Introduction
Resveratrol (3,5,4′-trihydroxystilbene) belongs to
the plant polyphenols from the group of stilbenes. Natural sources
of this substance are edible plants such as grapes, peanuts or
mulberries. Particularly high amounts of resveratrol can be found
in grape skins, therefore, it is considered to be the key compound
responsible for so-called 'French paradox'. In vitro, in
vivo and epidemiological studies show that there is a link
between red wine consumption in France and protection against
cardiovascular diseases, predominantly in individuals on a diet
that is high in saturated fats (1–3).
Because of its positive effect on human health, for many years
resveratrol has been of interest to many researchers all over the
world. Among its properties that deserve the highest attention are
anti-inflammatory, anti-viral, anti-bacterial, anti-aging and
anti-carcinogenesis activities, as well as reducing level of LDL
cholesterol. Inhibitory effect of resveratrol on all stages of
carcinogenesis was described. This was proved by numerous studies,
both pre-clinical (in vitro and in vivo) and clinical
(4–9). Resveratrol's mechanisms of action on
cancer cells are multidirectional. These can be direct interactions
by influencing certain genes, transcription factors and enzymes, as
well as indirect interactions affecting important biochemical
pathways in the cells. Dose, exposure time and cell type-dependent
anticancer effect of resveratrol was proved, among others, on cell
cycle, apoptosis, autophagy, adhesion and intercellular signalling,
detoxication and DNA repair processes (5,10–12).
Additionally, there have been reports that resveratrol can
sensitise cancer cells to cytostatics in various types of
chemotherapy resistance. Very low toxicity and protective effect on
normal cells are additional values of this polyphenol (13–16).
Multidrug resistance (MDR) is a serious problem in
chemotherapy. MDR phenomenon arises from insensitivity of cancer
cells to various types of drugs that often differ from each other
in structure and mode of action. Mechanisms of MDR can be of
extracellular nature, e.g. poor tumour vasculature or pH of
extracellular matrix, or intracellular. There are very many reasons
why cancer cells do not respond to chemotherapy. Usually,
intracellular mechanisms can be divided into classical and
atypical. The classical ones concern P-glycoprotein (P-gp), the
best known and the most often described ABC transporter associated
with MDR, which actively pump-out drug particles from the cell.
P-gp is multi-domain membrane protein having molecular mass of 170
kDa and encoded by ABCB1 gene. Overexpression of P-gp is the
main factor determining resistance of cancer cells to many
cytotoxic substances having different structures and properties
(17,18). Other causes of resistance are
considered 'atypical', however, it does not mean that they occur
only occasionally. Most of the time, several mechanisms of MDR are
active simultaneously. Examples are alterations in the level of
cellular pathways involved in various types of programmed cell
death (e.g. overexpression of anti-apoptotic proteins) or DNA
repair (e.g. proteins involved in mismatch repair, base or
nucleotide excision repair) and other molecular mechanisms (e.g.
level of topoisomerase II expression) (19–21).
Topoisomerase II (TopoII) is an enzyme playing an important role in
replication, transcription, recombination, as well as chromosome
structure and segregation. The main role of TopoII is cleavage of
both DNA strands and their movement, followed by ligation of
phosphodiester bonds. Therefore, it is molecular target point for
cytostatics. There are two isoforms of human TopoII, α and β
(22). Those enzymes are
overexpressed in numerous types of tumours. Unfortunately, lower
level of TopoII may cause resistance, particularly to anthracycline
group of drugs. It is also known that homozygous deletion of gene
encoding the α isoform of TOPO2A may significantly affect
the effectiveness of anthracycline therapy e.g. in breast cancer
(23,24).
In case of pancreatic cancer prognosis are
particularly poor, mainly because of late detection, rapid progress
of the disease and the resistance of cancer cells to chemotherapy
and radiotherapy (25,26). Morbidity to this type of cancer is
high, but survival is very low and in Europe it is estimated at 4.6
months from diagnosis. Recently it was proved that polyphenols,
including resveratrol, inhibit pancreatic cancer development at all
stages of tumour growth, during initiation, progression, metastasis
and invasion. Moreover, it may affect pancreatic cancer stem cells
by inhibition of pluripotency-maintaining factors and
epithelial-mesenchymal transition. Additionally it can sensitise
cancer cells to cytostatics (27,28).
The purpose of our studies was to evaluate in
vitro the effect of resveratrol on cells of human pancreatic
cancer, with particular attention being paid to the expression of
proteins responsible for resistance to cytostatics. Cellular models
used in the research are characterised by different mechanisms of
MDR, which gives the possibility to verify potential
multidirectional activity of resveratrol.
Materials and methods
Cell culture and drugs
Human pancreatic cancer cell line EPP85-181P (P),
parental cells and its drug-resistant derivatives were the in
vitro model system for the study. The cells were grown in L-15
medium (Lonza, Gdańsk, Poland) supplemented with 10% fetal bovine
serum (FBS; Lonza) and 10% FBS, 1 mM L-glutamine, 80 IE/l insulin,
6.25 mg/l fetuin, 2.5 mg/l transferrin, 1.1 g/l NaHCO3,
1 g/l glucose, 1% minimal essential vitamins (Sigma-Aldrich,
Darmstadt, Germany). The resistant cell line EPP85-181RDB (RDB) was
grown in the presence of 2.5 µg/ml daunorubicin (DB) and
EPP85-181RNOV (RNOV), 0.02 µg/ml mitoxantrone (MTX). Cell
culture was performed as previously described (29). In RDB cell line the basic mechanism
of resistance is the overexpression of P-gp, whereas in RNOV cell
line resistance is mainly caused by reduced level of TopoII. The
following agents were used: doxorubicin, mitoxantrone and
resveratrol (Sigma-Aldrich). The therapeutic dose of cytostatics
used in the experiments (the concentration of the cytostatic drug
in patient's blood 2 h after administration): DB 0.25 µg/ml
and MTX 0.02 µg/ml.
Cytotoxicity assay
In each assay, 1.5×104 cells of P, RDB
and RNOV lines were seeded in 96-well plates (TTP Techno Plastic
Products, Trasadingen, Switzerland) 24 h prior to the experiment.
Incubation with resveratrol (1–500 µM) was performed for 72
h. Cells were trypsinised and resveratrol cytotoxicity was examined
using sulforhodamine B (SRB) assay (30). Absorbance was quantified at 562 nm.
In order to determine IC50-value, the absorbance
difference of resveratrol untreated control cells was set to 100%.
Linear regressions were plotted and IC50-values were
calculated for each cell line.
Cell cycle analyses by flow cytometry
(FACS)
Cells of each cell line (2×105
cells/well) were cultured in 6-well tissue culture plates
(EuroClone, Milan, Italy) in specific Leibovitz L15 medium
(Sigma-Aldrich) for 24 h at 37°C, 5%, CO2. After 24 h,
cells were treated with resveratrol at a concentration of 30
µM (R30) and 50 µM (R50) for 72 h. Control and
resveratrol-treated cells were trypsinised with 0.25% Trypsin-EDTA
(Sigma-Aldrich), centrifuged (Thermo Fisher Scientific GmbH,
Dreieich, Germany) at 1,000 rpm for 5 min at room temperature, and
washed twice in PBS. Then, the cells were resuspended in ice-cold
PBS and fixed overnight in 70% ethanol at 4°C. Cells were pelleted
by centrifugation (1,000 rpm, 5 min, 4°C), washed twice in PBS and
resuspended in a small amount of phosphate-buffered saline at room
temperature. Samples of each cell line were mixed with propidium
iodide (PI) and RNase staining solution (Life Technologies,
Carlsbad, CA, USA) and incubated at 37°C in the dark for 30 min. PI
fluorescence was measured in the FL-2 channel of the BD FACSCanto
II flow cytometer (BD Biosciences, Temse, Belgium). Data from
minimum 20,000 events per sample were collected and calculated with
ModFit LT™ software, version 4.0.5 (Verity Software House, Inc.,
Topsham, ME, USA). The experiment was performed in 3 independent
replications.
Drug accumulation assay
Measurement of cellular daunorubicin accumulation
was performed by flow cytometry using FACSCanto II supported by BD
FACSDiva (version 6.1.3) analysis software (BD Biosciences, San
Jose, CA, USA).
The cells of the P and RDB cell lines were
transferred to 6-well plates (EuroClone), 4×105
cells/well. After 24-h incubation at 37°C and under 5%
CO2 atmosphere, the medium was removed from all wells.
Next, the culture medium was applied to the non-treated control
cells (C) and resveratrol at concentrations of R30 and R50
µM was added to the remaining wells. Cells were incubated
for 72 h at 37°C. Then daunorubicin 2.5 µg/ml was added and
after 2 h cells were harvested by trypsinisation, centrifuged
(1,000 rpm, 5 min, 24°C) and washed twice in PBS. Next, the cells
were resuspended in 200 µl ice-cold BSA buffer (1% bovine
serum albumin in 1X PBS) and stored on ice until the intracellular
fluorescence of daunorubicin was measured in the FL-2 channel. A
minimum of 100,000 cells were collected for each sample and the
experiments were performed in six independent replications. In
order to analyse the cell cycle, the total number of cells was
counted, obtaining the result of 20,000 events. Positive events,
from which the fluorescence signal was measured, represent the
population of cells that accumulated daunorubicin in their
interior. The experiment was performed in 6 independent
replications.
Immunofluorescence analyses
Each of the cell lines were cultured in densities of
6×103 cells/well on 8-well Merck Millicell EZ slides
(Merck Millipore, Darmstadt, Germany). After 24 h, cells were
treated with resveratrol (Sigma-Aldrich) at concentrations of R30
and R50 for 72 h. Afterwards, the slides were washed twice in PBS,
fixed in 4% formaldehyde in PBS (12 min, room temperature) and then
incubated with 0.2% Triton X-100 in PBS (10 min, room temperature).
The fixed cells were incubated overnight at 4°C with specific
antibodies. Detection of protein expression was performed by using
mouse monoclonal mAb against P-gp, clone C219 (1:2,000; Alexis
Biochemicals, Lausen, Switzerland), rabbit monoclonal TopoIIα,
clone D10G9 (Cell Signaling, Danvers, MA, USA) and rabbit
monoclonal TopoIIβ, clone EPR5377 (Novus Biologicals LLC,
Littleton, CO, USA). The primary antibodies were detected after 1 h
of incubation with a donkey anti-mouse or donkey anti-rabbit
secondary antibody, respectively, conjugated with Alexa Fluor 488
(Invitrogen, Carlsbad, CA, USA) at a dilution 1:2,000 in antibody
diluent (Dako, Glostrup, Denmark). Finally, the slides were washed
3 times in PBS and mounted on ProLong Gold Mounting Medium
(Invitrogen) with DNA intercalating dye
4,6-diamidino-2-phenylindole (DAPI) added to visualize the cell
nucleus. The analysis of the results was conducted under
fluorescent microscope, (Olympus BX51; Olympus, Tokyo, Japan)
magnification ×200. The data were collected using Cell-F software
(Olympus).
Western blot analyses
Cells were transferred to cell culture flasks (25
cm2), incubated for 72 h with resveratrol and/or
combinations of resveratrol and cytostatics at 37°C. Then, the
cells were harvested by trypsinisation and centrifuged (1,000 rpm,
5 min, 24°C), resuspended in PBS and washed twice.
Preparation of nuclear protein fraction for the
TopoIIβ evaluation: resveratrol-treated cells were suspended in
ice-chilled sterile isotonic buffer (50 mM Tris-HCl pH 7.6; 5 mM
MgCl2; 50 mM NaCl; 250 mM sucrose) and disrupted using
needle and syringe. After centrifugation (10 min, 12,000 g, 4°C)
pellet of cells was lysed in the ice-cold lysis buffer (50 mM
Tris-HCl pH 7.6; 250 mM NaCl; 1% Igepal NP-40; 0.5 mM PMSF and
protease inhibitor cocktail) and centrifuged as described above.
The supernatant was collected and protein concentration was
determined by BCA method (according to the manufacturer's protocol;
Thermo Fisher Scientific, Waltham, MA, USA).
Preparation of total cell lysate for the P-gp and
TopoIIα evaluation: whole cell extracts were prepared by lysing
resveratrol-treated cells in ice-cold lysis buffer (50 mM Tris-HCl,
pH 7.5; 250 mM NaCl; 0,1% Igepal NP-40; 0.5 mM PMSF and protease
inhibitor cocktail). After centrifugation (as described above), the
supernatant was collected and protein concentration was determined
by BCA method.
The cell lysates for the evaluation of TopoIIα and
TopoIIβ levels were denaturised (10 min, 95°C), while those for
P-gp were incubated for 20 min in room temperature and then for 10
min in 4°C. Equal amounts of proteins (30 µg) were subjected
to SDS-PAGE by using 10% Mini-PROTEAN TGX Ready Gels (Bio-Rad
Laboratories, Hempstead, UK) (31)
and transferred to a PVDF Immobilon-P membranes (Millipore,
Billerica, MA, USA) (32). After
blocking (P-gp: 1 h, room temperature, 1% BSA in TBS, 0.1%
Tween-20; TopoIIα: 1 h, room temperature, 4% BSA in TBS, 0.1%
Tween-20; TopoIIβ: overnight, 4°C, 4% BSA in TBS, 0.1% Tween-20),
membranes were incubated overnight at 4°C with specific antibodies:
the mouse anti-P-gp mAb (C-219, 1:300; Alexis Biochemicals, San
Diego, CA, USA), the rabbit anti-TopoIIα mAb (D10G9, 1:1,000; Cell
Signaling Technology) and the rabbit anti-TopoIIβ mAb (EPR5377,
1:10,000; Novus Biologicals). Horseradish peroxidase-labelled
secondary antibodies were incubated with the Immun-Star HRP
Substrate (Bio-Rad Laboratories), visualized with ChemiDoc XRS
Molecular Imager (Bio-Rad Laboratories) and normalized according to
the β-tubulin (the mouse mAb, ab6046; Abcam, Cambridge, MA, USA).
OD measurements of the protein bands were performed with the Image
Lab software (Bio-Rad Laboratories).
Statistical analysis
Statistical analysis was performed using the Prism
5.0 software (Graphpad Software, Inc., La Jolla, CA, USA). When two
groups of data were compared, the unpaired t-test was used. In
cases of 3 or more groups, the one-way ANOVA with post-hoc analysis
using the Dunnett's, Dunn's or Bonferroni multiple comparison tests
were applied to compare the data among tested cell lines and
experimental conditions. In all the analyses, the differences were
regarded as significant when P<0.05.
Results
Cytotoxicity and cell cycle
Based on resveratrol cytotoxicity tests,
IC50 was estimated for each of the studied cell lines to
be: P, 158 µM, RDB, 343 µM and NOV, 269 µM. On
the basis of statistical analysis it was determined that
significant (P<0.001) proliferation inhibition occurred for 30
µM resveratrol (in comparison to control), and then for 50
µM (in comparison to 30 µM). Those two
concentrations, 30 µM (R30) and 50 µM (R50), were
selected for further studies.
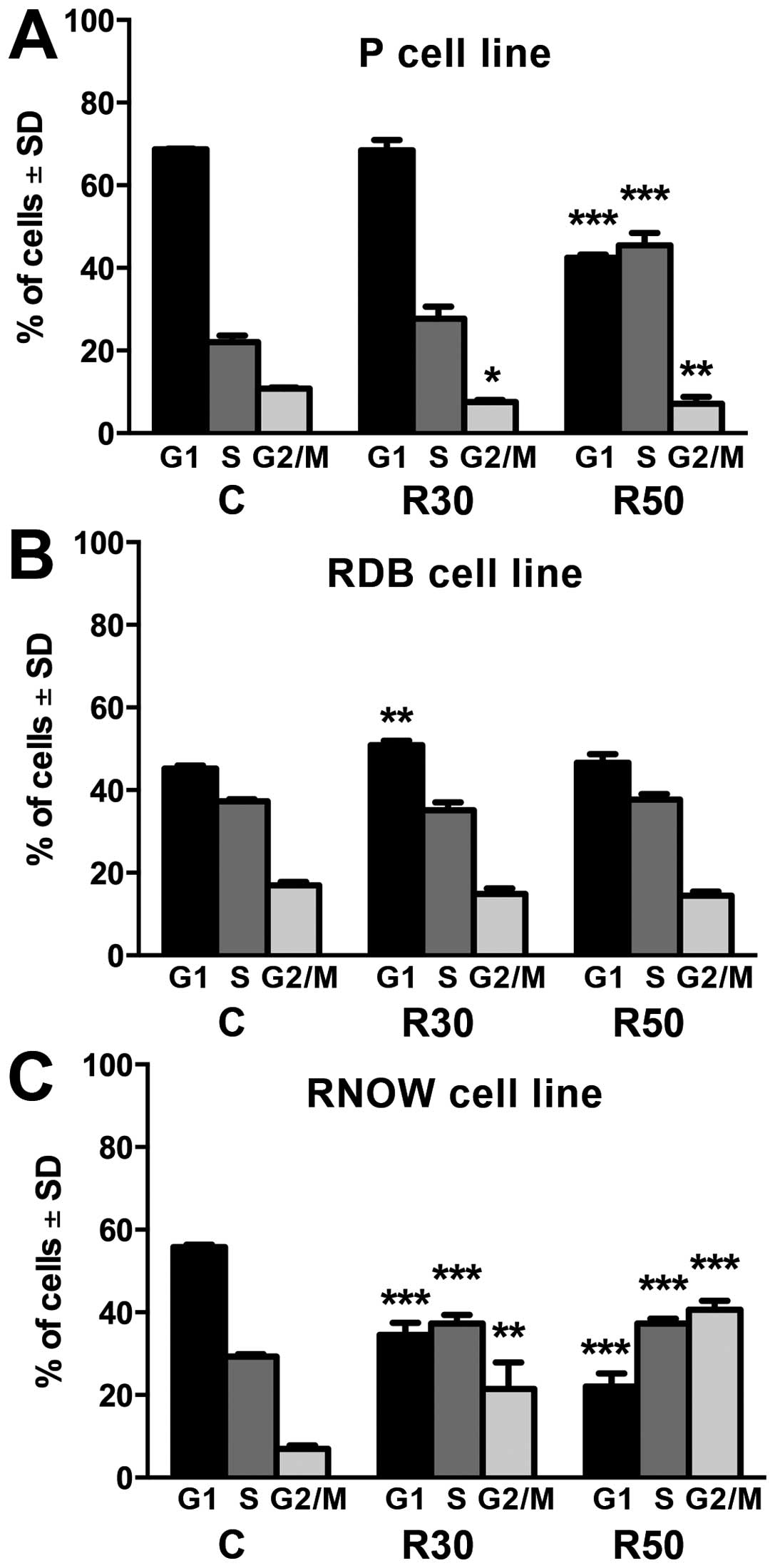
Cell cycle analysis (Fig. 1) showed that in case of cell line
sensitised upon administration of R50, the number of cells in S
phase increased in comparison to control (P<0.001) with
simultaneous decrease in other phases, particularly in G1. In
DB-resistant cell line (RDB), increase in the number of cells
arrested in G1 phase (P<0.01) upon incubation with R30 was
observed. In case of MTX-resistant line (RNOV), significant
increase in the number of cells in S phase (P<0.001) and G2/M
(P<0.01) was observed for R30 and R50 with simultaneous decrease
in G1 in comparison to control.
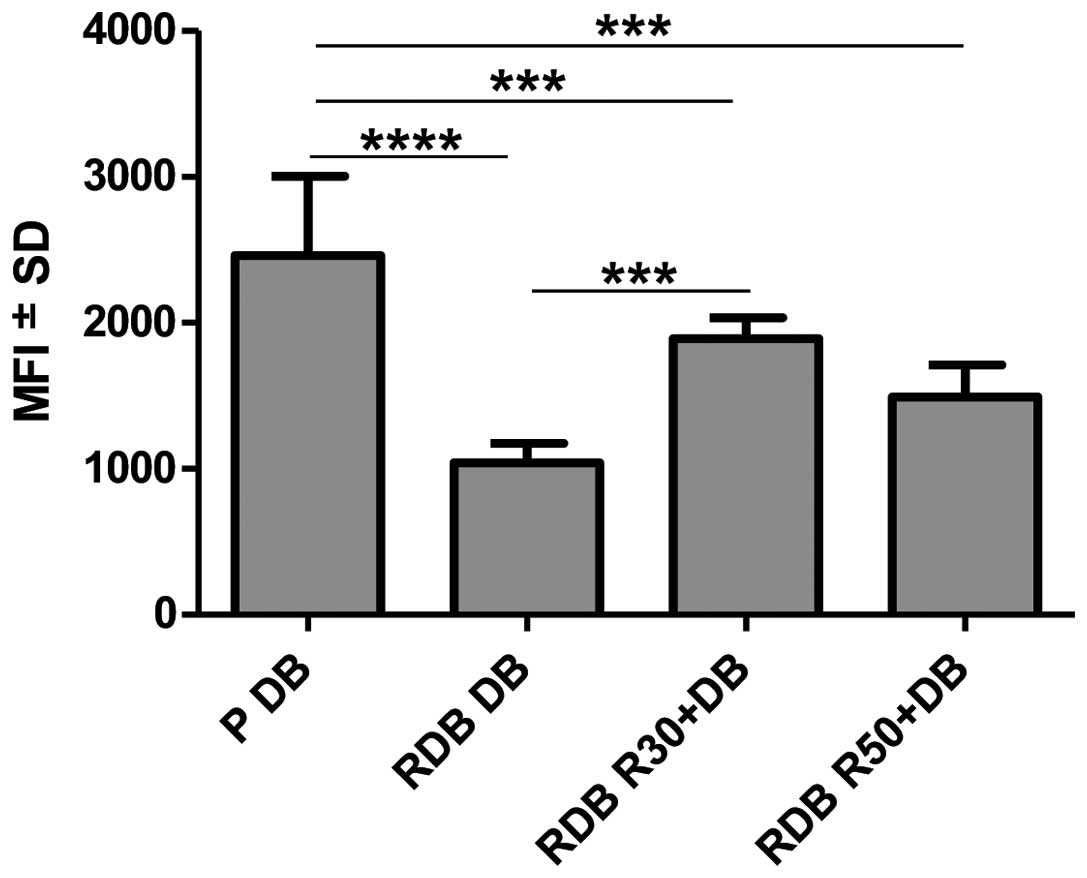
Daunorubicin accumulation (Fig. 2). DB accumulation in resistant cells
overexpressing P-gp is statistically significantly lower than in
sensitive cells (P<0.0001). Analysis showed that cytostatic
accumulation in RDB cells increased significantly following
resveratrol administration at concentration of 30 µM in
comparison to cells treated with cytostatic only (P<0.001).
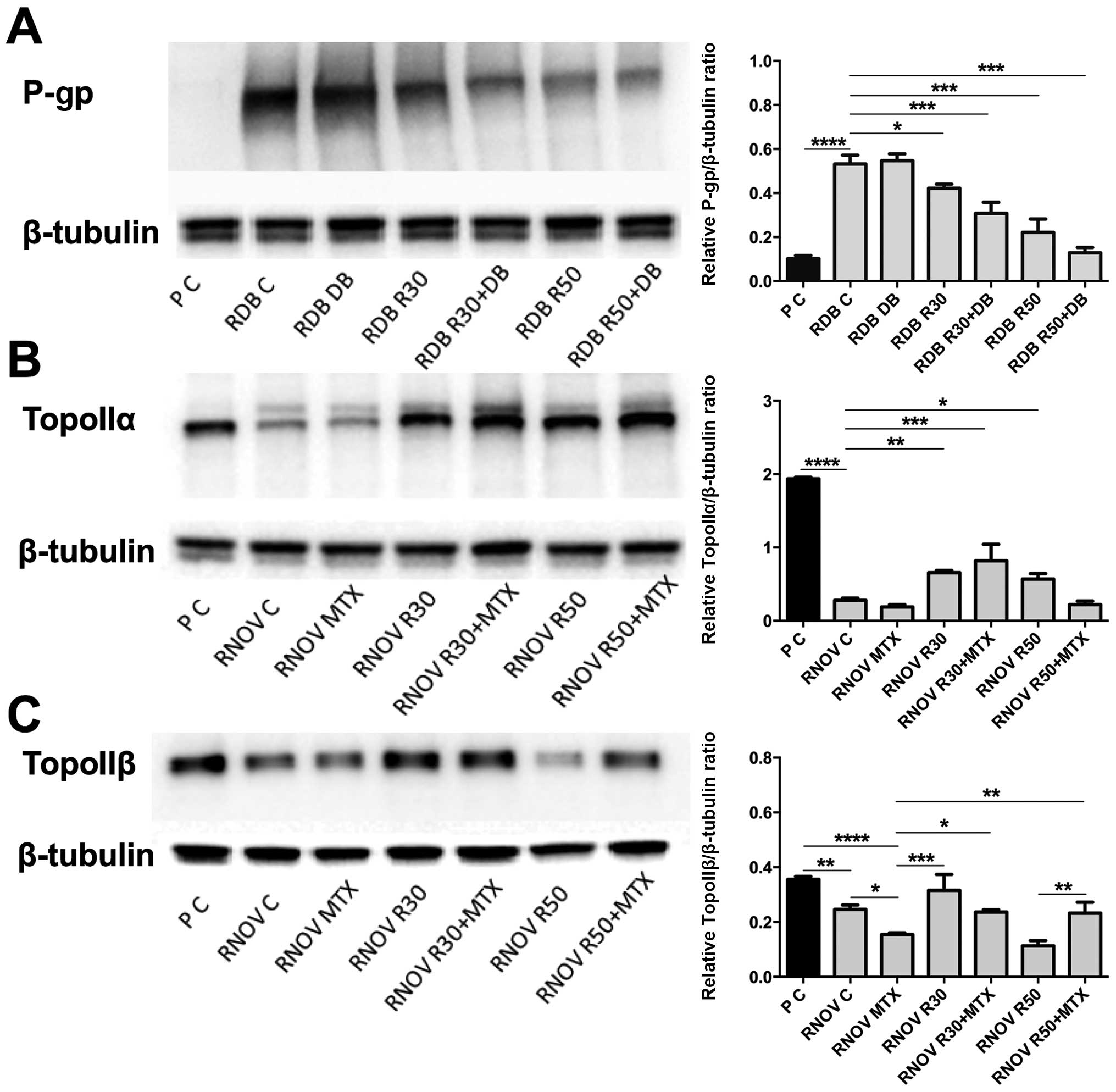
Expression level of resistance-related
proteins: P-gp, TopoIIα and TopoIIβ
Western blot method was used for the evaluation of
protein expression level. Comparison of P-gp level in P and RDB
cells showed high overexpression of this protein in DB-resistant
line (P<0.0001). After administration of R30, R50 and
combination of R30 + DB and R50 + DB, clear decrease in P-gp level
was noticed in the cells of RDB line: P<0.01 for R30 and
P<0.001 for others (Fig.
3A).
For RNOV line, markedly lower expression of TopoIIα
and TopoIIβ was observed in comparison to P line (P<0.0001). In
case of TopoIIα, resveratrol administration caused significant
increase in the level of analysed proteins in comparison to the
control: P<0.01 for R30, P<0.05 for R50 and P<0.001 for
R30 + MTX (Fig. 3B).
On the other hand, in resistant cells the level of
TopoIIβ differs significantly following cytostatic administration.
The analysis showed that in comparison to the cells untreated with
cytostatic during the experiment, protein level increased
significantly only after R30 administration, while in comparison to
the cells treated with cytostatic alone significant increase was
observed for R30 (P<0.001), R30 + MTX (P<0.05) and R50 + MTX
(P<0.01) in comparison with RNOV MTX. Clear difference is also
visible between R50 and R50 + MTX (P<0.01) (Fig. 3C).
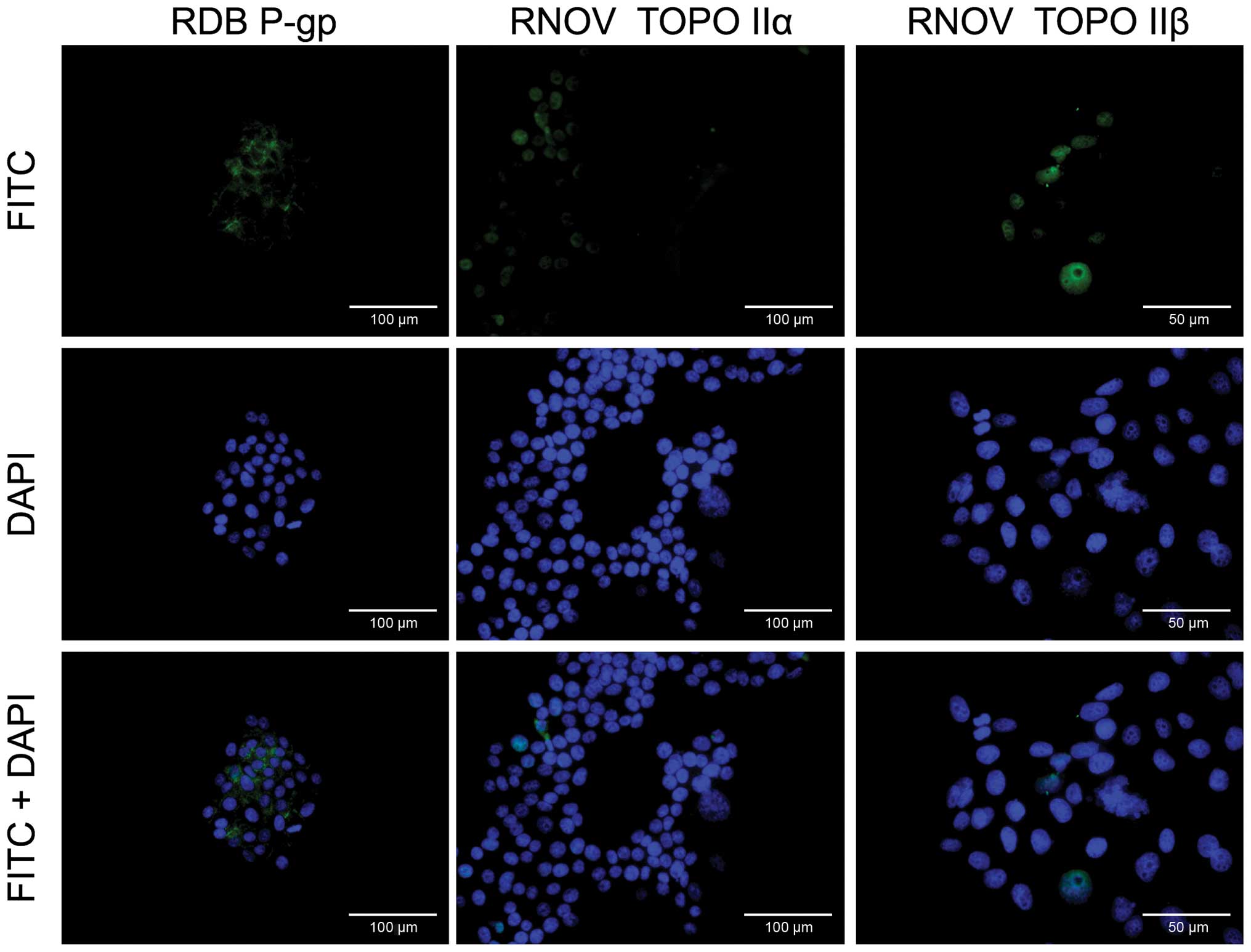
Immunofluorescence method was used to show high
expression of P-gp in the membranes of cells from line RDB, while
line RNOV is characterised by lower level of both α and β isoforms
of TopoII in cell nuclei in comparison to sensitive cell line P.
Localisation of individual proteins in resistant cell lines is
shown in Fig. 4.
Discussion
Based on the reports regarding potential effect of
resveratrol on cancer cell resistance, research was conducted on
pancreatic cancer cell lines characterised by different MDR
mechanisms. In case of RDB line this phenomenon is mainly
associated with P-gp overexpression in the cell membrane, leading
to active drug removal from cells. On the other hand, resistance of
RNOV cell line is due to the lowered level and activity of TopoII,
which is one of the targets for anthracy-clines (33).
Research on resveratrol cytotoxicity showed that
IC50 values are relatively high for all types of
analysed cancer cells. Statistical analysis of colorimetric test
and cell cycle confirmed that this compound effectively inhibits
cell proliferation also in low concentrations of 30 and 50
µM. Notably, for each of the analysed cell lines this occurs
in a different phase of cell cycle: S phase for sensitive cells, G1
for RDB, S and G2/M for RNOV. Cell cycle arrest caused by
resveratrol in different phases was previously described, among
others, as the effect on cell cycle regulating proteins e.g.
cyclins, XIAP, transcription factor NF-κB, p21/waf1 (14,34-36).
Inhibition in certain phase of cell cycle depends on the type of
the cancer and it is associated with both cytostatic-sensitive and
resistant cells e.g. chemoresistant melanoma B16, where inhibition
occurs in G1 phase and is associated with the effect on cyclin D1
and p53 protein (37,38).
The effect of polyphenols, including resveratrol,
was studied in KB-C2 cells. The level of P-gp was reduced together
with increased cytostatic accumulation (39,40).
In our research conducted on resistant pancreatic cancer cells we
showed significant increase in the accumulation of daunorubicin and
mitoxantrone under the influence of the polyphenol. However,
statistically significant changes were reported only in case of
cells resistant to daunorubicin, overexpressing P-gp (RDB). This
may indicate that the activity of this transporter is directly
affected by resveratrol. The level of P-gp in the membrane of RDB
cells is clearly higher than in the other two lines. After 72 h of
incubation with resveratrol, expression in resistant cells
decreased significantly in concentration-dependent manner.
Therefore, it can be assumed that in the studied cells resveratrol
significantly reduces the level of P-gp at post-translational
modification stage i.e. proteome or metabolome. Other polyphenols,
mainly quercetin, reduce not only P-gp level, but also expression
of its encoding gene, ABCB1, including cells of human
pancreatic and stomach cancer (41,42).
The research on the effect of resveratrol on TopoII
expression level is relatively new. It is known that in
glioblastoma U87 cell line resveratrol may unspecifically interact
both with DNA and TopoII, which was shown with the use of molecular
docking mechanisms. In the cells expressing topoisomerases on
normal or increased level resveratrol attaches to the cleavable
TopoII-DNA complex thus forming a network of hydrogen bonds and
causing its stabilisation (43),
thus, it acts similarly to cytostatics. Moreover, its effect on the
activity of TopoIIα was confirmed, which results in the loss of
capability to disentangle and remove DNA knots and supercoils.
Breakage of double-stranded DNA may be caused additionally by
prolongation of S-phase cell cycle as a result of histone H2AX
phosphorylation (44). The
enhancement of doxorubicin activity as a poison for TopoII was
recently described for colon cancer cell line, wherein used doses
of resveratrol were much higher than in our studies (45). In the cells with reduced expression
of TopoII daunorubicin has much lower effectiveness due to the
small number of cleavable complexes. By increasing the level of
TopoII, mainly its α isoform, resveratrol sensitises cells to
cytostatics. S-phase cell cycle inhibition was observed for studied
RNOV cells, which are characterised by reduced expression of
TopoII, and in P cell line. Upon resveratrol administration in RNOV
cell line significant increase in the level of expression of both
TopoII isomers was observed, in particular, at a lower
concentration of the polyphenol it suggests the effect of
resveratrol on so-called atypical mechanism of resistance.
In conclusion, resveratrol is a compound that
inhibits the cell cycle in studied human pancreatic cancer cell
lines, both sensitive and resistant to cytostatics. Moreover, it
affects MDR phenomenon caused by both P-gp overexpression and
atypical cases of resistance, such as reduced level of TopoII. It
proves that resveratrol has not only direct anticancer properties,
but it may also be use in sensitising cancer cells to chemotherapy
by multidirectional action on resistance-causing mechanisms.
Acknowledgments
We would like to thank H. Lage and M. Dietel
(Humboldt University Berlin, Charité Campus Mitte, Institute of
Pathology) for permission to use EPP85-181 cell lines. Bartosz Pula
was supported by the START stipend of Foundation for Polish
Science.
Abbreviations:
|
P
|
parental cells of human gastric
carcinoma line EPP85-181P
|
|
RDB
|
resistant cells of human gastric
carcinoma line EPP85-181RDB
|
|
RNOV
|
resistant cells of human gastric
carcinoma line EPP85-181RNOV
|
|
R30
|
resveratrol concentration of 30
µM
|
|
R50
|
resveratrol concentration of 50
µM
|
|
SRB
|
sulforhodamine B
|
|
P-gp
|
P-glycoprotein
|
|
TopoII
|
topoisomerase II
|
References
|
1
|
Liu BL, Zhang X, Zhang W and Zhen HN: New
enlightenment of French Paradox: Resveratrol's potential for cancer
chemoprevention and anti-cancer therapy. Cancer Biol Ther.
6:1833–1836. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meng C, Liu JL and Du AL: Cardioprotective
effect of resveratrol on atherogenic diet-fed rats. Int J Clin Exp
Pathol. 7:7899–7906. 2014.
|
|
3
|
Zheng JP, Ju D, Jiang H, Shen J, Yang M
and Li L: Resveratrol induces p53 and suppresses myocardin-mediated
vascular smooth muscle cell differentiation. Toxicol Lett.
199:115–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smoliga JM, Baur JA and Hausenblas HA:
Resveratrol and health: a comprehensive review of human clinical
trials. Mol Nutr Food Res. 55:1129–1141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: Preclinical and clinical studies.
Anticancer Res. 24(5A): 2783–2840. 2004.PubMed/NCBI
|
|
6
|
Athar M, Back JH, Tang X, Kim KH,
Kopelovich L, Bickers DR and Kim AL: Resveratrol: A review of
preclinical studies for human cancer prevention. Toxicol Appl
Pharmacol. 224:274–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park EJ and Pezzuto JM: The pharmacology
of resveratrol in animals and humans. Biochim Biophys Acta.
1852:1071–1113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Novelle MG, Wahl D, Diéguez C, Bernier M
and de Cabo R: Resveratrol supplementation: Where are we now and
where should we go? Ageing Res Rev. 21:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dhar S, Kumar A, Li K, Tzivion G and
Levenson AS: Resveratrol regulates PTEN/Akt pathway through
inhibition of MTA1/HDAC unit of the NuRD complex in prostate
cancer. Biochim Biophys Acta. 1853:265–275. 2015. View Article : Google Scholar
|
|
10
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Ma K, Qi T, Wei X, Zhang Q, Li G
and Chiu JF: P62 regulates resveratrol-mediated Fas/Cav-1 complex
formation and transition from autophagy to apoptosis. Oncotarget.
6:789–801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu XP, Xiong M, Xu CS, Duan LN, Dong YQ,
Luo Y, Niu TH and Lu CR: Resveratrol induces apoptosis of human
chronic myelogenous leukemia cells in vitro through p38 and
JNK-regulated H2AX phosphorylation. Acta Pharmacol Sin. 36:353–361.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fulda S and Debatin KM: Sensitization for
anticancer drug-induced apoptosis by the chemopreventive agent
resveratrol. Oncogene. 23:6702–6711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupta SC, Kannappan R, Reuter S, Kim JH
and Aggarwal BB: Chemosensitization of tumors by resveratrol. Ann
NY Acad Sci. 1215:150–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Türedi S, Yuluğ E, Alver A, Kutlu Ö and
Kahraman C: Effects of resveratrol on doxorubicin induced
testicular damage in rats. Exp Toxicol Pathol. 67:229–235. 2014.
View Article : Google Scholar
|
|
16
|
Zhang L, Guo X, Xie W, Li Y, Ma M, Yuan T
and Luo B: Resveratrol exerts an anti-apoptotic effect on human
bronchial epithelial cells undergoing cigarette smoke exposure. Mol
Med Rep. 11:1752–1758. 2015.
|
|
17
|
Gottesman MM and Ling V: The molecular
basis of multidrug resistance in cancer: The early years of
P-glycoprotein research. FEBS Lett. 580:998–1009. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colabufo NA, Contino M, Berardi F, Perrone
R, Panaro MA, Cianciulli A, Mitolo V, Azzariti A, Quatrale A and
Paradiso A: A new generation of MDR modulating agents with dual
activity: P-gp inhibitor and iNOS inducer agents. Toxicol In Vitro.
25:222–230. 2011. View Article : Google Scholar
|
|
19
|
Ullah MF: Cancer multidrug resistance
(MDR): A major impediment to effective chemotherapy. Asian Pac J
Cancer Prev. 9:1–6. 2008.PubMed/NCBI
|
|
20
|
Baguley BC: Multiple drug resistance
mechanisms in cancer. Mol Biotechnol. 46:308–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lage H: An overview of cancer multidrug
resistance: A still unsolved problem. Cell Mol Life Sci.
65:3145–3167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nitiss JL: DNA topoisomerase II and its
growing repertoire of biological functions. Nat Rev Cancer.
9:327–337. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burgess DJ, Doles J, Zender L, Xue W, Ma
B, McCombie WR, Hannon GJ, Lowe SW and Hemann MT: Topoisomerase
levels determine chemotherapy response in vitro and in vivo. Proc
Natl Acad Sci USA. 105:9053–9058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Järvinen TA and Liu ET: Topoisomerase
IIalpha gene (TOP2A) amplification and deletion in cancer - more
common than anticipated. Cytopathology. 14:309–313. 2003.
View Article : Google Scholar
|
|
25
|
Carrato A, Falcone A, Ducreux M, Valle JW,
Parnaby A, Djazouli K, Alnwick-Allu K, Hutchings A, Palaska C and
Parthenaki I: A Systematic review of the burden of pancreatic
cancer in europe: Real-world impact on survival, quality of life
and costs. J Gastrointest Cancer. 46:201–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boyle J, Czito B, Willett C and Palta M:
Adjuvant radiation therapy for pancreatic cancer: A review of the
old and the new. J Gastrointest Oncol. 6:436–444. 2015.PubMed/NCBI
|
|
27
|
Xu Q, Zong L, Chen X, Jiang Z, Nan L, Li
J, Duan W, Lei J, Zhang L, Ma J, et al: Resveratrol in the
treatment of pancreatic cancer. Ann NY Acad Sci. 1348:10–19. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shankar S, Nall D, Tang SN, Meeker D,
Passarini J, Sharma J and Srivastava RK: Resveratrol inhibits
pancreatic cancer stem cell characteristics in human and KrasG12D
transgenic mice by inhibiting pluripotency maintaining factors and
epithelial-mesenchymal transition. PLoS One. 6:e165302011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lage H, Jordan A, Scholz R and Dietel M:
Thermosensitivity of multidrug-resistant human gastric and
pancreatic carcinoma cells. Int J Hyperthermia. 16:291–303. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Skehan P, Storeng R, Scudiero D, Monks A,
McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S and Boyd MR:
New colorimetric cytotoxicity assay for anticancer-drug screening.
J Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Towbin H, Staehelin T and Gordon J:
Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: Procedure and some applications. Proc Natl
Acad Sci USA. 76:4350–4354. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lage H and Dietel M: Multiple mechanisms
confer different drug-resistant phenotypes in pancreatic carcinoma
cells. J Cancer Res Clin Oncol. 128:349–357. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Harikumar KB and Aggarwal BB: Resveratrol:
A multitargeted agent for age-associated chronic diseases. Cell
Cycle. 7:1020–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cui J, Sun R, Yu Y, Gou S, Zhao G and Wang
C: Antiproliferative effect of resveratrol in pancreatic cancer
cells. Phytother Res. 24:1637–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang G, Dai F, Yu K, Jia Z, Zhang A, Huang
Q, Kang C, Jiang H and Pu P: Resveratrol inhibits glioma cell
growth via targeting oncogenic microRNAs and multiple signaling
pathways. Int J Oncol. 46:1739–1747. 2015.PubMed/NCBI
|
|
37
|
Gatouillat G, Balasse E, Joseph-Pietras D,
Morjani H and Madoulet C: Resveratrol induces cell-cycle disruption
and apoptosis in chemoresistant B16 melanoma. J Cell Biochem.
110:893–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chin YT, Hsieh MT, Yang SH, Tsai PW, Wang
SH, Wang CC, Lee YS, Cheng GY, HuangFu WC, London D, et al:
Anti-proliferative and gene expression actions of resveratrol in
breast cancer cells in vitro. Oncotarget. 5:12891–12907. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nabekura T, Kamiyama S and Kitagawa S:
Effects of dietary chemopreventive phytochemicals on P-glycoprotein
function. Biochem Biophys Res Commun. 327:866–870. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Quan F, Pan C, Ma Q, Zhang S and Yan L:
Reversal effect of resveratrol on multidrug resistance in KBv200
cell line. Biomed Pharmacother. 62:622–629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Borska S, Sopel M, Chmielewska M, Zabel M
and Dziegiel P: Quercetin as a potential modulator of
P-glycoprotein expression and function in cells of human pancreatic
carcinoma line resistant to daunorubicin. Molecules. 15:857–870.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Borska S, Chmielewska M, Wysocka T,
Drag-Zalesinska M, Zabel M and Dziegiel P: In vitro effect of
quercetin on human gastric carcinoma: Targeting cancer cells death
and MDR. Food Chem Toxicol. 50:3375–3383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Leone S, Basso E, Polticelli F and Cozzi
R: Resveratrol acts as a topoisomerase II poison in human glioma
cells. Int J Cancer. 131:E173–E178. 2012. View Article : Google Scholar
|
|
44
|
Leone S, Cornetta T, Basso E and Cozzi R:
Resveratrol induces DNA double-strand breaks through human
topoisomerase II interaction. Cancer Lett. 295:167–172. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schroeter A and Marko D: Resveratrol
modulates the topoisomerase inhibitory potential of doxorubicin in
human colon carcinoma cells. Molecules. 19:20054–20072. 2014.
View Article : Google Scholar : PubMed/NCBI
|