Introduction
Colorectal cancer (CRC) ranks the 4th in the
mortality rates worldwide, and in China its incidence has been
increasing in recent years (1).
According to the Colon Cancer NCCN Guidelines (version 3.2014),
metastases occur in 50–60% CRC patients, and 80–90% of these
metastatic patients may have unresectable liver metastasis
(2). About 20% of CRC patients may
have synchronous liver metastases (3), which probably means worse prognosis
than the patients with metachronous liver metastases (4). Thus, it is crucial to explore the
metastatic predictors and to clarify the mechanism underlying liver
metastasis.
Protein tyrosine kinase-7 (PTK7), which is also
called Colon carcinoma kinase-4 (CCK4), is one of the receptor
protein tyrosine kinase (RPTK)-like molecules. A fragment of PTK7
was first cloned from normal melanocytes in 1993 (5). It is a transmembrane protein which
showed general homology with many tyrosine kinases but may lack
catalytical activity (6). PTK7 is
one of the co-receptors for the non-canonical WNT/PCP signaling
pathway, and it is involved in vertebrate embryogenesis (7). Aberrant expression of PTK7 has been
found in melanoma, renal clear carcinoma, gastric cancer,
epithelial ovarian carcinoma, and intrahepatic cholangiocarcinoma.
Several studies have suggested that PTK7 may be implicated in
carcinogenesis (8–12). However, whether the expression of
PTK7 correlates with development and progression of CRC remains
unclear.
Our study aimed to investigate the distinct
expression of PTK7 among non-tumorous colorectal mucosa, colonic
adenoma and colorectal carcinoma, and to clarify the correlation
between PTK7 expression and clinicopathological features or
prognosis of CRC patients, which helps to elucidate the mechanism
of CRC progression and provides a potential therapeutic target.
Materials and methods
Patients and samples
Two hundred and nine patients with CRC were included
in this study. They underwent surgical resection during 2004–2008
at Peking University Cancer Hospital, Beijing, China. Each tumor
specimen and paired non-cancerous mucosa were formalin-fixed within
30 min after resection, and then embedded with paraffin. Of the 209
patients, 203 have complete clinical records. Patients who received
neoadjuvant chemotherapy or radiation were excluded. All the
patients were followed up for at least 5 years after surgery,
wherein 12 were lost to follow-up. The clinicopathological features
of the 209 CRC patients are described in Table I. Another 14 pairs of CRC tissue and
paired non-cancerous mucosa were used for reverse transcription
polymerase chain reaction (RT-PCR) and quantitative real-time PCR
evaluation. These specimens were immediately frozen in liquid
nitrogen after resection, and were stored at −80°C until use.
Samples of colonic adenoma of 28 patients were provided by Daxing
Hospital Affiliated to Capital University of Medical Sciences (the
data of clinicopathological features of the 28 patients are not
shown). Informed consent was obtained from each patient. The
research Ethics Committee of Peking University Cancer Hospital and
that of Daxing Hospital approved this study.
 | Table IClinicopathological features of the
209 colorectal cancer patients. |
Table I
Clinicopathological features of the
209 colorectal cancer patients.
| Clinicopathological
features | No. of patients | % of patients |
|---|
| Gender |
| Male | 128 | 61.2 |
| Female | 81 | 38.8 |
| Age (years) |
| <60 | 94 | 45.0 |
| ≥60 | 115 | 55.0 |
| Tumor size (cm) |
| <5 | 113 | 54.3 |
| ≥5 – <8 | 73 | 35.1 |
| ≥8 | 22 | 10.6 |
| Tumor site |
| Right-side
colon | 54 | 25.8 |
| Transverse
colon | 15 | 7.2 |
| Left-side
colon | 61 | 29.2 |
| Rectum | 79 | 37.8 |
|
Differentiation |
| Well | 20 | 9.6 |
| Moderate | 153 | 73.2 |
| Poor | 36 | 17.2 |
| Depth of
invasion |
| T1 + T2 | 30 | 14.4 |
| T3 + T4 | 179 | 85.6 |
| Lymph node
metastasis |
| N0 | 89 | 42.6 |
| N1-2 | 120 | 57.4 |
| Distant
metastasis |
| M0 | 102 | 50.0 |
| M1 | 102 | 50.0 |
| TNM stage |
| I+II | 69 | 33.8 |
| III+IV | 135 | 66.2 |
| Vascular
invasion |
| Absent | 147 | 70.3 |
| Present | 62 | 29.7 |
RNA extraction and RT-PCR
Total RNA was extracted from 14 pairs of fresh
frozen tissues using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) following the manufacturer's instructions. Then
the RNA concentration was determined by NanoDrop 2000 (Thermo
Fisher Scientific, Waltham, MA, USA). Reverse transcription was
performed using the 5X All-In-One RT MasterMix (ABM, Inc.,
Richmond, BC, Canada). In brief, 1 µg of total RNA was added
into a 20-µl reaction volume, and then the mixture were
incubated at 25°C for 10 min, followed by synthesis at 42°C for 50
min, and termination reaction at 85°C for 5 min. Next, 2X Easy Taq
PCR SuperMix (Transgen Biotech, Beijing, China) was used to build
up a 30-µl reaction volume to carry out polymerase chain
reaction (PCR). The reaction conditions were as follows:
pre-denaturation at 94°C for 5 min; 40 cycles of denaturation at
94°C for 30 sec, annealing 30 sec at 60°C, and extension at 72°C
for 30 sec; and the last extension at 72°C for 10 min. The primer
sequences were as follows: PTK7 forward,
5′-CAGTTCCTGAGGATTTCCAAGAG-3′ and reverse, 5′-TGCATAGGGCCACCTTC-3′;
β-actin forward, 5′-TTAGTTGCGTTACACCCTTTC-3′ and reverse,
5′-ACCTTCACCGTTCCAGTTT-3′. PCR products were then separated by
electrophoresis of 2% agarose gel at 80 V for 40 min and evaluated
by UVP EC3 imaging system (UVP Inc., Upland, CA, USA).
Quantitative real-time PCR
The cDNA obtained by reverse transcription was
5-fold and 50-fold diluted for amplification of PTK7 and expressed
Alu repeats (EAR) (13),
respectively. The sequence of EAR primer was as follows: EAR
forward, 5′-GAGGCTGAGGCAGGAGAATCG-3′ and reverse,
5′-GTCGCCCAGGCTGGAGTG-3′. All reactions were performed in a 10
µl total volume containing 5 µl EvaGreen 2X qPCR
MasterMix (ABM, Inc.), 1 µl diluted cDNA and 0.1 µl
mixture of 10 µM forward primer and 10 µM reverse
primer. The amplification condition was set up as follows:
pre-denaturation at 95 °C for 10 min, followed by 45 cycles of 15
sec at 95°C and 1 min at 60°C. Melt curve stage was then performed
to confirm a single product formation. Each sample was performed in
triplicate. The mRNA expression level was assessed by calculating
the 2−ΔΔCt according to following steps: ΔCt were
determined as the cycle threshold (Ct) difference between PTK7 gene
and reference gene EAR; then ΔΔCt was calculated as the difference
between each individual sample and the average ΔCt value of
non-tumor mucosa group, and 2−ΔΔCt was calculated
thereafter.
CRC tissue microarray (TMA)
construction
All specimens were H&E stained and observed
under a microscope, representative cancerous tissue and paired
non-cancerous mucosae were matched to construct CRC TMA according
to the methods previously described (14).
Immunohistochemistry assay
The 4-µm thick slices were baked at 72°C for
1 h, and then dewaxed and rehydrated through xylene and alcohol
with graded concentrations. Hydrogen peroxide (3%) was used to
eliminate activity of endogenous peroxidase for 15 min. After PBS
washing 3 times, the antigen retrieval was performed in a pressure
cooker with EDTA (pH 8.0) (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China). After cooling to room
temperature, the sections were blocked with 5% skimmed milk at room
temperature for 1 h, followed by incubation with primary rabbit
polyclonal anti-PTK7 antibody (1:7,500, SAB3500340; Sigma-Aldrich,
St. Louis, MO, USA) at 4°C overnight. The ready-to-use EnVision™
reagent (EnVision™ detection system peroxidase/DAB, rabbit/mouse;
Dako, Glostrup, Denmark) was then used to bind the primary
antibody. The 3,3′-diaminobenzidine tetrahydrochloride kit (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd. was used to develop
substrates. After being counterstained with hematoxylin, the
sections were dehydrated with graded alcohol and xylene.
Evaluation of staining
The staining of PTK7 was examined and scored under a
microscope by two independent pathologists who were blinded to the
patient clinical data. Immunoreactivity score (IRS) was used to
assess the staining of PTK7. The percentage of positive cells (PP)
was scored as 0 (negative), 1 (<25%), 2 (25–75%), and 3
(>75%) respectively while the staining intensity (SI) was scored
as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). IRS was
determined as PP multiplied by SI, IRS=0 was determined to be
'negative' while IRS >0 was 'positive'.
Statistical analysis
SPSS statistics (version 22) was used to perform the
statistical analysis. Independent t-test was used to compare the
mRNA expression level of PTK7 between cancer tissue and
non-tumorous mucosa, and two-tailed χ2 test was used to
compare the expression of PTK7 protein in CRC TMA or adenoma.
Two-tailed χ2 test or Fisher's exact test were performed
to assess the correlation between PTK7 expression and the
clinicopathological parameters. Overall survival rates were
analyzed by Kaplan-Meier survival tests, and P-value was calculated
via log-rank test to evaluate the correlation of the patient
prognosis with PTK7 expression or other parameters. Multivariate
survival analysis was performed with Cox proportional hazards
regression model to identify the independent parameters affecting
overall survival. P<0.05 was considered significant.
Results
PTK7 mRNA expression in 14 paired fresh
frozen CRC tissues
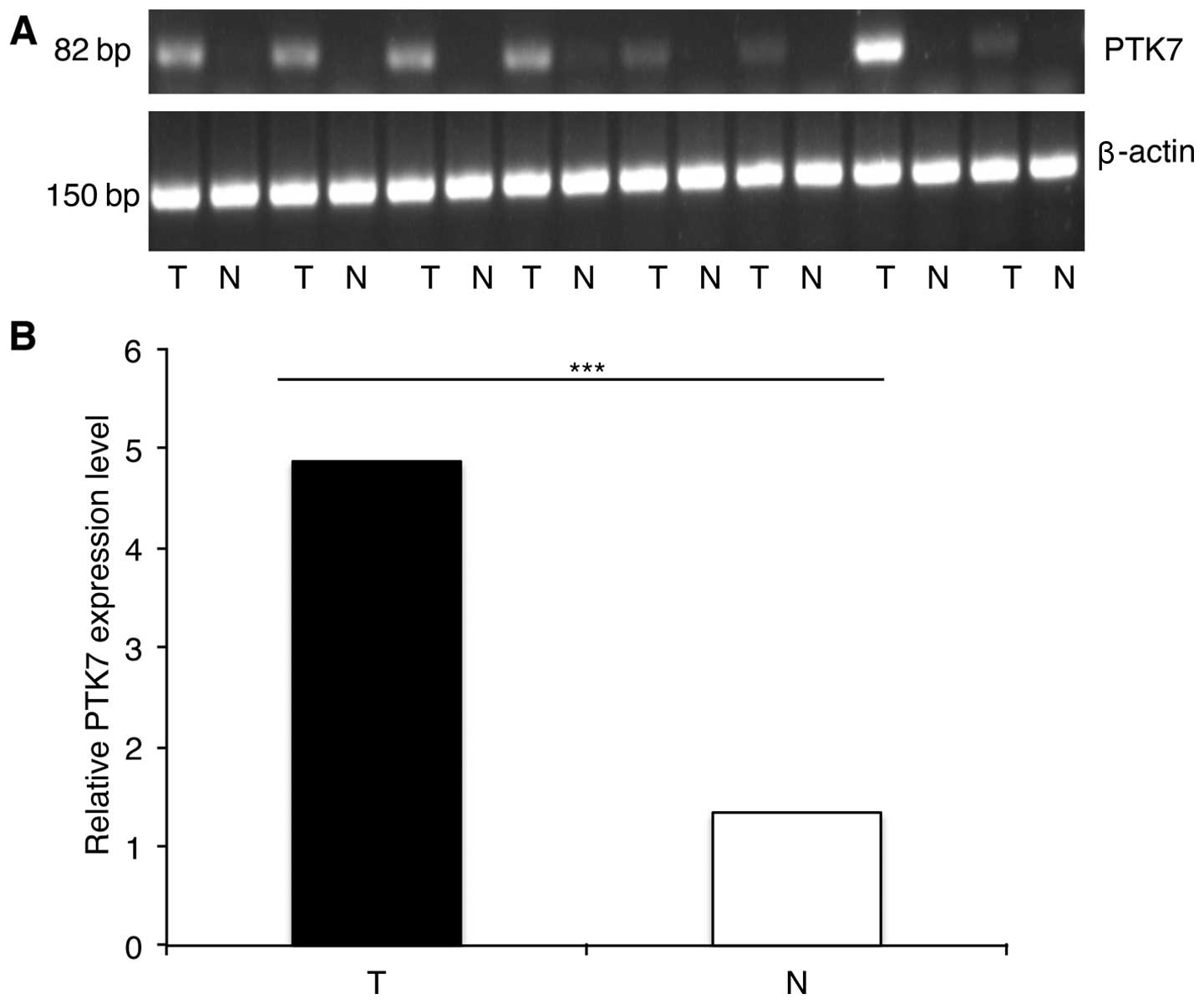
We examined PTK7 mRNA expression in 14 pairs of CRC
tissue and matched non-cancerous mucosa using RT-PCR and
quantitative real-time PCR. Of the 14 pairs, 12 pairs showed that
PTK7 expression was higher in tumor tissue than in non-cancerous
mucosa (Fig. 1), while the
remaining two pairs showed the opposite outcome. Independent t-test
based on 2−ΔΔCt further indicated that PTK7 mRNA
expression was significantly higher in CRC tissues than in matched
noncancerous mucosa (4.87±3.71 vs. 1.33±1.05; P<0.001).
Expression of PTK7 in colonic adenoma and
its association with clinicopathological features of adenoma
patients
We assessed PTK7 expression in sections of 28
patients with colonic adenoma, wherein normal colorectal mucosa was
taken along with the adenoma tissue for 27 patients. PTK7 was
positive in 29.6% normal mucosa and 75.0% adenoma tissues. PTK7
expression was markedly higher in colonic adenoma than in normal
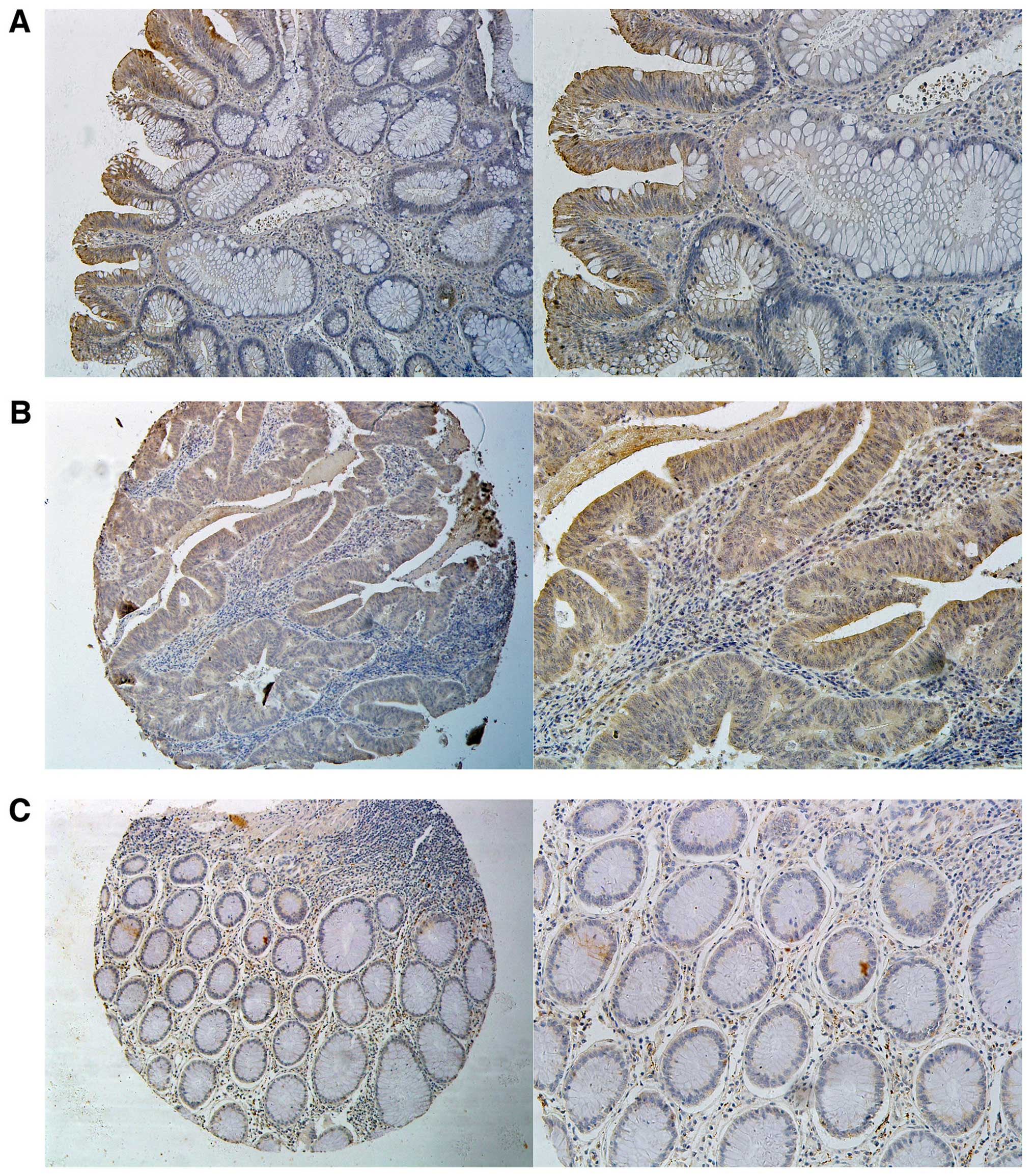
mucosa (75.0 vs. 29.6%; P=0.001). Immunohistochemistry showed a
cytoplasmic staining of representative expression of PTK7 (Fig. 2A).
Next, we assessed the correlation between PTK7
expression and the clinicopathological features of the adenoma
patients, and in these 28 patients, we did not find a correlation
between PTK7 expression and gender (P=0.198), age (P=0.198), site
(P=0.065) or stage (P=0.841) of adenoma patients (data not
shown).
Expression of PTK7 in CRC and its
correlation with clinicopathological features of CRC patients
Of the 209 cases in the immunohistochemistry assay,
7 tumor tissues and 8 non-cancerous mucosa tissues were
off-sectioned and cannot be assessed. In the remaining specimens,
PTK7 was positive in 138 of the 202 tumor tissues and 40 of the 201
non-cancerous mucosa tissues (68.3 vs. 19.9%; P<0.001),
respectively. Immunohistochemical staining showed that PTK7 protein
was predominantly localized in the cytoplasm (Fig. 2B and C).
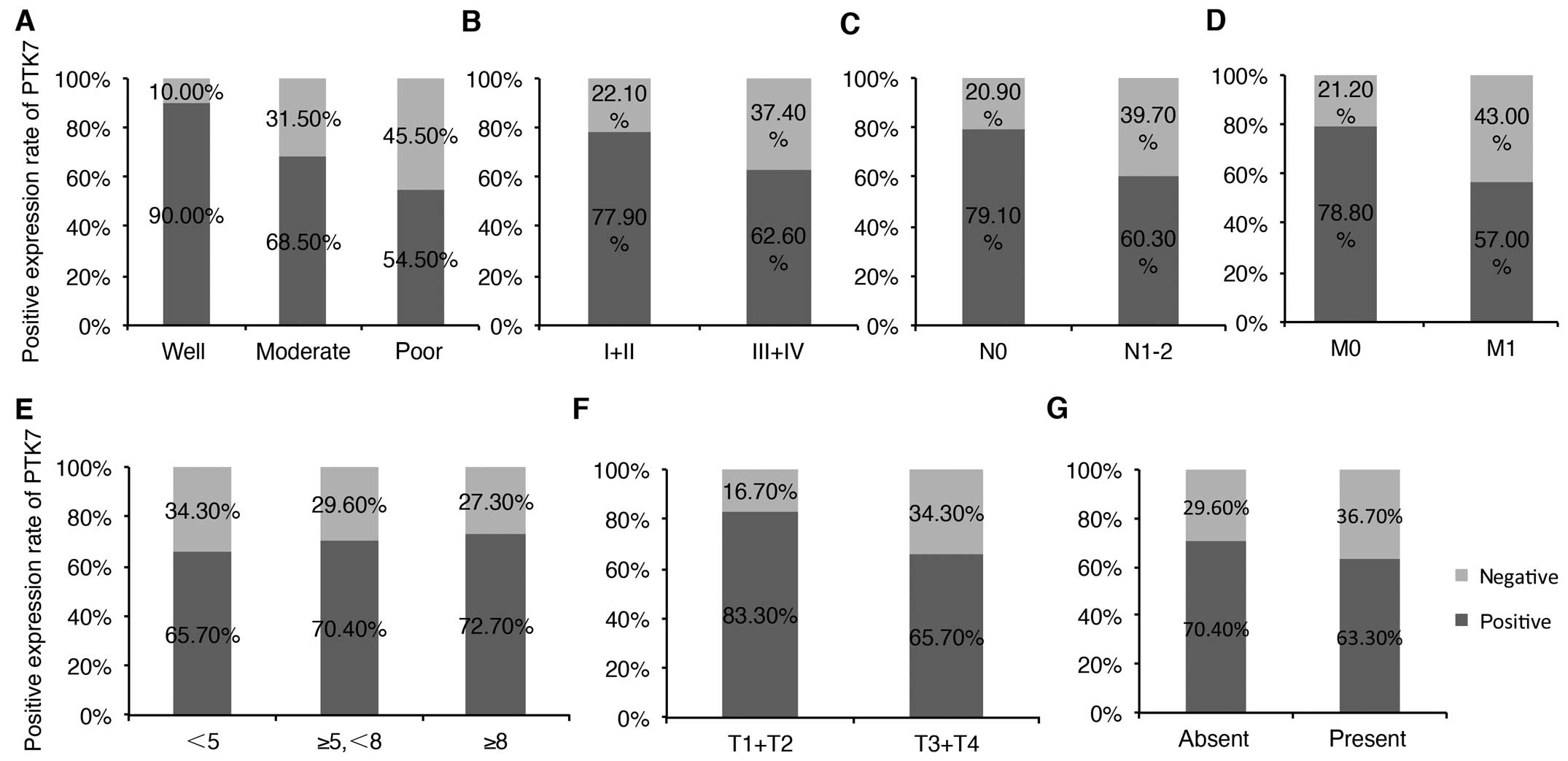
Among the 209 patients, PTK7 expression in tumor
samples was obviously higher in CRC patients with well
differentiation (P=0.027). For patients with well, moderate, poor
differentiation, PTK7 was 90, 68.5 and 54.5% positive, respectively
(Fig. 3A). Early TNM stage
(P=0.028; I+II vs. III+IV: 77.9 vs. 62.6%; Fig. 3B), and in patients without lymph
node metastasis (P=0.005; lymph node metastasis vs. no lymph node
metastasis: 79.1 vs. 60.3%; Fig.
3C) and distant metastasis (P=0.001; distant metastasis vs. no
distant metastasis: 78.8 vs. 57%; Fig.
3D). PTK7 expression was not significantly correlated with
gender, age, tumor size, tumor site, depth of invasion and vascular
invasion of CRC patients. For detailed information, see Table II. Expression of PTK7 between
tumors with diverse tumor size, depth of invasion and vascular
invasion were also compared as shown in Fig. 3E–G. PTK7 expression was a little
higher in tumors with larger size (from small size to large size,
the positive rate were 65.7, 70.4 and 72.7%, respectively; Fig. 3E), deeper tumor invasion (T1+T2 vs.
T3+T4: 83.3 vs. 65.7%; Fig. 3F) and
occurrence of vascular invasion (vascular invasion vs. without
vascular invasion: 70.4 vs. 63.3%; Fig.
3G).
 | Table IICorrelation between PTK7 expression
and clinicopathological features of the colorectal cancer
patients. |
Table II
Correlation between PTK7 expression
and clinicopathological features of the colorectal cancer
patients.
| Clinicopathological
features | No. of
patients | PTK7 expression
| P-value |
|---|
| Positive, n
(%) | Negative, n
(%) |
|---|
| Gender |
| Male | 125 | 91 (72.8) | 34 (27.2) | 0.081 |
| Female | 77 | 47 (61) | 30 (39.0) | |
| Age (years) |
| <60 | 88 | 56 (63.6) | 32 (36.4) | 0.209 |
| ≥60 | 114 | 82 (71.9) | 32 (28.1) | |
| Tumor size
(cm) |
| <5 | 108 | 71 (65.7) | 37 (34.3) | 0.715 |
| ≥5 – <8 | 71 | 50 (70.4) | 21 (29.6) | |
| ≥8 | 22 | 16 (72.7) | 6 (27.3) | |
| Tumor site |
| Right-side
colon | 52 | 39 (75.0) | 13 (25.0) | 0.535 |
| Transverse
colon | 15 | 11 (73.3) | 4 (26.7) | |
| Left-side
colon | 59 | 37 (62.7) | 22 (37.3) | |
| Rectum | 76 | 51 (67.1) | 25 (32.9) | |
|
Differentiation |
| Well | 20 | 18 (90.0) | 2 (10.0) | 0.027 |
| Moderate | 149 | 102 (68.5) | 47 (31.5) | |
| Poor | 33 | 18 (54.5) | 15 (45.5) | |
| Depth of
invasion |
| T1 + T2 | 30 | 25 (83.3) | 5 (16.7) | 0.055 |
| T3 + T4 | 172 | 113 (65.7) | 59 (34.3) | |
| Lymph node
metastasis |
| N0 | 86 | 68 (79.1) | 18 (20.9) | 0.005 |
| N1-2 | 116 | 70 (60.3) | 46 (39.7) | |
| Distant
metastasis |
| M0 | 99 | 78 (78.8) | 21 (21.2) | 0.001 |
| M1 | 100 | 57 (57.0) | 43 (43.0) | |
| TNM stage |
| I+II | 68 | 53 (77.9) | 15 (22.1) | 0.028 |
| III+IV | 131 | 82 (62.6) | 49 (37.4) | |
| Vascular
invasion |
| Absent | 142 | 100 (70.4) | 42 (29.6) | 0.322 |
| Present | 60 | 38 (63.3) | 22 (36.7) | |
Comparison of PTK7 expression in
non-tumorous mucosa, adenoma and CRC
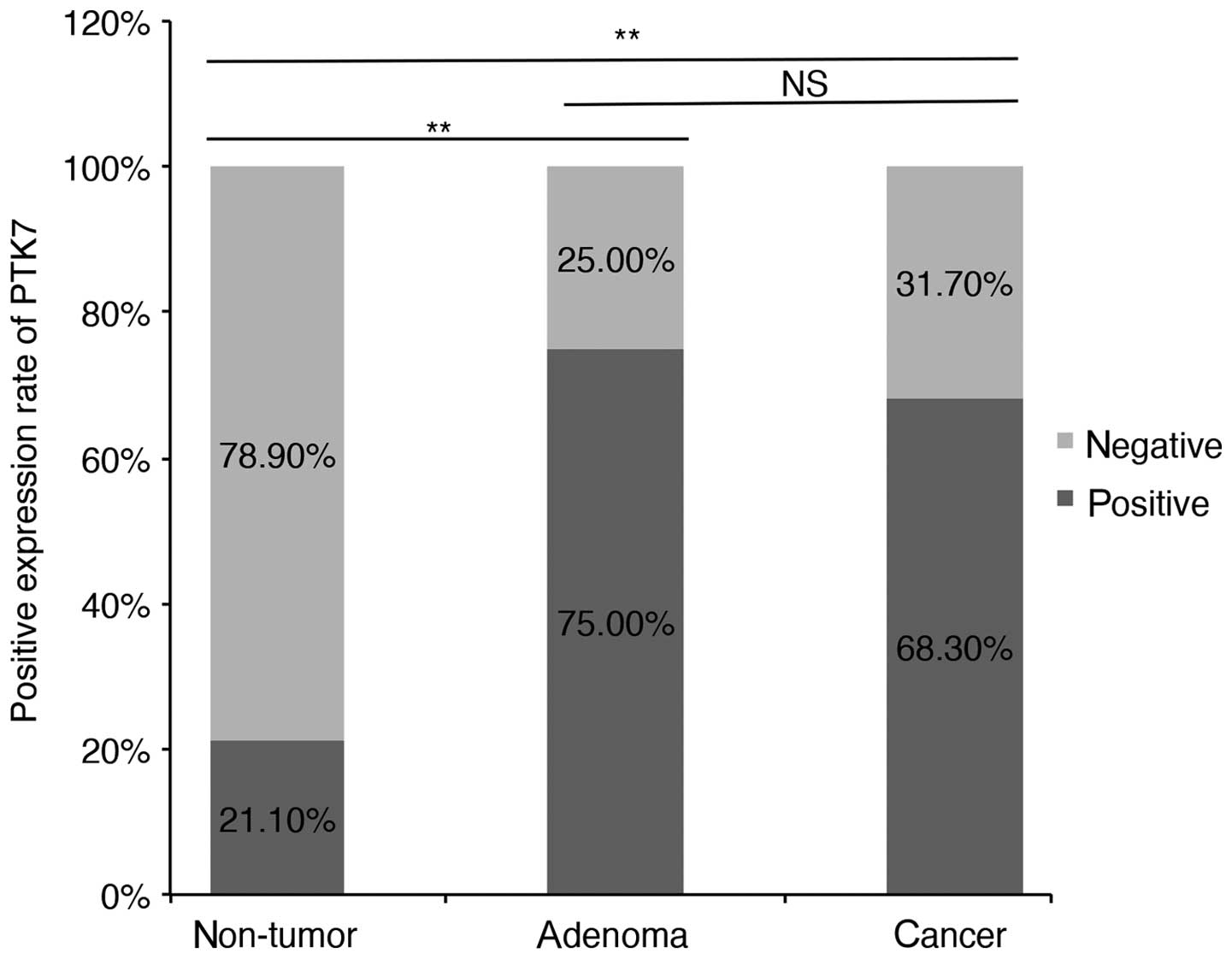
We further compared PTK7 expression in non-tumorous
mucosa, colonic adenoma and CRC (Table III). Normal colorectal mucosa
taken along with adenoma and the matched non-cancerous mucosa in
CRC TMA were consolidated for statistical analysis. As shown in
Fig. 4, PTK7 expression was lowest
in non-tumor mucosa (21.1%) and highest in adenoma group (75.0%).
PTK7 expression in adenoma was a little higher than it in malignant
tumor (75.0 vs. 68.3%; P=0.515).
 | Table IIIComparison of PTK7 expression in
non-tumorous mucosa, adenoma and CRC. |
Table III
Comparison of PTK7 expression in
non-tumorous mucosa, adenoma and CRC.
| Groups | Positive, n
(%) | Negative, n
(%) | Total |
|---|
| Non-tumorous | 48 (21.1) | 180 (78.9) | 228 |
| Adenoma | 21 (75) | 7 (25) | 28 |
| CRC | 138 (68.3) | 64 (31.7) | 202 |
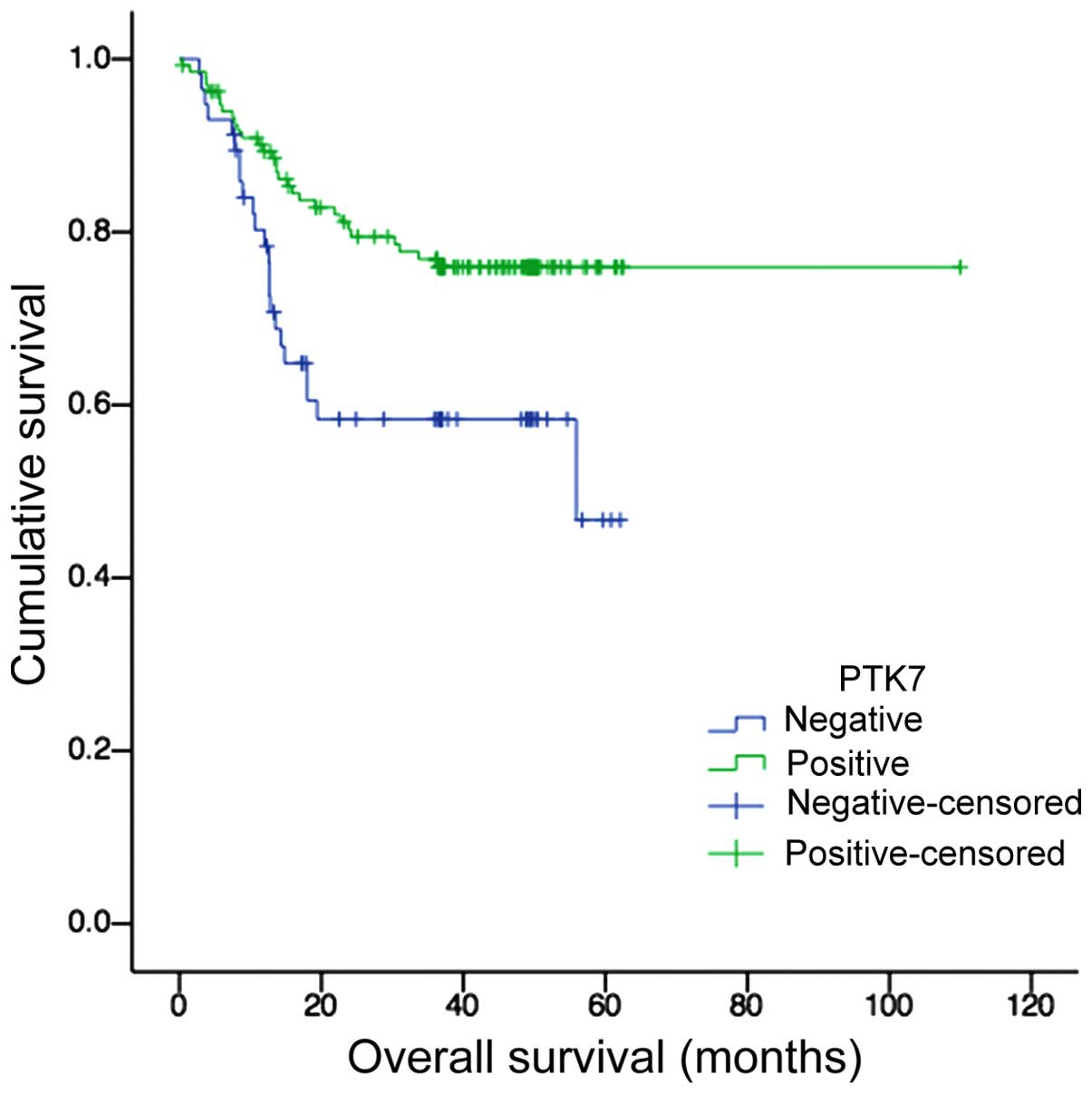
PTK7 expression was correlated with
better overall survival of CRC patients
Kaplan-Meier survival analysis and log-rank test
showed a significant correlation between PTK7 overexpression and
longer survival time (P=0.005, Fig.
5). The median survival time was 40.11±3.48 months for
PTK7-negative patients and 87.11±3.67 months for PTK7-positive
patients, respectively.
Various clinicopathological features that may affect
overall survival were evaluated by univariate survival analysis.
PTK7 was confirmed to be one of the prognostic factors (HR, 0.464;
95% CI, 0.269–0.8; P=0.006). Other prognostic factors included
tumor differentiation (P=0.006), tumor stage (P<0.001) and
vascular invasion (P<0.001). These prognostic factors were
included in Cox multivariate model. PTK7 was shown to be a novel
independent prognostic factor of overall survival (HR, 0.453; 95%
CI, 0.254–0.809; P=0.007). Vascular invasion was also shown to be
an independent prognostic factor (HR, 2.236; 95% CI, 1.158–4.318;
P=0.017). Detailed information are shown in Table IV.
 | Table IVCox proportional hazards regression
model analysis. |
Table IV
Cox proportional hazards regression
model analysis.
| Variables | Univariate analysis
| Multivariate
analysis
|
|---|
| Relative risk | 95% CI | P-value | Relative risk | 95% CI | P-value |
|---|
| Differentiation
(Well/moderate/poor) | 2.054 | 1.225–3.445 | 0.006 | | | 0.660 |
| Stage (I+II vs.
III+IV) | 58.917 | 6.427–540.077 | <0.001 | 403543.310 |
1.7×10−88–9.9×1098 | 0.906 |
| Vascular invasion
(Present vs. absent) | 4.953 | 2.869–8.550 | <0.001 | 2.236 | 1.158–4.318 | 0.017 |
| PTK7 expression
(Positive vs. negative) | 0.464 | 0.269–0.800 | 0.006 | 0.453 | 0.254–0.809 | 0.007 |
Discussion
PTK7 is a member of receptor tyrosine kinases
(RPTK)-like molecules. Recent studies have found that PTK7, as one
of the co-receptors of non-canonical WNT signals, was involved in
embryonic development (15), and it
regulates convergent extension, neural tube closure as well as
orientation of inner ear hair cells (7,16)
through WNT/PCP signaling pathway. In addition, PTK7 was found to
play a role in angiogenesis (17)
and epidermal wound repair (18) as
well as tumorigenesis.
PTK7 was generally regarded as an oncogene. Several
studies have found that PTK7 was overexpressed in erythroleukemia
cell line (6), AML (19), liposarcoma (20), gastric cancer (10), esophageal squamous cell carcinoma
(21), prostate cancer (22) and glioma (23). However, downregulation of PTK7 has
also been observed in several tumors including melanoma (8), clear cell renal cell carcinoma
(9), lung squamous cell carcinoma
(24), epithelial ovarian carcinoma
(12), and hepatocellular carcinoma
(25).
Our results have proved that PTK7 was overexpressed
in colorectal carcinoma both in mRNA level and protein level, which
is consistent with the results of Mossie et al (26) and Lhoumeau et al (27). Compared with these two studies, our
study included more patients and also evaluated the expression of
PTK7 in benign colonic adenoma, which has not been reported before.
PTK7 was expressed in 75% adenoma, which is a little higher than in
CRC tissues (68.3%). Moreover, in CRC tissues, PTK7 expression
ascended while the tumor size increased. The difference did not
reach a statistically significant level (P=0.515 and P=0.715,
respectively), but this phenomenon may be a clue that PTK7 was
involved in a process of transforming normal cells into tumor cells
and can promote cell growth. Overexpression of PTK7 in both colonic
adenoma and carcinoma indicated that PTK7 may be an oncogene.
We also found that lower PTK7 expression was
associated with poor-differentiation (P=0.036), lymph node
metastasis (P=0.005), distant metastasis (P=0.001) and advanced TNM
stage (P=0.028) of CRC patients. As shown in Fig. 3, PTK7 was 90, 68.5 and 54.5%
positive in well, moderately and poorly differentiated tumors,
respectively. The expression rate descended while the degree of
tumor malignancy increased. Similar trend can also be seen in tumor
stage, that is, PTK7 expression is relatively lower in tumors in
later stage. The correlation between PTK7 expression and tumor
clinicopathological features indicated a tumor suppressor gene
function of PTK7, which is consistent with the study of Wang et
al (12) in epithelial ovarian
carcinomas.
Furthermore, our data showed that overexpression of
PTK7 correlated with favorable prognosis, which is contrary to the
results of Lhoumeau et al (27), but similar with that in gastric
cancer (10). We classified the CRC
patients according to distant metastasis or not, PTK7 was expressed
in 78.8% patients of non-metastases group and 67.8% patients of
metastases group (P=0.049). PTK7 expression showed a gradually
decreasing tendency with tumor progression, which is consistent
with melanoma (8) and pulmonary
adenocarcinoma (28).
The reasons underlying the diverse functions of PTK7
in different tumors still remain unclear. As PTK7 has several
transcriptional variants, we infer that its diverse function and
distribution may be attributed to the different variants (29). Recent studies have shown different
functions of full-length PTK7 and its soluble fragment generated by
MT1-MMP. The enforced expression of full-length PTK7 can inhibit
tumor invasion due to actin cytoskeleton reorganization (30). Thus, more specific investigation may
be needed to elucidate the mechanism of PTK7 function.
In conclusion, we evaluated the expression of PTK7
protein in non-tumorous mucosa, benign colonic adenoma and
malignant colorectal carcinoma, and the trend of expression rate
appears rising early but declining along with the tumor
progression. Expression of PTK7 correlates with lymph node
metastasis and distant metastasis. CRC patients with higher PTK7
expression have better prognosis. Therefore, PTK7 may become a
candidate biomarker of colorectal carcinoma metastasis.
Acknowledgments
This study was supported by the International
Science and Technology Cooperation Program of China (approval no.
2013DFG32720), the Capital Health Research and Development of
Special Funds (approval no. 2016-2-2151), the Beijing Municipal
Natural Science Foundation (approval no. 7153161), and the National
Natural Science Funding (approval nos. 81441071, 61372028, 61571437
and 81272765).
Abbreviations:
|
PTK7
|
protein tyrosine kinase-7
|
|
CRC
|
colorectal cancer
|
|
TMA
|
tissue microarray
|
|
IRS
|
immunoreactivity score
|
References
|
1
|
Holmes D: A disease of growth. Nature.
521:S2–S3. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
NCCN Clinical Practice Guidelines in
Oncology: Colon Cancer (version 3.2014). National Comprehensive
Cancer Network; 2014
|
|
3
|
Muratore A, Zorzi D, Bouzari H, Amisano M,
Massucco P, Sperti E and Capussotti L: Asymptomatic colorectal
cancer with un-resectable liver metastases: Immediate colorectal
resection or up-front systemic chemotherapy? Ann Surg Oncol.
14:766–770. 2007. View Article : Google Scholar
|
|
4
|
Tsai MS, Su YH, Ho MC, Liang JT, Chen TP,
Lai HS and Lee PH: Clinicopathological features and prognosis in
resectable synchronous and metachronous colorectal liver
metastasis. Ann Surg Oncol. 14:786–794. 2007. View Article : Google Scholar
|
|
5
|
Lee ST, Strunk KM and Spritz RA: A survey
of protein tyrosine kinase mRNAs expressed in normal human
melanocytes. Oncogene. 8:3403–3410. 1993.PubMed/NCBI
|
|
6
|
Park SK, Lee HS and Lee ST:
Characterization of the human full-length PTK7 cDNA encoding a
receptor protein tyrosine kinase-like molecule closely related to
chick KLG. J Biochem. 119:235–239. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katoh M: WNT/PCP signaling pathway and
human cancer (Review). Oncol Rep. 14:1583–1588. 2005.PubMed/NCBI
|
|
8
|
Easty DJ, Mitchell PJ, Patel K, Flørenes
VA, Spritz RA and Bennett DC: Loss of expression of receptor
tyrosine kinase family genes PTK7 and SEK in metastatic melanoma.
Int J Cancer. 71:1061–1065. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Behbahani TE, Thierse C, Baumann C, Holl
D, Bastian PJ, von Ruecker A, Müller SC, Ellinger J and Hauser S:
Tyrosine kinase expression profile in clear cell renal cell
carcinoma. World J Urol. 30:559–565. 2012. View Article : Google Scholar
|
|
10
|
Lin Y, Zhang LH, Wang XH, Xing XF, Cheng
XJ, Dong B, Hu Y, Du H, Li YA, Zhu YB, et al: PTK7 as a novel
marker for favorable gastric cancer patient survival. J Surg Oncol.
106:880–886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin J, Ryu HS, Lee KB and Jang JJ: High
expression of protein tyrosine kinase 7 significantly associates
with invasiveness and poor prognosis in intrahepatic
cholangiocarcinoma. PLoS One. 9:e902472014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Li G, Yin Y, Wang J, Wang H, Wei
W, Guo Q, Ma H, Shi Q, Zhou X, et al: PTK7 protein is decreased in
epithelial ovarian carcinomas with poor prognosis. Int J Clin Exp
Pathol. 7:7881–7889. 2014.
|
|
13
|
Marullo M, Zuccato C, Mariotti C, Lahiri
N, Tabrizi SJ, Di Donato S and Cattaneo E: Expressed Alu repeats as
a novel, reliable tool for normalization of real-time quantitative
RT-PCR data. Genome Biol. 11:R92010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Guan XY, Dong B, Zhao M, Wu JH,
Tian XY and Hao CY: Expression of MMP-9 and WAVE3 in colorectal
cancer and its relationship to clinicopathological features. J
Cancer Res Clin Oncol. 138:2035–2044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung JW, Shin WS, Song J and Lee ST:
Cloning and characterization of the full-length mouse Ptk7 cDNA
encoding a defective receptor protein tyrosine kinase. Gene.
328:75–84. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu X, Borchers AG, Jolicoeur C, Rayburn H,
Baker JC and Tessier-Lavigne M: PTK7/CCK-4 is a novel regulator of
planar cell polarity in vertebrates. Nature. 430:93–98. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shin WS, Maeng YS, Jung JW, Min JK, Kwon
YG and Lee ST: Soluble PTK7 inhibits tube formation, migration, and
invasion of endothelial cells and angiogenesis. Biochem Biophys Res
Commun. 371:793–798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caddy J, Wilanowski T, Darido C, Dworkin
S, Ting SB, Zhao Q, Rank G, Auden A, Srivastava S, Papenfuss TA, et
al: Epidermal wound repair is regulated by the planar cell polarity
signaling pathway. Dev Cell. 19:138–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Müller-Tidow C, Schwäble J, Steffen B,
Tidow N, Brandt B, Becker K, Schulze-Bahr E, Halfter H, Vogt U,
Metzger R, et al: High-throughput analysis of genome-wide receptor
tyrosine kinase expression in human cancers identifies potential
novel drug targets. Clin Cancer Res. 10:1241–1249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gobble RM, Qin LX, Brill ER, Angeles CV,
Ugras S, O'Connor RB, Moraco NH, Decarolis PL, Antonescu C and
Singer S: Expression profiling of liposarcoma yields a multigene
predictor of patient outcome and identifies genes that contribute
to liposarcomagenesis. Cancer Res. 71:2697–2705. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shin WS, Kwon J, Lee HW, Kang MC, Na HW,
Lee ST and Park JH: Oncogenic role of protein tyrosine kinase 7 in
esophageal squamous cell carcinoma. Cancer Sci. 104:1120–1126.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Wang A, Qi S, Cheng S, Yao B and
Xu Y: Protein tyrosine kinase 7 (PTK7) as a predictor of lymph node
metastases and a novel prognostic biomarker in patients with
prostate cancer. Int J Mol Sci. 15:11665–11677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Q, Zhang C, Yuan J, Fu J, Wu M, Su J,
Wang X, Yuan X and Jiang W: PTK7 regulates Id1 expression in
CD44-high glioma cells. Neuro-oncol. 17:505–515. 2015. View Article : Google Scholar :
|
|
24
|
Kim JH, Kwon J, Lee HW, Kang MC, Yoon HJ,
Lee ST and Park JH: Protein tyrosine kinase 7 plays a tumor
suppressor role by inhibiting ERK and AKT phosphorylation in lung
cancer. Oncol Rep. 31:2708–2712. 2014.PubMed/NCBI
|
|
25
|
Hishida M, Inokawa Y, Takano N, Nishikawa
Y, Iwata N, Kanda M, Tanaka C, Kobayashi D, Yamada S, Nakayama G,
et al: Protein tyrosine kinase 7: A hepatocellular
carcinoma-related gene detected by triple-combination array. J Surg
Res. 195:444–453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mossie K, Jallal B, Alves F, Sures I,
Plowman GD and Ullrich A: Colon carcinoma kinase-4 defines a new
subclass of the receptor tyrosine kinase family. Oncogene.
11:2179–2184. 1995.PubMed/NCBI
|
|
27
|
Lhoumeau AC, Martinez S, Boher JM, Monges
G, Castellano R, Goubard A, Doremus M, Poizat F, Lelong B, de
Chaisemartin C, et al: Overexpression of the Promigratory and
Prometastatic PTK7 Receptor Is Associated with an Adverse Clinical
Outcome in Colorectal Cancer. PLoS One. 10:e01237682015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Endoh H, Tomida S, Yatabe Y, Konishi H,
Osada H, Tajima K, Kuwano H, Takahashi T and Mitsudomi T:
Prognostic model of pulmonary adenocarcinoma by expression
profiling of eight genes as determined by quantitative real-time
reverse transcriptase polymerase chain reaction. J Clin Oncol.
22:811–819. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jung JW, Ji AR, Lee J, Kim UJ and Lee ST:
Organization of the human PTK7 gene encoding a receptor protein
tyrosine kinase-like molecule and alternative splicing of its mRNA.
Biochim Biophys Acta. 1579:153–163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Golubkov VS, Chekanov AV, Cieplak P,
Aleshin AE, Chernov AV, Zhu W, Radichev IA, Zhang D, Dong PD and
Strongin AY: The Wnt/planar cell polarity protein-tyrosine kinase-7
(PTK7) is a highly efficient proteolytic target of membrane type-1
matrix metalloproteinase: Implications in cancer and embryogenesis.
J Biol Chem. 285:35740–35749. 2010. View Article : Google Scholar : PubMed/NCBI
|