Introduction
Soft tissue sarcomas (STS) are a heterogeneous group
of rare malignant tumors that account for ~1% of all adult
malignancies and occur in ~60% of all cases in the extremities
(1,2). According to the content of soft
tissues, the lower extremities are affected more frequently than
the upper extremities, with a ratio of ~4:1 (3,4).
In patients with primary STS of the extremities, the
therapy of choice involves limb-sparing surgical resection with
negative margins, usually followed by radiation treatment to
decrease local recurrence (4–6). There
have been several analyses of the prognostic factors influencing
overall survival (OS) in patients with extremity STS (7–11).
Among these factors, histologic grade, depth, anatomic site, tumor
size and histologic subtype are considered the most significant for
OS. The achievement of negative surgical margins in primary STS has
been determined to be an important factor for improving local
recurrence-free survival (LRFS). However, the implementation of
radical surgery in the distal region of the lower extremity is
often difficult due to the limited soft and bony tissue situation.
Here, attainment of negative surgical margins may require extensive
surgery and could result in considerable impairment of extremity
function, particularly in cases of large tumor size or localization
adjacent to critical anatomic structures. It is therefore important
to determine whether an aggressive surgical approach with the goal
of negative margins can be justified for STS at the distal lower
extremities.
In patients with extremity STS in general, the
impact of microscopic surgical margins on OS is still a subject of
debate. Large single-institutional studies investigating the
clinical significance of surgical margins have presented
inconsistent results (4,12–18).
Accordingly, smaller but more specific studies pertaining the
surgical treatment of STS in the distal lower extremities were not
able to establish an association between microscopic margins and OS
(19–23). Moreover, the follow-up data in these
studies were reported only inconsistently whereas longer follow-up
periods seem reasonable, especially for slow-growing STS subtypes.
Therefore, the question remains whether aggressive local disease
control would have a positive long-term impact on OS in patients
with STS at the distal lower extremities.
The aim of this study was to identify prognostic
indicators of survival and functional outcome in patients with STS
at the lower extremities by reviewing our institutional experience.
In particular, we focused on the effect of surgical margins on
disease outcome.
Patients and methods
Patients
Between September 1999 and May 2014, 157 patients
with STS of the distal lower extremities were treated surgically at
our institution. STS extending distally to the level of the knee
joints were included, but tumors involving the level of the knee
joints or located proximally were excluded. A total of 104 of the
157 patients presented with primary disease in our institution.
Forty-three patients were subsequently referred to our center after
incomplete resection or the diagnosis of recurrence at least 3
months after definitive surgery on the primary tumor which had been
performed at other institutions. From this group, we excluded 28
patients because essential data regarding the initial surgical
procedure, such as tumor size or margin status were not available.
Furthermore, 9 patients, including those from foreign countries,
were lost to follow-up. Thus, we restricted analyses to 120
participants with full information available on the outcome and
surgical margins at the initial procedure. They were assessed and
their clinicopathological characteristics are summarized in
Tables I and II. Patient follow-up was obtained from
our database, medical records and patient correspondence. The study
was approved by the local Ethics Committee.
 | Table IResults of univariate analyses to
determine factors predictive of overall survival in 120 patients
with soft tissue sarcomas of the distal lower extremities. |
Table I
Results of univariate analyses to
determine factors predictive of overall survival in 120 patients
with soft tissue sarcomas of the distal lower extremities.
| N | Estimated 1-year OS
(95% CI) | Estimated 2-year OS
(95% CI) | Estimated 5-year OS
(95% CI) | P-value
(log-rank)a |
|---|
| Age (years) |
| <60 | 57 | 100 (−) | 100 (−) | 94.0 (78.2–98.5) | 0.036 |
| ≥60 | 63 | 96.5 (86.6–99.1) | 88.6 (76.4–94.7) | 68.5 (53.1–79.8) | |
| Sex |
| Female | 60 | 100 (−) | 95.3 (82.7–98.8) | 90.0 (75.3–96.1) | 0.004 |
| Male | 60 | 96.5 (86.6–99.1) | 92.6 (81.5–97.2) | 71.1 (55.1–82.2) | |
| Tumor size (cm) |
| <5 | 62 | 98.2 (88.2–99.8) | 96.3 (86.0–99.1) | 84.8 (70.5–92.5) | 0.068 |
| ≥5 | 58 | 98.0 (86.6–99.7) | 91.1 (77.9–96.6) | 73.9 (56.6–85.2) | |
| Tumor depth |
| Epifascial | 60 | 100 (−) | 96.0 (85.1–99.0) | 83.5 (68.3–91.9) | 0.308 |
| Subfascial | 60 | 96.3 (85.9–99.1) | 91.7 (79.3–96.8) | 75.8 (59.3–86.3) | |
| Tumor site |
| Foot/ankle | 32 | 100 (−) | 96.6 (77.9–99.5) | 77.8 (54.3–90.2) | 0.479 |
| Lower leg | 88 | 97.4 (90.1–99.4) | 93.0 (84.0–97.0) | 80.8 (68.5–88.7) | |
| Grading |
| G1 | 16 | 100 (−) | 93.3 (61.3–99.0) | 86.2 (55.0–96.4) | 0.123 |
| G2 | 52 | 100 (−) | 97.4 (83.2–99.6) | 88.0 (70.9–95.3) | |
| G3 | 52 | 95.7 (83.7–98.9) | 90.7 (76.9–96.4) | 71.1 (53.7–82.9) | |
| Histologic
subset |
| Liposarcoma | 21 | 100 (−) | 100 (−) | 79.6
(48.9–93.0) | 0.315 |
|
Non-liposarcoma | 99 | 97.7
(91.2–99.4) | 92.5
(84.1–96.6) | 80.1
(68.5–87.8) | |
| NOS | 40 | 94.1
(78.5–98.5) | 90.9
(74.3–97.0) | 72.1
(51.5–85.1) | 0.229 |
| Non-NOS | 80 | 100 (−) | 95.4
(86.5–98.5) | 83.8
(71.0–91.3) | |
| Synovial
sarcoma | 18 | 100 (−) | 92.9
(59.1–99.0) | 82.5
(45.1–95.5) | 0.460 |
| Non-synovial
sarcoma | 102 | 97.8
(91.6–99.5) | 94.2
(86.5–97.5) | 79.6
(68.2–87.2) | |
|
Leiomyosarcoma | 14 | 100 (−) | 91.7
(53.9–98.8) | 91.7
(53.9–98.8) | 0.832 |
|
Non-leiomyosarcoma | 106 | 97.9
(91.8–99.5) | 94.3
(86.7–97.6) | 78.7
(67.5–86.5) | |
 | Table IIUnivariate analyses on overall
survival depending on treatment characteristics. |
Table II
Univariate analyses on overall
survival depending on treatment characteristics.
| N | Estimated 1-year OS
(95% CI) | Estimated 2-year OS
(95% CI) | Estimated 5-year OS
(95% CI) | P-value
(log-rank)a |
|---|
| Margin status after
primary resection |
| R0 | 108 | 98.0
(92.1–99.5) | 94.5
(87.2–97.7) | 80.5
(69.7–87.9) | 0.318 |
| R1 | 12 | 100 (−) | 88.9
(43.3–98.4) | 74.1
(28.9–93.0) | |
| Distance of closest
negative surgical margin at resection of the primary tumor (R0
group) (mm) |
| ≤1 | 20 | 94.4
(66.6–99.2) | 88.5
(61.4–97.0) | 50.3
(19.6–74.8) | 0.068 |
| >1 | 51 | 97.8
(85.3–99.7) | 92.6
(78.8–97.6) | 83.4
(66.4–92.3) | |
| ≤5 | 52 | 95.6
(83.7–98.9) | 90.4
(76.2–96.3) | 69.4
(49.9–82.5) | 0.981 |
| >5 | 19 | 100 (−) | 93.8
(63.2–99.1) | 87.1
(57.3–96.6) | |
| Wound closure after
primary resection |
| Primary
closure | 36 | 96.8
(79.2–99.5) | 96.8
(79.2–99.5) | 92.2
(71.5–98.0) | 0.887 |
| Non-primary
closure (plastic surgical soft tissue coverage) | 55 | 97.9
(86.1–99.7) | 90.7
(77.1–96.4) | 87.5
(72.2–94.7) | |
| Amputation |
| Yes | 29 | 100 (−) | 96.4
(77.2–99.5) | 59.4
(37.5–75.8) | 0.001 |
| No | 91 | 97.5
(90.3–99.4) | 93.0
(83.9–97.0) | 89.2
(78.5–94.8) | |
| Adjuvant
radiotherapy after primary resection |
| Yes | 60 | 98.2
(87.8–99.7) | 96.1
(85.2–99.0) | 80.1
(65.2–89.2) | 0.564 |
| No | 60 | 98.1
(87.1–99.7) | 91.7
(79.4–96.8) | 80.2
(63.8–89.7) | |
| Adjuvant
chemotherapy after primary resection |
| Yes | 12 | 91.7
(53.9–98.8) | 91.7
(53.9–98.8) | 73.3
(24.3–93.4) | 0.947 |
| No | 108 | 99.0
(93.0–99.9) | 94.4
(87.1–97.6) | 80.7
(69.9–88.0) | |
Treatment
The goal of surgical treatment for all patients was
function-preserving and limb-sparing resection of the primary tumor
with clear margins, according to the preoperative imaging results
in curative intent. Plastic reconstructive surgery involving split
thickness skin grafts, local or free flaps were used for the
coverage of resulting soft tissue defects. Based on prognostic
factors predicting an increased risk of disease progression,
several patients received adjuvant radiation and/or chemotherapy
using generally anthracycline-based regimens. The indication for
adjuvant radiation or chemotherapy was given at the discretion of
the interdisciplinary tumor board of either our institution or the
referring institutions.
Sixty patients received adjuvant radiotherapy after
resection of their primary tumor with a median overall dose of 58.9
gray (range, 36.0–70.0). Further four patients underwent first
adjuvant radiotherapy after initial or second local recurrence.
Twelve patients received adjuvant chemotherapy after resection of
the primary tumor. Eleven of them were treated with doxorubicin or
epirubicin combined with ifosfamide. One patient received
cyclophosphamide and vinblastine.
Histopathological classification
All tumors were diagnosed and classified using the
guidelines of the French Federation of Cancer Centres (FNCLCC) and
the latest World Health Organisation (WHO). All pathology slides
were analyzed or reviewed for consensus diagnosis by experienced
soft tissue pathologists. In specialized cases, an expert second
opinion was obtained in Germany (Professor Katenkamp, Jena).
Statistical analysis
All patients were retrospectively analyzed regarding
possible prognostic factors influencing survival (Tables I and II). OS was defined as the time period
from the date of surgery for primary disease to the date of death
from any cause. Survival rates were estimated according to the
Kaplan-Meier method with respective 95% confidence intervals (CIs)
and were compared using the log-rank test. Multivariate analyses
were performed using the Cox proportional hazards model. Variables
that were associated with P<0.1 in the univariate analysis were
included in the multivariate regression to assess independent
prognostic factors for OS. P<0.05 was considered statistically
significant. Mann-Whitney U test and Kruskal-Wallis test were used
to detect any correlations between functional outcome and different
tumor and treatment characteristics, respectively. The functional
outcome could be assessed through the Toronto extremity Salvage
Score questionnaire (TESS), Foot Function Index (FFI) and 36-Item
Short Form Health Survey (SF-36) at the cut-off date. The data
analysis was performed using the statistical program Stata (version
11.2; StataCorp, College Station, TX, USA).
Results
Patient characteristics and surgical
margins
The mean age at the time of primary diagnosis was
57.4 years (range, 16.5–89.2). There were 60 female and 60 male
individuals. Tumors were located in the lower legs in 87 patients
(72.5%); at the ankle region in 14 patients (11.7%); and in the
foot in 19 patients (15.8%). The distribution of the histologic
grading was G1 in 16 cases (13.3%), G2 in 52 (43.3%) and G3 in 52
(43.3%). In total, 37 patients had at least one local recurrence,
whereas 18 patients had two or more local recurrences (range,
2–10). Over time, 31 patients developed distant metastases. From
these patients 20 had pulmonary metastases. The Kaplan-Meier
estimated rate of 5-year distant metastasis-free survival after
primary diagnosis was 76.5% (95% CI: 66.5–83.9) for the entire
cohort.
In order to determine the impact of surgical
resection margins on survival, we analyzed the two following
variables. In 'margin status after primary resection', we assessed
OS depending on the resection status that was achieved at the
resection of the primary tumor in our or the referring institution.
The primary resection led to microscopically negative margins (R0)
in 108 patients (90.0%), whereas 12 patients (10.0%) were left with
microscopically positive margins (R1) and no patient with
macroscopically positive margins (R2).
In those patients with negative margins after
primary resection, we additionally assessed the impact of the clear
surgical margin width in 'distance of closest negative surgical
margin at resection of the primary tumor' which was available in 71
of 108 patients within the R0 subgroup.
Follow-up
As of November 2015 (cut-off date), the reverse
Kaplan-Meier estimate of median follow-up after primary diagnosis
was 6.3 years (95% CI: 5.3–9.2) (24). At the cut-off date, 80 patients had
no evidence of disease whereas 11 patients were alive with
metastatic disease and 4 patients with residual localized disease.
During follow-up, 18 patients died from disease and 7 patients from
other causes.
Recurrence-free time from primary
diagnosis to initial recurrence
The local recurrence-free survival (LRFS) was
calculated from the date of primary surgery to initial local
recurrence. The 5-year rate of LRFS was 65.0% (95% CI: 54.0–71.1)
for the entire cohort. Histologic subtypes had no significant
influence on LRFS. Notably, patients with adjuvant radiation tended
to have a prolonged LRFS compared with patients whose primary
tumors were not treated with radiation [5-year LRFS: 71.6%
(56.5–82.2) vs. 57.6% (40.5–71.3)], but the difference was not
statistically significant in the univariate analysis (P=0.159).
Microscopic margin status attained at the first oncological
resection had no statistical significant impact on LRFS [5-year
LRFS: R0 88.9% (43.3–98.4) vs. R1 63.1% (51.5–72.6); P=0.521].
Survival
In the entire series, the 5-year estimate of the OS
rate was 80.0% (95% CI: 69.6–87.1), whereas the estimated 5-year
rate of OS was 66.3% (95% CI: 44.8–81.0) for patients with local
recurrence. Patients who developed distant metastases had a 5-year
survival of 18.8% (95% CI: 5.0–39.5). The median survival time
after diagnosis of initial metastasis was 2.8 years (95% CI:
1.2–4.7).
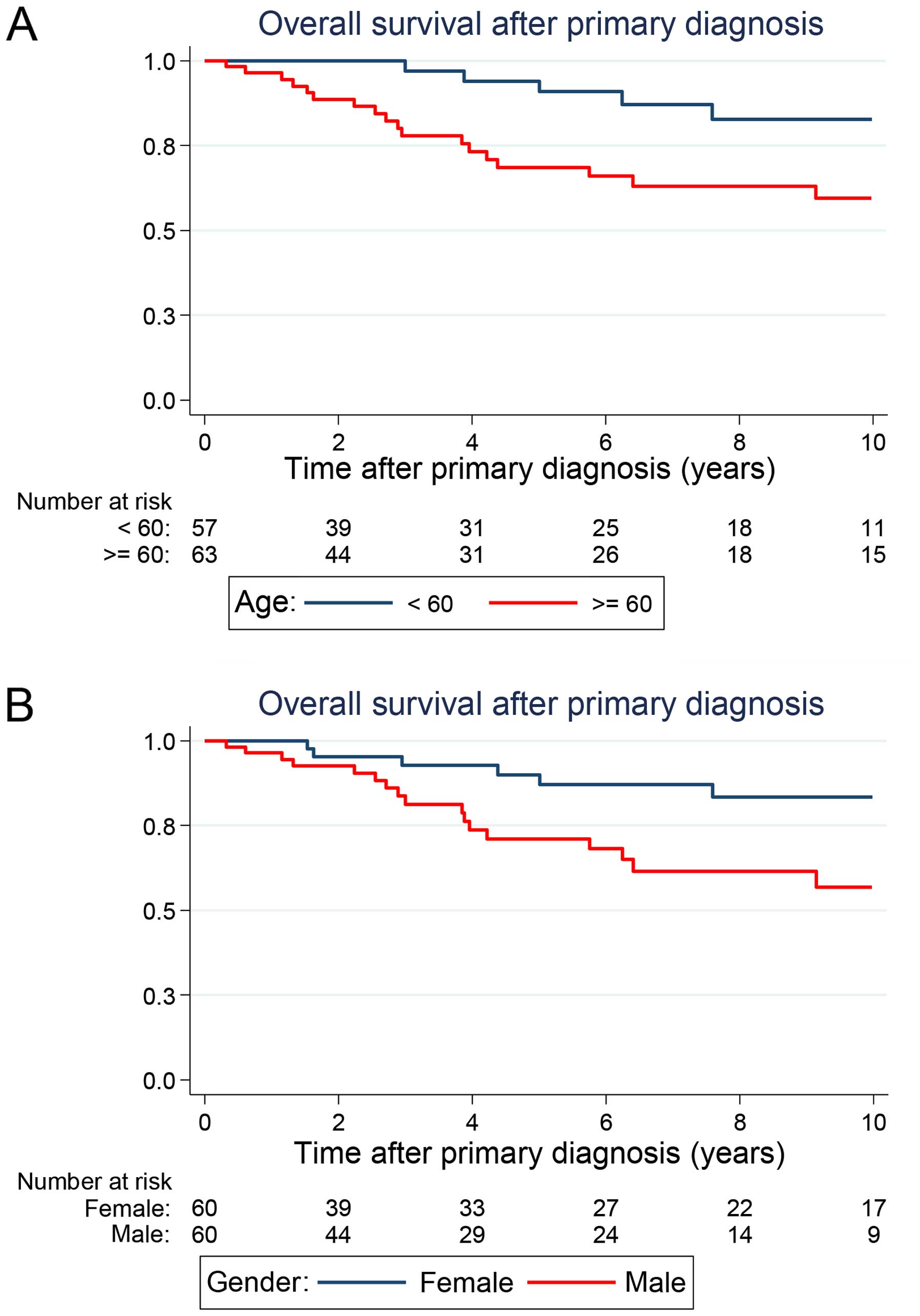
Univariate analysis of survival
Age and gender were found to have a prognostic
significance on OS (Table I,
Fig. 1A and B). Patients older than
60 years had a significantly diminished outcome when compared with
younger patients at the time point of primary diagnosis [5-year OS:
94.0% (78.2–98.5) vs. 68.5% (53.1–79.8); P=0.036]. In the entire
series, women had more favourable prognoses than men. The 5-year OS
rates were estimated to be 90.0% (95% CI: 75.3–96.1) for women and
71.1% (95% CI: 55.1–82.2) for men (P=0.004).
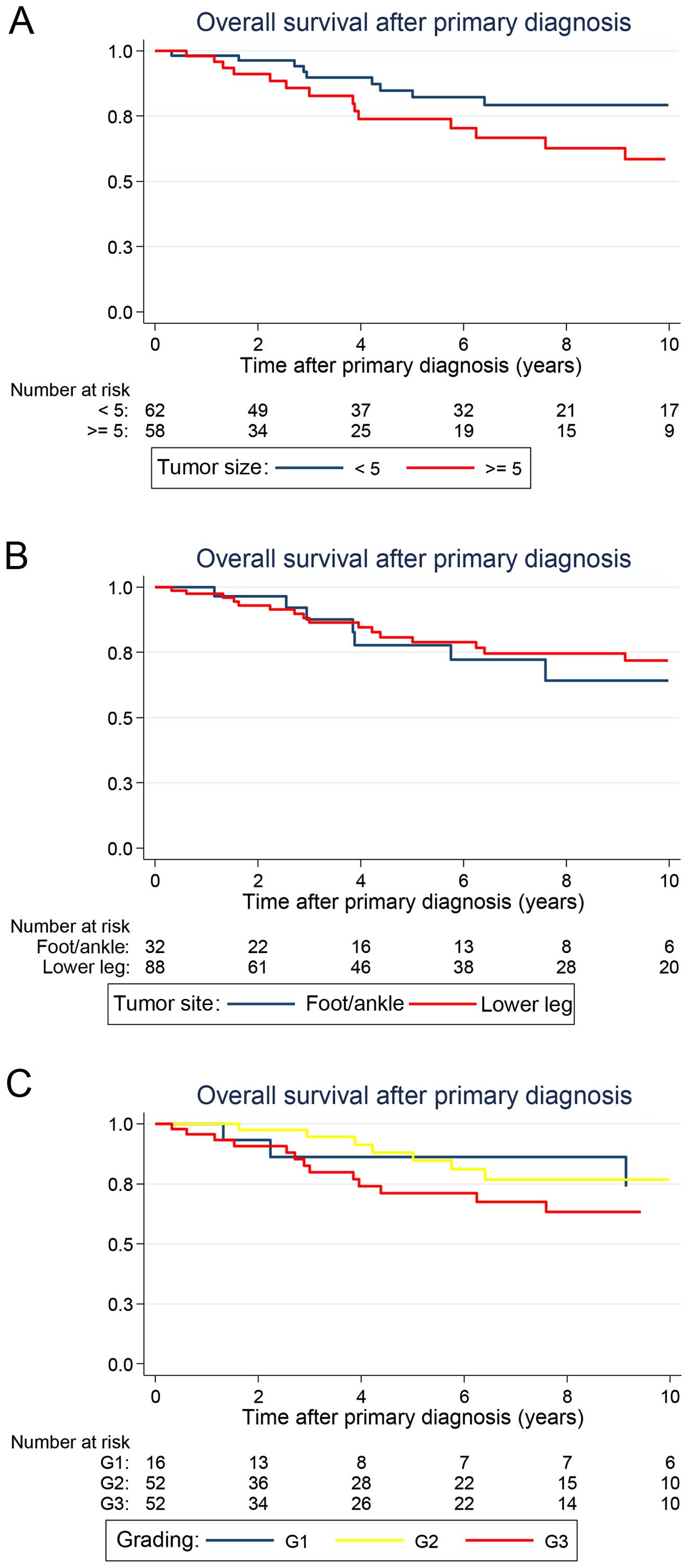
Patients with primary tumors <5 cm tended to have
an improved OS [5-year OS: 84.8% (95% CI: 70.5–92.5)] when compared
with patients with larger tumors [5-year OS: 73.9% (95% CI:
56.6–85.2)], although this survival distribution failed to reach
statistical significance in the univariate analysis, and a
borderline P-value was attained (P=0.068) (Fig. 2A). Notably, histologic grade as well
as tumor site and depth did not influence OS (Fig. 2B and C). Regarding the different
histologic subsets, all patients had comparable OS rates (Table II).
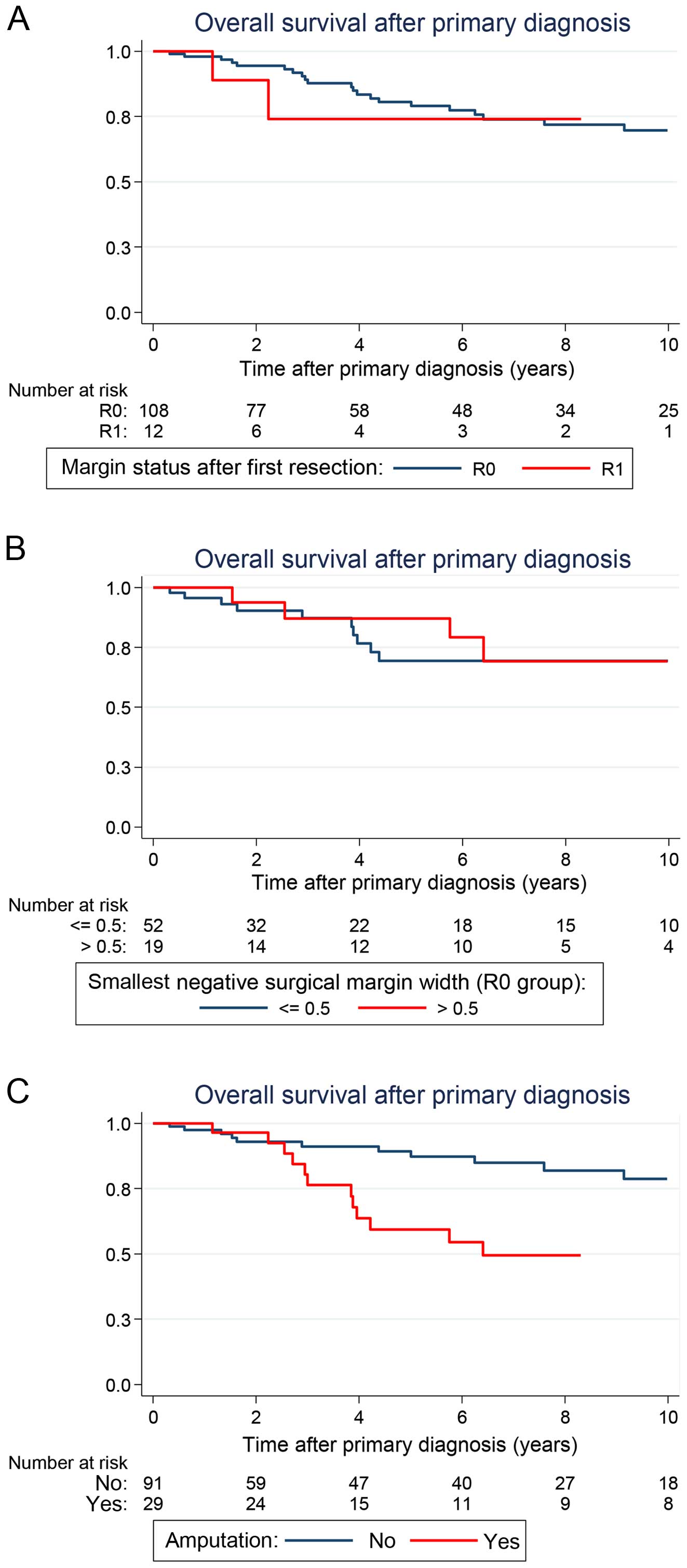
In the univariate analysis, the surgical margin
status attained at the resection of the primary tumor failed to
reach a prognostic significance. Remarkably, patients who underwent
only an incomplete resection with microscopically positive margins
(R1) had an outcome similar to patients who underwent a R0
resection of their primary tumor [5-year OS: 74.1% (28.9–93.0) vs.
80.5% (69.7–87.9); P=0.318] (Fig.
3A). Within the R0 group, the negative surgical margin width,
even under 1 mm in the closest distance to the tumor tissue, did
not influence the OS rates. Surgical margin widths ≤1 and >1 mm
led to similar outcomes, as well as ≤5 and >5 mm, respectively
(Table II, Fig. 3B). However, patients who had to
undergo an amputation during the course of disease had a
significantly worse outcome when compared with patients who were
not amputated [5-year OS: 59.4% (37.5–75.8) vs. 89.2% (78.5–94.8);
P=0.001] (Fig. 3C). Within the
cohort of patients who did not undergo amputation, primary wound
closure and plastic surgical soft tissue coverage with split
thickness skin grafting, local or free flaps led to a similar
outcomes [5-year OS: 92.2% (71.5–98.0) vs. 87.5% (72.2–94.7);
P=0.887]. Similar to findings for LRFS, adjuvant radiation after
initial oncologic resection did not alter OS [5-year OS: 80.1%
(65.2–89.2) vs. 80.2% (63.8–89.7); P= 0.564].
Multivariate analysis of survival
In the Cox model, significant prognostic factors for
OS were only gender and age (Table
III). The hazard ratio (HR) for death was 4.7 (95% CI:
1.01–21.84; P=0.048) for male patients and 3.77 (95% CI:
1.35–10.52; P=0.011) for patients older than 60 years. Amputation
was a significant indicator of diminished survival in the
univariate analysis, but this finding was not significant in the
multivariate analysis (P=0.071). Tumor size and negative surgical
margin width also failed to reach prognostic significance in the
multivariate analysis.
 | Table IIIResults of multivariate analysis on
overall survival according to Cox proportional hazards model. |
Table III
Results of multivariate analysis on
overall survival according to Cox proportional hazards model.
| Category
(reference) | Hazard ratio | 95% CI | P-value |
|---|
| Gender |
| Male (vs.
female) | 4.70 | 1.01–21.84 | 0.048 |
| Age |
| ≥60 years (vs.
<60 years) | 3.77 | 1.35–10.52 | 0.011 |
| Tumor size |
| ≥5 cm (vs. <5
cm) | 0.94 | 0.27–3.23 | 0.916 |
| Distance of closest
negative surgical margin at primary resection (R0 group) | 0.42 | 0.11–1.61 | 0.208 |
| >0.1 cm (vs.
≤0.1 cm) | | | |
| Amputation |
| Yes (vs. no) | 2.43 | 0.93–6.38 | 0.071 |
Postoperative function assessment
ESS, FFI and SF-36 were recorded a median of 7.4
years (range, 1.3–14.9) after the last surgical intervention. TESS
and SF-36 could be obtained from a total of 30 patients while FFI
was obtained from 27 patients with STS of the foot and ankle.
Adjuvant radiation led to slightly decreased TESS scores indicating
greater disabilities when compared with untreated patients,
although the difference was not statistically significant (Table IV). Furthermore, radiation
increased the FFI scores in a significant manner which inversely
indicates a decreased foot function in these patients. Primary
wound closure and plastic surgical soft tissue coverage led to
similar functional outcomes. Lower histologic grades were
associated with slightly better functional outcomes resulting in
higher TESS and lower FFI scores when compared with higher
histologic grades. However, this distribution failed to reach
statistical significance in our analysis.
 | Table IVCorrelation between functional
indices and different tumor/treatment characteristics. |
Table IV
Correlation between functional
indices and different tumor/treatment characteristics.
| TESS
| FFI
| SF-36
|
|---|
| N | Mean ± SD | P-value | N | Mean ± SD | P-value | N | Mean ± SD | P-value |
|---|
| Radiation |
| No | 12 | 70.3±9.6 | | 10 | 20.2±13.8 | | 11 | 60.0±30.8 | |
| Yes | 18 | 59.5±19.6 | | 17 | 38.9±26.0 | | 17 | 57.6±33.2 | |
| Total | 30 | 63.8±17.0 | 0.112a | 27 | 32.0±23.8 | 0.042a | 28 | 58.6±31.7 | 0.705a |
| Primary wound
closure |
| No | 10 | 60.3±18.0 | | 10 | 35.1±22.7 | | 9 | 55.0±35.3 | |
| Yes | 20 | 66.0±17.0 | | 17 | 31.2±25.4 | | 19 | 60.3±31.7 | |
| Total | 30 | 63.8±17.0 | 0.335a | 27 | 32.0±23.8 | 0.692a | 29 | 58.6±31.7 | 0.776a |
| Grading |
| G1 | 6 | 74.7±4.7 | | 4 | 21.7±16.1 | | 5 | 59.0±29.0 | |
| G2 | 13 | 62.9±18.7 | | 13 | 34.0±27.0 | | 12 | 62.1±36.3 | |
| G3 | 11 | 59.1±17.5 | | 10 | 33.4±22.9 | | 11 | 54.5±30.0 | |
| Total | 30 | 63.8±17.0 | 0.107b | 27 | 32.0±23.8 | 0.732b | 28 | 58.6±31.7 | 0.773 |
Discussion
In the present study, surgical margins attained at
the resection of the primary tumor did not influence LRFS and OS.
Resections with negative (R0) and microscopic positive margins (R1)
led to a similar outcome in our patient population. This finding is
in agreement with several studies assessing the impact of surgical
margins on local control and survival. Kim et al analyzed
numerous clinical and pathologic variables in 111 patients with
extremity STS and could not determine any prognostic significance
of positive margins on local recurrence and OS (25). Published in 2015 by Willeumier et
al, positive margins significantly decreased lRfS, but did not
influence the OS in 127 patients with extremity STS (26). A similar observation was made by
McKnee et al assessing the predictive role of surgical
margins in 111 extremity STS patients (27). To date, the largest study that
analyzed the outcome of distal extremity STS was reported by Lin
et al from the MD Anderson Cancer Center (19). In 115 patients with hand and foot
STS, positive margins were associated with an increased risk for
local recurrence, but did not diminish OS. In accordance to our
findings, STS histology, grading, size and localization were not
found to be significant prognostic factors of OS and amputations
did not result in an improved outcome. Similar observations were
made by Kozawa et al when analyzing 24 patients with foot
STS (23).
Nevertheless, these findings are contradictory to
several large studies as well. In 2010, Novais et al
demonstrated that positive margins increase the risk of local
recurrence and diminish the OS in 248 patients with extremity STS
(12). Potter et al
confirmed the adverse prognostic significance of positive margins
on LRFS and OS in 363 patients with extremity STS (28). In the Memorial Sloan-Kettering
Cancer Center (MSLKCC), Pisters et al assessed various
prognostic factors in 1,041 patients with extremity STS during a
median follow-up of 3.95 years and reported diminished LRFS and OS
rates in patients left with microscopic positive margins (10). However, another large study by
Gronchi et al from the Istituto Nazionale Tumori in Milan
analyzed the outcome of 911 patients with extremity STS presenting
a long-term median follow-up of 8.92 years (4). Notably, microscopic margin status
failed to reach statistical significance as an independent
predictive factor in this long-term survival analysis. Finally,
Kandel et al presented a meta-analysis including 32
retrospective and prospective studies in 2013 (29). Here, most studies failed to
establish a strong correlation between surgical margins and OS.
However, although the prognostic significance of
positive margins still remains unclear in extremity STS, the same
conclusions might be drawn. All recently published studies
suggested a less radical surgical approach with limb- and
function-sparing resections when feasible without leaving
microscopic positive margins. As indicated by Lin et al,
Kozawa et al and our current study, amputations did not
result in an improved OS in patients with distal extremity STS
(19,23). Moreover, none of the three studies
could identify any prognostic significance of positive margins on
OS in distal extremity STS. Finally, OS was not improved
significantly by wide and radical excisions in our series. Close
and wide negative margins had a similar outcome within the R0
resected subgroup. Although a trend in favor of negative margins
>1 mm was observed, no statistical significance could be
established. Notably, amputation during the course of disease was
associated with a significant diminished outcome in the univariate
analysis. However, it failed to reach statistical significance in
the multivariate analysis and it has to be stated that patients
that had to undergo amputation suffered from more aggressive and
local extensive tumors. Hence, the unfavorable outcome of
amputation was rather a result of the aggressive tumor biology than
the procedure itself. Taken together, these findings support a
surgical approach with more conservative resections that preserve
function and structure at the distal lower extremities.
In our survival analysis, only age and gender
emerged as independent predictors of OS. Tumor characteristics
including histology, size and depth did not affect OS. Noteworthy,
histologic grade also failed to reach prognostic significance in
our analysis. Regarding adjuvant treatment modalities, radiation
did not improve LRFS and OS in our patient population, but resulted
in impaired foot function. In accordance, Lin et al were not
able to detect any beneficial effects of radiation on LRFS and OS
in their hand and foot sarcoma patients (19). However, these findings have to be
interpreted with caution because of the relatively small number of
patients included in both studies assessing distal extremity STS.
In 2014, a randomized, prospective study conducted by the National
Cancer Institute in Bethesda included 141 patients with extremity
STS and revealed that patients who underwent limb-sparing surgery
with adjuvant radiation had a lower risk of local failure when
compared with patients who underwent surgery without radiation
(30). It therefore seems
reasonable to include adjuvant radiation when the potential for
local recurrence is elevated due to high tumor grade or aggressive
tumor progression.
Finally, the reservation must be made that our
series included only patients with STS that were suitable for
further surgical treatment. Patients with extensive tumors which
could not be approached surgically because of rapid disease
progression and therefore with less favourable outcome were not
assessed in this study. Hence, our findings are only applicable to
the selected group of patients where further surgical treatment was
possible and not to all patients with STS of the distal lower
extremities. This implies a study selection bias which has to be
acknowledged.
In conclusion, this study provides long-term
follow-up data that may help clinicians estimate the prognosis of
patients with STS of the distal lower extremities. Adverse
prognostic features include male gender and age >60 years at the
time point of primary diagnosis. The data from this study could not
underscore the long-term benefit of negative margins achieved at
the resection of the primary tumor or the recurring tumor. Although
reconstructive plastic surgery can frequently reduce functional
impairment at the distal lower extremities, the surgical approach
to attaining negative margins can be associated with considerable
morbidity and should, therefore, be weighed up carefully. Surgical
efforts should aim at function-sparing resections when feasible
with negative margins. Here, close negative margins seem to be
adequate. When the goal of achieving negative margins will require
major functional impairment of the extremity, a decision should be
made in each case based on the biology of the STS, the health
status of the patient and the decision of the informed patient.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Cli. 57:43–66.
2007. View Article : Google Scholar
|
|
2
|
Fernebro J, Bladström A, Rydholm A,
Gustafson P, Olsson H, Engellau J and Nilbert M: Increased risk of
malignancies in a population-based study of 818 soft-tissue sarcoma
patients. Br J Cancer. 95:986–990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Billingsley KG, Lewis JJ, Leung DH, Casper
ES, Woodruff JM and Brennan MF: Multifactorial analysis of the
survival of patients with distant metastasis arising from primary
extremity sarcoma. Cancer. 85:389–395. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gronchi A, Casali PG, Mariani L, Miceli R,
Fiore M, Lo Vullo S, Bertulli R, Collini P, Lozza L, Olmi P, et al:
Status of surgical margins and prognosis in adult soft tissue
sarcomas of the extremities: A series of patients treated at a
single institution. J Clin Oncol. 23:96–104. 2005. View Article : Google Scholar
|
|
5
|
Kaushal A and Citrin D: The role of
radiation therapy in the management of sarcomas. Surg Clin North
Am. 88:629–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singer S, Demetri GD, Baldini EH and
Fletcher CD: Management of soft-tissue sarcomas: An overview and
update. Lancet Oncol. 1:75–85. 2000. View Article : Google Scholar
|
|
7
|
Collin C, Godbold J, Hajdu S and Brennan
M: Localized extremity soft tissue sarcoma: An analysis of factors
affecting survival. J Clin Oncol. 5:601–612. 1987.PubMed/NCBI
|
|
8
|
Pisters PW and Pollock RE: Staging and
prognostic factors in soft tissue sarcoma. Semin Radiat Oncol.
9:307–314. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gaynor JJ, Tan CC, Casper ES, Collin CF,
Friedrich C, Shiu M, Hajdu SI and Brennan MF: Refinement of
clinicopathologic staging for localized soft tissue sarcoma of the
extremity: A study of 423 adults. J Clin Oncol. 10:1317–1329.
1992.PubMed/NCBI
|
|
10
|
Pisters PW, Leung DH, Woodruff J, Shi W
and Brennan MF: Analysis of prognostic factors in 1,041 patients
with localized soft tissue sarcomas of the extremities. J Clin
Oncol. 14:1679–1689. 1996.PubMed/NCBI
|
|
11
|
Matsumoto S, Ahmed AR, Kawaguchi N, Manabe
J and Matsushita Y: Results of surgery for malignant fibrous
histiocytomas of soft tissue. Int J Clin Oncol. 8:104–109. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Novais EN, Demiralp B, Alderete J, Larson
MC, Rose PS and Sim FH: Do surgical margin and local recurrence
influence survival in soft tissue sarcomas? Clin Orthop Relat Res.
468:3003–3011. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stojadinovic A, Leung DH, Hoos A, Jaques
DP, Lewis JJ and Brennan MF: Analysis of the prognostic
significance of microscopic margins in 2,084 localized primary
adult soft tissue sarcomas. Ann Surg. 235:424–434. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trovik CS, Bauer HC, Alvegård TA, Anderson
H, Blomqvist C, Berlin O, Gustafson P, Saeter G and Wallöe A:
Surgical margins, local recurrence and metastasis in soft tissue
sarcomas: 559 surgically-treated patients from the Scandinavian
Sarcoma Group Register. Eur J Cancer. 36:710–716. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eilber FC, Rosen G, Nelson SD, Selch M,
Dorey F, Eckardt J and Eilber FR: High-grade extremity soft tissue
sarcomas: Factors predictive of local recurrence and its effect on
morbidity and mortality. Ann Surg. 237:218–226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karakousis CP and Driscoll DL: Treatment
and local control of primary extremity soft tissue sarcomas. J Surg
Oncol. 71:155–161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sadoski C, Suit HD, Rosenberg A, Mankin H
and Efird J: Preoperative radiation, surgical margins, and local
control of extremity sarcomas of soft tissues. J Surg Oncol.
52:223–230. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stojadinovic A, Leung DH, Allen P, Lewis
JJ, Jaques DP and Brennan MF: Primary adult soft tissue sarcoma:
Time-dependent influence of prognostic variables. J Clin Oncol.
20:4344–4352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin PP, Guzel VB, Pisters PW, Zagars GK,
Weber KL, Feig BW, Pollock RE and Yasko AW: Surgical management of
soft tissue sarcomas of the hand and foot. Cancer. 95:852–861.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karakousis CP, De Young C and Driscoll DL:
Soft tissue sarcomas of the hand and foot: Management and survival.
Ann Surg Oncol. 5:238–240. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scully SP, Temple HT and Harrelson JM:
Synovial sarcoma of the foot and ankle. Clin Orthop Relat Res.
364:220–226. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Selch MT, Kopald KH, Ferreiro GA, Mirra
JM, Parker RG and Eilber FR: Limb salvage therapy for soft tissue
sarcomas of the foot. Int J Radiat Oncol Biol Phys. 19:41–48. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kozawa E, Nishida Y, Nakashima H, Tsukushi
S, Toriyama K, Kamei Y and Ishiguro N: Foot sarcomas: Factors
affecting oncological and functional outcomes. Oncol Lett. 3:82–88.
2012.PubMed/NCBI
|
|
24
|
Schemper M and Smith TL: A note on
quantifying follow-up in studies of failure time. Control Clin
Trials. 17:343–346. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim YB, Shin KH, Seong J, Roh JK, Kim GE,
Hahn SB and Suh CO: Clinical significance of margin status in
postoperative radiotherapy for extremity and truncal soft-tissue
sarcoma. Int J Radiat Oncol Biol Phys. 70:139–144. 2008. View Article : Google Scholar
|
|
26
|
Willeumier J, Fiocco M, Nout R, Dijkstra
S, Aston W, Pollock R, Hartgrink H, Bovée J and van de Sande M:
High-grade soft tissue sarcomas of the extremities: Surgical
margins influence only local recurrence not overall survival. Int
Orthop. 39:935–941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKee MD, Liu DF, Brooks JJ, Gibbs JF,
Driscoll DL and Kraybill WG: The prognostic significance of margin
width for extremity and trunk sarcoma. J Surg Oncol. 85:68–76.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Potter BK, Hwang PF, Forsberg JA, Hampton
CB, Graybill JC, Peoples GE and Stojadinovic A: Impact of margin
status and local recurrence on soft-tissue sarcoma outcomes. J Bone
Joint Surg Am. 95:e1512013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kandel R, Coakley N, Werier J, Engel J,
Ghert M and Verma S; Sarcoma Disease Site Group of Cancer Care
Ontario's Program in Evidence-Based Care: Surgical margins and
handling of soft-tissue sarcoma in extremities: A clinical practice
guideline. Curr Oncol. 20:e247–e254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beane JD, Yang JC, White D, Steinberg SM,
Rosenberg SA and Rudloff U: Efficacy of adjuvant radiation therapy
in the treatment of soft tissue sarcoma of the extremity: 20-year
follow-up of a randomized prospective trial. Ann Surg Oncol.
21:2484–2489. 2014. View Article : Google Scholar : PubMed/NCBI
|