Introduction
Pulmonary carcinoma is one of the most common types
of cancer and the leading cause of cancer-related deaths.
Approximately 90% of lung cancer deaths are the result of tumor
metastasis rather than of primary tumor proliferation (1). Tumor metastasis is a multi-step
process that requires the formation of new membrane extensions at
the leading edge of the cell, the formation of new adhesions to the
substrate and contractions at the trailing edge to move the cell
body forward. An increasing number of studies have shown that the
tumor microenvironment plays an important role in cancer
progression, especially in tumor metastasis.
Hypoxia is one of the most critical features of
tumor microenvironment, which is associated with stem cell
maintenance (2), cell
immortalization, glucose metabolism (3), epithelial-mesenchyme transition
(4), pH regulation (5), invasion and metastasis (6). The major regulator of hypoxic
responses is hypoxia-inducible factor-1 (HIF-1), which accumulates
at low oxygen levels and acts as a transcription factor for >100
target genes (7,8). However, the molecular basis for the
ability of cancer cells to migrate, invade and metastasize under
hypoxic conditions is still unclear.
A critical component of cell migration is thought to
be the actin-myosin force-generating machinery (9,10).
Myosin II is an actin-based motor protein that is important for
cell migration through its effects on adhesion, lamellar
protrusion, rear retraction, and polarity (11,12).
Using the actin network as a track, myosin II motors cross-link and
contract the actin network, thus generating forces (13). Myosin II molecules are comprised of
three pairs of peptides: two heavy chains of 230 kDa, two 20-kDa
regulatory light chains (RLCs) that regulate NM II activity and two
17-kDa essential light chains (ELCs) that stabilize the heavy-chain
structure. Isoforms of myosin II include myosin IIA, myosin IIB and
myosin IIC, which contain heavy chains encoded by the genes MYH9,
MYH10 and MYH14, respectively (14). Both myosin IIA and IIB are expressed
in most mammalian cells, where they fulfill different but
overlapping functions in cell migration. Myosin IIA localizes
throughout the cell, even in protrusions. This protein plays a
crucial role in rapid contractility, actin stress fiber formation,
focal adhesion formation and cell-cell junctions (12). However, the function of myosin IIA
in migration remains controversial. Several studies have indicated
that myosin IIA deficiency leads to faster cell speeds relative to
corresponding wild-type cells (12,15–17),
while some reports argued that myosin IIA accelerate the migration
speeds of breast cancer cells (18).
DT-13, a saponin of dwarf lilyturf tuber isolated
from Ophiopogon japonicus (Thunb.), was found to display
anti-tumor effects by inhibiting angiogenesis and proliferation
through the regulation of VEGF and PI3K/Akt signaling, as well as
anti-metastatic activity by inhibiting adhesion, migration and
invasion via inhibition of MMP-2/9 or tissue factor (19,20).
We previously found that myosin IIA specifically responded to DT-13
via affinity chromatography (21);
therefore, we hypothesized that myosin IIA may be a potential
target of DT-13 to inhibit tumor metastasis in lung cancer. As
hypoxia occurs during the deterioration of cancer, in this study,
we used a hypoxic model to study the mechanisms underlying the
anti-metastatic activity of DT-13.
Materials and methods
Cell culture, transfection, and
reagents
The cell line 95D was obtained from the Cell Bank of
the Institute of Biochemistry and Cellular Biology, Chinese Academy
of Sciences (Shanghai, China); 95D cells were maintained in
RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with
10% fetal bovine serum (FBS; Gibco), 80 U/ml penicillin, and 100
U/ml streptomycin. Cells were cultured in a humidified environment
with 5% CO2 at 37°C. The shMYH9 and negative control
shRNA were designed and synthesized by GenePharma (Shanghai,
China). The shRNAs were transfected into 95D cells using lentivirus
according to the manufacturer's instructions. DT-13 was a gift from
Professor Bo-Yang Yu (China Pharmaceutical University, China).
DT-13 was prepared at a stock concentration of 10 mM in 100% DMSO,
stored at −20°C, and diluted with phosphate-buffered solution (PBS)
before each experiment. The final DMSO concentration did not exceed
0.1% throughout the study. The pharmacological inhibitor
S(−)-blebbistatin (Merck) was used at a concentration of 100
µM. Phalloidin-FITC was obtained from Sigma. The following
antibodies were used at the dilution rate of 1:1,000: ERK1/2,
p-ERK1/2, c-Raf and p-c-Raf, β-actin, non-muscle myosin IIA,
paxillin, p-paxillin (Tyr 118) were obtained from Cell Signaling
Technology. Secondary anti-mouse IgG (Sigma) and anti-rabbit IgG
(Cell Signaling Technology) were diluted at the ratio of 1:2,500.
Immunoreactive proteins on membranes were visualized using ECL
western blot detection reagents (Amersham, UK).
Cell spreading assays
Quantitative cell spreading assays were carried out
in 96-well microtiter plates (Nunc, Naperville, IL, USA), which
were coated with fibronectin (20 µg/ml; Sigma) for 1 h at
room temperature and rinsed with PBS just before seeding the cells.
Briefly, cells were collected from the culture plates using
non-trypsin cell dissociation reagent and washed with PBS. Cell
suspensions (200 µl/well, 2×104 cells/ml) with or
without drugs were added to each well and incubated at 37°C for 30
min, 60 and 120 min.
Wound healing assay
95D cells were grown to confluency in 24-well
culture plates, after which a single linear wound was created
through the monolayers using a sterile pipette tip. Monolayers were
washed to remove cellular debris and placed in FBS-free media with
DT-13 (0.01, 0.1 and 1 µM), blebbistatin (100 µM) or
without any drugs. Sites at which wounds were to be measured were
marked on the undersurface of the wells to ensure that measurements
were taken at the same place (A defined area of the wound was
photographed). Wounds were imaged at 0 and 12 h under
phase-contrast microscopy.
Migration assay
The chemotactic motility of 95D cells was assayed
using a Transwell chamber migration system (Millipore, USA)
containing membranes with an 8-µm pore size. 95D cell
suspensions (5.0×104 cells per well) were added to the
upper chamber containing 1% FBS, while the lower chamber containing
10% FBS. DT-13 (0.01, 0.1 and 1 µM) or blebbistatin (100
µM) were added in the upper and lower chamber. After
incubation for 12 h under normoxic or hypoxic condition, the upper
surface of the membranes was swabbed to remove non-migrated cells,
and the cells attached onto the lower surface were fixed, stained,
and counted microscopically. The migrated cells were quantified by
manual counting, and five randomly chosen fields were analyzed for
each group.
Invasion assay
Invasion assays were performed by using commercially
available modified Boyden chambers (Millipore) layered with growth
factor-reduced Matrigel (BD Biosciences), and chambers were
incubated for 2 h at 37°C. An equal number (8×104 cells)
of 95D cells were suspended in the upper chamber [RPMI-1640 medium
with 1% fetal bovine serum (FBS)], whereas medium containing 10%
FBS was placed in the lower chamber. DT-13 (0.01, 0.1 and 1
µM) or blebbistatin (100 µM) were added in the upper
and lower chambers. After 12-h incubation under normoxic or hypoxic
condition, the cells on the upper surface of the membrane were
completely removed. Then, the membrane was fixed with 4%
paraformaldehyde for 10 min and stained with 0.1% crystal violet,
and the upper-chamber cells were cleared using a cotton swab. Cells
that invaded the Matrigel and reached the lower surface of the
membrane in five different fields were counted and averaged for
each group in a particular experiment. Each experiment was
performed in triplicate.
Immunofluorescence staining
95D cells were added into a 12-well plate with
4×104 cells per well, cells were treated with DT-13
(0.01, 0.1 and 1 µM) or blebbistatin (100 µM) under
normoxic and hypoxic conditions for 12 h, washed twice with PBS,
and fixed with 4% paraformaldehyde for 30 min. The fixed cells were
permeabilised with 0.1% Triton X-100 for 10 min at room
temperature, washed with TBST and blocked with blocking buffer (5%
BSA) at 37°C for 1 h and then incubated with primary antibody
(diluted to 1:300 in blocking buffer) at 37°C for 1 h, followed by
Alexa-conjugated secondary antibodies diluted to 1,000-fold in
blocking buffer and Hoechst 33342 (Beyotime) for 1 h at room
temperature in the dark. The cells were washed three times with
TBST before imaging on a confocal microscope.
Western blot analysis
95D cells were added into the 6-well plate with
4×105 cells per well, cells were treated with different
concentrations (0.01, 0.1 and 1 µM) of DT-13 after adhered
and then exposed to normoxia or hypoxia for 12 h. Cellular protein
extraction and western blot analysis were performed as previously
described. Total proteins (40 µg) were separated via 10%
SDS-PAGE and then electroblotted onto polyvinylidene difluoride
membranes (Millipore). After blocking with 5% BSA, 0.1% Tween-20 in
TBST at room temperature for 1 h, the blots were incubated
overnight with primary antibodies at 4°C. The blots were
subsequently washed and incubated with secondary antibodies for 1 h
at room temperature. Proteins were detected by enhanced
chemiluminescence using the ECL substrate (Amersham Pharmacia
Biotech).
Orthotopic implantation nude mouse
model
Six-week-old, female nude mice were used for
orthotopic implantation. 95D cells were suspended in 50% Matrigel
(BD) in PBS. A sample of 100 µl of 1×106
cells/mouse was injected into the right mouse lung to generate
tumors in a natural orthotopic site. One week later, the mice were
intra-gastrically administered with DT-13 (2.5, 5 and 10 mg/kg/1
body weight, daily) in 0.5% CMC-Na solution or with topotecan
hydrochloride which used as positive drug (2 mg/kg/1 body weight)
twice a week via tail-vein injection (8 mice per group). Mice in
the control group were intra-gastrically administered 0.5% CMC-Na
solution daily and this in vivo experiment was finished
after the mice in control group all died. Ethics approval was
received from the Committee of the Southeastern University.
Statistical analysis
The data represent at least three independent
experiments and are expressed as the means ± SEM. For statistical
analysis, One-way ANOVA or Student's t-test was used when
appropriate.
Results
DT-13 inhibits the effects of hypoxia on
95D cell migration and invasion
Lamellipodia formation is required for cancer cell
migration. To verify whether DT-13 suppresses lamellipodia
formation in lung cancer 95D cells, cells were treated with DT-13
(0.01, 0.1 and 1 µM) for 12 h and then plated onto a
fibronectin-coated 96-well plate. After adhering to the
fibro-nectin, we found that cells without DT-13 treatment showed
rapid, time-dependent lamellipodia formation, whereas this
extension was slower in the groups treated with DT-13 (0.01, 0.1
and 1 µM). These results suggested that DT-13 blocked the
spreading of 95D cells on fibronectin, as shown in Fig. 1A.
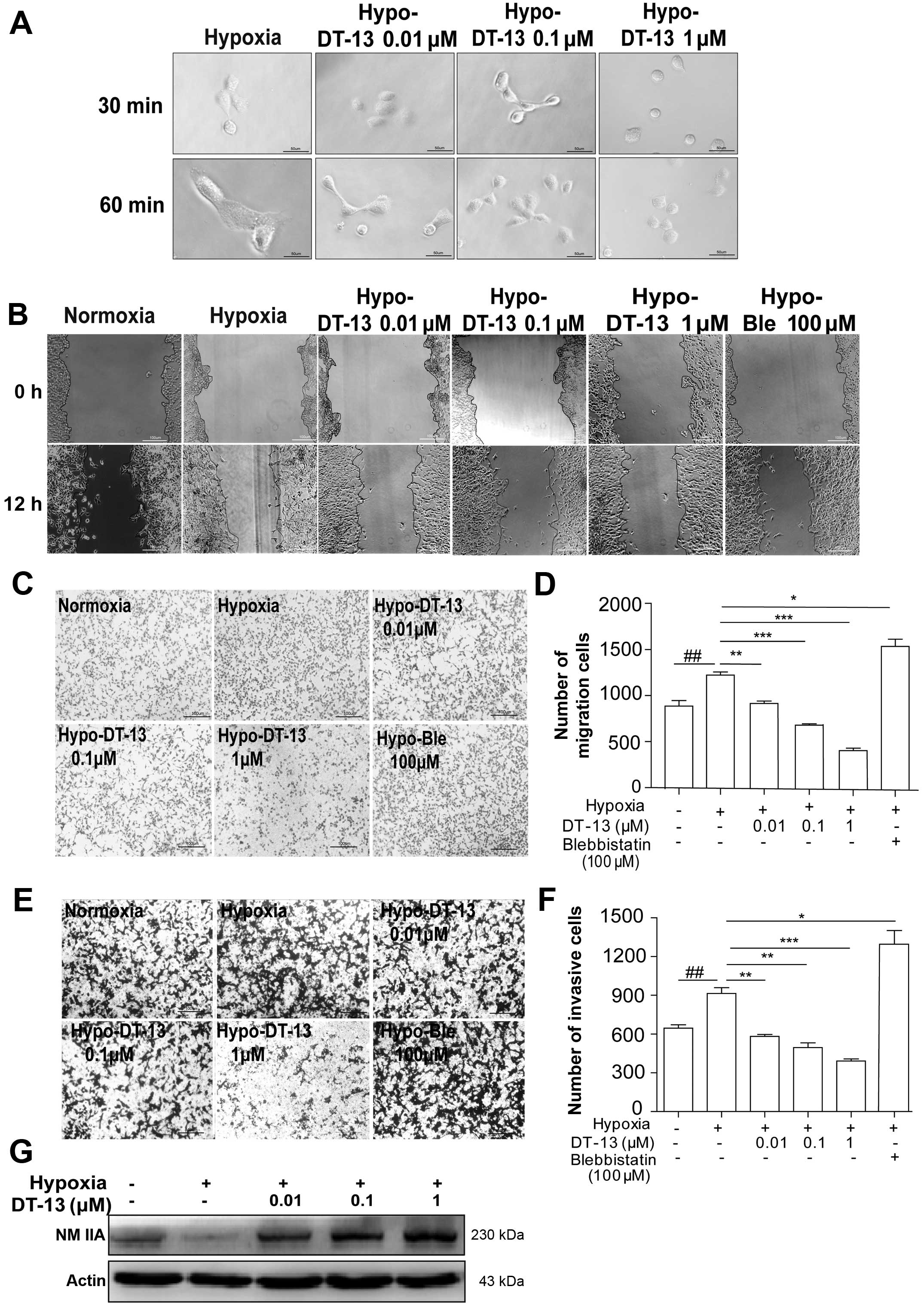 | Figure 1Inhibitory effects of DT-13 on 95D
cell migration and invasion under hypoxia. (A) Spreading assays
were used to examine the spreading ability of 95D cells treated
with 0.01, 0.1 and 1 µM DT-13 under hypoxia; images were
taken at 30 and 60 min after drug treatment. (B) Representative
images of cells were taken at 0 and 12 h after wounding, and cells
were treated with 0.01, 0.1 or 1 µM of DT-13 and 100
µM blebbistatin in serum-free medium for 12 h
(magnification, ×100). (C) Effects of DT-13 on 95D cell migration.
In the Transwell cell migration assay, cells were seeded in the
upper Transwell chamber and treated with DT-13 (0.01, 0.1 and 1
µM) and blebbistatin (100 µM) for 12 h in serum-free
medium. The lower chamber contained medium supplemented with 10%
FBS. The downward side of the membrane was stained with crystal
violet and Eosin Y and counted by an inverted microscope. (D)
Statistical analysis of 95D cell migration counted in 5 random
fields after Transwell migration. (E) Effects of DT-13 on 95D cell
invasion. In the Transwell cell invasion assay, cells were seeded
in the upper portion of Transwell chamber coated with Matrigel and
treated with DT-13 (0.01, 0.1 and 1 µM) and blebbistatin
(100 µM) for 12 h in serum-free medium; medium containing
10% FBS was placed in the lower chamber (magnification, ×100).
After 12 h of incubation, the downward side of the membrane was
stained and counted on an inverted microscope. (F) Statistical
analysis of 95D cell Matrigel invasion counted in 5 random fields.
#P<0.05, ##P<0.01, ###P<0.001 versus
normoxia; *P<0.05, **P<0.01,
***P<0.001 versus hypoxia. (G) Western blot analysis
of NMIIA expression in 95D cells after treated with DT-13 (0.01,
0.1 and 1 µM) for 12 h. |
Next, scratch migration assays and Transwell
migration assays were performed to evaluate the effects of DT-13 on
cell migration under hypoxia. We found that hypoxic conditions
enhanced the migration capacity of 95D cells compared with
normoxia; however, different concentrations of DT-13 noticeably
decreased tumor cell migration under hypoxia in a dose-dependent
manner (Fig. 1B, C and E). The
acquisition of an invasive phenotype of cancer cells is a critical
step for tumor progression. Matrigel-coated filters are widely used
to examine invasive migration through a three-dimensional
extracellular matrix. We found that hypoxia was necessary to
accelerate the invasion of 95D cells compared with normoxia;
however, after treatment of DT-13, we found that this cell invasion
activity was effectively decreased under hypoxia in a
dose-dependent manner (Fig. 1D and
F).
NMIIA, a conventional motor protein known to
generate intracellular contractile force and tension by associating
with F-actin, has been implicated in the regulation of many
cellular processes, including cell spreading, migration and
cytokinesis (12,17). Our previous study showed that DT-13
interacted specifically with NMIIA based on affinity chromatography
analysis (21), which led us to
hypothesize that NMIIA may be important for the anti-metastatic
effect of DT-13. Interestingly, we found that hypoxia led to the
downregulation of NMIIA, while DT-13 could upregulate NMIIA under
hypoxia (Fig. 1G). Furthermore, to
verify the effect of NMIIA on 95D cells migration and invasion in
depth, 100 µM blebbistatin was used as a pharmacological
inhibitor of myosin II, and we found that both the migration and
invasion of 95D cells were enhanced by treatment with blebbistatin
(Fig. 1B, C and E). These data
indicated that the inhibition of NMII accelerated the migration and
invasion of 95D cells, which was in agreement with previous studies
(16,22).
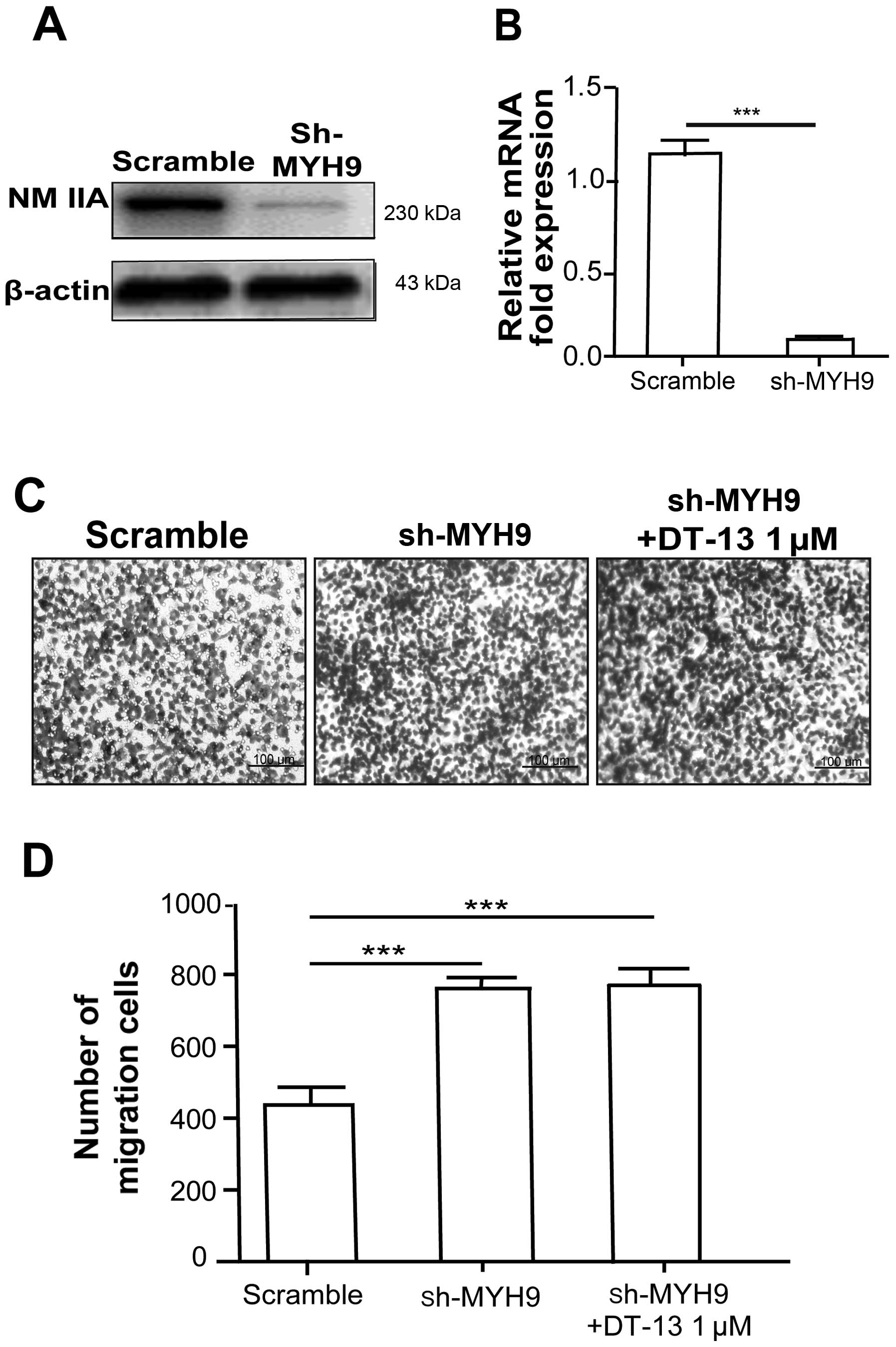
NMIIA is required for the inhibitory
effect of DT-13 on cancer cell metastasis
To verify whether NMIIA is responsible for the
inhibitory effect of DT-13 on cancer metastasis, the expression of
NMIIA in 95D cells was depleted by MYH9-targeted shRNA (Fig. 2A and B). Cells transfected with MYH9
shRNA significantly increased migration compared with sh-scramble
cells under hypoxia (Fig. 2C and
D). To further examine whether DT-13 inhibits 95D cell
migration through the motor activity of myosin IIA, shMYH9 cells
were treated with DT-13 (1 µM) in Transwell migration
assays. Our results showed that the inhibitory effect of DT-13 on
cell migration was abolished in shMYH9 cells. Together, these
results further indicated that the anti-migration effect of DT-13
occured through the upregulation of NMIIA.
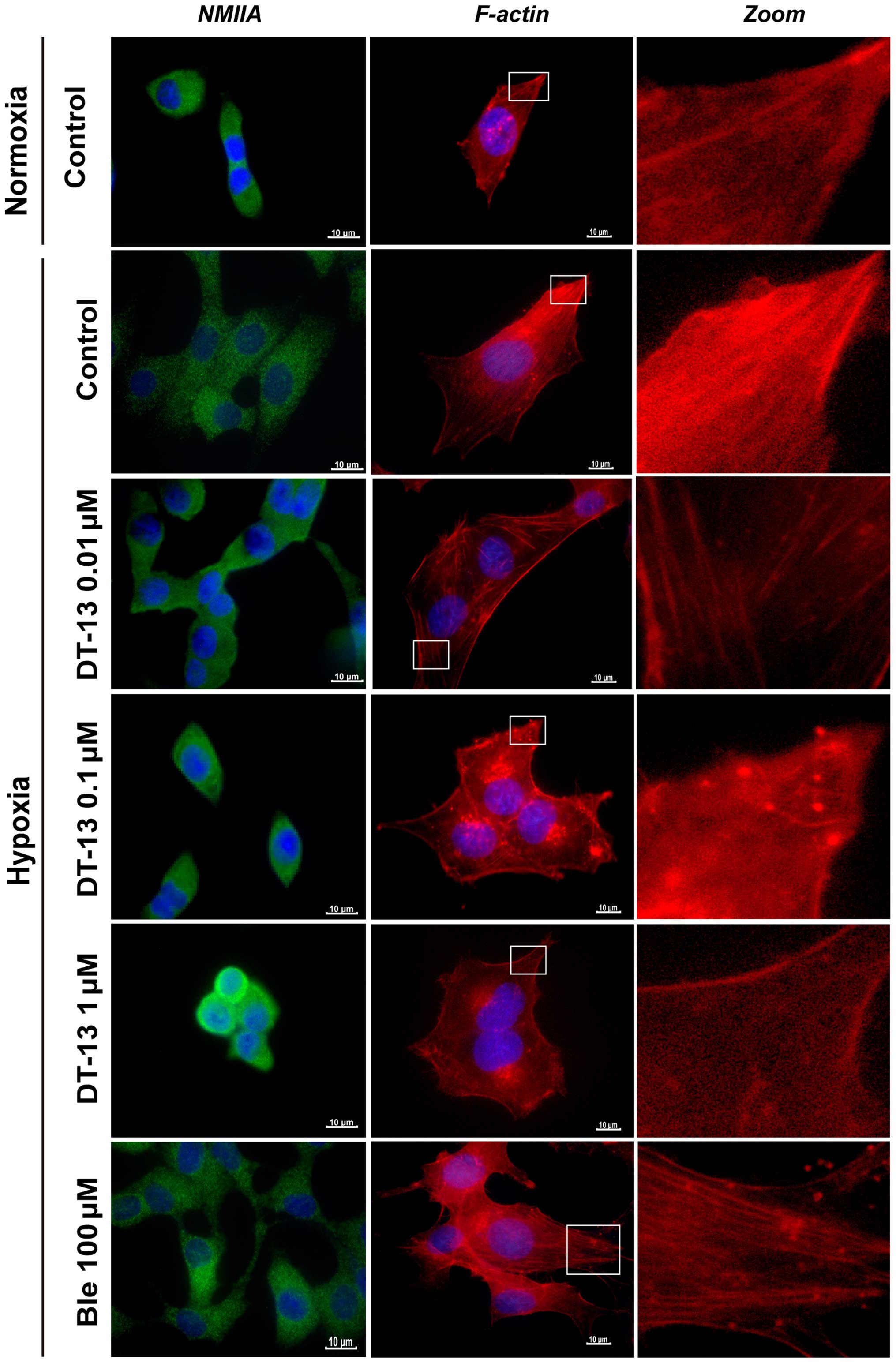
DT-13 attenuates migration by
redistributing NMIIA in 95D cells under hypoxia
NMIIA regulates the tension and contractility of
actin cytoskeleton. Previous studies (14) indicated that myosin IIA exerts a
retraction force to draw the cell cytoplasm closer to the nucleus
and attenuate cell movement. To explore whether DT-13 alters the
distribution of NMIIA in 95D cells, we used immunofluorescence
analysis to study the distribution of NMIIA. Compared with
normoxia, we observed a morphological reorganization of 95D cells
and redistribution of NMIIA under hypoxic conditions, with NMIIA
predominantly localizing to the cell periphery. Nevertheless, we
observed that NMIIA was expressed in the central region and
predominantly accumulated in the nuclear periphery after treatment
with DT-13. Thus, we hypothesize that DT-13 may promote NMIIA
accumulation in the perinuclear area to inhibit cell migration. To
verify this hypothesis, we treated 95D cells with blebbistatin (100
µM) under hypoxia and observed the redistribution of NMIIA
to the cell periphery (Fig. 3).
Collectively, these observations suggested that DT-13 affected the
distribution of NMIIA and thus has a major role in the inhibition
of 95D cell migration.
DT-13 regulates actin cytoskeletal
rearrangement in 95D cells
The dynamic regulation of the filamentous actin
(F-actin) cytoskeleton is crucial for cell migration, and myosin
IIA is involved in the formation of actin stress fibers. Therefore,
we analyzed whether DT-13 alters cellular F-actin arrangement under
hypoxia.
First, we compared the formation of F-actin in 95D
cells in two different environments (normoxia and hypoxia) for 12 h
and observed cytoskeletal variations by staining for F-actin with
phalloidin-FITC. We found that hypoxic environments stimulated the
remodeling of actin filaments and also changed the cellular
morphology; moreover, the formation of F-actin in 95D cells was
accelerated under hypoxia compared to normoxia. However, after
treatment with DT-13, we observed a noticeable disruption of actin
stress fibers and loss of actin stress fibers in 95D cells
(Fig. 3). In contrast to this
observation, treatment with blebbistatin (100 µM) instead of
DT-13 for 12 h had the opposite effect, resulting in a significant
increase in the number of stress fibers with polymerization at the
leading edge. These findings indicated that DT-13 may inhibit cell
migration through reducing the formation of F-actin in 95D
cells.
DT-13 inhibits 95D cell migration and
invasion via repression of the Raf-ERK1/2 signaling pathway
Given that DT-13 could impair 95D cell migration and
invasion by regulating NMIIA, we next investigated the potential
mechanisms involved. As NMIIA associates with FA proteins, we
explored whether the expression of FA proteins and the related
adhesion signaling pathway was modulated.
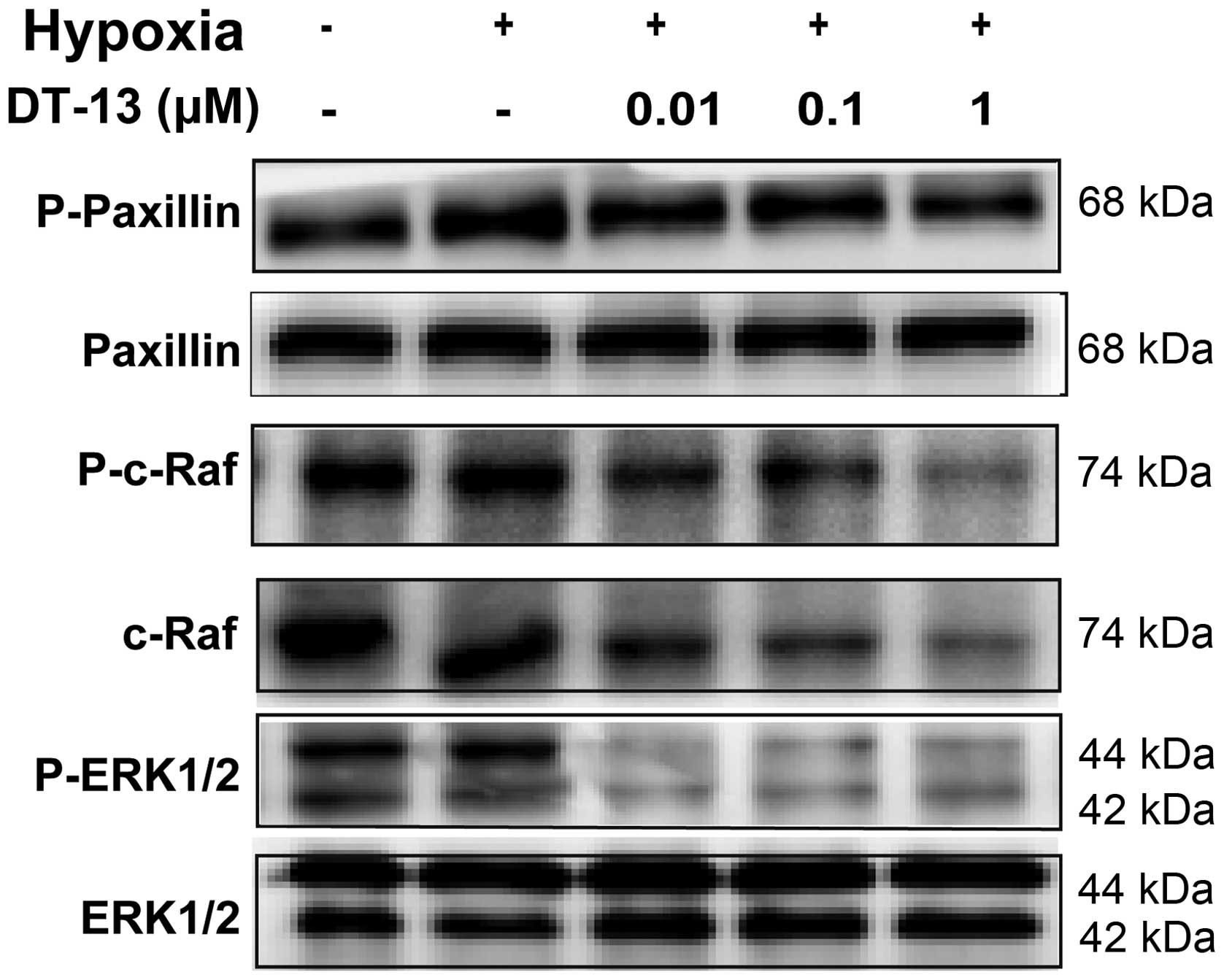
Thus, immunoblot analysis was performed to examine a
possible effect of DT-13 on p-paxillin and paxillin under hypoxic
condition, which have been shown to be downstream effectors of
NMIIA. Our results showed that the level of p-paxillin was
upregulated under hypoxia, while DT-13 reversed the hypoxic effect
(Fig. 4).
Multiple NMIIA downstream effectors have been shown
to mediate the migration behavior of epithelial cells by activating
the Raf-ERK1/2 signaling cascade (25). To assess whether DT-13 increased the
expression of NMIIA under hypoxia via Raf-ERK1/2 signaling, we
conducted immunoblot analysis and showed an association between
DT-13 and the Raf-ERK1/2 pathway. As p-c-Raf and p-ERK1/2 were
significantly downregulated after treatment with DT-13 in 95D cells
under hypoxia, while total c-Raf levels were also downregulated
(Fig. 4). Cumulatively, these
observations suggested that Raf-ERK1/2 inhibition was responsible
for the suppression of migration and invasion by DT-13 treatment in
95D cells.
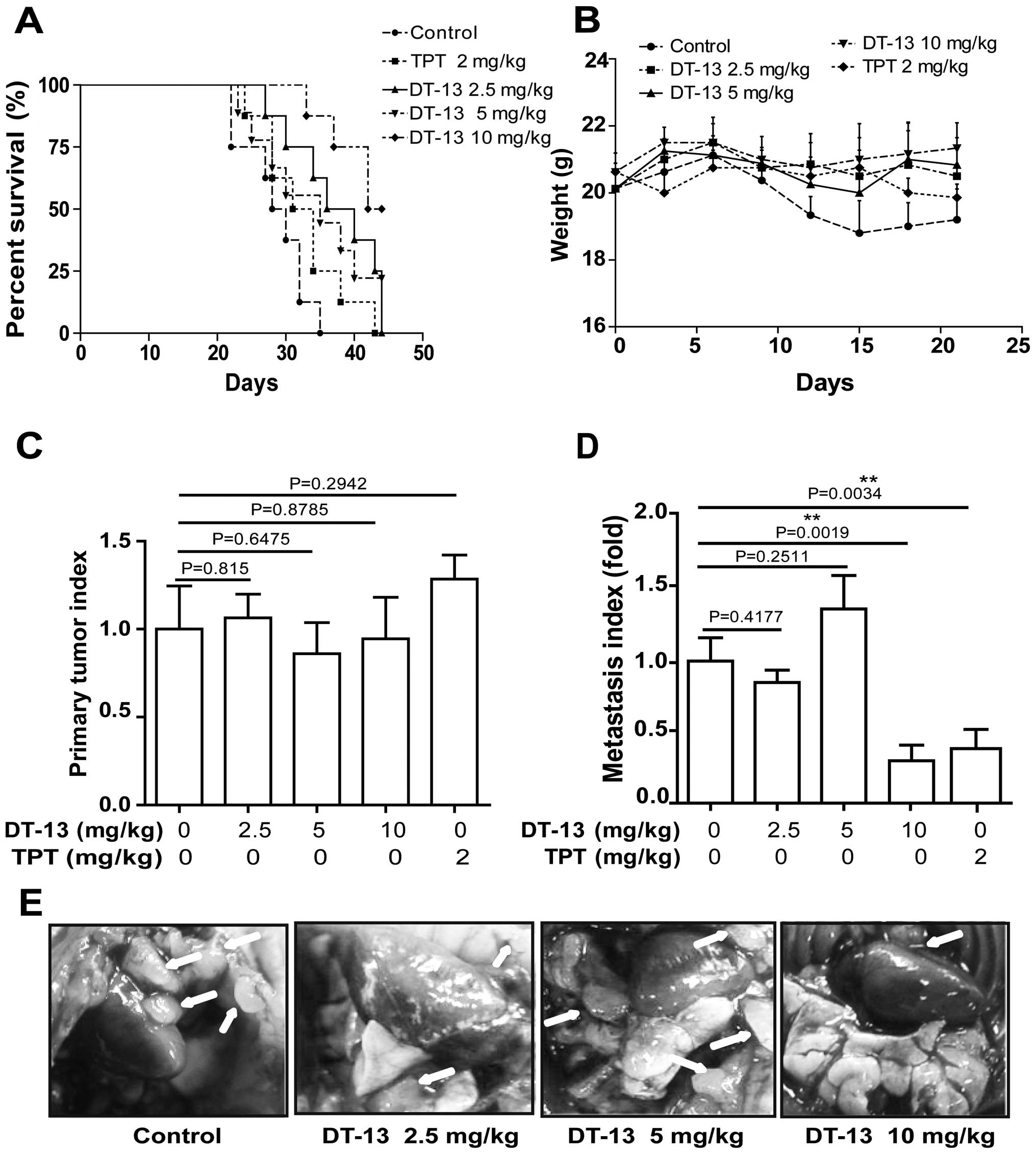
DT-13 inhibits human lung cancer 95D cell
metastasis in vivo
To further evaluate the efficacy of DT-13 in a
clinically relevant non-small cell lung cancer (NSCLC) model,
Matrigel-embedded 95D cells were implanted into mouse lungs at a
natural orthotopic site that provides a biologically appropriate
environment for tumor growth, invasion and metastasis. The survival
rate was significantly increased after treatment with DT-13 (10
mg/kg) (P=0.0014) and DT-13 (2.5 mg/kg) (P=0.0148) compared with
control group (Fig. 5A). Then, we
observed that the weight of the mice in the group without DT-13
treatment gradually decreased compared to the DT-13-treated group
(Fig. 5B). Further analysis of the
orthotopic lung-weight/mouse-weight ratio showed that DT-13 had no
effect on tumor growth of the primary cancer (Fig. 5C). However, the metastasis index
indicated that 10 mg/kg DT-13 treatment significantly impeded the
metastatic spread of cancer to other lung areas (P=0.0019) and that
2.5 mg/kg DT-13 treatment slightly inhibited metastasis (P=0.4177),
while the medium dose exhibited pro-metastatic effect but without
statistical difference (Fig. 5D).
In addition, we also found that tumor metastasis along the parietal
peritoneum in DT-13 treated groups was significantly decreased
compared with the control group (Fig.
5E).
Discussion
In this study, we first showed that hypoxia leads to
the redistribution of NMIIA to the cell periphery and strongly
downregulates NMIIA protein expression in 95D cells compared with
normoxia. Furthermore, we found the Raf-ERK1/2 signaling is
involved in regulating NMIIA and its downstream molecular paxillin
under hypoxia. Moreover, DT-13 reverses the hypoxic effect via
greatly inhibiting Raf-ERK1/2 signaling and further regulating
NMIIA.
According to previous studies, both NMIIA and NMIIB
can bind and contract actin filaments to generate force. However,
NMIIA and NMIIB have different cellular localizations, which lead
to different functions. NMIIA primarily localizes to the front,
protruding edge and is required for adhesion maturation. NMIIA is
also required for inward cellular contractility, the formation of
actin stress fibers, and morphogenic cell clustering (23). Controversially, previous research
suggested that NMIIA deficiency leads to faster cell migration in
multiple cell types (18,22), whereas other studies argued that
myosin IIA overexpression is implicated in enhanced cancer cell
migration and metastasis (24–26).
However, this controversy is lessened by recent reports on the
roles of myosin IIA in the posttranscriptional stabilization of p53
activity and the repression of squamous cell carcinoma in mice
(22).
It has been reported that the absence of actin
stress fibers in NMIIA deficient cells may increase the
concentration of cytoplasmic actin monomers, which in turn promotes
actin polymerization at the leading edge, and NMIIA-deficient cells
have fewer cell-extracellular matrix adhesions, thus requiring less
contractile force to achieve de-adhesion, which is an essential
step in cell migration (27). The
present study showed that hypoxia downregulates NMIIA expression
and induces the redistribution of NMIIA to the cellular peripheries
thus possibly weakening their anchoring force, and this further
accelerates the formation of F-actin in 95D cells. Our results
showed that DT-13 reverses the effect of hypoxia by lessening the
formation of F-actin. Moreover, we found that DT-13 inhibits human
lung cancer 95D cells spreading on fibronectin or migration and
invasion in vitro, and these findings are consistent with
orthotopic lung cancer metastasis in vivo. Our findings
strongly support the notion that DT-13 upregulates NMIIA and
disrupts actin stress fibers, which are key factors associated with
cell migration. Firthermore, we also verified these findings by
knocking down MYH9 in 95D cells or using blebbistain to inhibit
NMIIA, which all accelerate cell migration (Fig. 2C and D).
Paxillin is a component of focal adhesion complexes,
which mediates focal adhesion assembly and cell motility (28). In this study, we found that hypoxia
activates p-paxillin, while DT-13 downregulates the expression of
p-paxillin. This effect may be the result of DT-13-induced NMIIA
upregulation, while NMIIA may increase the anchoring forces
generated through NMIIA to FAS. Furthermore, we discovered that the
Raf-ERK1/2 signaling pathway is the major pathway through which
DT-13 exerts the cell migration-inhibitory effect. On the one hand,
upregulation of NMIIA expression by DT-13 treatment induces
significant inactivation of ERK by decreasing phospho-ERK1/2 levels
and inhibits c-Raf kinase under hypoxia. On the other hand, as
ERK1/2 is known to complex and colocalize with paxillin (13), DT-13 downregulates paxillin and
ERK1/2 may inhibit the binding of ERK1/2 to paxillin, and further
inhibit the migration of 95D cells, but this hypothesis still need
to be confirmed. Besides paxillin, previous studies reported that
calpain and MMPs are involved in the Raf-ERK1/2 signaling pathway
regulating NMIIA (29), and our
previous results showed that DT-13 could repress the metastasis of
human lung cancer A549 cells by inhibiting MMPs (20). Therefore, we speculate the MMPs are
involved in the anti-metastatic effect of DT-13 in 95D cells.
Thus, it is necessary to clarify how DT-13
suppresses 95D cell metastasis by regulating NMIIA under hypoxia.
Hypoxia is a potent inducer of HIF-1α expression, and HIF-1α plays
a critical role in tumor progression and dissemination. Our
previous report showed DT-13 could inhibit the expression of
HIF-1α, thus we hypothesize DT-13 may indirectly regulate NMIIA by
the inhibition of HIF-1α. In this study, we showed hypoxia
redistributes NMIIA to the cell periphery, which reduces the
adhesion effect, while NMIIA accumulated in the nuclear periphery
after treated with DT-13. Therefore, these results demonstrate that
DT-13 may directly regulate NMIIA under hypoxic condition.
In conclusion, this study demonstrated, hypoxia
promotes the migratory potential of in vitro cultured 95D
cells by down-regulating NMIIA expression and redistributing NMIIA
to the periphery of 95D cells via rearrangement of F-actin and
activation of Raf-ERK1/2 signaling pathway. The experiments further
indicated the potential of DT-13 to hamper metastasis in
vitro and in vivo, acting through upregulation of NMIIA
and redistribution NMIIA to the nuclear periphery, further
disrupting actin stress fibers and inhibiting the Ras-ERK1/2
signaling pathway. This conclusion is also supported by MYH9
knockdown cells or blebbistatin treated 95D cells. Thus, NMIIA may
play an important role in the anti-metastatic process of DT-13.
However, further studies are required to determine whether NMIIB,
MMPs and calpain are involved in the anti-metastatic effect of
DT-13, and more in-depth experiments, such as changes of NMIIA
in vivo after treatment with DT-13, and clinic trials also
need to be performed. In general, this study provides the first
demonstration that DT-13 inhibits 95D cell migration and invasion
through the activation of NMIIA via inhibition of Ras-ERK1/2
signaling pathway. Therefore, our findings offer new insights into
the potential significance of DT-13 in lung cancer.
Abbreviations:
|
DT-13
|
saponin monomer of dwarf lilyturf
tuber
|
|
NMIIA
|
non-muscle myosin IIA
|
|
F-actin
|
filamentous actin
|
|
ERK1/2
|
extracellular signal-regulated
kinase1/2
|
|
FAs
|
focal adhesions
|
Acknowledgments
This study was supported by the National Natural
Science Fund nos. 81302794, 81102853 and 81573456.
References
|
1
|
Edwards BK, Noone AM, Mariotto AB, Simard
EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA,
et al: Annual Report to the Nation on the status of cancer,
1975–2010, featuring prevalence of comorbidity and impact on
survival among persons with lung, colorectal, breast, or prostate
cancer. Cancer. 120:1290–1314. 2014. View Article : Google Scholar
|
|
2
|
Wang Y and Liu Y, Malek SN, Zheng P and
Liu Y: Targeting HIF1α eliminates cancer stem cells in
hematological malignancies. Cell Stem Cell. 8:399–411. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luo W, Hu H, Chang R, Zhong J, Knabel M,
O'Meally R, Cole RN, Pandey A and Semenza GL: Pyruvate kinase M2 is
a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell.
145:732–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mak P, Leav I, Pursell B, Bae D, Yang X,
Taglienti CA, Gouvin LM, Sharma VM and Mercurio AM: ERbeta impedes
prostate cancer EMT by destabilizing HIF-1alpha and inhibiting
VEGF-mediated snail nuclear localization: Implications for Gleason
grading. Cancer Cell. 17:319–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Swietach P, Vaughan-Jones RD and Harris
AL: Regulation of tumor pH and the role of carbonic anhydrase 9.
Cancer Metastasis Rev. 26:299–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan DA and Giaccia AJ: Hypoxia, gene
expression, and metastasis. Cancer Metastasis Rev. 26:333–339.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Semenza GL: Hypoxia-inducible factors in
physiology and medicine. Cell. 148:399–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Wong CCL, Wei H, Gilkes DM,
Korangath P, Chaturvedi P, Schito L, Chen J, Krishnamachary B,
Winnard PT Jr, et al: HIF-1-dependent expression of
angiopoietin-like 4 and L1CAM mediates vascular metastasis of
hypoxic breast cancer cells to the lungs. Oncogene. 31:1757–1770.
2012. View Article : Google Scholar
|
|
9
|
Estecha A, Sánchez-Martín L, Puig-Kröger
A, Bartolomé RA, Teixidó J, Samaniego R and Sánchez-Mateos P:
Moesin orchestrates cortical polarity of melanoma tumour cells to
initiate 3D invasion. J Cell Sci. 122:3492–3501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poincloux R, Collin O, Lizárraga F, Romao
M, Debray M, Piel M and Chavrier P: Contractility of the cell rear
drives invasion of breast tumor cells in 3D Matrigel. Proc Natl
Acad Sci USA. 108:1943–1948. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dahan I, Yearim A, Touboul Y and Ravid S:
The tumor suppressor Lgl1 regulates NMII-A cellular distribution
and focal adhesion morphology to optimize cell migration. Mol Biol
Cell. 23:591–601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vicente-Manzanares M, Ma X, Adelstein RS
and Horwitz AR: Non-muscle myosin II takes centre stage in cell
adhesion and migration. Nat Rev Mol Cell Biol. 10:778–790. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clark K, Langeslag M, Figdor CG and van
Leeuwen FN: Myosin II and mechanotransduction: A balancing act.
Trends Cell Biol. 17:178–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Betapudi V: Myosin II motor proteins with
different functions determine the fate of lamellipodia extension
during cell spreading. PLoS One. 5:e85602010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doyle AD, Kutys ML, Conti MA, Matsumoto K,
Adelstein RS and Yamada KM: Micro-environmental control of cell
migration - myosin IIA is required for efficient migration in
fibrillar environments through control of cell adhesion dynamics. J
Cell Sci. 125:2244–2256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, van Grunsven LA, Van Rossen E,
Schroyen B, Timmermans JP, Geerts A and Reynaert H: Blebbistatin
inhibits contraction and accelerates migration in mouse hepatic
stellate cells. Br J Pharmacol. 159:304–315. 2010. View Article : Google Scholar :
|
|
17
|
Liu Z, Van Rossen E, Timmermans JP, Geerts
A, van Grunsven LA and Reynaert H: Distinct roles for non-muscle
myosin II isoforms in mouse hepatic stellate cells. J Hepatol.
54:132–141. 2011. View Article : Google Scholar
|
|
18
|
Betapudi V, Licate LS and Egelhoff TT:
Distinct roles of nonmuscle myosin II isoforms in the regulation of
MDA-MB-231 breast cancer cell spreading and migration. Cancer Res.
66:4725–4733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao R, Sun L, Lin S, Bai X, Yu B, Yuan S
and Zhang L: The saponin monomer of dwarf lilyturf tuber, DT-13,
inhibits angio-genesis under hypoxia and normoxia via
multi-targeting activity. Oncol Rep. 29:1379–1386. 2013.PubMed/NCBI
|
|
20
|
Zhang YY, Liu JH, Kou JP, Yu J and Yu BY:
DT-13, a steroidal saponin from Liriope muscari L. H. Bailey,
suppresses A549 cells adhesion and invasion by inhibiting MMP-2/9.
Chin J Nat Med. 10:436–440. 2012.
|
|
21
|
Yu XW, Lin S, Du HZ, Zhao RP, Feng SY, Yu
BY, Zhang LY, Li RM, Qian CM, Luo XJ, et al: Synergistic
combination of DT-13 and topotecan inhibits human gastric cancer
via myosin IIA-induced endocytosis of egf receptor in vitro and in
vivo. Oncotarget. doi: 10.18632. 2016.
|
|
22
|
Schramek D, Sendoel A, Segal JP, Beronja
S, Heller E, Oristian D, Reva B and Fuchs E: Direct in vivo RNAi
screen unveils myosin IIa as a tumor suppressor of squamous cell
carcinomas. Science. 343:309–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Z, Ho CH and Grinnell F: The different
roles of myosin IIA and myosin IIB in contraction of 3D collagen
matrices by human fibroblasts. Exp Cell Res. 326:295–306. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Casalou C, Seixas C, Portelinha A, Pintado
P, Barros M, Ramalho JS, Lopes SS and Barral DC: Arl13b and the
non-muscle myosin heavy chain IIA are required for circular dorsal
ruffle formation and cell migration. J Cell Sci. 127:2709–2722.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Derycke L, Stove C, Vercoutter-Edouart AS,
De Wever O, Dollé L, Colpaert N, Depypere H, Michalski JC and
Bracke M: The role of non-muscle myosin IIA in aggregation and
invasion of human MCF-7 breast cancer cells. Int J Dev Biol.
55:835–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia ZK, Yuan YC, Yin N, Yin BL, Tan ZP and
Hu YR: Nonmuscle myosin IIA is associated with poor prognosis of
esophageal squamous cancer. Dis Esophagus. 25:427–436. 2012.
View Article : Google Scholar
|
|
27
|
Jorrisch MH, Shih W and Yamada S: Myosin
IIA deficient cells migrate efficiently despite reduced traction
forces at cell periphery. Biol Open. 2:368–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Chen Y, Wadham C, McCaughan GW,
Keane FM and Gorrell MD: Dipeptidyl peptidase 9 subcellular
localization and a role in cell adhesion involving focal adhesion
kinase and paxillin. Biochim Biophys Acta. 1853:470–480. 2015.
View Article : Google Scholar
|
|
29
|
Babbin BA, Koch S, Bachar M, Conti MA,
Parkos CA, Adelstein RS, Nusrat A and Ivanov AI: Non-muscle myosin
IIA differentially regulates intestinal epithelial cell restitution
and matrix invasion. Am J Pathol. 174:436–448. 2009. View Article : Google Scholar : PubMed/NCBI
|