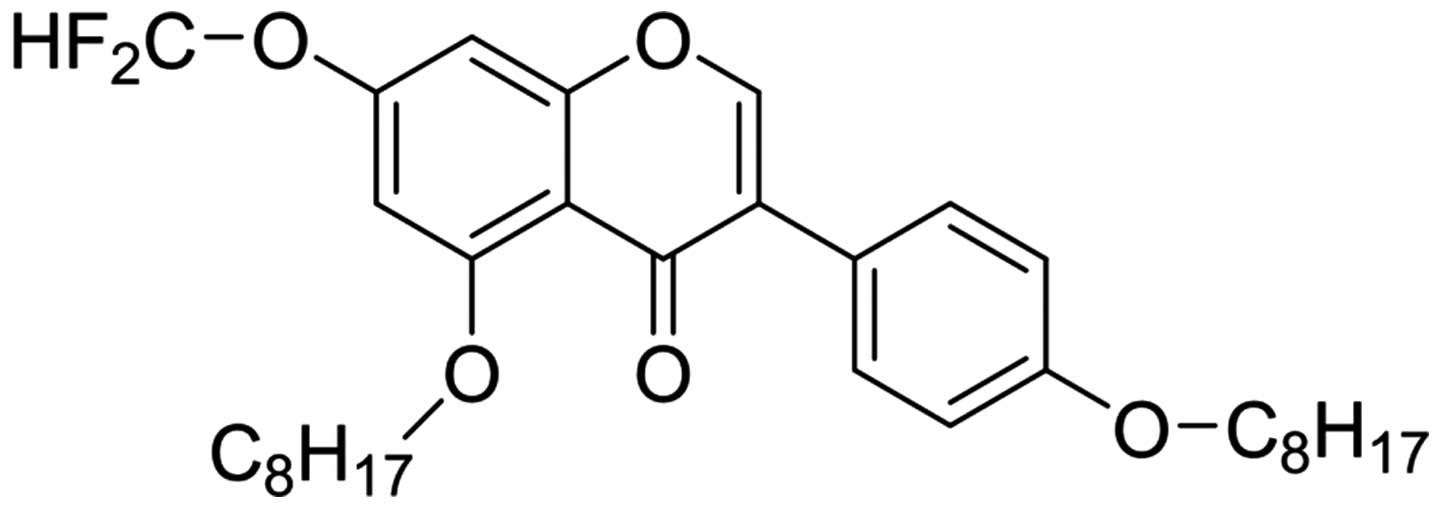
Compound 1 was synthesized and obtained using
methods from the patent application (31) and was identified by a combination of
NMR and mass spectral data and by comparison of these to the
published literature.
Compound 1, yellow powder; EI-MS, m/z 544.1;
1H NMR (500 MHz, CDCl3): 0.88–0.92 (6H, m),
1.26–1.55 (24H, m), 1.77–1.83 (2H, m), 1.90–1.96 (2H, m), 3.98 (2H,
J=6.5 Hz), 4.06 (2H, J=6.5 Hz), 6.53 (1H, d, J=2.0 Hz), 6.65 (1H,
t, J=72.5 Hz), 6.69 (1H, d, J=2.0 Hz), 6.94 (2H, d, J=8.5 Hz), 7.44
(2H, d, J=8.5 Hz), 7.77 (1H, s); 13C NMR (125 MHz,
CDCl3): 14.1, 22.6, 22.7, 25.8, 26.0, 28.8, 29.1, 29.2,
29.3, 29.4, 29.5, 31.8, 68.0, 69.9, 98.3, 99.1, 112.6, 113.2,
114.4, 115.2, 117.3, 123.6, 126.5, 130.3, 150.3, 154.8, 159.0,
159.1, 161.4 and 175.2. The 1H-NMR data were consistent
with the literature (31) and
13C-NMR data were reported for the first time. Thus,
compound 1 was identified as 7-difluoromethoxyl-5,4′-di-n-octyl
genistein and named DFOG (Fig.
1).
Characteristics of GCSLCs derived from
the SGC-7901 cell line
In order to enrich GCSLCs from human GC SGC-7901
cells, a stem cell conditioned medium suspension culture method was
used. Under these conditions, the cells grew as non-adherent,
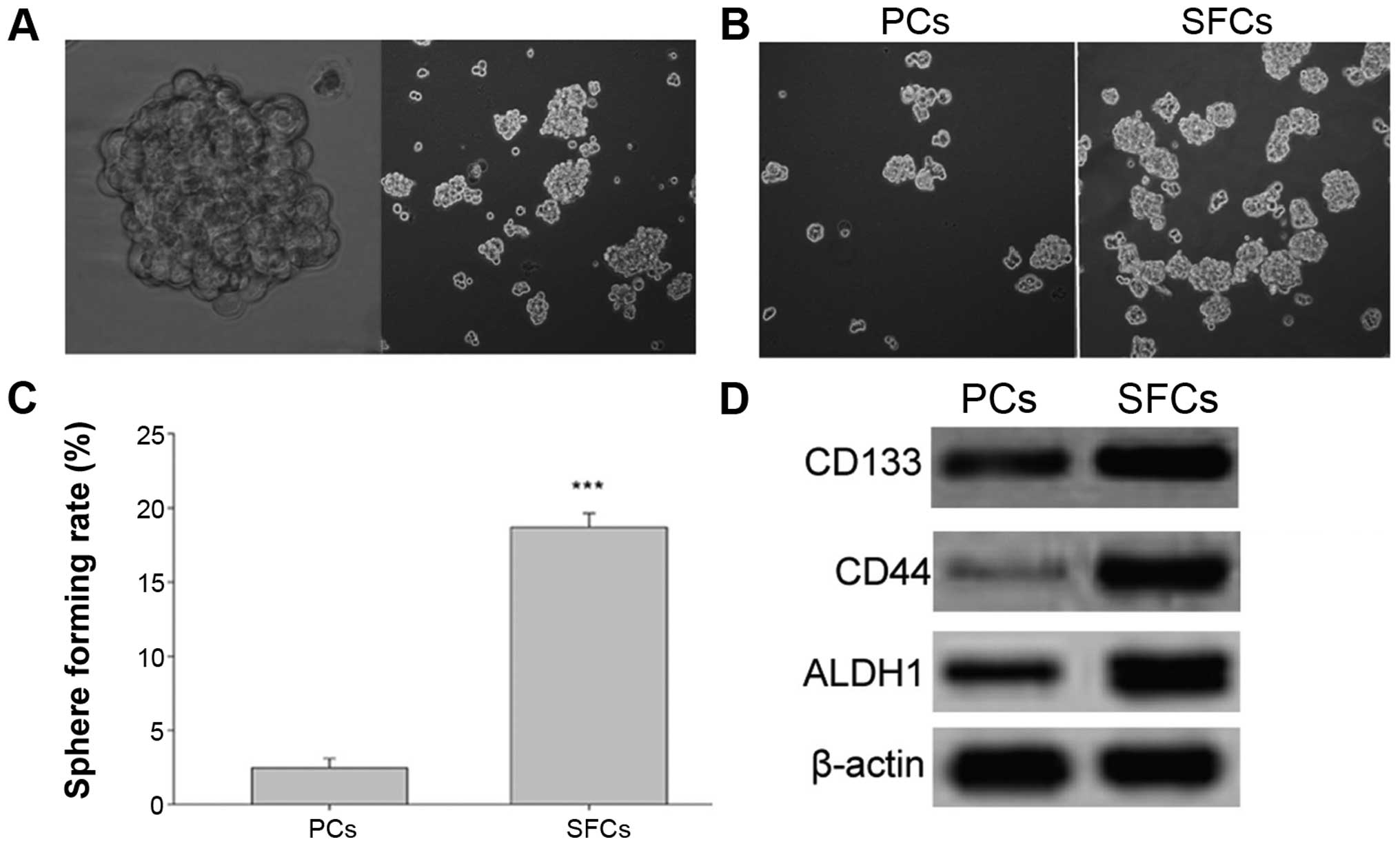
three-dimensional sphere clusters. Fig.
2A shows the anchorage-independent spheres that formed in the
SGC-7901 cells. After 5 days of incubation, the SFCs from the
SGC-7901 cell line were found to generate more and larger spheroid
colonies compared with that noted in the PCs (Fig. 2B and C).
Next, western blotting was performed to identify
protein expression of the gastric CSC markers [cluster of
differentiation CD133, CD44 and aldehyde dehydrogenase 1 (ALDH1)].
The results showed enrichment of CD133+,
CD44+ and ALDH1-high populations in the SFCs derived
from the SGC-7901 cells compared with the PCs (Fig. 2D). These results indicated that SFCs
from the SGC-7901 cells cultured in stem cell-conditioned medium
possessed GCSLC properties.
GCSLCs from the SGC-7901 cell line show
mesenchymal cell characteristics
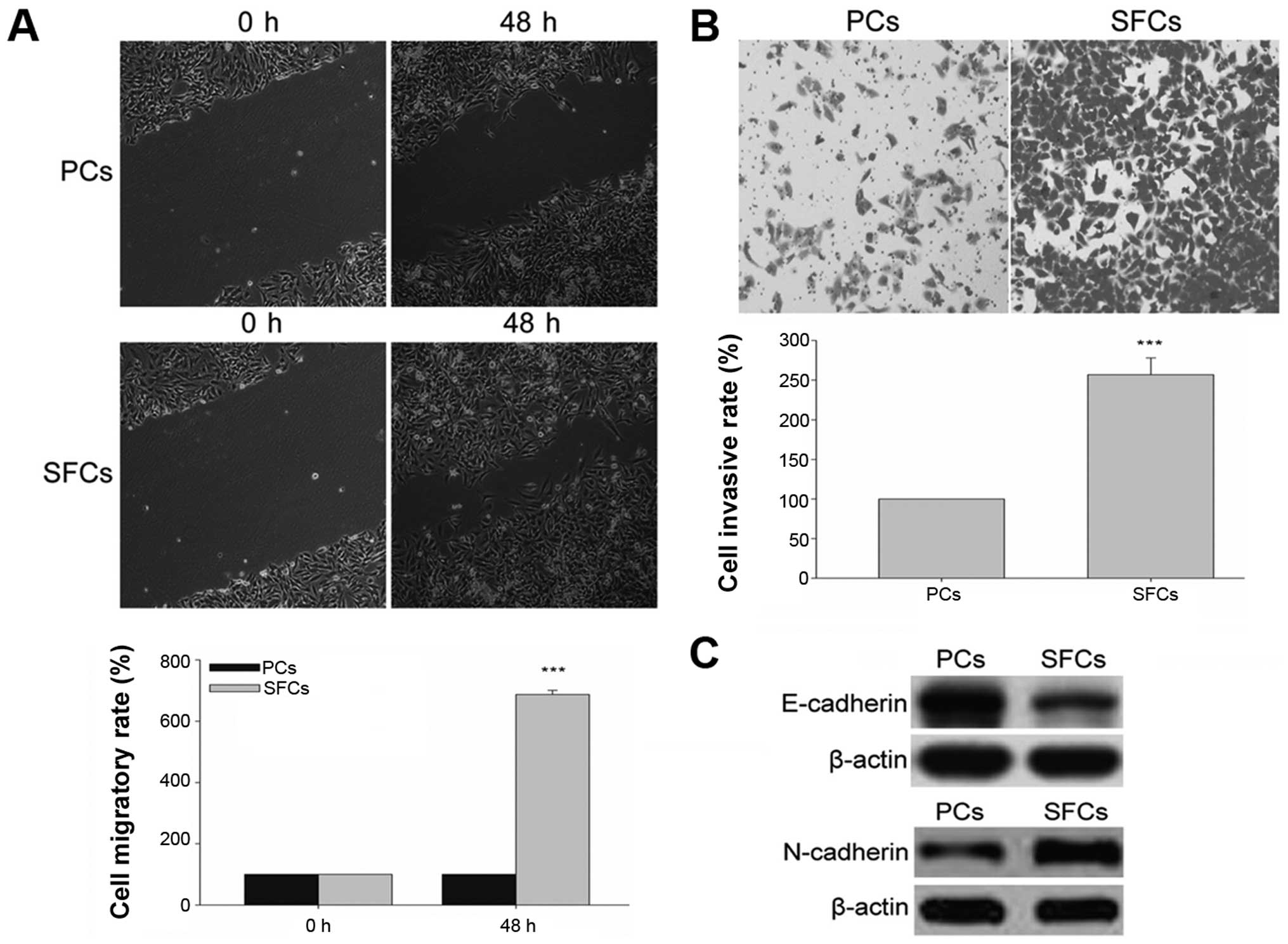
CSCs have higher migratory and invasion capacities,
which facilitate metastasis and growth. The migration and invasion
capabilities of GCSLCs and PCs were evaluated by scratch method and
Transwell chamber invasion assay in vitro, respectively. The
results demonstrated that GCSLCs showed increased migratory and
invasive capabilities than these capacities noted in the PCs
(Fig. 3A and B). CSCs are also
thought to facilitate metastasis through EMT characteristics
related to the mobility of cells. We evaluated the protein
expression of a known mesenchymal phenotype cell biomarker
(N-cadherin) and an epithelial phenotype cell biomarker
(E-cadherin) by western blot analysis. The results demonstrated
that the relative protein level of N-cadherin was highly expressed
in the GCSLCs, while that of E-cadherin was weakly expressed
(Fig. 3C).
DFOG inhibits the self-renewal of GCSLCs
derived from the SGC-7901 cell line
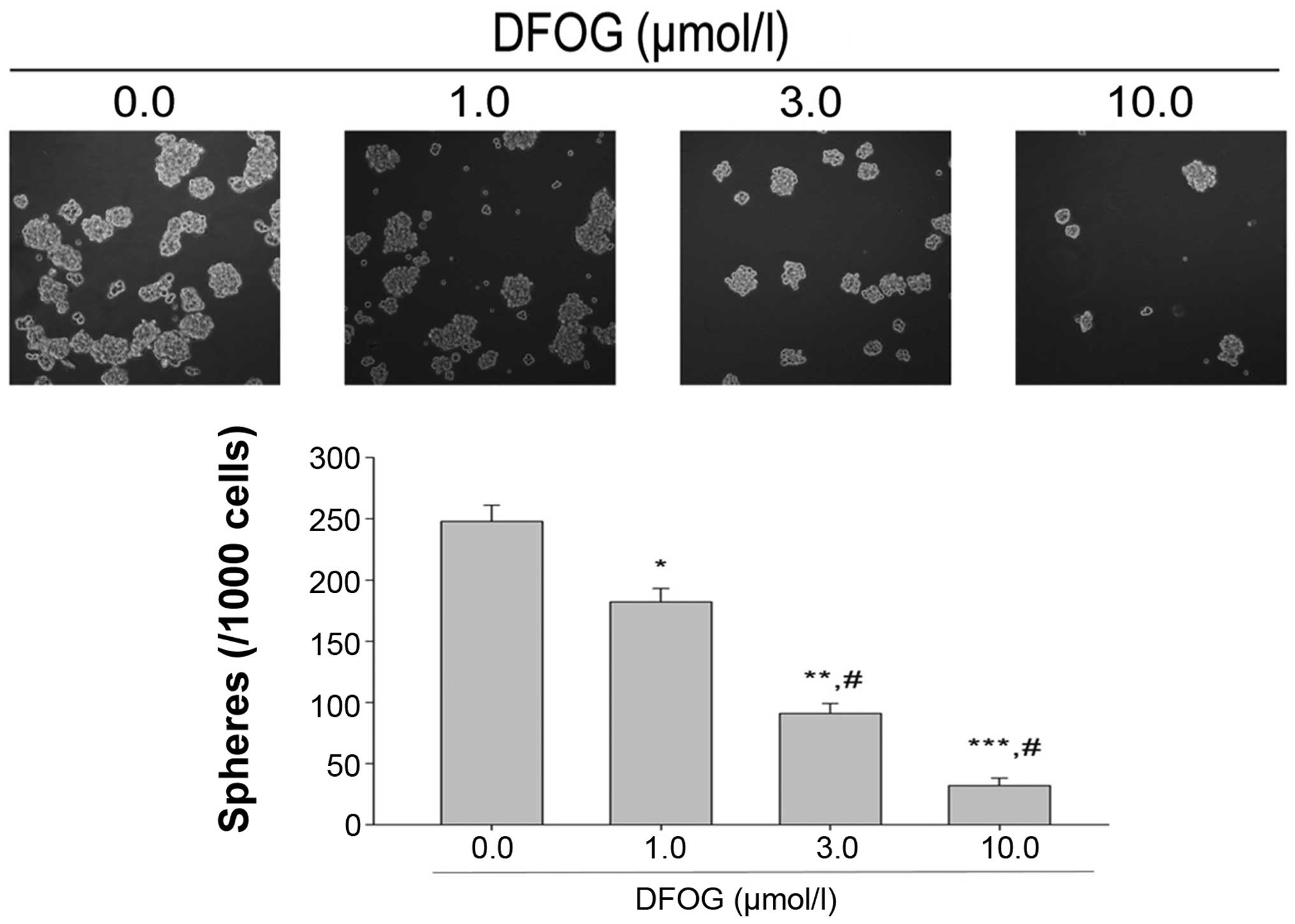
Tumor sphere assay is used to identify stem cells in
in vitro assays. We examined the tumor sphere formation
capacity of SGC-7901 cells following treatment of DFOG. The results
showed that DFOG (1.0, 3.0 and 10.0 µmol/l) reduced the
number of SFCs derived from the SGC-7901 cells in a
concentration-dependent manner (Fig.
4), indicating that DFOG is able to preferentially suppress the
self-renewal of GCSLCs.
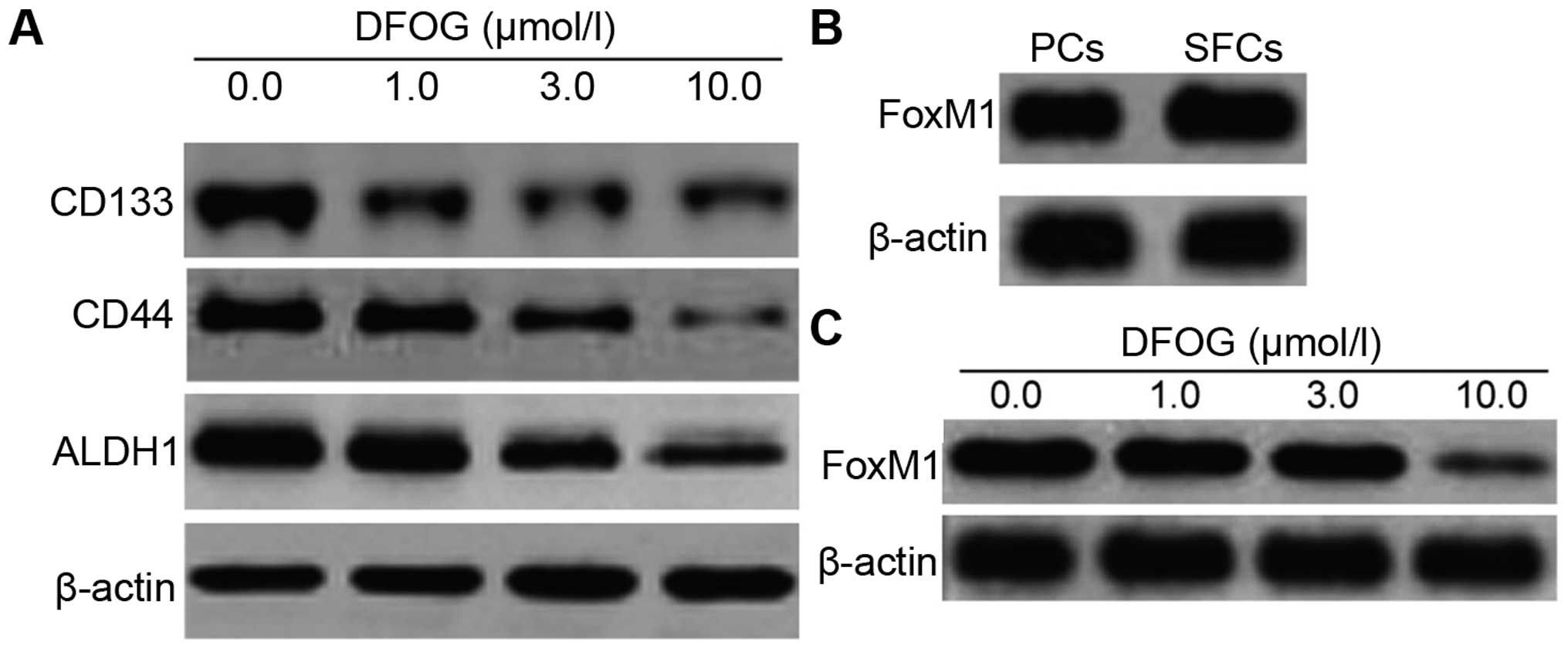
DFOG downregulates the expression of CSC
markers and FoxM1 in GCSLCs derived from the SGC-7901 cell
line
CD133, CD44 and ALDH1 are used as cancer stem cell
markers in many types of tumor cells including GC. To investigate
the effect of DFOG on GCSLC surface marker expression including
CD44, CD133 and ALDH1, we incubated GCSLCs with DFOG (1.0, 3.0 and
10.0 µmol/l) and DMSO as a control. The expression levels of
CD44, CD133 and ALDH1 in the GCSLCs were significantly suppressed
following treatment with DFOG compared with the spheres that were
untreated (Fig. 5A). A previous
study demonstrated that overexpression of FoxM1 led to EMT by the
acquisition of the EMT phenotype and downregulation of FoxM1 led to
the inhibition of EMT in GC cell lines (21). Therefore, the present study compared
the level of FoxM1 protein expression in the PCs and SFCs and
evaluated the inhibitory effect of DFOG on FoxM1. The results
revealed that FoxM1 expression was higher in the SFCs in comparison
with that in the PCs (Fig. 5B).
Furthermore, as shown in Fig. 5C,
the expression of FoxM1 in the SFCs was significantly reduced by
DFOG in a dose-dependent manner.
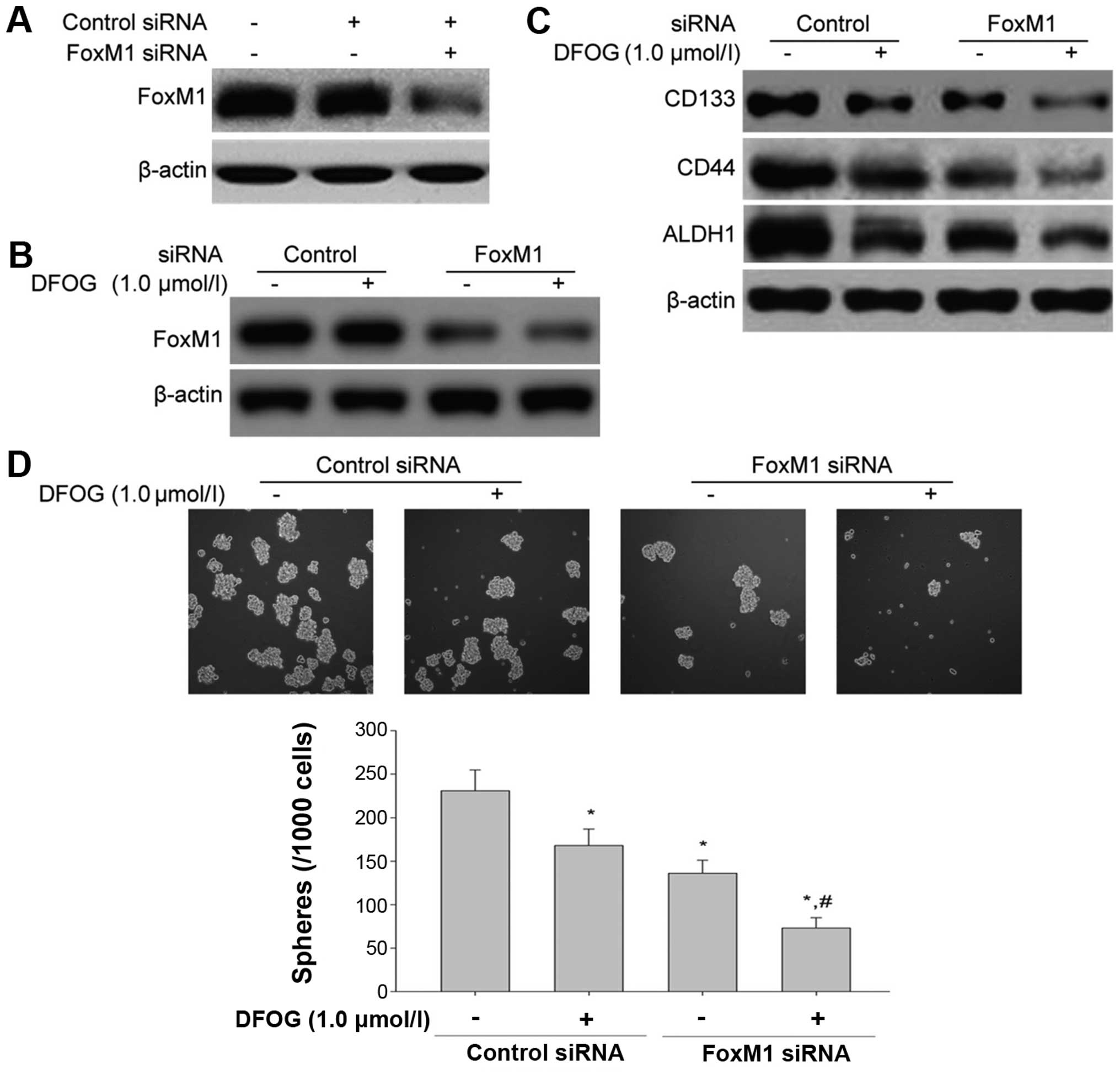
Transfection of FoxM1 siRNA enhances the
inhibitory effects of DFOG on the expression of FoxM1, CSC markers
and the self-renewal in GCSLCs derived from the SGC-7901 cell
line
We next transfected FoxM1 siRNA into the GCSLCs to
confirm the inhibitory effect of DFOG on FoxM1 expression in GC
cells and the effect of FoxM1 on the characteristics of GCSLCs. The
GCSLCs were transfected with either scramble siRNA or FoxM1 siRNA.
The expression levels of FoxM1 and CSC markers including CD133,
CD44 and ALDH1 were assessed using western blot analysis. The
results showed that the protein expression level of FoxM1 was
inhibited following FoxM1 knockdown (Fig. 6A). Furthermore, after transfection
of FoxM1 siRNA, the inhibitory effects of DFOG on the expression of
FoxM1 and CSC markers (CD133, CD44 and ALDH1) were markedly
enhanced compared with the scramble siRNA control group (Fig. 6B and C). The knockdown of FoxM1 also
suppressed the sphere-forming capability of the GCSLCs and
suppressed the inhibition of the self-renewal of GCSLCs
synergistically together with DFOG (Fig. 6D).
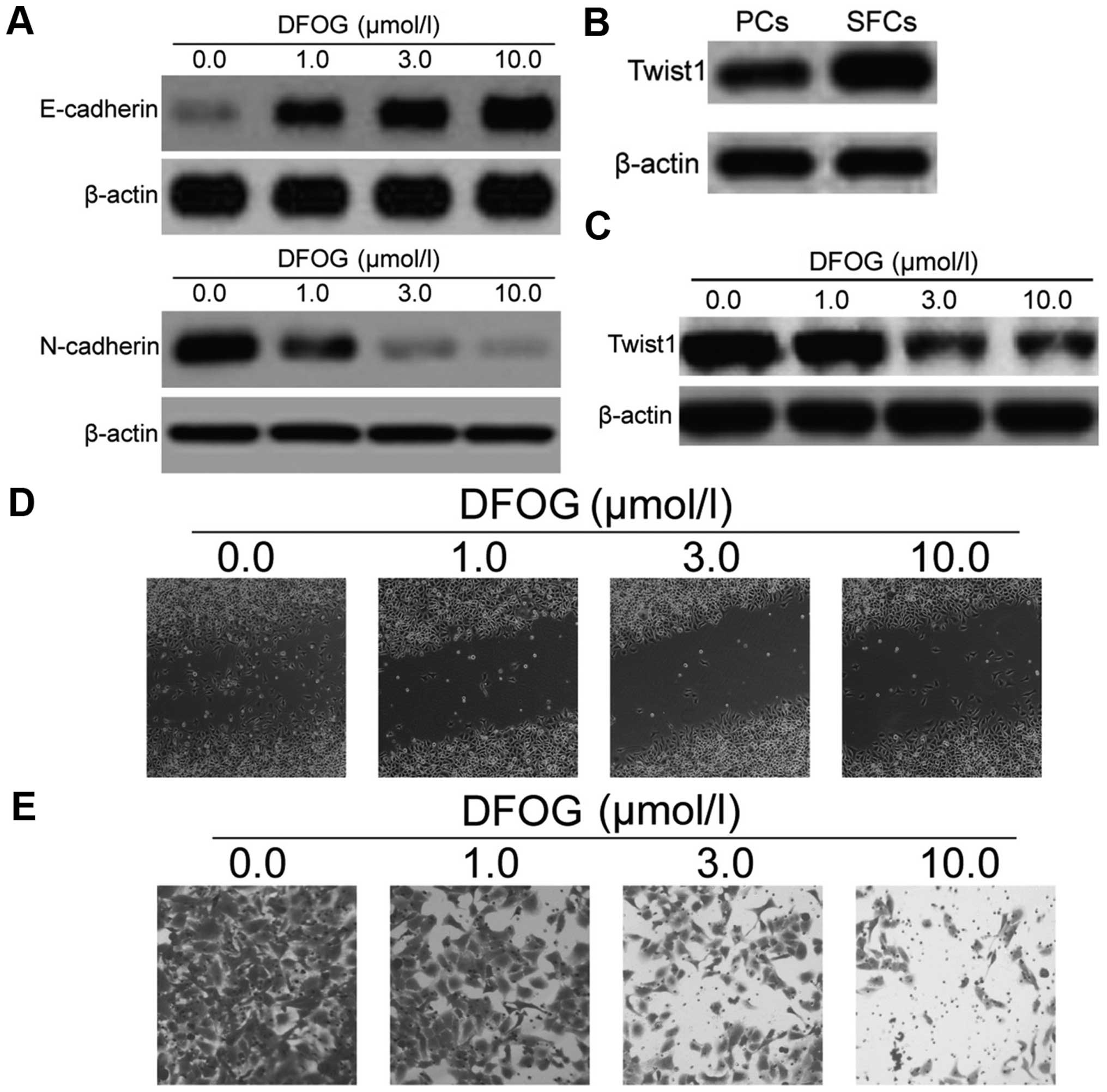
DFOG reverses EMT, as well as decreases
the migration and invasion of GCSLCs derived from the SGC-7901 cell
line
To investigate the effects of DFOG on the EMT
process of GCSLCs, the protein levels of E-cadherin, N-cadherin and
the Twist1 (EMT-related transcription factors) were measured. The
results from the western blot analysis demonstrated that DFOG
upregulated the protein level of E-cadherin and downregulated the
protein level of N-cadherin (Fig.
7A). The protein expression level of Twist1 in the GCSLCs was
higher than that in the PCs (Fig.
7B), while DFOG also dose-dependently suppressed the expression
of Twist1 (Fig. 7C). These results
clearly demonstrated that DFOG could reverse EMT, relying on
inhibition of the EMT phenotypic biomarkers and Twist1. Migration
and invasion abilities are important characteristics of CSCs
responsible for tumor metastasis and growth. CSCs are assumed to
have higher migration capacity than normal cancer cells and Twist1
is closely associated with cancer cell migration and invasion.
Functionally, the relative migratory and invasive cell numbers of
GCSLCs were significantly decreased when compared to these numbers
in the negative control, suggesting that DFOG reduced cell
migration and invasion in the GCSLCs (Fig. 7D and E). The above results showed
that DFOG inhibited the expression of Twist1, leading to the
inhibition of cancer cell migration and invasion.
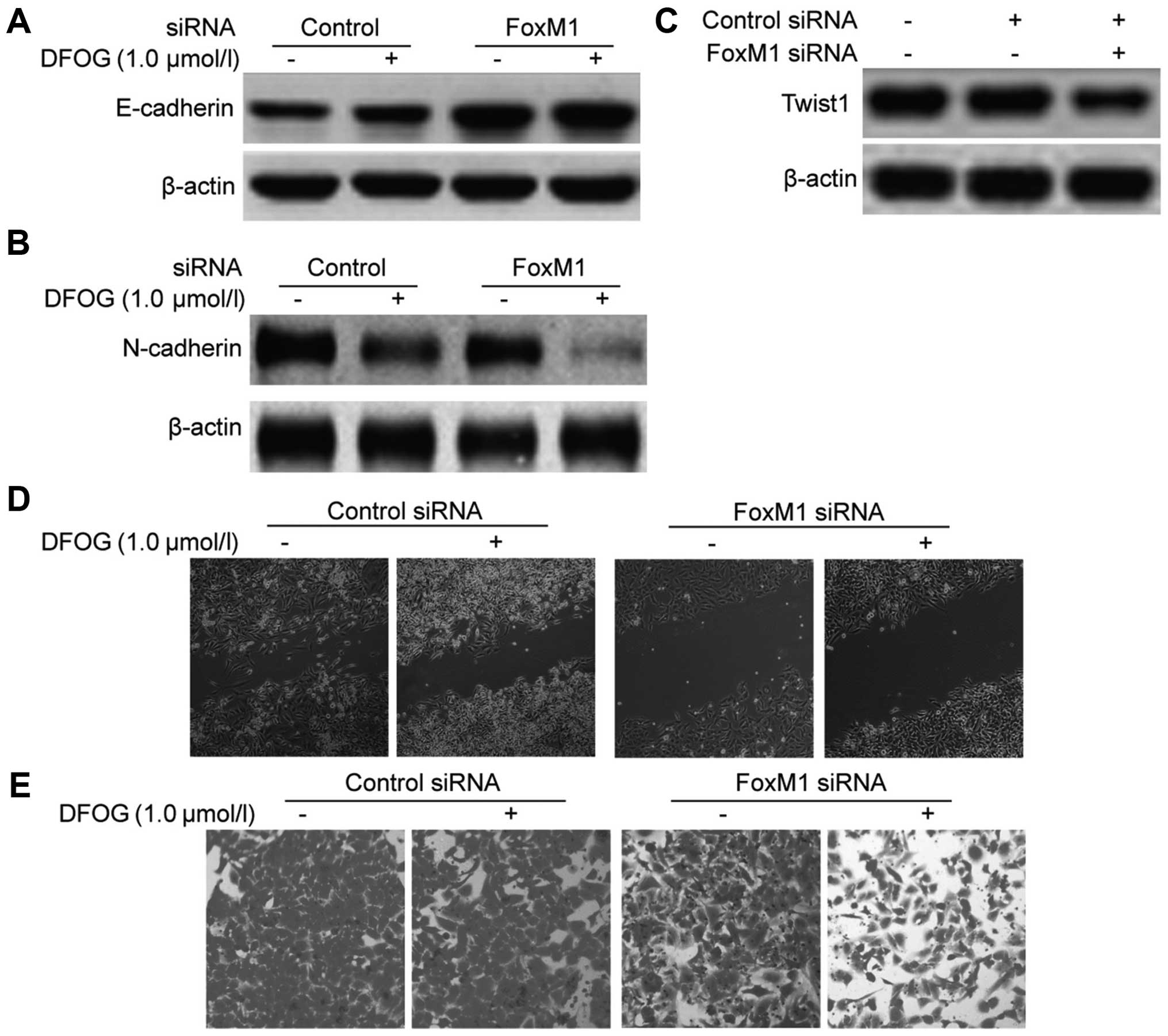
Transfection of FoxM1 siRNA cooperates
with DFOG to reverse EMT
To examine whether forced knockdown of FoxM1 can
cooperate with DFOG to reverse EMT, the protein expression of EMT
biomarkers (E-cadherin and N-cadherin) and Twist1 was measured by
western blot analysis. As compared to the negative control groups,
silencing by FoxM1 siRNA increased the protein expression of
E-cadherin (Fig. 8A) and decreased
the protein expression of N-cadherin and Twist1 (Fig. 8B and C), suggested that knockdown of
FoxM1 could reverse EMT. Moreover, transfection of FoxM1 siRNA also
enhanced the upregulation of E-cadherin protein expression
(Fig. 7A) and the downregulation of
N-cadherin and Twist1 protein expression (Fig. 8B and C) caused by DFOG,
demonstrating that transfection of FoxM1 siRNA can cooperate with
DFOG to reverse the EMT process. Following transfection of FoxM1
siRNA, the influence on the migratory and invasive capabilities of
the GCSLCs were evaluated. The results showed that transfection of
FoxM1 siRNA suppressed the migration and invasion of GCSLCs and
also enhanced the inhibitory effect of DFOG on the migration and
invasion of GCSLCs compared with the control group (Fig. 8D and E). These results confirmed
that transfection of FoxM1 siRNA and DFOG can synergistically
reverse the EMT process of GCSLCs.
Discussion
In the present study, it was confirmed that SFCs
derived from the SGC-7901 cell line possessed superactive
self-renewal capacity in vitro when compared with that noted
in the parental cells. It was also found that CD133+,
CD44+ and ALDH-high populations were enriched in the
tumor spheroid cells from the SGC-7901 cells, which exhibited the
characteristics of GCSCs such as invasion capacity and EMT, and
were therefore identified as GCSLCs.
Previous studies have confirmed that FoxM1 plays an
important role in the regulation of cancer stem cell (CSC)
properties and EMT in various types of cancers. Meng et al
reported that overexpression of FoxM1 promotes EMT and metastasis
of hepatocellular carcinoma (32).
Miao et al reported that downregulation of FoxM1 leads to
the inhibition of EMT in GC cells (21). Bao et al discovered that
overexpression of FoxM1 led to EMT and a cancer stem cell phenotype
in pancreatic cancer cells (22).
In our previous study, we found that DFOG inhibited ovarian and GC
cell growth by downregulation of FoxM1 (30). The present study uncovered, for the
first time, that DFOG can inhibit the function and properties of
GCSLCs through downregulation of FoxM1.
Twist1 is reported as one of the major inducers of
the EMT process and also acts as an EMT biomarker in CSCs. Ren
et al demonstrated that overexpression of Twist in HCC cell
line SMMC-7721 promoted the generation of a hepatocellular cancer
stem cell (HSC) phenotype through upregulation of the expression of
the biomarkers CD133 and CD44 (33). He et al reported that
casticin inhibited EMT in liver CSCs from the SMMC-7721 cell line
by downregulating Twist (34). Our
results showed that overexpression of Twist protein in GCSLCs were
significantly suppressed by exposure to DFOG consistent with the
increased expression of E-cadherin and decreased expression of
N-cadherin. These results revealed that the EMT process in GCSLCs
was suppressed by DFOG. In addition, Qian et al reported
that Twist1 promoted GC cell proliferation through upregulation of
FoxM1, which suggested that Twist1 and FoxM1 can interact with each
other to affect the function and properties of CSCs (35). Our current results indicated that
DFOG was capable to reverse the EMT phenotype by downregulation of
FoxM1 and further downregulation of Twist1 based on different
mechanism when compared with that of FoxM1 siRNA.
Overall, our findings further clarify the anticancer
effects of 7-difluoromethoxyl-5,4′-di-n-octyl genistein (DFOG), a
novel synthetic genistein analogue. DFOG eliminated stem-like
characteristics of GCSLCs and reversed EMT, partially due to the
downregulation of FoxM1 and EMT-related proteins (Twist1), and
attenuated the migratory and invasive abilities of GCSLCs. DFOG may
be potentially effective in preventing GC by targeting CSCs.
Acknowledgments
The present study was financially supported by
grants from the National Natural Science Foundation of China (nos.
31400311 and 81172375), the Program for Excellent Talents of Hunan
Normal University (no. ET1508), the Project of Hunan Provincial
Natural Science Foundation (no. 13JJ3061), the Scientific Research
Fund of Hunan Normal University (nos. 140668 and 140666), and the
Construct Program of the Key Discipline of Basic Medicine in Hunan
Province.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ignatova TN, Kukekov VG, Laywell ED,
Suslov ON, Vrionis FD and Steindler DA: Human cortical glial tumors
contain neural stem-like cells expressing astroglial and neuronal
markers in vitro. Glia. 39:193–206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu SC, Ping YF, Yi L, Zhou ZH, Chen JH,
Yao XH, Gao L, Wang JM and Bian XW: Isolation and characterization
of cancer stem cells from a human glioblastoma cell line U87.
Cancer Lett. 265:124–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim Y, Wu Q, Hamerlik P, Hitomi M, Sloan
AE, Barnett GH, Weil RJ, Leahy P, Hjelmeland AB and Rich JN:
Aptamer identification of brain tumor-initiating cells. Cancer Res.
73:4923–4936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen K, Huang YH and Chen JL:
Understanding and targeting cancer stem cells: Therapeutic
implications and challenges. Acta Pharmacol Sin. 34:732–740. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang J, Zhang Y, Chuai S, Wang Z, Zheng
D, Xu F, Zhang Y, Li C, Liang Y and Chen Z: Trastuzumab Herceptin)
targets gastric cancer stem cells characterized by CD90 phenotype.
Oncogene. 31:671–682. 2012. View Article : Google Scholar
|
|
12
|
Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y,
Zhao L, Qu H, Fan Y and Wu C: Antagonism of miR-21 reverses
epithelial-mesenchymal transition and cancer stem cell phenotype
through AKT/ERK1/2 inactivation by targeting PTEN. PLoS One.
7:e395202012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li K, Dan Z and Nie YQ: Gastric cancer
stem cells in gastric carcinogenesis, progression, prevention and
treatment. World J Gastroenterol. 20:5420–5426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Ma L, Xu J, Liu C, Zhang J, Liu J,
Chen R and Zhou Y: Spheroid body-forming cells in the human gastric
cancer cell line MKN-45 possess cancer stem cell properties. Int J
Oncol. 42:453–459. 2013.
|
|
15
|
Kim IM, Ackerson T, Ramakrishna S,
Tretiakova M, Wang IC, Kalin TV, Major ML, Gusarova GA, Yoder HM,
Costa RH, et al: The Forkhead Box m1 transcription factor
stimulates the proliferation of tumor cells during development of
lung cancer. Cancer Res. 66:2153–2161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng J, Wang L, Li Q, Li W, Björkholm M,
Jia J and Xu D: FoxM1 is up-regulated in gastric cancer and its
inhibition leads to cellular senescence, partially dependent on
p27kip1. J Pathol. 218:419–427. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Banerjee S, Kong D, Li Y and
Sarkar FH: Downregulation of Forkhead Box M1 transcription factor
leads to the inhibition of invasion and angiogenesis of pancreatic
cancer cells. Cancer Res. 67:8293–8300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Laoukili J, Stahl M and Medema RH: FoxM1:
At the crossroads of ageing and cancer. Biochim Biophys Acta.
1775:92–102. 2007.
|
|
19
|
Wang IC, Ustiyan V, Zhang Y, Cai Y, Kalin
TV and Kalinichenko VV: Foxm1 transcription factor is required for
the initiation of lung tumorigenesis by oncogenic
KrasG12D. Oncogene. 33:5391–5396. 2014. View Article : Google Scholar
|
|
20
|
Yu G, Zhou A, Xue J, Huang C, Zhang X,
Kang SH, Chiu WT, Tan C, Xie K, Wang J, et al: FoxM1 promotes
breast tumorigenesis by activating PDGF-A and forming a positive
feedback loop with the PDGF/AKT signaling pathway. Oncotarget.
6:11281–11294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miao L, Xiong X, Lin Y, Cheng Y, Lu J,
Zhang J and Cheng N: Down-regulation of FoxM1 leads to the
inhibition of the epithelial-mesenchymal transition in gastric
cancer cells. Cancer Genet. 207:75–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bao B, Wang Z, Ali S, Kong D, Banerjee S,
Ahmad A, Li Y, Azmi AS, Miele L and Sarkar FH: Over-expression of
FoxM1 leads to epithelial-mesenchymal transition and cancer stem
cell phenotype in pancreatic cancer cells. J Cell Biochem.
112:2296–2306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ning YX, Li QX, Ren KQ, Quan MF and Cao
JG: 7-Difluoromethoxyl-5,4′-di-n-octyl genistein inhibits ovarian
cancer stem cell characteristics through the downregulation of
FOXM1. Oncol Lett. 8:295–300. 2014.PubMed/NCBI
|
|
24
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khan MA, Chen HC, Zhang D and Fu J: Twist:
A molecular target in cancer therapeutics. Tumour Biol.
34:2497–2506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bourgeois P, Stoetzel C, Bolcato-Bellemin
AL, Mattei MG and Perrin-Schmitt F: The human H-twist gene is
located at 7p21 and encodes a B-HLH protein that is 96% similar to
its murine M-twist counterpart. Mamm Genome. 7:915–917. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vesuna F, Lisok A, Kimble B and Raman V:
Twist modulates breast cancer stem cells by transcriptional
regulation of CD24 expression. Neoplasia. 11:1318–1328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J and Zhou BP: Activation of β-catenin
and Akt pathways by Twist are critical for the maintenance of EMT
associated cancer stem cell-like characters. BMC Cancer. 11:492011.
View Article : Google Scholar
|
|
29
|
Xiang HL, Liu F, Quan MF, Cao JG and Lv Y:
7-difluoromethoxyl-5,4′-di-n-octylgenistein inhibits growth of
gastric cancer cells through downregulating forkhead box M1. World
J Gastroenterol. 18:4618–4626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ning Y, Luo C, Ren K, Quan M and Cao J:
FOXO3a-mediated suppression of the self-renewal capacity of
sphere-forming cells derived from the ovarian cancer SKOV3 cell
line by 7-difluoromethoxyl-5,4′-di-n-octyl genistein. Mol Med Rep.
9:1982–1988. 2014.PubMed/NCBI
|
|
31
|
Cao JG, Cao XZ and Xiang HL: Patent CN
201210591131.2. Filed. December 22–2012
|
|
32
|
Meng FD, Wei JC, Qu K, Wang ZX, Wu QF, Tai
MH, Liu HC, Zhang RY and Liu C: FoxM1 overexpression promotes
epithelial-mesenchymal transition and metastasis of hepatocellular
carcinoma. World J Gastroenterol. 21:196–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ren KQ, Cao XZ, Liu ZH, Guo H, Quan MF,
Liu F, Jiang L, Xiang HL, Deng XY and Cao JG:
8-Bromo-5-hydroxy-7-methoxychrysin targeting for inhibition of the
properties of liver cancer stem cells by modulation of Twist
signaling. Int J Oncol. 43:1719–1729. 2013.PubMed/NCBI
|
|
34
|
He M, Cao XC, He GC, Sheng XF, Ai XH and
Wu YH: Casticin inhibits epithelial-mesenchymal transition of liver
cancer stem cells of the SMMC-7721 cell line through downregulating
Twist. Oncol Lett. 7:1625–1631. 2014.PubMed/NCBI
|
|
35
|
Qian J, Luo Y, Gu X, Zhan W and Wang X:
Twist1 promotes gastric cancer cell proliferation through
up-regulation of FoxM1. PLoS One. 8:e776252013. View Article : Google Scholar : PubMed/NCBI
|