Introduction
Bone metastasis is significantly related to
cancer-related mortality, and is a common complication in patients
with advanced prostate cancer. Once tumor cells metastasize to
bone, there are no effective treatments for the affected patients
and the prognosis is usually poor (1,2). Solid
tumors are considered to live in a special pathophysiologic
microenvironment different from normal tissues (3). Increasing evidence indicates that the
unique tumor microenvironment are involved in tumor initiation,
growth, progression, invasion and metastasis (4,5).
Although the importance of acidic tumor microenvironment in
sustaining tumor progression and metastasis is widely recognized,
the underlying mechanisms are still poorly understood, and
additional studies are urgently required to better understand the
role of acidic tumor microenvironment in tumor metastasis.
Extracellular acidosis (low pH) due to hypoxia
(6), excessive glycolysis (7), hyperexpression of carbonic anhydrase
(8) and poor perfusion (9) is a tumor microenvironmental stressor.
Extracellular pH (pHe) is significantly lower than neighboring
normal tissues in many tumors and may decrease to a lower level
with the enlargement of tumor volume (6,9). This
pathological microenvironment promotes malignant progression and
metastasis by activating several intracellular signaling pathways
(10,11). In addition, acidic tumor
microenvironment may blunt the effectiveness of antitumor therapy
(12). Consequently, a more
thorough understanding of the role of acidic tumor microenvironment
in bone metastasis may help to develop more effective
therapeutics.
Accumulating evidence suggests that cancer stem
cells (CSCs), a small number of cancer cells with stem cell
properties, have the potential of unlimited proliferation,
self-renewal and multipotent differentiation. CSCs have been
reported to regulate cancer cells from the original tumor to a
distant organ (13), although the
underlying mechanisms remain unclear. Previous study showed that
acidic pHe can promote a stem cell-like phenotype in glioma
(14). However, whether acidic
tumor microenvironment promotes prostate cancer bone metastasis by
regulating the cancer stem cell characteristics of prostate cancer
remains unclear.
Acidosis has also been reported to increase the
production of proteinases and pro-angiogenic factors in tumor
cells, which are generally believed to accelerate the invasion and
metastasis of tumor cells (15,16).
The matrix metalloproteinases (MMPs) secreted by the tumor and
stroma cells may facilitate tumor growth, invasion and metastasis
through degrading the basement membranes and extracellular matrix
(ECM) (17). It is reported that
the expression of MMP-9 was significantly higher in the PCa than
that of the normal adjacent tissues, and there was significant
correlation between MMP-9 expression and clinicopathological stage
(18). Moreover, compared with
normal prostatic glands, angiogenic factor vascular endothelial
growth factor (VEGF) expression and microvessel density is
significantly higher in the premalignant and malignant tissues
(19). In addition, the expression
of VEGF significantly correlates with metastasis of PCa (20). However, whether acidic stress can
affect the cytokine expression in PCa and then affect the tumor
invasiveness and new vasculature formation is still unknown.
Tumor-associated neovasculature is an important
process for solid tumor progression and metastasis. Despite the
significant progress that has been made in the application of
antiangiogenic drugs for the treatment of cancer, the
antiangiogenic therapy remains transient and with insufficient
efficacy (21). Emerging evidence
indicates that circulating bone marrow-derived endothelial
progenitor cells (BM-EPCs) are recruited into the tumor
microenvironment during tumor vasculogenesis and ultimately promote
metastatic spreading through newly formed blood vessels (22,23).
In addition, VEGF plays a more important role in the recruitment of
BM-EPCs to tumor neovascularization sites and promote
vasculogenesis of BM-EPC (24,25).
Taken together, these studies suggest an important relationship
between VEGF and BM-EPCs in the formation of tumor vessel. Although
the positive role of acidic tumor microenvironmentin promoting
tumor progression and metastasis has been extensively investigated,
little is known as to its effect on BM-EPCS-mediated
vasculogenesis. Understanding the roles of acidic microenvironment
in tumor vessel formation is crucial for designing new
anti-angiogenic drugs to treat solid malignancies.
In the present study, we investigated the effect of
acidic extracellular microenvironment on PC-3 stem cell
characteristics and cell invasiveness after incubation in acidic
medium (AM) or neutral medium (NM). We isolated human bone
marrow-derived EPCs to determine the effect of PC-3 CMAM
on BM-EPCs functions. Additionally, we further investigated the
mechanisms involved in PC-3 CMAM-inducted vasculogenesis
of BM-EPC.
Materials and methods
Cell culture and acidic/NM
preparation
Human prostate cancer cell line PC-3 was purchased
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and the cells were cultured in RPMI-1640 medium supplemented with
10% fetal bovine serum (FBS) (both from HyClone, Logan, UT, USA),
streptomycin (100 µg/ml) and penicillin (100 U/ml). PC-3
cells were incubated in a humidified incubator (21% O2,
5% CO2, 37°C). The pH value of complete medium was
measured by pH and adjusted to 6.5 AM or 7.4 NM with hydrochloric
acid or sodium hydroxide.
Conditioned medium preparation and
enzyme-linked immunosorbent assay (ELISA)
PC-3 cells were cultured in 150 cm2
flasks in RPMI-1640 complete medium. Upon reaching 70–80%
confluency, cells were washed twice with phosphate-buffered saline
(PBS) and were then cultured in acidic (pH 6.5, 1% FBS) or neutral
(pH 7.4, 1% FBS) medium for additional 48 h. Subsequently, AM CM
(CMAM) and NM CM (CMNM) were harvested and
filtered. The MMP-9 and VEGF level of CMs were detected by ELISA
kit (R&D systems, Minneapolis, MN, USA) according to the
manufacturer's instructions. Thereafter, the CMs were stored at
−80°C for further use.
Spheroid formation assay
The spheroid formation assay was performed as we
previously described (26,27). In brief, PC-3 cells were incubated
in AM or NM for 48 h, then, single cell suspensions (400
cells/well) were added into 6-well non-adherent plates (Corning,
Corning, NY, USA) and grown in serum-free spheroid formation media
consisting of RPMI-1640 medium supplemented with 10 ng/ml of bFGF,
20 ng/ml of EGF and 2% B27 (all from Invitrogen and sigma). After 2
weeks, spheroids (diameter >100 µm) were counted and
captured under a light microscope (magnification, ×100).
Colony formation assay
Briefly, after incubating in AM or NM for 48 h, PC-3
cell suspensions (300 cells/well) were plated in 65-mm Petri dishes
and incubated for 15 days. Subsequently, the dishes were washed
twice with PBS before being fixed with 4% paraformaldehyde, and
then stained with 0.1% crystal violet. The colonies (≥50
cells/colony) were counted.
Cell viability and invasion assay
After incubating in AM or NM for 48 h, PC-3 cell
suspensions (1×104 cells/well) were plated into 96-well
plates. After 24 h, cell viability was examined by a Cell Counting
Kit-8 (CCK-8; Dojindo, Shanghai, China) following the
manufacturer's instructions. Transwell system (Corning Costar,
Acton, MA, USA) with polycarbonate filters (8-µm pore size;
6.5 mm diameter) was used for invasion assay. PC-3 cells were
incubated in AM or NM for 48 h before experiments. Next, cells were
resuspended in serum-free RPMI-1640 medium with or without
anti-MMP-9 antibody (5 µg/ml) or general MMP inhibitor
(GM6001; 15 µmol/l) (both from Chemicon, Temecula, CA, USA).
Cell suspensions (200 µl) (1×106 cells/ml) were
plated onto the upper chambers, and then 600 µl RPMI-1640
complete medium (10% FBS) was added to the lower chamber. After 48
h, the non-invading cells on the upper surface of upper chamber
were wiped off by cotton swab. After that, the invading cells on
the lower surface were washed twice with PBS before being fixed
with 4% paraformaldehyde for 20 min, and then stained with 0.1%
crystal violet for 15 min. The invasive cells were counted and
captured at a magnification of ×100.
Isolation and cultivation of BM-EPCs
The present study was approved by the Medical Ethics
Committee of the First Affiliated Hospital of Sun Yat-sen
University (approval no. 2008-55). BM-EPCs in our experiments were
isolated from human bone marrow, which was extracted from lumbar
vertebral body of patients (12 donors; age range, 42–63 years; mean
age, 52.1 years) in the First Affiliated Hospital of sun Yat-sen
University. All the informed patients underwent lumbar fusion due
to lumber degenerative diseases without tumor, hematological and
metabolic diseases. This procedure of isolation and cultivation of
BM-EPCs were performed as we previously described (28). BM-EPCs at passages 2–3 were used in
the present study.
BM-EPC cell viability and migration
assay
BM-EPCs (1×104 cells/well) were incubated
in EGM-2 into 96-well plates for 24 h. Then, cells were exposed to
NM, CMAM and CMNM (added 1% FBS to NM and
CMs, and modulated pH to 7.4). After 36 h, cell viability was
evaluated by a CCK-8 according to the manufacturer's instructions.
Cell migration was also evaluated by Transwell system with
polycarbonate filters (8-µm pore size, 6.5 mm diameter).
First, BM-EPCs were resuspended in serum-free RPMI-1640 medium.
Cell suspensions (200 µl) (1×106 cells/ml) were
plated onto the upper chambers, and then 600 µl NM,
CMAM and CMNM (added 1% FBS to NM and CMs,
and modulated pH to 7.4) was added to the lower chamber. After 16
h, the non-migrating cells on the upper surface of upper chamber
were wiped off by a cotton swab. The cells on the lower surface
were washed twice with PBS before being fixed with 4%
paraformaldehyde for 20 min, and then stained with 0.1% crystal
violet for 15 min. The migrated cells were counted and captured at
a magnification of ×100.
BM-EPC tube formation assay
In Vitro Angiogenesis Assay kit (Chemicon) was used
to evaluate the tube formation ability of BM-EPCs according to the
manufacturer's instructions. Briefly, after thawing at 4°C,
Matrigel was added to 96-well plate, and then incubated at 37°C for
30 min. Next, cells were re-suspended in NM, CMAM and
CMNM (added 1% FBS to NM and CMs, and modulated pH to
7.4). Then cell suspensions (1×104 cells/well) were
plated onto Matrigel-precoated 96-well plates and then incubated at
37°C for 16–18 h. The capillary-like structures were examined and
the number of closed network units was counted under a light
microscope (magnification, ×100).
Western blot analysis
Briefly, after washing twice with ice-cold PBS,
cells were lysed with extraction buffer (Novagen, Merck, Darmstadt,
Germany) for 30 min on ice. Next, the lysate was centrifuged
(12,000 × g for 10 min at 4°C), and protein concentration was
measured with Bradford reagent (Bio-Rad, Hercules, CA, USA). Total
proteins (30 µg) were separated on 10% SDS-polyacrylamide
gels, and then transferred to a polyvinylidene difluoride membrane.
After blocking with 5% fat-free milk powder, transferred blots were
incubated with primary antibodies: CD133, purchased from Miltenyi
Biotech (Auburn, CA, USA); CD44, purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA); Oct4, Klf4, GAPDH,
phospho-VEGFR2, phospho-P38, phospho-AKT, VEGFR2, P38 and AKT, were
purchased from Cell Signaling Technology (Beverly, MA, USA).
Statistical analysis
All experiments in the present study were performed
in triplicate. All experimental data are shown as the mean ±
standard deviation (SD). SPSS software (version 13.0; SPSS, Inc.,
Chicago, IL, USA) was used for data analysis. Statistical
differences between the groups were performed by Student's t-test.
A P-value <0.05 was regarded as statistically significant.
Results
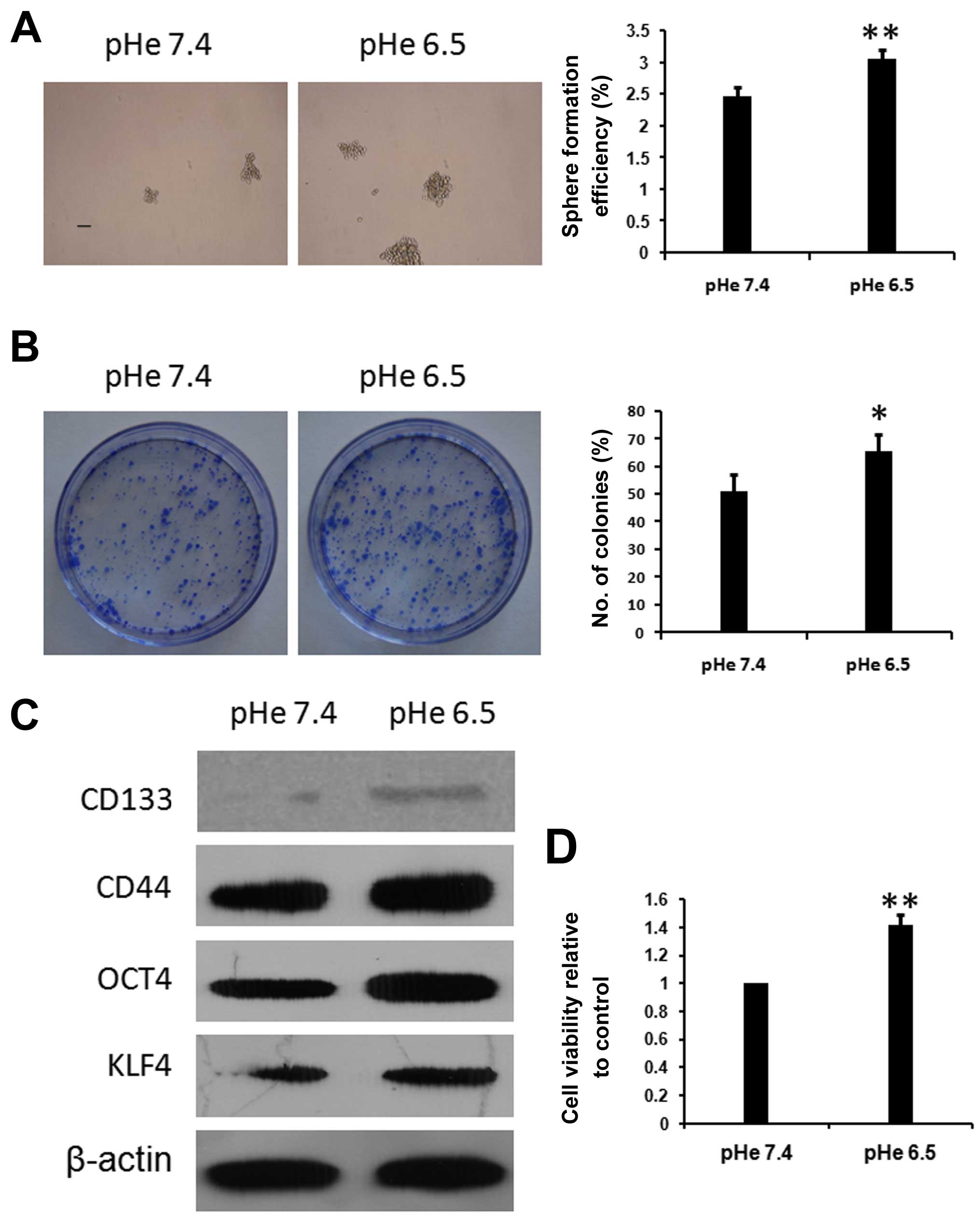
Acidic pHe promotes PC-3 stem cell
characteristics
To test whether acidic extracellular
microenvironment promotes PC-3 cell metastasis is through targeting
cancer stem cell-like characteristics, we first investigated
whether acidic pHe regulates the expression of prostate CSC
stemness-related markers in the PC-3 cell line. PC3 cells were
incubated in AM (pH 6.5) or NM (pH 7.4) for 48 h, then the
expression of CSC stemness-related markers (including CD133, CD44,
Oct4 and Klf4) in PC-3 cells was examined by western blotting. As
shown in Fig. 1C, CD44, Oct4 and
KLF4 were significantly upregulated after 48 h incubation in AM (pH
6.5). CD133 protein expression also increased after incubation in
AM (pH 6.5), although this effect was relatively less obvious.
Results showed that acidic pHe may be involved in regulating the
prostate stem cell characteristics. This was further confirmed by
spheres and colony formation assays of PC-3 cells. Results showed
the sizes and numbers of spheres in non-adherent culture
significantly increased when cells were pre-treated with AM
(Fig. 1A). In addition, colony
forming efficiency and cell viability significantly increased when
cells were pre-treated with AM (Fig.
1B). These results suggest that acidic extracellular
microenvironment is involved in regulating the stemness of PC-3
cells.
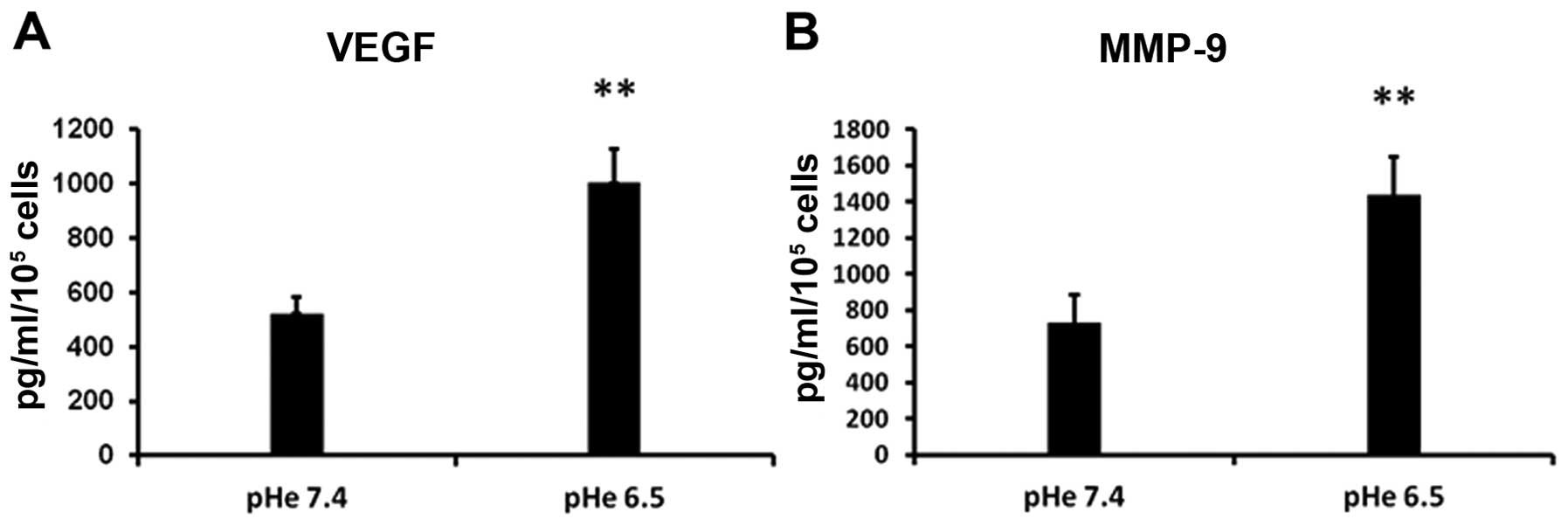
Acidic pHe induces MMP-9 and VEGF
secretion
It is now well confirmed that MMP-9 perform central
roles in tumor progression and metastasis (29). In addition, VEGF has been reported
to participate in the recruitment of BM-EPCs to tumor
neovascularization sites and play critical roles in regulating
vasculogenesis of BM-EPCs (25).
Therefore, we estimated whether acidic stress can affect the
cytokine expression in PC-3 cells. PC-3 cells were incubated in AM
or NM for 48 h, then the CMs were harvested. The secretion of MMP-9
and VEGF in CMs was measured by ELISA. Compared with
CMNM, the expression of MMP-9 and VEGF is significantly
higher in CMAM (Fig. 2).
These results suggest that acidic tumor microenvironment may
stimulate the secretion of many proteolytic enzymes such as MMP-9
and VEGF, which ultimately contribute to tumor invasion, metastasis
and vasculogenesis.
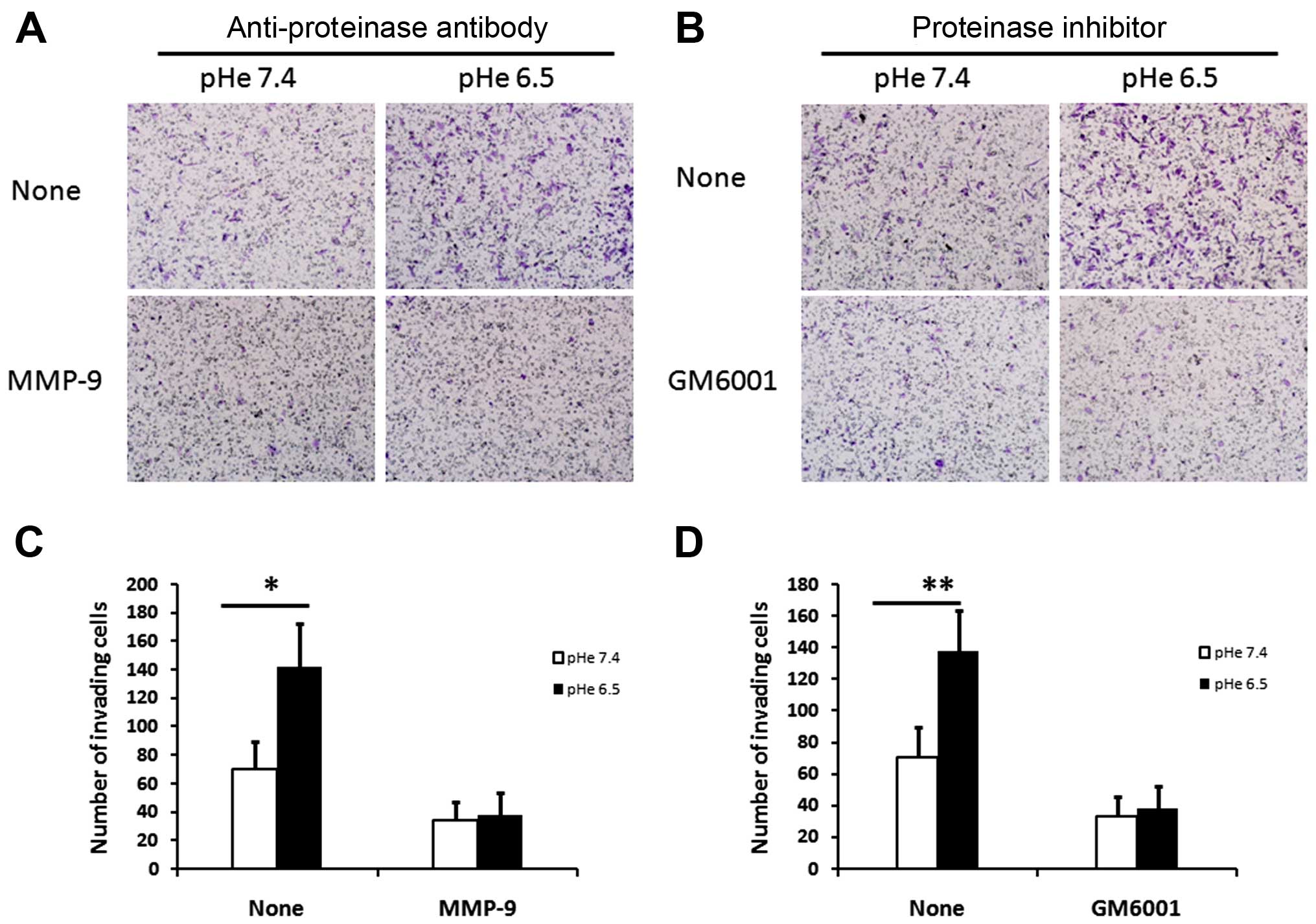
MMP-9 is involved in acid-induced
invasion of PC-3 cells
It is reported that upregulation of MMP-9 expression
increase the capacity of invasion in prostate cancer cell line
(30,31). Furthermore, the production of MMP-9
in CMAM was higher than that in the CMNM
(Fig. 2B). It seems that acidic
extracellular may induce MMP-9 expression in PC-3 cells and then
promote cell invasiveness. Therefore, in our experiments,
anti-MMP-9 antibody and the general MMP inhibitor (GM6001) were
used to further assess the role of MMP-9 in acid-regulated cell
invasiveness. As expected, in the absence of anti-MMP-9 antibody or
GM6001, PC-3 cells showed higher invasion activities after 48 h
incubation in AM than that in the NM (Fig. 3A and C). Notably, the addition of
anti-MMP-9 antibody or GM6001 significantly decreased the invasive
ability of PC-3 cells, which were pre-treated in AM or NM for 48 h.
However, in the presence of anti-MMP-9 antibody or GM6001, there
was no significant differences in the invasive ability between
cells pre-treated with AM and cells pre-treated with NM. Taken
together, these results revealed that upregulation of MMP-9 in PC-3
cells is involved in the acidy-induced invasion of PC-3 cells.
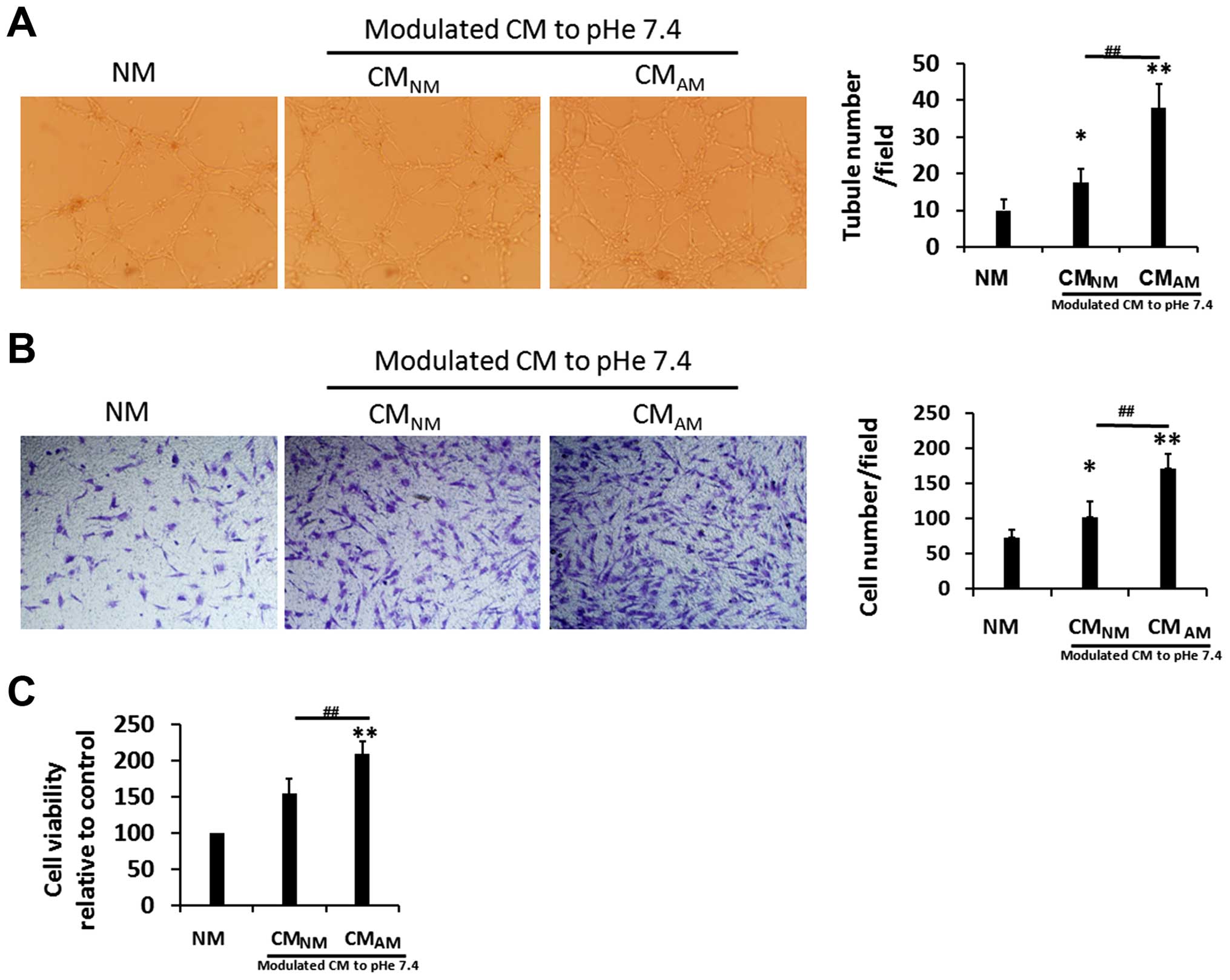
PC-3 CMAM promotes cell
viability, migration and tube formation of BM-EPCs
To observe the possible effects of PC-3 CM on the
vasculogenesis of BM-EPC, we examined whether CMs or NM modulate
cell viability, migration and tube formation of BM-EPCs. We found
that CMAM and CMNM significantly enhanced
cell viability and migration of BM-EPCs than NM, and the effects on
cell viability and migration was more obvious in CMAM
(Fig. 4B and C). Similarly,
CMAM and CMNM promoted capillary tubule
formation of BM-EPCs rather than NM, and more markedly in
CMAM (Fig. 4A). These
data suggest that PC-3 CMAM can promote vasculogenesis
of BM-EPCs.
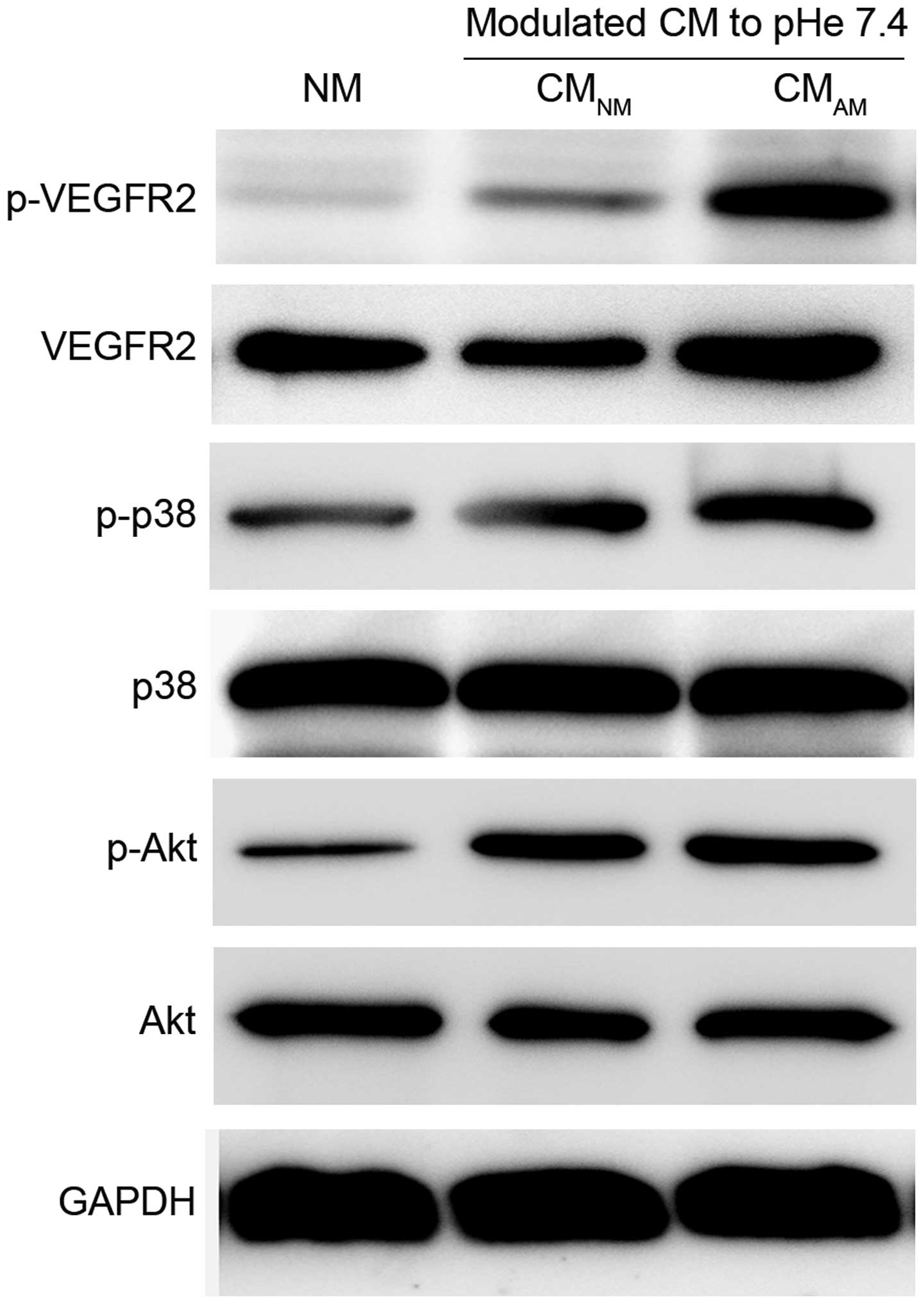
PC-3 CMAM induces activation
of VEGFR2-, Akt- and p38-phosphorylation
VEGF/VEGFR signaling pathway has been confirmed to
play central roles in pathological angiogenesis, and binding of
VEGFR2 with VEGF can activate numerous downstream signal pathways,
including Akt and p38, which sequentially promote endothelial cell
growth, migration and tube formation (32–34).
Therefore, we further evaluated whether these signaling pathways
are involved in the PC-3 CMAM-induced vasculogenesis of
BM-EPCs. Western blotting showed the expression levels of
phosphorylated VEGFR2, phosphorylated Akt and phosphorylated P38
was significantly increased in CMAM group compared with
CMNM and NM groups (Fig.
5). PC-3 CMAM showed obvious promotion effect on
phosphorylated AKT and P38. These data revealed that PC-3
CMAM promoted VEGFR2 signal through activation of AKT
and p38, then induced vasculogenesis of BM-EPCs.
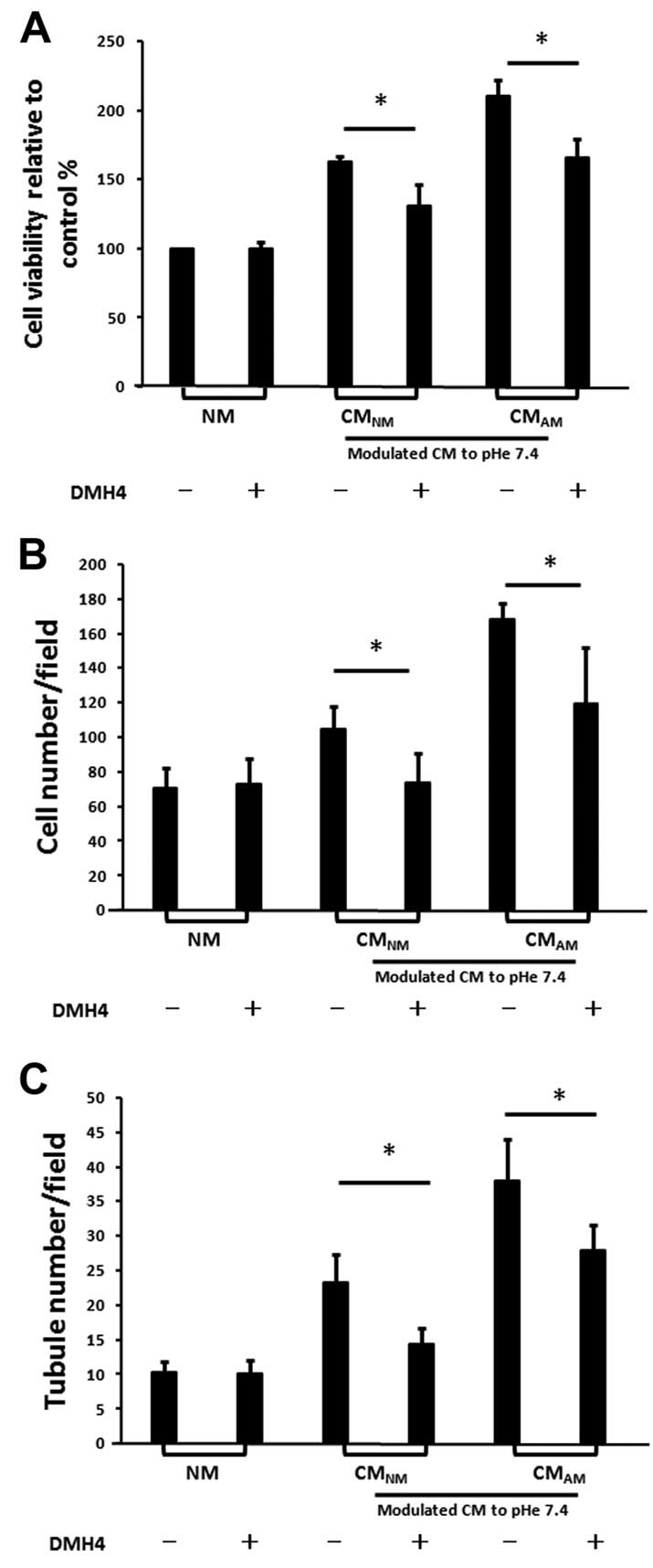
CMAM-induced vasculogenesis of
BM-EPC is partly reduced by the inhibition of VEGFR2 with DMH4
VEGF has been reported to play critical roles in
regulating vasculogenesis of BM-EPCs (24,25).
As we previously described, the production of VEGF in
CMAM was higher than that in CMNM (Fig. 2A). Taken together, it suggests that
VEGF may play an important role in CM-induced vasculogenesis of
BM-EPCs. We further assessed the effects of DMH4 on CM-induced
vasculogenesis of BM-EPCs (Fig. 6).
We found that the addition of DMH4 to the CMs reduced the cell
viability, migration and tube formation of BM-EPCs. The results
further confirm that acidic pHe may promote BM-EPCs-mediated
vasculogenesis by stimulating the secretion of VEGF in PC-3
cells.
Discussion
Many types of cancer are reported to be
characterized by the presence of cancer stem cells (CSCs), residing
in acidic microenvironment, and these rare cell subpopulations are
currently considered responsible for tumor initiation, maintenance
and post-therapeutic recurrence. Additionally, CSCs are considered
to be involved in bone metastasis of breast (35,36)
and prostate cancer (37,38). Acidic tumor microenvironment seems
to promote a stem cell-like phenotype in glioma (14). Although the importance of tumor
microenvironment in tumor metastasis is recognized, whether acidic
tumor microenvironment promotes prostate cancer bone metastasis by
enhancing the cancer stem-like cell characteristics of prostate
cancer remains unclear.
In the present study, the PC-3 cell line which
derives from a metastatic bone of prostate cancer was used as a
cell model to investigate the possible effect of acidic
extracellular microenvironment on the bone metastasis in prostate
cancer. We found that acidic pHe enhanced the PC-3 cell
proliferation and tumor sphere formation. Furthermore, acidic pHe
increased the expression of CSC stemness-related markers (including
CD133, CD44, Oct4 and Klf4) in PC-3 cells. These findings
demonstrate that acidic pHe positively regulate the stem cell
characteristics of PC-3 cells. Importantly, these stem cell-like
cancer cells are considered to be the initial factor in tumor
progression and distant metastasis (39). Thus, our results revealed that
acidic extracellular microenvironment may promote prostate cancer
bone metastasis by enhancing the cancer stem cell characteristics.
However, further studies are necessary to uncover the exact
mechanism of how acidic pHe enhances PC-3 stem cell
characteristics.
It is now well confirmed that the MMPs perform
central roles in tumor progression and metastasis (29,40).
In addition, MMPs are considered to be promising biomarkers in the
diagnosis and prognosis of cancers (41). Given the critical roles that MMPs
play in cancer progression and metastasis, MMP inhibitors have been
developed to treat cancers (42).
Previous studies showed that upregulation of MMP-9 expression
increase the capacity of invasion and metastasis in a prostate
cancer cell line (30). In
addition, it is reported that the MMP-9 was significantly higher in
PCa than that of normal adjacent prostate, and its expression
closely correlated with clinicopathological stage (18). In the present study, we found that
acidic pHe increased the secretion of MMP-9 as well as PC-3
invasiveness, and the capacity of cell invasiveness promoted by
acidic pHe can be repressed by MMP-9 antibodies or MMP-9
inhibitors. The results of the present study indicated that acidic
pHe may promote cell invasiveness through stimulating upregulation
of MMP-9. Recently, it is reported that acidic microenvironment
significantly enhances invasiveness of PC-3 cells by stimulating
the secretion of cathepsin B (43).
These observations are consistent with other reports that
proteolytic enzymes are closely related to the invasion and
metastasis of many tumors (44,45).
Therefore, it seems that acidic tumor microenvironment may
stimulate the secretion of many proteolytic enzymes such as MMP-9,
which ultimately contributes to tumor invasion and metastasis.
Accumulative evidence illustrated that circulating
BM-EPCs are recruited around the tumor by growth factors and
chemokines and contribute to an important component of tumor
microenvironment (22,23). Importantly, the crucial role of
BM-EPCs in mediating the tumor-associated neovasculature has been
deeply investigated (46,47). Furthermore, VEGF has been reported
to participate in the recruitment of BM-EPCs to tumor
neovascularization sites and play critical roles in regulating
vasculogenesis of BM-EPCs (25).
VEGF/VEGFR signaling pathway has been confirmed to
play central roles in pathological angiogenesis, and various
anti-angiogenic drugs have been developed to fight cancer by
blocking this pathway (25,48). Previous studies demonstrated that
two types of VEGFR (VEGFR1 and VEGFR2) were expressed in BM-EPC
(49). Circulating
VEGFR2+ BM-EPCs are proved to correlate with tumor
metastasis (50). VEGFR2 can
activate many downstream signal pathways, including Akt and p38,
which sequentially promote endothelial cell growth, migration and
tube formation (32,33). In our experiments, the
phosphorylation of VEGFR2, Akt and p38 in BM-EPCs was increased by
PC-3 CM and the phosphorylation level was more significant when
PC-3 cells were cultured at pHe 6.5. This finding indicates that
PC-3 CMAM promotes VEGF-induced vasculogenesis of
BM-EPCs, which leads to activating the phosphorylation of VEGFR-2,
Akt and P38.
In the present study, we found that PC-3 CMs
enhanced proliferation and migration of BM-EPCs rather than NM, and
the positive impacts on BM-EPCs were more significant when PC-3
cells were cultured at pHe 6.5. Similar impact was found in
CM-induced tube formation of BM-EPCs. On the other hand, these
positive roles of PC-3 CMs on BM-EPCs were partly reduced by DMH4.
The results of the present study revealed that acidic pHe may
promote BM-EPCs-mediated vasculogenesis by stimulating the
secretion of VEGF in PC-3 cells.
In conclusion, acidic tumor microenvironment may
have the potential to modulate the prostate cancer metastasized to
bone by enhancing cancer stem cell characteristics, cell
invasiveness and VEGF-induced vasculogenesis of BM-EPCs. Given the
critical roles that acidic tumor microenvironment plays in tumor
growth and metastasis, anticancer strategies should be designed to
selectively target acidic tumor microenvironment.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81402227), the
National Basic Research Program of China [973 Program
(2012CB619105)], and the Guangdong Natural Science Foundation (no.
2014A030310157).
References
|
1
|
Piccioli A, Maccauro G, Spinelli MS,
Biagini R and Rossi B: Bone metastases of unknown origin:
Epidemiology and principles of management. J Orthop Traumatol.
16:81–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Doctor SM, Tsao CK, Godbold JH, Galsky MD
and Oh WK: Is prostate cancer changing?: Evolving patterns of
metastatic castration-resistant prostate cancer. Cancer.
120:833–839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Polyak K, Haviv I and Campbell IG:
Co-evolution of tumor cells and their microenvironment. Trends
Genet. 25:30–38. 2009. View Article : Google Scholar
|
|
4
|
Gribben J, Rosenwald A, Gascoyne R and
Lenz G: Targeting the microenvironment. Leuk Lymphoma. 51(Suppl 1):
S34–S40. 2010. View Article : Google Scholar
|
|
5
|
Whipple CA: Tumor talk: understanding the
conversation between the tumor and its microenvironment. Cancer
Cell Microenviron. 2:e7732015.PubMed/NCBI
|
|
6
|
Chiche J, Brahimi-Horn MC and Pouysségur
J: Tumour hypoxia induces a metabolic shift causing acidosis: A
common feature in cancer. J Cell Mol Med. 14:771–794. 2010.
View Article : Google Scholar
|
|
7
|
Gillies RJ, Robey I and Gatenby RA: Causes
and consequences of increased glucose metabolism of cancers. J Nucl
Med. 49(Suppl 2): 24S–42S. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pastorekova S, Zatovicova M and Pastorek
J: Cancer-associated carbonic anhydrases and their inhibition. Curr
Pharm Des. 14:685–698. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vaupel P, Kallinowski F and Okunieff P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors: A review. Cancer Res.
49:6449–6465. 1989.PubMed/NCBI
|
|
10
|
Riemann A, Schneider B, Gündel D, Stock C,
Gekle M and Thews O: Acidosis promotes metastasis formation by
enhancing tumor cell motility. Adv Exp Med Biol. 876:215–220. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song J, Ge Z, Yang X, Luo Q, Wang C, You
H, Ge T, Deng Y, Lin H, Cui Y, et al: Hepatic stellate cells
activated by acidic tumor microenvironment promote the metastasis
of hepatocellular carcinoma via osteopontin. Cancer Lett.
356:713–720. 2015. View Article : Google Scholar
|
|
12
|
Gerweck LE, Vijayappa S and Kozin S: Tumor
pH controls the in vivo efficacy of weak acid and base
chemotherapeutics. Mol Cancer Ther. 5:1275–1279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Wu JB, Li Q, Shigemura K, Chung LW
and Huang WC: SREBP-2 promotes stem cell-like properties and
metastasis by transcriptional activation of c-Myc in prostate
cancer. Oncotarget. 7:12869–12884. 2016.PubMed/NCBI
|
|
14
|
Hjelmeland AB, Wu Q, Heddleston JM,
Choudhary GS, MacSwords J, Lathia JD, McLendon R, Lindner D, Sloan
A and Rich JN: Acidic stress promotes a glioma stem cell phenotype.
Cell Death Differ. 18:829–840. 2011. View Article : Google Scholar :
|
|
15
|
Rofstad EK, Mathiesen B, Kindem K and
Galappathi K: Acidic extracellular pH promotes experimental
metastasis of human melanoma cells in athymic nude mice. Cancer
Res. 66:6699–6707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsubara T, Diresta GR, Kakunaga S, Li D
and Healey JH: Additive influence of extracellular pH, oxygen
tension, and pressure on invasiveness and survival of human
osteosarcoma cells. Front Oncol. 3:1992013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hidalgo M and Eckhardt SG: Development of
matrix metalloproteinase inhibitors in cancer therapy. J Natl
Cancer Inst. 93:178–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cardillo MR, Di Silverio F and Gentile V:
Quantitative immunohistochemical and in situ hybridization analysis
of metalloproteinases in prostate cancer. Anticancer Res.
26:973–982. 2006.PubMed/NCBI
|
|
19
|
Pallares J, Rojo F, Iriarte J, Morote J,
Armadans LI and de Torres I: Study of microvessel density and the
expression of the angiogenic factors VEGF, bFGF and the receptors
Flt-1 and FLK-1 in benign, premalignant and malignant prostate
tissues. Histol Histopathol. 21:857–865. 2006.PubMed/NCBI
|
|
20
|
Wegiel B, Bjartell A, Tuomela J, Dizeyi N,
Tinzl M, Helczynski L, Nilsson E, Otterbein LE, Härkönen P and
Persson JL: Multiple cellular mechanisms related to cyclin A1 in
prostate cancer invasion and metastasis. J Natl Cancer Inst.
100:1022–1036. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shang B, Cao Z and Zhou Q: Progress in
tumor vascular normalization for anticancer therapy: Challenges and
perspectives. Front Med. 6:67–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Palma M and Naldini L: Role of
haematopoietic cells and endothelial progenitors in tumour
angiogenesis. Biochim Biophys Acta. 1766:159–166. 2006.PubMed/NCBI
|
|
23
|
Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi
JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA,
Benezra R, et al: Bone marrow-derived endothelial progenitor cells
are a major determinant of nascent tumor neovascularization. Genes
Dev. 21:1546–1558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li B, Sharpe EE, Maupin AB, Teleron AA,
Pyle AL, Carmeliet P and Young PP: VEGF and PlGF promote adult
vasculogenesis by enhancing EPC recruitment and vessel formation at
the site of tumor neovascularization. FASEB J. 20:1495–1497. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SH, Jeong D, Han YS and Baek MJ:
Pivotal role of vascular endothelial growth factor pathway in tumor
angiogenesis. Ann Surg Treat Res. 89:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang S, Guo W, Tang Y, Ren D, Zou X and
Peng X: miR-143 and miR-145 inhibit stem cell characteristics of
PC-3 prostate cancer cells. Oncol Rep. 28:1831–1837.
2012.PubMed/NCBI
|
|
27
|
Huang S, He P, Peng X, Li J, Xu D and Tang
Y: Pristimerin inhibits prostate cancer bone metastasis by
targeting PC-3 stem cell characteristics and VEGF-induced
vasculogenesis of BM-EPCs. Cell Physiol Biochem. 37:253–268. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Folkins C, Shaked Y, Man S, Tang T, Lee
CR, Zhu Z, Hoffman RM and Kerbel RS: Glioma tumor stem-like cells
promote tumor angiogenesis and vasculogenesis via vascular
endothelial growth factor and stromal-derived factor 1. Cancer Res.
69:7243–7251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shuman Moss LA, Jensen-Taubman S and
Stetler-Stevenson WG: Matrix metalloproteinases: Changing roles in
tumor progression and metastasis. Am J Pathol. 181:1895–1899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aalinkeel R, Nair BB, Reynolds JL, Sykes
DE, Mahajan SD, Chadha KC and Schwartz SA: Overexpression of MMP-9
contributes to invasiveness of prostate cancer cell line LNCaP.
Immunol Invest. 40:447–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Q, Diao X, Sun J and Chen Z:
Regulation of VEGF, MMP-9 and metastasis by CXCR4 in a prostate
cancer cell line. Cell Biol Int. 35:897–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kowshik J, Giri H, Kishore TK, Kesavan R,
Vankudavath RN, Reddy GB, Dixit M and Nagini S: Ellagic acid
inhibits VEGF/VEGFR2, PI3K/Akt and MAPK signaling cascades in the
hamster cheek pouch carcinogenesis model. Anticancer Agents Med
Chem. 14:1249–1260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koch S and Claesson-Welsh L: Signal
transduction by vascular endothelial growth factor receptors. Cold
Spring Harb Perspect Med. 2:a0065022012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang Y, Huang B, Sun L, Peng X, Chen X and
Zou X: Ginkgolide B promotes proliferation and functional
activities of bone marrow-derived endothelial progenitor cells:
Involvement of Akt/eNOS and MAPK/p38 signaling pathways. Eur Cell
Mater. 21:459–469. 2011.PubMed/NCBI
|
|
35
|
D'Amico L, Patanè S, Grange C, Bussolati
B, Isella C, Fontani L, Godio L, Cilli M, D'Amelio P, Isaia G, et
al: Primary breast cancer stem-like cells metastasise to bone,
switch phenotype and acquire a bone tropism signature. Br J Cancer.
108:2525–2536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Balic M, Lin H, Young L, Hawes D, Giuliano
A, McNamara G, Datar RH and Cote RJ: Most early disseminated cancer
cells detected in bone marrow of breast cancer patients have a
putative breast cancer stem cell phenotype. Clin Cancer Res.
12:5615–5621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li H and Tang DG: Prostate cancer stem
cells and their potential roles in metastasis. J Surg Oncol.
103:558–562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Monteiro J and Fodde R: Cancer stemness
and metastasis: Therapeutic consequences and perspectives. Eur J
Cancer. 46:1198–1203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Michl M, Heinemann V, Jung A, Engel J,
Kirchner T and Neumann J: Expression of cancer stem cell markers in
metastatic colorectal cancer correlates with liver metastasis, but
not with metastasis to the central nervous system. Pathol Res
Pract. 211:601–609. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Purcell WT, Rudek MA and Hidalgo M:
Development of matrix metalloproteinase inhibitors in cancer
therapy. Hematol Oncol Clin North Am. 16:1189–1227. 2002.
View Article : Google Scholar
|
|
41
|
Hadler-Olsen E, Winberg JO and
Uhlin-Hansen L: Matrix metalloproteinases in cancer: Their value as
diagnostic and prognostic markers and therapeutic targets. Tumour
Biol. 34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang JS, Lin CW, Su SC and Yang SF:
Pharmacodynamic considerations in the use of matrix
metalloproteinase inhibitors in cancer treatment. Expert Opin Drug
Metab Toxicol. 12:191–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gao L, Fang YQ, Zhang TY, Ge B, Tang RJ,
Huang JF, Jiang LM and Tan N: Acidic extracellular microenvironment
promotes the invasion and cathepsin B secretion of PC-3 cells. Int
J Clin Exp Med. 8:7367–7373. 2015.PubMed/NCBI
|
|
44
|
Buckley JJ and Jessen JR: Matrix
metalloproteinase function in non-mammalian model organisms. Front
Biosci. 7:168–183. 2015. View
Article : Google Scholar
|
|
45
|
Zhang X, Huang S, Guo J, Zhou L, You L,
Zhang T and Zhao Y: Insights into the distinct roles of MMP-11 in
tumor biology and future therapeutics (Review). Int J Oncol.
48:1783–1793. 2016.PubMed/NCBI
|
|
46
|
Gao D, Nolan D, McDonnell K, Vahdat L,
Benezra R, Altorki N and Mittal V: Bone marrow-derived endothelial
progenitor cells contribute to the angiogenic switch in tumor
growth and metastatic progression. Biochim Biophys Acta.
1796:33–40. 2009.PubMed/NCBI
|
|
47
|
Moschetta M, Mishima Y, Sahin I, Manier S,
Glavey S, Vacca A, Roccaro AM and Ghobrial IM: Role of endothelial
progenitor cells in cancer progression. Biochim Biophys Acta.
1846:26–39. 2014.PubMed/NCBI
|
|
48
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hoffmann BR, Wagner JR, Prisco AR, Janiak
A and Greene AS: Vascular endothelial growth factor-A signaling in
bone marrow-derived endothelial progenitor cells exposed to hypoxic
stress. Physiol Genomics. 45:1021–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Farace F, Gross-Goupil M, Tournay E,
Taylor M, Vimond N, Jacques N, Billiot F, Mauguen A, Hill C and
Escudier B: Levels of circulating CD45 (dim)CD34 (+)VEGFR2 (+)
progenitor cells correlate with outcome in metastatic renal cell
carcinoma patients treated with tyrosine kinase inhibitors. Br J
Cancer. 104:1144–1150. 2011. View Article : Google Scholar : PubMed/NCBI
|