Introduction
Breast cancer is one of the most aggressive cancers
among females worldwide (1).
Radiotherapy, surgery and molecular-targeted drug therapies have
been applied in the treatment of breast cancer. However, these
conventional therapies cause serious side-effects, deeply affecting
the quality of life of patients (2). Therefore, there is an urgent need to
search for safe and effective drugs for the treatment of breast
cancer.
Growing evidence suggests that matrix
metalloproteinases (MMPs) play an important role in cell invasion
and metastasis, since they essentially degrade the extracellular
matrix (ECM) (3). Among the MMPs,
the protein level and activity of MMP-2 (gelatinase A, 72 kDa) and
MMP-9 (gelatinase B, 92 kDa) are frequently increased in metastatic
carcinomas, including colon, lung and breast (4–6). In
cancer cells, increased MMP-2 and MMP-9 activity and expression
require constitutive mitogen-activated protein kinase (MAPK)
activity, which includes p38 MAPK, c-Jun N-terminal kinase (JNK)
and extracellular signal-regulated kinase (ERK) (7–10). The
expression of MMP-2 and MMP-9 also depends on the activity of
activator protein-1 (AP-1) and nuclear factor-κB (NF-κB), which are
the downstream factors of the MAPK signaling pathway (11).
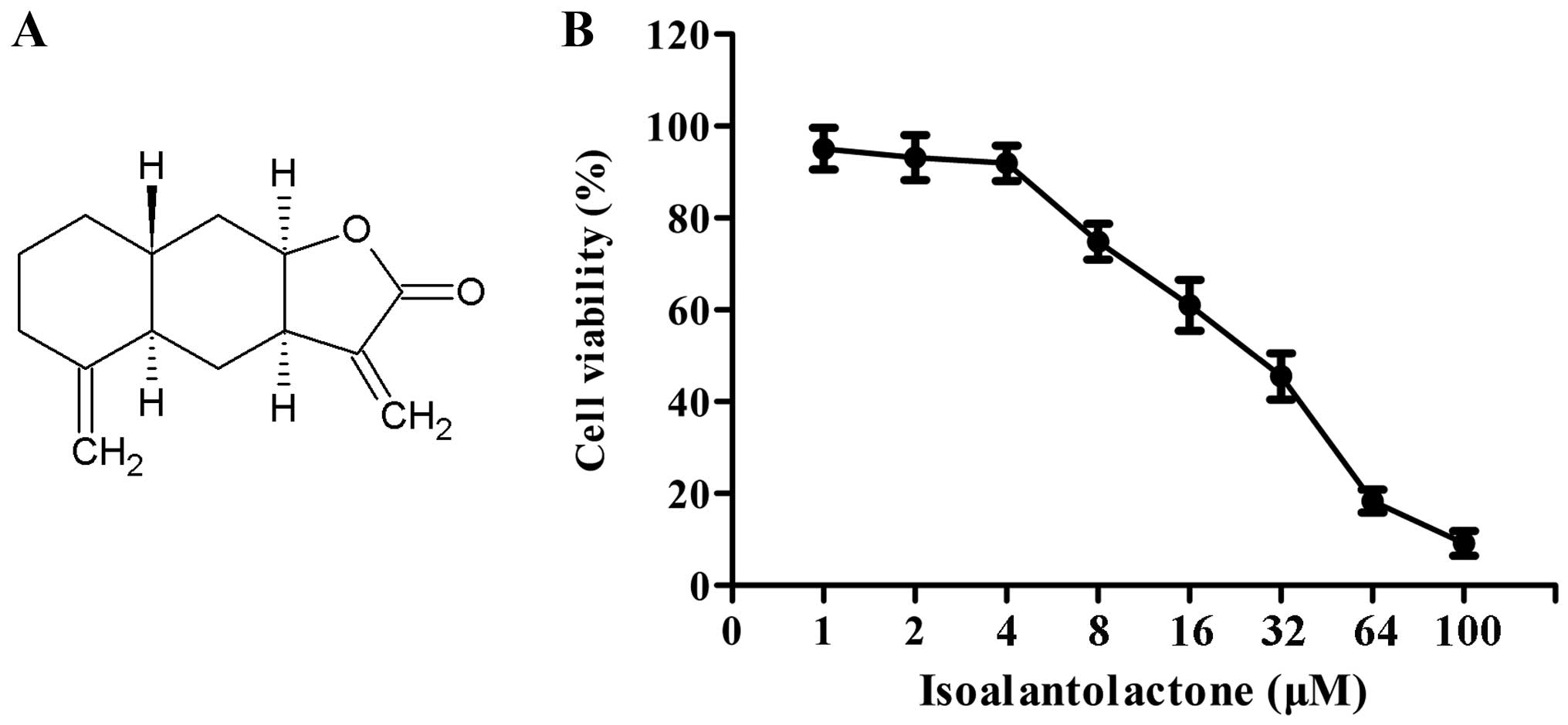
Isoalantolactone (Fig.
1A), a sesquiterpene lactone isolated from the flowering plant
Inula helenium L., has been shown to hold various
pharmacological activities, including antitrypanosomal,
anti-apoptosis, anti-microbial activities (12–15).
It has also been reported that isoalantolactone possesses
anticancer activity in several types of cancer cells, such as neck
squamous cell carcinoma, prostate cancer and gastric adenocarcinoma
cells (16–18). However, the ability of
isoalantolactone to inhibit the migration and invasion of
metastatic breast cancer cells remains unknown, and the underlying
mechanism responsible for these effects is unclear. In this study,
we investigated the effects of isoalantolactone on the viability,
adhesion, migration and invasion of a highly metastatic human
breast cancer cell line MDA-MB-231, and further explored the
underlying molecular mechanism of action.
Materials and methods
Chemicals and drugs
Isoalantolactone (purity, ≥98%) was obtained from
Sigma Chemical Co. (St. Louis, MO, USA), and it was dissolved in
dimethyl sulfoxide (DMSO) as primary stock solution. The solution
was stored at −20°C, and diluted with medium for further study.
During the process of the experiment, the final concentration of
DMSO was <0.5%. The primary antibodies for MMP-2, MMP-9, p38
MAPK, p-p38 MAPK, JNK, p-JNK, p-ERK, ERK, NF-κB p65 and β-actin
were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Secondary antibodies were from Amersham Biosciences (Freiburg,
Germany). L-15 medium, fetal bovine serum (FBS),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
trypsin, Tween-20, and sodium dodecyl sulfate (SDS) were purchased
from Sigma-Aldrich. Matrigel was purchased from BD Biosciences (San
Jose, CA, USA). Other reagents were obtained from commercial
sources.
Cell culture
Human breast cancer cell line, MDA-MB-231, was
purchased from the Cell Bank of the Shanghai Institute of Cell
Biology (Shanghai, China). The cells were maintained in L-15 medium
supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin at 37°C with 5% CO2 in a humidified
atmosphere.
Cell viability assay
Cell viability was determined by MTT assay (19). MDA-MB-231 cells were seeded at a
density of 1×104 cells/well (100 μl) and treated
with various concentrations of isoalantolactone for 24 h.
Subsequently, the medium was removed and 10 μl of MTT
solution (5 mg/ml) was added to each well for an additional 4 h. At
the end of the incubation, 100 μl DMSO was added for 10 min
after the removal of medium, owing to the fact that the number of
viable cells was directly proportional to the production of
formazan, which can be solubilized by DMSO. The optimal density
(OD) was measured at 570 nm on a SpectraMax 190 (Molecular Devices,
Sunnyvale, CA, USA). All the experiments were repeated three
times.
Cell adhesion assay
After being incubated with different concentrations
of isoalantolactone at 37°C for 24 h, MDA-MB-231 cells were
digested by 0.25% trypsin and centrifuged at 2,000 × g for 2 min.
Then, the cells were seeded into a 96-well plate (1×104
cells/well), each of which was coated with 30 μg Matrigel
(BD Biosciences) to form a basement membrane. At the end of the
incubation for 1 h, the medium was discarded, and the cells were
washed with 100 μl phosphate-buffered saline (PBS) for three
times to remove the non-adherent cells. Subsequently, MTT (5 mg/ml)
in L-15 medium was added into each well and the attached cells were
incubated at 37°C for 4 h. DMSO (100 μl) was added into each
well, and shaken for 10 min. The following processes were performed
using the same method as for measurement of cell viability. The
cell adhesion rate was calculated using the formula: Cell adhesion
rate = (OD drug/OD blank) × 100%.
Wound-healing assay
The wound-healing assay was performed as described
previously (20). MDA-MB-231 cells
were seeded into 12-well plates (1×105 cells/well) in
L-15 medium supplement. Cells were grown to nearly 80% confluency
overnight and were then scratched using a 200-μl pipette
tip. The suspended cells were washed away and the wounded cell
monolayer was incubated in FBS-free medium with isoalantolactone
and/or other agents for 24 h. The cells were visualized under a
IX73 microscope (Olympus, Tokyo, Japan) at 0 and 24 h. After
treatment, the relative wound area was analyzed using Image-Pro
Plus 6.0 software (Media Cybernetics, Rockville, MD, USA). Three
independent experiments were performed.
Cell invasion assay
The effect of isoalantolactone on the invasion of
MDA-MB-231 cells was evaluated in vitro as described
previously (21). Matrigel (5
mg/ml; Becton Dickinson, Bedford, MA, USA) was applied to 8-mm pore
size polycarbonate membrane filters of a Transwell chamber (Corning
Costar, Cambridge, MA, USA) at 37°C for 1 h. MDA-MB-231 cells
(1×105 cells/well) were seeded on the upper part of the
chamber. The bottom chamber was filled with 500 μl of L-15
medium containing 10% FBS. After treatment with isoalantolactone
and/or other agents for 24 h, the cells penetrating through the
Matrigel and migrating to the lower chamber were fixed with
paraformaldehyde, and stained with 0.1% crystal violet, and
photographed. Invasion of the MDA-MB-231 cells was defined in terms
of the total number of invasive cells in five randomly selected
fields.
RNA extraction and quantitative real-time
PCR
After being treated with isoalantolactone and/or
other agents, cellular RNA was extracted and reversely transcripted
to determine the expression of MMP-2 and MMP-9. According to the
manufacturer's protocols (Invitrogen, Carlsbad, CA, USA), total RNA
was isolated from the MDA-MB-231 cells using TRIzol reagent
(Invitrogen Life Technologies, Waltham, MA, USA), followed by DNase
I digestion (Thermo Fisher Scientific, Waltham, MA, USA).
Complementary DNA was synthesized using PrimeScript™ RT reagent kit
(Fermentas, Germany).
Real-time PCR was performed to detect mRNA levels,
along with the ABI PRISM 7900 sequence detection system (Applied
Biosystems, Foster City, CA, USA). Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was chosen as an internal standard. Primer
sequences (5′→3′) and probe numbers were as follows: MMP-2 forward,
TTG ACG GTA AGG ACG GAC TC and reverse, CAT ACT TCA CAC GGA CCA CTT
G; MMP-9 forward, TTC CAG TAC CAA GAC AAA GCC and reverse, CAC GGT
TGA AGC AAA GAA GG; and GAPDH forward, CAC CCA CTC CTC CAC CTT TGA
C and reverse, GCA ACT GTG AGG AGG GGA GAT T. Results were
normalized to the GAPDH expression level, and relative quantitation
of MMP-2 and MMP-9 was analyzed using the comparative
2−ΔΔCt method.
Preparation of cytosolic and nuclear
fractions
MDA-MB-231 cells were treated with or without
isoalantolactone for 24 h. Then, the cells were washed three times
in ice-cold PBS followed by lysis in 1% Triton X-100 lysis buffer
(10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.5% Nonidet P-40, 1
mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride). The
cytosolic fraction was collected and centrifuged at 14,000 × g for
15 min at 4°C, and the nuclear pellets were resuspended in 1%
Triton X-100 lysis buffer containing 400 mM NaCl. The nuclear
extract was recovered after centrifugation at 14,000 × g for 15 min
at 4°C.
Western blot analysis
After whole-cell lysates were obtained, cytosolic
and nuclear extracts were isolated, and equal quantities of protein
were loaded onto 10% SDS-polyacrylamide gels for separation and
then were transferred to a PVDF membrane blocked with 5% skim milk.
Subsequently, primary antibodies were used and then the membrane
was washed for secondary antibody staining. Antibody binding was
detected by enhanced chemiluminescence (Amersham Life Sciences,
Amersham, UK). The quantification of proteins was analyzed by
Image-Pro Plus (IPP) software.
Immunocytochemical analysis
Immunocytochemical assay was performed to determine
the NF-κB p65 protein level in the nucleus according to a previous
method (22). MDA-MB-231 cells were
grown on glass coverslips and incubated with isoalantolactone for
24 h. Then, the cells were fixed with fresh 4% formaladehyde at
room temperature for 90 min and washed twice with PBS. The cells
were permeabilized with 0.1% Triton X-100 for 20 min, and treated
with 5% bovine serum albumin (BSA; Santa Cruz Biotechnology Inc.).
Primary antibody, NF-κB p65 (1:400), was diluted for application
for the incubation of cells, followed by the secondary antibody. An
Elivison two-step method was carried out for the
immunohistochemical staining and image were captured using a DM2500
optical microscope. The positive expression was analyzed by IPP
software.
Gelatin zymography assay
MDA-MB-231 cells were cultured with or without
isoalantolactone (1, 2, or 4 μM) in serum-free media for 24
h, and then the supernatants were collected. The activities of
MMP-2 and MMP-9 were estimated by gelatin zymography assay as
previously described (23).
Briefly, conditioned medium was electrophoresed on a 8% SDS-PAGE
gel containing 0.1% gelatin. The gels were washed with 2.5% (v/v)
Triton X-100 at room temperature, and then incubated in reaction
buffer [50 mM Tris-Cl (pH 7.6), 10 mM CaCl2, 200 mM
NaCl] at 37°C overnight to digest the gelatin. Finally,
enzyme-digested regions were observed as clear bands against a blue
background. Negatively stained bands were considered as the zones
of enzymatic activity.
Luciferase reporter gene assay
The effect of isoalantolactone on NF-κB-dependent
reporter gene transcription was analyzed by NF-κB-luciferase assay.
MDA-MB-231 cells (5×105 cells/well) were plated in
6-well plates and transiently transfected by the Liposome 2000
method with the pNF-κB-luc plasmid reporter gene (0.5 μg;
Beyotime) and β-galactosidase (90 ng). After transfection for 24 h,
the cells were treated with isoalantolactone (1, 2 or 4 μM)
for an additional 24 h. Cells were harvested for measuring
β-galactosidase activity and luciferase activity. Relative
luciferase activity was normalized to the β-galactosidase value to
correct transfection efficacy.
Statistical analysis
All data in this study were taken from three
independent experiments and are expressed as means ± standard
deviation (SD). The statistical significance was analyzed using the
one-way analysis of variance (ANOVA) with the Statistical Package
for the Social Sciences (SPSS, 13.0) software. Differences were
considered significant at p<0.05.
Results
Effect of isoalantolactone on the
viability of the MDA-MB-231 cells
The effect of isoalantolactone on the cell viability
of the MDA-MB-231 cells was determined by MTT assay. MDA-MB-231
cells were treated with isoalantolactone for 24 h at various
concentrations (1–100 μM). As depicted in Fig. 1B, isoalantolactone did not show any
cytotoxic effect on the MDA-MB-231 cells at a dose <4 μM.
Therefore, we chose 1, 2 and 4 μM of isoalantolactone in the
following experiments.
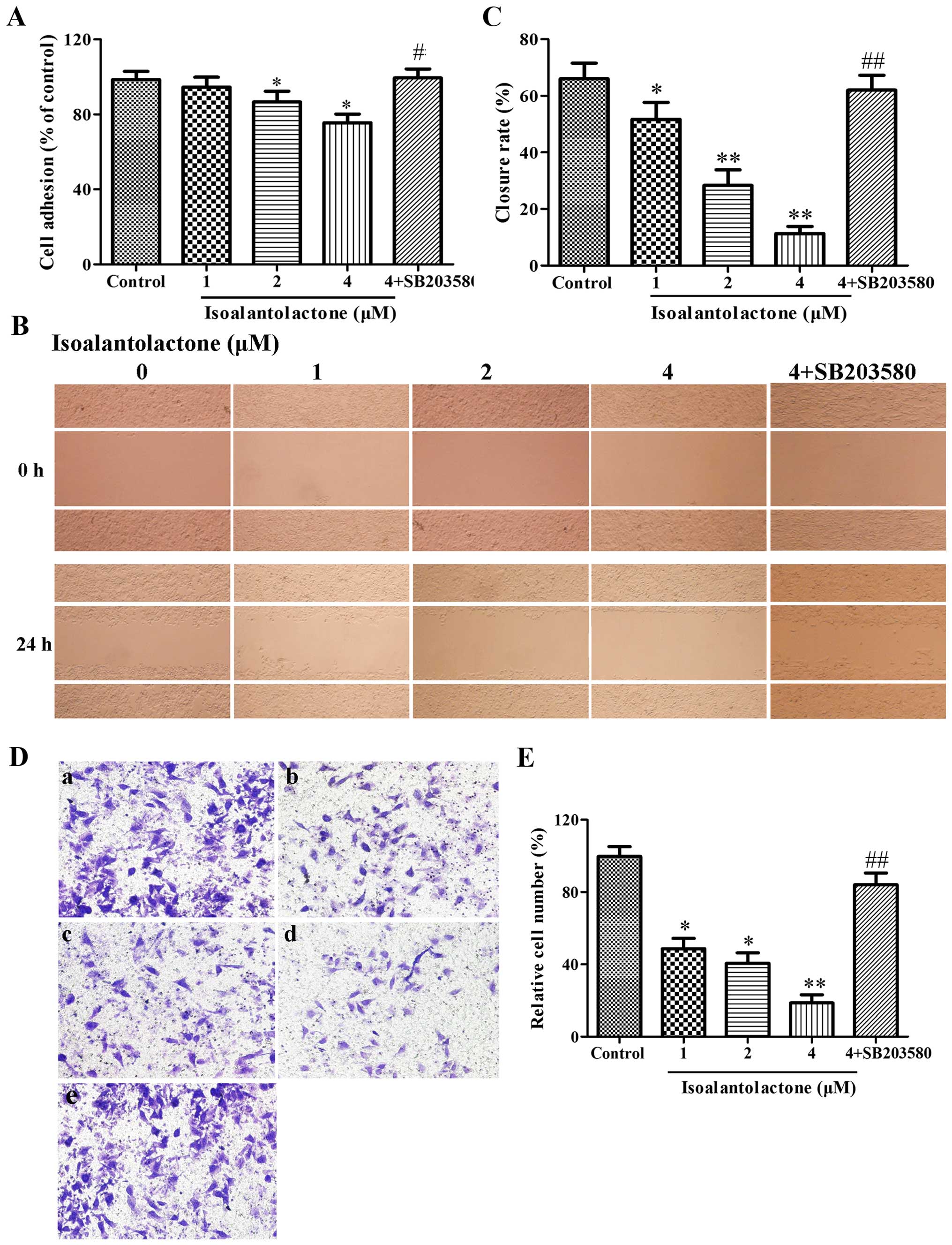
Isoalantolactone suppresses the adhesion,
migration and invasion of MDA-MB-231 cells in vitro
We examined the effect of isoalantolactone on the
adhesion ability of the MDA-MB-231 cells by MTT assay. As shown in
Fig. 2A, the number of adhesive
cells was markedly decreased following the treatment of
isoalantolactone in a concentration-dependent manner, when compared
with the blank control. This result indicated that isoalantolactone
suppressed the invasiveness of the MDA-MB-231 cells by decreasing
cell adhesion.
To investigate the effect of isoalantolactone on
MDA-MB-231 cell migration, wound-healing assay was performed. The
closure rate of MDA-MB-231 cells was determined by IPP software. As
depicted in Fig. 2B and C, the
MDA-MB-231 cells covered most of the wound area after a 24-h
incubation in the control blank group. The wound closure rates of
the groups treated with isoalantolactone at 1, 2 and 4 μM
were 51.67±6.03, 28.33±5.51 and 11.33±2.52%, respectively. These
closure rates were all significantly smaller than the rate noted in
the untreated group. Subsequently, we estimated the invasive
ability of the MDA-MB-231 cells incubated in the presence or
absence of isoalantolactone by the Transwell chamber assay. As
shown in Fig. 2D and E,
isoalantolactone inhibited the invasion of MDA-MB-231 cells in a
concentration-dependent manner. To confirm the role of p38 MAPK in
the inhibition of isoalantolactone on MDA-MB-231 cells, we added
p38 MAPK (4 μM) to cells with isoalantolactone (4
μM). Then, the inhibiton of isoalantolactone on adhesion,
migration and invasion was significantly reversed (p<0.05,
p<0.01). Overall, these results indicated that isoalantolactone
decreased the adhesive, migratory, and invasive ability of highly
metastatic MDA-MB-231 cells in vitro through the p38 MAPK
signaling pathway.
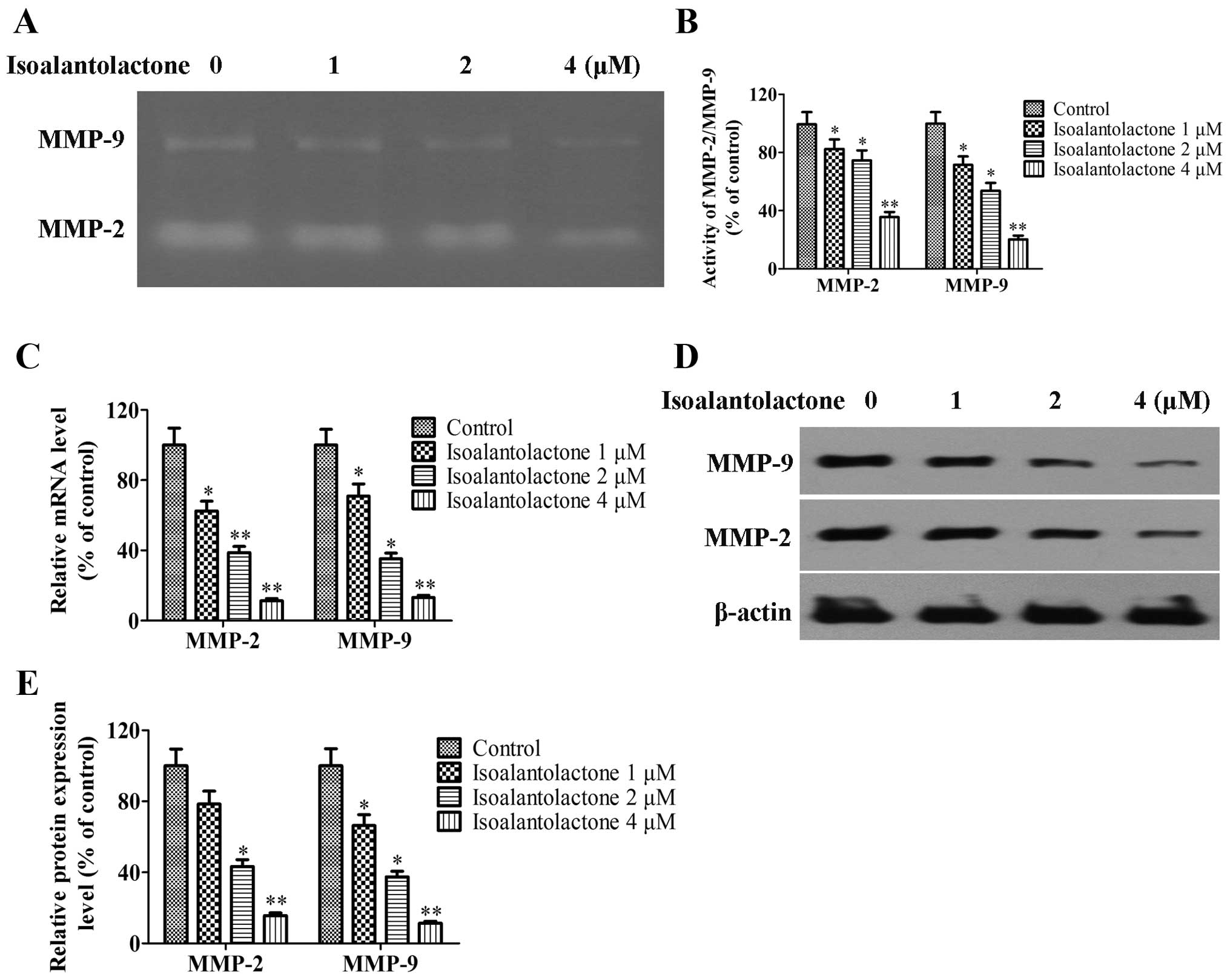
Isoalantolactone downregulates the
proteolytic activities and expression of MMP-2 and MMP-9 in
MDA-MB-231 cells
To investigate the anti-invasive mechanism of
isoalantolactone, the activity, protein expression and mRNA levels
of MMP-2 and MMP-9 in the isoalantolactone-treated MDA-MB-231 cells
were determined. As shown in Fig.
3, the activity as well as the protein and mRNA levels of MMP-2
and MMP-9 in the isoalantolactone-treated cells were downregulated
in a concentration-dependent manner. These results indicated that
isoalantolactone inhibited the invasion ability of MDA-MB-231 cells
via suppression of the activity and expression of MMP-2 and
MMP-9.
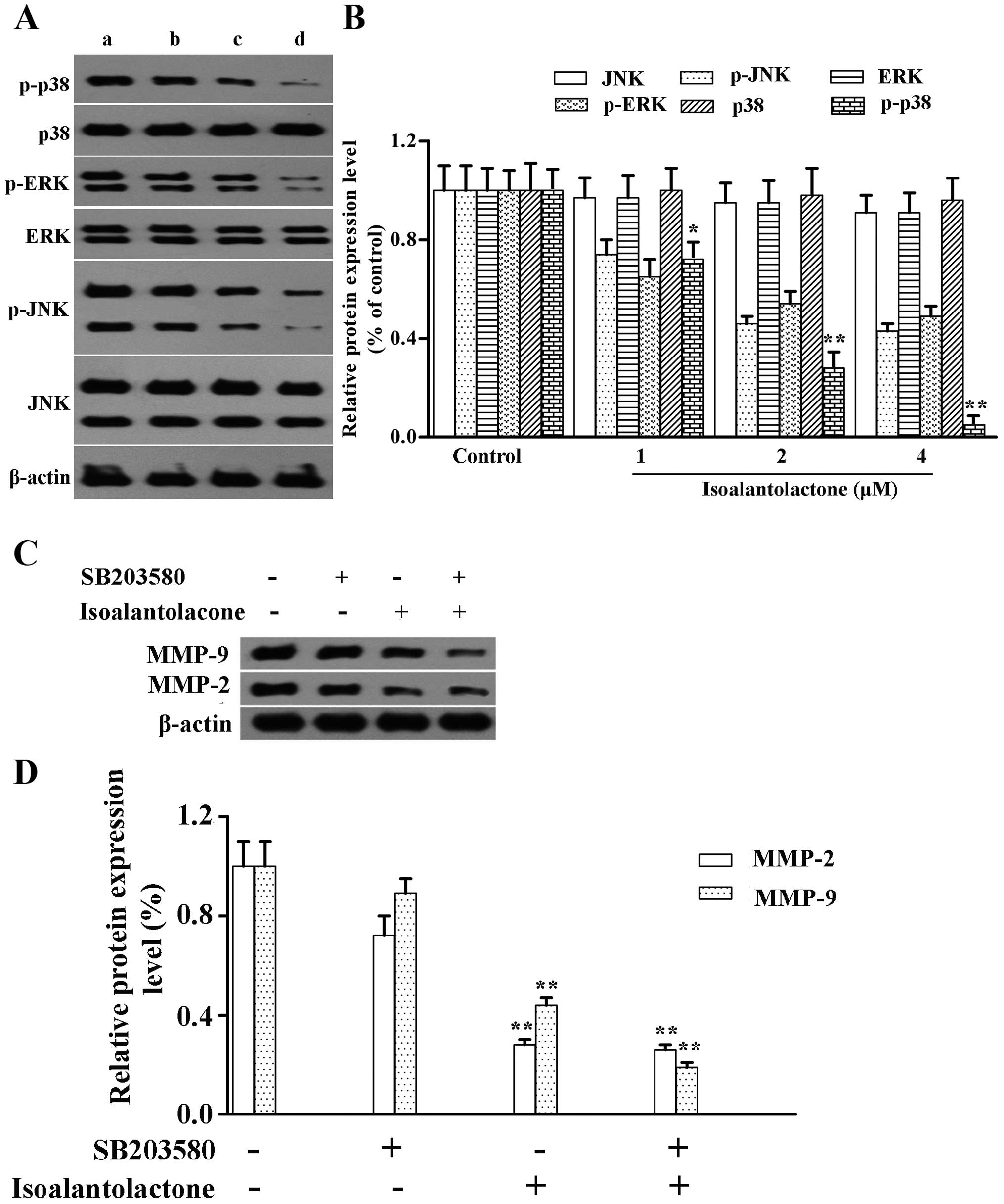
Isoalantolactone suppresses p38 MAPK
activity
MAPK superfamily members, including p38, ERK and
JNK, play important roles in cell invasion (24). To demonstrate whether
isoalantolactone inhibits invasion by regulating the MAPK signaling
pathway, we determined the protein expression of phosphorylated
p38, ERK, JNK in the isoalantolactone-treated MDA-MB-231 cells.
Western blotting indicated that isoalantolactone downregulated the
protein expression of p-p38 MAPK in a concentration-dependent
manner with only a slight effect on the protein expression of
p-ERK1/2 and p-JNK1/2 (Fig. 4A and
B). The total protein levels were not affected. The
observations revealed that isoalantolactone specifically suppressed
p38 MAPK activity.
To explore the possible functional relationship
between p38 MAPK and MMP-2 and MMP-9, MDA-MB-231 cells were treated
with SB203580 (p38 MAPK-specific inhibitor) with or without
isoalantolactone for 24 h. Western blot analysis indicated that the
inhibitor decreased the protein expression of MMP-2 and MMP-9 that
was increased by isoalantolactone (Fig.
4C and D). The results indicated that isoalantolactone
downregulated the expression of MMP-2 and MMP-9 proteins and in
vitro invasion via blocking the activation of p38 MAPK.
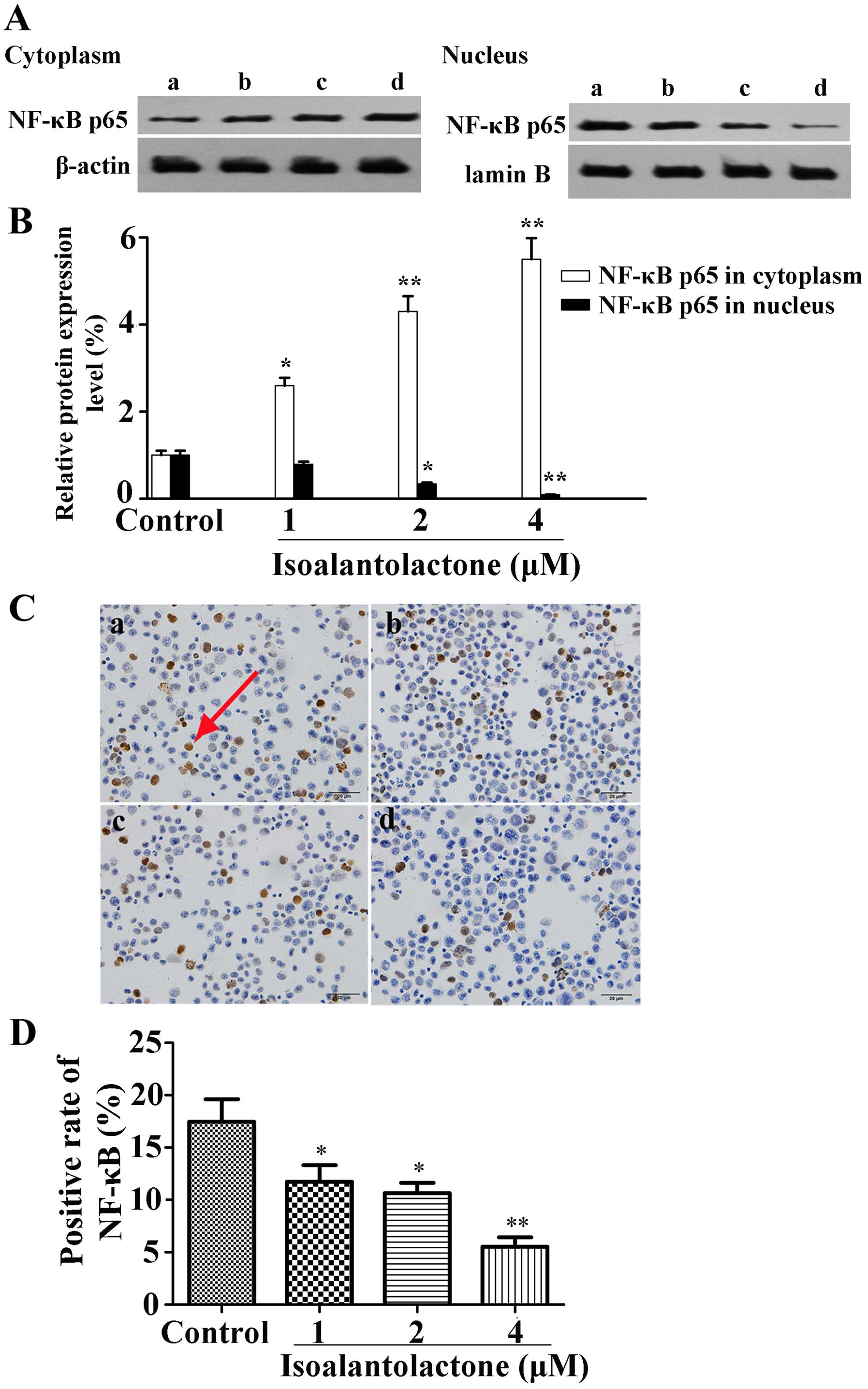
Isoalantolactone inhibits NF-κB p65
activation in the MDA-MB-231 cells
To demonstrate the involvement of the NF-κB pathway,
the effect of isoalantolactone on NF-κB p65 protein expression in
the MDA-MB-231 cells was determined using western blot analysis. As
shown in Fig. 5A and B,
isoalantolactone markedly blocked the translocation of NF-κB p65
from the cytoplasm into the nucleus. In addition,
immunocytochemical analysis was performed to clarify the
translocation of NF-κB p65 to the nucleus. As shown in Fig. 5C and D, isoalantolactone inhibited
the level of NF-κB p65 protein in the nucleus in a dose-dependent
manner.
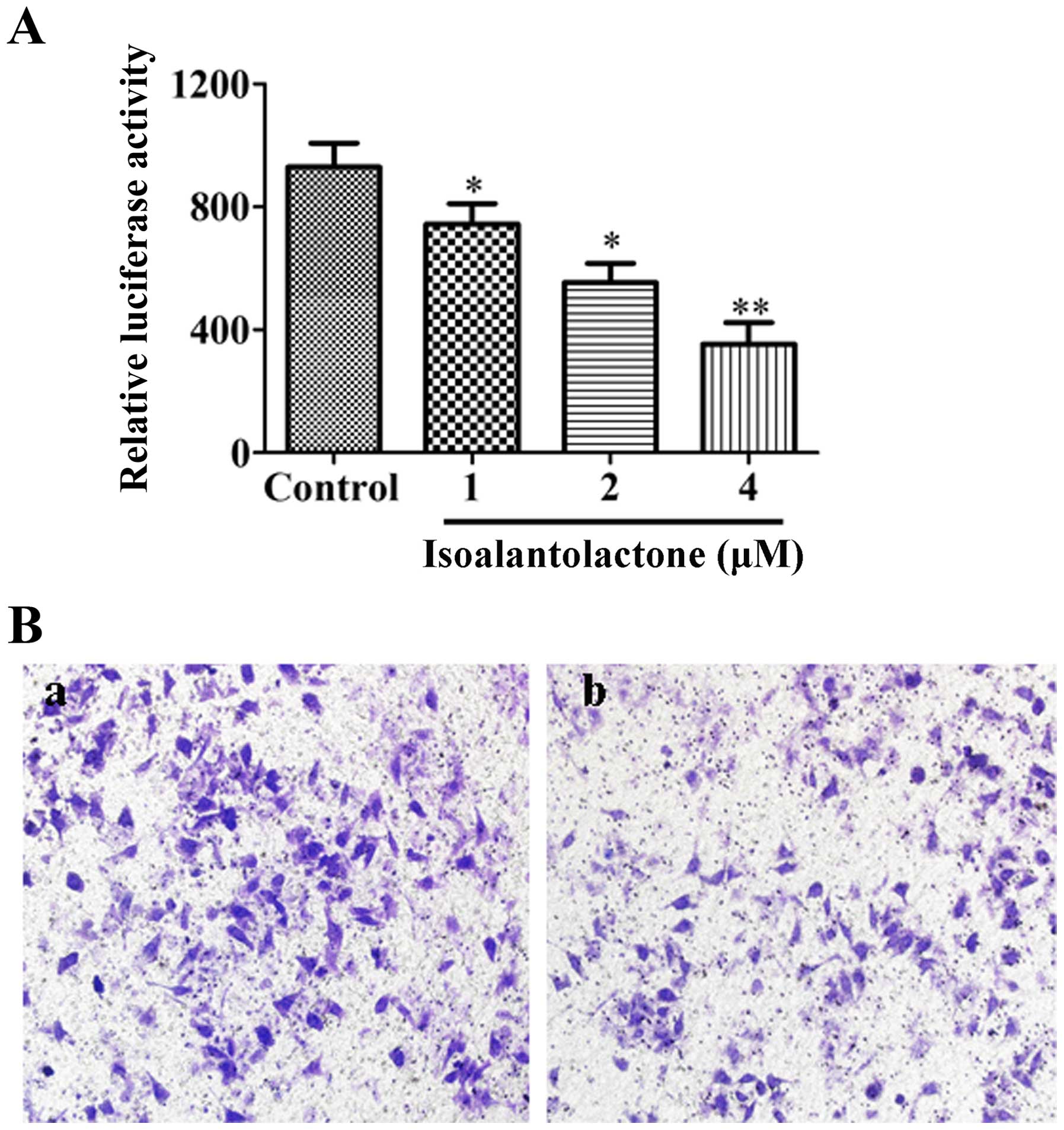
Effect of isoalantolactone on
NF-κB-mediated transcriptional activity
The effect of isoalantolactone on NF-κB-mediated
transcriptional activity was assessed. As shown in Fig. 6A, isoalantolactone significantly
reduced luciferase activity compared with that in the control cells
in a dose-dependent manner. To demonstrate the essential role of
NF-κB signaling in the inhibitory effect of isoalantolactone on
invasion, MDA-MB-231 cells after transfection were treated with 4
μM isoalantolactone. The result indicated that
isoalantolactone inhibited cell invasion (37% decrease, Fig. 6B), which was weaker than the
inhibitory effect of isoalantolactone on the non-transfected cells
(Fig. 2D and E).
Discussion
Metastasis is the primary cause of the
cancer-related high mortality rate worldwide (25,26).
Therefore, the search for agents which can be employed to
effectively inhibit cancer metastasis is urgent (27). Due to drug resistance and toxic
side-effects of current chemotherapy, herbal medicine has attracted
the attention of scientists. Isoalantolactone is a natural
sesquiterpene lactone extracted from Inula helenium.
Recently published studies have demonstrated that isoalantolactone
possesses anticancer activity against osteosarcoma U2OS, UM-SCC-10A
and SGC-7901 cells (13,16,18).
In the present study, we found that isoalantolactone potently
suppressed cell adhesion, migration and invasion at concentrations
with no effect on cell viability to exert its anticancer effect
(Figs. 1 and 2). Our study suggests that
isoalantolactone may be a promising candidate agent against the
metastasis of breast cancer.
MMPs, a family of well-characterized structurally
related zinc-dependent proteases, play a pivotal role in tissue
remodeling (28–30). Among the MMP members, MMP-2 and
MMP-9 are commonly related to tumor migration, invasion, and
metastasis in various human cancers, due to their capacities to
degrade type IV collagen which is an important component of the
basement membrane, leading to increased cell motility (31). Previous studies have confirmed that
high levels and activation of MMP-2 and MMP-9 significantly enhance
cancer cell invasion (32). Our
findings indicated that isoalantolactone downregulated the activity
and expression of MMP-2 and MMP-9 at the protein and mRNA levels in
a dose-dependent manner in MDA-MB-231 cells (Fig. 3), suggesting that isoalantolactone
inhibited cancer cell invasion via a decrease in MMP-2 and MMP-9,
thus resulting in failure of tissue remodeling via the ECM.
The MAPK-mediated signaling pathway is closely
associated with cell invasion and metastasis during the development
and progression of tumors (33,34).
It was recently reported that the MAPK signaling pathway is
involved in the modulation of MMP-2 and MMP-9 levels in cancer
cells (35). In the present study,
we found that isoalantolactone decreased the levels of p-p38 MAPK,
but did not reduce the expression of p-ERK1/2 or p-JNK1/2 (Fig. 4A and B). Additionally, SB203580 (a
specific inhibitor of p38 MAPK) reduced the expression of MMP-2 and
MMP-9 proteins and suppressed MDA-MB-231 cell invasion to the same
degree as did isoalantolactone (Fig.
4C–E). The results were in line with previous studies, which
demonstrated that p38 MAPK activity was needed for
12-O-tetradecanoylphorbol-13-acetate (TPA)-, hepatitis C
virus (HCV)-, HOXA10 overexpression-induced increase of MMP in
cancer cells (9,36,37).
Thus, isoalantolactone inhibits the invasion of MDA-MB-231 cells
via blocking the activity of p38 MAPK.
It was reported that activation of the p38 MAPK
pathway activates NF-κB, which promotes NF-κB translocation to the
nucleus and then regulates NF-κB-dependent MMP transcription
(38). Activated NF-κB by p38 MAPK
induced the invasion of prostate cancer cells by inhibiting MMP-9
expression (39). Our results
indicated that isoalantolactone inhibited the translocation of
NF-κB p65 to the nucleus (Fig. 5).
Therefore, isoalantolactone decreased the level of MMPs and had an
anti-invasive effect.
In summary, the present study demonstrated for the
first time that isoalantolactone treatment resulted in the
inhibition of adhesion, migration, and invasion of highly
metastatic MDA-MB-231 breast cancer cells in vitro.
Isoalantolactone reduced the level and activation of MMP-2 and
MMP-9 by blocking the p38 MAPK signaling pathway and NF-κB p65
transcriptional activity. These results provide experimental
evidence for further development of isoalantolactone as a potent
agent against MDA-MB-231 cells and might be used as a promising
chemopreventive agent for the treatment of breast cancer.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81403121).
References
|
1
|
Carvalho I, Milanezi F, Martins A, Reis RM
and Schmitt F: Overexpression of platelet-derived growth factor
receptor alpha in breast cancer is associated with tumour
progression. Breast Cancer Res. 7:R788–R795. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Côme C, Laine A, Chanrion M, Edgren H,
Mattila E, Liu X, Jonkers J, Ivaska J, Isola J, Darbon JM, et al:
CIP2A is associated with human breast cancer aggressivity. Clin
Cancer Res. 15:5092–5100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng X, Gu J, Zhang M, Yuan J, Zhao B,
Jiang J and Jia X: Astragaloside IV inhibits migration and invasion
in human lung cancer A549 cells via regulating PKC-α-ERK1/2-NF-κB
pathway. Int Immunopharmacol. 23:304–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mohammad MA, Zeeneldin AA, Abd Elmageed
ZY, Khalil EH, Mahdy SM, Sharada HM, Sharawy SK and Abdel-Wahab AH:
Clinical relevance of cyclooxygenase-2 and matrix
metalloproteinases (MMP-2 and MT1-MMP) in human breast cancer
tissue. Mol Cell Biochem. 366:269–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim TD, Song KS, Li G, Choi H, Park HD,
Lim K, Hwang BD and Yoon WH: Activity and expression of
urokinase-type plasminogen activator and matrix metalloproteinases
in human colorectal cancer. BMC Cancer. 6:2112006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bodey B, Bodey B Jr, Gröger AM, Siegel SE
and Kaiser HE: Invasion and metastasis: The expression and
significance of matrix metalloproteinases in carcinomas of the
lung. In Vivo. 15:175–180. 2001.PubMed/NCBI
|
|
7
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trusolino L and Comoglio PM:
Scatter-factor and semaphorin receptors: Cell signalling for
invasive growth. Nat Rev Cancer. 2:289–300. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noh EM, Park YJ, Kim JM, Kim MS, Kim HR,
Song HK, Hong OY, So HS, Yang SH, Kim JS, et al: Fisetin regulates
TPA-induced breast cell invasion by suppressing matrix
metalloproteinase-9 activation via the PKC/ROS/MAPK pathways. Eur J
Pharmacol. 764:79–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia P, Zhang R and Ge G: C/EBPβ mediates
TNF-α-induced cancer cell migration by inducing MMP expression
dependent on p38 MAPK. J Cell Biochem. 116:2766–2777. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu ZL, Liu Q, Xiao B, Zhou J, Zhang JG
and Li Y: The vascular protective properties of kinsenoside
isolated from Anoectochilus roxburghii under high glucose
condition. Fitoterapia. 86:163–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmidt TJ, Brun R, Willuhn G and Khalid
SA: Antitrypanosomal activity of helenalin and some structurally
related sesquiterpene lactones. Planta Med. 68:750–751. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di W, Khan M, Rasul A, Sun M, Sui Y, Zhong
L, Yang L, Zhu Q, Feng L and Ma T: Isoalantolactone inhibits
constitutive NF-κB activation and induces reactive oxygen
species-mediated apoptosis in osteosarcoma U2OS cells through
mitochondrial dysfunction. Oncol Rep. 32:1585–1593. 2014.PubMed/NCBI
|
|
14
|
Stojanović-Radić Z, Comić Lj, Radulović N,
Blagojević P, Denić M, Miltojević A, Rajković J and
Mihajilov-Krstev T: Antistaphylococcal activity of Inula helenium
L. root essential oil: Eudesmane sesquiterpene lactones induce cell
membrane damage. Eur J Clin Microbiol Infect Dis. 31:1015–1025.
2012. View Article : Google Scholar
|
|
15
|
Kumar A, Kumar S, Kumar D and Agnihotri
VK: UPLC/MS/MS method for quantification and cytotoxic activity of
sesquiterpene lactones isolated from Saussurea lappa. J
Ethnopharmacol. 155:1393–1397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu M, Zhang H, Hu J, Weng Z, Li C, Li H,
Zhao Y, Mei X, Ren F and Li L: Isoalantolactone inhibits UM-SCC-10A
cell growth via cell cycle arrest and apoptosis induction. PLoS
One. 8:e760002013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rasul A, Di J, Millimouno FM, Malhi M,
Tsuji I, Ali M, Li J and Li X: Reactive oxygen species mediate
isoalantolactone-induced apoptosis in human prostate cancer cells.
Molecules. 18:9382–9396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rasul A, Khan M, Yu B, Ali M, Bo YJ, Yang
H and Ma T: Isoalantolactone, a sesquiterpene lactone, induces
apoptosis in SGC-7901 cells via mitochondrial and
phosphatidylinositol 3-kinase/Akt signaling pathways. Arch Pharm
Res. 36:1262–1269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang C, Chi YL, Wang PY, Wang YQ, Zhang
YX, Deng J, Lv CJ and Xie SY: miR-511 and miR-1297 inhibit human
lung adenocarcinoma cell proliferation by targeting oncogene TRIB2.
PLoS One. 7:e460902012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu W, He X, Kong J and Ye B: mir-373
affects human lung cancer cells' growth and its E-cadherin
expression. Oncol Res. 20:163–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Albini A, Benelli R, Noonan DM and Brigati
C: The 'chemoinvasion assay': A tool to study tumor and endothelial
cell invasion of basement membranes. Int J Dev Biol. 48:563–571.
2004. View Article : Google Scholar
|
|
22
|
Feng L, Zhu MM, Zhang MH, Wang RS, Tan XB,
Song J, Ding SM, Jia XB and Hu SY: Protection of glycyrrhizic acid
against AGEs-induced endothelial dysfunction through inhibiting
RAGE/NF-κB pathway activation in human umbilical vein endothelial
cells. J Ethnopharmacol. 148:27–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Ling Y, Chen Y, Li CL, Feng F, You
QD, Lu N and Guo QL: Flavonoid baicalein suppresses adhesion,
migration and invasion of MDA-MB-231 human breast cancer cells.
Cancer Lett. 297:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang S, Liu Q, Zhang Y, Liu K, Yu P, Liu
K, Luan J, Duan H, Lu Z, Wang F, et al: Suppression of growth,
migration and invasion of highly-metastatic human breast cancer
cells by berbamine and its molecular mechanisms of action. Mol
Cancer. 8:812009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu E, Zhao J, Ma J, Wang C, Zhang C, Jiang
H, Cheng J, Gao R and Zhou X: miR-146b-5p promotes invasion and
metastasis contributing to chemoresistance in osteosarcoma by
targeting zinc and ring finger 3. Oncol Rep. 35:275–283. 2016.
|
|
27
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: Multifunctional contributors to tumor
progression. Mol Med Today. 6:149–156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan C and Boyd DD: Regulation of matrix
metalloproteinase gene expression. J Cell Physiol. 211:19–26. 2007.
View Article : Google Scholar
|
|
30
|
Kim JH, Yang YI, Ahn JH, Lee JG, Lee KT
and Choi JH: Deer (Cervus elaphus) antler extract suppresses
adhesion and migration of endometriotic cells and regulates MMP-2
and MMP-9 expression. J Ethnopharmacol. 140:391–397. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang SF, Chen MK, Hsieh YS, Yang JS,
Zavras AI, Hsieh YH, Su SC, Kao TY, Chen PN and Chu SC:
Antimetastatic effects of Terminalia catappa L. on oral cancer via
a down-regulation of metastasis-associated proteases. Food Chem
Toxicol. 48:1052–1058. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Puricelli L, Dell'Aica I, Sartor L,
Garbisa S and Caniato R: Preliminary evaluation of inhibition of
matrix-metalloprotease MMP-2 and MMP-9 by Passiflora edulis and P.
foetida aqueous extracts. Fitoterapia. 74:302–304. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Niu J, Huang Y and Zhang L: CXCR4
silencing inhibits invasion and migration of human laryngeal cancer
Hep-2 cells. Int J Clin Exp Pathol. 8:6255–6261. 2015.PubMed/NCBI
|
|
34
|
Zhan Y, Zhang H, Liu R, Wang W, Qi J and
Zhang Y: Eupolyphaga sinensis Walker ethanol extract suppresses
cell growth and invasion in human breast cancer cells. Integr
Cancer Ther. 15:102–112. 2016. View Article : Google Scholar
|
|
35
|
Chen Y, Zheng L, Liu J, Zhou Z, Cao X, Lv
X and Chen F: Shikonin inhibits prostate cancer cells metastasis by
reducing matrix metalloproteinase-2/-9 expression via AKT/mTOR and
ROS/ERK1/2 pathways. Int Immunopharmacol. 21:447–455. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu L, Zhang Q, Wu K, Chen X, Zheng Y, Zhu
C and Wu J: Hepatitis C virus NS3 protein enhances cancer cell
invasion by activating matrix metalloproteinase-9 and
cyclooxygenase-2 through ERK/p38/NF-κB signal cascade. Cancer Lett.
356:470–478. 2015. View Article : Google Scholar
|
|
37
|
Cui XP, Qin CK, Zhang ZH, Su ZX, Liu X,
Wang SK and Tian XS: HOXA10 promotes cell invasion and MMP-3
expression via TGFβ2-mediated activation of the p38 MAPK pathway in
pancreatic cancer cells. Dig Dis Sci. 59:1442–1451. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim D, Kim S, Koh H, Yoon SO, Chung AS,
Cho KS and Chung J: Akt/PKB promotes cancer cell invasion via
increased motility and metalloproteinase production. FASEB J.
15:1953–1962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu P, Cai F, Liu X and Guo L: Sesamin
inhibits lipopolysaccharide-induced proliferation and invasion
through the p38-MAPK and NF-κB signaling pathways in prostate
cancer cells. Oncol Rep. 33:3117–3123. 2015.PubMed/NCBI
|