Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy
arising from the epithelium of the nasopharynx, the epicenter of
which is frequently observed at the pharyngeal recess, from where
the tumor invades adjacent anatomical spaces or organs (1). NPC is highly endemic in the regions of
Southeast Asia, Northern Africa and the Middle East. Radiotherapy,
the standard treatment for NPC, is effective in controlling early
tumors with a good prognosis (2);
however, treatment success largely depends on tumor stage, which,
at the point of diagnosis, has often reached advanced disease.
Although modern practice involves the strategy of combining
chemotherapy with radiotherapy and intensity-modulated radiotherapy
(IMRT), retrospective prognosis reports of patients treated with
these therapeutic schemes have revealed that ~5–15% of NPC patients
still have local recurrence, and 15–30% may experience failure at
distant sites (3–5). Another challenge confounding treatment
options is that surviving NPC patients frequently suffer late
adverse events that greatly affect their quality of life, including
cervical subcutaneous fibrosis, hearing loss, skin dystrophy,
xerostomia and radiation-induced sarcoma of the head and neck
(RISHN) (6,7). Therefore, it is of great clinical
value to explore and develop more effective and less toxic
therapeutic agents for the treatment of NPC.
In normal physiology, cells that have been
improperly stimulated for survival are eliminating by apoptosis, a
protective mechanism against neoplastic development that can be
mediated by several different pathways (8). The most commonly modulated type of
cell death in cancers is the mitochondrial pathway of apoptosis,
also called the intrinsic pathway (9). This pathway is strictly controlled by
proteins of the Bcl-2 family which induce mitochondrial outer
membrane permeabilization (MOMP) (10), and are subdivided into the
anti-apoptotic proteins (Bcl-2 and Bcl-xL), the pro-apoptotic
proteins (Bax and Bak), and the BH3-only proteins (Bim, Bad and
Puma). Anti-apoptotic Bcl-2 proteins promote cell proliferation,
while oligomerization of Bak and Bax proteins are the two primary
activators of MOMP (8). An increase
in the Bax/Bcl-2 ratio is critical for apoptosis and can enhance
the membrane permeability of mitochondria (11). The mitochondrial apoptosis pathway
provides an effective means of inducing cell death, and many drugs
and macromolecules intended for cancer therapy have been targeted
to this pathway.
Previous epidemiological studies have indicated that
intake of fruits and vegetables may be associated with a reduced
risk for various types of cancers (12). The active ingredients of grape seed
extract (GSE) are grape seed proanthocyanidins (GSPs), which occur
in dimers, trimers, tetramers and oligomers/polymers of monomeric
catechins and/or (−)-epicatechins, and are responsible for the
various aesthetic and taste-related qualities of red wine (13). GSE is commonly consumed as a dietary
supplement, and is sold in the form of capsules or tablets (100–500
mg) as an over-the-counter product in the US. It has been
demonstrated that GSPs have anticancer properties in vitro
or in vivo in various types of cancers such as pancreatic
(14), colorectal (15), cervical (16) and lung cancer (17). Furthermore, both toxicity testing in
rats, as well as genotoxicity testing, have shown that GSPs have
low toxicity and no genotoxic potential (18). Nevertheless, the antitumor potential
of GSPs in NPC has rarely been reported.
In the present study, we aimed to investigate the
chemotherapeutic potential of GSPs by examining their effect on the
human-derived NPC CNE-2 cell line. Based on the reported antitumor
effect, we sought to obtain a deeper understanding of the ability
of GSPs to treat NPC, and elucidate the specific mechanism by which
it does so, with the ultimate aim of providing a useful reference
for clinical medication.
Materials and methods
Materials
Primary antibodies were obtained from the following
vendors: antibodies specific for Bax, Bcl-2, Bcl-xL, Bim, Bad,
pro-caspase-3, cleaved caspase-3, PARP, cleaved PARP and β-actin
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA); the horseradish peroxidase (HRP)-conjugated goat anti-rabbit
IgG secondary antibodies were purchased from Bio-Rad (Hercules, CA,
USA). The Annexin V-FITC/propidium iodide (PI) apoptosis detection
and cell cycle assay kits were purchased from Vazyme Biotech, Inc.
(Nanjing, Jiangsu, China). The JC-1 mitochondrial membrane
potential (MMP) detection kit, Cell Counting Kit-8 (CCK-8), Hoechst
33258 staining kit, and all other chemicals were purchased from
Beyotime Institute of Biotechnology (Haimen, Jiangsu, China). The
GSPs used in the present study were purchased from Jingke Chemical,
Inc. (Shanghai, China) (lot no. MUST-14081604; purity, 95%).
Cell culture
The CNE-2 human NPC cell line was obtained from the
Institute of Biochemistry and Molecular Biology, Guangdong Medical
University (Guangdong, China). The cells were cultured in RPMI-1640
medium (Gibco-BRL, Carlsbad, CA, USA) supplemented with 10% (v/v)
fetal bovine serum (Sijiqing, Hangzhou, Zhejiang, China), 100 U/ml
penicillin and 100 μg/ml streptomycin (Beyotime Institute of
Biotechnology). Cells were maintained in an incubator with a
humidified atmosphere of 5% CO2 at 37°C, subcultured and
allowed to reach 70–80% confluency, before beginning
experimentation.
Cell proliferation and colony formation
assays
Cell proliferation was evaluated using the CCK-8 and
colony formation assays. CNE-2 cells were seeded in 96-well plates
at a density of 5,000 cells/well, and incubated for 24 h. The cells
were then treated with 5, 10, 20, 40 and 60 μg/ml GSPs or
control for 6, 12 and 24 h. At the end of the stipulated time,
CCK-8 was added to each well according to the manufacturer's
instructions, and the absorbance was recorded at 450 nm using a
microplate reader (Bio-Rad). The effect of GSPs on cell viability
was calculated in terms of the percent of the control, arbitrarily
assigned as having 100% viability. All tests were carried out in
triplicate, and IC50 values were analyzed using GraphPad
Prism 5.0. For the colony formation assays, CNE-2 cells were seeded
at 500 cells/well on 6-well plates and treated with 1.25, 2.5 and 5
μg/ml GSPs or control, followed by culture for 10 days in an
incubator with a humidified atmosphere of 5% CO2 at
37°C. Visible colonies gradually emerged, and after the 10-day
culture period, were stained with crystal violet and counted under
a dissection microscope.
Cell cycle analysis
CNE-2 cells were treated with different
concentrations of GSPs (0, 10, 20 and 40 μg/ml, the same
below) for 12 h, collected, and then fixed with 75% cold ethanol
overnight at −20°C. Next, the cells were washed twice with cold
phosphate-buffered saline (PBS), incubated with RNase in a 37°C
water bath for 30 min, and finally incubated with PI on ice, in the
dark, as described in the Cell Cycle Assay kit (Vazyme Biotech Co.,
Ltd.) instructions. Cell cycle distribution was then analyzed with
a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA)
equipped with CellQuest software.
Fluorescence microscopy
Apoptotic nuclear morphology was assessed by
staining the cells with the fluorescent DNA-binding dye Hoechst
33258. After a 12-h treatment with GSPs, CNE-2 cells were fixed and
washed twice with PBS, and then stained with Hoechst 33258 for 30
min in the dark at room temperature. Following two washes with PBS,
the nuclear morphology was observed under a fluorescence microscope
(Nikon Corp., Tokyo, Japan).
Flow cytometric detection of
apoptosis
GSP-induced apoptosis was also analyzed by flow
cytometry, utilizing the Annexin V-FITC apoptosis detection kit
(Vazyme Biotech Co., Ltd.) in accordance with the manufacturer's
protocol. Briefly, CNE-2 cells were seeded in 6 cm-well plates at a
density of 2×105 cells, and cultured for 24 h, at which
point they were treated with GSPs for 12 h. Following treatment,
the cells were harvested, washed twice with cold PBS, and incubated
with Annexin V-FITC and PI for 10 min in the dark. The stained
cells were analyzed on a FACSCanto II flow cytometer equipped with
CellQuest software. Experiments were repeated in triplicate.
Assay for MMP
The MMP of CNE-2 cells was determined using a MMP
assay kit with JC-1 (Beyotime Institute of Biotechnology),
according to the manufacturer's instructions. Briefly, the cells
were subjected to treatment with GSPs for 12 h, after which JC-1
staining solution was added to the plates and incubated at 37°C for
20 min. JC-1 can then gather in the matrix of normal mitochondria
as J-aggregates, emitting red fluorescence, but cannot accumulate
in mitochondria with lower MMP and in that situation the JC-1
remains in a monomeric form, emitting green fluorescence. The
collapse of the MMP is reflected by the color change of the dye
from red to green, as analyzed by flow cytometry, in this case a
FACSCanto II equipped with CellQuest software.
Protein extraction and western blot
analysis
CNE-2 cells treated for 24 h with or without GSPs at
the indicated concentrations were harvested and washed with
ice-cold PBS, and then lysed on ice for 30 min using RIPA buffer
supplemented with 20 mM Tris (pH 7.5), 1% Triton X-100, 150 mM NaCl
and 1% of two types of protein inhibitors; phenylmethanesulfonyl
fluoride (PMSF) and protein phosphatase inhibitor. Cellular
extracts were clarified by centrifugation (Eppendorf, Hamburg,
Germany) at 15,294 × g at 4°C for 15 min, and protein
concentrations were determined using the BCA assay (Beyotime
Institute of Biotechnology). The extracted proteins were then
subjected to western blot analysis, resolved on 10% Tris-glycine
gels and transferred onto a polyvinylidene fluoride (PVDF) membrane
(Immobilon-P; Millipore, Billerica, MA, USA). After blocking the
non-specific binding sites with 5% skim milk for 1 h, the membranes
were incubated overnight at 4°C with specific primary antibodies.
The membranes were incubated with the appropriate horseradish
peroxidase-conjugated secondary antibody and then immunoreactive
protein membranes were visualized using enhanced chemiluminescence
(ECL). The protein bands were analyzed using a ChemiDoc XRS
transilluminator (Bio-Rad). All blots shown are representative of
three independent experiments.
Statistical analysis
All quantitative data are presented as mean ± SD of
triplicate independent experiments. The statistical analyses were
performed by one-way analysis of variance (ANOVA) using SPSS v.17.0
software. A p-value <0.05 was considered to indicate a
statistically significant result.
Results
GSPs suppress the proliferation of NPC
CNE-2 cells
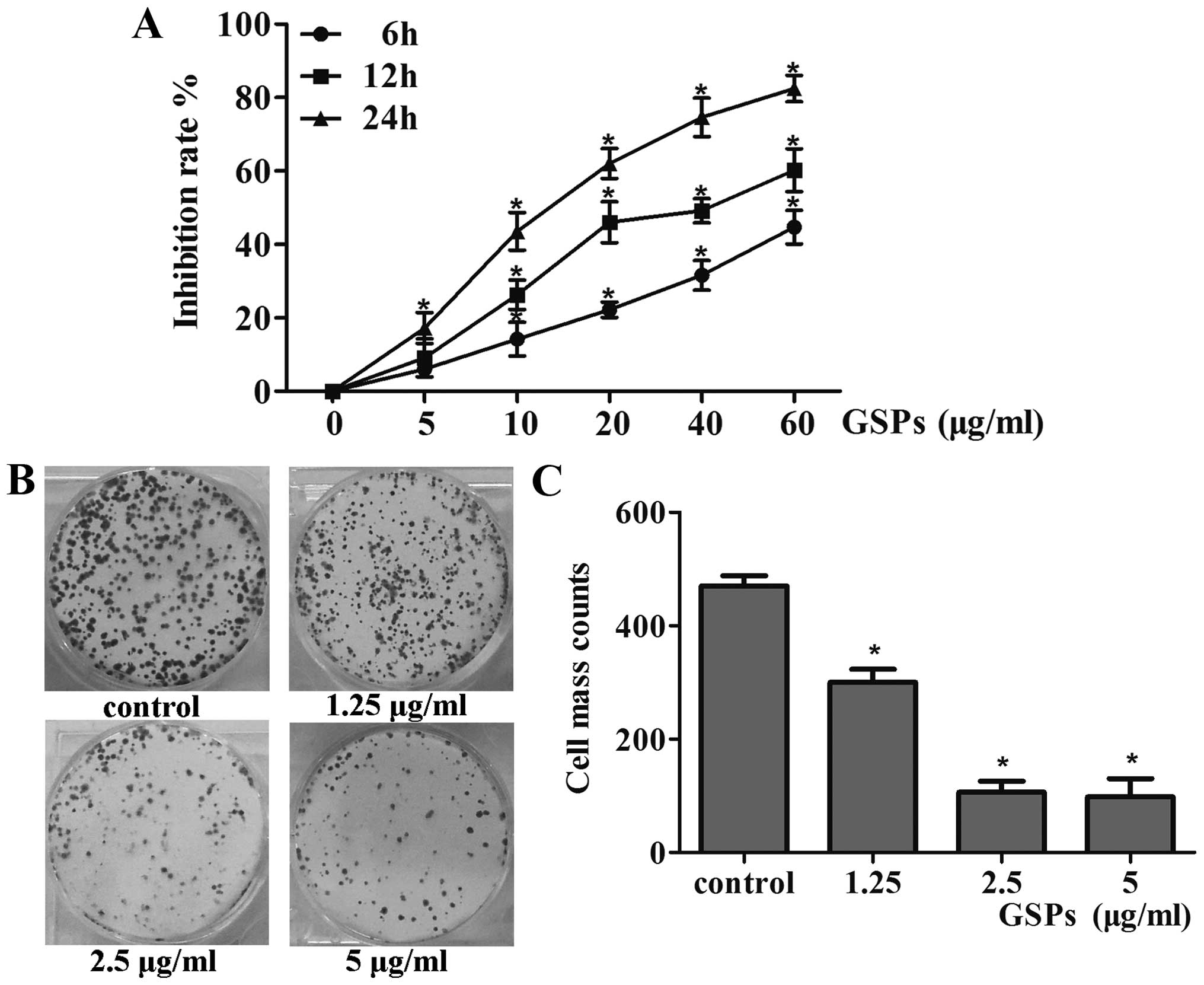
GSPs significantly inhibited the survival of CNE-2
cells in a dose-dependent manner, with ranges observed from 6 to
44% following 6 h of exposure, 9–60% after 12 h, and 17–82% after
24 h (p<0.05), as shown in Fig.
1A. The observed effect also exhibited a time-dependent manner
(p<0.05). Additionally, the half maximal inhibitory
concentration (IC50 value) of GSPs in CNE-2 cells was
80.19 μg/ml at 6 h, 34.78 μg/ml at 12 h, and 14.48
μg/ml at 24 h. Based on these results, most subsequent
experiments were carried out at 10, 20 and 40 μg/ml for 12
h. Further confirmation of the growth-suppressive property of GSPs
was obtained using the colony formation assay, the results of which
indicated that GSPs could reduce the colony forming efficiency of
CNE-2 cells by 76.5, 38.66 and 19.16% after pre-treatment with
1.25, 2.5 and 5 μg/ml GSPs, respectively (p<0.05)
(Fig. 1B and C). In combination,
these results confirmed the inhibitory effects of GSPs in NPC
cells.
GSPs induce G2/M phase cell cycle arrest
in CNE-2 cells
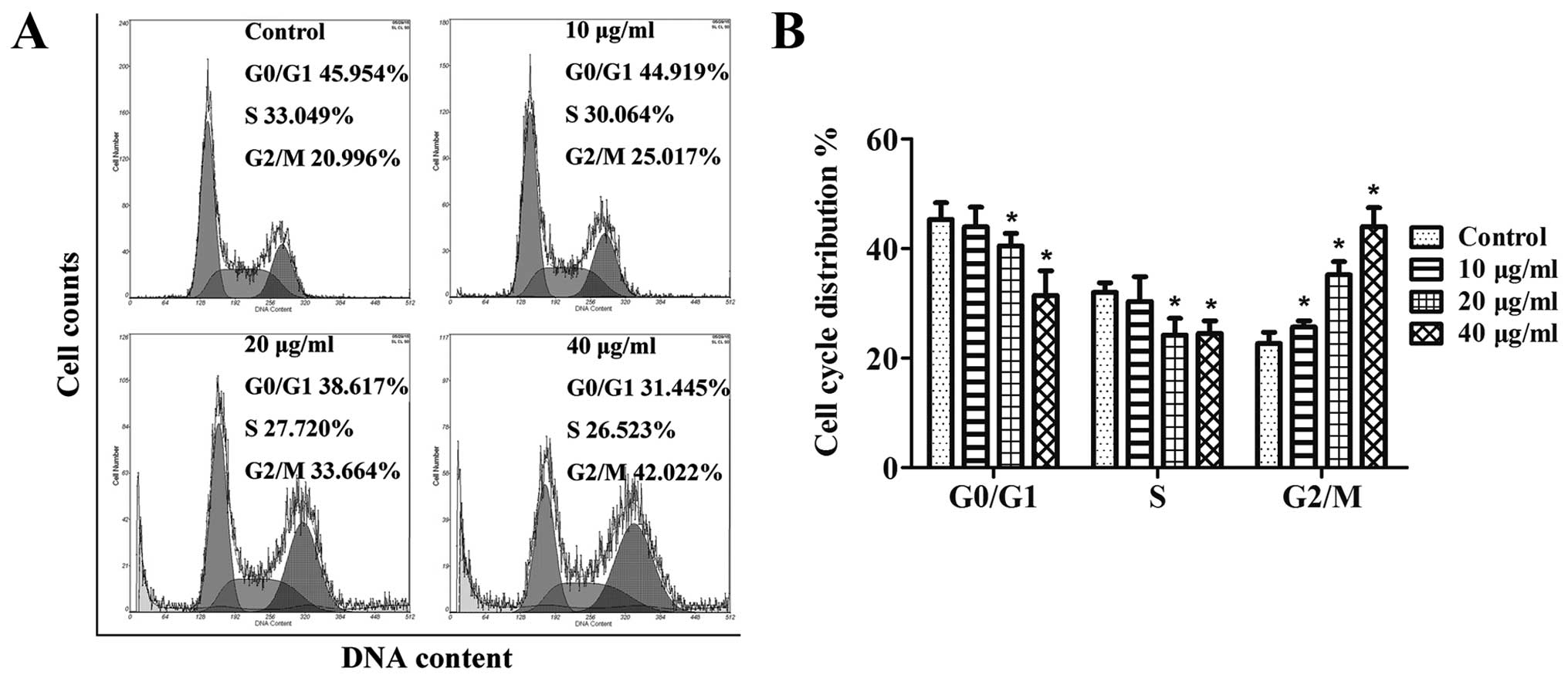
Based on the significant inhibitory effect GSPs
exerted on the growth of CNE-2 cells, we next aimed to determine
whether the possible mechanism was related to the effect that GSPs
had on the cell cycle progression of the CNE-2 cells. The results
in Fig. 2A indicated that in NPC
cells treated with GSPs, there was an obvious increase in the
percentage of cells in G2/M phase at all the concentrations tested;
10 μg/ml (25%, p<0.05), 20 μg/ml (33.6%,
p<0.05) and 40 μg/ml (42%, p<0.05) when compared to
the control (non-GSP-treated) group (20.9%). Summarized in Fig. 2B are the results of cell cycle
distribution analysis for each tested dose of the GSPs, which
suggest that the inhibitory effect of the GSPs on the proliferation
of CNE-2 cells may be associated with induction of G2/M phase
arrest.
GSPs induce apoptosis of NPC CNE-2
cells
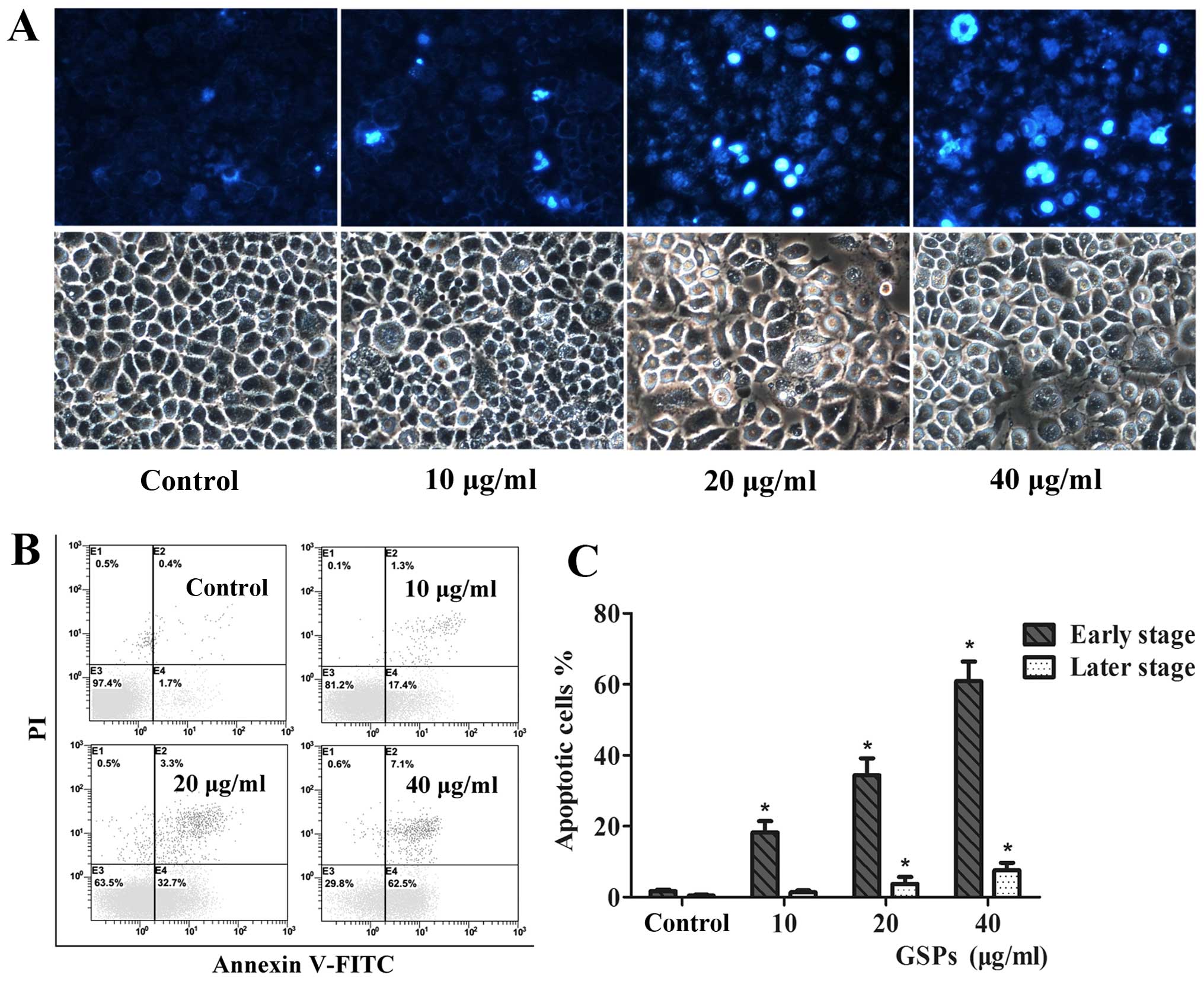
To determine the underlying mechanisms by which GSPs
act to reduce NPC cell survival, we next investigated the
morphological changes observed in cell nuclei induced by GSPs with
Hoechst 333258 staining. CNE-2 cells treated with varying
concentrations of GSPs for 12 h displayed classic changes,
including chromatin agglutination, karyopyknosis and apoptotic body
formation (Fig. 3A). Contrastingly,
cells in the untreated control group displayed characteristics of
almost normal cells, suggesting that GSPs induced the apoptosis of
the NPC cells.
In order to provide further evidence that GSPs do
induce apoptosis, quantitative analysis of apoptosis in the CNE-2
cells was analyzed by flow cytometry. Following a 12-h treatment
with various doses of GSPs, the cells were stained with Annexin
V-FITC/PI double dye and then typed as either early-stage (Annexin
V+ and PI−) or late-stage apoptotic (Annexin
V+ and PI+), as respectively shown in the E3
and E4 quadrants of the FACS histograms in Fig. 3B. The percentage of the total
apoptotic population (including early- and late-stage) of the CNE-2
cells are further summarized in Fig.
3C, and were as follows: 2.1% in control cells (early-stage,
0.4% and late-stage, 1.7%), 18.7% in cells treated with 10
μg/ml GSPs (early-stage, 1.3% and late-stage, 17.4%;
p<0.05), 36% in cells treated with 20 μg/ml GSPs
(early-stage, 3.3% and late-stage, 32.7%, p<0.05), and 69.6% in
cells treated with 40 μg/ml GSPs (early-stage, 7.1% and
late-stage, 62.5%; p<0.05). Together, these results demonstrated
that GSPs induced a significant increase in NPC CNE-2 cell
apoptosis, both at the early and late stage (p<0.05).
GSPs induce disruption of MMP in CNE-2
cells
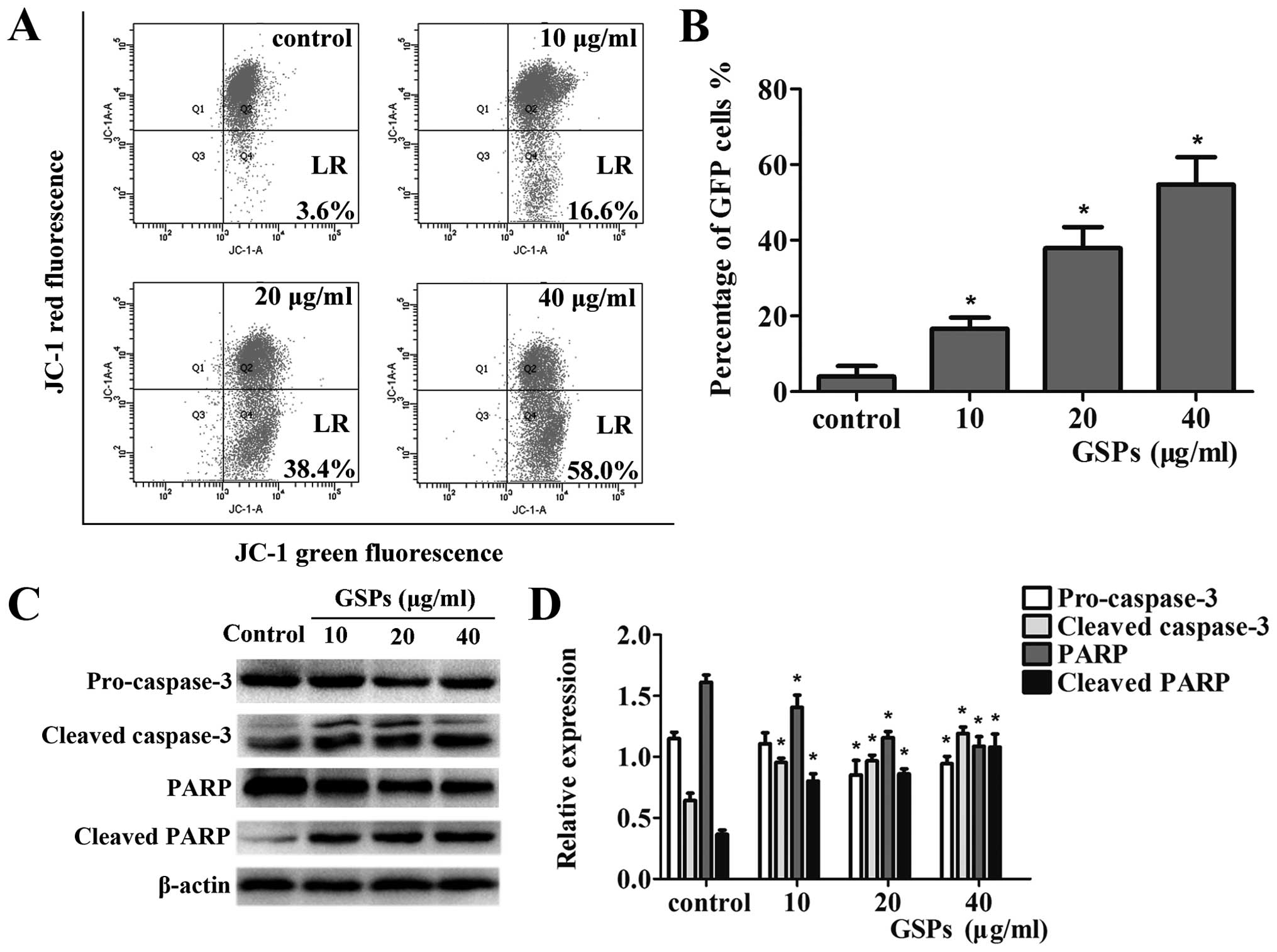
The depletion of mitochondrial transmembrane
potential (MMP) (Δψm) is a hallmark of cellular apoptosis, linked
to the initiation and activation of the apoptotic process in cells
(10). Staining with the cationic
lipophilic dye JC-1 was used to investigate the integrity of the
mitochondrial membrane of the cells, so that the effect of GSPs on
the MMP in CNE-2 cells could be determined. As shown in Fig. 4A, the staining resulted in a
significant increase in green fluorescence-positive cells; 3.6% of
the control group, 16.6% of the 10 μg/ml group, 38.4% of the
20 μg/ml group, and 58.0% of the 40 μg/ml group. The
data revealed that treatment of CNE-2 cells with different
concentrations of GSPs resulted in a dose-dependent increase in MMP
levels (p<0.05) (Fig. 4B). Thus,
we concluded that it was possible that the apoptotic effect
observed in CNE-2 cells following GSP treatment is connected with
the mitochondrial pathway and warrants further study.
GSPs activate caspase-3 and
poly(ADP-ribose) polymerase (PARP)
The release of apoptotic factors into the cytosol
leads to the cleavage of caspase-3, which then cleaves various
target proteins, including PARP, ultimately resulting in apoptotic
cell death (24). As shown in Fig.
4C, data from the western blot analysis revealed a reduction in
the levels of pro-caspase-3 and total PARP with a concomitant
increase in the levels of cleaved caspase-3 and PARP (p<0.05).
The relative expression levels of these proteins were also
calculated through normalization to β-actin (Fig. 4D). These results indicated that the
apoptosis of CNE-2 cells induced by GSPs may involve the activation
of the caspase-3 pathway.
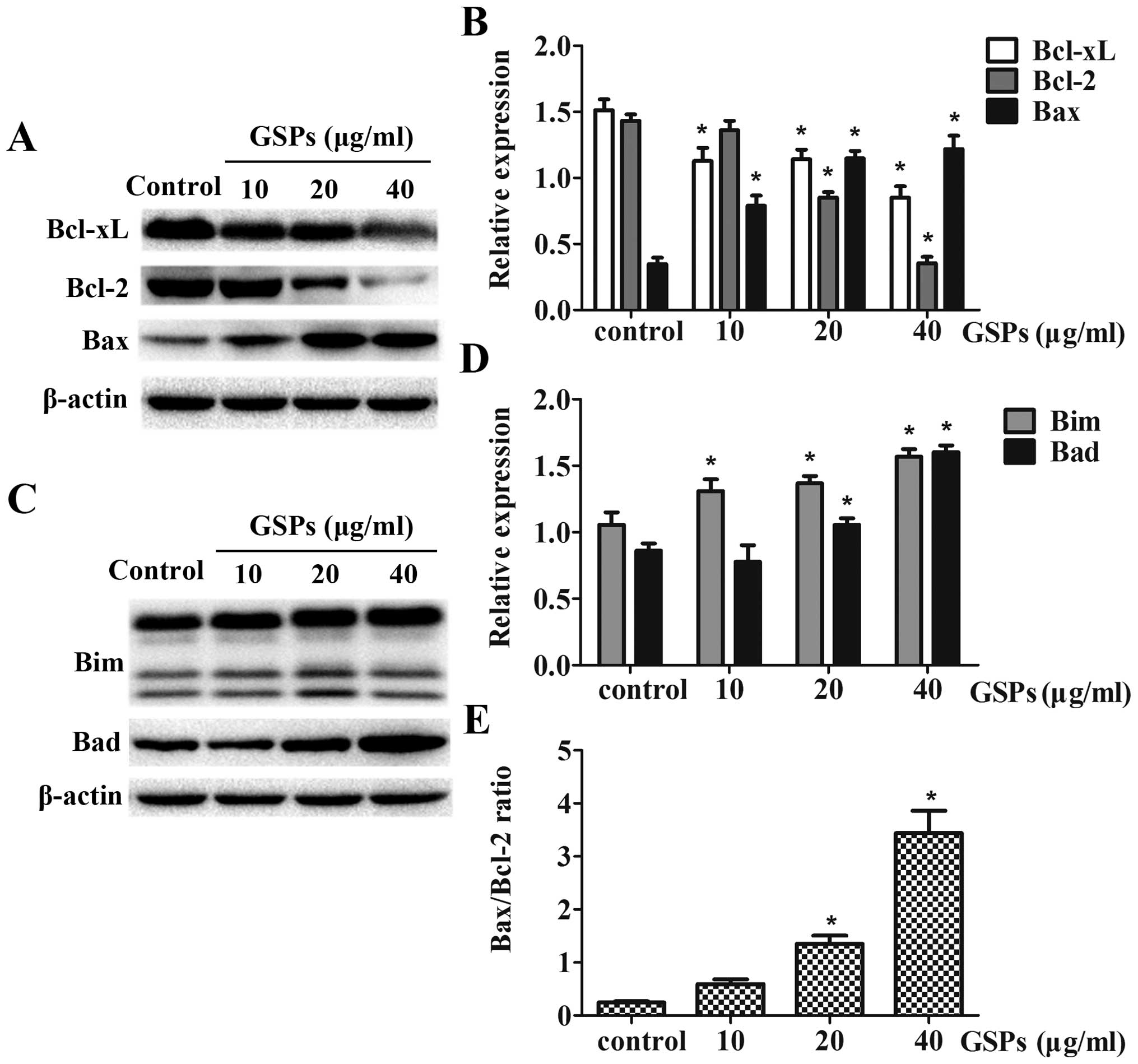
GSPs affect the protein expression of the
Bcl-2 family
To assess whether or not treatment with GSPs induced
cell death in NPC cells by affecting key regulators of apoptosis
within the mitochondrial pathway, the expression of the proteins,
Bax, Bcl-2, Bcl-xL, Bim and Bad was examined using western blot
analysis. Treatment of CNE-2 cells with GSPs for 12 h resulted in a
dose-dependent reduction in protein expression of Bcl-2 and Bcl-xL,
whereas the expression levels of Bax, Bim and Bad were upregulated
with increasing concentrations of GSPs (Fig. 5). GSP treatment also resulted in an
obvious increase in the ratio of Bax/Bcl-2, which is crucial in
determining the survival or death of cells following an apoptotic
stimulus (Fig. 5E). The relative
expression levels of these proteins were calculated by
normalization to β-actin expression, and were analyzed by Image
gray analysis software, which revealed a dose-dependent trend.
Discussion
Interest in the biology of grape seed extract first
originated with reports that moderate red wine consumption may be
associated with a low incidence of coronary heart disease, as
observed in the population of France. More recently, GSPs have been
extensively studied for their potential anticancer or
chemopreventative properties. Dhanalakshmi et al observed
that GSPs could inhibit the nuclear factor-κB (NF-κB) pathway and
induce the apoptosis of DU145 prostate carcinoma cells (19). It has also been found that dietary
intake of GSPs was effective in inhibiting the development of
UV-induced skin tumors in SKH-1 hairless mice, which was associated
with the inhibition of oxidative stress and inflammatory responses
(20). Furthermore, it has been
demonstrated that GSPs exert anticancer effects against cervical
cancer through the mitochondrial apoptosis pathway (16). However, a nasopharyngeal carcinoma
(NPC)-specific effect or mechanism of GSPs have not yet been seen.
In the present study, we first found that GSPs could attenuate the
viability of CNE-2 cells in a dose- and time-dependent manner,
leading to significant cell cycle arrest at the G2/M phase
(Figs. 1 and 2). This suggested that blocking cell cycle
progression could be an effective strategy to halt the development
of NPC.
It has been reported that apoptosis can effectively
prevent cancer development through the culling of cells that are at
risk of transformation, and that evasion of apoptosis is a
characteristic present in numerous cancers that exhibit therapeutic
resistance (21). Thus, it can be
reasonably argued that the best treatment for cancer is to induce
cellular apoptosis. In the present study, our findings revealed
that GSPs could notably induce apoptosis in CNE-2 cells accompanied
by morphological changes in the cell nuclei (Fig. 3). In the presence of oncogenic
stress, the mitochondrial pathway is triggered and the activity of
the anti-apoptotic Bcl-2 proteins is suppressed, followed by the
oligomerization of Bak and Bax proteins to induce MOMP (11). The present study has shown that
CNE-2 cells expressed significantly less Bcl-2 and Bcl-xL proteins,
but more Bax protein, suggesting that the Bax/Bcl-2 ratio was
markedly increased in GSP-induced apoptosis (Fig. 5A and E). Furthermore, GSPs depleted
the mitochondrial membrane potential (MMP) to a very low level
(Fig. 4A). Importantly, the
activation of Bax and Bak requires direct interaction with several
BH3-only proteins, while the other BH3-only proteins promote death
indirectly by neutralizing the activity of anti-apoptotic Bcl-2
proteins (22). Our results also
indicated that exposure to GSPs increased the expression levels of
the BH3-only proteins Bim and Bad in CNE-2 cells (Fig. 5C). Moreover, proteins in the
intermembrane space responsible for the activation of caspases were
released following MOMP. Cleaved caspase-3 can then go on to cleave
hundreds of different substrates in parallel, including PARP, all
within minutes, leading to rapid cell death with the characteristic
morphological hallmarks (23). Our
data demonstrated that GSPs decreased the expression of
pro-caspase-3 and PARP, whereas the levels of cleaved-caspase-3 and
cleaved-PARP were increased (Fig.
4C). When considered together, these findings lend support to
our hypothesis that, in NPC CNE-2 cells, GSPs induced apoptosis via
the mitochondrial pathway.
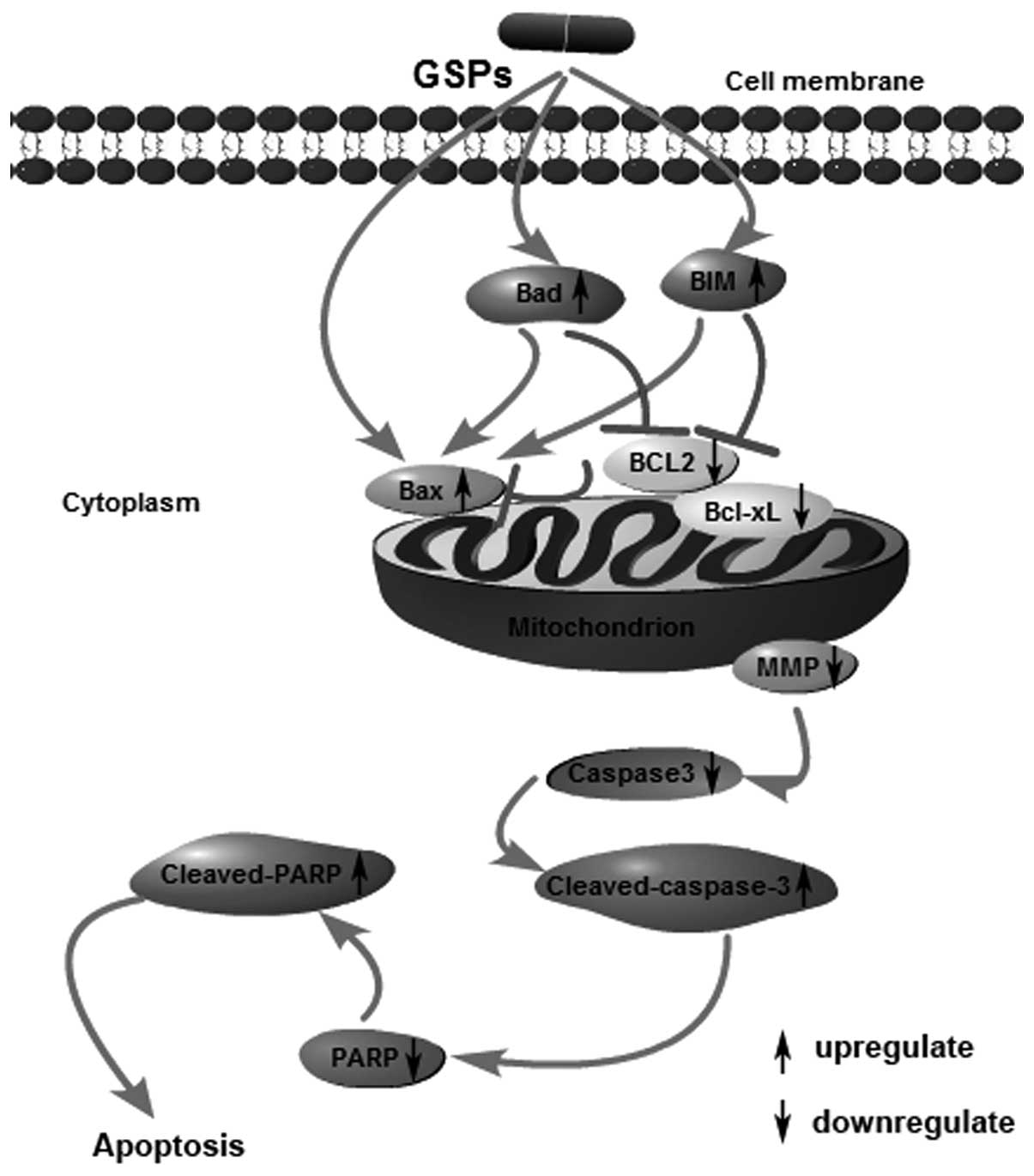
Upon consideration of our results as well as other
previous research, we speculated that GSPs could induce the
apoptosis of human NPC CNE-2 cells through the mitochondrial
pathway, characterized by stimulating cell membrane receptors to
upregulate the expression of Bax, Bim and Bad, while downregulating
the expression of Bcl-2 and Bcl-xL, as shown in Fig. 6. The loss of MMP revealed that the
integrity of the mitochondrial membrane was destroyed.
Subsequently, the release of proteins in the intermembrane space
activated caspase-3, which cleaved poly(ADP-ribose) polymerase
(PRAP), resulting in cell apoptosis. Thus, GSPs appear to be
plausible candidates as a bioactive phytochemical effective for the
treatment of NPC. In summation, the results of the present study
have demonstrated the chemotherapeutic potential of GSPs based on
their effects on the induction of apoptosis in NPC CNE-2 cells.
However, the specific components of GSPs responsible for this
induction of apoptosis still need to be identified, and our future
aims are focused on this goal as well as determining whether GSPs
also exert an inhibitory effect in vivo.
Acknowledgments
The present study was financially supported by
grants from the National Natural Science Foundation of China (no.
81272434), the Traditional Chineses Medicine Bureau of Guangdong
Province (no. 2014154), and the Cultivation Foundation of Guangdong
Medical University (M2014003).
References
|
1
|
Chua ML, Wee JT, Hui EP and Chan AT:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016. View Article : Google Scholar
|
|
2
|
Lee AW, Sze WM, Au JS, Leung SF, Leung TW,
Chua DT, Zee BC, Law SC, Teo PM, Tung SY, et al: Treatment results
for nasopharyngeal carcinoma in the modern era: The Hong Kong
experience. Int J Radiat Oncol Biol Phys. 61:1107–1116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee AW, Ng WT, Chan LL, Hung WM, Chan CC,
Sze HC, Chan OS, Chang AT and Yeung RM: Evolution of treatment for
nasopharyngeal cancer - success and setback in the
intensity-modulated radiotherapy era. Radiother Oncol. 110:377–384.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin S, Pan J, Han L, Guo Q, Hu C, Zong J,
Zhang X and Lu JJ: Update report of nasopharyngeal carcinoma
treated with reduced-volume intensity-modulated radiation therapy
and hypothesis of the optimal margin. Radiother Oncol. 110:385–389.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yi J, Huang X, Gao L, Luo J, Zhang S, Wang
K, Qu Y, Xiao J and Xu G: Intensity-modulated radiotherapy with
simultaneous integrated boost for locoregionally advanced
nasopharyngeal carcinoma. Radiat Oncol. 9:562014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng Y, Han F, Xiao W, Xiang Y, Lu L,
Deng X, Cui N and Zhao C: Analysis of late toxicity in
nasopharyngeal carcinoma patients treated with intensity modulated
radiation therapy. Radiat Oncol. 10:172015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei Z, Xie Y, Xu J, Luo Y, Chen F, Yang Y,
Huang Q, Tang A and Huang G: Radiation-induced sarcoma of head and
neck: 50 years of experience at a single institution in an endemic
area of nasopharyngeal carcinoma in China. Med Oncol. 29:670–676.
2012. View Article : Google Scholar
|
|
8
|
Strasser A, Cory S and Adams JM:
Deciphering the rules of programmed cell death to improve therapy
of cancer and other diseases. EMBO J. 30:3667–3683. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lopez J and Tait SW: Mitochondrial
apoptosis: Killing cancer using the enemy within. Br J Cancer.
112:957–962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Westphal D, Dewson G, Czabotar PE and
Kluck RM: Molecular biology of Bax and Bak activation and action.
Biochim Biophys Acta. 1813:521–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hockenbery D, Nuñez G, Milliman C,
Schreiber RD and Korsmeyer SJ: Bcl-2 is an inner mitochondrial
membrane protein that blocks programmed cell death. Nature.
348:334–336. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurahashi N, Inoue M, Iwasaki M, Tanaka Y,
Mizokami M and Tsugane S; JPHC Study Group: Vegetable, fruit and
antioxidant nutrient consumption and subsequent risk of
hepatocellular carcinoma: A prospective cohort study in Japan. Br J
Cancer. 100:181–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katiyar SK and Athar M: Grape seeds: Ripe
for cancer chemo-prevention. Cancer Prev Res. 6:617–621. 2013.
View Article : Google Scholar
|
|
14
|
Prasad R, Vaid M and Katiyar SK: Grape
proanthocyanidin inhibit pancreatic cancer cell growth in vitro and
in vivo through induction of apoptosis and by targeting the
PI3K/Akt pathway. PLoS One. 7:e430642012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Derry MM, Raina K, Balaiya V, Jain AK,
Shrotriya S, Huber KM, Serkova NJ, Agarwal R and Agarwal C: Grape
seed extract efficacy against azoxymethane-induced colon
tumorigenesis in A/J mice: Interlinking miRNA with cytokine
signaling and inflammation. Cancer Prev Res. 6:625–633. 2013.
View Article : Google Scholar
|
|
16
|
Chen Q, Liu XF and Zheng PS: Grape seed
proanthocyanidins (GSPs) inhibit the growth of cervical cancer by
inducing apoptosis mediated by the mitochondrial pathway. PLoS One.
9:e1070452014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh T, Sharma SD and Katiyar SK: Grape
proanthocyanidins induce apoptosis by loss of mitochondrial
membrane potential of human non-small cell lung cancer cells in
vitro and in vivo. PLoS One. 6:e274442011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nandakumar V, Singh T and Katiyar SK:
Multi-targeted prevention and therapy of cancer by
proanthocyanidins. Cancer Lett. 269:378–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dhanalakshmi S, Agarwal R and Agarwal C:
Inhibition of NF-kappaB pathway in grape seed extract-induced
apoptotic death of human prostate carcinoma DU145 cells. Int J
Oncol. 23:721–727. 2003.PubMed/NCBI
|
|
20
|
Sharma SD, Meeran SM and Katiyar SK:
Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative
stress and activation of mitogen-activated protein kinases and
nuclear factor-kappaB signaling in in vivo SKH-1 hairless mice. Mol
Cancer Ther. 6:995–1005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kelly GL and Strasser A: The essential
role of evasion from cell death in cancer. Adv Cancer Res.
111:39–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ku B, Liang C, Jung JU and Oh BH: Evidence
that inhibition of BAX activation by BCL-2 involves its tight and
preferential interaction with the BH3 domain of BAX. Cell Res.
21:627–641. 2011. View Article : Google Scholar
|
|
23
|
Park D and Dilda PJ: Mitochondria as
targets in angiogenesis inhibition. Mol Aspects Med. 31:113–131.
2010. View Article : Google Scholar
|