Introduction
Colorectal cancer (CRC) is the third most common
cancer in males and the second in females; it also remains the
third leading cause of cancer-related deaths (1). Innovative therapeutic strategies have
greatly improved the long-term survival of CRC patients over the
past decade; however, a significant proportion of patients suffer
from drug resistance, relapse and poor outcomes (2,3). The
development of CRC is a complex process involving a series of
genetic and epigenetic alterations (4). Thus, understanding the molecular
mechanisms of CRC carcinogenesis and progression are extremely
crucial to improve CRC diagnosis and treatment.
Thyroid hormones [such as triiodothyroine
(T3)] regulate the growth, development and
differentiation in vertebrates (5).
T3 binds to specific high affinity receptors [thyroid
receptors (TRs)] which belong to the superfamily of nuclear
receptors (6). They function as
ligand-modulated transcription factors by binding to thyroid
hormone response elements (TREs) located in the promoter regions of
target genes (7). Two human TR
genes, TRα and TRβ, respectively, are located on human chromosomes
17 and 3. By alternative splicing and different promoter usage,
these two genes yield at least four proteins: thyroid hormone
receptor α1 (TRα1), TRα2, thyroid hormone receptor β1 (TRβ1), and
TRβ2 (8). Most tissues of the human
body express TRs, but there is differential expression of the TR
isoforms. TRα1 predominates in skeletal muscle and brown fat, TRα2
in brain, TRβ1 in brain, liver and kidney, and TRβ2 in the central
nervous system and developing retina (9–11).
Aberrant expression or mutation of TRs are common
events in human cancer (12).
Somatic mutation of TRs have been found in human hepatocellular
carcinoma (13), renal clear cell
carcinoma (14,15), breast cancer (16), pituitary tumors (17,18)
and thyroid cancer (19). Moreover,
an increasing number of studies indicate that TRs are potent
suppressors of tumorigenesis, invasiveness, and metastasis
(20). Mice devoid of functional
TRs (TRα1−/−, TRβ1−/−) spontaneously develop
follicular thyroid cancer and metastasis to the lung (21). In hepatocarcinoma cells transfected
with TR, T3 was found to downregulate expression of
pituitary tumor-transforming gene 1 (PTTG1) and inhibit cell growth
(22). Martínez-Iglesias et
al indentified that expression of TRβ1 in hepatocarcinoma and
breast cancer cells reduced tumor growth and caused partial
mesenchymal-to-epithelial cell transition (20). These findings suggest that TRs may
act as tumor suppressors and constitute novel therapeutic targets
in cancer.
In this study, we report that TRβ1 expression was
decreased in human CRC tissues compared to that in normal controls.
In two CRC cell lines overexpressing TRβ1, the cell proliferation
and migration was suppressed. The PI3K/Akt signaling pathway plays
an important role in tumor progression. It has been reported that
Akt is over-activated in thyroid cancer in humans and in mice
carrying mutated TRβ genes (23,24).
Here, we identified that PI3K/Akt signaling was inactivated by TRβ1
expression in CRC tissues and cells.
Materials and methods
Clinical samples
A total of 222 CRC tissue sections and adjacent
normal colorectal mucosal tissues were collected from January 2008
to December 2010 at Renji Hospital, School of Medicine, Shanghai
Jiao Tong University. All of the samples were formalin-fixed and
paraffin-embedded. Important clinical data, such as tumor size,
lymphatic metastasis, histological grade were collected from the
medical records of 100 patients. In addition, 29 pairs of fresh CRC
and adjacent specimens were available and snap-frozen in liquid
nitrogen immediately after surgery and stored at −80°C until use.
All of the human materials were obtained with informed approval of
the World Health Organization Collaborating Center for Research in
Human Production (authorized by the Shanghai Municipal
Government).
Cell culture
Two human CRC cell lines HCT116 (ATCC CCL-247) and
SW620 (ATCC CCL-228) were obtained from the Shanghai Institute of
Biochemistry and Cell Biology, Chinese Academy of Science
(Shanghai, China), and separately cultured in RPMI-1640 medium and
Dulbecco's modified Eagle's medium (DMEM) (Gibco, Carlsbad, CA,
USA) supplemented with 10% (v/v) fetal bovine serum (FBS) and 1%
antibiotics (both from Gibco, Grand Island, NY, USA) at 37°C in a
humidified incubator under 5% CO2 condition.
Tissue microarray (TMA) and
immunohistochemistry (IHC) staining
TMA was constructed by Suzhou Xinxin Biotechnology
Co., Ltd. (Suzhou, China). Cores with a 2-mm diameter were
collected from individual paraffin-embedded sections and arranged
in the recipient paraffin blocks. Then, 5-µm thick sections
were placed on superfrost charged glass microscope slides.
Prepared slides were deparaffinized in xylene, and
rehydrated in a series of graded ethanol. The antigens were
retrieved in 0.01 M sodium citrate buffer (pH 6.0) using a
microwave oven, and 3% hydrogen peroxide was used to block
endogenous peroxidase activity. After washing for 60 min in PBS
with 10% BSA to prevent non-specific binding, the tissue slides
were incubated with the primary antibody for TRβ1 (Clone J51, 1:50
dilution; Santa Cruz Biotechnology, Inc., USA) or pAkt (Ser473)
(#2118-1, 1:50 dilution; Abcam, USA) overnight at 4°C. On the next
day, the tissues were incubated with species-specific secondary
antibodies (1:1,000; Abcam) for 60 min at room temperature.
Immunostaining was carried out using a DAB substrate kit (Thermo
Fisher Scientific, USA), followed by immersing into hematoxylin for
nuclear counterstaining. A staining index was obtained as the
intensity of positive staining (negative, 0; weak, 1; moderate, 2;
or strong, 3 scores) and the proportion of immunopositive cells of
interest was scored (<25%, 1; 25–50%, 2; >50–75%, 3; ≥75%,
4). All scores were subdivided according to the median values of
the study cohort into two categories: low expression (< median)
and high expression (≥ median).
Quantitative real-time PCR
Total RNA from the human tissues or CRC cells was
extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and
reversely transcribed through PrimeScript RT-PCR (Takara Bio, Inc.,
Shiga, Japan) according to the protocol. Real-time PCR analyses
were performed with SYBR Premix Ex Taq (Takara Bio, Inc.) on a 7500
Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) at
the recommended thermal cycling settings: one initial cycle at 95°C
for 30 sec followed by 40 cycles of 5 sec at 95°C and 31 sec at
60°C. The primers were as follows: human TRβ1 forward,
5′-TTACAGCCTGGGACAAACCG-3′ and reverse, 5′-GCGACATTCCTGGCACTGAT-3′;
human β-actin forward, 5′-AGTTGCGTTACACCCTTTCTTG-3′ and reverse,
5′-CACCTTCACCGTTCCAGTTTT-3′. The relative expression of TRβ1 was
calculated and normalized using the 2−ΔΔCt method
relative to β-actin. Independent experiments were conducted in
triplicate.
Lentiviral overexpression
The lentiviral expression system was obtained from
System Biosciences (SBI; Mountain View, CA, USA). The cDNA encoding
human TRβ1 was amplified and cloned into pCDH-CMV-MCS-EF1-Puro, and
then the expression vector was co-transfected with packaging
vectors psPAX and pMD2.G at a ratio of 3:2:1 into 293T cells using
X-tremeGENE HP (Roche Diagnostics, GmbH, Mannheim, Germany).
Lentiviruses were harvested at 48 and 72 h after transfection, and
virus titers were determined. Target cells (1×105),
including HCT116 and SW620 cells, were infected with
1×106 recombinant lentivirus-transducing units in the
presence of 6 µg/ml polybrene (Sigma-Aldrich, St. Louis, MO,
USA).
Dual-Luciferase reporter assay
TRE-activated firefly luciferase reporter vector
(TRE-luc) was a kind gift from Dr Hao Ying at the Institute for
Nutritional Sciences (SIBS), Chinese Academy of Sciences. The
Renilla luciferase vector (pRL-TK) (Promega, Madison, WI,
USA), driven by an HSV-TK promoter, was used as an internal
control. HCT116 or SW620 cells were seeded in 96-well plates and
co-transfected with TRE-luc (0.1 µg/well) and Renilla
control plasmids (0.01 µg/well) following the manufacturer's
instructions. After 48 h, the CRC cells were treated with 10 nM
3,3′,5-triiodo-L-thyronine (T3) (Sigma-Aldrich) with
dH2O as a control, and incubated for further 24 h.
Luciferase activities were measured on a luminometer using the
Dual-Luciferase reporter assay system (Promega) according to the
manufacturer's instructions.
Cell viability and colony formation
assay
Cells were seeded into a 96-well plate at 2,000
cells/well with 100 µl complete medium and cultured at 37°C.
A total of 10 µl Cell Counting Kit-8 (CCK-8) (WST-8; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) solution was added
to each well after 24, 48, 72, 96 and 120 h, respectively. After 1
h of incubation, WST-8 was metabolized to produce a colorimetric
dye that is detected at OD450 nm by using a PowerWave XS microplate
reader (BioTek Instruments, Inc.). The experiment was performed in
triplicate and repeated twice. To determine clonogentic ability,
the cells were seeded into a 6-well plate at 1,000 cells/well and
cultured for 14 days. Cell colonies were then stained with crystal
violet (Beyotime Institute of Biotechnology, Shanghai, China) and
counted.
Migration assay
Cell migration assays were performed using Transwell
chambers (BD Biosciences, Bedford, MA, USA). Cells
(5×105) in 200 µl serum-free DMEM were seeded in
the upper chamber and 800 µl medium supplemented with 10%
FBS was added to the lower chamber. The migrated cells were fixed
and stained with 0.1% (w/v) crystal violet 48 h later. Five
randomly selected fields were photographed and the numbers were
counted.
Western blot analysis
Cells were lysed in RIPA buffer containing 1 mM PMSF
and protease inhibitor cocktail. The protein concentrations were
measured by using a BCA Protein Assay kit (Pierce Biotechnology,
Inc., Rockford, IL, USA). Then protein samples were separated on
10% SDS-PAGE gels under reducing condition and transferred onto
nitrocellulose membranes (Millipore Corp., Billerica, MA, USA).
After blocking in phosphate-buffered saline/Tween-20 (PBST)
containing 1% BSA at room temperature for 1 h, the membranes were
incubated overnight at 4°C with the primary antibodies. The
following antibodies were used: anti-TRβ1 (1:500 dilution; Santa
Cruz Biotechnology, Inc.), anti-Akt (#1081-1, 1:1,000 dilution),
antiphospho-Akt (Ser473) (#2118-1, 1:1,000 dilution) (both from
Abcam), and anti-β-actin (1:10,000 dilution, Sigma-Aldrich). After
washing with PBST, the membranes were incubated with
species-specific secondary antibodies. Bound secondary antibodies
were revealed by Odyssey imaging system (LI-COR Biosciences,
Lincoln, NE, USA).
Statistical analysis
Data were analyzed using the Statistical Package for
the Social Sciences (SPSS) version 16 (SPSS, Inc. Chicago, IL,
USA). The Pearson's Chi-square test was used to analyze the
relationship between TRβ1 expression and clinicophathological
characteristics. The two-tailed Student's t-test was used for
comparison between two groups. P<0.05 was considered to indicate
a statistically significant result.
Results
TRβ1 expression is decreased in human
CRCs
We firstly analyzed the normalized mRNA expression
of TRβ1 from The Cancer Genome Atlas (TCGA) data of 20 cancer
types. TRβ1 expression was decreased in colon adenocarcinoma (COAD)
and rectum adenocarcinoma (READ), the data of which were obtained
from 41 and 9 pairs of tumor samples and normal tissues,
respectively (Fig. 1A). To confirm
the TRβ1 expression level in CRC, we analyzed two independent
microarray datasets (GSE20916 and GSE9348) of healthy and CRC
patients from the NCBI Gene Expression Omnibus (GEO)
dataset-record. The results also showed that TRβ1 expression was
significantly downregulated in the tumor specimens compared with
that in the normal colorectal mucosal specimens in these two
datasets (fold change was −4.169 and −5.858 respectively, Fig. 1B).
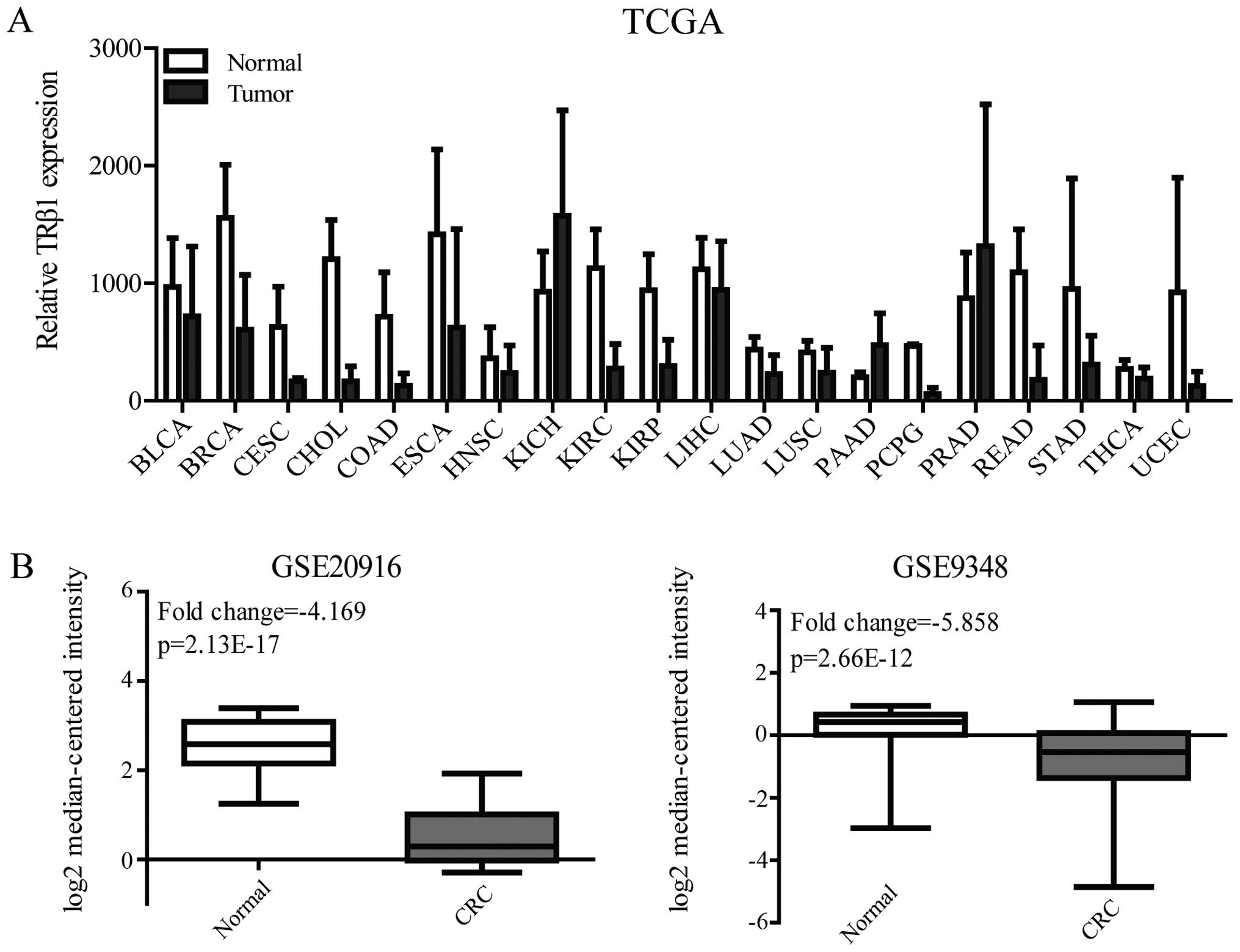 | Figure 1TRβ1 expression in TCGA and GEO
datasets. (A) TRβ1 mRNA expression in paired tumor vs. normal
tissues from the TCGA data. (B) TRβ1 mRNA expression in CRC tumor
and normal mucosal tissues obtained from GSE20916 and GSE9348.
TRβ1, thyroid hormone receptor β1; TCGA, The Cancer Genome Atlas;
GEO, Gene Expression Omnibus; BLCA, bladder urothelial carcinoma;
BRCA, breast invasive carcinoma; CESC, cervical squamous cell
carcinoma and endocervical adenocarcinoma; CHOL,
cholangiocarcinoma; COAD, colon adenocarcinoma; ESCA, esophageal
carcinoma; HNSC, head and neck squamous cell carcinoma; KICH,
kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP,
kidney renal papillary cell carcinoma; LIHC, liver hepatocellular
carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell
carcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma
and paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum
adenocarcinoma; STAD, stomach adenocarcinoma; THCA, thyroid
carcinoma; UCEC, uterine corpus endometrial carcinoma; CRC,
colorectal cancer. |
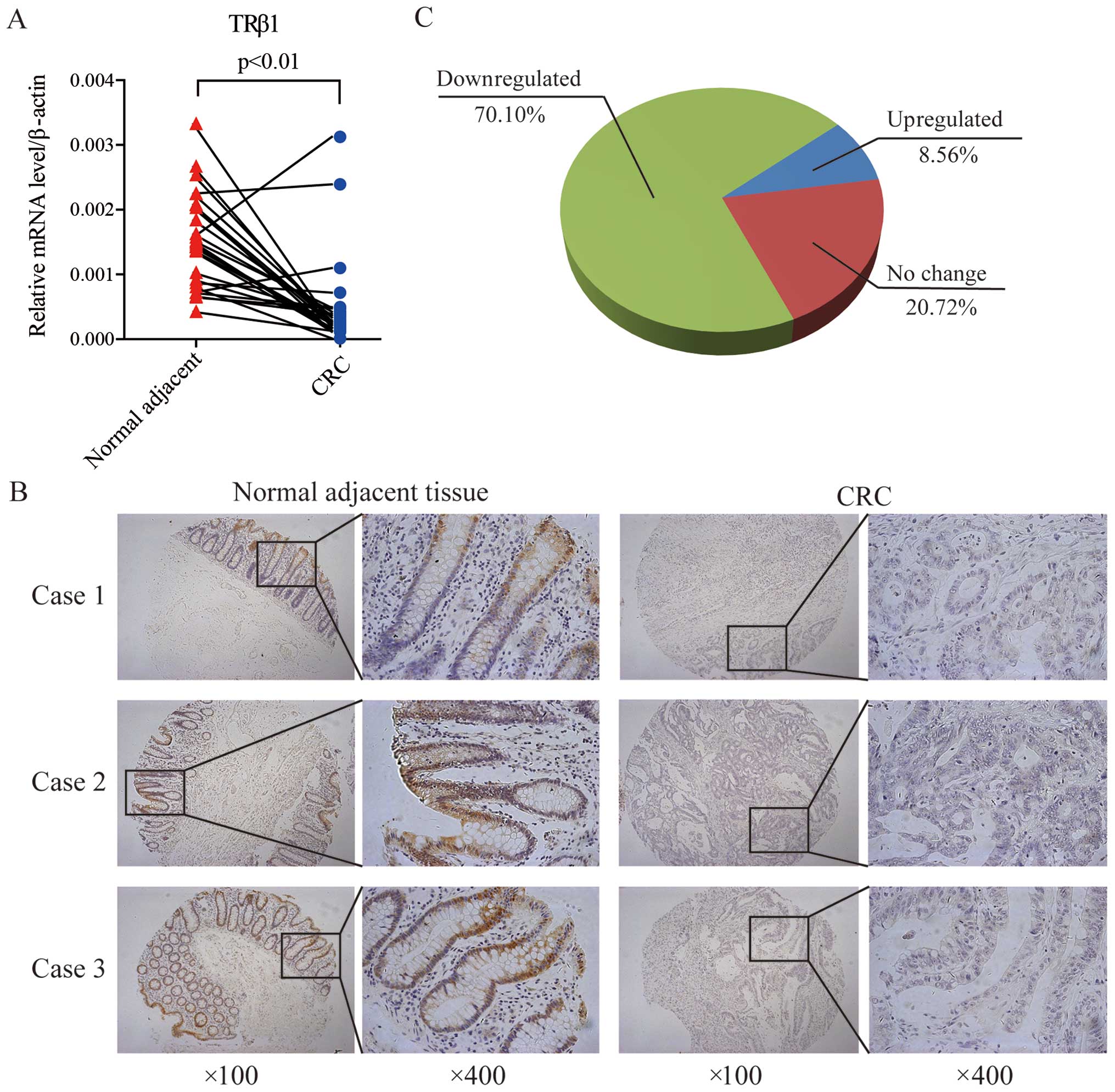
Furthermore, we measured the TRβ1 mRNA and protein
levels in CRC tissues. Twenty-nine pairs of CRC and corresponding
adjacent normal mucosal tissues were collected and subjected to
quantitative real-time PCR. TRβ1 expression was decreased in 89.7%
(26/29) of the CRC patients at the mRNA level, consistent with the
data from the TCGA and GEO datasets (Fig. 2A). Using a CRC-TMA containing 222
pairs of CRC specimens and corresponding normal colorectal mucosal
tissues, TRβ1 protein expression was detected by IHC staining. In
normal mucosa, TRβ1 was localized predominantly in the nucleus and
cytoplasm of surface epithelium cells, and it was weaker in the
crypt bases (Fig. 2B). In
carcinomas, TRβ1 expression was less prevalent (Fig. 2B), and it was downregulated in
70.10% (157/222) of the CRC patients (Fig. 2C).
These results suggest that attenuated expression of
TRβ1 may contribute to CRC carcinogenesis and progression.
Association between TRβ1 expression and
the clinicopathological features of CRC
The basic clinical characteristics of 100 CRC
patients are summarized in Table I.
The Chi-square test was used to analyze correlations between TRβ1
protein expression and clinicopathological parameters in the CRC
cases. The results indicated that TRβ1 expression was significantly
correlated with tumor size (p=0.045). No significant difference was
found in age, gender, lymphatic metastasis, histological grade and
P53 staining between the two groups (Table I).
 | Table ICorrelation of TRβ1 expression with
clinicopathological characteristics of the 100 colorectal cancer
patients. |
Table I
Correlation of TRβ1 expression with
clinicopathological characteristics of the 100 colorectal cancer
patients.
| Clinicopathological
features | Total | Expression of TRβ1
| P-value
(χ2 test) |
|---|
| Low | High |
|---|
| Gender |
| Male | 56 | 39 | 17 | 0.554 |
| Female | 44 | 33 | 11 | |
| Age (years) |
| ≤60 | 28 | 18 | 10 | 0.284 |
| >60 | 72 | 54 | 18 | |
| Tumor size
(cm) |
| ≤5 | 67 | 44 | 33 | 0.045 |
| >5 | 33 | 28 | 5 | |
| Lymphatic
metastasis |
| Yes | 33 | 26 | 7 | 0.289 |
| No | 67 | 46 | 21 | |
| Histological grade
(WHO) |
| G1 | 15 | 11 | 4 | 0.907 |
| G2 | 80 | 57 | 23 | |
| G3 | 5 | 4 | 1 | |
| P53 staining |
| Yes | 38 | 30 | 8 | 0.226 |
| No | 62 | 42 | 20 | |
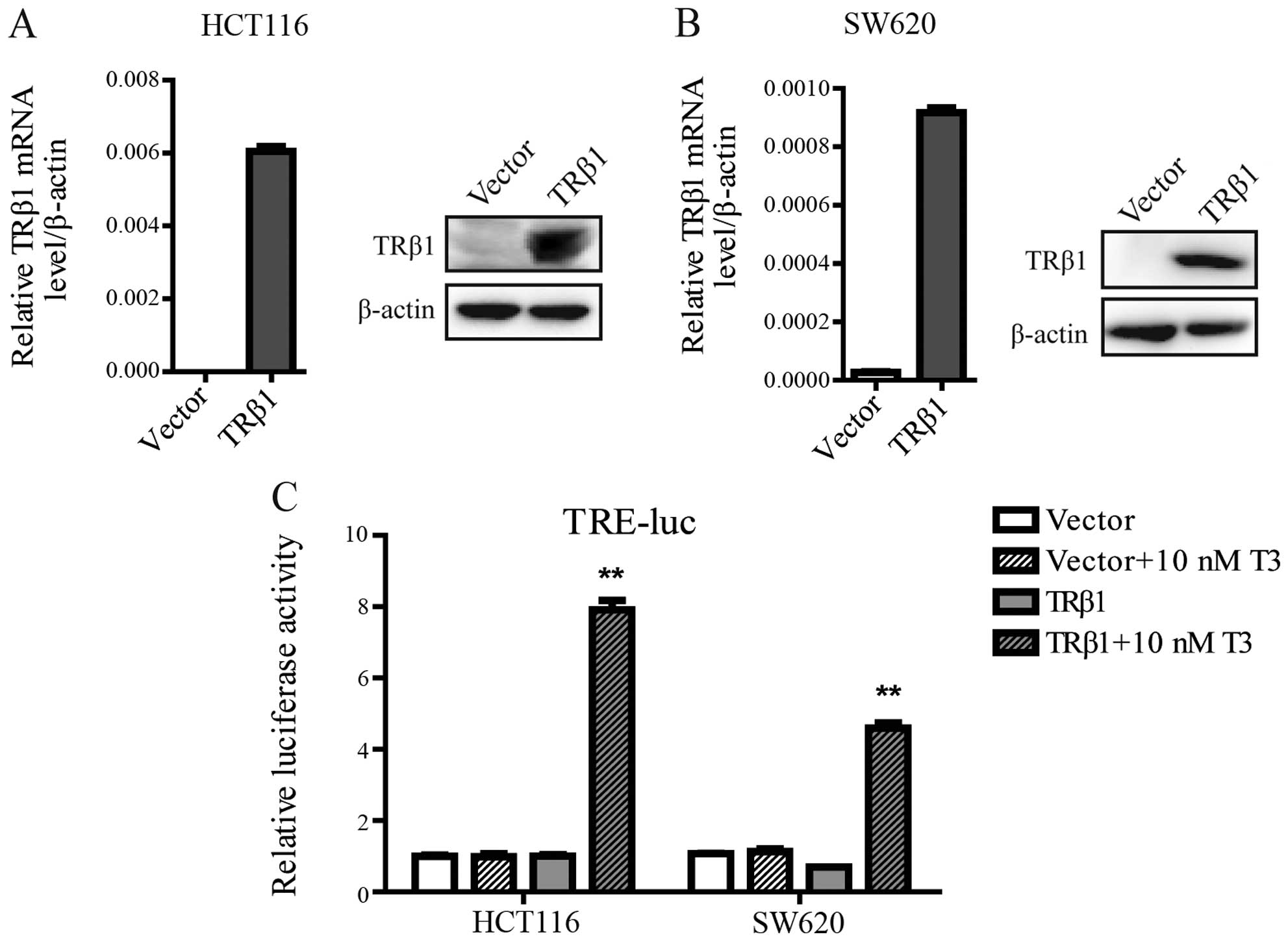
Overexpression of TRβ1 inhibits CRC cell
proliferation
To identify the function of TRβ1 in CRC, we
established stable cell lines by a lentivirus carrying the TRβ1
gene in HCT116 and SW620 cells, which exhibit a low endogenous
level of TRβ1. Stable HCT116 and SW620 cells transfected with an
empty vector were used as controls. TRβ1 was overexpressed in the
lenti-TRβ1-infected cells as characterized both by quantitative
RT-PCR and western blot analysis (Fig.
3A and B). To ensure that ectopic TRβ1 was capable to drive
gene transcription, we used a luciferase reporter vector containing
TRE. The TRE-driven luciferase expression readily responded to
T3 in both the HCT116 and SW620 cells stably
overexpressing TRβ1 (Fig. 3C).
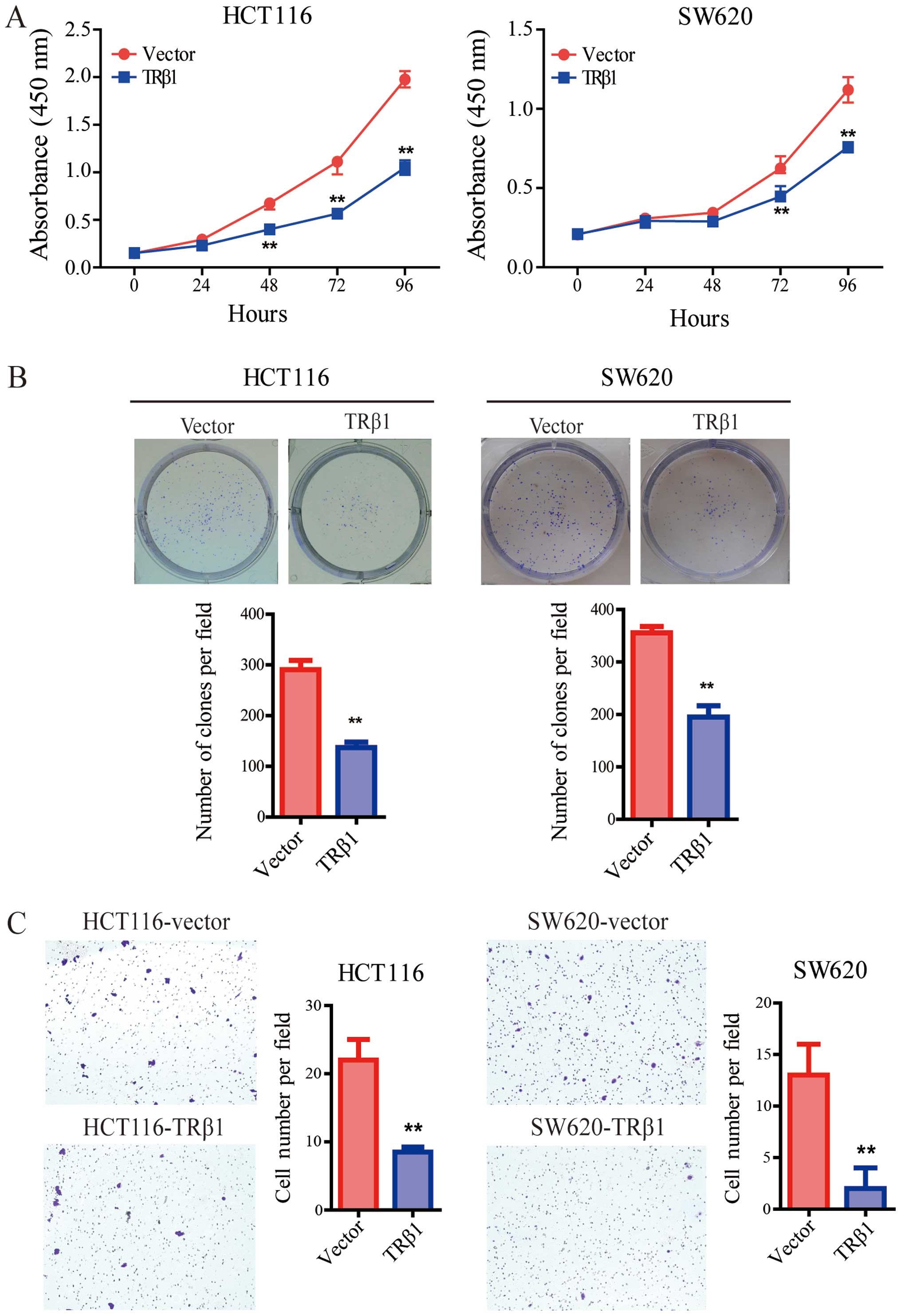
CCK-8 assay was performed to verify the role of TRβ1
in the two stable CRC cell lines. Compared with the control cells,
overexpression of TRβ1 significantly inhibited the proliferation of
the HCT116 and SW620 cells (Fig.
4A). Consistently, TRβ1 suppressed the colony formation of CRC
cells (Fig. 4B).
Overexpression of TRβ1 suppresses the
migration of CRC cells
To further evaluate the effect of TRβ1 on CRC
metastasis, we used Transwell assay. Our findings showed that the
number of migrated cells in the TRβ1-overexpressing group was
clearly decreased compared with the number in the control group
(Fig. 4C).
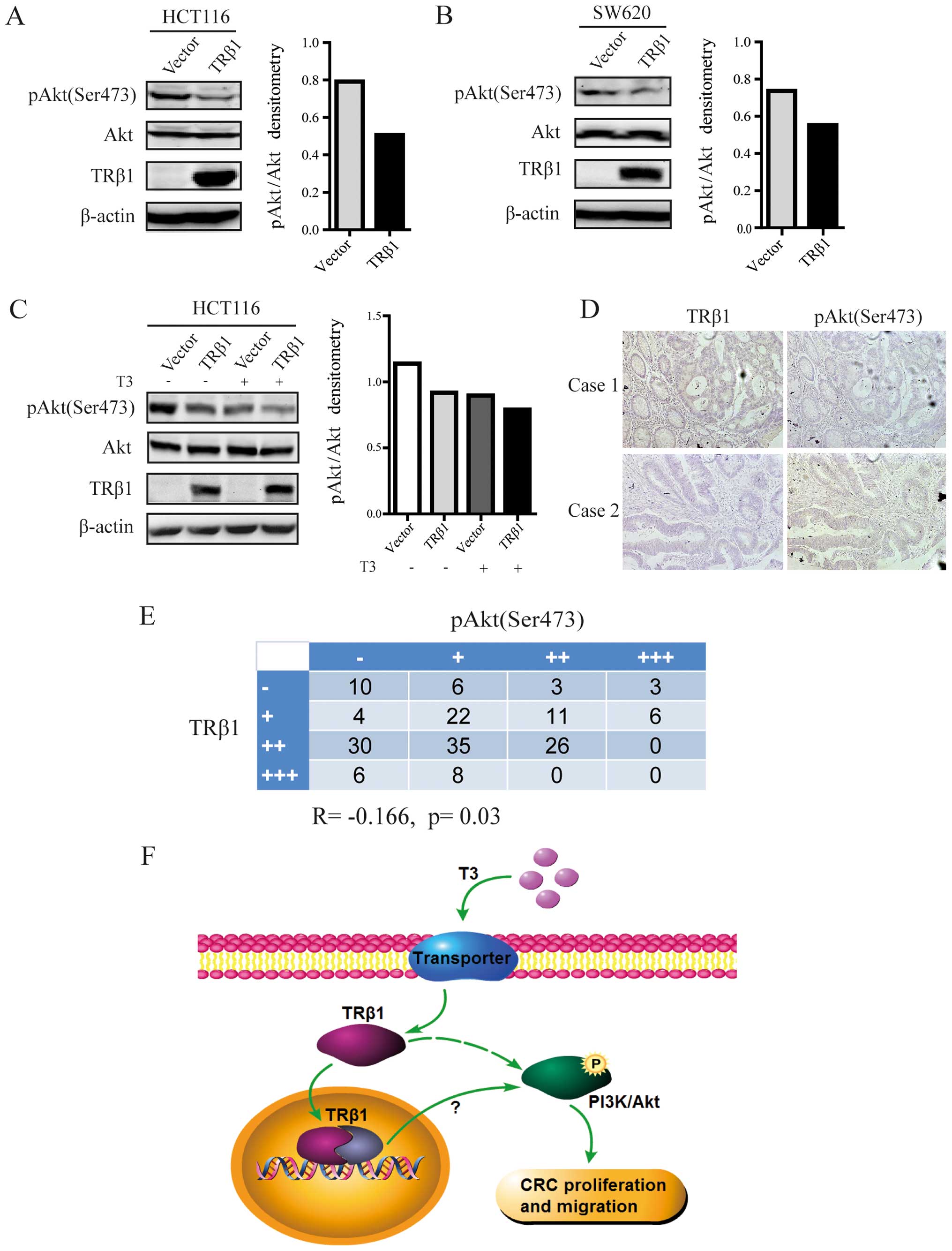
Overexpression of TRβ1 inhibits PI3K/Akt
signaling in CRC cells
To elucidate the underlying mechanism of
TRβ1-suppressed CRC cell proliferation and migration, we examined
whether PI3K/Akt, which plays an important role in tumor
progression (25), was involved in
the function of TRβ1 in CRC cells. As shown in Fig. 5A and B, overexpression of TRβ1 led
to significant decreases in the level of phosphorylated Akt with no
change in total Akt expression in both the HCT116 and SW620 cells.
When TRβ1-expressing HCT116 cells were treated with T3,
the decrease in pAkt expression was more obvious (Fig. 5C).
Furthermore, the protein expression level of
phosphorylated Akt was detected by IHC from the CRC-TMA. We found
that phospho-Akt expression was inversely correlated with TRβ1
expression in 170 CRC tissues (R=−0.166, p=0.03, Fig. 5D and E). This result suggests a
possible mechanism - TRβ1 inhibits CRC cell proliferation and
migration by suppressing PI3K/Akt signaling.
Taken together, our results demonstrated that
overexpression of TRβ1 suppressed the activation of the PI3K/Akt
signaling pathway and inhibited the proliferation and migration of
CRC cells (Fig. 5F).
Discussion
In the present study, we found that TRβ1 mRNA and
protein were downregulated in cancer tissues compared with the
levels in their corresponding normal tissues as detected by qRT-PCR
and IHC staining, and TRβ1 expression was significantly correlated
with tumor size. In vitro cellular experiments demonstrated
that overexpression of TRβ1 inhibited the proliferation and
migration of CRC cells. Moreover, the Akt signaling pathway was
suppressed by ectopic TRβ1 in the CRC tissues and cells. All our
data suggest that TRβ1 acts as a tumor suppressor in CRC
progression. For the first time to the best of our knowledge, the
functional and clinical significance of TRβ1 expression was studied
in CRC.
Aberrant expression or mutations of TRs are common
events in human cancer, and TRs also have an important role in
tumor progression in cancer cell lines (20,22)
and experimental animal models (26–28),
suggesting that these receptors may be involved in human cancer.
Horkko et al reported that TRβ1 is always present in normal
colorectal mucosal epithelium but less frequent in CRC, and its
expression is associated with the presence of K-ras
mutations. However, the role of TRβ1 in the progression of CRC
remains unknown. In this study, the TRβ1 expression pattern was
analyzed in datasets (TCGA, GSE20916 and GSE9348) and in human
tissues by qRT-PCR and IHC. Consistent with the findings of Horkko
et al (30), we confirmed
that TRβ1 expression was decreased in the CRC tissues compared with
normal mucosal tissues. Further study revealed that tumor size was
closely correlated with TRβ1 expression. Yet, more clinical samples
and data are needed to identify the association between TRβ1 and
overall survival (OS) and disease-free survival (DFS).
Furthermore, the biological functions of TRβ1 in CRC
were detected by cell viability and migration assays. We found that
overexpression of TRβ1 significantly suppressed CRC cell
proliferation and migration. Yet, the in vivo role of TRβ1
in CRC carcinogenesis remains undetermined to date. CRC mouse
models induced by genetic alterations (e.g., APCmin/+
mice) or by chemical carcinogens are needed to reveal the
pathological role of TRβ1 in colonic carcinogenesis.
The PI3K/Akt signaling pathway modulates the
function of numerous substrates involved in the regulation of cell
survival, cell cycle progression and cellular growth, and
components of this pathway are frequently altered in human cancers.
In the present study, we found that overexpression of TRβ1 resulted
in obvious decreased activation of Akt phosphorylation. However, it
remains unconfirmed whether the effect of TRβ1 on cell survival and
migration are mediated by the PI3K/Akt pathway. Moreover, TRs
physically interact with the regulatory p85α subunit of PI3K to
modulate downstream signaling pathway in thyroid cancer (21,29).
Whether or not TRβ1 inhibits Akt phosphorylation by direct
interaction with PI3K in CRC remains unknown. TRβ1 also acts as a
transcriptional factor and may regulate a series of target genes in
CRC cells. Which target gene is involved in the suppression of cell
viability and migration by TRβ1, and whether or not these target
genes regulate CRC progression via the PI3K/Akt signaling pathway
require further investigation.
In summary, this is the first study to report that
TRβ1 plays a critical role in CRC by regulating the PI3K/Akt
pathway. Future functional experiments with CRC mouse models are
needed to clarify the role of TRβ1 in CRC progression and
metastasis. Although the detailed mechanism remains to be
delineated, this preliminary study provides further knowledge of
the biological functions of TRβ1 in CRC and suggests that TRβ1
could be a potential target for CRC therapeutics.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (81472678), the Shanghai
Natural Science Foundation (13ZR1440100) and the State Key
Laboratory of Oncogenes and Related Genes (91-1511). We thank Dr
Hao Ying for providing the luciferase reporter vector.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rovcanin B, Ivanovski I, Djuric O, Nikolic
D, Petrovic J and Ivanovski P: Mitotic crossover - an evolutionary
rudiment which promotes carcinogenesis of colorectal carcinoma.
World J Gastroenterol. 20:12522–12525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oppenheimer JH: The molecular basis of
thyroid hormone action: Scattered pieces of jigsaw puzzle. Prog
Clin Biol Res. 74:45–55. 1981.PubMed/NCBI
|
|
6
|
Mangelsdorf DJ, Thummel C, Beato M,
Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M,
Chambon P, et al: The nuclear receptor superfamily: The second
decade. Cell. 83:835–839. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muñoz A and Bernal J: Biological
activities of thyroid hormone receptors. Eur J Endocrinol.
137:433–445. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng SY: Multiple mechanisms for
regulation of the transcriptional activity of thyroid hormone
receptors. Rev Endocr Metab Disord. 1:9–18. 2000. View Article : Google Scholar
|
|
9
|
Bradley DJ, Towle HC and Young WS III:
Spatial and temporal expression of alpha- and beta-thyroid hormone
receptor mRNAs, including the beta 2-subtype, in the developing
mammalian nervous system. J Neurosci. 12:2288–2302. 1992.PubMed/NCBI
|
|
10
|
Lazar MA: Thyroid hormone receptors:
Multiple forms, multiple possibilities. Endocr Rev. 14:184–193.
1993.PubMed/NCBI
|
|
11
|
Tagami T, Nakamura H, Sasaki S, Miyoshi Y
and Imura H: Estimation of the protein content of thyroid hormone
receptor alpha 1 and beta 1 in rat tissues by western blotting.
Endocrinology. 132:275–279. 1993.PubMed/NCBI
|
|
12
|
González-Sancho JM, García V, Bonilla F
and Muñoz A: Thyroid hormone receptors/THR genes in human cancer.
Cancer Lett. 192:121–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin KH, Shieh HY, Chen SL and Hsu HC:
Expression of mutant thyroid hormone nuclear receptors in human
hepatocellular carcinoma cells. Mol Carcinog. 26:53–61. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Puzianowska-Kuznicka M, Nauman A, Madej A,
Tanski Z, Cheng S and Nauman J: Expression of thyroid hormone
receptors is disturbed in human renal clear cell carcinoma. Cancer
Lett. 155:145–152. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamiya Y, Puzianowska-Kuznicka M, McPhie
P, Nauman J, Cheng SY and Nauman A: Expression of mutant thyroid
hormone nuclear receptors is associated with human renal clear cell
carcinoma. Carcinogenesis. 23:25–33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Silva JM, Domínguez G, González-Sancho JM,
García JM, Silva J, García-Andrade C, Navarro A, Muñoz A and
Bonilla F: Expression of thyroid hormone receptor/erbA genes is
altered in human breast cancer. Oncogene. 21:4307–4316. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Safer JD, Colan SD, Fraser LM and
Wondisford FE: A pituitary tumor in a patient with thyroid hormone
resistance: A diagnostic dilemma. Thyroid. 11:281–291. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ando S, Sarlis NJ, Oldfield EH and Yen PM:
Somatic mutation of TRbeta can cause a defect in negative
regulation of TSH in a TSH-secreting pituitary tumor. J Clin
Endocrinol Metab. 86:5572–5576. 2001.PubMed/NCBI
|
|
19
|
Puzianowska-Kuznicka M, Krystyniak A,
Madej A, Cheng SY and Nauman J: Functionally impaired TR mutants
are present in thyroid papillary cancer. J Clin Endocrinol Metab.
87:1120–1128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martínez-Iglesias O, Garcia-Silva S,
Tenbaum SP, Regadera J, Larcher F, Paramio JM, Vennström B and
Aranda A: Thyroid hormone receptor beta1 acts as a potent
suppressor of tumor invasiveness and metastasis. Cancer Res.
69:501–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu XG, Zhao L, Willingham MC and Cheng
SY: Thyroid hormone receptors are tumor suppressors in a mouse
model of metastatic follicular thyroid carcinoma. Oncogene.
29:1909–1919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen RN, Huang YH, Yeh CT, Liao CH and Lin
KH: Thyroid hormone receptors suppress pituitary tumor transforming
gene 1 activity in hepatoma. Cancer Res. 68:1697–1706. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ringel MD, Hayre N, Saito J, Saunier B,
Schuppert F, Burch H, Bernet V, Burman KD, Kohn LD and Saji M:
Overexpression and overactivation of Akt in thyroid carcinoma.
Cancer Res. 61:6105–6111. 2001.PubMed/NCBI
|
|
24
|
Kim CS, Vasko VV, Kato Y, Kruhlak M, Saji
M, Cheng SY and Ringel MD: AKT activation promotes metastasis in a
mouse model of follicular thyroid carcinoma. Endocrinology.
146:4456–4463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dent P: Crosstalk between ERK, AKT, and
cell survival. Cancer Biol Ther. 15:245–246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki H, Willingham MC and Cheng SY: Mice
with a mutation in the thyroid hormone receptor beta gene
spontaneously develop thyroid carcinoma: A mouse model of thyroid
carcinogenesis. Thyroid. 12:963–969. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guigon CJ, Zhao L, Willingham MC and Cheng
SY: PTEN deficiency accelerates tumour progression in a mouse model
of thyroid cancer. Oncogene. 28:509–517. 2009. View Article : Google Scholar
|
|
28
|
Lu C, Willingham MC, Furuya F and Cheng
SY: Activation of phosphatidylinositol 3-kinase signaling promotes
aberrant pituitary growth in a mouse model of thyroid-stimulating
hormone-secreting pituitary tumors. Endocrinology. 149:3339–3345.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Furuya F, Lu C, Guigon CJ and Cheng SY:
Nongenomic activation of phosphatidylinositol 3-kinase signaling by
thyroid hormone receptors. Steroids. 74:628–634. 2009. View Article : Google Scholar
|
|
30
|
Hörkkö TT, Tuppurainen K, George SM,
Jernvall P, Karttunen TJ and Mäkinen MJ: Thyroid hormone receptor
beta1 in normal colon and colorectal cancer-association with
differentiation, polypoid growth type and K-ras mutations. Int J
Cancer. 118:1653–9. 2006. View Article : Google Scholar
|