Introduction
Esophageal cancer (EC) is the seventh most common
malignant tumor in the world, the mortality of which is ranked
sixth among the malignant tumors worldwide (1); its incidence has increased
significantly in recent years (2),
especially in China. Despite the clinical advances in diagnosis and
treatment improvement for malignant tumors, EC remains one of the
leading causes of cancer-associated mortality. The overall 5-year
survival rate for all patients with EC is <20% (3). Owing to its aggressive nature and poor
response to chemotherapy, EC cure is still a problem (4). The incidence and treatment rates of EC
will continue to increase substantially with the development of an
aging population and the social economic development in China.
Therefore, research to identify and develop more effective
molecular biomarkers to prevent or treat EC is urgently needed.
Further studies have reported that transglutaminase 3 (TGM3) is
significantly downregulated in ECs (5–8).
The transglutaminase 3 (TGM 3) enzyme is an enzyme
with the ability to catalyze the irreversible cross-linking of
peptide-bound glutamine residues either with peptide-bound lysines
or with primary amines (5). It is
encoded by the TGM3 gene and widely expressed in the small
intestine, brain, skin and mucosa (6). In the skin and mucosa, TGM3 is
predominantly expressed in the suprabasal layers of the stratified
squamous epithelium (7,8). It has been demonstrated that TGM3 is
required for the cross-linking of the structural protein
trichohyalin and the keratin intermediate filaments to form a rigid
structure within the inner root sheath cells (9,10).
Recent studies have revealed that downregulation of the TGM3
gene is closely linked with a variety of human cancer types,
including laryngeal carcinoma, esophageal and oral squamous cell
carcinoma (OSCC) (11–13). Moreover, Uemura et al
(12) identified TGM3 as a novel
prognostic indicator in ESCC; the prognostic performance of TGM3
was confirmed by immunohistochemistry in 76 ESCC cases. In
addition, Mendez et al (14)
reported that the TGM3 gene is differentially expressed in
node-positive and node-negative primary tumors in patients with
OSCC, implying that decreased TGM3 expression might contribute to
the metastatic potential of OSCC. However, the biological function
and molecular mechanism of the TGM3 gene in cancer
initiation and progression have not been reported. In addition,
whether the TGM3 gene might be a valuable diagnostic or
therapeutic biomarker for cancer, especially for EC, needs to be
further investigated.
The nuclear factor kappa B (NF-κB) transcription
factor family is composed of p50, p52, RelA/p65, c-rel and Rel B.
The homodimers and heterodimers of these molecules are sequestered
in the cytoplasm in an inactive form by the inhibitor of kappa B
(IκB). Upon stimulation, the IκB kinase complex (IKK)
phosphorylates the κB inhibitor, which then releases NF-κB and
allows its phosphorylation, nuclear translocation, binding and
subsequent activation of target genes involved in the regulation of
cell proliferation, survival, angiogenesis and metastasis (15). Constitutively active NF-κB is common
in human cancer cell lines as well as tumor tissues derived from
patients, but is rare in normal cells (16). In some types of human cancer, there
is strong evidence of NF-κB being involved in cancer progression
(17), thus, making NF-κB and its
downstream signals promising targets for therapeutic intervention.
However, the role of the NF-κB signaling pathway in the
tumorigenesis of human EC is not fully understood.
In the present study, we confirmed that the
expression levels of TGM3 are downregulated in EC cell lines
compared with normal primary esophageal epithelial cells, and
tissue specimens compared with paired adjacent normal tissues, by
means of RT-PCR and western blotting. We further evaluated the
effect of ectopic TGM3 expression in the SKGT-4, KYSE-510, OE33 and
OE21 cell lines. We provide the first evidence that the ectopic
expression of TGM3 in EC cell lines inhibits cell proliferation and
migration and induces apoptosis in cancer cells in vitro. In
addition, we demonstrate that the NF-κB signaling pathway is a
direct target of TGM3 and show that TGM3 functions as an oncogene
by activating the NF-κB signaling pathway. These results suggest
that TGM3 might be a candidate tumor suppressor contributing to EC
and could act as a valuable prognostic predictor for patients with
EC.
Materials and methods
Tissue samples
Fifty-eight pairs of primary EC tissue samples and
adjacent non-cancerous tissues (located >3 cm away from the
tumor) were obtained from the Department of Oncology, The Second
Affiliated Hospital, Zhengzhou University (Zhengzhou, China)
between June 2014 and December 2015. All subjects were diagnosed
and confirmed by a pathological evaluation. None of the subjects
received any biotherapy or chemotherapy treatment before
recruitment to this study. The study was approved by the
Institutional Ethics Committee of the Second Affiliated Hospital,
Zhengzhou University and all of the patients provided written
informed consent in accordance with the institutional
guidelines.
Cell culture and transfection
The EC cell lines SKGT-4 and KYSE-510 were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). OE33 and OE21 were obtained from the European Collection of
Animal Cell Cultures (ECACC; Porton Down, Salisbury, UK). All EC
cell lines were grown in RPMI-1640 medium supplemented with 10%
filtered fetal calf serum, 100 units/ml penicillin, 100
μg/ml streptomycin and 2 mM L-glutamine. In addition, the
normal human esophageal epithelial cell line HEEC was grown in
Dulbecco's modified Eagle's medium (DMEM) with 10% FBS. All cells
were cultured in a humidified atmosphere of 5% CO2 at
37°C.
Bay11-7082, a specific inhibitor of NF-κB signaling,
was purchased from CsA (Biomol, Plymouth Meeting, PA) and diluted
in culture medium to obtain the desired concentration. Recombinant
human TGM3 was obtained from Abcam Inc. (Cambridge, MA, USA), and
the rabbit anti-TGM3 antibody was from Adipo Bioscience Inc. (Santa
Clara, CA, USA). EC cells were transfected with recombinant human
TGM3 (10 μg) or TGM3 antibody (1:400; Neomarkers, Fremond,
CA, USA) with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions; EC cells without any
treatment were used as controls.
RNA isolation and quantitative real-time
PCR (qRT-PCR)
Total RNA was isolated using the TRIzol reagent
(Invitrogen Life Technologies). The concentration of RNA were
determined using a NanoDrop ND-1000 instrument (Thermo Fisher
Scientific, Waltham, MA, USA), and aliquots of the samples were
stored at −80°C. For reverse transcription, cDNA was synthesized by
using a reverse transcription kit (Promega, Madison, WI, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
was performed (StepOne; Applied Biosystems, Foster City, CA, USA)
using SYBR-Green (Takara, Shiga, Japan). All PCR experiments were
performed in triplicate. The TGM3 primer sequences are as follows:
sense: 5′-TCAACTGGCAGACGGCCTTCA-3′ and antisense
5′-GTACCGTCCTATGGGTGCGCT-3′.
Western blot analysis
Total protein was extracted using RIPA lysis buffer
and the concentrations in different samples were determined using
the BCA kit (Beyotime Institute of Biotechnology, Haimen, China).
An equivalent amount of protein (30 μg) from different
samples was separated by SDS-PAGE, and then transferred onto a
nitrocellulose membrane (Millipore, Bedford, MA, USA). The membrane
was blocked with 3.0% non-fat milk at room temperature for 1 h and
then blotted with rabbit anti-TGM3 antibody or mouse anti-β-actin
antibody (1:10,000) purchased from Sigma (San Francisco, CA, USA)
overnight at 4°C. After incubation with horseradish
peroxidase-conjugated secondary antibodies (1:2,000; Bioss,
Beijing, China) for 1 h at room temperature, the protein complexes
was detected using enhanced chemiluminescence reagents (Pierce,
Rockford, IL, USA). β-actin was used as the internal control. The
signal intensity on the scanned images was analyzed using Image-Pro
Plus 6.0 software.
Cell proliferation assay
Cells were seeded in 96-well plates at
6×103 cells/well and the surviving fractions were
determined at 0, 24, 48, 72 and 96 h using the MTT assay, as
previously described (18). The
absorbance of each well was measured with a spectrophotometer
(Bio-Rad Laboratories, Hercules, CA, USA) at 570 nm. Each
experiment was performed in triplicate (19).
Cell migration and invasion assays
EC cells were grown to confluence in 12-well plastic
dishes and treated with recombinant human TGM3 protein or a
scramble peptide. Then, 24 h after transfection, linear scratch
wounds (in triplicate) were created on the confluent cell
monolayers using a 200 μl pipette tip. To remove cells from
the cell cycle prior to wounding, cells were maintained in
serum-free media. A total of 10 areas were selected randomly from
each well, and the cells in three wells from each group were
quantified.
For the invasion assays, 24 h after transfection,
1×105 cells in serum-free media were seeded in Transwell
migration chambers. The upper chamber of the Transwell inserts was
coated with Matrigel (Sigma-Aldrich, St. Louis, MO, USA). Medium
containing 20% FBS was added to the lower chamber. After 24 h, the
non-invading cells were removed with cotton wool. Invasive cells
located on the lower surface of the chamber were stained with
May-Grunwald-Giemsa stain (Sigma-Aldrich) and counted using a
microscope (Olympus Corp., Tokyo, Japan). From these images, the
number of invasive cells was counted. Experiments were
independently repeated three times.
Apoptosis assay
Apoptosis was assayed by an Annexin V apoptosis
detection kit (BD Biosciences, San Diego, CA, USA). Following
transfection for 72 h, the cells were collected and detected using
an Annexin V fluorescein isothiocyanate kit (FITC) according to the
manufacturer's instructions. In brief, cells transfected with the
recombinant human vasostatin-2 or vasostatin-2 antibody were
resuspended in 100 ml binding buffer, at a density of
1×106 cells/ml, then incubated with Annexin V-FITC and
PI for 15 min. The cells were analyzed with Beckman CXP software on
a FC-500 flow cytometer (Beckman Coulter, Pasadena, CA, USA) within
1 h of cell collection.
ELISA analysis
The levels of p65 concentrations in culture medium
were determined by ELISA (Tanjin Biotechnology Co., Shanghai,
China) following the manufacturer's instructions. The absorbance
was assessed at 450 nm using a 680XR microplate reader (Bio-Rad
Laboratories).
Statistical analysis
The differences were analyzed using Student's t-test
for two groups and examined by the χ2 test. A P-value
<0.05 was considered to indicate statistically significant
differences. Data were processed as mean ± standard deviation (SD).
All statistical analyses were performed using SPSS 16.0 software
(SPSS, Inc., Chicago, IL, USA).
Results
TGM3 is downregulated in EC tissues and
cell lines
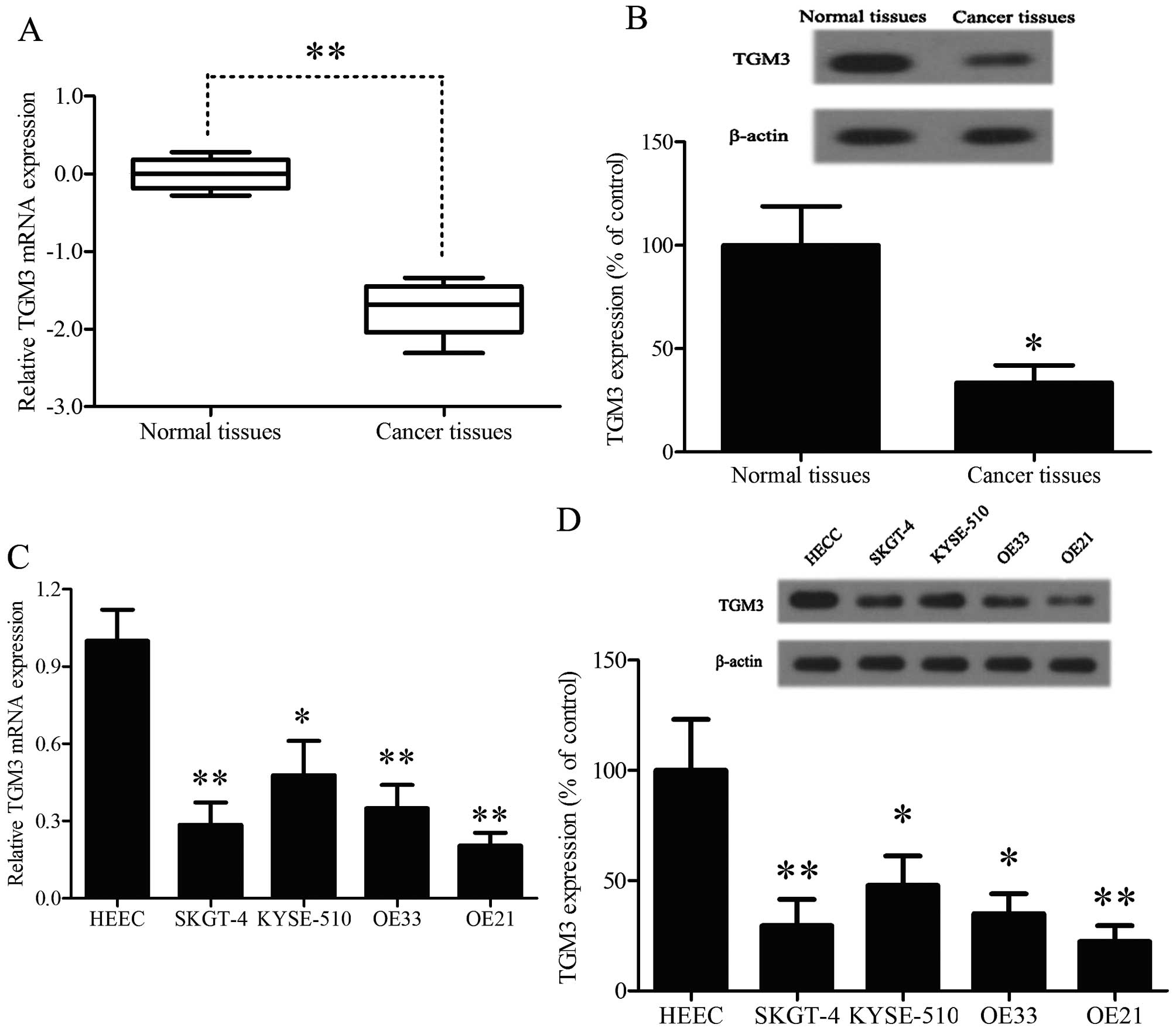
In order to confirm the potential roles of TGM3 in
EC, RT-PCR was performed to investigate the mRNA levels of TGM3 in
58 paired EC specimens compared to the corresponding adjacent
non-cancerous tissues (NCTs) (Fig.
1A). The TGM3 mRNA levels in the EC specimens were
significantly lower than those of non-cancerous tissues. Western
blot assay was performed to investigate the protein levels of TGM3
in EC specimens, same as above, the protein levels of TGM3 were
significantly downregulated in the EC specimens compared to the
corresponding adjacent non-cancerous tissues (Fig. 1B). In addition, RT-PCR and western
blot assay were performed to investigate the expression level of
four EC cell lines and a normal human esophageal epithelial cell
line. The TGM3 mRNA levels in the EC cell lines were significantly
lower than those of normal esophageal epithelial cells (Fig. 1C). Correspondingly, the protein
levels of TGM3 were markedly downregulated in the four EC cell
lines compared with the levels in normal epithelial cells using
western blot analysis (Fig. 1D).
These data show that the expression of TGM3 is universally
downregulated in EC cells and patients, indicating that a change of
TGM3 expression may affect the development of EC.
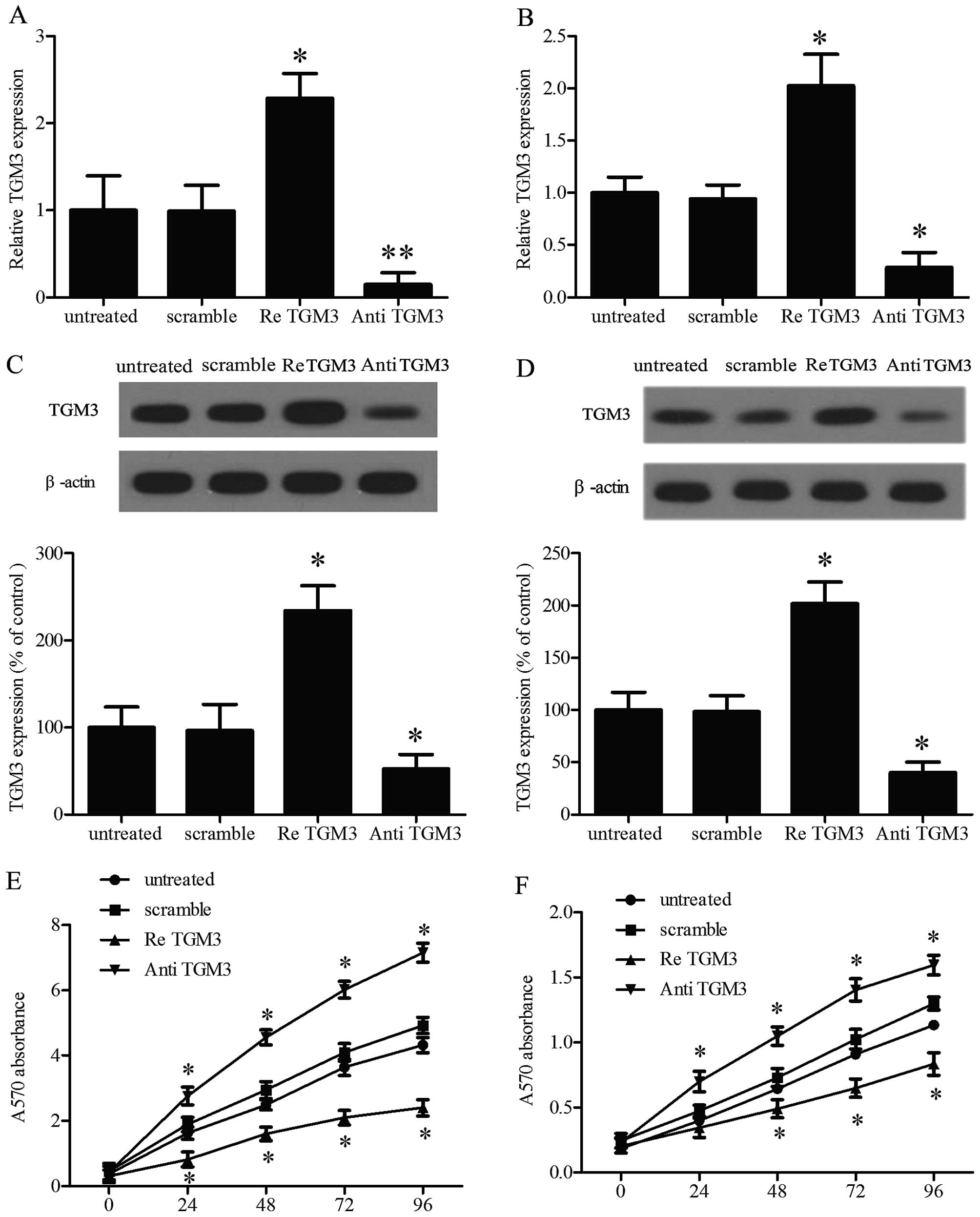
Ectopic expression of TGM3 directly
affects cell proliferation in vitro
It is well-known that cell proliferation is a key
event in the formation and development of cellular oncogenesis. In
order to investigate the role of TGM3 in EC cell proliferation,
TGM3 was overexpressed or repressed in SKGT-4 cells and KYSE-510
cells by transfection with recombinant human TGM3 or TGM3 antibody,
qRT-PCR (Fig. 2A and B) and western
blot analysis (Fig. 2C and D)
evaluation demonstrated TGM3 expression in SKGT-4 and KYSE-510 cell
lines. The MTT assay showed that overexpression of TGM3 inhibited
cell proliferation. Conversely, downregulating the expression of
TGM3 accelerated cell proliferation in comparison with the control
group (Fig. 2E and F). Similar data
were also obtained in the other two cell lines (data not
shown).
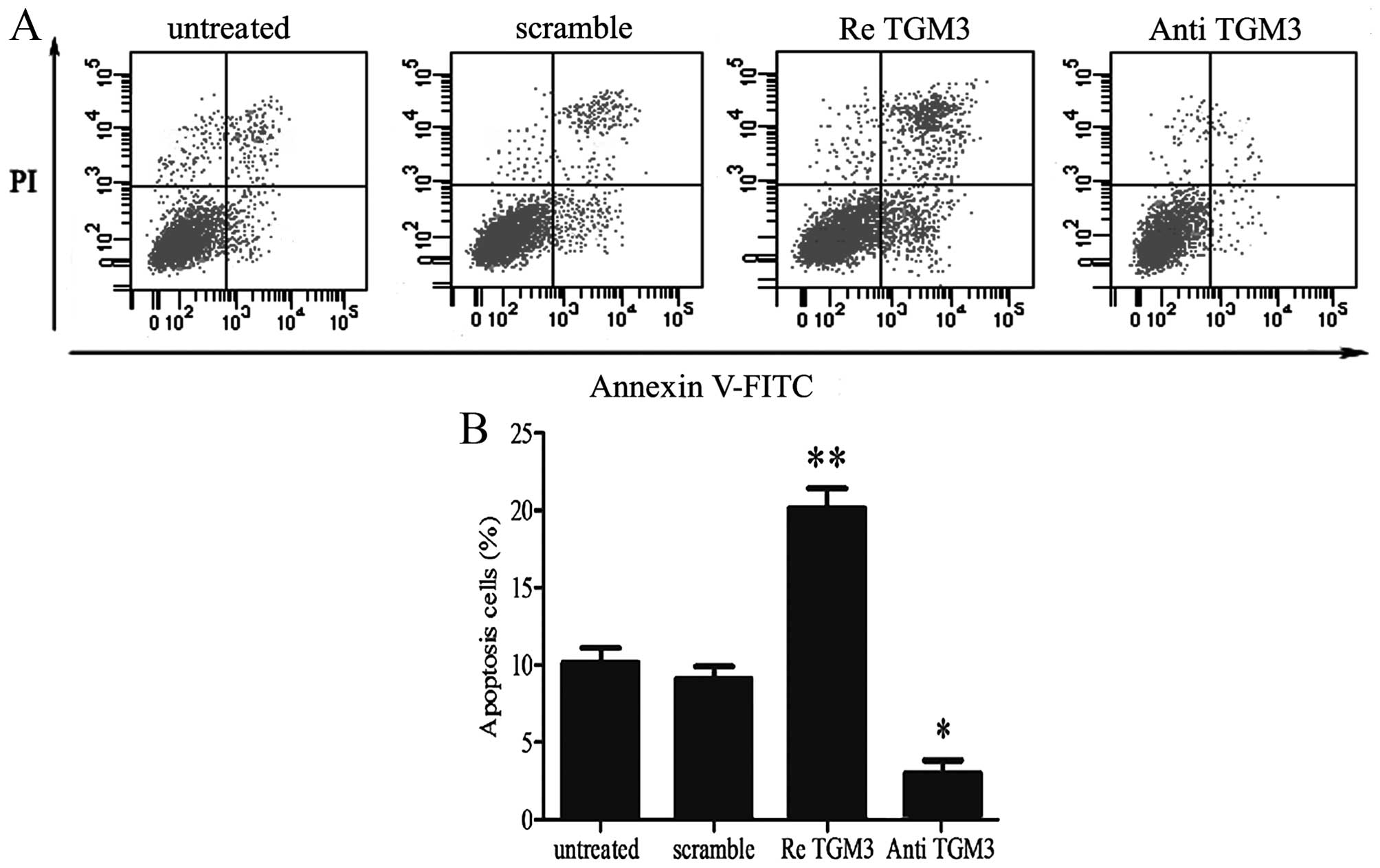
Ectopic expression of TGM3 protein
affects cell apoptosis in EC cells
One of the hallmarks of cancer is its ability to
evade apoptosis (20). To
investigate the effect of TGM3 on EC cell apoptosis, we performed a
series of experiments in EC cell lines, which were stimulated by
transfection with recombinant human TGM3 or TGM3 antibody to obtain
TGM3 protein ectopic expression. We then investigated the effect of
TGM3 on EC cell apoptosis using Annexin V-FITC staining. As shown
in Fig. 3A, overexpression of TGM3
significantly increased apoptosis in SKGT-4 cells. The percentage
of apoptotic cells increased to 9.8% after transfection with
recombinant human TGM3. Suppressed expression of TGM3 increased
apoptosis in SKGT-4 cells (Fig.
3B). Overall, these data indicate that TGM3 may play the role
of promoting apoptosis in EC cells.
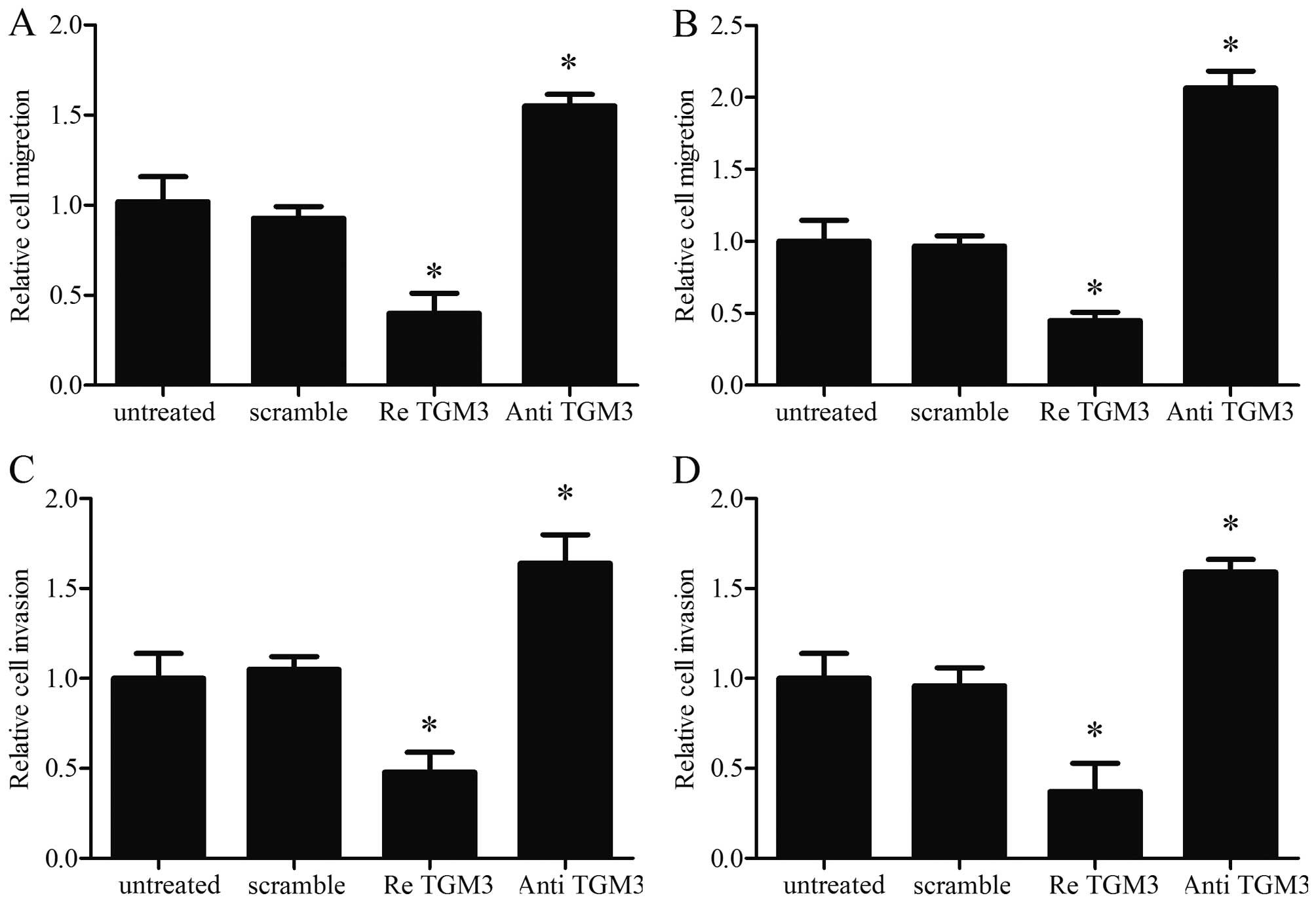
Ectopic expression of TGM3 directly
affects cell migration and invasion in vitro
To analyze the role of TGM3 in cell migration and
invasion, which are the key determinants of malignant progression
and metastasis, scratch assays and Transwell assays were performed
in the SKGT-4 and KYSE-510 cell lines. Cells treated with
recombinant human TGM3 were distinctively less migratory and
invasive; however, cells treated with the TGM3 antibody were
distinctively more migratory and invasive than untreated cells at
48 h after scratching (Fig. 4).
These results indicate that TGM3 plays an important role in
regulating the proliferation of EC cells and strongly suggest that
introduction of TGM3 could inhibit cellular oncogenesis by
suppressing the migration and invasion of EC cells.
Overexpression of TGM3 promotes
activation of the NF-κB signaling pathway
The NF-κB signaling pathway has been demonstrated to
play an important role in cellular energy homeostasis and reported
to be extensively involved in cell proliferation, migration and
differentiation (21–23). To further explore the mechanisms
involved in TGM3 induced tumor cell growth and apoptosis, we
analyzed the NF-κB pathway at the protein level via ELISA and
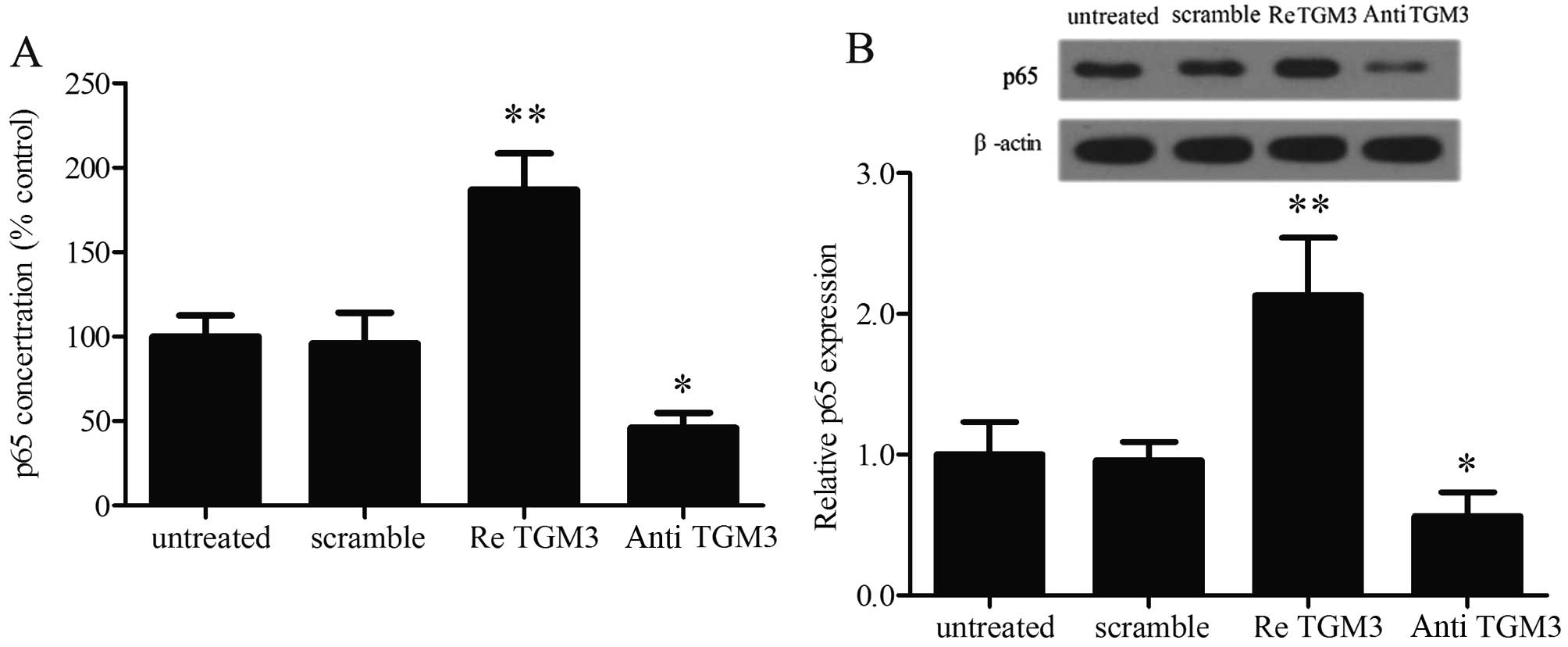
western blotting. The results show that elevated p65 concentrations
in culture medium of SKGT-4 cells were found with TGM3
overexpression (Fig. 5A).
Furthermore, the protein expression of p65 was also found to be
markedly increased after TGM3 pre-treatment (Fig. 5B); the same effect was observed in
the other three cell lines. These results suggest that TGM3 induces
activation of the NF-κB pathway.
TGM3 modulates EC cell growth and
apoptosis by targeting the NF-κB signaling pathway
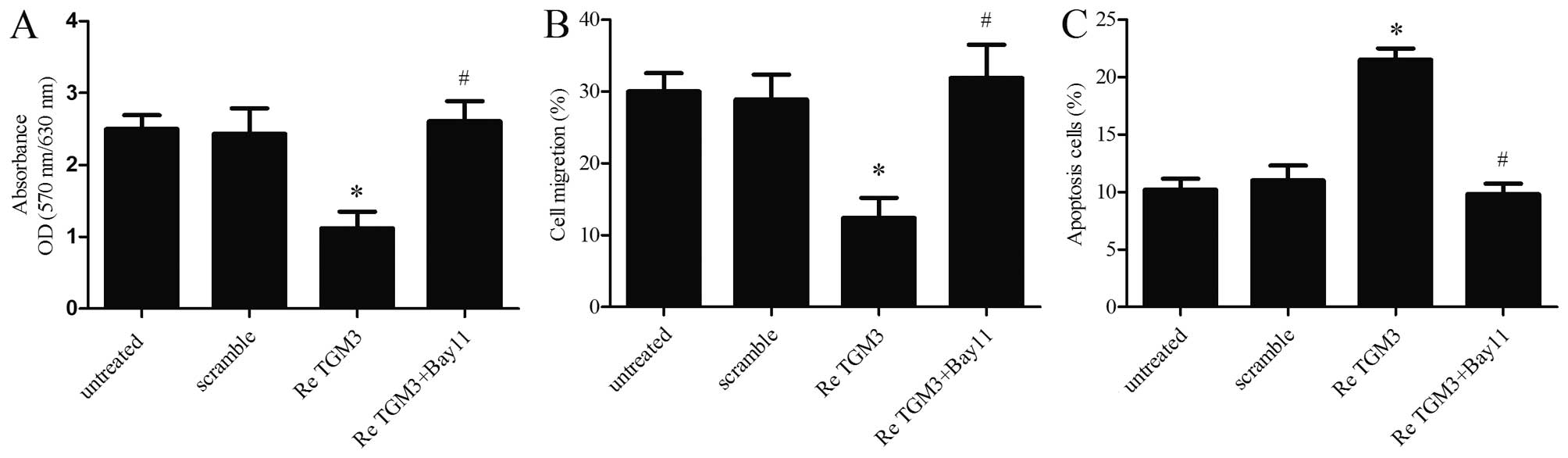
To further delineate the effect of NF-κB on TGM3
modulated EC cell growth and apoptosis, the NF-κB signaling
inhibitor Bay11-7082 (20 mol/l) was used. After a series of
functional restoration assays, we found that the decrease in SKGT-4
cell proliferation (Fig. 6A) and
migration (Fig. 6B) induced by TGM3
upregulation was markedly strengthened following Bay11-7082
stimulation. Moreover, disruption of NF-κB signaling by Bay11-7082
treatment also significantly decreased the apoptosis rate (Fig. 6C). These results indicate that TGM3
may repress EC cell proliferation and migration and promote
apoptosis by modulating the NF-κB signaling pathway.
Discussion
A variety of treatments is currently available for
EC. The choice of treatment is crucial, recurrent or distant
metastases as the limitations of conventional treatments,
therefore, it is necessary to search for novel approaches.
Recently, several research groups have reported certain genes and
signaling molecules that are potentially involved in EC initiation
and progression (24,25). These molecular targets may provide
significant clues for early diagnosis, prognosis and new targeted
therapies (26–28). Previous studies have found that TGM3
expression is downregulation in head and neck cancer, laryngeal
carcinoma and oral squamous cell carcinoma (29–32),
other reports found that TGM3 expression is decreased in EC tissues
compared with normal tissues (29).
However, the exact function and mechanisms of TGM3 in EC are
largely unknown. In this study, we verified this finding by
assessing the mRNA and protein levels of TGM3 in four EC cell lines
and 58 EC specimens. We found that the levels of this TGM3 were
much lower in EC tissues and cell lines compared with adjacent
controls. Our findings are consistent with previous reports, these
results allowed us to speculate that the overexpression of TGM3 may
confer a survival advantage to EC patients. Since metastasis is the
major cause of morbidity and mortality in EC patients, we
investigated the roles of TGM3 in cell migration and invasion of EC
cell lines. Transglutaminase (TGM) enzymes are widespread in both
plants and animals, they are a family of calcium-dependent enzymes
that catalyze the formation of isopeptide bonds (34,35).
TGM3 is expressed predominantly during the late stages of the
terminal differentiation of the epidermis and in certain cell types
of hair follicles (36). Although
TGM3 downregulation has been found in many cases, however, little
information is available concerning its function and mechanisms
involvement in EC. The results of this study indicate that TGM3
plays an important role in regulating the proliferation of EC cells
and strongly suggest that the introduction of TGM3 could inhibit
cellular oncogenesis by suppressing the migration and invasion of
EC cells. We consider TGM3 to be a strong biomarker candidate of
EC, because its expression clearly correlated with cancer
progression in our study and it has also been shown to be
potentially relevant to ESCC (esophageal squamous cell carcinoma)
(37).
Further studies identified that TGM3 promotes
activation of the NF-κB signaling pathway. Recently, abundant
studies have confirmed the important roles of NF-κB signaling in
cancer development and progression, including proliferation,
survival, angiogenesis and metastasis (38). The significance of NF-κB and its
therapeutic value as a target for cancer therapy have been
investigated in several types of human cancer (39,40).
Only a handful of studies to date have explored the involvement of
NF-κB activation in EC (41–43) in
which the NF-κB status correlated positively with metastasis,
resistance to chemotherapy and patient survival (44–46).
The present study broadens our understanding of the roles that TGM3
plays in EC by targeting NF-κB signaling and thus indicates that it
can be a powerful target for cancer therapy.
In conclusion, this study showed that TGM3
expression is low in EC tissues and cell lines, this result is
consistent with the previous studies. We also found that in EC cell
lines overexpression of TGM3 inhibits cell proliferation and
induces apoptosis by activation of NF-κB signaling pathway. As the
TGM3 expression level is associated with EC cell growth process,
promotion of TGM3 expression in EC tissues may inhibit tumor cell
amplification. Therefore, the present study may provide a
therapeutic strategy for patients with EC.
Acknowledgments
The present study is supported by grants from the
Key Scientific Research Project of Henan Province (14A310017) and
the Key Project of Henan Provincial Department of Science and
Technology (142102310311).
References
|
1
|
Kamangar F1, Qiao YL, Schiller JT, Dawsey
SM, Fears T, Sun XD, Abnet CC, Zhao P, Taylor PR and Mark SD: Human
papillomavirus serology and the risk of esophageal and gastric
cancers: results from a cohort in a high-risk region in China. Int
J Cancer. 119:579–584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2012. CA Cancer J Clin.
62:283–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gaur P, Kim MP and Dunkin BJ: Esophageal
cancer: Recent advances in screening, targeted therapy, and
management. J Carcinog. 13:112014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahvazi B, Kim HC, Kee SH, Nemes Z and
Steinert PM: Three-dimensional structure of the human
transglutaminase 3 enzyme: Binding of calcium ions changes
structure for activation. EMBO J. 21:2055–2067. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hitomi K, Horio Y, Ikura K, Yamanishi K
and Maki M: Analysis of epidermal-type transglutaminase (TGase 3)
expression in mouse tissues and cell lines. Int J Biochem Cell
Biol. 33:491–498. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hitomi K: Transglutaminases in skin
epidermis. Eur J Dermatol. 15:313–319. 2005.PubMed/NCBI
|
|
8
|
Hitomi K, Presland RB, Nakayama T,
Fleckman P, Dale BA and Maki M: Analysis of epidermal-type
transglutaminase (transglutaminase 3) in human stratified epithelia
and cultured keratinocytes using monoclonal antibodies. J Dermatol
Sci. 32:95–103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalinin AE, Kajava AV and Steinert PM:
Epithelial barrier function: Assembly and structural features of
the cornified cell envelope. BioEssays. 24:789–800. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eckert RL, Sturniolo MT, Ann-Marie B,
Monica R and Rorke EA: Transglutaminase function in epidermis. JJ
Invest Dermatol. 124:481–492. 2005. View Article : Google Scholar
|
|
11
|
Guang HE, Zhao Z, Weineng FU, Sun X,
Zhenming XU and Sun K: Study on the loss of heterozygosity and
expression of transglutaminase 3 gene in laryngeal carcinoma.
Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 19:120–123. 2002.In
Chinese.
|
|
12
|
Uemura N, Nakanishi YH, Saito S, Nagino M,
Hirohashi S and Kondo T: Transglutaminase 3 as a prognostic
biomarker in esophageal cancer revealed by proteomics. Int J
Cancer. 124:2106–1255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Negishi A, Masuda M, Ono M, Honda K,
Shitashige M, Satow R, Sakuma T, Kuwabara H, Nakanishi Y, Kanai Y,
et al: Quantitative proteomics using formalin-fixed
paraffin-embedded tissues of oral squamous cell carcinoma. Cancer
Sci. 100:1605–1611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Méndez E, Fan W, Choi P, Agoff SN, Whipple
M, Farwell DG, Futran ND, Weymuller EA Jr, Zhao LP and Chen Cl:
Tumor-specific genetic expression profile of metastatic oral
squamous cell carcinoma. Head Neck. 29:803–814. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Viatour P, Merville MP, Bours V and
Chariot A: Phosphorylation of NF-kappaB and IkappaB proteins:
Implications in cancer and inflammation. Trends Biochem Sci.
30:43–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li B, Li YY, Tsao SW and Cheung AL:
Targeting NF-kappaB signaling pathway suppresses tumor growth,
angiogenesis, and metastasis of human esophageal cancer. Mol Cancer
Ther. 8:2635–2644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bassères DS and Baldwin AS: Nuclear
factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic
initiation and progression. Oncogene. 25:6817–6830. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Liu X, Jin H, Guo X, Xia L, Chen
Z, Bai M, Liu J, Shang X, Wu K, et al: miR-206 inhibits gastric
cancer proliferation in part by repressing cyclinD2. Cancer Lett.
332:94–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cotter TG: Apoptosis and cancer: The
genesis of a research field. Nat Rev Cancer. 9:501–507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Darius W, Ilja M, Margitta E, Christian K
and Barbara K: Tumor necrosis factor alpha triggers proliferation
of adult neural stem cells via IKK/NF-kappaB signaling. BMC
Neurosci. 7:1–18. 2006. View Article : Google Scholar
|
|
22
|
Palumbo R, Galvez BG, Pusterla T, De
Marchis F, Cossu G, Marcu KB and Bianchi ME: Cells migrating to
sites of tissue damage in response to the danger signal HMGB1
require NF-kappaB activation. J Cell Biol. 179:33–40. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Y and Zhou BP:
TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and
invasion. Br J Cancer. 102:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong GS, Lee JS, Park YY, Klein-Szanto AJ,
Waldron TJ, Cukierman E, Herlyn M, Gimotty P, Nakagawa H and Rustgi
AK: Periostin cooperates with mutant p53 to mediate invasion
through the induction of STAT1 signaling in the esophageal tumor
microenvironment. Oncogenesis. 2:e592013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaz AM, Luo Y, Dzieciatkowski S, Chak A,
Willis JE, Upton MP, Leidner RS and Grady WM: Aberrantly methylated
PKP1 in the progression of Barrett's esophagus to esophageal
adenocarcinoma. Genes Chromosomes Cancer. 51:384–393. 2012.
View Article : Google Scholar
|
|
26
|
Shigaki H, Baba Y, Watanabe M, Murata A,
Ishimoto T, Iwatsuki M, Iwagami S, Nosho K and Baba H: PIK3CA
mutation is associated with a favorable prognosis among patients
with curatively resected esophageal squamous cell carcinoma. Clin
Cancer Res. 19:2451–2459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shah AK, Cao KA, Choi E, Chen D, Gautier
B, Nancarrow D, Whiteman DC, Saunders NA, Barbour AP, Joshi V, et
al: Serum glycoprotein biomarker discovery and qualification
pipeline reveals novel diagnostic biomarker candidates for
esophageal adenocarcinoma. Mol Cell Proteomics. 14:3023–3039. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Isozaki Y, Hoshino I, Nohata N, Kinoshita
T, Akutsu Y, Hanari N, Mori M, Yoneyama Y, Akanuma N, Takeshita N,
et al: Identification of novel molecular targets regulated by tumor
suppressive miR-375 induced by histone acetylation in esophageal
squamous cell carcinoma. Int J Oncol. 41:985–994. 2012.PubMed/NCBI
|
|
29
|
Uemura N, Nakanishi Y, Kato H, Saito S,
Nagino M, Hirohashi S and Kondo T: Transglutaminase 3 as a
prognostic biomarker in esophageal cancer revealed by proteomics.
Int J Cancer. 124:2106–2115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu X, Cao W, Wang X, Zhang J, Lv Z, Qin X,
Wu Y and Chen W: TGM3, a candidate tumor suppressor gene,
contributes to human head and neck cancer. Mol Cancer. 12:1512013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Zhou Y, Wan J and Liu Z: Expression
of TGM3 protein and its significance in laryngeal carcinoma. Lin
Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 26:101–103. 2012.In
Chinese. PubMed/NCBI
|
|
32
|
Ye H, Yu T, Temam S, Ziober BL, Wang J,
Schwartz JL, Mao L, Wong DT and Zhou X: Transcriptomic dissection
of tongue squamous cell carcinoma. BMC Genomics. 9:692008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kondoh N, Ishikawa T, Ohkura S, Arai M,
Hada A, Yamazaki Y, et al: Gene expression signatures that classify
the mode of invasion of primary oral squamous cell carcinomas. Mol
Carcinog. 47:744–756. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lorand L and Graham RM: Transglutaminases:
Crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell
Biol. 4:140–156. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Griffin M, Casadio R and Bergamini CM:
Transglutaminases: Nature's biological glues. Biochem J.
368:377–396. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Candi E, Schmidt R and Melino G: The
cornified envelope: A model of cell death in the skin. Nat Rev Mol
Cell Biol. 6:328–340. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu W, Yu ZC, Cao WF, Ding F and Liu ZH:
Functional studies of a novel oncogene TGM3 in human esophageal
squamous cell carcinoma. World J Gastroenterol. 12:3929–3932. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sourbier C, Danilin S, Lindner V, Steger
J, Rothhut S, Meyer N, Jacqmin D, Helwig JJ, Lang H and Massfelder
T: Targeting the nuclear factor-kappaB rescue pathway has promising
future in human renal cell carcinoma therapy. Cancer Res.
67:11668–11676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Robe PA, Bentires-Alj M, Bonif M, Rogister
B, Deprez M, Haddada H, Khac MT, Jolois O, Erkmen K, Merville MP,
et al: In vitro and in vivo activity of the nuclear factor-kappaB
inhibitor sulfasalazine in human glioblastomas. Clin Cancer Res.
10:5595–5603. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Abdel-Latif MMM, O'Riordan J, Windle HJ,
Carton E, Ravi N, Kelleher D and Reynolds JV: NF-kappaB activation
in esophageal adenocarcinoma: Relationship to Barrett's metaplasia,
survival, and response to neoadjuvant chemoradiotherapy. Ann Surg.
239:491–500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Abdel Latif MM, O'Riordan JN, Kelleher D
and Reynolds JV: Activated nuclear factor-kappaB and cytokine
profiles in the esophagus parallel tumor regression following
neoadjuvant chemoradiotherapy. Dis Esophagus. 18:246–252. 2005.
View Article : Google Scholar
|
|
43
|
Konturek PC, Nikiforuk A, Kania J, Raithel
M, Hahn EG and Mühldorfer S: Activation of NFkappaB represents the
central event in the neoplastic progression associated with
Barrett's esophagus: A possible link to the inflammation and
overexpression of COX-2, PPARgamma and growth factors. Dig Dis Sci.
49:1075–1083. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Izzo JG, Correa AM, Wu TT, Malhotra U,
Chao CK, Luthra R, Ensor J, Dekovich A, Liao Z, Hittelman WN, et
al: Pretherapy nuclear factor-kappaB status, chemoradiation
resistance, and metastatic progression in esophageal carcinoma. Mol
Cancer Ther. 5:2844–2850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Izzo JG, Malhotra U, Wu TT, Ensor J,
Luthra R, Lee JH, Swisher SG, Liao Z, Chao KS, Hittelman WN, et al:
Association of activated transcription factor nuclear factor kappab
with chemoradiation resistance and poor outcome in esophageal
carcinoma. J Clin Oncol. 24:748–754. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Izzo JG, Malhotra U, Wu TT, Luthra R,
Correa AM, Swisher SG, Hofstetter W, Chao KS, Hung MC and Ajani JA:
Clinical biology of esophageal adenocarcinoma after surgery is
influenced by nuclear factor-kappaB expression. Cancer Epidemiol
Biomarkers Prev. 16:1200–1205. 2007. View Article : Google Scholar : PubMed/NCBI
|