Introduction
Human bladder transitional cell carcinoma (BTCC) is
one of the most frequent malignant tumors and its incidence is
increasing worldwide (1). Despite
many advances in cancer diagnosis and treatment in recent decades,
the outcome of patients with advanced BTCC remains poor. There is,
therefore, a tremendous and urgent need for elucidating the
underlying molecular mechanisms of BTCC tumorigenesis and for
developing novel therapeutic strategies, including treatments aimed
at specific molecular targets.
MicroRNAs (miRNAs), which are short, highly
conserved non-coding RNAs, regulate the expression of messenger
RNAs (mRNAs) by binding to their 3′-untranslated region (3′-UTR)
(2,3). miRNAs play a critical role in
processes related to cancer by acting as oncogenes or tumor
suppressors in the regulation of carcinogenesis, metastatic
capacity and drug resistance. Low expression of miR-497 has been
observed in breast, colorectal and cervical cancers (4). Recent studies have shown that miR-497
suppresses angiogenesis and invasion of ovarian cancer by targeting
vascular endothelial growth factor A (5). Li et al reported that miRNA-497
modulates gastric cancer cell proliferation and invasion by
repressing eIF4E (6). Notably, a
recent study by Itesako et al found that miR-497
significantly inhibited cancer cell proliferation, migration and
invasion in two bladder cell lines by targeting BIRC5 and WNT7A
(7). However, the relationship
between miR-497 and the prognosis of BTCC patients, and whether
miR-497 targets other target genes to regulate BTCC cell growth and
invasion are undefined.
Transcription factor E2F3, a key regulator of G1/S
phase transition, plays major roles in regulating cell cycle
progression (8,9). Previous studies have shown that E2F3
overexpression is critical for the growth and survival of bladder
cancer cells, and is generally correlated with poor outcomes
(3,10). Furthermore, BTCC patients with
higher levels of E2F3 also show rates of higher mortality compared
with patients with lower levels of E2F3 (3,11),
suggesting that E2F3 is important for BTCC development. To date,
several miRNAs, such as miR-34a, miR-20a, miR-125b and miR-217,
have been confirmed to suppress cell growth and survival by
targeting E2F3 in tumor cells (3,12–16).
However, the relationship between miR-497 and E2F3 in BTCC is
poorly understood.
In the present study, we found that miR-497 was
significantly downregulated in BTCC cell lines and tissues.
Furthermore, we identified E2F3 as a target gene of miR-497 and
showed that upregulation of miR-497 inhibited cell proliferation
and invasion by directly downregulating E2F3 expression in T24 and
UM-UC-3 cells in vitro. Our data indicated that miR-497
functions as a tumor suppressor in BTCC development and serves as a
prognostic marker for BTCC.
Materials and methods
Cell lines and culture
Human BTCC cell lines (T24, 5637, BIU-87 and
UM-UC-3) and a human bladder urothelium cell line (SV-HUC-1) were
purchased from the Chinese Science Institute (Shanghai, China). All
cell lines were maintained in high glucose Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS) (both from Gibco, Gaithersburg, MD, USA). Cells were
incubated at 37°C in a humidified atmosphere with 5%
CO2.
Tissue specimens
Eighty paired tissue samples from BTCCs and the
corresponding adjacent normal tissues were collected from the
Department of Pathology of Shengjing Hospital. All tissue specimens
were confirmed by pathology. The collection and use of tissues
followed procedures that were in accordance with the ethical
standards formulated in the Declaration of Helsinki. Patients
provided informed consent prior to tissue collection. The present
study was approved by the Institutional Research Ethics Committee
of China Medical University.
Transfection of miRNA mimics and small
interfering RNAs
An miR-497 mimic (referred to as miR-497) and
negative control (NC) duplex were used for further gain-of-function
experiments. A small interfering RNA (siRNA) duplex (siE2F3)
targeting E2F3 mRNA was used for RNA interference experiments. All
RNA duplexes and oligos were obtained from RiboBio (Guangzhou,
China).
Cells (5×105) were seeded into 6-well
plates (Nest Biotechnology, Hong Kong, China). At 70% confluency,
the cells were subjected to transfection using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA) following the manufacturer's
instructions (17). The sequences
of the RNA duplexes and oligos were as follows: miR-497 mimics,
5′-CAGCAGCACACUGUGGUUUGU-3′; NC, 5′-UUGUACUACACAAAAGUACUG-3′;
siE2F3, 5′-UAACCUUUGAUUCUCUGAAUCCUCG-3′.
Real-time quantitative RT-PCR
Total RNA was extracted from cells or tissues using
an All-in-One microRNA extraction kit (GeneCopoeia, Rockville, MD,
USA), according to the manufacturer's protocol. Expression of
miR-497 was measured using a one-step qRT-PCR kit (EzOmics SYBR
qPCR kit) as previously described (18). An miR-497 stem-loop primer (U6
primer) and EzOmics SYBR qPCR kit were obtained from Biomics
Biotechnology Inc. (Jiangsu, China). Real-time PCR was performed
with the StepOnePlus Real-Time PCR instrument. The primers were as
follows: miR-497,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACAAA-3′ (stem-loop
primer), 5′-CGCCAGCAGCACACTGTGG-3′ (sense), and
5′-GTGCAGGGTCCGAGGT-3′ (anti-sense); U6,
5′-GTCCTATCCAGTGCAGGGTCCGAGGTGCACTGGATACGACAAAATATGGAAC-3′
(stem-loop primer), 5′-TGCGGGTGCTCGCTTCGCAGC-3′ (sense), and
5′-CCAGTGCAGGGTCCGAGGT-3′ (antisense). U6 was used as an internal
control. All results are representative of three independent
assays, and the expression levels of miR-497 were calculated by the
2−ΔΔCt method.
Cell Counting Kit-8 assay
Cell proliferation was measured using a Cell
Counting Kit-8 assay (CCK-8) (Solarbio, Beijing, China). Cells
transfected with miR-497 mimics, NC, siRNAs and relative controls
were seeded in 96-well plates (Corning, Corning, NY, USA) at
2×103 cells/well. At 0, 24, 48 and 72 h, 10 µl
CCK-8 reagent was added to each well, After 2 h of incubation, the
absorbance was measured at 450 nm as previously described (3). All results are representative of three
independent assays.
Colony formation assay
After transfection, 300 cells were seeded into
6-well plates containing 2 ml DMEM supplemented with 10% FBS/well
and incubated at 37°C in a humidified atmosphere with 5%
CO2 for 7–10 days. After staining with a 0.1% crystal
violet solution for 10 min, the numbers of colonies were counted.
All results are representative of three independent assays.
Cell migration and invasion assays
At 90% confluency, a sterile pipette tip was used to
make a scratch through each well. Cells were photographed under a
microscope at 0 and 24 h for comparison. We used Transwell invasion
assays to evaluate the invasive ability of cells as previously
reported (3). An inverted
microscope was used to observed cell invasion. The invasion assay
was terminated when the cells crossed into the lower well. After
the Matrigel was scraped off, the number of cells in the bottom
well was counted. Images of cells stained with
4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) were obtained.
Each treatment was performed in triplicate.
Dual-luciferase reporter assays
Dual-luciferase reporter assays were performed as
previously reported (18). Briefly,
T24 cells were seeded in 96-well plates and co-transfected with a
pMir-Report luciferase vector, pRL-TK Renilla luciferase
vector and miR-497 mimics, as previously reported (17). After 48 h, the luciferase activities
were determined with the Dual-Luciferase Reporter Assay system
(Promega, Madison, WI, USA). Renilla luciferase activity was
used as an internal control. After normalization to Renilla
luciferase activity, the firefly luciferase activity was analyzed.
All results are representative of three independent assays.
E2F3 rescue experiments
The E2F3-coding sequence excluding the 3′-UTR was
inserted into a pReceiver vector (GeneCopoeia) to construct the
pReceiver-E2F3 vector. The BTCC cells were co-transfected with
miR-497 or NC and pReceiverE2F3 or an empty pReceiver vector. BTCC
cells were harvested at 48 h after transfection. Cell proliferation
and invasion were determined and E2F3 protein expression was
analyzed by western blotting as described above.
Western blot analysis
Proteins were isolated from the cells or tissues by
mechanical disruption and a Mammalian Cell Lysis kit
(Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer's
instructions. The proteins were separated on 1 mm NuPage Novex 10%
Bis-Tris gels using a NuPage MOPS SDS Buffer kit (Life
Technologies, Carlsbad, CA, USA) followed by electrotransfer to
0.2-mm nitrocellulose membranes (Pall, Port Washington, WI, USA).
Non-specific binding sites were blocked with 5% bovine serum
albumin in phosphate-buffered saline (PBS) for 1 h at room
temperature. The membranes were then incubated with a diluted
primary antibody (1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA) at 4°C overnight. After three washes with PBS
containing 0.5% Tween-20, the membranes were incubated with a
diluted secondary antibody (GE Healthcare, Buckinghamshire, UK) at
room temperature for 2 h. Signals were visualized with enhanced
chemiluminescent reagent (Amersham Biosciences, Piscataway, NJ,
USA). As a protein loading control, the blots were stripped and
stained for GAPDH using an anti-GAPDH antibody (1:2,000; Abcam,
Cambridge, MA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). All statistical analyses were performed by one-way analysis
of variance (ANOVA) (SPSS 18.0). miRNA target prediction and the
associated mRNA pathway analysis were performed using ingenuity
pathway analysis and TargetScan. Differences between treatments
were assessed using Fisher's least significant difference test.
Significant differences were considered at P<0.05.
Results
miR-497 is downregulated in BTCC cell
lines and tissues
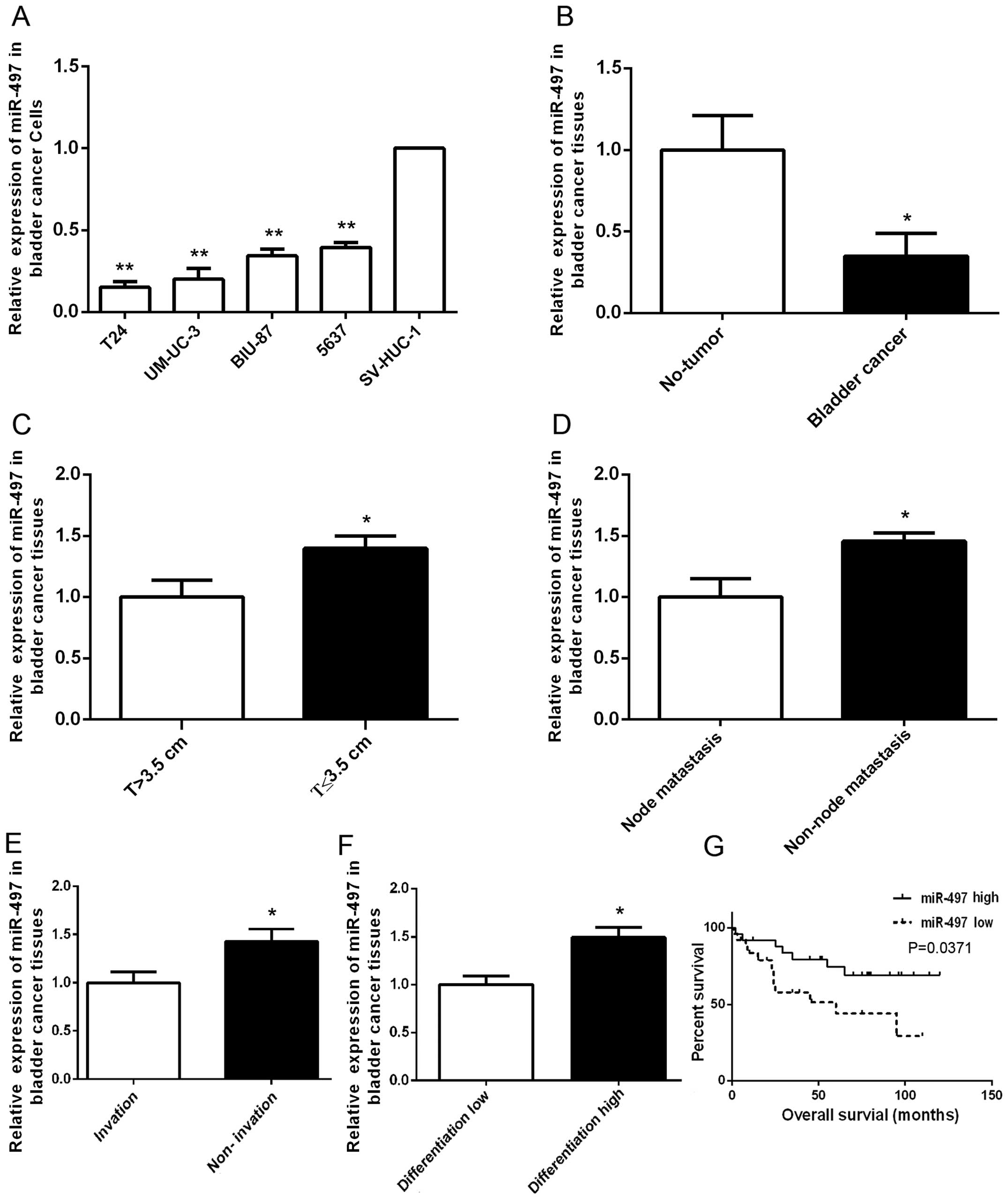
We detected the expression of miR-497 in four human
BTCC cell lines (T24, 5637, BIU-87 and UM-UC-3) by RT-PCR and found
that miR-497 was downregulated in BTCC cell lines T24, 5637, BIU-87
and UM-UC-3 compared with that noted in a normal bladder epithelial
cell line, SV-HUC-1 (Fig. 1A). The
levels of miR-497 were also detected in 80 human BTCC specimens and
adjacent normal tissues by RT-PCR. The results showed that miR-497
was downregulated in the human BTCC specimens compared with that
noted in their adjacent normal tissues (Fig. 1B). Moreover, a low level of miR-497
expression was correlated with tumor size (Fig. 1C), node metastasis (Fig. 1D), invasion (Fig. 1E), differentiation (Fig. 1F) and poor prognosis (Fig. 1G).
miR-497 suppresses BTCC progression in
vitro
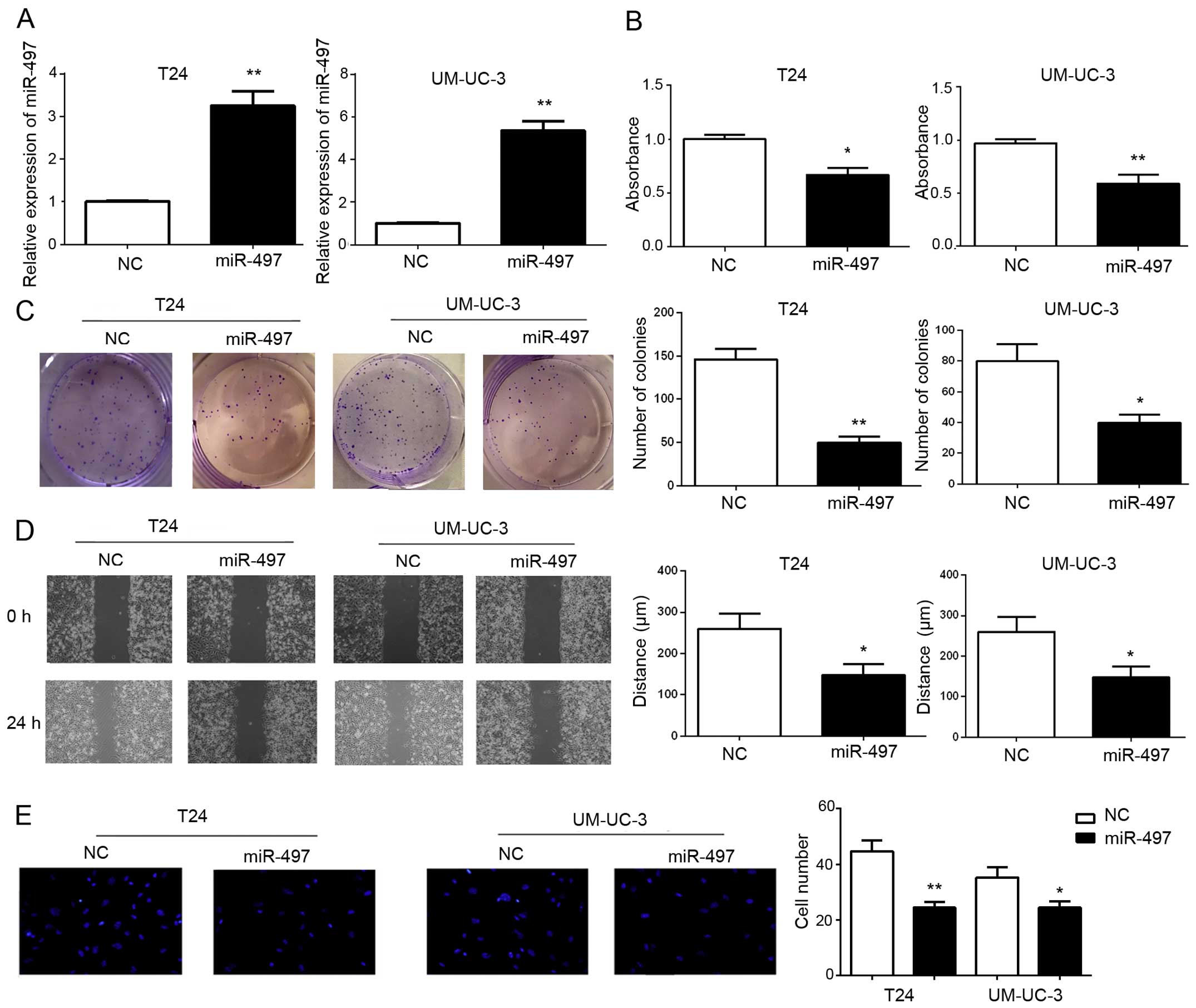
To examine the role of miR-497 in cell
proliferation, migration and invasion, T24 and UM-UC-3 cell lines
were transfected with miR-497 mimics or NC. Increased expression of
miR-497 upon transfection was confirmed by RT-PCR (Fig. 2A). As shown in Fig. 2B, ectopic miR-497 expression
suppressed proliferation of the BTCC cells as analyzed by the CCK-8
assay. Consistent with the effects on cell proliferation, the
capacity for colony formation by both cell lines was robustly
compromised by miR-497 transfection compared with the corresponding
control cells (Fig. 2C). As shown
in Fig. 2D, the migratory ability
of the miR-497 mimic groups was lower than that of the NC groups.
Similar to the effect on cell migration, the capacity for invasion
of both cell lines was significantly decreased by miR-497
transfection compared with that noted in the corresponding control
cells (Fig. 2E). Taken together,
our results revealed that miR-497 inhibited BTCC cell
proliferation, migration and invasion in vitro.
miR-497 directly targets E2F3 in BTCC
cells
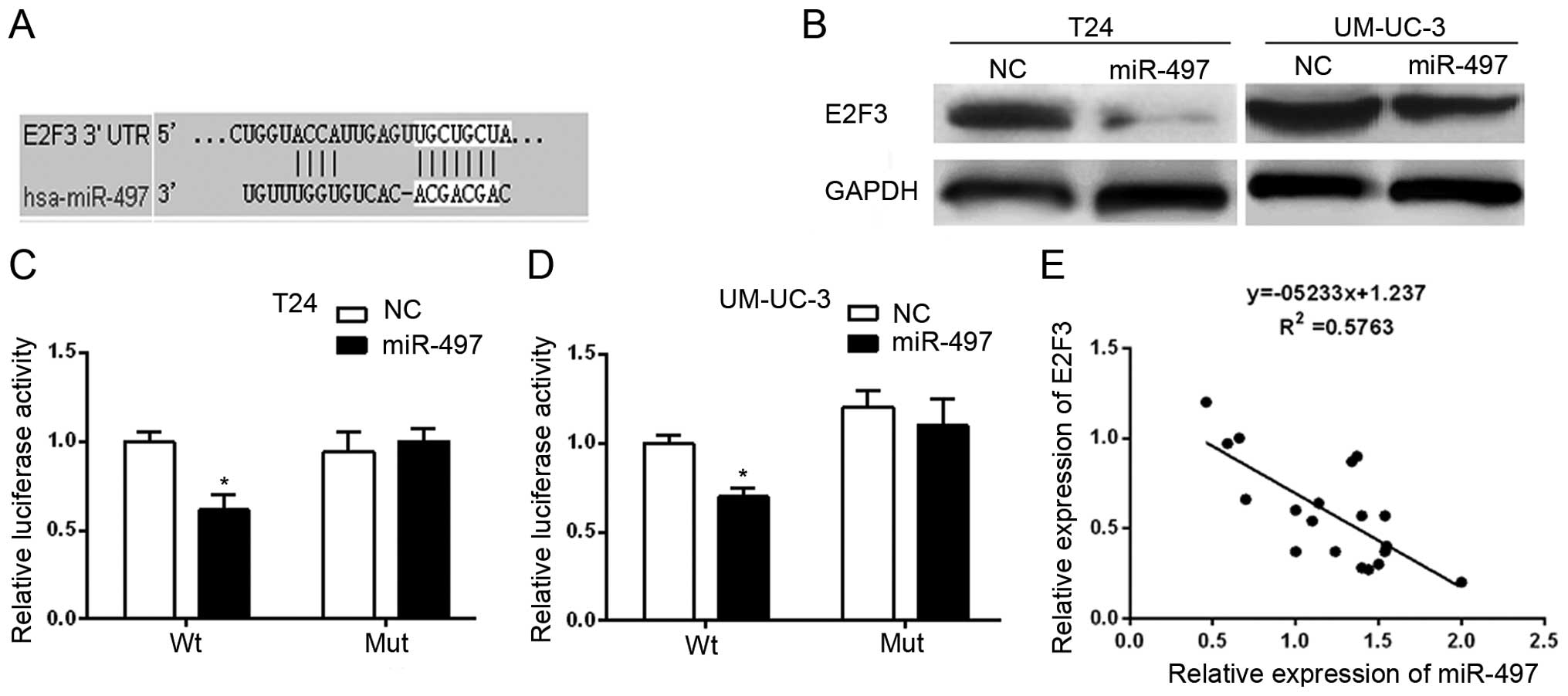
Next, we identified the miR-497 target gene to gain
further insight into the molecular mechanisms of miR-497 in BTCC
proliferation, migration and invasion. The public
database-TargetScan (http://www.targetscan.org) was used to predict the
potential target of miR-497. Due to a critically conserved binding
site, E2F3 was selected for further examination (Fig. 3A).
To investigate the relationship between miR-497 and
E2F3, the protein expression of E2F3 was measured in BTCC cell
lines with differential expression of miR-497. E2F3 protein levels
in T24 and UM-UC-3 cells were significantly decreased after
overexpression of miR-497 mimics (Fig.
3B). To confirm whether E2F3 is a direct target of miR-497, we
constructed a dual-luciferase reporter vector with the putative
E2F3 3′UTR target site for miR-497, which was downstream of the
luciferase gene (pMir-E2F3-Wt), and a deletion mutant of 7 bp in
the seed region (pMir-E2F3-Mut). As shown in Fig. 3C and D, luciferase activity assays
showed that miR-497 significantly suppressed the activity of Wt,
but not Nut reporters in the T24 and UM-UC-3 cells. In addition,
the relative expression levels of E2F3 were inversely correlated
with the relative expression levels of miR-497 in the BTCC tissues
(Fig. 3E). These results strongly
demonstrated the specificity of miR-497 to target E2F3.
siE2F3 inhibits the proliferation,
migration and invasion of BTCC cells
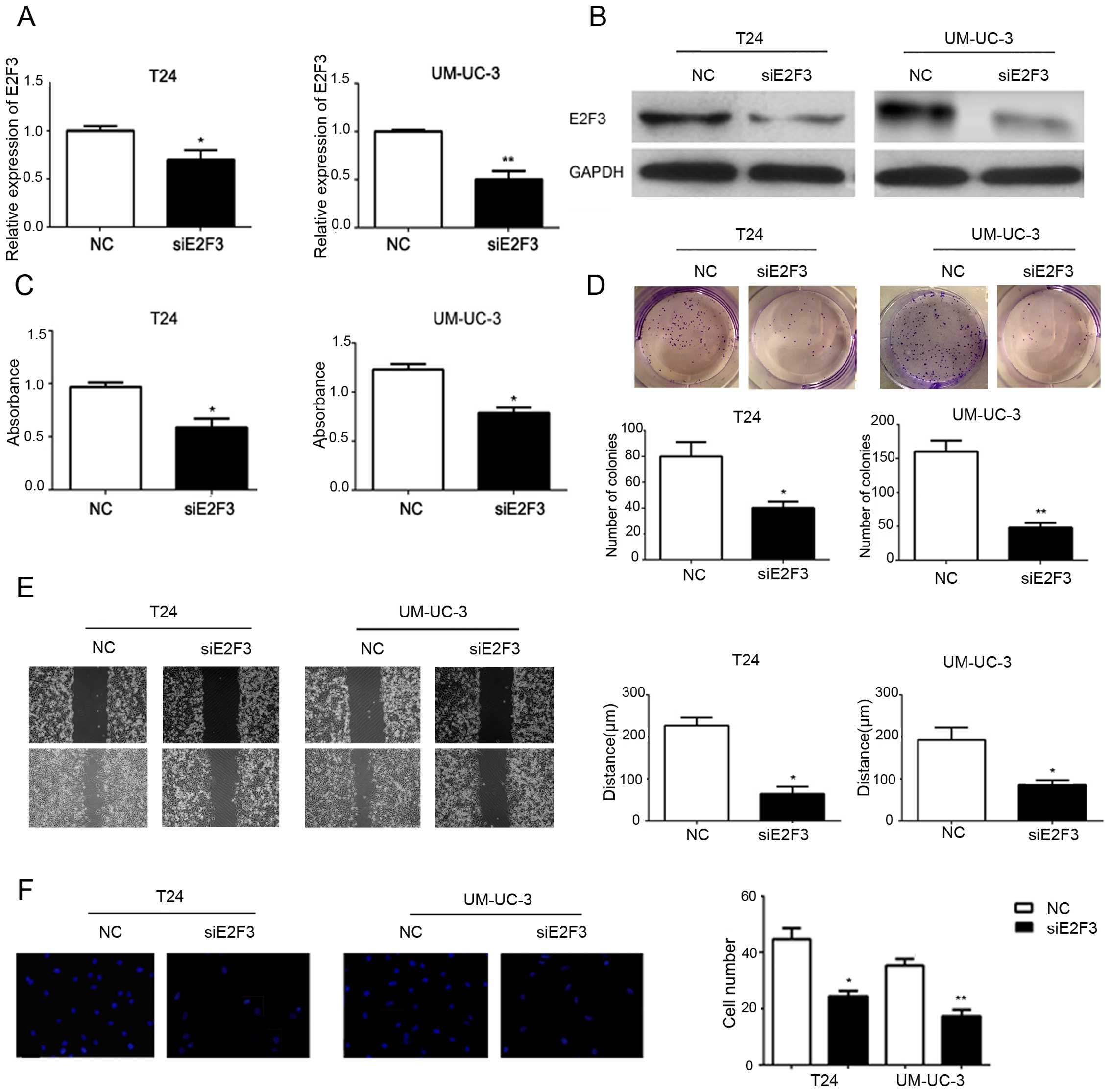
As shown in Fig. 4A and
B, compared with NC, siE2F3 decreased the expression of E2F3 at
both the mRNA and protein levels. To reveal the biological function
of E2F3, siE2F3 was stably transfected into the T24 and UM-UC-3
cells. As a result, we found that siE2F3 could reproduce an effect
on cell proliferation, migration and invasion similar to that of
miR-497 (Fig. 4C–F).
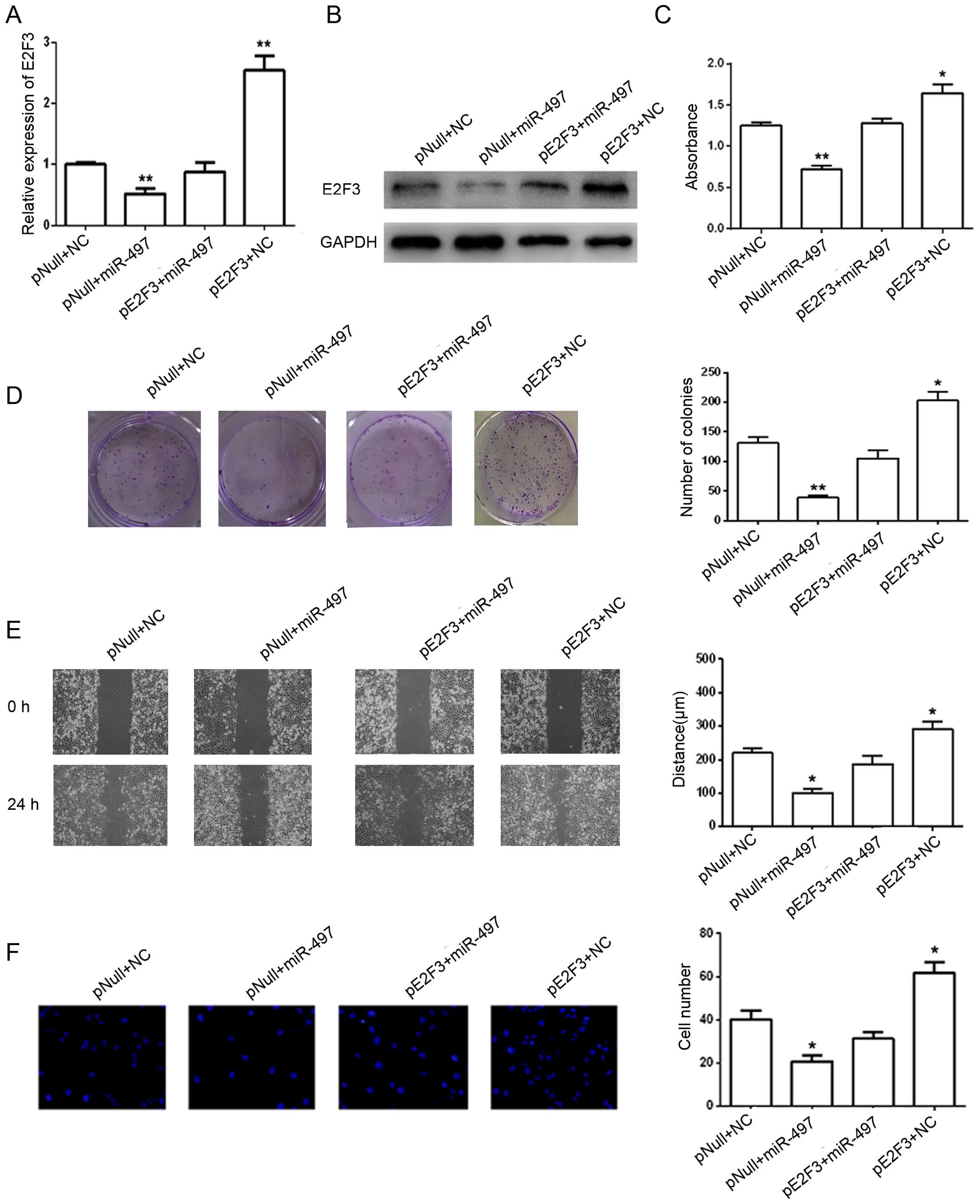
Restoration of E2F3 partially rescues
miR-497-induced inhibition of the proliferation, migration and
invasion of BTCC cells
To further explore whether overexpression of E2F3
could rescue the suppression of cell proliferation, migration and
invasion induced by miR-497, we inserted the E2F3-coding sequence
excluding the 3-UTR into the pReceiver vector, and found that E2F3
expression levels were enhanced by transfection in the T24 cells
(Fig. 5A). Cell proliferation,
migration and invasion were evaluated in cells co-transfected with
both miR-497 mimics or NC and pReceiver-E2F3 or an empty pReceiver
vector. Our results indicated that overexpression of E2F3 could
partially rescue miR-497-induced inhibition of cell proliferation,
migration and invasion (Fig.
5B–F).
Discussion
Recent studies have demonstrated the potential of
miRNAs as promising prognostic biomarkers (2,3).
Targeting miRNAs is an effective approach for the treatment of
advanced cancers (2–4). Moreover, aberrant expression of miRNAs
has been identified in bladder cancer, which plays crucial roles in
tumorigenesis and cancer progression. miR-497 is a recently
discovered miRNA that is downregulated in bladder, gastric and
breast cancers (19–24). In the present study, we found that
miR-497 was markedly downregulated in the BTCC cell lines. Our
results indicate that low expression of miR-497 is significantly
associated with BTCC progression.
We also analyzed the relative expression of miR-497
in BTCC tissues. miR-497 expression was decreased in cancer
tissues, and the low expression of miR-497 was significantly
associated with tumor size, node metastasis, invasion,
differentiation, as well as a poor prognosis. These results imply
that miR-497 expression may function as an independent prognosis
factor that is significantly associated with the overall survival
rates of BTCC patients and BTCC progression. Our results were
consistent with a previous study by Zhao et al who showed
that downregulation of miR-497 is associated with a poor prognosis
in renal cancer (25). Further
studies demonstrated that upregulation of miR-497 significantly
decreased cell proliferation, the colony formation rate, wound
healing and migration rates, indicating that miR-497 inhibits the
proliferation, migration and invasion of BTCC cells.
Next, we explored the molecular mechanism by which
miR-497 acts as a suppressor in BTCC progression. Luciferase
reporter assays and western blotting were employed to confirm that
E2F3 is direct target of miR-497 in BTCC cells. E2F3, which is
known as a transcription factor, has a central role in linking cell
cycle proteins, such as cyclins, cyclin-dependent kinases and pRB,
to the expression of genes involved in cell growth and survival
(26–29).
E2F3 gene amplification and protein overexpression
have been extensively studied in bladder cancer (10). High levels of E2F3 expression have
been observed in approximately one-third of BTCC with E2F3
overexpression increasing with tumor grade and stage (10). Hence, the mechanism of E2F3
upregulation, except for gene RNA inference, recent reports showed
several miRNAs (miR-141, miR-199a-5p, miR-125 and miR-200c) may be
involved (30–34). In the present study, western blot
analysis and RT-PCR demonstrated downregulation of E2F3 by miR-497,
which inhibited the cell proliferation, migration and invasion of
BTCC cells. To test whether miR-497 inhibits proliferation,
migration and invasion of BTCC cells through targeting E2F3, the
expression of E2F3 was knocked down using specific siRNAs. siE2F3
could emulate the effects of miR-497 overexpression on cell growth,
migration and invasion. All these results suggest that miR-497 may
act as a suppressor of BTCC cells by inhibiting the expression of
E2F3. Therefore, we proposed that downregulation of miR-497 may
enhance the proliferation and invasion of BTCC cells and
subsequently facilitate the development of BTCC through
upregulating the expression of E2F3. The rescue effect of E2F3
expression partly reversed the inhibition of cell proliferation,
migration and invasion induced by miR-497. In the present study, we
demonstrated the direct role of E2F3 in the proliferation,
migration and invasion of BTCC cells, which were regulated by
miR-497. The specific mechanism will be revealed in a future
study.
In conclusion, our data demonstrated altered
expression of miR-497 in human BTCC cell lines and BTCC tissues,
and that low levels of miR-497 correlate with poor prognoses in
patients. Our data also showed that miR-497 suppresses the
proliferation and invasion of BTCC cells, possibly through
modulating the target gene E2F3. These findings suggest that
miR-497 may be valuable biomarker for BTCC progression, and the
miR-497-E2F3 axis may be a novel therapeutic target for BTCC.
Abbreviations:
|
BTCC
|
bladder transitional cell
carcinoma
|
|
miRNAs
|
microRNAs
|
|
miR-497
|
microRNA-497
|
Acknowledgments
The present study was supported by research grants
from the National Natural Science Foundation of China (no.
81372723), and the Key Urology Laboratory Foundation of Shenyang
City of China (F13-293-1-00).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget.
6:8474–8490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu L, Qiu M, Tan G, Liang Z, Qin Y, Chen
L, Chen H and Liu J: miR-200c inhibits invasion, migration and
proliferation of bladder cancer cells through down-regulation of
BMI-1 and E2F3. J Transl Med. 12:3052014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo Q, Li X, Gao Y, Long Y, Chen L, Huang
Y and Fang L: MiRNA-497 regulates cell growth and invasion by
targeting cyclin E1 in breast cancer. Cancer Cell Int. 13:952013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang W, Ren F, Wu Q, Jiang D, Li H and Shi
H: MicroRNA-497 suppresses angiogenesis by targeting vascular
endothelial growth factor A through the PI3K/AKT and MAPK/ERK
pathways in ovarian cancer. Oncol Rep. 32:2127–2133.
2014.PubMed/NCBI
|
|
6
|
Li W, Jin X, Deng X, Zhang G, Zhang B and
Ma L: The putative tumor suppressor microRNA-497 modulates gastric
cancer cell proliferation and invasion by repressing eIF4E. Biochem
Biophys Res Commun. 449:235–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Itesako T, Seki N, Yoshino H, Chiyomaru T,
Yamasaki T, Hidaka H, Yonezawa T, Nohata N, Kinoshita T, Nakagawa
M, et al: The microRNA expression signature of bladder cancer by
deep sequencing: The functional significance of the miR-195/497
cluster. PLoS One. 9:e843112014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vimala K, Sundarraj S, Sujitha MV and
Kannan S: Curtailing overexpression of E2F3 in breast cancer using
siRNA (E2F3)-based gene silencing. Arch Med Res. 43:415–422. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hurst CD, Tomlinson DC, Williams SV, Platt
FM and Knowles MA: Inactivation of the Rb pathway and
overexpression of both isoforms of E2F3 are obligate events in
bladder tumours with 6p22 amplification. Oncogene. 27:2716–2727.
2008. View Article : Google Scholar :
|
|
10
|
Olsson AY, Feber A, Edwards S, Te Poele R,
Giddings I, Merson S and Cooper CS: Role of E2F3 expression in
modulating cellular proliferation rate in human bladder and
prostate cancer cells. Oncogene. 26:1028–1037. 2007. View Article : Google Scholar
|
|
11
|
Shen H, Morrison CD, Zhang J, Underwood W
III, Yang N, Frangou C, Eng K, Head K, Bollag RJ, Kavuri SK, et al:
6p22.3 amplification as a biomarker and potential therapeutic
target of advanced stage bladder cancer. Oncotarget. 4:2124–2134.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang L, Luo J, Cai Q, Pan Q, Zeng H, Guo
Z, Dong W, Huang J and Lin T: MicroRNA-125b suppresses the
development of bladder cancer by targeting E2F3. Int J Cancer.
128:1758–1769. 2011. View Article : Google Scholar
|
|
13
|
Oeggerli M, Tomovska S, Schraml P,
Calvano-Forte D, Schafroth S, Simon R, Gasser T, Mihatsch MJ and
Sauter G: E2F3 amplification and overexpression is associated with
invasive tumor growth and rapid tumor cell proliferation in urinary
bladder cancer. Oncogene. 23:5616–5623. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Welch C, Chen Y and Stallings RL:
MicroRNA-34a functions as a potential tumor suppressor by inducing
apoptosis in neuroblastoma cells. Oncogene. 26:5017–5022. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sylvestre Y, De Guire V, Querido E,
Mukhopadhyay UK, Bourdeau V, Major F, Ferbeyre G and Chartrand P:
An E2F/miR-20a autoregulatory feedback loop. J Biol Chem.
282:2135–2143. 2007. View Article : Google Scholar
|
|
16
|
Su J, Wang Q, Liu Y and Zhong M: miR-217
inhibits invasion of hepatocellular carcinoma cells through direct
suppression of E2F3. Mol Cell Biochem. 392:289–296. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu Y, Zhao F, Wang Z, Song Y, Luo Y, Zhang
X, Jiang L, Sun Z, Miao Z and Xu H: MicroRNA-335 acts as a
metastasis suppressor in gastric cancer by targeting Bcl-w and
specificity protein 1. Oncogene. 31:1398–1407. 2012. View Article : Google Scholar :
|
|
18
|
Iseki H, Takagi M, Kuroda Y, Katsuda K,
Mikami O, Tsunemitsu H and Yamakawa M: Application of a
SYBR®Green one step real-time RT-PCR assay to detect
type 1 porcine reproductive and respiratory syndrome virus. J Vet
Med Sci. 76:1411–1413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Wang T, Cao Z, Huang H, Li J, Liu W,
Liu S, You L, Zhou L, Zhang T, et al: MiR-497 downregulation
contributes to the malignancy of pancreatic cancer and associates
with a poor prognosis. Oncotarget. 5:6983–6993. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao WY, Wang Y, An ZJ, Shi CG, Zhu GA,
Wang B, Lu MY, Pan CK and Chen P: Downregulation of miR-497
promotes tumor growth and angiogenesis by targeting HDGF in
non-small cell lung cancer. Biochem Biophys Res Commun.
435:466–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Li B, Li L and Wang T:
MicroRNA-497 suppresses proliferation and induces apoptosis in
prostate cancer cells. Asian Pac J Cancer Prev. 14:3499–3502. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen L, Li J, Xu L, Ma J, Li H, Xiao X,
Zhao J and Fang L: miR-497 induces apoptosis of breast cancer cells
by targeting Bcl-w. Exp Ther Med. 3:475–480. 2012.PubMed/NCBI
|
|
23
|
Guo ST, Jiang CC, Wang GP, Li YP, Wang CY,
Guo XY, Yang RH, Feng Y, Wang FH, Tseng HY, et al: MicroRNA-497
targets insulin-like growth factor 1 receptor and has a tumour
suppressive role in human colorectal cancer. Oncogene.
32:1910–1920. 2013. View Article : Google Scholar :
|
|
24
|
Flavin RJ, Smyth PC, Laios A, O'Toole SA,
Barrett C, Finn SP, Russell S, Ring M, Denning KM, Li J, et al:
Potentially important microRNA cluster on chromosome 17p13.1 in
primary peritoneal carcinoma. Mod Pathol. 22:197–205. 2009.
View Article : Google Scholar
|
|
25
|
Zhao X, Zhao Z, Xu W, Hou J and Du X:
Down-regulation of miR-497 is associated with poor prognosis in
renal cancer. Int J Clin Exp Pathol. 8:758–764. 2015.PubMed/NCBI
|
|
26
|
Chang S, Gao L, Yang Y, Tong D, Guo B, Liu
L, Li Z, Song T and Huang C: miR-145 mediates the antiproliferative
and gene regulatory effects of vitamin D3 by directly targeting
E2F3 in gastric cancer cells. Oncotarget. 6:7675–7685. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanai D, Ueda A, Akagi T, Yokota T and
Koide H: Oct3/4 directly regulates expression of E2F3a in mouse
embryonic stem cells. Biochem Biophys Res Commun. 459:374–378.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen PH, Shih CM, Chang WC, Cheng CH, Lin
CW, Ho KH, Su PC and Chen KC: MicroRNA-302b-inhibited E2F3
transcription factor is related to all trans retinoic acid-induced
glioma cell apoptosis. J Neurochem. 131:731–742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
An Q, Wang Y, An R, Li Y, Yao T, Zhai B
and Sun X: Association of E2F3 expression with clinicopathological
features of Wilms' tumors. J Pediatr Surg. 48:2187–2193. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cooper CS, Nicholson AG, Foster C, Dodson
A, Edwards S, Fletcher A, Roe T, Clark J, Joshi A, Norman A, et al:
Nuclear overexpression of the E2F3 transcription factor in human
lung cancer. Lung Cancer. 54:155–162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tao T, Liu D, Liu C, Xu B, Chen S, Yin Y,
Ang L, Huang Y, Zhang X and Chen M: Autoregulatory feedback loop of
EZH2/miR-200c/E2F3 as a driving force for prostate cancer
development. Biochim Biophys Acta. 1839:858–865. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song C, Wu G, Xiang A, Zhang Q, Li W, Yang
G, Shi X, Sun S and Li X: Over-expression of miR-125a-5p inhibits
proliferation in C2C12 myoblasts by targeting E2F3. Acta Biochim
Biophys Sin. 47:244–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xue J, Niu YF, Huang J, Peng G, Wang LX,
Yang YH and Li YQ: miR-141 suppresses the growth and metastasis of
HCC cells by targeting E2F3. Tumour Biol. 35:12103–12107. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JM, Heo MJ, Lee CG, Yang YM and Kim
SG: Increase of miR-199a-5p by protoporphyrin IX, a photocatalyzer,
directly inhibits E2F3, sensitizing mesenchymal tumor cells to
anticancer agents. Oncotarget. 6:3918–3931. 2015. View Article : Google Scholar : PubMed/NCBI
|