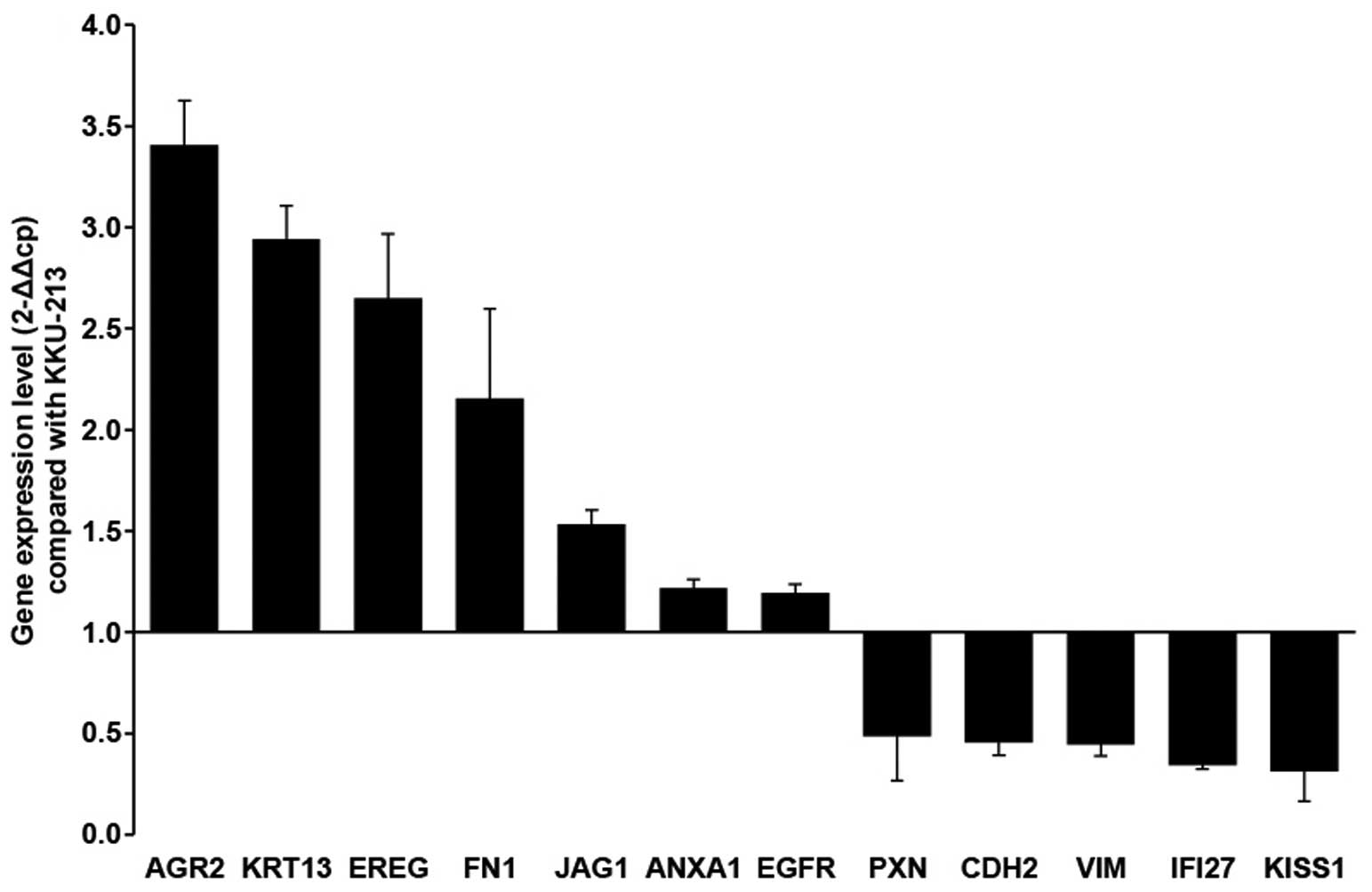
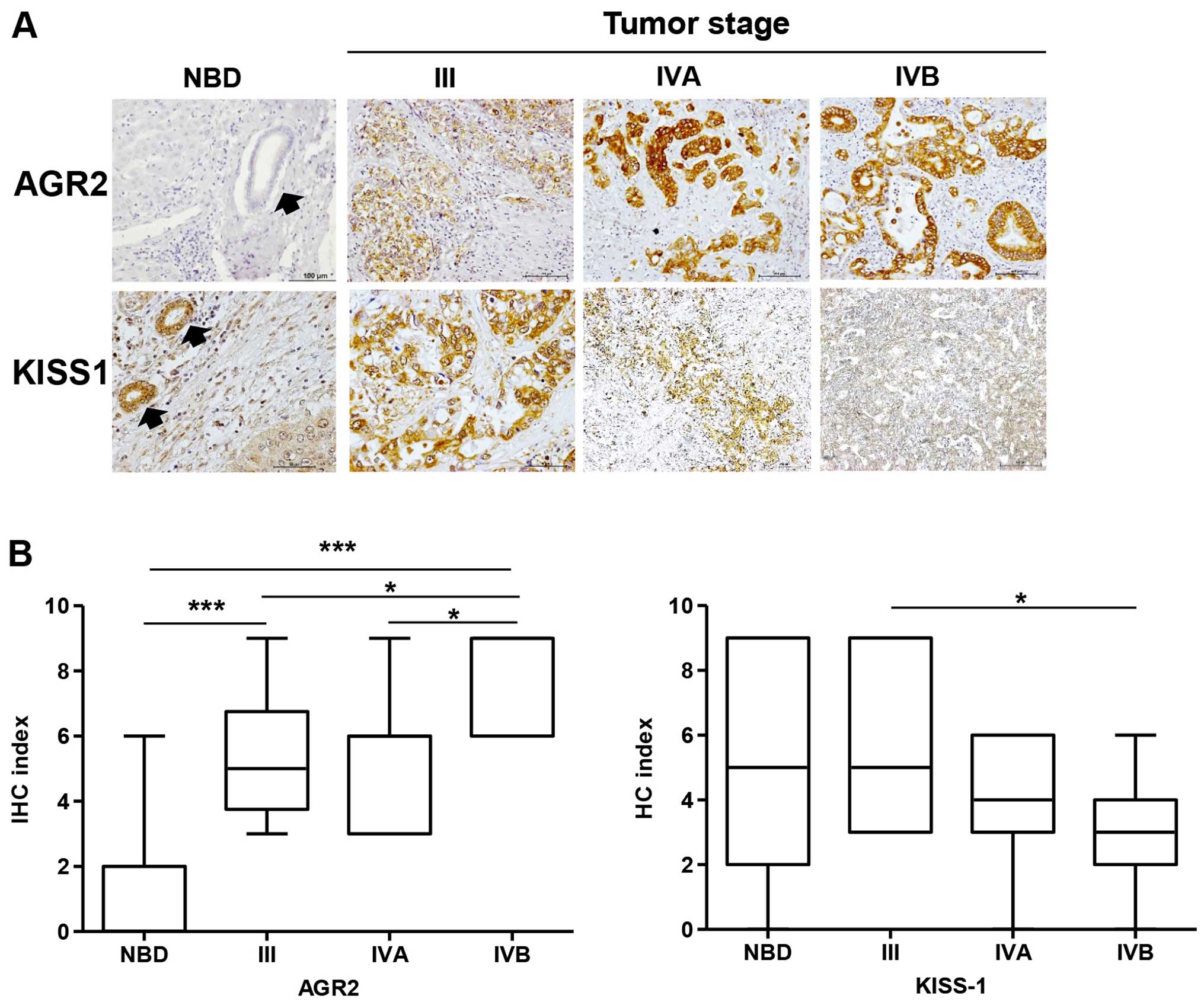
|
1
|
Fidler IJ: The pathogenesis of cancer
metastasis: The 'seed and soil̓ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
3
|
Wakahara T, Tsukamoto T, Kitamura S,
Watanabe A, Tsujimura T, Nakamura Y, Toyokawa A, Onishi N, Hamabe
Y, Mukai H, et al: Metastatic colon cancer from intrahepatic
cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 12:415–418.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamamoto M, Takasaki K and Yoshikawa T:
Lymph node metastasis in intrahepatic cholangiocarcinoma. Jpn J
Clin Oncol. 29:147–150. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuroki T, Fukuda K, Tajima Y, Matsuzaki S,
Kitajima T, Furui J and Kanematsu T: Parapapillary
choledochoduodenal fistula associated with cholangiocarcinoma. J
Hepatobiliary Pancreat Surg. 12:143–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takahashi I, Nakamura Y, Suzuki Y,
Mitsuhashi N, Niibe H and Osada J: Case report: Bone metastasis
from cholangiocarcinoma showing unusual accumulation on bone
scintigraphy and 67Ga scintigraphy. Br J Radiol. 67:303–305. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li SQ, Liang LJ, Hua YP, Peng BG, He Q, Lu
MD and Chen D: Long-term outcome and prognostic factors of
intrahepatic cholangiocarcinoma. Chin Med J. 122:2286–2291.
2009.
|
|
8
|
Sripa B, Leungwattanawanit S, Nitta T,
Wongkham C, Bhudhisawasdi V, Puapairoj A, Sripa C and Miwa M:
Establishment and characterization of an opisthorchiasis-associated
cholangiocarcinoma cell line (KKU-100). World J Gastroenterol.
11:3392–3397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okada S, Harada H, Ito T, Saito T and Suzu
S: Early development of human hematopoietic and acquired immune
systems in new born NOD/Scid/Jak3null mice intrahepatic
engrafted with cord blood-derived CD34+ cells. Int J
Hematol. 88:476–482. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patterson MK Jr: Measurement of growth and
viability of cells in culture. Methods Enzymol. 58:141–152. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grenman R, Burk D, Virolainen E, Buick RN,
Church J, Schwartz DR and Carey TE: Clonogenic cell assay for
anchorage-dependent squamous carcinoma cell lines using limiting
dilution. Int J Cancer. 44:131–136. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uthaisar K, Seubwai W, Srikoon P,
Vaeteewoottacharn K, Sawanyawisuth K, Okada S and Wongkham S:
Cepharanthine suppresses metastatic potential of human
cholangiocarcinoma cell lines. Asian Pac J Cancer Prev. 13(Suppl):
S149–S154. 2012.
|
|
13
|
Kan J, Thomson S, Argast GM, O'Connor ME,
Robinson M, Feng B, Heyer J, Chiu MI and Nicoletti R: Use of EMT
gene signatures in cancer drug discovery, diagnostics and
treatment. (US Patent Application Publication. Pub. No.:
US2012/0302572 A1). 2012
|
|
14
|
Li F, Tiede B, Massagué J and Kang Y:
Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res.
17:3–14. 2007. View Article : Google Scholar
|
|
15
|
Mishra L, Banker T, Murray J, Byers S,
Thenappan A, He AR, Shetty K, Johnson L and Reddy EP: Liver stem
cells and hepatocellular carcinoma. Hepatology. 49:318–329. 2009.
View Article : Google Scholar :
|
|
16
|
Sirisinha S, Tengchaisri T, Boonpucknavig
S, Prempracha N, Ratanarapee S and Pausawasdi A: Establishment and
characterization of a cholangiocarcinoma cell line from a Thai
patient with intrahepatic bile duct cancer. Asian Pac J Allergy
Immunol. 9:153–157. 1991.PubMed/NCBI
|
|
17
|
Rattanasinganchan P, Leelawat K,
Treepongkaruna SA, Tocharoentanaphol C, Subwongcharoen S,
Suthiphongchai T and Tohtong R: Establishment and characterization
of a cholangiocarcinoma cell line (RMCCA-1) from a Thai patient.
World J Gastroenterol. 12:6500–6506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goodman ZD, Terracciano LM and Wee A: 14 -
Tumours and tumour-like lesions of the liver. MacSween's Pathology
of the Liver. Burt AD, Portmann BC and Ferrell LD: 6th edition.
Churchill Livingstone; Edinburgh: pp. 761–851. 2012, View Article : Google Scholar
|
|
19
|
Asai T, Ueda T, Itoh K, Yoshioka K, Aoki
Y, Mori S and Yoshikawa H: Establishment and characterization of a
murine osteosarcoma cell line (LM8) with high metastatic potential
to the lung. Int J Cancer. 76:418–422. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue
Q, Chen J, Gao DM and Bao WH: Establishment of cell clones with
different metastatic potential from the metastatic hepatocellular
carcinoma cell line MHCC97. World J Gastroenterol. 7:630–636.
2001.
|
|
21
|
Barroga EF, Kadosawa T, Okumura M and
Fujinaga T: Establishment and characterization of the growth and
pulmonary metastasis of a highly lung metastasizing cell line from
canine osteosarcoma in nude mice. J Vet Med Sci. 61:361–367. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su Y, Luo X, He BC, Wang Y, Chen L, Zuo
GW, Liu B, Bi Y, Huang J, Zhu GH, et al: Establishment and
characterization of a new highly metastatic human osteosarcoma cell
line. Clin Exp Metastasis. 26:599–610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou Y, Zou Q, Ge R, Shen F and Wang Y: The
critical role of CD133+CD44+/high tumor cells
in hematogenous metastasis of liver cancers. Cell Res. 22:259–272.
2012. View Article : Google Scholar
|
|
24
|
Thanan R, Pairojkul C, Pinlaor S,
Khuntikeo N, Wongkham C, Sripa B, Ma N, Vaeteewoottacharn K,
Furukawa A, Kobayashi H, et al: Inflammation-related DNA damage and
expression of CD133 and Oct3/4 in cholangiocarcinoma patients with
poor prognosis. Free Radic Biol Med. 65:1464–1472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo W and Frenette PS: Alternative CD44
splicing in intestinal stem cells and tumorigenesis. Oncogene.
33:537–538. 2014. View Article : Google Scholar
|
|
26
|
Hajra KM and Fearon ER: Cadherin and
catenin alterations in human cancer. Genes Chromosomes. Cancer.
34:255–268. 2002.
|
|
27
|
Skubitz AP: Adhesion molecules. Cancer
Treat Res. 107:305–329. 2002.PubMed/NCBI
|
|
28
|
Adam PJ, Boyd R, Tyson KL, Fletcher GC,
Stamps A, Hudson L, Poyser HR, Redpath N, Griffiths M, Steers G, et
al: Comprehensive proteomic analysis of breast cancer cell
membranes reveals unique proteins with potential roles in clinical
cancer. J Biol Chem. 278:6482–6489. 2003. View Article : Google Scholar
|
|
29
|
Dumartin L, Whiteman HJ, Weeks ME,
Hariharan D, Dmitrovic B, Iacobuzio-Donahue CA, Brentnall TA,
Bronner MP, Feakins RM, Timms JF, et al: AGR2 is a novel surface
antigen that promotes the dissemination of pancreatic cancer cells
through regulation of cathepsins B and D. Cancer Res. 71:7091–7102.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vanderlaag KE, Hudak S, Bald L,
Fayadat-Dilman L, Sathe M, Grein J and Janatpour MJ: Anterior
gradient-2 plays a critical role in breast cancer cell growth and
survival by modulating cyclin D1, estrogen receptor-α and survivin.
Breast Cancer Res. 12:R322010. View Article : Google Scholar
|
|
31
|
Park K, Chung YJ, So H, Kim K, Park J, Oh
M, Jo M, Choi K, Lee EJ, Choi YL, et al: AGR2, a mucinous ovarian
cancer marker, promotes cell proliferation and migration. Exp Mol
Med. 43:91–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Z, Hao Y and Lowe AW: The
adenocarcinoma-associated antigen, AGR2, promotes tumor growth,
cell migration, and cellular transformation. Cancer Res.
68:492–497. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu D, Rudland PS, Sibson DR,
Platt-Higgins A and Barraclough R: Human homologue of cement gland
protein, a novel metastasis inducer associated with breast
carcinomas. Cancer Res. 65:3796–3805. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chanda D, Lee JH, Sawant A, Hensel JA,
Isayeva T, Reilly SD, Siegal GP, Smith C, Grizzle W, Singh R, et
al: Anterior gradient protein-2 is a regulator of cellular adhesion
in prostate cancer. PLoS One. 9:e899402014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lepreux S, Bioulac-Sage P and Chevet E:
Differential expression of the anterior gradient protein-2 is a
conserved feature during morphogenesis and carcinogenesis of the
biliary tree. Liver Int. 31:322–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim SJ, Kim DH, Kang D and Kim JH:
Expression of anterior gradient 2 is decreased with the progression
of human biliary tract cancer. Tohoku J Exp Med. 234:83–88. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hata K, Dhar DK, Watanabe Y, Nakai H and
Hoshiai H: Expression of metastin and a G-protein-coupled receptor
(AXOR12) in epithelial ovarian cancer. Eur J Cancer. 43:1452–1459.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Prentice LM, Klausen C, Kalloger S, Köbel
M, McKinney S, Santos JL, Kenney C, Mehl E, Gilks CB, Leung P, et
al: Kisspeptin and GPR54 immunoreactivity in a cohort of 518
patients defines favourable prognosis and clear cell subtype in
ovarian carcinoma. BMC Med. 5:332007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dhar DK, Naora H, Kubota H, Maruyama R,
Yoshimura H, Tonomoto Y, Tachibana M, Ono T, Otani H and Nagasue N:
Downregulation of KiSS-1 expression is responsible for tumor
invasion and worse prognosis in gastric carcinoma. Int J Cancer.
111:868–872. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guan-Zhen Y, Ying C, Can-Rong N, Guo-Dong
W, Jian-Xin Q and Jie-Jun W: Reduced protein expression of
metastasis-related genes (nm23, KISS1, KAI1 and p53) in lymph node
and liver metastases of gastric cancer. Int J Exp Pathol.
88:175–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Masui T, Doi R, Mori T, Toyoda E, Koizumi
M, Kami K, Ito D, Peiper SC, Broach JR, Oishi S, et al: Metastin
and its variant forms suppress migration of pancreatic cancer
cells. Biochem Biophys Res Commun. 315:85–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nagai K, Doi R, Katagiri F, Ito T, Kida A,
Koizumi M, Masui T, Kawaguchi Y, Tomita K, Oishi S, et al:
Prognostic value of metastin expression in human pancreatic cancer.
J Exp Clin Cancer Res. 28:92009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kostadima L, Pentheroudakis G and Pavlidis
N: The missing kiss of life: Transcriptional activity of the
metastasis suppressor gene KiSS1 in early breast cancer. Anticancer
Res. 27:2499–2504. 2007.PubMed/NCBI
|
|
44
|
Stark AM, Tongers K, Maass N, Mehdorn HM
and Held-Feindt J: Reduced metastasis-suppressor gene
mRNA-expression in breast cancer brain metastases. J Cancer Res
Clin Oncol. 131:191–198. 2005. View Article : Google Scholar
|
|
45
|
Marot D, Bieche I, Aumas C, Esselin S,
Bouquet C, Vacher S, Lazennec G, Perricaudet M, Kuttenn F, Lidereau
R, et al: High tumoral levels of Kiss1 and G-protein-coupled
receptor 54 expression are correlated with poor prognosis of
estrogen receptor-positive breast tumors. Endocr Relat Cancer.
14:691–702. 2007. View Article : Google Scholar : PubMed/NCBI
|