Introduction
In recent decades, an integrated approach to cancer
treatment that includes surgery, chemotherapy, radiotherapy, gene
therapy and immune therapy, has become a reasonable therapeutic
strategy (1). Oncolytic virotherapy
using agents such as Newcastle disease virus (NDV) is one of the
new biological strategies for gene and immune therapy and has been
tested against many different cancers including gastric cancer,
skin tumors and several solid cancers (2–4). NDV,
which is a member of the Paramyxoviridae family, is a non-segmented
negative-strand RNA virus (NNSV). The NDV genes encode six major
structural proteins, including nucleoprotein (NP), phosphoprotein
(P), matrix protein (M), fusion protein (F),
hemagglutinin-neuraminidase (HN) and large (L) RNA-dependent RNA
polymerase, in the following order 3′-NP-P-M-F-HN-L-5′. Oncolysis,
apoptosis and enhancing innate immunity are the key mechanisms
capacitating NDV as an agent against malignant cells (5). NDV virus can be propagated in
embryonated chicken eggs, harvested from the allantoic fluid, and
quantified by hemagglutination, NDV has been actively developed and
evaluated as a vaccine vector for the control of human and animal
diseases, these characteristics made the NDV virus easy medium to
obtain (6–9).
Interferon-λ1 (IFN-λ1), also known as interleukin
(IL)-29, is a recently discovered cytokine of the type III IFN
family (10,11). It is thought to have biologic
properties similar to the type I IFNs. Unlike IFN-α, the receptor
for IFN-λ1 expresses on a limited number of normal cells including
dendritic cells, T cells, and intestinal epithelial cells (12,13).
Leukemia cells and colon, prostate, pancreatic, lung, hepatoma,
glioblastoma, and breast cancer cells have also been shown to
express IFNLR1 receptor (14–18).
It is widely accepted that cytokines associated with a T helper
cell subtype 1 (Th1) exhibit tumour-suppressive activity, whereas T
helper cell subtype 2 (Th2) cytokines can promote tumour
progression (14,19–21).
IFN-λ1 primarily inhibits IL-13 production and gives rise to IFN-γ
production in PBMCs (22). IFN-λ1
receptor IFNLR1 expresses on both naive and memory CD4+
T cells (23). It is also reported
when cultured with IL-29 the cytokine tumor microenvironment is
skewed towards a Th1 response that results in reducing tumor growth
(24).
In the present study, we used an established reverse
genetics system (8) to generate a
recombinant NDV LaSota viruses named as human IFN-λ1 recombinant
adenovirus (rl-hIFN-λ1), we incerted IFN-λ1 gene into a lentogenic
recombinant LaSota (NDV) genome at the position between the P and M
genes. We believe that this new recombinant virus can express
IFN-λ1 protein stably allowing us to consider it to br a promising
agent for cancer therapy. Our group studied the antitumor effect of
the rL-hIFN-λ1 on gastric adenocarcinoma cell lines compared with
NDV. Furthermore, in this study we also investigated whether the
rL-hIFN-λ1 is capable of regulating the IFN-γ secretion (the Th1
response) and the IL-13 secretion (the Th2 response) indicating the
change of Th1/Th2 balance in the tumor microenvironment that
results in inhibiting tumor growth.
Materials and methods
Cells and viruses
The BSR cells (Harbin Veterinary Research Institute)
for virus rescue, and the human gastric adenocarcinoma cell lines
SGC-7901 and AGS were purchased from the Cancer Cell Repository
(Shanghai Cell Bank, Shanghai, China; 2010-02-20) and the American
Type Culture Collection (ATCC; Rockville, MD, USA). Gastric
adenocarcinoma cells SGC-7901 and AGS cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA,
USA) containing 10% fetal bovine serum (FBS) at 37°C in a
humidified 5% CO2 incubator. Cell culture reagents were
acquired from Gibco (Grand Island, NY, USA). When the cells reached
50–70% confluence, they were infected with rL-hIFN-λ1 or NDV for
further study. The NDV LaSota strain and the recombiant viruses
were propagated and titrated in 9-day-old SPF embryonated chicken
eggs. The NDV strain LaSota, SPF eggs and anti-NDV serum was
provided by the Harbin Veterinary Research Institute, Chinese
Academy of Agricultural Sciences. Human PBMCs were isolated from
peripheral blood of volunteer donor with density gradient
centrifugation over Histopaque-1077 (Sigma-Aldrich, St. Louis, MO,
USA). Cells were collected and washed twice in RPMI-1640 medium,
and then were resuspended at a final density of 106/ml
in RPMI-1640 medium supplemented with 10% heat-inactivated fetal
calf serum (FCS) both from Invitrogen. Con-A stimulations were
performed in 24-well plates using 1×106 whole PBMCs with
5 µg/ml Con-A (Sigma-Aldrich) in a final volume of 2 ml.
Materials
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tet-razolium bromide
(MTT) was purchased from Amresco (Solon, OH, USA). Hoechst 33342
were obtained from Sigma-Aldrich. All PCR primers were purchased
from Shanghai Sangon Biological Engineering Technology and Services
Co., Ltd. (Shanghai, China). Total RNA from viral or tumour cells
were extracted with TRIzol reagent (Invitrogen). Anti-cleaved
caspase-3 was from ImmunoWay Biotechnology (Newark, DE, USA).
Anti-IFN-λ1 was from Santa Cruz Biotechnology, Inc. (Santa Cruz,
CA). Anti-IFNλ-R1 was from R&D Systems, Inc. (Minneapolis, MN,
USA). HRP-conjugated goat anti-rabbit, HRP-conjugated goat
anti-mouse and FITC-conjugated goat anti-mouse antibodies were
purchased from CWBio (Shanghai, China). HRP-conjugated goat
anti-rabbit, HRP-conjugated goat anti-mouse, and FITC-conjugated
goat anti-mouse antibodies were purchased from CWBio.
HRP-conjugated rabbit anti-chicken antibody was from EarthOx Life
Sciences (Millbrae, CA, USA), and Cy3-conjugated rabbit
anti-chicken antibody was purchased from KPL, Inc. (Gaithersburg,
MD, USA). The polyvinylidene difluoride (PVDF) membrane and
Luminata were provided by Millipore (Billerica, MA, USA). Trypsin,
and EDTA-2Na were offered by Gibco. A mitochondrial transmembrane
potential analysis kit was from Nanjing KeyGen Biotech Co., Ltd.
(Nanjing, China). All other supplies for cell culture were obtained
from Corning Costar (Corning, NY, USA).
Construction of full-length NDV plasmid
and virus rescue
To construct a full-length recombinant genomic cDNA.
The cDNA of the IFN-λ1 genes was amplified from a previous study
(25). The primers for
amplification of human IFN-λ1 gene are shown in (Table I). These primers included the gene
start and termination sequences of the NDV genome and the Kozak
sequence. The amplified human IFN-λ1 gene was inserted into plasmid
pBluescriptII Ks(+) then tested with nucleotide sequence analysis,
the newly recombinant plasmid was named as pBS. IFN-λ1. The
amplified human IFN-λ1 gene was digested by PmeI and then
inserted into the NDV genome cDNA through a unique PmeI site
in the P-M intergenic region at nucleotide position 3165 of the NDV
genome (8) to build the new
recombinant plasmid named pBRN.hIFN-λ1 (Fig. 2).
 | Table IPrimer sequences used in the
study. |
Table I
Primer sequences used in the
study.
| Gene name | Primer sequence
(5′–3′)
|
|---|
| Forward | Reverse |
|---|
| IFN-λ1 |
GACTgtttaaacTTAGAAAAAATACGGGTA
GAAgtgccaccatggctgcagcttggaccgt |
GACTgtttaaactcaggtggactcagggtgggttg |
| IFNLR1 |
ACCTATTTTGTGGCCTATCAGAGCT |
CGGCTCCACTTCAAAAAGGTAAT |
| NDV-PMEI |
GGAAATCAGGAAAATCAAGCGCCT |
AGAATCAAAGTACAGCCCAAT |
The rescue of recombinant NDV viruses from cloned
cDNA was as follows: BSR cells infected with A modified virus
expressing T7 RNA polymerase at a multiplicity of infection (MOI)
of 1.0 and then transfected with pBowing in a six-well plate with
pBRN.hIFN-λ1 together with 3 helper plasmids [pBS-NP (0.4
µg), pBS-P (0.2 µg), and pBS-L (0.2 µg)].
After 16 h of incubation at 37°C, the medium was replaced with 2 ml
Opti-MEM (Invitrogen) containing 0.5 µg of TPCK-trypsin and
the cells were incubated for another 3 days at 37°C. The
supernatant was then inoculated into the allantoic cavities of
9-day-old embryonated SPF eggs. After 72 h of incubation at 37°C,
the allantoic fluid was harvested and the virus was identified by
hemagglutination assay using 1% chicken red blood cells. The
resultant recombinant viruses were designated as rL-hIFN-λ1.
Growth characteristics of the recombinant
viruses
Virus growth was determined both in cell culture and
embryonated chicken eggs. Confluent monolayers of SGC cells were
infected with rl, rL-hIFN-λ1 in 96-well plates at a MOI of 1.0.
Every 12-h post-infection, the cellular monolayers of SGC cells
were harvested by freeze-thawing three times. Virus titers were
determined as 50% tissue culture-infective dose
(TCID50). Two hundred microliters of the recombinant
virus dilution containing 104 TCID50 per ml
was inoculated into the allantoic cavity of 9-day-old embryonated
chicken eggs. After three days, the allantoic fluids of inoculated
eggs were harvested and clarified by centrifugation at 1,000 × g
for 10 min. Supernatants were used for titration as described
above.
Immunofluorescence analysis
Tumour cells were cultured in 24-well plates for 24
h as previously described, infected with rL-hIFN-λ1 at a MOI of
1.0, treated with NDV and phosphate-buffered saline (PBS) as
controls. Then, at 24-h post-infection, the cells were fixed in 4%
paraformaldehyde at 4°C overnight, followed by immunofluorescence
staining with antibodies against NDV, IFN-λ1 and Hoechst 33342
staining. These stained SGC cells were monitored using
immunofluorescence microscopy.
MTT analysis
To measure the proliferation level, the survial of
cells was assessed with MTT with six replicates for NDV, rL-hIFN-λ1
and PBS. At 12, 24, 36, 48, 60 and 72-h post-infection, cell
survival was determined by incubating the cells with MTT.
Absorbance at 490 nm was determined with ELISA microplate readers
(BioTek Instruments, Inc., Winooski, VT, USA).
RNA isolation and RT-PCR analysis
Total RNA of virus or tumor cells was extracted from
the cultured cells using TRIzol reagent. First-strand cDNA
synthesis was performed using oligo(dT) primers and M-MLV reverse
transcriptase. The primer set for the IFN-λ1 gene and the IFNLR1
gene were shown in (Table I). All
the procedures were based on manufacturer's instructions.
TUNEL assay
Cells were cultured in 24-well plates with slides
and 10% FBS DMEM at 37°C with 5% CO2. Then, the SGC
cells were infected with rL-hIFN-λ1 and NDV within the logarithmic
growth phase and fixed in 4% paraformaldehyde. After fixing, the
cells were stained according to the manufacturer's instructions.
The slides were observed and imaged under an optical microscope.
The apoptotic index (AI) was calculated as the number of apoptotic
cells/(the number of apoptotic cells + the number of non-apoptotic
cells) × 100%.
Enzyme-linked immunoabsorbent assay
Gastric adenocarcinoma cells in six-well plates were
infected with NDV or rL-hIFN-λ1 virus at MOI of 1.0. Then the
supernatant was harvested at 0, 6, 12, 24, 36 and 48-h
post-infection. The expression levels of IFN-λ1 in the supernatant
were measured by means of enzyme immune assay as described by human
IFN-λ1 platinum kit (eBioscience, San Diego, CA, USA). The OD450 of
the samples were determined and plotted as a standard curve. The
standard curve was generated by serially diluting the stock enzyme
IFN-λ1 in dilution buffer supplied by the kit.
To measure the cytokine protein, cytokine levels
within supernatants was measured with ELISA. Antibodies for IFN-λ1,
and IFN-γ were purchased from eBioscience and for IL-13 from
R&D Systems, Inc. Manufacturer's instructions were followed.
Standards and culture supernatants were plated in triplicate.
Western blot assay
SGC and AGS cells with or without treatment were
lysed in RIPA lysis buffer with a protease inhibitor cocktail
(Santa Cruz Biotechnology, Inc.). The protein concentrations were
measured using a BCA kit (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). An equal amount of protein from each sample was loaded
onto a 10% polyacrylamide gel and separated by electrophoresis.
Then, the proteins were transferred to PVDF membrane (Millipore,
Temecula, CA, USA). The membranes were blocked for 1 h in 5% BSA,
incubated with primary antibodies against specific proteins (i.e.,
caspase-3 for apoptosis, anti-NDV for infection, anti-hIFN-λl for
expression of recombiant gene, anti-IFNLR1 for detection of IFNλ-l
receptor components and β-actin as a control) and then incubated
with HRP-conjugated secondary antibodies. The protein bands were
scanned using a Typhoon 9400 variable mode imager (Amersham
Biosciences, Buckinghamshire, UK) and detected by Pierce ECL Plus
substrate (Thermo Fisher Scientific, Inc.).
Flow cytometry assay using Annexin V
Annexin V/propidium iodide (PI) double-staining was
used to detect membrane events according to the manufacturer's
instructions (Biotech, Beijing, China). Flow cytometric analysis of
labeled cells was performed using a FACSort flow cytometer
(FACSCalibur) and data were analyzed using CellQuest software (both
from BD Biosciences, Franklin Lakes, NJ, USA). The cytogram of the
four quadrants in the figure was used to distinguish normal
(Annexin V−/PI+), early apoptotic (Annexin
V+/PI−), late apoptotic (Annexin
V+/PI+), and necrotic cells (Annexin
V−/PI+). The sum of early and late apoptosis
was presented as total apoptosis. Finally, staining of the cells
was observed with a fluorescence microscope. All experiments were
carried out in triplicate.
Immunoelectron microscopy
Purified virus particles were bound to 200-mesh
Formvar carbon-coated nickel grids (Electron Microscopy Science,
Hatfield, PA, USA). For immunolabeling, grids were blocked in PBS
containing 2% globulin-free BSA (Sigma-Aldrich) and incubated with
chicken anti-NDV polyclonal IgG. Grids were then washed in blocking
solution and incubated in goat anti-chicken IgG conjugated to 10-nm
gold beads (Sigma-Aldrich). The grids received a final wash,
followed by negative staining with 1% phosphotungstic acid. They
were examined under a model H7500 transmission electron microscope
(Hitachi High Technologies America, Inc., Schaumburg, IL, USA) at
80 kV. Images were obtained by using an XR100 digital camera system
(Advanced Microscopy Techniques, Danvers, MA, USA).
Statistical analysis
The data comparisons were performed using one-way
analysis of variance (ANOVA) in SPSS v17.0 software. p<0.05 and
p<0.01 were considered statistically significant. All
experiments were repeated at least three times.
Results
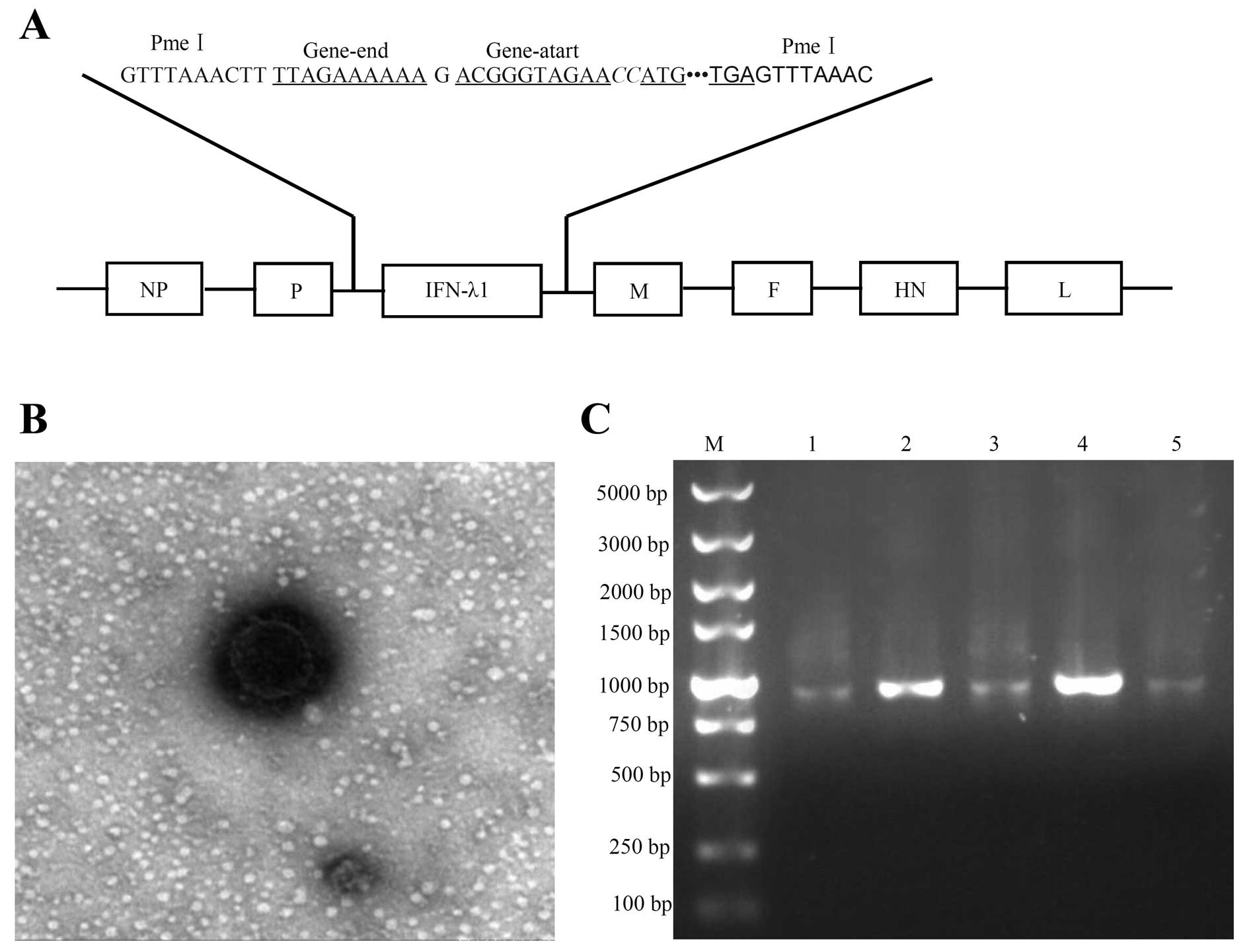
Generation of recombinant NDV LaSota
strain expressing human IFN-λ1 gene
Recombinant NDV expressing the human IFN-λ1 was
generated by inserting the IFN-λ1 gene in the genomic cDNA of the
NDV LaSota strain (Fig. 1A). For
virus recovery, the heterogeneous gene carrying plasmid was
transferred into 9–11 days old SPF embryonated chicken eggs for
efficient virus propagation. After incubation for 5 days, the
allantoic fluids were harvested and analyzed in a rapid plate HA
test using chicken erythrocytes and observed by electron microscopy
(Harbin Veterinary Research Institute) (Fig. 1B). The obtained viruses were named
as rL-hIFN-λ1. The presence and accuracy of the inserted IFN-λ1
gene, the sequences from rL-hIFN-λ1 genome were confirmed by
nucleotide sequence analysis (data not shown), the sequences
contained whole IFN-λ1 genome were cloned by RT-PCR (Fig. 1C) by primers NDV-PMEI (Table I) set above and blow the PMEI set on
NDV. The results showed they represented the correct sequences
successfully inserted into the NDV genome, and mutation did not
occur in rL-hIFN-λ1.
Growth characteristics of the recombinant
virus
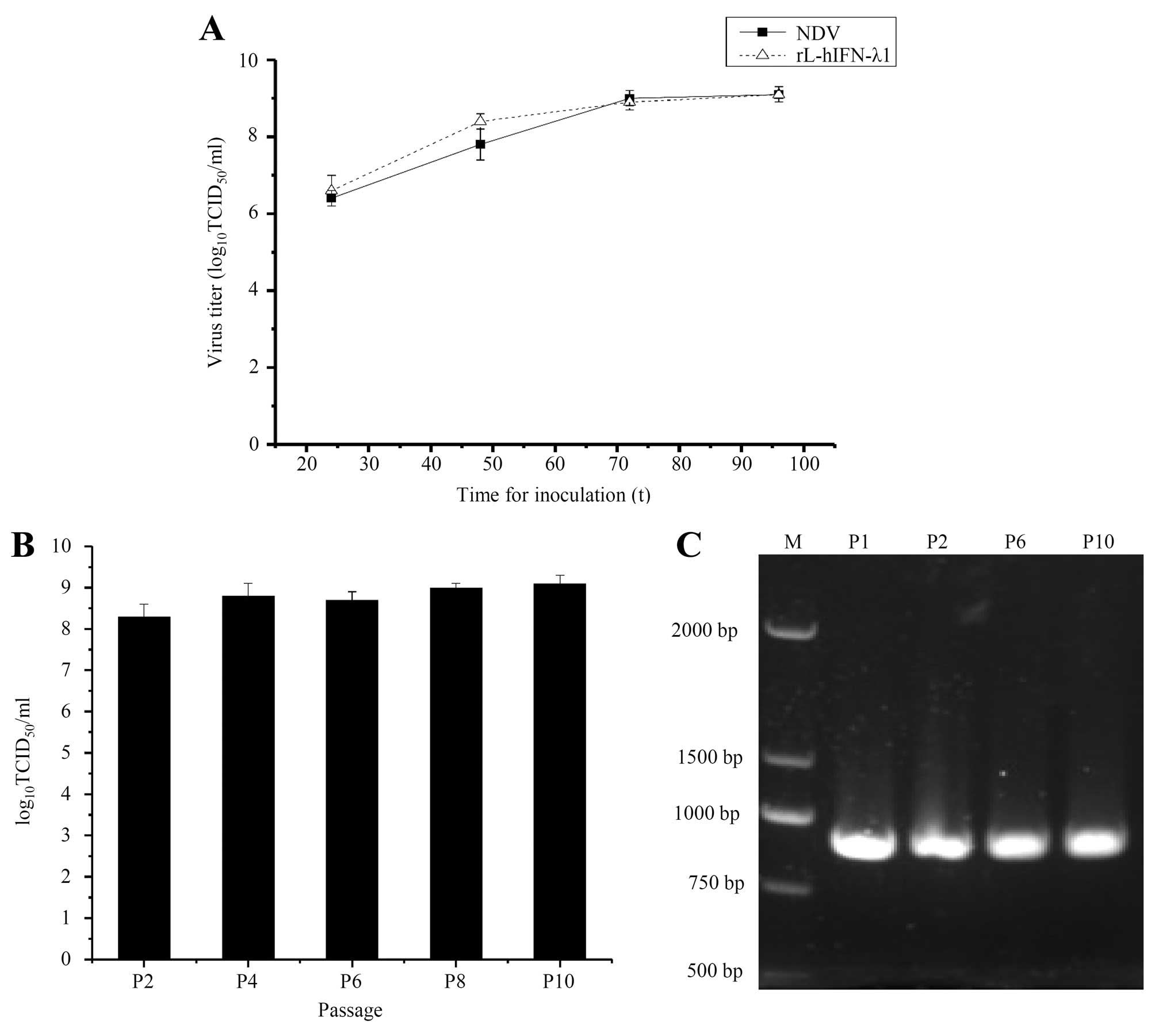
To compare the growth characteristics of the
recombinant viruses rL-hIFN-λ1 and NDV, viral titers of the
supernatants collected from infected SGC cells at 0, 24, 36, 48 and
72 h were determined by TCID50. The growth properties of
rL-hIFN-λ1 in the SGC cells were similar to those of the parental
virus NDV (Fig. 2A). The result
demonstrated that the recombinant viruses had mostly retained the
growth characteristics of the parental virus in the SGC cells.
The allantoic cavities of 10-day-old embryonated SPF
chicken eggs were inoculated with 104 TCID50
of rL-hIFN-λ1. The allantoic fluid was harvested after 4-day
incubation. The viral titers was determined in duplicate by
end-point titration. The results of the replication of rL-hIFN-λ1
indicated that the amount of the recombinant virus remains similar
after 10 passages (Fig. 2B). After
10 passages, the presence of the human IFN-λ1 gene was confirmed by
RT-PCR (Fig. 2C) and nucleotide
sequence analysis (data not shown).
Human gastric carcinoma cell lines and
human peripheral blood mononuclear cells express IFN-λ1 specific
receptor
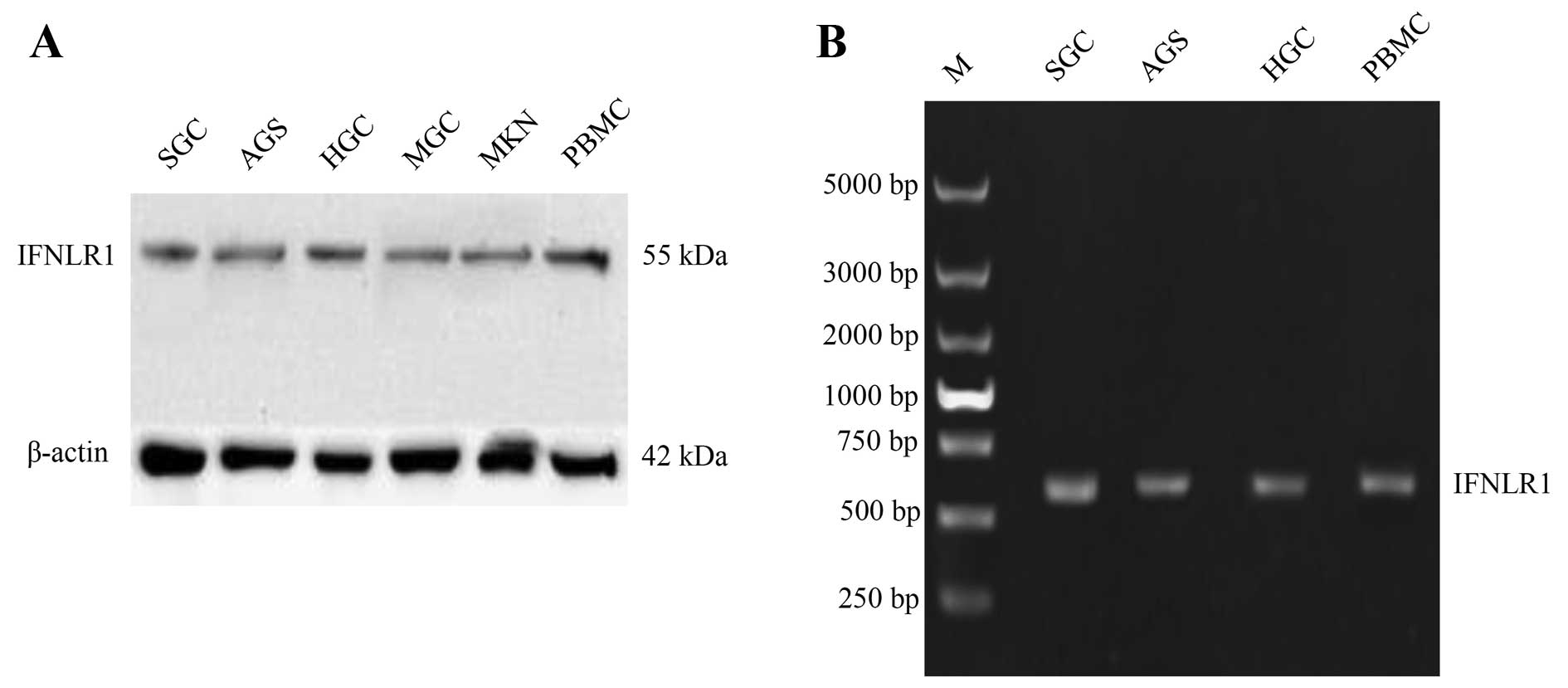
To determine the expression of IFN-λ receptor
specific receptor IFNLR1 in human gastric cancer cell lines. We
used RT-PCR assays and western blot analysis to analyze mRNA and
protein expression of IFNLR1, respectively. The IFNLR1 was
expressed in all examined gastric cancer cell lines, while the
expression level of mRNA or protein was divided vividly among cell
lines (Fig. 3), the SGC and AGS
gastric cells expressed more IFNLR1 compared with other gastric
tumor cells. For this reason we chose these two cell lines for our
further study. The IFN-λ receptor IFNLR1 expressed in gastric
cancer cell lines showed potential as a target for IFN-λ1 antitumor
therapy. We found that PBMCs (human peripheral blood mononuclear
cells) also expressed IFNLR1, which allowed us to study the
antitumor immune response of rL-hIFN-λ1.
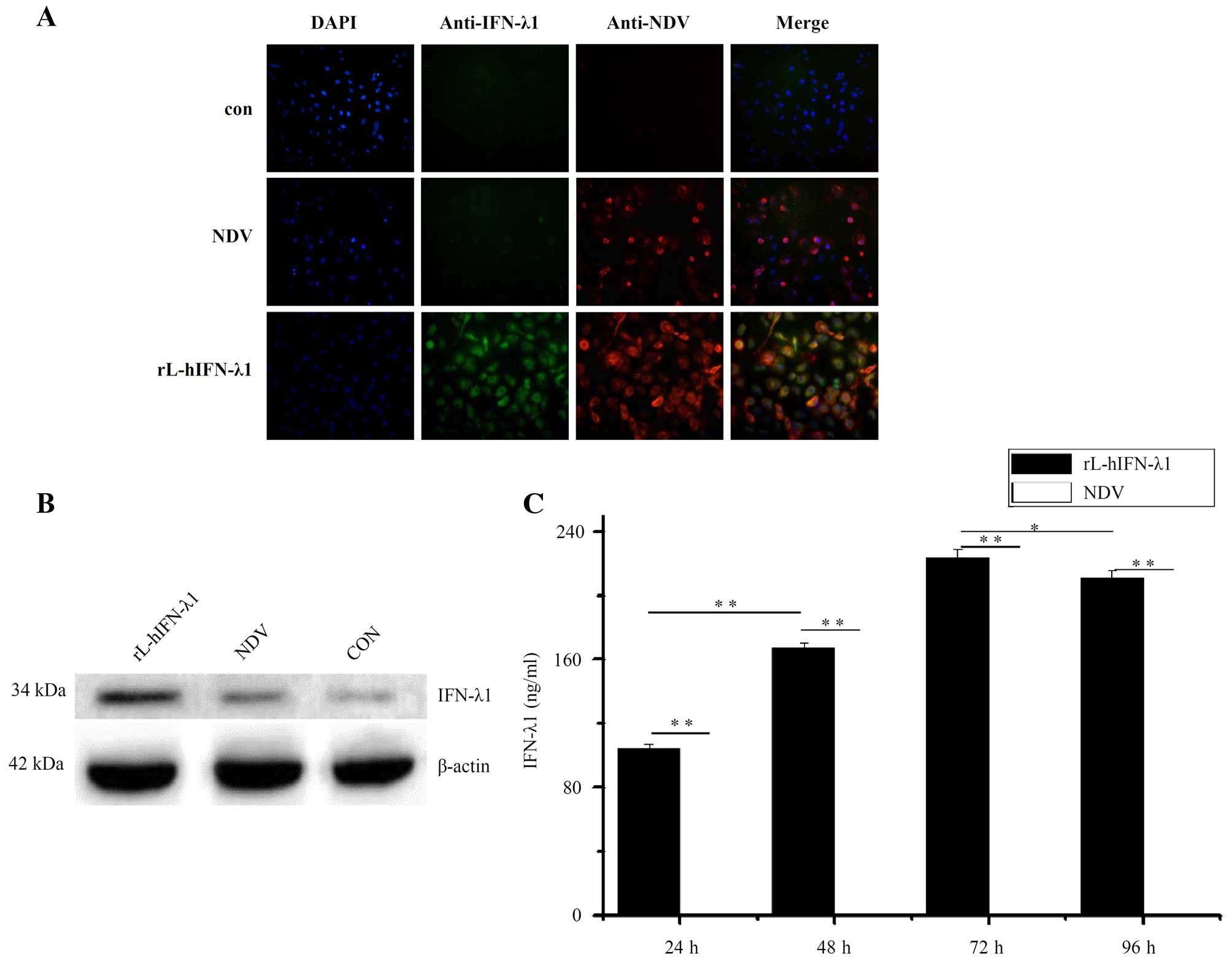
Expression of IFN-λ1 by
rL-hIFN-λ1-infected gastric adenocarcinoma cells
To define whether the IFN-λ1 gene integrated in the
NDV genome, the infected gastric tumor cell lines SGC were tested
for production of the protein IFN-λ1. The presence of IFN-λ1 genes
in the viral genome and expression in virus-infected cells were
confirmed as positive for all passage generations using indirect
immunofluorescence (Fig. 4A) and
western blot analysis (Fig. 4B). In
the indirect immunofluorescence the IFN-λ1 protein (green) was
highly expressed in the rL-hIFN-λ1-infected group, while the NDV
protein (red) was expressed in both the rL-hIFN-λ1-infected group
and NDV-infected group. Neither IFN-λ1 nor NDV protein was
expressed in the control group.
To define whether the IFN-λ1 gene integrated in the
NDV genome leads to the production of biologically active IFN-λ1 in
supernatants of rL-hIFN-λ1-infected gastric tumor cells, we
measured the quantitation of IFN-λ1 by ELISA. The supernatants of
tumour cells were harvested 24, 48, 72 and 96 h after the
infection. The IFN-λ1 expression reached the highest level at 72 h
to 223 ng/ml in the supernatants of rL-hIFN-λ1-infected SGC cells,
whereas the SGC cells infected with NDV did not release any IFN-λ1
into the supernatants (Fig 4C).
The proliferation changes of SGC cells
infected with rL-hIFN-λ1
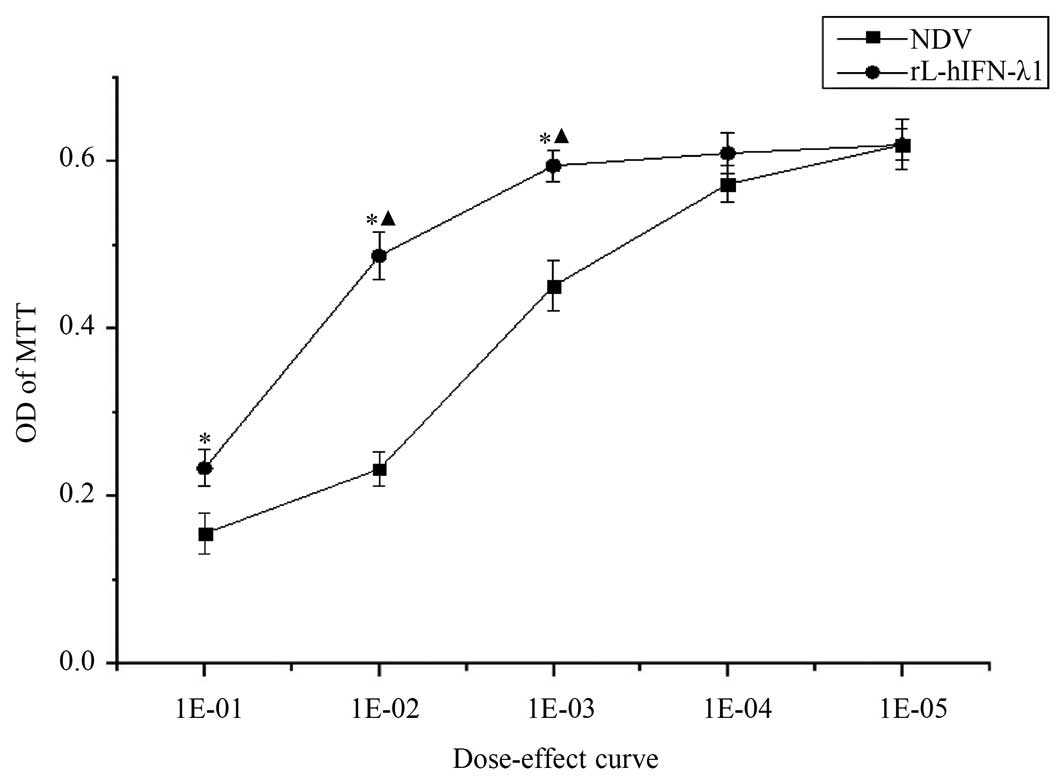
To elucidate the proliferation changes of
rL-hIFN-λ1-infected gastric adenocarcinoma cells, MTT assay was
utilized to portray dose-response curves. After 24 h of rL-hIFN-λ1
or NDV infection, the gastric tumour cells were assessed by MTT
assay. OD of MTT in the rL-hIFN-λ1-infected group was weaker
compared with that in the NDV-infected group, suggested that the
rL-hIFN-λ1-infected group had a greater inhibition ratio. Moreover,
the inhibition ratio increased over the dose after infection
(Fig. 5).
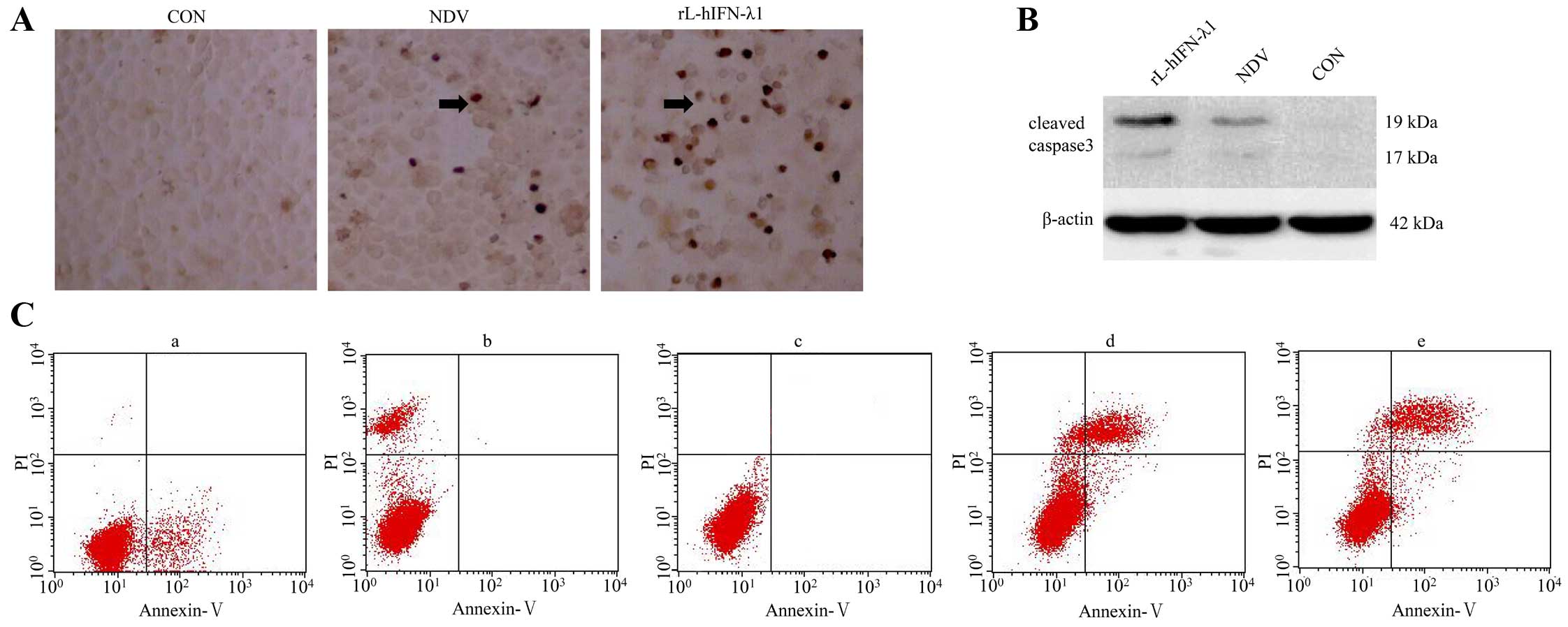
rL-hIFN-λ1 induces apoptosis in gastric
tumor cells
The recombinant virus rL-hIFN-λ1 was designed for
cancer therapeutics, and apoptosis was measured in gastric
carcinoma cell lines.
TUNEL assay kit was used for monitoring apoptosis.
The results revealed that the number of apoptotic cells and the AI
was much higher in the rL-hIFN-λ1 compared with NDV group, the
negative control group showed much less apoptosis (p<0.01)
(Fig. 6A).
Furthermore, the levels of cleaved caspase-3, a key
protein to indicate apoptosis, were measured by western blot
analysis at 24 h after virus infecting tumour cell lines. The
expression levels of cleaved caspase-3 proteins were upregulated in
the rL-hIFN-λ1 group compared with the other NDV group and the
negative control group (Fig.
6B).
Flow cytometry analysis showed that early apoptosis
of cells was higher in the rL-hIFN-λ1-infecting group than that in
NDV group, early apoptosis was not detected in the control group
(Fig. 6C).
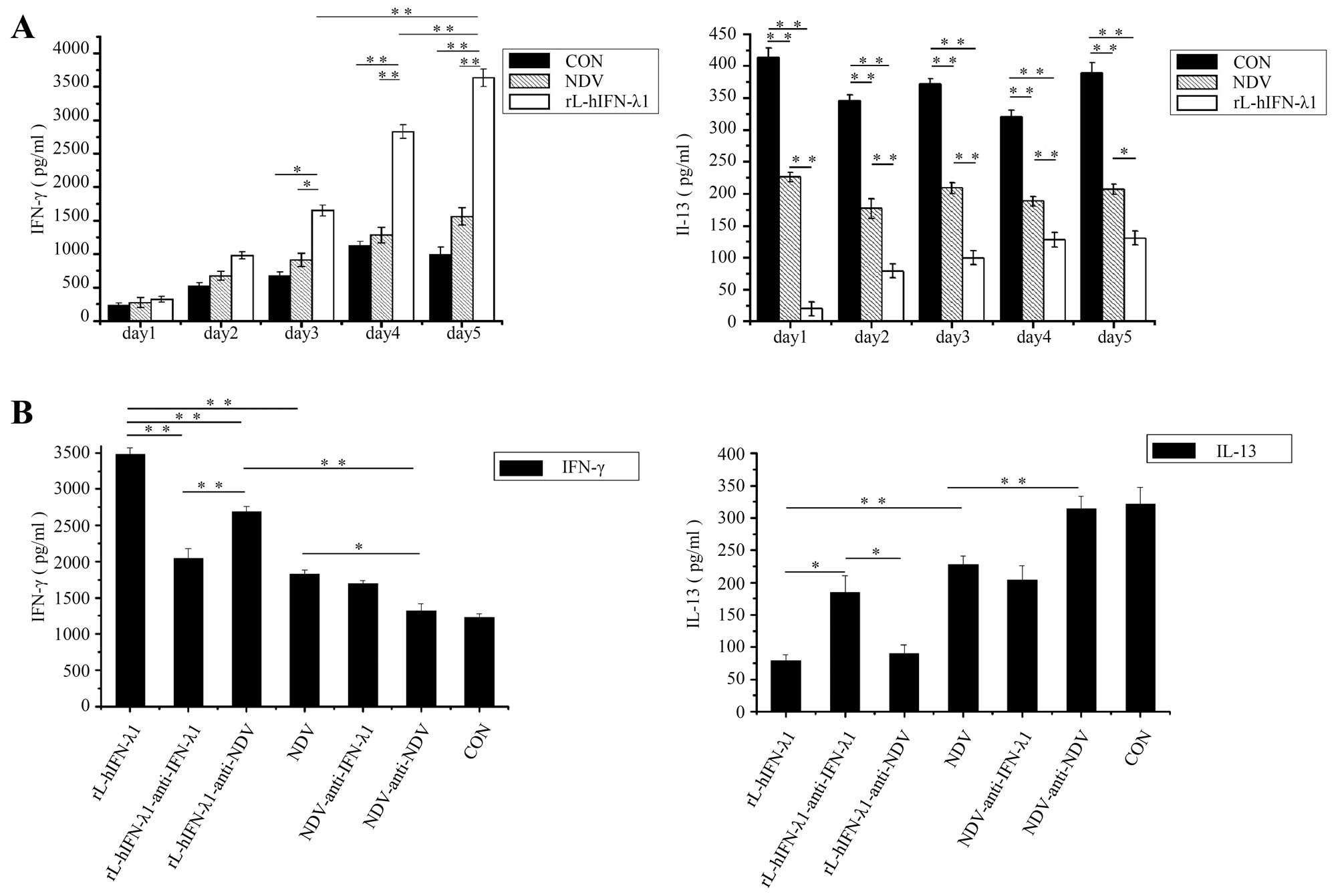
rL-hIFN-λ1 modulates the human Th1/Th2
response in the tumor microenvironment
Th1/Th2 balance is confirmed to play an important
role in tumor growth, the high expression of Th1 response and
reduction is capable of inducing antitumor immunity to prevent
cancer cell lines in preclinical study (14,27)
and gastric cancer in clinical research (28). To determine whether rL-hIFN-λ1 could
elevate the secretion of chemokines of tumor microenvironment to
promote antitumor immune responses, SGC cells were infected with
rL-hIFN-λ1 or NDV, after cultured for 72 h, supernatants were
collected to stimulate PBMCs for 5 days, then the production as
quantified of T-cell cytokines IFN-γ (representative of Th1
responses) and IL-13 (representative of Th2 responses) by ELISA
everyday. Our studies revealed that the Th1 cytokine IFN-γ
increased significantly and the Th2 cytokine IL-13 was highly
decreased in the rL-hIFN-λ1 group, as compared with NDV and control
groups (p<0.01) (Fig. 7A). To
confirm whether the Th1/Th2 immune response change was related to
cytokine IFN-λ1 or the NDV viral, anti-IFN-λ1 or anti-NDV was added
during the cell culture with the former supernatants, then cytokine
levels were tested after PBMCs cultured for 5 days. The Th1
response was enhanced, and the Th2 response inhibited much more in
the anti-IFN-λ1 group than in the anti-NDV group, NDV group and
control group showed no significant change. The results
demonstrated that the tumor microenvironment cytokine skewed
towards a Th1 response duo to the high dose of IFN-λ1 expressed by
the rL-hIFN-λ1-infected tumour cells (Fig. 7B).
Discussion
Oncolytic viral therapy is a method that harnesses
the natural ability of a virus to infect, duplicate and lyse a host
cell as part of its natural life cycle (29). NDV, an oncolytic virus, has been
confirmed to possess the capablity to inhibit malignant cells via
multiple mechanisms (30). In
recent years, NDV has been shown to induce the immune system to
eliminate tumor cells (31), and
NDV strains can selectively replicate up to 10,000 times better in
tumor cells than in normal cells (32). With the development of reverse
genetics, modification of the viral genome for NDV as well as
introduction of foreign sequences has become possible (33).
IFN-λ1 (IL-29), a recently discovered cytokine,
accompanies type I IFN in the signaling pathways and differs wildly
in tissue responsiveness. It is known that the epithelial cells of
most tissues express IFNLR1 and are responsive to IFN-λ in mice
(34,35). Most of the tumor cells are
epithelial in origin. The tumor cells express IFNLR1 and are
responsive to IFN-λ1. On the other hand, IFN-λ1 receptor also
expressed in both naive and memory CD4+ T cells
(22). T cells are capable of
responding to IFN-λ1 with altering cytokine production, it allows
IFN-λ1 to alter the balance of Th1 and Th2 immune responses, which
could be potential agents for cancer therapy (27,36,37).
IFN-λ1 has also been shown to exert antitumor effects in both
murine and human models (24).
Due to the above advantages of tumour therapy, we
inserted human IFN-λ1 gene into the NDV viral genome, in such a way
that the viral infection of tumor cells leads to the expression of
IFN-λ1. We propose that the recombinant virus rL-hIFN-λ1 may have
antitumor therapy potential. At the start of this study, there was
no study on genetically engineering recombined NDV expressing
IFN-λ1 genes to improve the effect of cancer therapy.
In the present study, we confirmed the stability of
growth characteristics of our recombinant virus was as much as the
NDV virus passing 10 generations in the embryonated SPF eggs. The
results have also shown the high dose stable expression of IFN-λ1
gene in rL-hIFN-λ1 infected tumour cell lines at 24 h reaching the
highest levels at 72 h.
In recent years, some studies have confirmed
apoptosis as the dominant key to NDV-related cell death (29,38–41).
It was demonstrated in our previous study that NDV caused gastric
adenocarcinoma SGC-7901 and AGS cell death via apoptosis (42). The mechanism behind NDV oncolytic
effect have been investigated. It was recently found that its
apoptosis inducing effect is presumably exerted through ER-mediated
cellular stress mechanism (43–45).
Our previous study also found in addition to apoptosis, that NDV
can also induce autophagy, ERs and mitochondrial dysfunction
(42).
The new generated recombinant NDV LaSota strain is
designed as a potential candidate for a viral vector in cancer
therapy in human, we further found that the high levels of IFN-λ1
receptor IFNLR1 was expressed in the gastric tumor cell lines SGC
and AGS, which indicated that gastric cancer could be a potential
target for IFN-λ1 and rL-hIFN-λ1 therapy. The TUNEL assay, western
blot assay and the Annexin V flow cytometric analysis all confirmed
that the number of apoptotic cells and the AI were markedly higher
in the rL-hIFN-λ1 and NDV groups compared with control group, and
rL-hIFN-λ1 group exhibited a higher AI compared with NDV
groups.
The high dose of cytokine expressed by the
rL-hIFN-λ1 infecting tumour cells had pro-tumor Th2 immune
responses and in favor of antitumor Th1 responses in PBMCs, which
generate an antitumour environment making the rL-hIFN-λ1 a
protential antitumor agent for immunotherapeutic approaches.
In conclusion, rL-hIFN-λ1 inhibited the growth of
gastric cancer cell lines which contained the IFNλ-R1 receptors and
accelerated apoptosis to a certain extent. The recombinant
rL-hIFN-λ1 virus modulated Th1/Th2 immune response to change the
tumor microenvironment to an antitumour cytokine which led to the
reducing of tumor growth. The present study is expected to provide
an experimental basis for further mechanism research and clinical
application of rL-hIFN-λ1 in gastric adenocarcinoma therapy,
considered that the recombinant NDV LaSota expressing human IFN-λ1
named as rL-hIFN-λ1 could be a potential viral agent for gastric
tumor therapy.
Acknowledgments
The authors would like to thank Zhijian Zhang and
Ai-Hua Gong from Jiangsu University (Jiangsu, China) for kindly
providing suggestions of the experiments performed. We thank all
our laboratory members for their help. The present study was
supported by the Natural Science Foundation of Jiangsu Province
(grant no. BK20151333) and the Social Development Fund of
Zhenjiang, China (SH2014046).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khalighinejad N, Hariri H, Behnamfar O,
Yousefi A and Momeni A: Adenoviral gene therapy in gastric cancer:
A review. World J Gastroenterol. 14:180–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Janke M, Peeters B, de Leeuw O, Moorman R,
Arnold A, Fournier P and Schirrmacher V: Recombinant Newcastle
disease virus (NDV) with inserted gene coding for GM-CSF as a new
vector for cancer immunogene therapy. Gene Ther. 14:1639–1649.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beutner U, Lorenz U, Illert B, Rott L,
Timmermann W, Vollmers HP, Müller-Hermelink HK, Thiede A and
Ulrichs K: Neoadjuvant therapy of gastric cancer with the human
monoclonal IgM antibody SC-1: Impact on the immune system. Oncol
Rep. 19:761–769. 2008.PubMed/NCBI
|
|
5
|
Krishnamurthy S and Samal SK: Nucleotide
sequences of the trailer, nucleocapsid protein gene and intergenic
regions of Newcastle disease virus strain Beaudette C and
completion of the entire genome sequence. J Gen Virol.
79:2419–2424. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bukreyev A and Collins PL: Newcastle
disease virus as a vaccine vector for humans. Curr Opin Mol Ther.
10:46–55. 2008.PubMed/NCBI
|
|
7
|
Bukreyev A, Skiadopoulos MH, Murphy BR and
Collins PL: Nonsegmented negative-strand viruses as vaccine
vectors. J Virol. 80:10293–10306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ge J, Deng G, Wen Z, Tian G, Wang Y, Shi
J, Wang X, Li Y, Hu S, Jiang Y, et al: Newcastle disease
virus-based live attenuated vaccine completely protects chickens
and mice from lethal challenge of homologous and heterologous H5N1
avian influenza viruses. J Virol. 81:150–158. 2007. View Article : Google Scholar :
|
|
9
|
Ge J, Tian G, Zeng X, Jiang Y, Chen H and
Bua Z: Generation and evaluation of a Newcastle disease virus-based
H9 avian influenza live vaccine. Avian Dis. 54(Suppl 1): 294–296.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elliott S, Egrie J, Browne J, Lorenzini T,
Busse L, Rogers N and Ponting I: Control of rHuEPO biological
activity: The role of carbohydrate. Exp Hematol. 32:1146–1155.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sinclair AM and Elliott S:
Glycoengineering: The effect of glycosylation on the properties of
therapeutic proteins. J Pharm Sci. 94:1626–1635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheppard P, Kindsvogel W, Xu W, Henderson
K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C,
Roraback J, et al: IL-28, IL-29 and their class II cytokine
receptor IL-28R. Nat Immunol. 4:63–68. 2003. View Article : Google Scholar
|
|
13
|
Sommereyns C, Paul S, Staeheli P and
Michiels T: IFN-lambda (IFN-lambda) is expressed in a
tissue-dependent fashion and primarily acts on epithelial cells in
vivo. PLoS Pathog. 4:e10000172008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haabeth OA, Lorvik KB, Hammarström C,
Donaldson IM, Haraldsen G, Bogen B and Corthay A: Inflammation
driven by tumour-specific Th1 cells protects against B-cell cancer.
Nat Commun. 2:2402011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mohty AM, Grob JJ, Mohty M, Richard MA,
Olive D and Gaugler B: Induction of IP-10/CXCL10 secretion as an
immunomodulatory effect of low-dose adjuvant interferon-alpha
during treatment of melanoma. Immunobiology. 215:113–123. 2010.
View Article : Google Scholar
|
|
16
|
Pertl U, Luster AD, Varki NM, Homann D,
Gaedicke G, Reisfeld RA and Lode HN: IFN-gamma-inducible protein-10
is essential for the generation of a protective tumor-specific CD8
T cell response induced by single-chain IL-12 gene therapy. J
Immunol. 166:6944–6951. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tominaga M, Iwashita Y, Ohta M, Shibata K,
Ishio T, Ohmori N, Goto T, Sato S and Kitano S: Antitumor effects
of the MIG and IP-10 genes transferred with poly
[D,L-2,4-diaminobutyric acid] on murine neuroblastoma. Cancer Gene
Ther. 14:696–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang P, Yang X, Xu W, Li K, Chu Y and
Xiong S: Integrating individual functional moieties of CXCL10 and
CXCL11 into a novel chimeric chemokine leads to synergistic
antitumor effects: A strategy for chemokine-based
multi-target-directed cancer therapy. Cancer Immunol Immunother.
59:1715–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Egeter O, Mocikat R, Ghoreschi K,
Dieckmann A and Röcken M: Eradication of disseminated lymphomas
with CpG-DNA activated T helper type 1 cells from nontransgenic
mice. Cancer Res. 60:1515–1520. 2000.PubMed/NCBI
|
|
20
|
Müller-Hermelink N, Braumüller H, Pichler
B, Wieder T, Mailhammer R, Schaak K, Ghoreschi K, Yazdi A, Haubner
R, Sander CA, et al: TNFR1 signaling and IFN-gamma signaling
determine whether T cells induce tumor dormancy or promote
multistage carcinogenesis. Cancer Cell. 13:507–518. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shankaran V, Ikeda H, Bruce AT, White JM,
Swanson PE, Old LJ and Schreiber RD: IFNgamma and lymphocytes
prevent primary tumour development and shape tumour immunogenicity.
Nature. 410:1107–1111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai J, Megjugorac NJ, Gallagher GE, Yu RY
and Gallagher G: IFN-lambda1 (IL-29) inhibits GATA3 expression and
suppresses Th2 responses in human naive and memory T cells. Blood.
113:5829–5838. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sargurupremraj M, Pukelsheim K, Hofer T
and Wjst M: Intermediary quantitative traits - an alternative in
the identification of disease genes in asthma? Genes Immun. 15:1–7.
2014. View Article : Google Scholar
|
|
24
|
Stiff A and Carson Iii W: Investigations
of interferon-lambda for the treatment of cancer. J Innate Immun.
7:243–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bu X, Wang M, Zhang J, Liu J, Jia L, Liang
B and Yan Y: Recombinant adenovirus expressing hIFN-λ1 inhibits
gastric adenocarcinoma cell line SGC-7901 proliferation. Oncol
Lett. 11:287–292. 2016.PubMed/NCBI
|
|
26
|
Wyatt LS, Moss B and Rozenblatt S:
Replication-deficient vaccinia virus encoding bacteriophage T7 RNA
polymerase for transient gene expression in mammalian cells.
Virology. 210:202–205. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Burkart C, Arimoto K, Tang T, Cong X, Xiao
N, Liu YC, Kotenko SV, Ellies LG and Zhang DE: Usp18 deficient
mammary epithelial cells create an antitumour environment driven by
hypersensitivity to IFN-λ and elevated secretion of Cxcl10. EMBO
Mol Med. 5:967–982. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reichard KW, Lorence RM, Cascino CJ,
Peeples ME, Walter RJ, Fernando MB, Reyes HM and Greager JA:
Newcastle disease virus selectively kills human tumor cells. J Surg
Res. 52:448–453. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Foucault C, Mordant P, Grand B, Achour K,
Arame A, Dujon A, Le Pimpec Barthes F and Riquet M: Unexpected
extensions of non-small-cell lung cancer diagnosed during surgery:
Revisiting exploratory thoracotomies and incomplete resections.
Interact Cardiovasc Thorac Surg. 16:667–672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vähä-Koskela MJ, Heikkilä JE and Hinkkanen
AE: Oncolytic viruses in cancer therapy. Cancer Lett. 254:178–216.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hossain A, Radwan FF, Doonan BP, God JM,
Zhang L, Bell PD and Haque A: A possible cross-talk between
autophagy and apoptosis in generating an immune response in
melanoma. Apoptosis. 17:1066–1078. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lam HY, Yeap SK, Rasoli M, Omar AR, Yusoff
K, Suraini AA and Alitheen NB: Safety and clinical usage of
Newcastle disease virus in cancer therapy. J Biomed Biotechnol.
2011:7187102011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakaya T, Cros J, Park MS, Nakaya Y, Zheng
H, Sagrera A, Villar E, García-Sastre A and Palese P: Recombinant
Newcastle disease virus as a vaccine vector. J Virol.
75:11868–11873. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lasfar A, Abushahba W, Balan M and
Cohen-Solal KA: Interferon lambda: A new sword in cancer
immunotherapy. Clin Dev Immunol. 2011:3495752011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Steen HC and Gamero AM: Interferon-lambda
as a potential therapeutic agent in cancer treatment. J Interferon
Cytokine Res. 30:597–602. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jordan WJ, Eskdale J, Srinivas S, Pekarek
V, Kelner D, Rodia M and Gallagher G: Human interferon lambda-1
(IFN-lambda1/IL-29) modulates the Th1/Th2 response. Genes Immun.
8:254–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding S, Khoury-Hanold W, Iwasaki A and
Robek MD: Epigenetic reprogramming of the type III interferon
response potentiates antiviral activity and suppresses tumor
growth. PLoS Biol. 12:e10017582014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Elankumaran S, Rockemann D and Samal SK:
Newcastle disease virus exerts oncolysis by both intrinsic and
extrinsic caspase-dependent pathways of cell death. J Virol.
80:7522–7534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hrabák A, Csuka I, Bajor T and Csatáry LK:
The cytotoxic antitumor effect of MTH-68/H, a live attenuated
Newcastle disease virus is mediated by the induction of nitric
oxide synthesis in rat peritoneal macrophages in vitro. Cancer
Lett. 231:279–289. 2006. View Article : Google Scholar
|
|
40
|
Bian H, Fournier P, Peeters B and
Schirrmacher V: Tumor-targeted gene transfer in vivo via
recombinant Newcastle disease virus modified by a bispecific fusion
protein. Int J Oncol. 27:377–384. 2005.PubMed/NCBI
|
|
41
|
Lorence RM, Rood PA and Kelley KW:
Newcastle disease virus as an antineoplastic agent: Induction of
tumor necrosis factor-alpha and augmentation of its cytotoxicity. J
Natl Cancer Inst. 80:1305–1312. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bu XF, Wang MB, Zhang ZJ, Zhao YH, Li M
and Yan YL: Autophagy is involved in recombinant Newcastle disease
virus (rL-RVG)-induced cell death of stomach adenocarcinoma cells
in vitro. Int J Oncol. 47:679–689. 2015.PubMed/NCBI
|
|
43
|
Fábián Z, Töröcsik B, Kiss K, Csatary LK,
Bodey B, Tigyi J, Csatary C and Szeberényi J: Induction of
apoptosis by a Newcastle disease virus vaccine (MTH-68/H) in PC12
rat phaeochromo-cytoma cells. Anticancer Res. 21(1A): 125–135.
2001.PubMed/NCBI
|
|
44
|
Fábián Z, Csatary CM, Szeberényi J and
Csatary LK: p53-independent endoplasmic reticulum stress-mediated
cytotoxicity of a Newcastle disease virus strain in tumor cell
lines. J Virol. 81:2817–2830. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fábián Z, Vecsernyés M, Pap M and
Szeberényi J: The effects of a mutant p53 protein on the
proliferation and differentiation of PC12 rat phaeochromocytoma
cells. J Cell Biochem. 99:1431–1441. 2006. View Article : Google Scholar : PubMed/NCBI
|