Introduction
Breast cancer is the leading cause of cancer
morbidity and mortality in women worldwide. Based on early
diagnosis and treatment, the 5-year survival for patients with
breast cancer has made noticeable improvement. Breast cancer still
accounts for 25% of all cancer cases and 15% of all cancer-related
deaths among females (1). Although
estrogen receptor (ER), progesterone receptor (PR) and human
epidermal growth factor receptor 2 (HER2)/neu protein are useful
biomarkers in breast cancer diagnosis and treatment, the discovery
of new biomarkers related to breast cancer can help build a deeper
and comprehensive understanding of this disease.
Adenylyl cyclase-associated protein (CAP) is an
evolutionarily highly conserved protein in all eukaryotes including
mammals and was first identified in yeast as a protein that
regulates both the actin cytoskeleton and the Ras/cAMP pathway
(2,3). The actin cytoskeleton plays an
important role in cellular functions such as morphogenesis,
cytokinesis and cell migration. An aberrant actin cytoskeleton
usually underlies oncogenesis and cancer metastasis (4,5). In
mammals, cells have two CAP isoforms, CAP1 and 2. Compared with
CAP1, CAP2 has a more restricted expression pattern and is found
mainly in skeletal muscle, cardiac muscle, brain and skin (6). At present, there are few studies on
the relationship between CAPs and tumors. CAP1 overexpression was
found in hepatocellular carcinoma (HCC), breast and ovarian cancers
(7–9). Shibata et al reported that CAP2
is upregulated in HCC and is related to multistage
hepatocarcinogenesis (10). Masugi
et al found that CAP2 overexpression is a novel prognostic
marker in malignant melanoma and its expression appears to increase
stepwise during tumor progression (11).
However, the clinical significance of CAP2 in breast
cancer remains unclear. In this study, we examined the CAP2
expression in breast cancer cell lines and tissue samples, and
revealed its clinicopathological and prognostic significance.
Materials and methods
Cell lines
Human breast cancer cell lines, including
MDA-MB-435, MDA-MB-453, MCF-7, T47D, and SK-BR-3 were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY,
USA) supplemented with 10% fetal calf serum (HyClone, Logan, UT,
USA) at 37°C in 5% CO2. The procedure for primary
culture of human mammary epithelial cells (HMECs) is similar to
that for human nasopharyngeal epithelial cells (12).
Patients and specimens
This study was conducted using a total of 126
paraffin-embedded primary breast cancer samples from patients who
were histopathologically diagnosed and underwent curative resection
at the Third Affiliated Hospital of Sun Yat-sen University between
March 2001 and December 2012. None of the patients received any
type of neoadjuvant therapy and all of them underwent curative
surgery. The clinical information of these cases is summarized in
Table I. The follow-up time of the
breast cancer cohort ranged from 2 to 131 months, and the median
follow-up time was 111 months. Of these 126 breast cancer patients,
paired adjacent non-cancerous tissues (the adjacent non-cancerous
tissue was defined as at least 2 cm distant from the edge of the
tumor) were obtained from 30 patients. Twenty normal breast tissues
were obtained from patients that underwent mammaplasty.
 | Table ICorrelation of CAP2 expression with
clinicopathologic features of the breast cancer cases. |
Table I
Correlation of CAP2 expression with
clinicopathologic features of the breast cancer cases.
| Characteristics | Total
(n=126)
n (%) | CAP2 expression
| P-value |
|---|
Low (n=89)
n (%) | High (n=37)
n (%) |
|---|
| Age (years) | | | | 0.626 |
| ≥60 | 37 (29.37) | 25 (67.6) | 12 (32.4) | |
| <60 | 89 (70.63) | 64 (71.9) | 25 (28.1) | |
| Clinical stage | | | | 0.223 |
| I | 10
(7.94) | 6
(60.0) | 4
(40.0) | |
| II | 76 (60.32) | 58 (76.3) | 18 (23.7) | |
| III | 40 (31.75) | 25 (62.5) | 15 (37.5) | |
| T classification | | | | 0.506 |
| T1 | 26 (20.63) | 20 (76.9) | 6
(23.1) | |
| T2 | 87 (69.05) | 60 (69.0) | 27 (31.0) | |
| T3 | 13 (10.32) | 9
(69.2) | 4
(30.8) | |
| N classification | | | | 0.588 |
| N0 | 49 (38.89) | 35 (71.4) | 14 (28.6) | |
| N1 | 39 (30.95) | 29 (74.4) | 10 (25.6) | |
| N2 | 30 (23.81) | 21 (70.0) | 9
(30.0) | |
| N3 | 8
(6.35) | 4
(50.0) | 4
(50.0) | |
| Differentiation | | | | 0.448 |
| Well | 13 (10.32) | 11 (84.6) | 2
(15.4) | |
| Moderate | 94 (74.60) | 64 (68.1) | 30 (31.9) | |
| Poor | 19 (15.08) | 14 (73.7) | 5
(26.3) | |
| ER expression | | | | 0.122 |
| Negative | 45 (35.71) | 28 (62.2) | 17 (37.8) | |
| Positive | 81 (64.29) | 61 (75.3) | 20 (24.7) | |
| PR expression | | | | 0.005 |
| Negative | 54 (42.86) | 31 (57.4) | 23 (42.6) | |
| Positive | 72 (57.14) | 58 (80.6) | 14 (19.4) | |
| HER2
expression | | | | 0.536 |
| Negative | 90 (71.43) | 65 (72.2) | 25 (27.8) | |
| Positive | 36 (28.57) | 24 (66.7) | 12 (33.3) | |
Clinicopathological classification and staging were
determined according to the American Joint Committee on Cancer
(AJCC, 7th edition) criteria. Patient consent to the use of these
clinical specimens for research purposes was gained prior and the
protocol was approved by the Institutional Research Ethics
Committee of Sun Yat-sen University. Ten paired breast cancer and
adjacent non-cancerous tissues were collected immediately after
surgery for real-time PCR and western blotting.
Real-time quantitative-polymerase chain
reaction (RT-PCR) analysis
Total RNA samples were extracted from cell lines and
primary breast tumor materials using TRIzol reagent (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer's instructions.
Extracted RNA was pretreated with RNase-free DNase. Two micrograms
of RNA from each sample were used for cDNA synthesis. For the PCR
amplification of CAP2 cDNA, an initial amplification step using
CAP2-specific primers was performed with a denaturation at 95°C for
10 min, followed by 28 denaturation cycles at 95°C for 60 sec, then
primer annealing at 58°C for 30 sec, and then a primer extension
phase at 72°C for 30 sec. Upon completion of these cycling steps, a
final extension at 72°C for 5 min was carried out before the
reaction mixture was stored at 4°C. Then real-time PCR was
performed to determine the fold increase of CAP2 mRNA in each of
the breast tumor and paired normal breast tissues from the same
patient. The primer sequences were as follows: CAP2 sense,
5′-GCCGCCTGGAGTCGCTGTC-3′ and antisense,
5′-AAAACTCGGCCACCATACTGTCCA-3′. GAPDH (sense,
5′-TGTTGCCATCAATGACCCC-3′ and antisense, 5′-CTCCACGACGTACTCAGC-3′)
was used as an internal control. The primers were designed by
Primer Express Software v2.0 (Applied Biosystems).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an
internal control, and all experiments were performed in
triplicate.
Western blotting
Cells at 70–80% confluency were washed twice by
ice-cold phosphate-buffered saline (PBS) and then lysed on ice by
radioimmunoprecipitation assay (RIPA; Cell Signaling Technology,
Inc., Danvers, MA) buffer which contained complete protease
inhibitor cocktail (Roche Applied Science, Mannheim, Germany).
Fresh tissue samples were ground into powder in liquid nitrogen and
lysed by SDS-PAGE sample buffer. A total of 20 µg of protein
samples was separated on 10.5% SDS polyacrylamide gels and then
transferred to PVDF membranes (Immobilon-P; Millipore, Billerica,
MA, USA). The membranes were then blocked with 5% fat-free milk in
Tris-buffered saline with 0.1% Tween-20 (TBST) for 1 h at room
temperature. PVDF membranes were incubated with anti-CAP2 antibody
(1:1,000, 15865-1-AP; Proteintech Group, Inc., Rosemont, IL, USA)
overnight at 4°C, and then with horseradish peroxidase-conjugated
goat anti-rabbit IgG (SC-2004; Santa Cruz Biotechnology, Inc.).
CAP2 expression was detected by ECL Western Blotting detection
reagent (Amersham/GE Healthcare Life Sciences) according to the
manufacturer's instructions. GAPDH (1:1,000; Proteintech Group,
Inc.) was used as loading control.
Immunohistochemical (IHC) analysis
IHC staining was performed to study altered protein
expression in 126 human breast cancer tissues, 30 paired adjacent
non-cancerous tissues, and 20 normal breast tissues. Briefly,
4-µm-thick paraffin sections of the tissue were
deparaffinized with xylene and rehydrated. Antigenic retrieval was
performed by submerging the slides into EDTA antigenic retrieval
buffer and microwaving. In order to quench endogenous peroxidase
activity, the slides were treated with 3% hydrogen peroxide in
methanol, and then incubated with 1% bovine serum albumin to block
non-specific binding. After that, sections were incubated with
anti-CAP2 rabbit polyclonal antibody (1:100, bs-1616R; Beijing
Bioss Biosynthesis Biotechnology Co., Ltd.; Beijing, China) at 4°C
overnight. Normal goat serum was used as a negative control. The
tissue sections were incubated with a biotinylated anti-rabbit
secondary antibody after being washed 3 times, followed by further
incubation with streptavidin-horseradish peroxidase complex (both
from Abcam). Slides were immersed in 3-amino-9-ethyl carbazole and
then counterstained with 10% Mayer's hematoxylin, finally
dehydrated and mounted in Crystal Mount.
As for evaluation of immunostaining, the degree of
immunostaining was viewed and scored separately by two
pathologists, who were blinded to the histopathological
characteristics and patient information of the samples. Scores
given by the two independent pathologists were averaged for further
comparative evaluation of CAP2 expression. The intensity of CAP2
staining was graded according to the following criteria: 0, no
staining; 1, weak staining (light yellow); 2, moderate staining
(yellow brown); 3, strong staining (brown). The percentage of
stained tumor cells was scored as follows: 0, no positive tumor
cells; 1, 1–25% positive tumor cells; 2, 26–50% positive tumor
cells; 3, 51–75% positive tumor cells; 4, >75% positive tumor
cells.
The staining score was calculated as the product of
the proportion of positive tumor cells and the staining intensity
score. The expression level of CAP2 was defined as follows: '−'
(score 0, negative), '+' (score 1–4, weakly positive), '++' (score
5–8, positive), '+++' (score 9–12, strongly positive). Cut-off
values for CAP2 were chosen on the basis of the heterogeneity using
log-rank test with respect to overall survival (OS). The optimal
cut-off value was estimated as follows: a staining index score of
≥8 was used to define tumors with high CAP2 expression and <8
indicated low CAP2 expression.
Statistical analysis
The duration from the date of each patient's
randomization to the date of death for any cause or the censoring
of the patient at the last follow-up date was defined as OS. All
the statistical analyses were conducted using the SPSS 20.0
statistical software packages. The differences in CAP2 expression
between breast cancer tissues, adjacent non-cancerous tissues and
normal breast tissues were analyzed by Chi-square test. Survival
curves were plotted by Kaplan-Meier method and compared using the
log-rank test. The relationship between CAP2 expression and other
clinicopathological characteristics was analyzed by Chi-square test
and Fisher's exact test. Bivariate correlations between the
clinicopathological characteristics were calculated by Spearman's
rank correlation coefficients. Clinicopathological characteristics
used to predict prognosis in clinical practice were evaluated by
univariate and multivariate Cox regression analyses. The chosen
type of Cox model for univariate analysis was the enter method, and
for multivariate analysis was forward method. A p-value <0.05
was considered to indicate a statistically significant result.
Results
CAP2 is overexpressed in breast cancer
cell lines
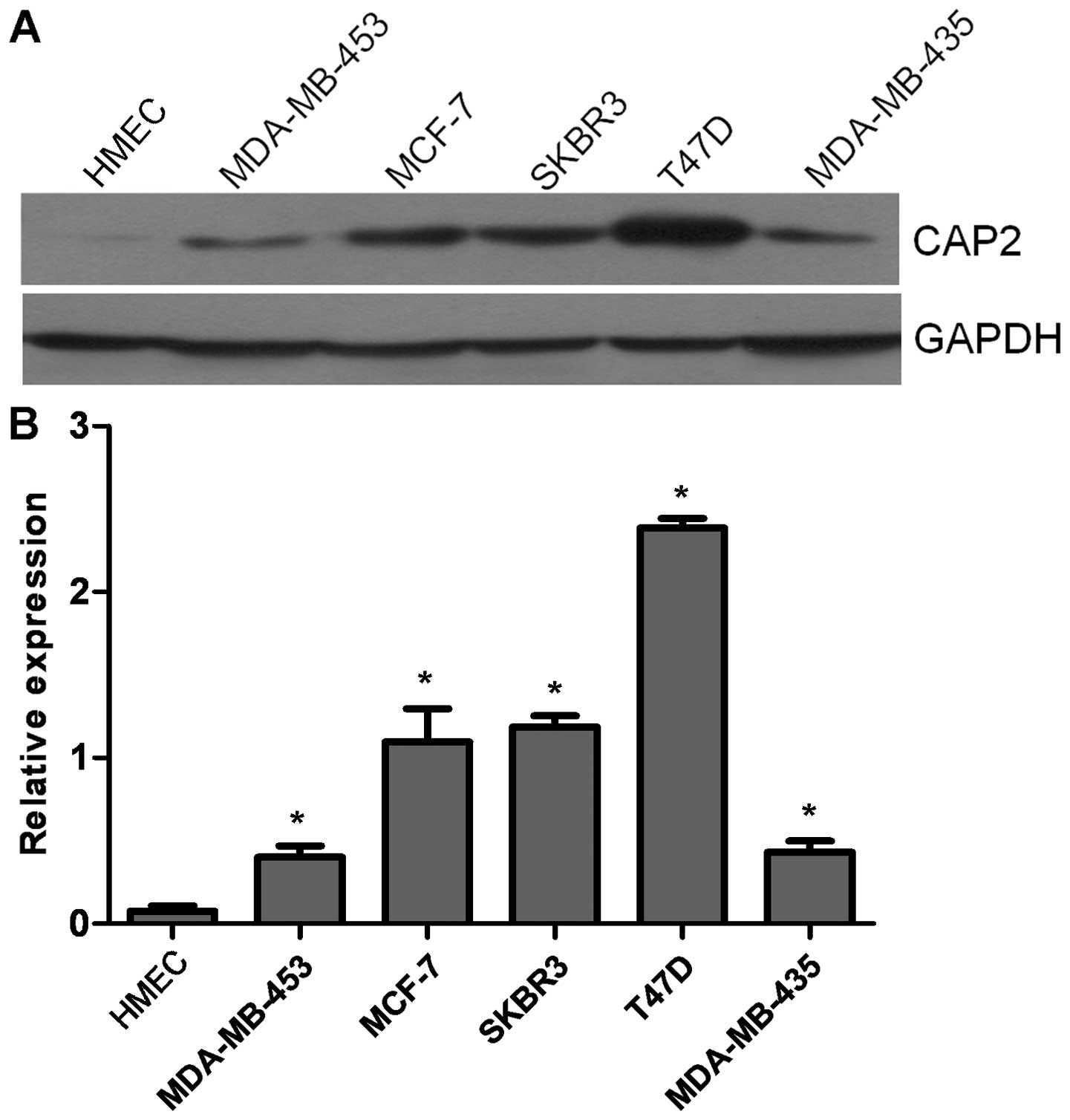
We used western blotting to evaluate the expression
level of CAP2 protein in the breast cancer cell lines and normal
HMECs. The expression levels of CAP2 were determined in five breast
cancer cell lines (MDA-MB-453, MCF-7, SK-BR-3, T47D and MDA-MB-435)
and were compared with CAP2 expression levels in primary cultured
normal HMECs. CAP2 protein was highly expressed in all five breast
cancer cell lines and only weakly expressed in the HMECs (Fig. 1A and B).
CAP2 is overexpressed in breast cancer
tissues
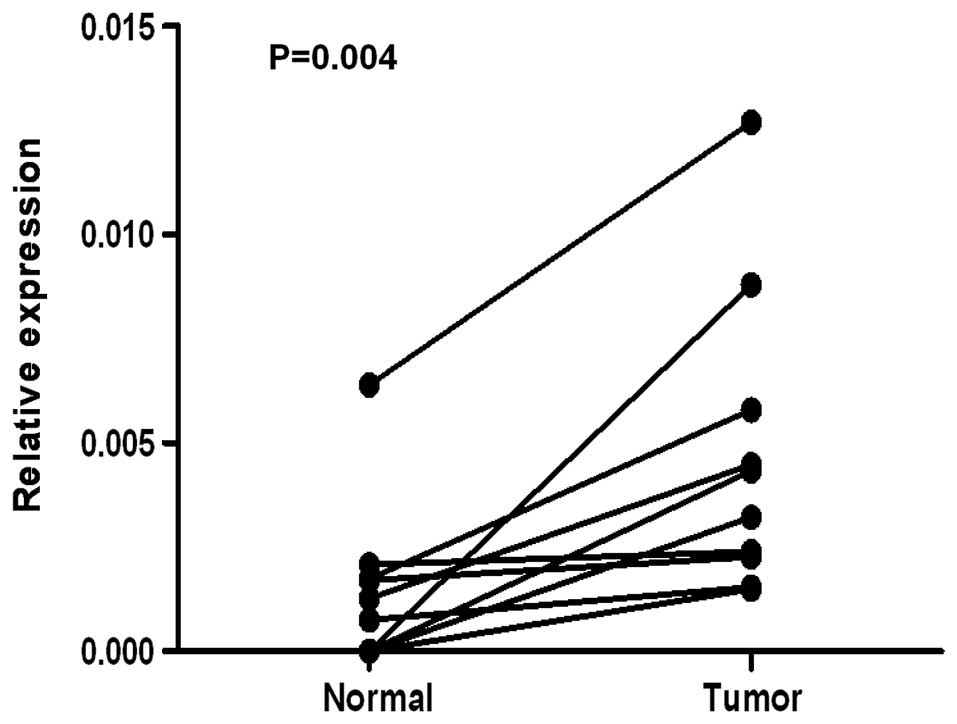
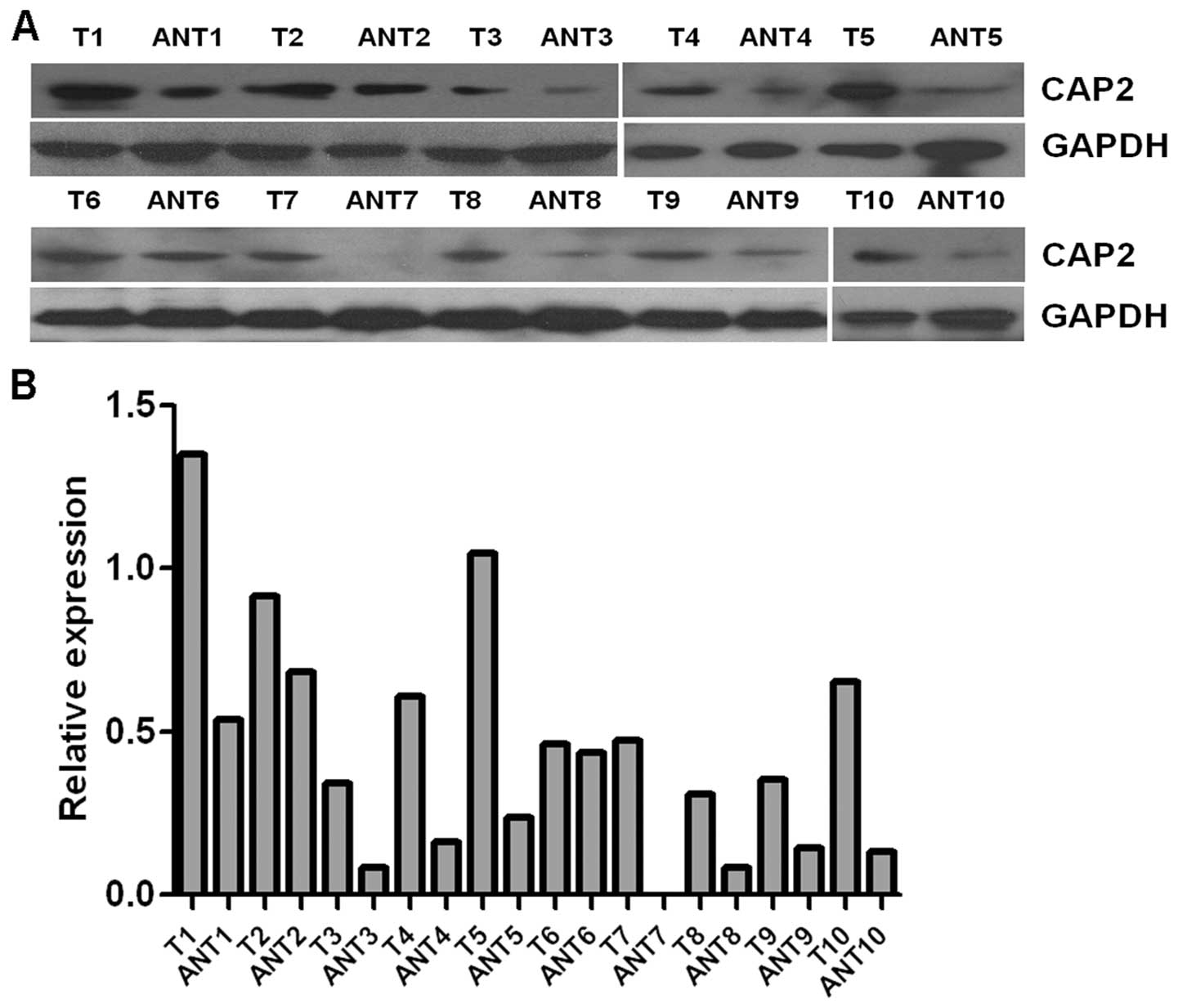
To determine whether CAP2 is also highly expressed
in human breast cancer samples, we performed RT-PCR and western
blotting on 10 breast tumor samples and adjacent non-cancerous
tissues. As illustrated in Fig. 2,
CAP2 mRNA was expressed at higher levels in all of the 10 breast
cancer tissues than that noted in the adjacent non-cancerous
tissues, with the differential expression level ranging from 4.7-
to 49.4-fold. Consistent with these data, CAP2 protein was also
found to be upregulated in the fresh breast cancer tissues compared
with that found in the adjacent non-cancerous tissues (Fig. 3). For immunostaining results,
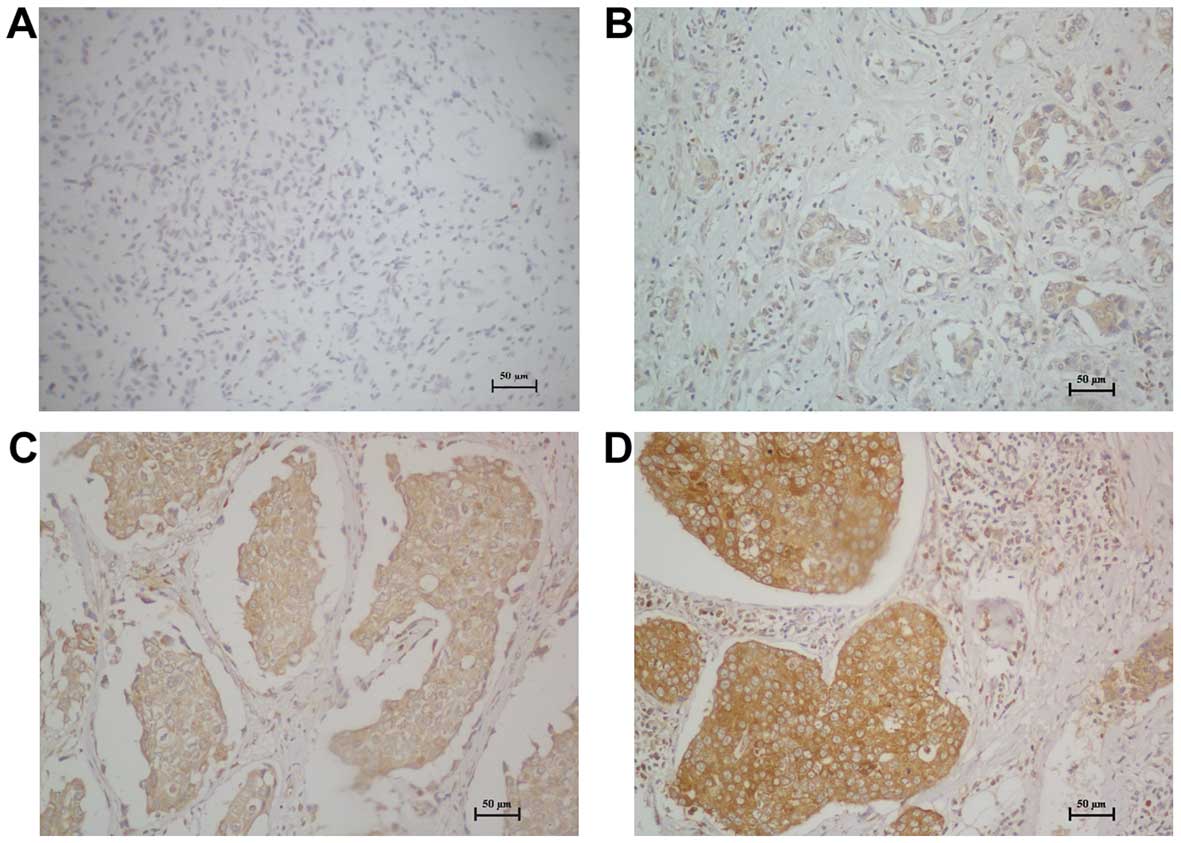
overexpression of CAP2 was observed in 29.37% (37/126) of the
breast cancer patients. CAP2 protein staining was weak or no
staining was observed in the adjacent non-tumor tissues and normal
breast tissues; only 6.67% (2/30) in the adjacent non-tumor tissues
and 5% (1/20) in normal breast tissues. The difference between the
breast cancer group and the adjacent non-tumor group was
statistically significant (χ2=6.658, p=0.01). The
difference between the breast cancer group and the normal breast
tissue group was statistically significant (χ2=5.322,
p=0.01). But the difference between the adjacent non-tumor group
and normal breast tissue group was not statistically significant
(χ2=0.059, p=0.651).
CAP2 overexpression is associated with
breast cancer clinical features
For better understanding of the potential roles of
CAP2 in breast cancer development and progression, we investigated
the status of CAP2 expression in 126 paraffin-embedded archived
breast cancer tissues by IHC staining, including 10 stage I, 76
stage II, and 40 stage III tumors. Among the 126 samples, high CAP2
protein expression was detected in 37 samples (29.37%) and weak or
no staining was observed in 89 tumor samples (70.63%, Table I). As shown in Fig. 4, CAP2 was highly expressed in breast
cancer tissues. In contrast, no signals or only weak signals were
detected in adjacent non-cancerous and normal breast tissues. The
subcellular location of CAP2 was mainly at the cytoplasm.
We further analyzed the correlation between CAP2
expression and the clinicopathological characteristics of the
breast cancer patients. As summarized in Table I, there were no significant
correlations between the expression of CAP2 protein and patient
age, clinical stage, T classification, N classification,
differentiation, ER expression levels or HER2 in patients with
breast cancer. However, the CAP2 expression was markedly associated
with PR expression levels (p=0.005).
Association between CAP2 expression and
patient survival
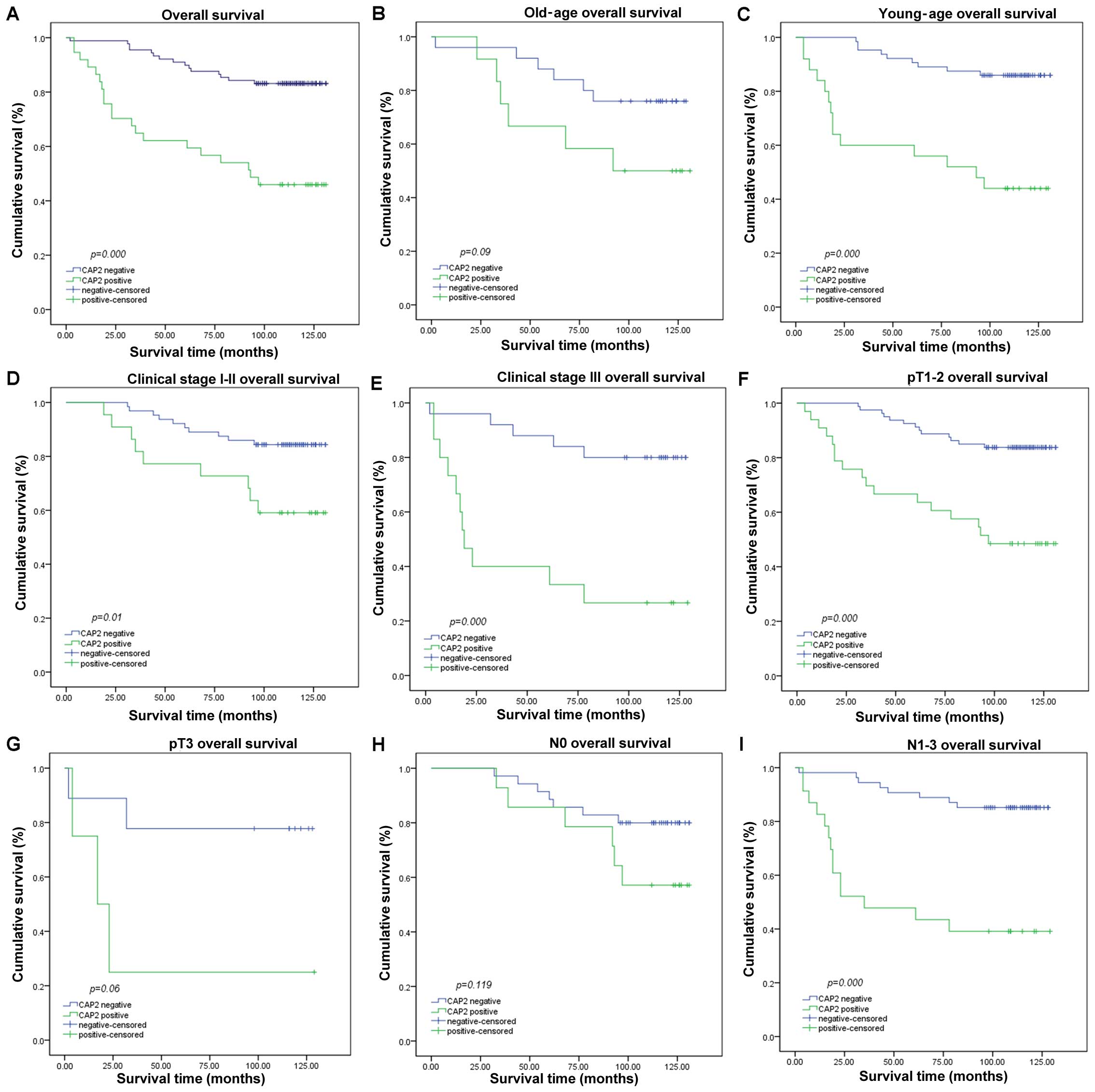
Survival analysis showed a clear negative
correlation between CAP2 protein expression level and the OS of
patients with breast cancer (p<0.001, Fig. 5A). In addition, Cox regression
revealed that CAP2 expression, clinical stage, and PR expression
were independent prognostic factors for OS (Table II).
 | Table IICox regression analysis of various
prognostic parameters for all patients. |
Table II
Cox regression analysis of various
prognostic parameters for all patients.
| Factors | Univariate
| Multivariate
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) | | | | |
| <60 | Reference | | | |
| ≥60 | 1.259
(0.626–2.531) | 0.518 | – | – |
| Clinical stage | | | | |
| I | Reference | 0.045 | Reference | |
| II | 2.612
(0.349–19.573) | 0.350 | 3.199
(0.422–24.240) | 0.26 |
| III | 5.436
(0.721–41.011) | 0.101 | 6.479
(0.847–49.541) | 0.072 |
| T
classification | | | | |
| T1 | Reference | 0.296 | | |
| T2 | 1.601
(0.613–4.182) | 0.337 | – | – |
| T3 | 2.682
(0.776–9.269) | 0.119 | – | – |
| N
classification | | | | |
| N0 | Reference | 0.123 | | |
| N1 | 0.694
(0.277–1.739) | 0.435 | – | – |
| N2 | 1.695
(0.759–3.784) | 0.198 | – | – |
| N3 | 2.407
(0.784–7.389) | 0.125 | – | – |
|
Differentiation | | | | |
| Well | Reference | 0.187 | | |
| Moderate | 4.036
(0.548–29.743) | 0.171 | – | – |
| Poor | 6.255
(0.782–50.025) | 0.084 | – | – |
| ER expression | | | | |
| Negative | Reference | | Reference | |
| Positive | 0.387
(0.199–0.753) | 0.005 | 0.412
(0.21–0.805) | 0.01 |
| PR expression | | | | |
| Negative | Reference | | | |
| Positive | 0.334
(0.166–0.671) | 0.002 | – | – |
| HER2
expression | | | | |
| Negative | Reference | | | |
| Positive | 1.006
(0.483–2.095) | 0.987 | – | – |
| CAP2
expression | | | | |
| Low | Reference | | Reference | |
| High | 4.375
(2.236–8.562) | 0.001 | 4.821
(2.442–9.518) | 0.001 |
Furthermore, we analyzed the prognostic value of
CAP2 in selective patient subgroups stratified by patient age,
tumor grade, T and N classification, respectively. For patients
<60 years of age, the expression of CAP2 was strongly associated
with OS duration (Fig. 5C; log-rank
test, p<0.001), but not for patients >60 years of age
(Fig. 5B; log-rank test, p=0.09).
The expression of CAP2 was strongly associated with OS duration of
the patients with both early-stage tumors (stage I; log-rank test,
p=0.01) and late-stage tumors (stage II and III; log-rank test,
p<0.001) (Fig. 5D and E).
However, when it was evaluated according to T and N classification,
the impact on the outcome associated with the expression of CAP2
continued to be more favorable only in T1-2 subgroups (Fig. 5F; log-rank test, p<0.001) and
N1-3 subgroup (Fig. 5I; log-rank
test, p<0.001) but not in the T3 subgroup (Fig. 5G; log-rank test, p=0.06) and N0
subgroup (Fig. H; log-rank test,
p=0.119).
Discussion
CAPs are conserved in all eukaryotes. CAP2 is part
of the actin cytoskeleton, which regulates cell shape, cell
motility and muscle contraction. The cytoskeleton is assembled by
polymerization of globular actin (G-actin) monomers into
filamentous actin (F-actin). The balance of F- and G-actin is
coordinated by actin-binding proteins (13). There are two CAP homologs in
mammals, CAP1 and CAP2. CAP1 is widely expressed in most cells and
tissues, while CAP2 expression is restricted to the brain, skin,
skeletal muscle, cardiac muscle and testis (6,14). At
the subcellular level, CAP2 is found in the cytoplasm although,
unlike other isoforms, some CAP2 exists in the nucleus. It is
likely that CAP1 and CAP2 complement each other in some cellular
functions, but CAP2 may have unique roles, especially in skeletal
and cardiac muscles (6).
Overexpression of CAP1 has been found in cancers,
including pancreatic, breast, ovarian, lung, esophageal, and liver
cancers (7,9,15–17).
However, to date, only a few studies in hepatocellular and
malignant melanoma have focused on CAP2 (10,11,18).
Shibata et al reported that CAP2 is upregulated in early HCC
and even greater overexpression is observed in progressive HCC.
They believe that the functional link between mitogen-activated
protein kinase and cyclic AMP might be related to proliferative
activity and carcinogenesis through CAP2 overexpression in HCC
(10). Masugi et al reported
that CAP2 overexpression is a novel prognostic marker in malignant
melanoma. CAP2 expression seems to increase stepwise during tumor
progression. IHC analysis of CAP2 could be helpful for histological
identification of highly aggressive components in melanoma tissues
and for early detection of aggressive subpopulations in clinical
melanomas (11).
In this study, we present new evidence that the
upregulation of CAP2 is associated with poor prognosis in breast
carcinoma patients with both early- and late-stage disease. Our
results clearly showed that elevation of CAP2 protein expression
was observed in all of the five breast cancer cell lines, while the
expression in non-tumorous HMECs was relatively very low. Then, we
found that breast cancer lesions displayed higher CAP2 expression
at the mRNA and protein levels as compared with adjacent
non-cancerous tissues. Thus, we consider that CAP2 is an important
molecular marker of breast cancer and can facilitate precise
diagnoses. At present the precise roles of CAP2 in human cancers
are still obscure. Cancer cell motility and metastasis depend on
cell migration, adhesion and morphological change. Therefore,
dynamic actin cytoskeleton reorganization and remodeling obviously
increases and CAP may play a determinant role in these cell
processes (19,20). CAP2 overexpression in breast cancer
may reflect the aberrant regulation of actin dynamics. However, in
order to understand the precise signaling pathways of CAP2 in
breast cancer further studies are needed.
We further analyzed the relationship between the
expression of CAP2 and clinical characteristics of patients with
breast cancer. There was a significant correlation between CAP2 and
PR expression levels. Meanwhile, there were no significant
correlations between the expression of CAP2 protein and patient
age, clinical stage, T classification, N classification,
differentiation, ER expression levels or HER2.
Reports have confirmed the prognostic value of CAP2
in human cancers. Fu et al reported that in a large cohort
of 520 patients with HCC, CAP2 expression was significantly
associated with overall and disease-free survival, and CAP2 was as
an independent factor for prognostic prediction (21). CAP2 overexpression was also found to
be a novel prognostic marker in malignant melanoma (11). However, the prognostic implication
of CAP2 in breast cancer has not been investigated. In our study,
patients in the high CAP2 expression group had a 45.95% cumulative
10-year survival rate, which was significantly lower than that of
patients with low CAP2 expression levels (83.15%). Multivariate
analysis revealed that CAP2 expression might be an independent
prognostic indicator for OS in breast cancer patients (Table II). This finding indicates the
possibility of using high expression levels of CAP2 as a predictor
for prognosis and survival. Interestingly, subgroup analysis
revealed that CAP2-overexpression patients exhibited a
significantly poor prognosis among patients whose tumors
demonstrated the features of young age, early T stage and lymph
node metastasis, respectively.
In conclusion, to the best of our knowledge, this is
the first report addressing CAP2 expression and its
clinicopathological and prognostic significance in breast cancer.
Our findings suggest that CAP2 is upregulated in breast cancer and
associated with the expression of PR. Multivariate analysis
revealed that CAP2 might be an independent biomarker for the
prediction of breast cancer prognosis and survival. Therefore,
testing the CAP2 protein level may be helpful for stratifying
patients for novel therapeutic strategy and establishing a rational
treatment selection criteria for breast cancer patients. Further
investigation is also needed to investigate the molecular mechanism
of CAP2 involvement in the development and progression of breast
cancer.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (81502268), the Guangdong
Provincial Natural Science Foundation (2015A030313182,
2015A030310126), the Guangzhou Medical and Health Technology
Program (20141A011075, 20151A011068), and Key Clinical Disciplines
of Guangdong Province (20111219).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Makkonen M, Bertling E, Chebotareva NA,
Baum J and Lappalainen P: Mammalian and malaria parasite
cyclase-associated proteins catalyze nucleotide exchange on G-actin
through a conserved mechanism. J Biol Chem. 288:984–994. 2013.
View Article : Google Scholar :
|
|
3
|
Fedor-Chaiken M, Deschenes RJ and Broach
JR: SRV2, a gene required for RAS activation of adenylate cyclase
in yeast. Cell. 61:329–340. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olson MF and Sahai E: The actin
cytoskeleton in cancer cell motility. Clin Exp Metastasis.
26:273–287. 2009. View Article : Google Scholar
|
|
5
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peche V, Shekar S, Leichter M, Korte H,
Schröder R, Schleicher M, Holak TA, Clemen CS, Ramanath-Y B,
Pfitzer G, et al: CAP2, cyclase-associated protein 2, is a dual
compartment protein. Cell Mol Life Sci. 64:2702–2715. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Cui X, Hu B, Lu C, Huang X, Cai J,
He S, Lv L, Cong X, Liu G, et al: Upregulated expression of CAP1 is
associated with tumor migration and metastasis in hepatocellular
carcinoma. Pathol Res Pract. 210:169–175. 2014. View Article : Google Scholar
|
|
8
|
Yu XF, Ni QC, Chen JP, Xu JF, Jiang Y,
Yang SY, Ma J, Gu XL, Wang H and Wang YY: Knocking down the
expression of adenylate cyclase-associated protein 1 inhibits the
proliferation and migration of breast cancer cells. Exp Mol Pathol.
96:188–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hua M, Yan S, Deng Y, Xi Q, Liu R, Yang S,
Liu J, Tang C, Wang Y and Zhong J: CAP1 is overexpressed in human
epithelial ovarian cancer and promotes cell proliferation. Int J
Mol Med. 35:941–949. 2015.PubMed/NCBI
|
|
10
|
Shibata R, Mori T, Du W, Chuma M, Gotoh M,
Shimazu M, Ueda M, Hirohashi S and Sakamoto M: Overexpression of
cyclase-associated protein 2 in multistage hepatocarcinogenesis.
Clin Cancer Res. 12:5363–5368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masugi Y, Tanese K, Emoto K, Yamazaki K,
Effendi K, Funakoshi T, Mori M and Sakamoto M: Overexpression of
adenylate cyclase-associated protein 2 is a novel prognostic marker
in malignant melanoma. Pathol Int. 65:627–634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song LB, Zeng MS, Liao WT, Zhang L, Mo HY,
Liu WL, Shao JY, Wu QL, Li MZ, Xia YF, et al: Bmi-1 is a novel
molecular marker of nasopharyngeal carcinoma progression and
immortalizes primary human nasopharyngeal epithelial cells. Cancer
Res. 66:6225–6232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ono S: The role of cyclase-associated
protein in regulating actin filament dynamics - more than a
monomer-sequestration factor. J Cell Sci. 126:3249–3258. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bertling E, Hotulainen P, Mattila PK,
Matilainen T, Salminen M and Lappalainen P: Cyclase-associated
protein 1 (CAP1) promotes cofilin-induced actin dynamics in
mammalian nonmuscle cells. Mol Biol Cell. 15:2324–2334. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamazaki K, Takamura M, Masugi Y, Mori T,
Du W, Hibi T, Hiraoka N, Ohta T, Ohki M, Hirohashi S, et al:
Adenylate cyclase-associated protein 1 overexpressed in pancreatic
cancers is involved in cancer cell motility. Lab Invest.
89:425–432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li M, Yang X, Shi H, Ren H, Chen X, Zhang
S, Zhu J and Zhang J: Downregulated expression of the
cyclase-associated protein 1 (CAP1) reduces migration in esophageal
squamous cell carcinoma. Jpn J Clin Oncol. 43:856–864. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie SS, Tan M, Lin HY, Xu L, Shen CX, Yuan
Q, Song XL and Wang CH: Overexpression of adenylate
cyclase-associated protein 1 may predict brain metastasis in
non-small cell lung cancer. Oncol Rep. 33:363–371. 2015.
|
|
18
|
Effendi K, Yamazaki K, Mori T, Masugi Y,
Makino S and Sakamoto M: Involvement of hepatocellular carcinoma
biomarker, cyclase-associated protein 2 in zebrafish body
development and cancer progression. Exp Cell Res. 319:35–44. 2013.
View Article : Google Scholar
|
|
19
|
Kirfel G, Rigort A, Borm B and Herzog V:
Cell migration: Mechanisms of rear detachment and the formation of
migration tracks. Eur J Cell Biol. 83:717–724. 2004. View Article : Google Scholar
|
|
20
|
Zhou GL, Zhang H and Field J: Mammalian
CAP (Cyclase-associated protein) in the world of cell migration:
Roles in actin filament dynamics and beyond. Cell Adhes Migr.
8:55–59. 2014. View Article : Google Scholar
|
|
21
|
Fu J, Li M, Wu DC, Liu LL, Chen SL and Yun
JP: Increased expression of CAP2 indicates poor prognosis in
hepatocellular carcinoma. Transl Oncol. 8:400–406. 2015. View Article : Google Scholar : PubMed/NCBI
|