Introduction
Gliomas are the most common primary brain tumors,
and they constitute approximately 30% of all brain and central
nervous system tumors and 80% of all malignant brain tumors
(1). Less than 3% of glioma
patients are still alive at 5 years after diagnosis (2). Treatment for brain gliomas is a
combined approach, using surgery, radiation therapy and
chemotherapy. However, limited by the location of the tumor, glioma
is difficult to be resected completely by surgery. As a result,
radiotherapy and chemotherapy are necessary to increase the
survival time of patients (3,4).
Temozolomide (TMZ), which is able to cross the
blood-brain barrier effectively, has become the most effective
chemotherapy agent for glioma. A clinical study demonstrated that
radiotherapy and adjuvant TMZ treatment leads to an increased
median survival time of approximately 14.6 months (5,6).
Unfortunately, however, the response rate of TMZ treatment is not
satisfactory, and the response is usually accompanied by
chemotherapy resistance and high rates of toxicity such as severe
nausea, vomiting, genotoxic, teratogenic and fetotoxic effects
(7). There has been a shift from
management with traditional, non-specific cytotoxic chemotherapies
to treatment with molecular-specific targeted therapies that are
used either alone or in combination with traditional chemotherapy
and radiation therapy. Thus, more effective and less toxic agents
for glioma are urgently required. Researchers have conducted
laboratory and clinical studies to investigate whether it may be
possible to enhance the anticancer potency of TMZ by combination
with other pharmacologic agents. In preclinical trials, it was
found that chloroquine increased the chemosensitivity of glioma
cells to TMZ (8). A laboratory
study indicated that TMZ killed brain tumor cells more efficiently
when epigallocatechin gallate (EGCG), a component of green tea, was
added (9). More recently, use of
the novel oxygen diffusion-enhancing compound trans sodium
crocetinate (TSC) when combined with TMZ and radiation therapy has
been investigated in preclinical studies and a clinical trial is
currently underway (10).
Clinical studies have shown that depressive disorder
is common among patients with advanced cancer, and anti-depression
treatment in cancer patients not only improves depressive symptoms,
but also enhances immunologic function, thereby improving the
quality of life of cancer patients (11,12).
Thus, anti-depressants are routinely prescribed to cancer patients
with depression. As a representative of selective serotonin
reuptake inhibitors (SSRIs), fluoxetine (FLT) is one of the most
commonly used anti-depressants (13). While increased attention has been
given to the direct effects of antidepressants on tumor cells,
recent preclinical studies suggest that the use of FLT may be
associated with a reduced colon cancer risk (14–16).
Actually, a growing body of evidence has demonstrated that FLT
possesses antitumor activity in different cancer cell types, such
as hepatocellular carcinoma (17),
OC2 human oral cancer (18) and
human epithelial ovarian cancer OVCAR-3 cell lines (19). In addition, FLT was also found to
have a synergistic effect with anticancer drugs and can overcome
drug resistance (20–22). However, the downstream apoptotic
signaling pathway involved in FLT-induced cell death remains
unknown.
In the present study, we determined the in
vitro cytotoxicity of FLT and explored the underlying
mechanisms involved in its effects against glioma cells, and
assessed the potential synergism of FLT and TMZ in inhibiting the
growth of C6 glioma cells. These findings may provide a new
therapeutic strategy to achieve anti-glioma synergism.
Materials and methods
Chemicals and antibodies
FLT and TMZ were obtained from Sigma-Aldrich (St.
Louis, MO, USA). The antibodies against phospho-PERK, CHOP and
caspase-3 were obtained from Cell Signaling Technology (Danvers,
MA, USA), The antibodies against PERK, eIF2α, phospho-eIF2α, ATF4,
ATF6 and GADD34 were obtained from Abcam (Cambridge, UK). The
antibody against β-actin and horseradish peroxidase-conjugated
anti-mouse or anti-rabbit IgG were obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA).
Cell culture
Cells were purchased from the Chinese Academy of
Sciences Cell Bank (Shanghai, China). The cells were routinely
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal bovine serum and 100 U/ml penicillin and
streptomycin (all from Invitrogen, Carlsbad, CA, USA) in a
humidified incubator with 5% CO2 at 37°C.
CCK-8 assay
Cell viability assay was analyzed using a Cell
Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan).
Briefly, the cells were plated on a 96-well culture plate at a
density of 6,000 cells/well and were cultured overnight. The cells
were then incubated in fresh culture medium containing FLT and/or
TMZ at various concentrations for 24 h. The cell viability was then
assessed according to the manufacturer's instructions.
Apoptosis assay
C6 cells were seeded in 96-well microplates at 3,500
cells/well and treated with FLT for 24 h. Then, 50 μl of
MitoTracker/Hoechst solution (Cellomics, Pittsburgh, PA, USA) was
added to each well, and the cells were incubated at 37°C for 30
min. Next, the cells were fixed with 100 μl of the fixation
solution, without removing the medium and the incubation continued
in a fume hood at room temperature for 10 min. The plate was sealed
after being washed once and was run on the ArrayScan high-content
screening (HCS) reader (Cellomics). The nuclear size and intensity
of Hoechst fluorescence were also calculated and analyzed by Cell
Motility BioApplication software.
Flow cytometric analysis
C6 cells were seeded at 4.0×105
cells/well in 6-well plates and treated with FLT for 24 h, and then
washed twice with cold PBS and re-suspended at a concentration of
1×106 cells/ml. Next, 5 μl FITC-Annexin V and 10
μl PI (both from BD Biosciences) were added into 100
μl of the solution. After that, the cells were mixed gently
and incubated for 15 min at room temperature in the dark. Finally,
1 ml PBS was added and the samples were detected by flow cytometric
analysis (Beckman Coulter, Brea, CA, USA) within 1 h.
Caspase-3 activity measurements
C6 cells were seeded on a 96-well microplate at a
density of 3,000 cells/well and treated with FLT at 24 h. Then, 100
μl of Caspase-Glo® 3/7 reagent (Promega, Madison,
WI, USA) was added to each sample after allowing the plates to
equilibrate at room temperature. The contents of the wells were
gently mixed for 0.5–2 min and incubated at room temperature for 1
h. The luminescence intensities were then analyzed using a
multimode reader (Tecan, Männedorf, Switzerland) and the enzyme
activities were expressed as relative luminescence units.
Measurement of the mitochondrial membrane
potential
C6 cells were seeded on a 96-well plate at a density
of 3,000 cells/well and exposed to the drugs for 24 h. Fifty
microliters of MitoTracker/Hoechst solution (Cellomics Inc.) was
added to each well, and the cells were incubated for 30 min at
37°C. Next, we added 100 μl of the fixation solution
directly to each well without removing the medium and incubation
was carried out in a fume hood at room temperature for 10 min. Then
we aspirated the supernatant and washed the plate once with 150
μl wash buffer. Images were acquired on the
ArrayScan® HCS system; the intensity of red fluorescence
represented the MMP of the cells.
Quantitative real-time RT-PCR
C6 cells were plated on a 6-well plate at a density
of 4×105 cells/well, and exposed to the drugs for 24 h.
Total RNA extraction was isolated from the cells using an RNeasy
Mini kit (Qiagen, Valencia, CA, USA). The first strand cDNA
synthesis was carried out using RevertAid™ First Strand cDNA
Synthesis kit (Fermentas, Burlington, CA, USA). The RT mixture was
mixed with Quanti Fast SYBR Green PCR Kit (Qiagen, Hilden, Germany)
and gene-specific primers in a final volume of 20 μl. The
following oligonucleotide primers were used as specific for the
gene CHOP (5′-CTGGAAGCCTGGTATGAGGA-3′ and
5′-AGGTGCTTGTGACCTCTGCT-3′) and gene GADD34 (5′- CCTTGATGTG
GAAGCCCAAAGTT-3′ and 5′-TCCACTTCTTGCTCTCTAAGGCCAT-3′) (Invitrogen).
The expression levels of the genes were normalized with that of
β-actin (5′-CCCATCTATGAGGGTTACGC-3′ and 5′-TTAATGTCACGCACGATTTC-3′)
housekeeping gene product as an endogenous reference. The
quantitative real-time RT-PCR was performed in ABI 7500 Fast
Real-Time PCR (Life Technologies, Carlsbad, CA, USA). The
amplification conditions were as follows: hold for 15 min at 95°C,
followed by 40 cycles of 60°C for 30 sec and 59°C for 31 sec. The
final results were expressed as fold differences in target gene
expression related to both the endogenous control gene expression
and the calibrator was determined using the 2−ΔΔCT
method.
CHOP protein analysis
C6 cells were seeded on a 96-well plate at a density
of 3,000 cells/well and exposed to FLT for 24 h. After fixation and
permeabilization, 50 μl of the mouse monoclonal anti-CHOP
antibody (Abcam) solution was added to each sample, which was
incubated for 1.5 h at 37°C. Next, 50 μl of the secondary
antibody conjugated with the fluorophore Alexa Fluor®
555/Hoechst solution (Cellomics) was added and incubated at room
temperature for 1 h. Then the plate was washed twice with 100
μl of blocking buffer. The plate was read on an HCS system
reader. The fluorescence intensity represented the level of CHOP
protein in the C6 cells.
Western blot analysis
C6 cells were cultured on a 6-well plate at a
density of 4×105 cells/well overnight. Cells were
treated with FLT for 24 h. Then we aspirated media, washed the
plate with ice-cold 1X PBS for twice and lysed cells with RIPA
buffer. Total cell lysates were separated by 10 or 12% SDS-PAGE and
electrotransferred to PVDF membrane (Millipore, Bedford, MA, USA).
The membranes were blocked in blocking buffer (5% nonfat milk in
TBS containing 0.1% Tween-20) for 2 h at room temperature. After
washing in TBST buffer [10 mM Tris-HCl (pH 8.3), 0.05% Tween-20)]
(Tween-20; Sigma-Aldrich, St. Louis, MO, USA), the membranes ware
incubated overnight at 4°C with the primary antibodies against
GADD34 (1:500) and β-actin (1:1,000) (both from Santa Cruz
Biotechnology) as control. Then the membranes were incubated with
horseradish peroxidase-conjugated anti-mouse IgG (1:5,000;
Zhongshan, Beijing,China) at room temperature for 1 h. The
immunoblots were visualized using a chemiluminescence detection kit
(Pierce Chemical, Rockford, IL, USA).
Small interference RNA experiments
The CHOP-specific siRNAs
(5′-UCAAGGAAGAACUAGGAAATT-3′ and 5′-UUUCCUAGUUCUUCCUUGATT-3′) and
scrambled siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACG
UGACACGUUCGGAGAATT-3′) were designed and synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China). C6 cells were plated in a
12-well plate or in a 96-well plate 1 day before transfection.
After 60% confluency was achieved, siRNA was transfected into the
culture plates using Lipofectamine 2000 (Invitrogen Life
Technologies) following the manufacturer's instructions. Scrambled
siRNA was used as a negative control. The knockdown effect was
measured by real-time RT-PCR and HCS. Then, the cells were treated
with FLT for 24 h. After a 24-h incubation, the cells were washed
with medium and used for caspase-3 activity measurement and CCK-8
assays.
Statistical analysis
Data are presented as means ± SEM and were analyzed
by analysis of variance (ANOVA) followed by Dunnett's test, where
P<0.05 indicated a significant difference.
Results
FLT inhibits the growth of glioma cell
lines
FLT is well recognized for its anti-proliferative
activity. To determine the effect of FLT on the growth of glioma
cells, various glioma cell lines including C6,U87-MG, U373 and U251
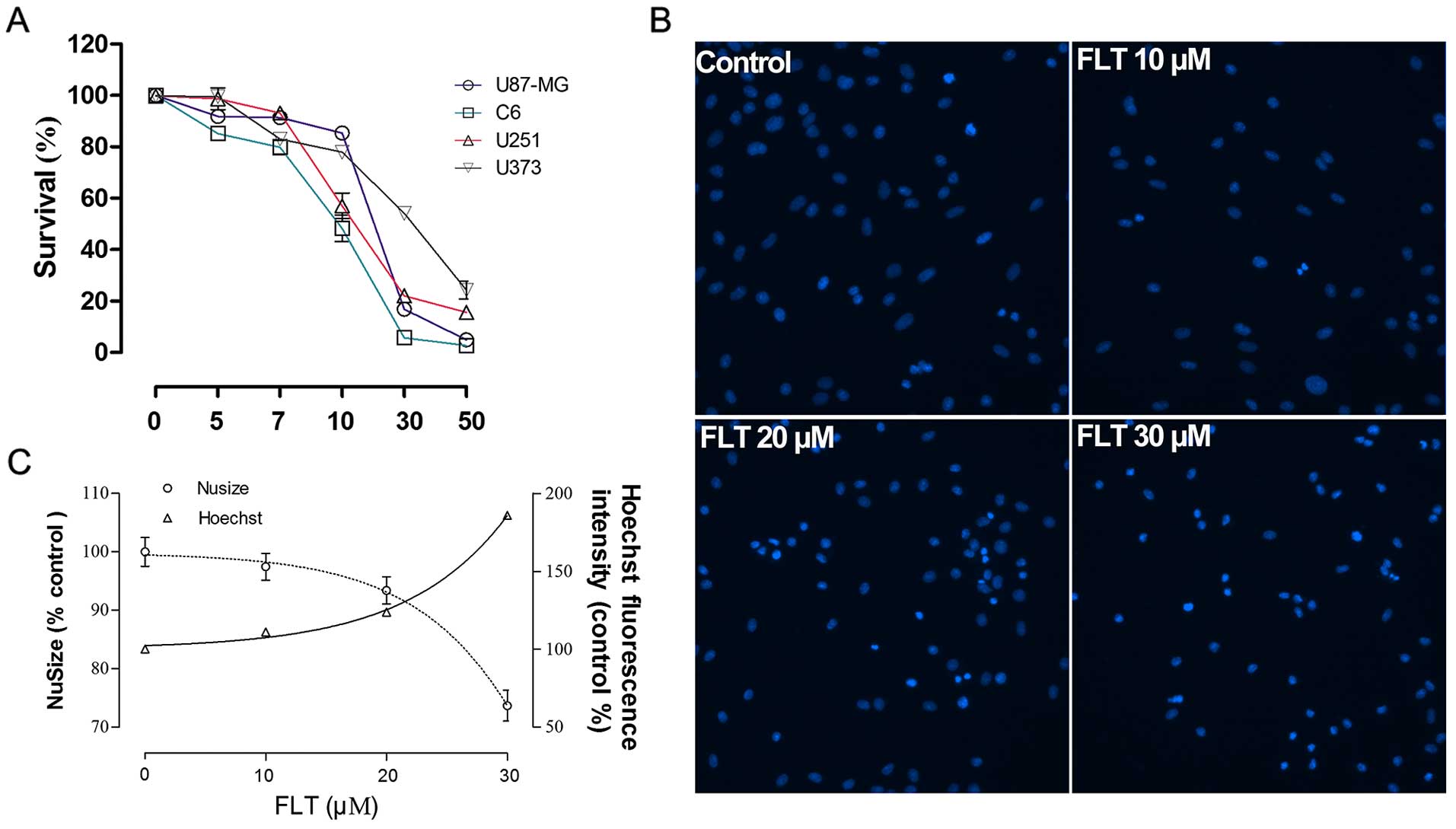
glioma cells were used to exam the growth inhibitory ability of FLT
by using a CCK-8 assay. All the four glioma cells showed
significant inhibition of cell proliferation upon FLT treatment in
a dose-dependent manner (Fig. 1A).
The IC50 levels of FLT for C6, U87-MG, U373 and U251
cells were 14.7, 21.8, 48.5 and 22.9 μM, respectively. In
particular, C6 cells had a highly sensitive response to the
addition of FLT. Thus, C6 cells were selected for further
study.
FLT induces apoptotic cell death in C6
glioma cells
To determine whether FLT influences C6 cell
apoptosis, we examined the effects of FLT on cell apoptosis. The
results showed that FLT treatment for 24 h decreased the nuclear
size of the C6 cells and increased the Hoechst fluorescence
intensity in the nuclei in a dose-dependent manner. The nuclei of
the C6 cells were significantly condensed at the FLT concentration
of 10 μM with the concomitant enhanced intensity of Hoechst
fluorescence in the nuclei, showing the typical phenotype of
apoptosis. This effect was more pronounced with the increase in the
drug concentration to above 20 μM (Fig. 1B and C). To further determine
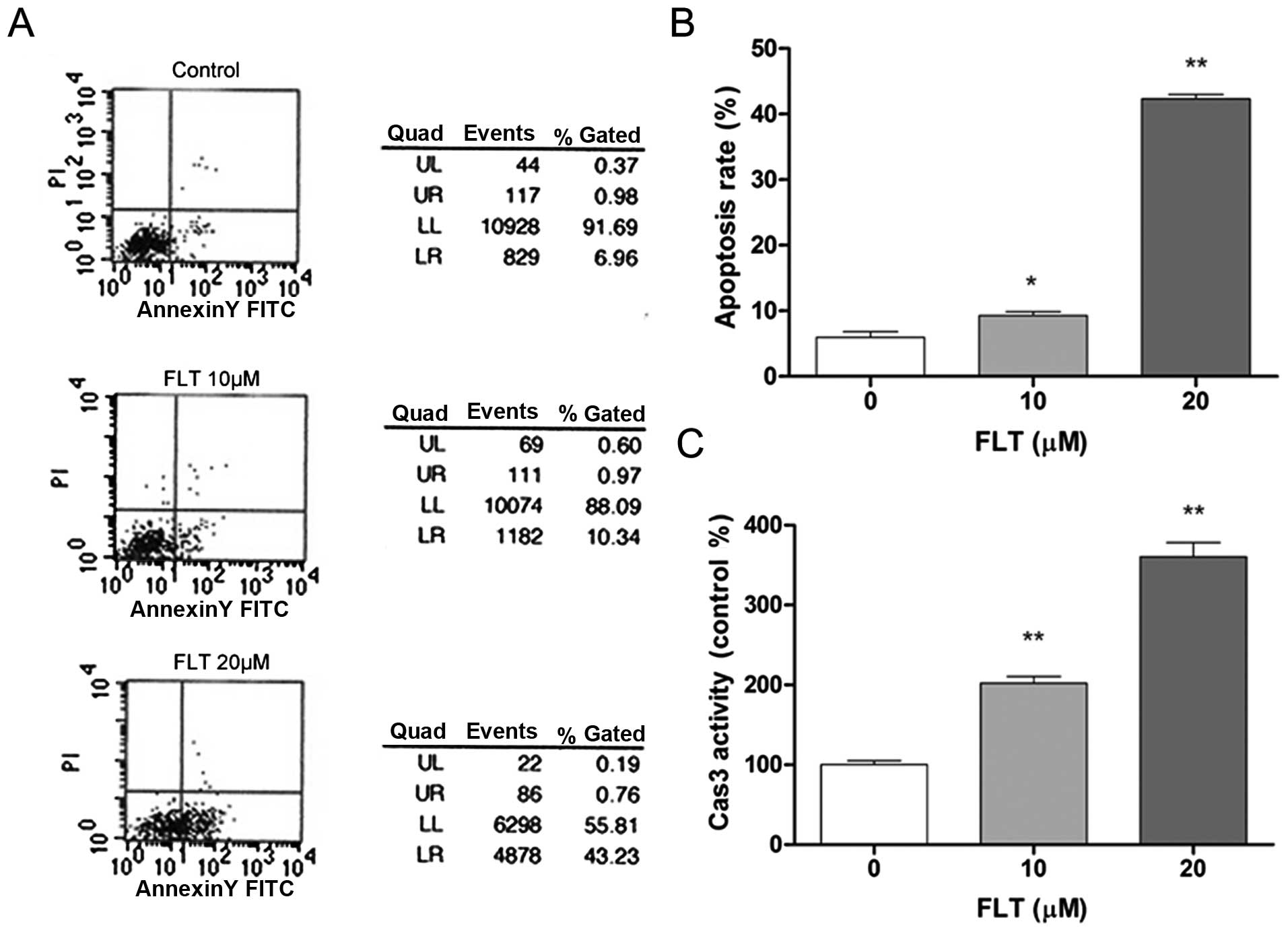
whether FLT influences cell apoptosis, flow cytometry was
performed. As shown in Fig. 2A and
B, the early apoptosis rate of the C6 cells following FLT (0,
10, 20 μM) treatment by flow cytometry was 6.5, 9.21 and
45.3%, respectively, which demonstrated that FLT significantly
increased the percentage of apoptotic cells (Fig. 2A and B). We further assessed the
activation of a key apoptosis mediator caspase-3. After FLT
treatment, the enzyme activities of caspase-3 in the C6 cells were
evidently enhanced (Fig. 2C). These
results indicated that FLT can effectively promote the apoptosis of
C6 cells.
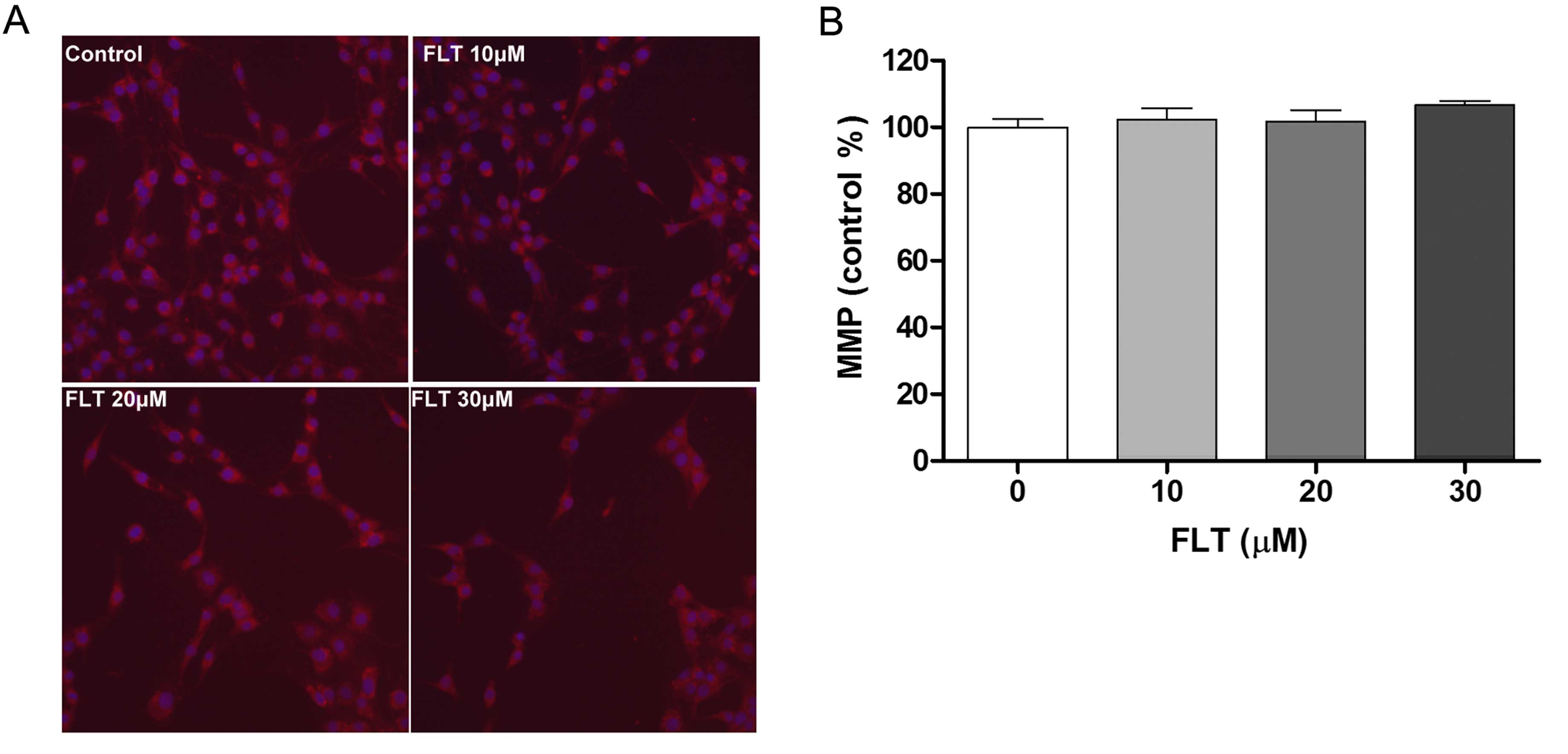
FLT shows no effect on mitochondrial
membrane potential
In order to gain further insight into the mechanism
of apoptosis exerted by FLT on C6 cells, we performed a series of
experiments in the C6 cells. Since mitochondrial damage represents
an event extensively associated with apoptosis, MitoTracker red
fluorescence representing MMP was used through the HCS System.
Incubation of the C6 cells with FLT did not affect the MitoTracker
red fluorescence intensity (Fig.
3). The results showed that FLT may induce cell programmed
death through a non-mitochondrial signaling pathway.
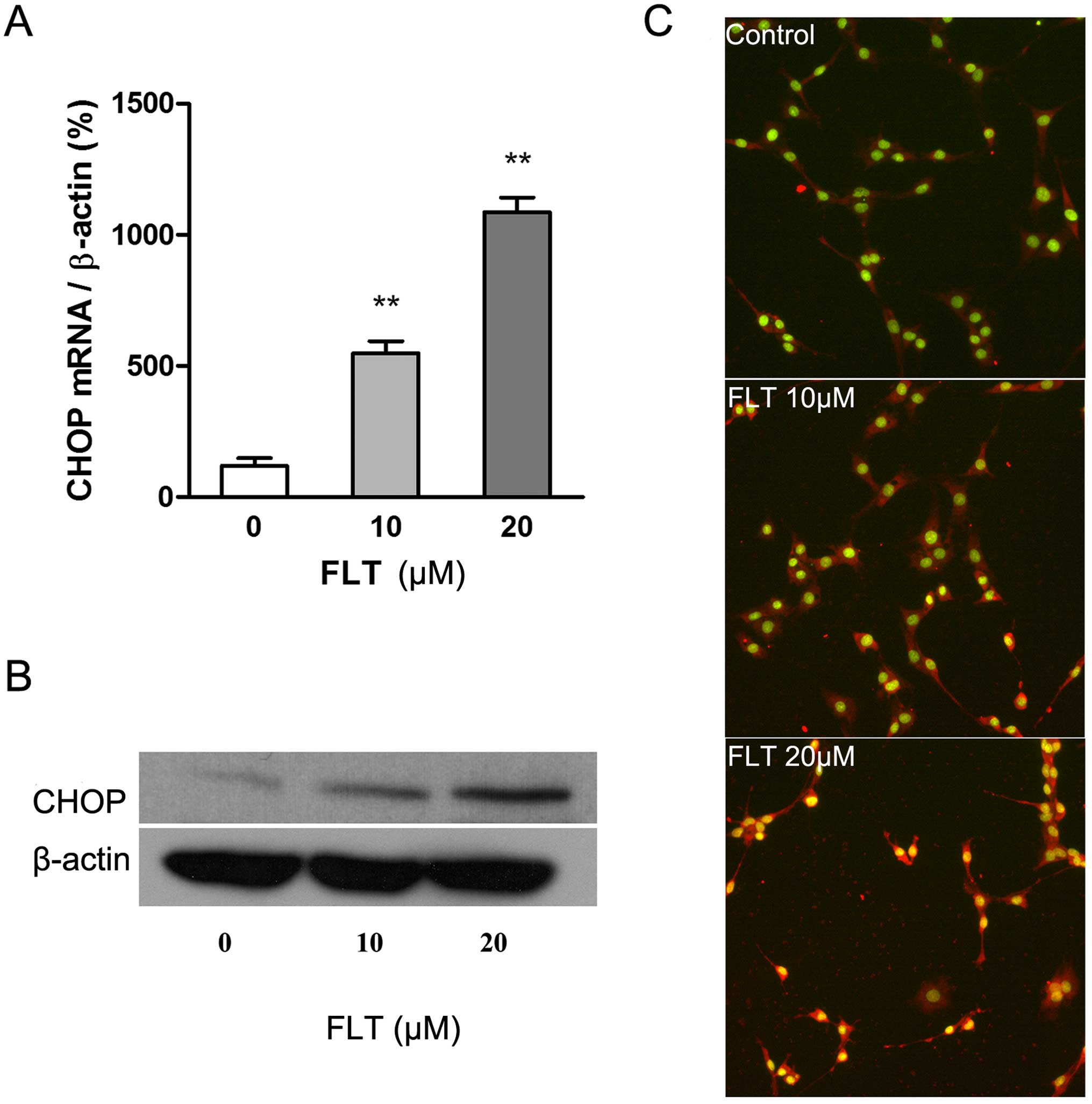
FLT induces C6 cell apoptosis through the
endoplasmic reticulum apoptotic pathway
Next, we focused on the endoplasmic reticulum stress
(ERS) apoptotic pathway. Firstly we examined the effects of FLT on
CHOP, a hallmark of ERS, by real-time RT-PCR, western blotting and
HCS, respectively. The results showed that 10 μM FLT
treatment for 24 h significantly increased the transcription
activation of CHOP mRNA (P<0.05), and the effect was more
pronounced under the higher drug concentration (20 μM)
(P<0.01) (Fig. 4A). CHOP protein
is present in the cytosol under non-stressed conditions. It can be
robustly induced under cell stress conditions and thereafter
translocates into nuclei (Fig. 4C).
The result of western blotting showed that treatment of FLT (20
μM, 24 h) significantly increased the expression of CHOP
protein (Fig. 4B).
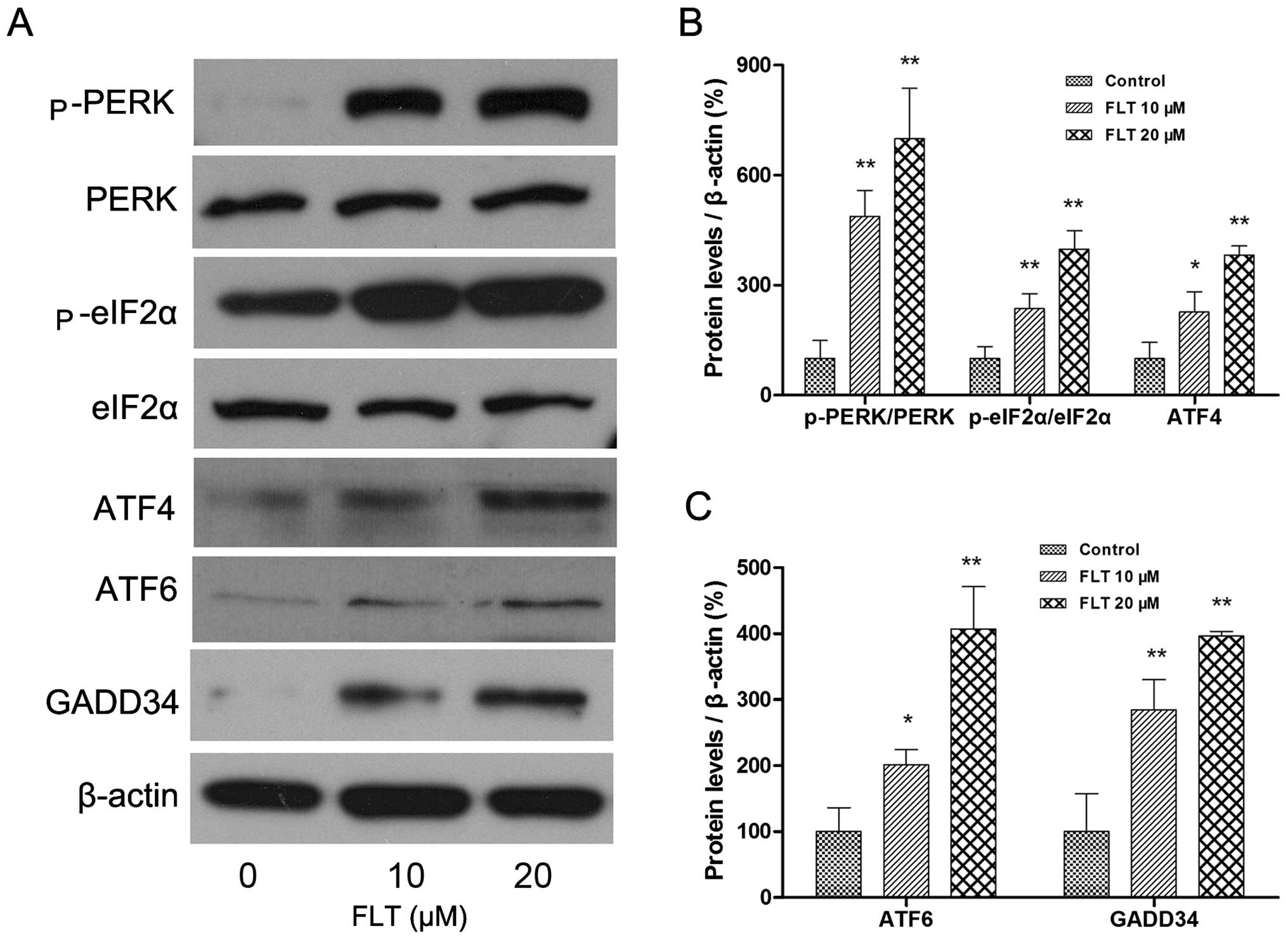
Then, we investigated the upstream and downstream
signaling pathway of CHOP. The 3 principal unfolded protein
response (UPR) receptors involved are PERK, ATF6 and Ire1, all of
which induce expression of CHOP. The protein levels of
PERK-eIF2α-ATF4 and ATF6 were examined by western blotting. The
results showed that FLT treatment (10 and 20 μM) markedly
increased the autophosphorylation of PERK and eIF2α. In addition,
ATF4, the downstream target of eIF2α was increased as well.
Meanwhile, another ER stress sensor ATF6 was also markedly
upregulated following FLT treatment (Fig. 5). Concomitantly, the protein
expression of GADD34, one of the transcriptional downstream
effector genes of CHOP activation, was also upregulated (Fig. 5).
FLT sensitizes glioma cells to TMZ by
activating CHOP
To explore the possibility of synergistic
cytotoxicity, C6 cells were treated with various concentrations of
FLT and TMZ, either alone or in combination. Cell viability was
determined by CCK-8 assay and synergistic effects between the two
drugs were evaluated using the CompuSyn 2.0 software (ComboSyn,
Inc., Paramus, NJ, USA) (23).
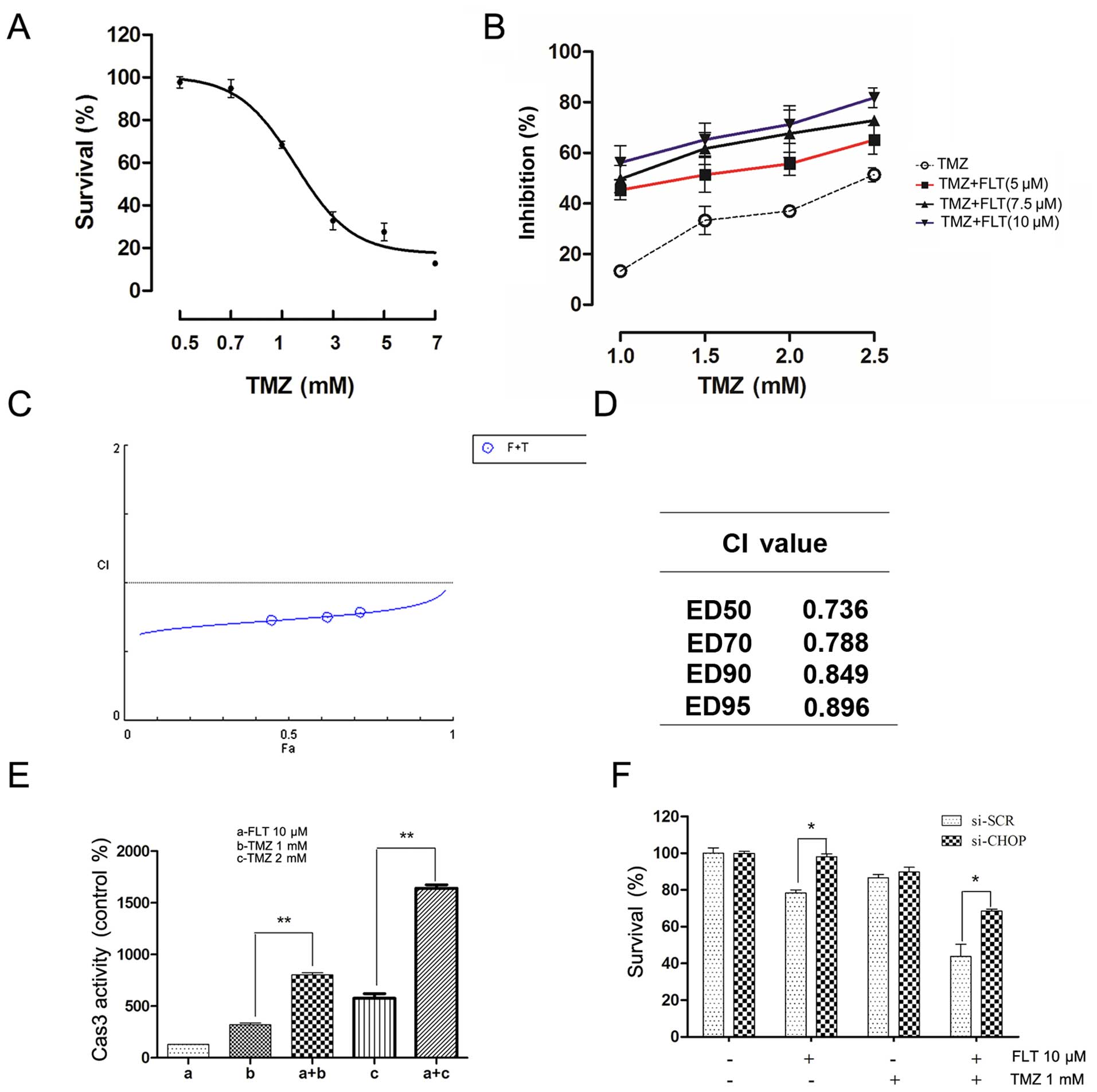
First, the effect of TMZ alone on the viability of the C6 cells was
examined. As shown in Fig. 6A, TMZ
dose-dependently reduced cell viability after a 24-h treatment. In
the TMZ treatment group, the 24-h IC50 was 2.23 mM.
Then, the C6 cells were treated in culture with a combination of
FLT and TMZ at various concentrations for 24 h. The combination of
FLT and TMZ at certain drug concentrations led to marked effects in
decreasing the viability of the C6 cells (Fig. 6B). Synergistic analysis is presented
with a Fa-CI plot and CI values (Fig.
6C and D). It was observed that the combination of FLT and TMZ
produced a significant synergistic effect and had a CI value below
1.0 (range, 0.736–0.896). The synergism was particularly obvious at
lower inhibitory concentrations, whereas little impact was observed
at higher proliferation suppression. The synergistic effects of FLT
and TMZ were also determined in caspase-3 activity analysis
following treatment with TMZ and FLT in the C6 cells (Fig. 6E). Knockdown of CHOP expression by
CHOP-specific siRNA was used to further confirm the role of CHOP in
the synergistic effect of FLT. CHOP-specific siRNA effectively
downregulated CHOP mRNA and protein levels (24). We did not observe enhanced
cytotoxicity following treatment with TMZ plus FLT compared to TMZ
alone in the C6 cells with CHOP depletion (Fig. 6F). These results suggested that FLT
may sensitize glioma cells to TMZ by activating CHOP.
Discussion
Fluoxetine is one of the most commonly used
antidepressants for improving the mood of patients with depressive
disorder mainly though inhibiting selective serotonin reuptake. In
addition, it is also used to treat chronic pain and other diseases
(25,26). Actually, studies have shown that FLT
exhibits a series of target-off biological actions such as
upregulation of T-cell mediated antitumor immunity,
anti-proliferative and pro-apoptotic effects in cancer cells
(17–19,27).
Since FLT can easily pass through the blood-brain barrier, and has
a high concentration in the brain, its direct action on brain
tumors is a 'hot' issue. FLT was reported to induce brain tumor
apoptosis and even enhance the sensitivity of chemotherapeutic
drugs in cancer cells (20).
However the underlying mechanisms of apoptosis induced by FLT are
not clear.
In the present study, we first found that exposure
to FLT significantly and dose-dependently inhibited the
proliferation of various glioma cell lines. Among these, C6 cells
were most sensitive to FLT treatment. Therefore, whether exposure
to FLT would trigger apoptosis was analyzed in C6 cells through
subsequent experiments. HCS assay showed that the typical apoptotic
morphological indicators such as nuclear shrinkage and DNA
condensation appeared in the C6 cells following FLT treatment
(Fig. 1). Meanwhile, flow
cytometric analysis also found that FLT induced early apoptotic
events in a dose-dependent manner, and apoptotic percentage of 20
μM FLT was ~45.3%. Simultaneously, caspase-3 activity was
greatly activated (Fig. 2),
suggesting involvement of the caspase-dependent pathway in the
pro-apoptotic effect. However, further study found there was no
notable change in MMP after FLT treatment, indicating an
alternative apoptotic pathway involved in the apoptotic action of
FLT. The ERS apoptotic pathway, which differs from the
mitochondrial apoptosis pathway, is involved in the apoptotic
induction of various anti-carcinogens and chemical compounds.
Therefore, we next focused on the ERS apoptotic pathway.
The endoplasmic reticulum serves many general
functions, including the folding and transport of protein (28,29).
Disturbances in calcium regulation, redox regulation, glucose
deprivation, and the overexpression of proteins can lead to an
endoplasmic reticulum stress response (ER stress) and severe ERS
may trigger the apoptosis pathway (30). CHOP i s considered to be a crucial
regulator of ER stress-related apoptotic signaling (31). Therefore, whether exposure to FLT
triggers the ER stress response was analyzed in subsequent
experiments. We first determined the effects of FLT on CHOP. We
found that a 24-h FLT treatment greatly activated CHOP, which
demonstrated that ER stress was elicited by FLT, Then, we further
explored the upstream and downstream of the CHOP signaling pathway.
The ER stress response triggered under stress conditions is
mediated through three main sensors, namely inositol requiring
element-1 (IRE-1), protein kinase-like ER kinase (PERK) and
activating transcription factor 6 (ATF6) (32). The PERK-eIF2α-ATF4 pathway and ATF6
pathway are the two main branches that activate CHOP.
Simultaneously, CHOP mediates cell apoptosis through the induction
of genes such as Gadd34 and Ero1α (32). As expected, western blot results
indicated that FLT treatment increased PERK phosphorylation
concomitant with the stimulation of eIF2α phosphorylation, and the
levels of ATF4 and ATF6 protein were also increased. Upregulation
of Gadd34 expression was also observed in the C6 cells
exposed to FLT. All the results indicated that the endoplasmic
reticulum (ER) stress-related apoptotic pathway was responsible for
FLT-induced apoptosis in the C6 cells. FLT may induce C6 cell
apoptosis through the PERK-eIF2α-ATF4-Chop and ATF6-Chop signaling
pathways.
TMZ is the most effective chemotherapeutic drug in
glioma cancer therapy. As a second generation of DNA methylating
agents, its cytotoxicity is reported to be mainly mediated through
adduction of a methyl group to the O6 position of
guanine in genomic DNA. However, more and more experiments support
that the antitumor effects induced by TMZ to non-DNA targets may
also contribute to its action (33–35).
Despite the high TMZ potential, progression of disease and
recurrence are still observed. It is a key issue to find a strategy
by which to enhance the antitumor action of TMZ. A large number of
clinical studies have demonstrated that anti-depression treatment
in cancer patients not only improves depressive symptoms, but also
enhances immunologic function, thereby improving the quality of
life of cancer patients. Thus, anti-depression treatment has become
an important means for adjuvant therapy of cancer. Meanwhile,
studies from our and other laboratories have demonstrated that FLT
inhibits glioma cell proliferation and induces apoptosis.
Therefore, we evaluated whether FLT could improve the sensitivity
of C6 glioma cells to the combination chemotherapy. The CI method
was used to analyze the type of drug interactions in combination
chemotherapy. The combination index (CI) is a parameter that
indicates whether the interaction of 2 or more drugs is synergistic
(CI <1), additive (CI =1), or antagonistic (CI >1). Our study
showed that FLT exerted a synergistic effect on glioma cells when
combined with TMZ.
Accumulating data suggest that multi-targeted
therapy may produce greater benefits than those observed with
single-targeted therapies. The mechanism of FLT may be different
from TMZ. Thus, we postulate that FLT as distinguished from TMZ may
be partly responsible for their synergistic cytocidal effect in C6
cells. An in vivo study is now being undertaken in our
laboratory to evaluate the synergistic effect in inhibiting the
growth of tumor xenografts in mice, and we are also trying to
identify potential predictive biomarkers with respect to the drug
interaction.
To summarize, we showed that FLT is a potential
anticancer drug that acts by activating an ERS-related apoptotic
pathway in the treatment of glioma. In addition, our findings
suggest that FLT exerts a synergistic effect on glioma cells when
combined with TMZ. Further studies are needed to elucidate the role
of the ERS-related apoptotic pathway in the synergistic effect of
FLT and TMZ.
Acknowledgments
We gratefully thank the National Natural Science
Foundation of China (nos. 81302202, 81272683, 81441111) and the
Natural Science Foundation of Shandong Province (no. ZR2011HQ055)
for the financial support.
References
|
1
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohgaki H and Kleihues P: Epidemiology and
etiology of gliomas. Acta Neuropathol. 109:93–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang F, Huang Q and Zhou LY: Analysis of
the treatment of gliomas with SEC therapy combined with
radiochemotherapy. Eur Rev Med Pharmacol Sci. 19:2400–2405.
2015.PubMed/NCBI
|
|
4
|
Quick A, Patel D, Hadziahmetovic M,
Chakravarti A and Mehta M: Current therapeutic paradigms in
glioblastoma. Rev Recent Clin Trials. 5:14–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al European Organisation for Research and Treatment
of Cancer Brain Tumour and Radiation Oncology Groups; National
Cancer Institute of Canada Clinical Trials Group: Effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neyns B, Tosoni A, Hwu WJ and Reardon DA:
Dose-dense temozolomide regimens: Antitumor activity, toxicity, and
immunomodulatory effects. Cancer. 116:2868–2877. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hori YS, Hosoda R, Akiyama Y, Sebori R,
Wanibuchi M, Mikami T, Sugino T, Suzuki K, Maruyama M, Tsukamoto M,
et al: Chloroquine potentiates temozolomide cytotoxicity by
inhibiting mitochondrial autophagy in glioma cells. J Neurooncol.
122:11–20. 2015. View Article : Google Scholar
|
|
9
|
Zhang Y, Wang SX, Ma JW, Li HY, Ye JC, Xie
SM, Du B and Zhong XY: EGCG inhibits properties of glioma stem-like
cells and synergizes with temozolomide through downregulation of
P-glycoprotein inhibition. J Neurooncol. 121:41–52. 2015.
View Article : Google Scholar
|
|
10
|
Sheehan J, Cifarelli CP, Dassoulas K,
Olson C, Rainey J and Han S: Trans-sodium crocetinate enhancing
survival and glioma response on magnetic resonance imaging to
radiation and temozolomide. J Neurosurg. 113:234–239. 2010.
View Article : Google Scholar
|
|
11
|
Park EM and Rosenstein DL: Depression in
adolescents and young adults with cancer. Dialogues Clin Neurosci.
17:171–180. 2015.PubMed/NCBI
|
|
12
|
Lauer AL: Treatment of anxiety and
depression in adolescents and young adults with cancer. J Pediatr
Oncol Nurs. 32:278–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perez-Caballero L, Torres-Sanchez S, Bravo
L, Mico JA and Berrocoso E: Fluoxetine: A case history of its
discovery and preclinical development. Expert Opin Drug Discov.
9:567–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kannen V, Marini T, Turatti A, Carvalho
MC, Brandão ML, Jabor VA, Bonato PS, Ferreira FR, Zanette DL, Silva
WA Jr, et al: Fluoxetine induces preventive and complex effects
against colon cancer development in epithelial and stromal areas in
rats. Toxicol Lett. 204:134–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koh SJ, Kim JM, Kim IK, Kim N, Jung HC,
Song IS and Kim JS: Fluoxetine inhibits NF-κB signaling in
intestinal epithelial cells and ameliorates experimental colitis
and colitis-associated colon cancer in mice. Am J Physiol
Gastrointest Liver Physiol. 301:G9–G19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kannen V, Hintzsche H, Zanette DL, Silva
WA Jr, Garcia SB, Waaga-Gasser AM and Stopper H: Antiproliferative
effects of fluoxetine on colon cancer cells and in a colonic
carcinogen mouse model. PLoS One. 7:e500432012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mun AR, Lee SJ, Kim GB, Kang HS, Kim JS
and Kim SJ: Fluoxetine-induced apoptosis in hepatocellular
carcinoma cells. Anticancer Res. 33:3691–3697. 2013.PubMed/NCBI
|
|
18
|
Lin KL, Chou CT, Cheng JS, Chang HT, Liang
WZ, Kuo CC, Chen IL, Tseng LL, Shieh P, Wu RF, et al: Effect of
fluoxetine on [Ca2+]i and cell viability in OC2 human
oral cancer cells. Chin J Physiol. 57:256–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee CS, Kim YJ, Jang ER, Kim W and Myung
SC: Fluoxetine induces apoptosis in ovarian carcinoma cell line
OVCAR-3 through reactive oxygen species-dependent activation of
nuclear factor-kappaB. Basic Clin Pharmacol Toxicol. 106:446–453.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song T, Li H, Tian Z, Xu C, Liu J and Guo
Y: Disruption of NF-κB signaling by fluoxetine attenuates MGMT
expression in glioma cells. Onco Targets Ther. 8:2199–2208.
2015.
|
|
21
|
Cloonan SM and Williams DC: The
antidepressants maprotiline and fluoxetine induce Type II
autophagic cell death in drug-resistant Burkitt's lymphoma. Int J
Cancer. 128:1712–1723. 2011. View Article : Google Scholar
|
|
22
|
Zhou T, Duan J, Wang Y, Chen X, Zhou G,
Wang R, Fu L and Xu F: Fluoxetine synergys with anticancer drugs to
overcome multidrug resistance in breast cancer cells. Tumour Biol.
33:1299–1306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bijnsdorp IV, Giovannetti E and Peters GJ:
Analysis of drug interactions. Methods Mol Biol. 731:421–434. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma J, Qiu Y, Yang L, Peng L, Xia Z, Hou
LN, Fang C, Qi H and Chen HZ: Desipramine induces apoptosis in rat
glioma cells via endoplasmic reticulum stress-dependent CHOP
pathway. J Neurooncol. 101:41–48. 2011. View Article : Google Scholar
|
|
25
|
Bundeff AW and Woodis CB: Selective
serotonin reuptake inhibitors for the treatment of irritable bowel
syndrome. Ann Pharmacother. 48:777–784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dharmshaktu P, Tayal V and Kalra BS:
Efficacy of antidepressants as analgesics: A review. J Clin
Pharmacol. 52:6–17. 2012. View Article : Google Scholar
|
|
27
|
Frick LR, Rapanelli M, Arcos ML, Cremaschi
GA and Genaro AM: Oral administration of fluoxetine alters the
proliferation/apoptosis balance of lymphoma cells and up-regulates
T cell immunity in tumor-bearing mice. Eur J Pharmacol.
659:265–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koch GL: The endoplasmic reticulum and
calcium storage. BioEssays. 12:527–531. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Klausner RD and Sitia R: Protein
degradation in the endoplasmic reticulum. Cell. 62:611–614. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu H, Ng BS and Thibault G: Endoplasmic
reticulum stress response in yeast and humans. Biosci Rep.
34:e001182014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar
|
|
32
|
Sano R and Reed JC: ER stress-induced cell
death mechanisms. Biochim Biophys Acta. 1833:3460–3470. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roos WP, Batista LF, Naumann SC, Wick W,
Weller M, Menck CF and Kaina B: Apoptosis in malignant glioma cells
triggered by the temozolomide-induced DNA lesion
O6-methylguanine. Oncogene. 26:186–197. 2007. View Article : Google Scholar
|
|
34
|
Zhang WB, Wang Z, Shu F, Jin YH, Liu HY,
Wang QJ and Yang Y: Activation of AMP-activated protein kinase by
temozolomide contributes to apoptosis in glioblastoma cells via p53
activation and mTORC1 inhibition. J Biol Chem. 285:40461–40471.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zou Y, Wang Q and Wang W: MutL homolog 1
contributes to temozolomide-induced autophagy via
ataxia-telangiectasia mutated in glioma. Mol Med Rep. 11:4591–4596.
2015.PubMed/NCBI
|