Introduction
Multiple myeloma (MM), the second most common
hematological malignancy following non-Hodgkin's lymphoma (NHL),
contribute to 13% of all malignancies and 1% of all neoplasias
(1). MM is characterized by bone
lesions, anemia, susceptibility to infections, renal failure and
hypercalcemia, caused by proliferation of clonal plasma cells in
the bone marrow (2). Recently, some
agents such as thalidomide, lenalidomide, and bortezomib have
prolonged overall survival of MM patients, but MM remains an
incurable disease and eventually almost all patients relapse and
become resistant to the chemical treatment. Therefore, there is an
urgent need to explore new therapeutic agent to improve the
survival of MM patients.
In MM, angiogenesis takes place in the
microenvironment and that is strongly correlated to disease
progression and poor prognosis (3).
Hypoxia inducible factor 1α (HIF-1α) and vascular endothelial
growth factor (VEGF) are known to play important roles in
angiogenesis and tumor progression (4). It is hypoxic in the bone marrow
microenvironment of MM patients (5), and HIF-1α has been regarded as the
most important factor promoting angiogenesis by upregulating
pro-angiogenic factors such as VEGF (6). In MM, VEGF is secreted not only by
myeloma cells but also bone marrow stem cells (BMSC) (7). The activation of extracellular
signal-regulated kinases 1/2 (ERK1/2) and phosphatidylinositol
3-kinase/protein kinase B (PI3K/AKT) pathway are critical for
myeloma cells to express HIF-1α and VEGF (8).
Fucoidan is a kind of marine drug, and its
anticancer function has been focused on. It is clear that Fucoidan
has potential to inhibit the proliferation of cancer cells
(9–11). Furthemore, Fucoidan suppressed
neovascularization of human umbilical vein endothelial cells
(HUVECs) in vitro and tumor-bearing animal models (9,10,12,13).
Previously, we found that Fucoidan was able to reduce MM cell
escape caused by various chemotherapy drugs, which might prevent
the formation of MRD (minimal residual desease) and occurrence of
relapse (14). However, whether
Fucoidan has the ability to suppress HIF-1α/VEGF expression in
human multiple myeloma cells still remains unknown. Thus, in this
study, we investigated whether Fucoidan can significantly inhibit
angiogenesis of MM both in MM cells and a xenograft mouse
model.
Materials and methods
Reagents
Fucoidan was purchased from Sigma-Aldrich (St.
Louis, MO, USA), and dissolved in PBS (Sigma) and sterilized by a
0.22-mm syringe filter (Millipore, Carrigtwohill, Ireland). Total
and phospho-specific antibodies against AKT, and ERK1/2 were
purchased from Cell Signaling Technology (Beverly, MA, USA).
Antibodies against HIF-1α and VEGF were obtained from Abcam
(Cambridge, UK).
Animals
Four to six-week-old female severe combined
immunodeficiency/non-obese diabetic (NOD/SCID) mice (HFK
Bioscience, Beijing, China), with body weight 15–18 g, were housed
under individual ventilated cages (IVC) in a room maintained at
constant temperature under 12-h light and darkness cycle at the
Laboratory Animal Center of Chongqing Medical University. All
procedures associated with animals was reviewed and approved by the
Institutional Animal Care and Use Committee of Chongqing Medical
University.
Cell culture and induction of
hypoxia
Human MM cell lines (RPMI-8226 and U266) were kept
frozen in our laboratory and human umbilical vein endothelial cells
(HUVECs) were kindly provided by Dr Qifu Li (Department of
Geriatrics, The First Affiliated Hospital of Chongqing Medical
University). All cell lines were maintained with RPMI-1640
(Sigma-Aldrich, St. Louis, MO, USA) containing 10% fetal bovine
serum (Gibco, Grand Island, NY, USA), penicillin (100 U/ml), and
streptomycin (100 μg/ml) (Beyotime, Bejing, China) and
subsequently incubated at 37°C in humid air with 19% O2,
5% CO2. For hypoxia induction, cells were incubated in a
hypoxic chamber with a gas mixture (94% N2, 5%
CO2 and 1% O2) before being treated with
Fucoidan.
Collection of conditioned medium
(CM)
Condition media (CM) was collected as described
(15). Briefly, 5×105/ml
RPMI-8226 and U266 cells were cultured with different
concentrations of Fucoidan (0, 25, 50, 100 and 200 μg/ml)
for 72 h under normoxic and hypoxic conditions. After treatment,
cells were recollected and CM was collected, and centrifuged at
12,000 rpm for 10 min under 4°C. CM was either used immediately or
stored at −20°C.
ELISA
CM of each group were collected as above, human VEGF
ELISA kit (Boster, Wuhan, China) was used to measure the
concentration of VEGF according to the manufacturer's protocol.
Then the plate was read immediately at 450 nm on a microplate
reader (Amersham Pharmacia Biotech, USA), and the concentration was
determined by calculating formula from a standard curve. The
experiment was performed three times.
Tube formation assay
The ability of HUVECs induced by CM was assessed by
the tube formation assay. Matrigel (50 μl/well, BD
Pharmingen, San Diego, CA, USA) was added into a 96-well plate and
incubated for 30 min at 37°C. HUVECs (2×104 cells/well)
were suspended in CM (100 μl/well) and plated on top of
Matrigel, then incubated for 8 h and the capillary-like structures
were observed under a microscope. Three random microscopic fields
were photographed to evaluate the capillary-like structure
formation (×200), and the tube areas were quantified by using
Image-Pro Plus software 5.0 (Media Cybernetics, MD, USA).
Transwell migration assay
The effect of Fucoidan on migration of HUVECs was
demonstrated in 24-well Transwell cell culture chambers with the
upper chamber containing filters. HUVECs (2×104) were
plated in the upper chambers, followed by cocultivation with 50% CM
in the lower chamber. Incubating the plate for 6 h at 37°C, media
in the upper chamber were sucked out and cells on the surface of
the upper chamber were removed gently with a cotton swab. Cells on
the lower surface of the filters were fixed with 4% poly
paraformaldehyde solution for 20 min, stained with 0.1% crystal
violet solution for 10 min, then washed by PBS twice. Five random
microscopic fields were photographed (×200), and the number of
HUVECs were counted by using Image-Pro Plus software 5.0 (Media
Cybernetics).
Western blot analysis
After being cultured with Fucoidan for 72 h under
normoxic and hypoxic conditions, cells were collected and washed
with ice-cold PBS twice. Cell extracts were prepared with cell
lysis buffer mixed with protease and phosphatase inhibitors (KeyGen
Biotech, Nanjing, China). Total protein concentration was
determined using the BCA protein assay kit (Beyotime). Aliquots of
proteins (60 μg/lane) were separated in 12% SDS-PAGE and
transferred onto PVDF membrane (Bio-Rad, Hercules, CA, USA), then
blocked with 5% non-fat milk for 2 h. Membranes were incubated with
primary antibodies overnight at 4°C as follows: HIF-1α and VEGF
(Abcam, dilution: 1:1,000 and 1:600), AKT, phospho-AKT, ERK 1/2,
and phospho-ERK 1/2 (Cell Signaling Technology, dilution: 1:1,000),
and β-actin (Boster, dilution: 1:2,000) was used as the internal
control. After washing with TBS-Tween-20, the membranes were
incubated with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit or mouse IgG as the secondary antibody (Boster,
dilution: 1:2,000) for 2 h at 37°C. The protein bands were
visualized using an enhanced chemiluminescence (ECL, Beyotime) kit
according to the manufacturer's instructions.
Mouse xenograft model
The NOD/SCID mice were injected subcutaneously with
1×107 RPMI-8226 cells which were suspended in 100
μl of serum-free RPMI-1640 medium, together with 100
μl Matrigel in the right flank (16). To prevent leakage, a cotton swab was
held cautiously for 1 min over the site of injection. The weight of
the mice and tumor size were measured and recorded. Tumors were
measured with vernier caliper every other day and volumes were
calculated according to the formula: V = a2b/2,
where a is the major axis and b is the minor axis.
When the volume was measurable, the mice (n=6/group) were assigned
to three groups randomly, group I as the control one treated with
same volume of PBS, groups II and III treated by intraperitoneal
injection with 10, 50 mg/kg Fucoidan every two days. After 3 weeks
of treatments, the mice were sacrificed by cervical dislocation and
the tumors were removed and measured. Fixing the tumors with 4%
poly paraformaldehyde solution for 48 h, embedded in paraffin,
sectioned at 4 μm, and stained with hematoxylin and eosin
(H&E).
TUNEL assay for apoptotic cells in
vivo
Apoptosis of tumor tissue sections were detected
using terminal deoxynucleotidyl transferase (TdT)-mediated
dUTP-digoxigenin nick-end labeling (TUNEL) assays with a TUNEL
apoptosis assay kit (Beyotime). Tumor tissue sections were dewaxed,
rehydrated through graded alcohols to water. Then, the sections
were incubated with 3% H2O2 for 10 min at
37°C and washed with PBS three times. The sections were blocked
with goat serum for 10 min. TUNEL assays were then performed
according to the manufacturer's instructions.
Immunohistochemistry
For microvessel density (MVD) assay,
immunohistochemical staining was performed by using IHC kit
(Zhongshan Gold Bridge Bio, Beijing, China) according to the
manufacturer's protocol. After the sections were dewaxed and
antigen were repaired, the sections were incubated with Primary
rabbit antihuman CD34 antibody (Zhongshan Gold Bridge Bio,
dilution: 1:150) overnight at 4°C. Then the sections were stained
with a streptavidin-peroxidase system, the signal was visualized by
using 3, 3′-diaminobenzidine (DAB) and counterstaining was done
with hematoxylin. Areas of neovascularization were counted at high
power magnification (×400) by two investigators in a blinded manner
using Image-Pro Plus 5.0 (Media Cybernetics).
Statistical analysis
The data was expressed as mean ± SD of a
representative experiment in triplicate. Data were analyzed using
One-way ANOVA and Student's t-test. The level of significance was
indicated as P<0.05 and P<0.01.
Results
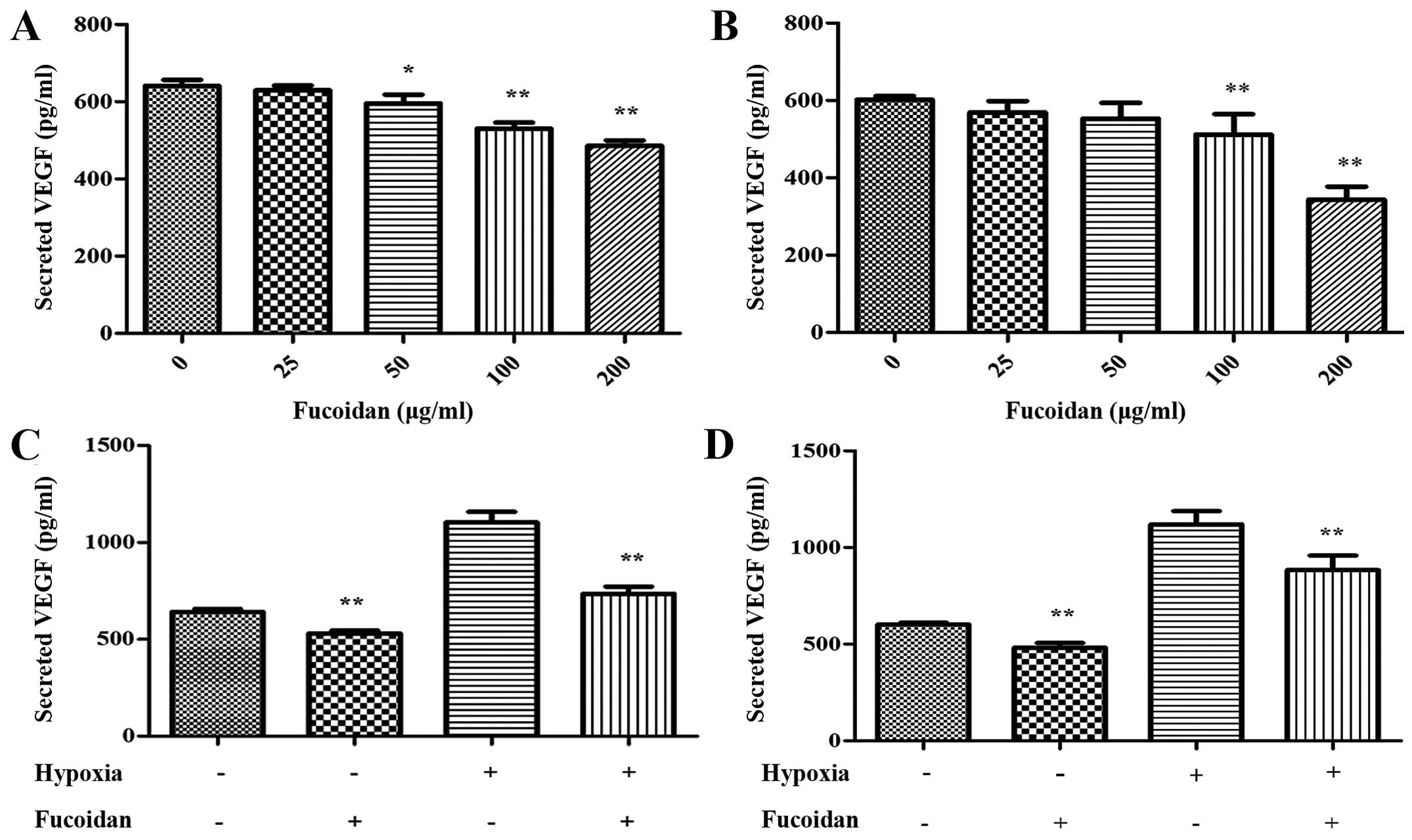
Fucoidan suppresses secretion of VEGF in
myeloma cells
The VEGF secreted by myeloma cells is essential to
angiogenesis, so we quantified the concentration of VEGF in the CM
by ELISA assay. Under normoxic condition, the secretion of VEGF was
decreased dose-dependently (Fig. 1A and
B). As shown in Fig. 1C and D,
it was obvious that the secretion of VEGF was increased under
hypoxia condition, at the same time, Fucoidan at 100 μg/ml
could lead to the suppression of VEGF secrete apparently in both
myeloma cell lines.
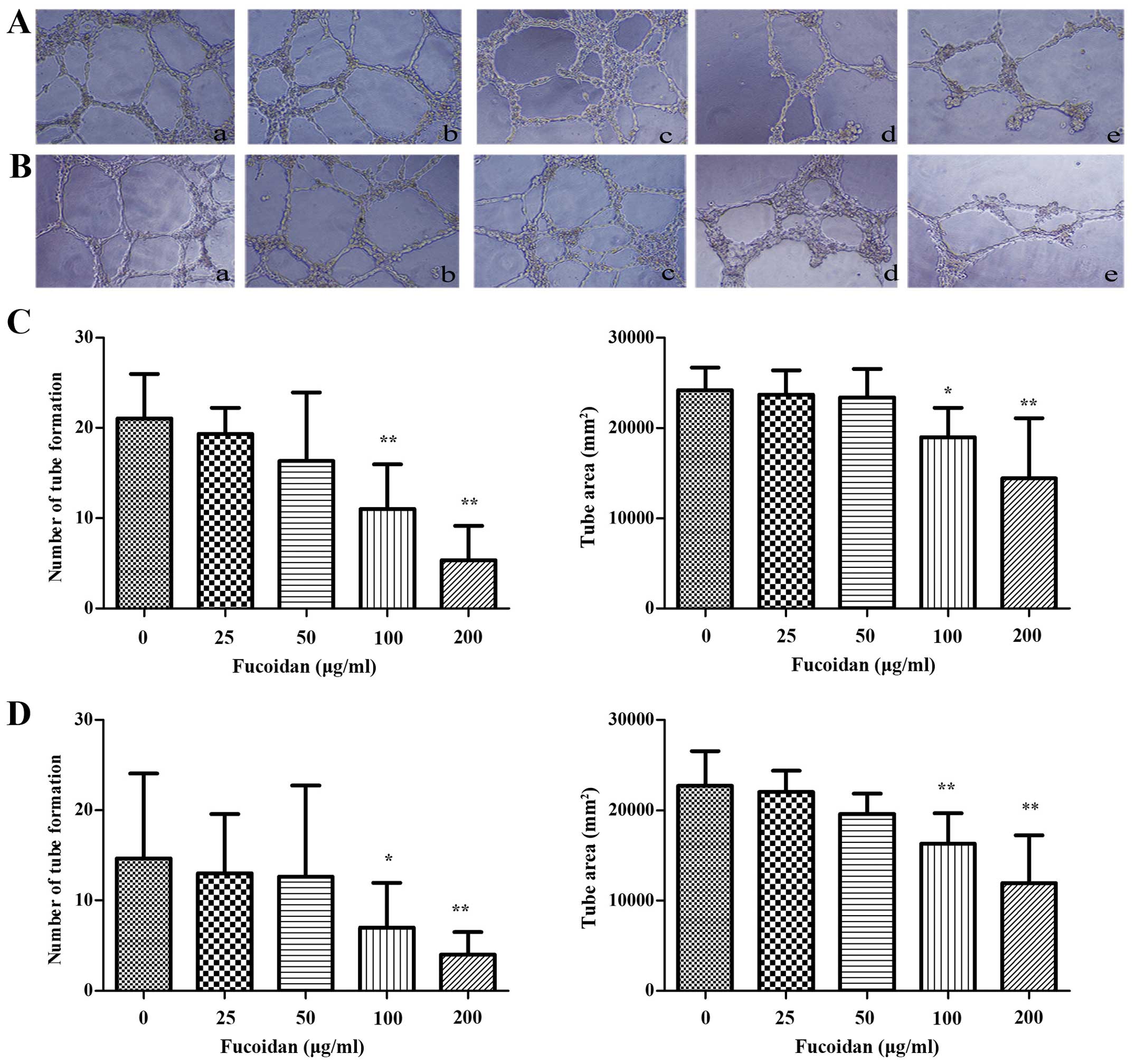
Fucoidan decreases tube formation of
HUVECs in vitro
To investigate the effect of Fucoidan on HUVECs
in vitro, tube formation assay was conducted. HUVECs
resuspended in the CM of multiple myeloma cells were seeded onto
Matrigel and incubated for 6 h. HUVECs spread and formed
capillary-like structures. Compared with the control group, number
and tube size of capillary structures stimulated by CM from myeloma
cells pretreated with Fucoidan was reduced, the tubes were thinner
and highly disconnected (P<0.01) (Fig. 2).
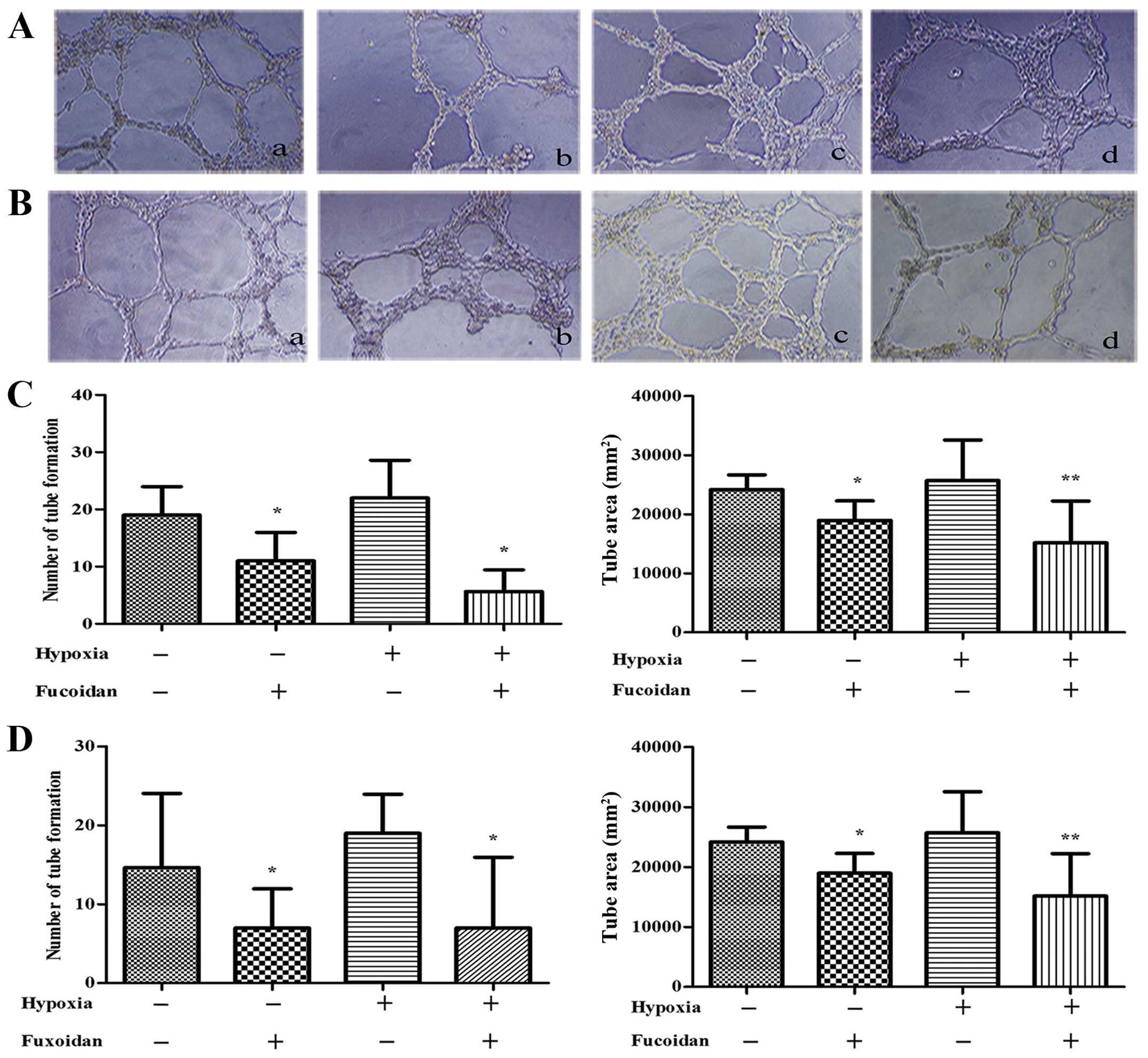
When HUVECs pretreated with CM from hypoxic
condition, the HUVECs spread and formed tubes better than that with
CM from normoxic condition (P<0.01) (Fig. 3).
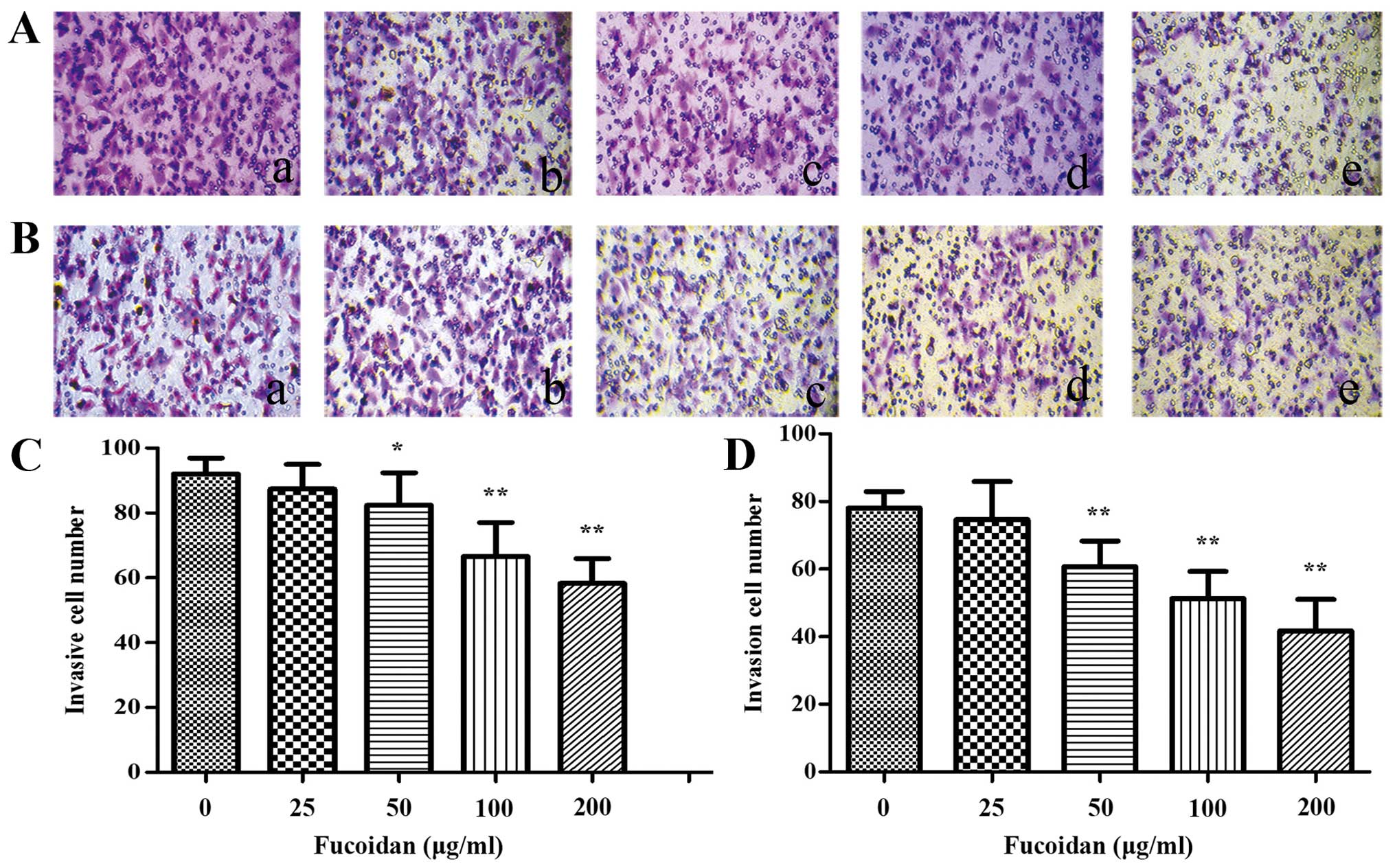
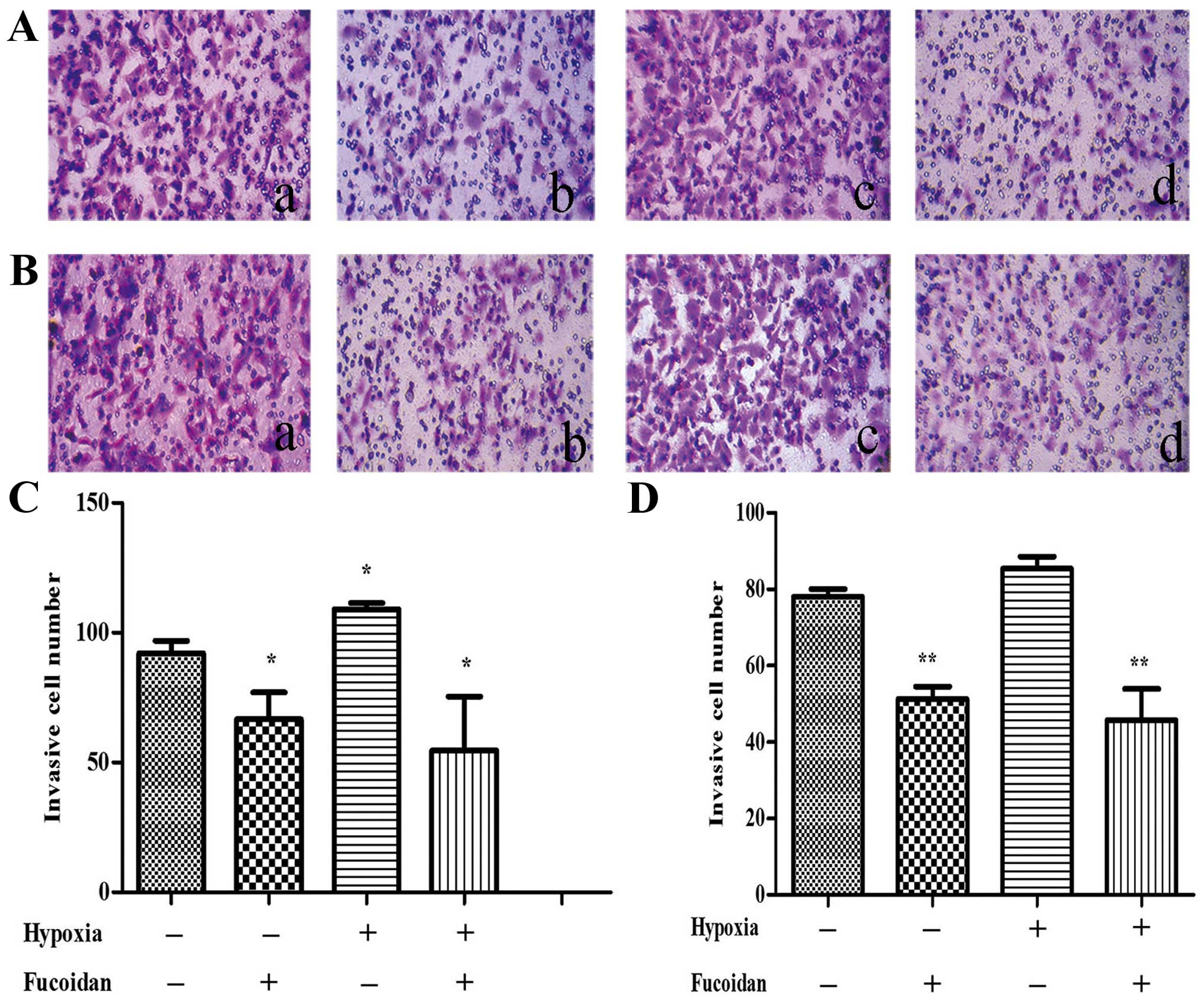
Fucoidan inhibits migration of HUVECs
cocultured with CM of multiple myeloma cells
To assess the potential effect of Fucoidan in HUVECs
migration, a Transwell migration assay was performed. After treated
with CM from normoxic condition, the results indicated that less
HUVECs migrated to the lower chamber when the myeloma cells were
cultured with CM from the high dose-Fucoidan pretreated, similar
results were obtained in both myeloma cells (P<0.01) (Fig. 4). CM from hypoxic condition promoted
HUVECs migration, when pretreated with Fucoidan at 100
μg/ml, HUVECs migration was significantly inhibited
(P<0.01) (Fig. 5).
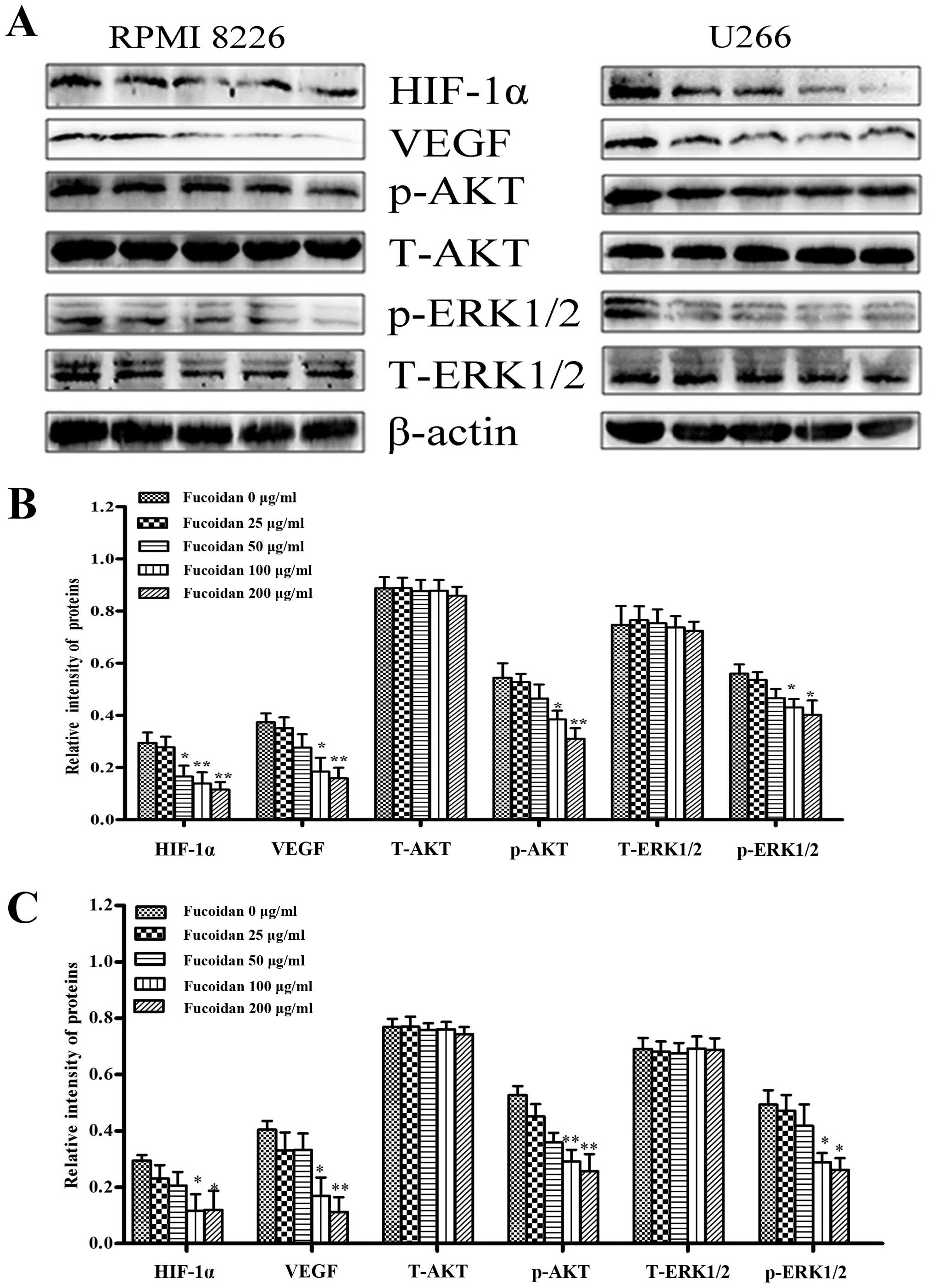
Effect of Fucoidan on the expression of
various proteins involved in angiogenesis
After myeloma cells were treated with Fucoidan under
normoxic condition, HIF-1α and VEGF were inhibited when Fucoidan
was increased. To ascertain which signaling pathways were involved
in regulating VEGF secretion and expression of multiple myeloma
cells by Fucoidan, we focused on the level of phosphorylation of
AKT and ERK1/2. It was shown that the expression of p-AKT and
p-ERK1/2 were significantly decreased when Fucoidan was increased
(Fig. 6).
We further evaluated the effect by western blot
analysis. The results showed that hypoxia induced higher levels of
HIF-1α and VEGF in myeloma cells, and so did p-AKT and p-ERK1/2.
Then, we determined whether Fucoidan reduces angiogenesis induced
by myeloma cells. Treated with Fucoidan 100 μg/ml, it is
obvious that HIF-1α, VEGF and p-AKT were inhibited in both kinds of
myeloma cells, but p-ERK1/2 changed little, as shown in Fig. 7.
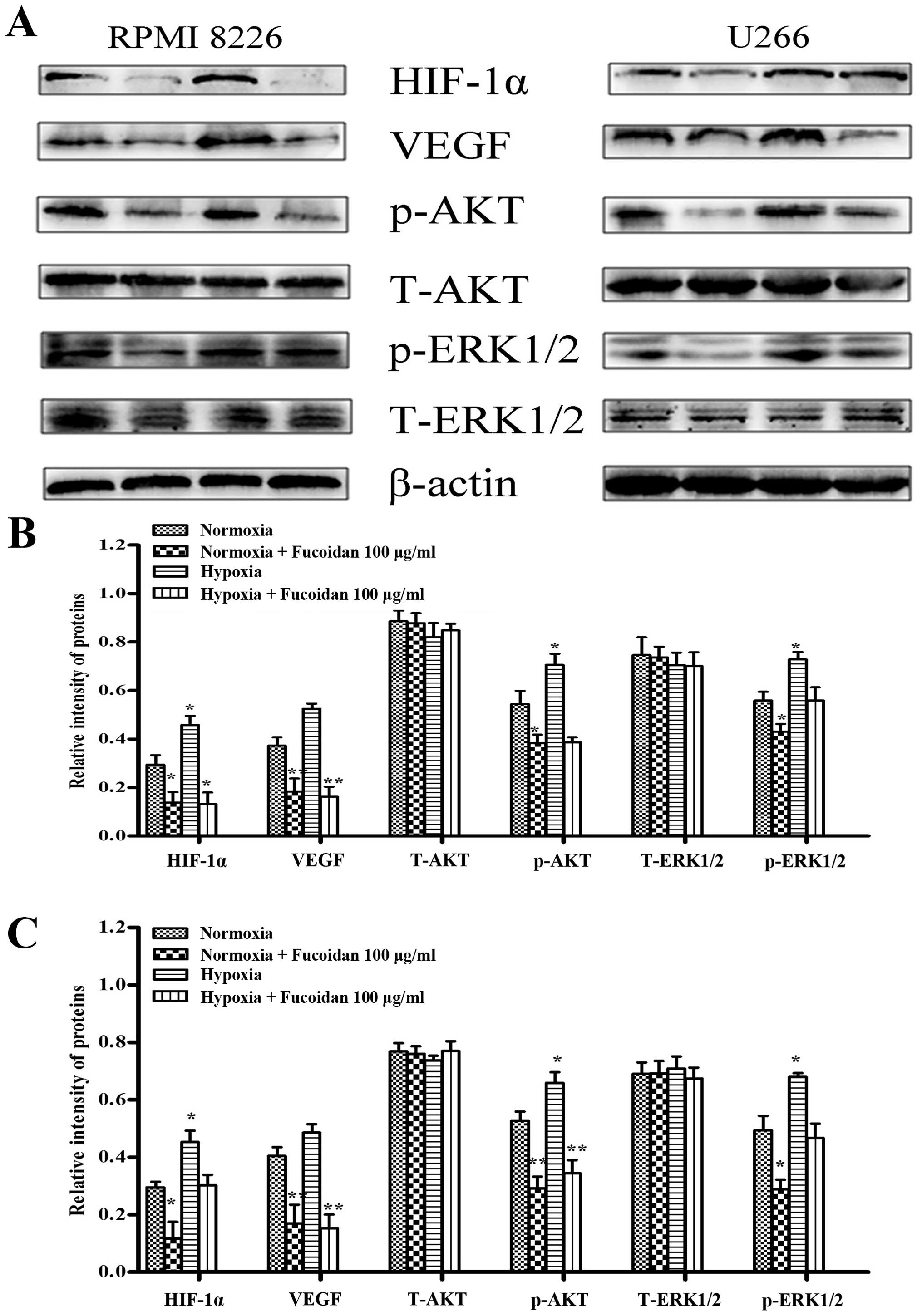 | Figure 7Fucoidan affected the expression of
various proteins involved in angiogenesis under two conditions. (A)
RPMI-8226 and U266 cells were treated with Fucoidan under normoxic
and hypoxic conditions. (B) For quantity of RPMI-8226 cells, it
showed that the expressions of HIF-1α, VEGF, p-AKT and p-ERK1/2
were increased without pretreatment, but not when pretreated with
Fucoidan at 100 μg/ml; VEGF and p-AKT expression increased
under hypoxic condition, and the treatment of Fucoidan at 100
μg/ml inhibited VEGF, p-AKT, but not HIF-1α and p-ERK1/2.
Bars are the mean ± SD (n=3). The comparisons were made relative to
β-actin, and the different levels of significance are indicated as
*P<0.05, **P<0.01. (C) For quantity of
U266 cells, it showed that the expressions of HIF-1α, p-AKT and
p-ERK1/2 were increased without pretreatment, but not when
pretreated with Fucoidan at 100 μg/ml; VEGF and p-AKT
expression increased under hypoxic condition, and the treatment of
Fucoidan at 100 μg/ml inhibited VEGF, p-AKT, but not HIF-1α
and p-ERK1/2. Bars are the mean ± SD (n=3). The comparisons were
made relative to untreated controls, and the different levels of
significance are indicated as *P<0.05,
**P<0.01. |
Fucoidan markedly decreases tumor growth
in a xenograft mouse model of human MM
We also assessed the efficacy of Fucoidan in
vivo using a mouse model of human MM. NOD/SCID mice were given
subcutaneous inoculations into the right flank with
1×107 RPMI-8226 cells and then Fucoidan was given to
mice by i.p. every two days. At the end of the treatment, the
average tumor volume and weight in the Fucoidan treated group (50
mg/kg body weight) was significantly smaller and lighter than that
in group I and II (Fig. 8A and C).
In addition, more necrotic tissues and disorderly and irregular
tumor cell arrangements were observed in H&E stains of
Fucoidan-treated mice, compared with untreated groups (Fig. 8B). TUNEL assay showed that the
apoptotic cells were increased significantly in treated mice
(Fig. 8D). Immunohistochemistry for
CD34+ on tumor sections showed a significant reduction
in the number of CD34+ vessels within group III (median
number of vessels positive for CD34+ per vision 8 vs 2,
P<0.05) (Fig. 8E). Taken all
together, these results demonstrated that Fucoidan inhibited tumor
growth in vivo, induced MM-cell apoptosis, and decreased the
number of MVD.
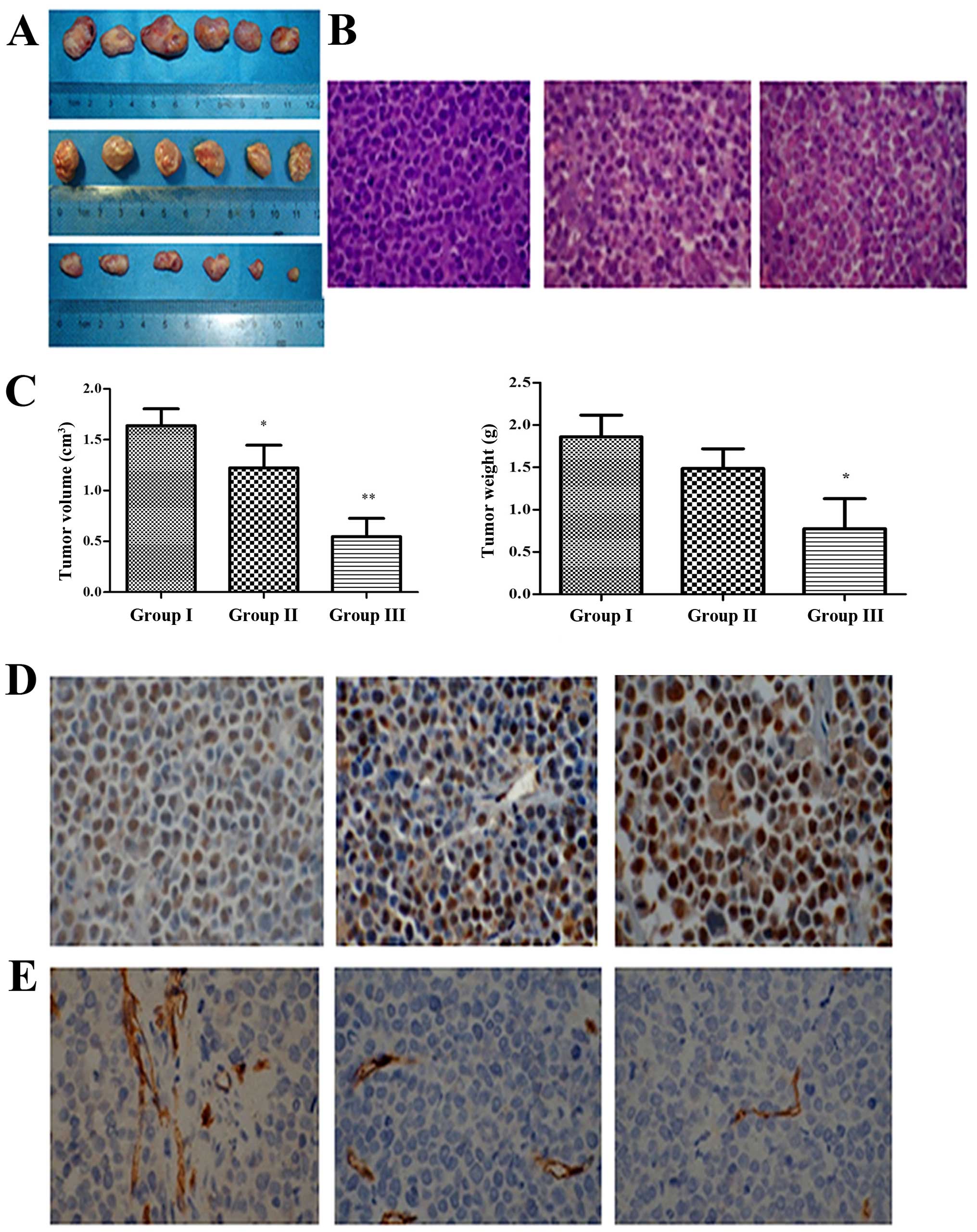 | Figure 8Fucoidan interfered with tumor growth
in xenograft mouse model. (A) Multiple myeloma cells xenograft
mouse model was built (n=6), group I was given PBS (100 μL,
i.p., every two days), group II and III were given Fucoidan (10 and
50 mg/kg body weight, i.p., every two days) for 3 weeks.
Subcutaneous tumor tissue removed from the mice after the
treatment. (B) H&E staining showed that more disorder and
irregular tumor cells arrangement in group III (×400). (C) Tumor
volume and weight in mice were measured (n=6). Columns, mean; bars,
SD, *p<0.05, **p<0.01. (D) TUNEL assay
showed that cell apoptosis rate was increased significantly by
treatment with Fucoidan (×400). (E) MVD assay showed that the
number of CD34+ vessels within mice of group III were
decreased significantly (×400). |
Discussion
Fucoidan, extracted from brown algae species, is a
polysaccharide and consists of sulfated fucose residues (17). As a promising anticancer agent, it
has been shown that Fucoidan could inhibit the growth of a wide
variety of tumor cells, but it is still unclear whether Fucoidan
has any impact on multiple myeloma-induced angiogenesis. Therefore,
we investigated the effects of Fucoidan on the inducing capability
of multiple myeloma cells.
Angiogenesis is a complex process and essential for
cancer progression and metastasis. In multiple myeloma patients,
microvessel density in the bone marrow was significantly higher
(18). We directly investigated the
tube formation and invasion of HUVECs. We found that the number and
the area of tube were significantly decreased when myeloma cells
were pre-treated with Fucoidan. Furthermore, we compared the tube
area between CM collected under normoxia and hypoxia, regardless
whether treated with Fucoidan or not. It showed that Fucoidan had
the same efficacy even though the environment changed. During
angiogenesis processes, migration is a key step for the formation
of new blood vessels (19). We next
performed Transwell chamber migration assay, the results displayed
a similar tendency. That suggesting that the use of Fucoidan can
interfere with neovascularization.
Bone marrow microenvironment is also hypoxic in many
hematological malignancies, such as non-Hodgkin lymphoma (20), acute myeloid leukemia (21), chronic lymphocytic leukemia
(22), and MM (23). The HIF-1α/VEGF/VEGF-receptor pathway
is upregulated in MM cases and linked with increased angiogenesis.
HIF1α is an important transcription factor directly regulating the
expression of the VEGF gene (24).
Previous studies have shown that HIF-1α inhibition can block
angiogenesis (25), but under the
opposite assumption, it promotes (26). Therefore, HIF-1α may be a target to
control MM cell-derived angiogenesis. Here, our results suggested
that Fucoidan inhibited the expression of HIF-1α protein in a
dose-dependent manner in RPMI-8226 and U266 cells under normoxia.
Hypoxia induced HIF-1α accumulation, at the same time, HIF-1 could
be inhibited when treated with Fucoidan.
To further clarify the mechanism of Fucoidan
inhibiting angiogenesis induced by myeloma cells, the expression of
VEGF protein was detected. Results showed that VEGF protein
expression decreased with the treatment of increasing Fucoidan, and
angiogenesis decreased accordingly. Hypoxia can stimulate the
activation of PI3K/AKT pathway, the increase of HIF-1α synthesis is
associated with activated PI3K/AKT signaling. ERK can be activated
by hypoxia and may be involved in the response to hypoxia (27). It should be considered that the
possible crosstalk among these pathways, AKT and ERK pathways
activated by VEGF and VEGF secretion can be reduced by inhibition
of AKT or ERK protein kinase activity (7,28). In
multiple myeloma, downregulation of p-ERK1/2 activity reduces
myeloma-induced angiogenesis by inhibiting VEGF secretion (29). Our results showed that p-AKT and
p-ERK1/2 were inactivated in a dose-dependent manner under
normoxia. Under hypoxia, p-AKT was activated and also, Fucoidan had
the capability to inhibit it. Whereas p-ERK1/2 was not apparently
activated, there was no sign that p-ERK1/2 was restrained even
treated with Fucoidan. It was not possible to tell whether PI3K/AKT
pathway was more important than ERK1/2.
A xenograft myeloma tumor model in NOD/SCID mice was
set up. As the result show, consecutive administration of Fucoidan
for 21 days significantly reduced the tumor volume and weight. In
H&E and TUNEL staining, the tumor cells were induced to
apoptosis. In addition, CD34+ MVD expression in tumor
sections were decreased. This confirms that tumor
neovascularization was inhibited by Fucoidan, making the findings
in vitro more certain.
In conclusion, these results demonstrate that
Fucoidan can prevent angiogenesis induced by myeloma cells. It may
be related to the ability to downregulate HIF-1α/VEGF protein
levels under normoxia and hypoxia. The inhibition of HIF-1α/VEGF
protein expression is possibly associated with the suppression of
PI3K/AKT pathway in both conditions, but it is remarkable that
p-ERK1/2 is not obviously attenuated under hypoxia. Taken together,
this study implies that Fucoidan might be a new potential agent for
human multiple myeloma therapy.
Acknowledgments
We would like to thank Dr Weixue Tang, Ms. Xiaoju
Wang and Mr. Jie Liu for their excellent technical assistance, Dr
Qifu Li (Department of Geriatrics, The First Affiliated Hospital of
Chongqing Medical University) for HUVEC cells. This study was done
in the First Affiliated Hospital of Chongqing Medical University
Central Lab. This study was supported by the Key Subject of
Chongqing Public Health Bureau (grant no. 2013-1-013).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Röllig C, Knop S and Bornhäuser M:
Multiple myeloma. Lancet. 385:2197–2208. 2015. View Article : Google Scholar
|
|
3
|
Ribatti D, Mangialardi G and Vacca A:
Antiangiogenic therapeutic approaches in multiple myeloma. Curr
Cancer Drug Targets. 12:768–775. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gacche RN and Meshram RJ: Targeting tumor
micro-environment for design and development of novel
anti-angiogenic agents arresting tumor growth. Prog Biophys Mol
Biol. 113:333–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Azab AK, Hu J, Quang P, Azab F,
Pitsillides C, Awwad R, Thompson B, Maiso P, Sun JD, Hart CP, et
al: Hypoxia promotes dissemination of multiple myeloma through
acquisition of epithelial to mesenchymal transition-like features.
Blood. 119:5782–5794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giatromanolaki A, Bai M, Margaritis D,
Bourantas KL, Koukourakis MI, Sivridis E and Gatter KC: Hypoxia and
activated VEGF/receptor pathway in multiple myeloma. Anticancer
Res. 30:2831–2836. 2010.PubMed/NCBI
|
|
7
|
Medinger M, Fischer N and Tzankov A:
Vascular endothelial growth factor-related pathways in
hemato-lymphoid malignancies. J Oncol. 2010:7297252010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang XM, Wang YS, Zhang J, Li Y, Xu JF,
Zhu J, Zhao W, Chu DK and Wiedemann P: Role of PI3K/Akt and MEK/ERK
in mediating hypoxia-induced expression of HIF-1alpha and VEGF in
laser-induced rat choroidal neovascularization. Invest Ophthalmol
Vis Sci. 50:1873–1879. 2009. View Article : Google Scholar
|
|
9
|
Han YS, Lee JH and Lee SH: Antitumor
effects of Fucoidan on human colon cancer cells via activation of
Akt signaling. Biomol Ther (Seoul). 23:225–232. 2015. View Article : Google Scholar
|
|
10
|
Delma CR, Somasundaram ST, Srinivasan GP,
Khursheed M, Bashyam MD and Aravindan N: Fucoidan from Turbinaria
conoides: A multifaceted 'deliverable' to combat pancreatic cancer
progression. Int J Biol Macromol. 74:447–457. 2015. View Article : Google Scholar
|
|
11
|
Yoshimoto M, Higaki K, Nanba E and
Ikeguchi M: Anti-proliferation activity of Fucoidan in MKN45
gastric cancer cells and downregulation of phosphorylated ASK1, a
cell cycle-regulated kinase. Yonago Acta Med. 58:1–7.
2015.PubMed/NCBI
|
|
12
|
Zhu C, Cao R, Zhang SX, Man YN and Wu XZ:
Fucoidan inhibits the growth of hepatocellular carcinoma
independent of angiogenesis. Evid Based Complement Alternat Med.
2013:6925492013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xue M, Ge Y, Zhang J, Wang Q, Hou L, Liu
Y, Sun L and Li Q: Anticancer properties and mechanisms of fucoidan
on mouse breast cancer in vitro and in vivo. PLoS One.
7:e434832012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lv J, Xiao Q, Wang L, Liu X, Wang X, Yang
Z, Zhang H and Dong P: Fucoidan prevents multiple myeloma cell
escape from chemotherapy-induced drug cytotoxicity. Fitoterapia.
84:257–263. 2013. View Article : Google Scholar
|
|
15
|
Yang YC, Chen PN, Wang SY, Liao CY, Lin
YY, Sun SR, Chiu CL, Hsieh YS, Shieh JC and Chang JT: The
differential roles of Slit2-exon 15 splicing variants in
angiogenesis and HUVEC permeability. Angiogenesis. 18:301–312.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Podar K, Raab MS, Zhang J, McMillin D,
Breitkreutz I, Tai YT, Lin BK, Munshi N, Hideshima T, Chauhan D, et
al: Targeting PKC in multiple myeloma: In vitro and in vivo effects
of the novel, orally available small-molecule inhibitor enzastaurin
(LY317615.HCl). Blood. 109:1669–1677. 2007. View Article : Google Scholar
|
|
17
|
Kwak JY: Fucoidan as a marine anticancer
agent in preclinical development. Mar Drugs. 12:851–870. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen H, Shi L, Yang X, Li S, Guo X and Pan
L: Artesunate inhibiting angiogenesis induced by human myeloma
RPMI8226 cells. Int J Hematol. 92:587–597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Masiero M, Simões FC, Han HD, Snell C,
Peterkin T, Bridges E, Mangala LS, Wu SY, Pradeep S, Li D, et al: A
core human primary tumor angiogenesis signature identifies the
endothelial orphan receptor ELTD1 as a key regulator of
angiogenesis. Cancer Cell. 24:229–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Minoia C, Quero C, Asselti M, Galise I,
Marzano AL, Iacobazzi A, Rana A, Merchionne F, Serratì S, De Tullio
G, et al: Changes in angiogenesis and hypoxia-inducible factor-1α
protein expression in relapsed/refractory indolent non-Hodgkin
lymphomas. Br J Haematol. 163:640–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Drolle H, Wagner M, Vasold J, Kütt A,
Deniffel C, Sotlar K, Sironi S, Herold T, Rieger C and Fiegl M:
Hypoxia regulates proliferation of acute myeloid leukemia and
sensitivity against chemotherapy. Leuk Res. 39:779–785. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huelsemann MF, Patz M, Beckmann L,
Brinkmann K, Otto T, Fandrey J, Becker HJ, Theurich S, von
Bergwelt-Baildon M, Pallasch CP, et al: Hypoxia-induced p38 MAPK
activation reduces Mcl-1 expression and facilitates sensitivity
towards BH3 mimetics in chronic lymphocytic leukemia. Leukemia.
29:981–984. 2015. View Article : Google Scholar
|
|
23
|
Muz B, de la Puente P, Azab F, Luderer M
and Azab AK: Hypoxia promotes stem cell-like phenotype in multiple
myeloma cells. Blood Cancer J. 4:e2622014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hashimoto T and Shibasaki F:
Hypoxia-inducible factor as an angiogenic master switch. Front
Pediatr. 3:332015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Storti P, Bolzoni M, Donofrio G, Airoldi
I, Guasco D, Toscani D, Martella E, Lazzaretti M, Mancini C,
Agnelli L, et al: Hypoxia-inducible factor (HIF)-1α suppression in
myeloma cells blocks tumoral growth in vivo inhibiting angiogenesis
and bone destruction. Leukemia. 27:1697–1706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan GC: Hypoxic exosomes promote
angiogenesis. Blood. 124:3669–3670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen TL, Zhu GL, Wang JA, Wang Y, He XL
and Jiang J: Apoptosis of bone marrow mesenchymal stem cells caused
by hypoxia/reoxygenation via multiple pathways. Int J Clin Exp Med.
7:4686–4697. 2014.
|
|
28
|
Giuliani N, Lunghi P, Morandi F, Colla S,
Bonomini S, Hojden M, Rizzoli V and Bonati A: Downmodulation of ERK
protein kinase activity inhibits VEGF secretion by human myeloma
cells and myeloma-induced angiogenesis. Leukemia. 18:628–635. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Menu E, Kooijman R, Van Valckenborgh E,
Asosingh K, Bakkus M, Van Camp B and Vanderkerken K: Specific roles
for the PI3K and the MEK-ERK pathway in IGF-1-stimulated
chemotaxis, VEGF secretion and proliferation of multiple myeloma
cells: Study in the 5T33MM model. Br J Cancer. 90:1076–1083. 2004.
View Article : Google Scholar : PubMed/NCBI
|