Introduction
Bladder cancer is the fifth most common cancer, with
a lifetime incidence rate of 1 in 26 for men in the US. The
American Cancer Society stated that ~74,000 new cases were
diagnosed in the year 2015; of these cases, ~16,000 were expected
to die from the disease (1). The
incidence of bladder cancer in males is 4 times higher than it is
in females, and it is 2 times higher in white people than in black
people for unknown reasons (1). The
initiation and progression of bladder cancer is affected by various
risk factors such as genetic alterations, and exposure to chemicals
and carcinogens in cigarette smoke (1,2).
Pathologically, transitional cell carcinoma (TCC) makes up ~90% of
cases, and the other 10% consists of neoplastic lesions including
squamous cell carcinoma, adenocarcinoma, sarcoma and small cell
carcinoma. Although early detection followed by surgical resection
extends the chances of survival, the 5-year survival rate for
patients with advanced-stage bladder cancer is significantly less
than 10% (1).
Bladder cells are transformed by a multistep process
which includes genetic alterations such as chromosomal
abnormalities (3), loss of tumor
suppressor genes (4,5) and amplification of oncoproteins
(6). Loss of tumor suppressor genes
such as p53 or Rb is common in early stages, while amplification of
oncoproteins is more common in advanced stages. However, the
particular mutations that give rise to bladder cancer are not yet
clearly understood, and even the mutational status of tumor
suppressors is unclear. Despite its high impact on public health,
there have been relatively few studies on the mechanisms of
tumorigenesis of bladder cancer compared to other types of cancers.
Therefore, it is critically important to identify molecular targets
in order to find novel therapeutic options for this deadly
disease.
MicroRNAs (miRs) are small non-coding RNA fragments
that regulate gene expression by binding to the 3′-untranslated
regions (3′-UTRs) of their target genes (7). When normal bladder epithelia are
transformed, as evidenced by alterations in protein expression,
dysregulation of miRs is also observed. Previous studies showed
that miRs act as either oncogenes or tumor suppressors in bladder
cancer. Oncogenic miRs are mainly upregulated (8–10) and
tumor-suppressive miRs are downregulated (11–13).
Similarly, our previous studies demonstrated that miR-20b-induced
p21WAF1-mediated G1 phase cell cycle arrest of bladder
cancer EJ cells, suggesting that the miR has a tumor suppressive
function (14). In the present
study, we reported that miR-892b inhibits proliferation, migration
and invasion of bladder cancer EJ cells by regulating the
p19ARF/cyclin D1/CDK6 and Sp-1/MMP-9 cascade.
Materials and methods
Materials
Polyclonal antibodies against cyclin E, CDK2, CDK6,
cyclin D1, p53, p19ARF, p21WAF1, p27KIP1 and GAPDH were purchased
from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-MMP-9
polyclonal antibody was obtained from Chemicon International
(Billerica, MA, USA). miR-892b (5′-CACUGGCUCCUUUCUGGGUAGA-3′) and
miR-892b inhibitor were designed and synthesized by Genolution
Pharmaceuticals, Inc. (Seoul, Korea).
Cell cultures
Human bladder carcinoma cell lines (EJ, 5637 and
T24) were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). The cells were maintained in Dulbecco's
modified Eagle's medium (DMEM) with glucose supplemented with 10%
fetal calf serum, L-glutamine and antibiotics (Biological
Industries, Beit Haemek, Israel) at 37°C in a 5% CO2
humidified incubator. Normal human urothelial cells (HUCs) were
purchase from ScienCell Research Laboratories (Carlsbad, CA, USA).
The cells were maintained in urothelial cell medium with
supplements according to the manufacturer's protocol.
Quantitative real-time RT-PCR
(qRT-PCR)
Quantification of miRNA expression was performed
using a Rotor-Gene™ 6000 as previously described (15). Real-time PCR assays were carried out
using the miScript PCR Starter kit (Qiagen Korea, Seoul, Korea).
The PCR reaction was performed in a final volume of 20 µl
(10 µl 2X QuantiTect SYBR-Green PCR Master Mix, 2 µl
10X miScript Universal Primer (U6), 2 µl 10 pmol forward
primer, 2 µl template cDNA and RNase-free water). Real-time
PCR conditions were as follows: 1 cycle of initial activation for
15 min at 95°C, followed by 50 cycles of 15 sec at 94°C for
denaturation, annealing for 30 sec at 55°C and extension for 30 sec
at 70°C. The melting program was performed at 70–99°C at a heating
rate of 1°C/5 sec. Spectral data were captured and determined using
Rotor-Gene™ Real-Time Analysis Software 6.0, Build 14. All
experiments were carried out in triplicate. U6 was used as a
control to normalize the quantity of miRNA.
Bioinformatics analysis
Screening for possible targets of miR-892b was
performed with the miRanda algorithm (http://www.microrna.org/microrna/home.do) and the NCBI
mRNA database (NCBI mRNA DB: http://www.ncbi.nlm.nih.gov/).
Cell proliferation
Cell proliferation was measured using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay as previously described (16). Cell morphology was photographed
using phase-contrast microscopy.
Transfection
Cells were transfected with miR-892b and the
miR-892b inhibitor using Lipofectamine 2000 transfection reagent
(Invitrogen Corp., Carlsbad, CA, USA) according to the
manufacturer's protocol.
Flow cytometric cell cycle analysis
Cells were harvested and fixed in 70% ethanol. After
being washed with ice-cold phosphate-buffered saline (PBS), cells
were incubated with RNase (1 mg/ml) followed by propidium iodide
(50 mg/ml). The phase distribution of the cell cycle was determined
by a Becton-Dickinson FACStar flow cytometer equipped with
Becton-Dickinson Cell Fit software.
Immunoblot analysis
Preparation and quantitation of protein lysates were
performed as previously described (14). Lysates were electrophoresed on a 10%
polyacrylamide gel (SDS-PAGE) under denaturing conditions and
transferred to nitrocellulose membranes (Hybond; Amersham Corp.,
Arlington Heights, IL, USA). The blots were blocked with 5%
(wt/vol) non-fat dry milk in Tris-buffered saline (TBS) [10 mM
Tris-HCl (pH 8.0), 150 mM NaCl], and then, the membranes were
incubated with primary antibodies at 4°C overnight. The blots were
then incubated with peroxidase-conjugated secondary antibodies for
90 min. A chemiluminescence reagent kit (Amersham Corp.) was
utilized for the detection of the western blot analyses. The
experiments were repeated at least 3 times.
Immunoprecipitation and immune complex
kinase assays
Cell lysates were prepared using ice-cold lysis
buffer as previously described (14). Briefly, after centrifugation of the
lysates at 10,000 × g for 5 min, the supernatants were precipitated
by protein A sepharose beads pre-coated with the indicated
antibodies at 4°C for 2 h. The immunoprecipitated proteins on the
beads were washed 4 times with 1 ml of lysis buffer and twice with
a kinase buffer (14). Finally,
pellets were re-suspended in 25 µl of kinase buffer
containing 1 µg glutathione S-transferase (GST)-pRb
C-terminal (pRb amino acids 769–921) fusion protein (Santa Cruz
Biotechnology), 20 µM/l ATP and 5 µCi of
[γ32P]-ATP (4,500 µCi/mmol; ICN). Subsequently,
re-suspended pellets were incubated at 30°C for 20 min with
occasional mixing. The kinase reactions were terminated by the
addition of 25 µl of 2X Laemmli sample buffer and were
heated at 100°C for 5 min. Samples were resolved on 10%
SDS-polyacrylamide gels, which were then dried. Radioactive bands
were visualized. The migration of GST-pRb was determined using
Coomassie blue staining.
Wound-healing migration assay
Cells (3×105) were seeded into 6-well
plates in 2 ml medium and were grown to 90% confluency. A clear
area was created with a 2 mm pipette tip. After 3 washes with PBS,
the plates were incubated at 37°C in serum-free medium. Migration
of cells into the clear area was analyzed and photographed using an
inverted microscope (magnification, ×40).
Invasion assay
Invasion assays were performed using an invasion
assay kit (Cell Biolabs, Inc., San Diego, CA, USA) according to the
manufacturer's instructions. Cells (2.5×104) were
re-suspended in serum-free medium and plated in the upper chamber.
Media with 10% FBS was added to the lower chamber as a
chemoattractant. After 24 h of incubation, cells in the lower
chamber were stained and photographed. Cell invasion was evaluated
using a commercial cell invasion assay kit (Chemicon
International).
Gelatin zymography
Culture supernatants were resolved on a
polyacrylamide gel containing 1 mg/ml gelatin. Gels were washed
with 2.5% Triton X-100 at room temperature for 2 h followed by
incubation at 37°C overnight in a buffer containing 10 mM
CaCl2, 150 mM NaCl and 50 mM Tris-HCl, pH 7.5. The gel
was stained with 0.2% Coomassie blue and photographed with a light
box. Areas of gelatinase activity were visible as clear bands in a
dark blue field.
Preparation of nuclear extracts and
EMSA
Nuclear proteins were extracted as previously
described (17). Briefly, cells
were washed, scraped and suspended in a buffer [10 mM HEPES (pH
7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT and 0.5 mM
phenylmethylsulfonyl fluoride (PMSF)]. The cells were lysed with
0.5% NP40. The homogenates were then centrifuged, and the nuclear
pellets were extracted with an ice-cold high-salt buffer (20 mM
HEPES pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT and 1 mM
PMSF). After centrifugation, the supernatants containing the
nuclear extracts were prepared. Protein concentrations were
measured using Bradford reagent (Bio-Rad). An electrophoretic
mobility shift assay (EMSA) was performed as previously described
(17). In brief, the
oligonucleotides spanning the MMP-9 cis element of interest
were end-labeled with 32P-ATP by T4 polynucleotide
kinase (Promega, Madison, WI, USA). Nuclear extracts (10–20
µg) were incubated with a radio-labeled oligonucleotide
probe (10,000 cpm) at 4°C for 20 min in a binding buffer solution
(25 mM HEPES buffer pH 7.9, 0.5 mM EDTA, 0.5 mM DTT, 0.05 M NaCl,
and 2.5% glycerol, and 2 µg poly dI/dC). The DNA-protein
complex was resolved at 4°C on a 6% polyacrylamide gel containing a
TBE running buffer (89 mM Tris, 89 mM boric acid and 1 mM EDTA).
The gel was rinsed, dried and then exposed to X-ray film for 10 h.
The sequences for the oligonucleotides were as follows: AP-1,
CTGACCCCTGAGTCAGCACTT; NF-κB, CAGTGGAATTCCCCAGCC; and Sp-1,
GCCCATTCCTTCCGCCCCCAGATGAAGCAG.
Statistical analysis
Where appropriate, data are expressed as mean ± SE.
Data were evaluated by factorial ANOVA and a Fisher's least
significant difference test where appropriate. Statistical
significance was set at P<0.05.
Results
Basal level of miR-892b expression is low
in bladder cancer cells
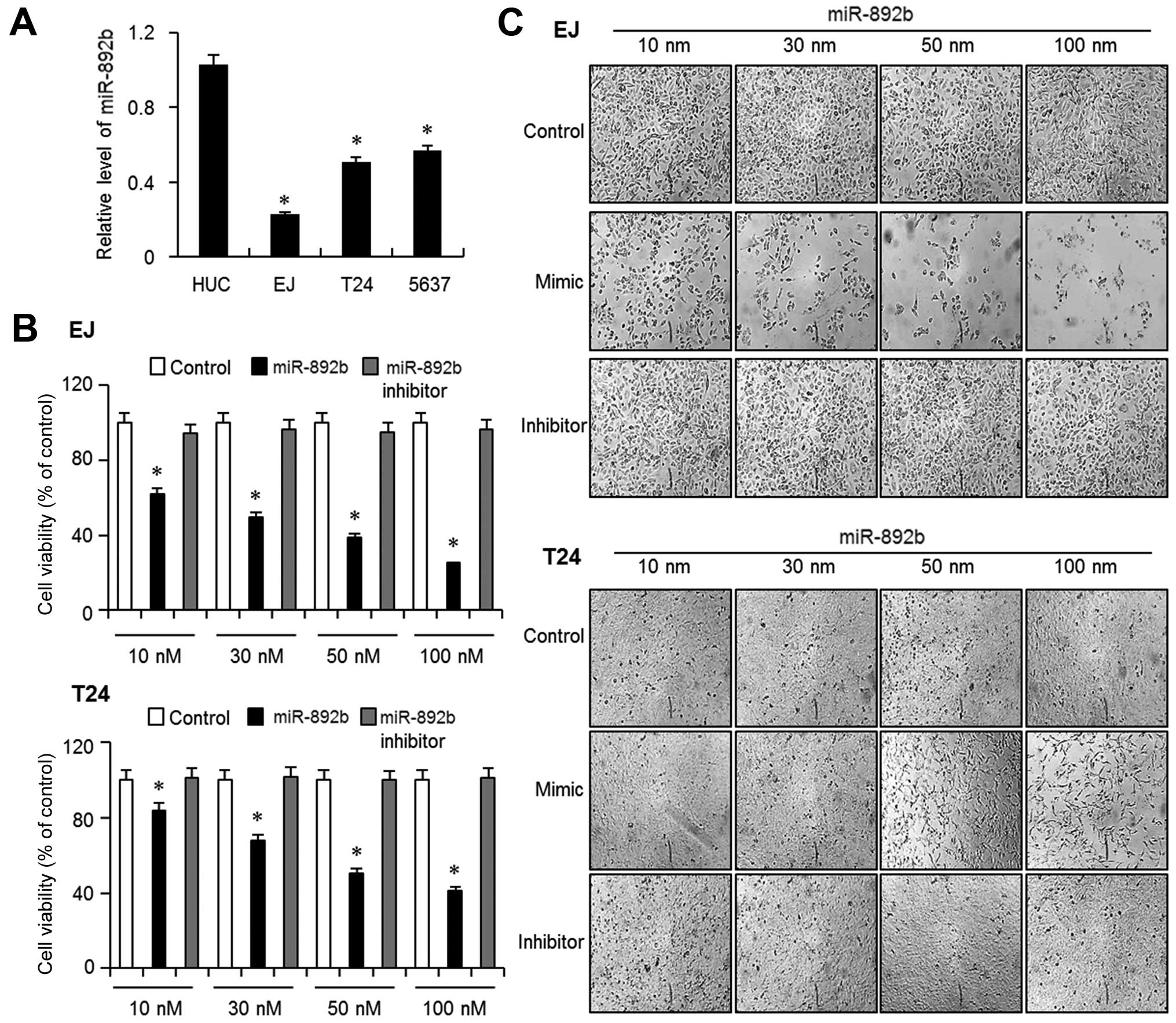
The aim of the present study was to verify the role
of miR-892b in bladder cancer. We first measured the basal
expression level of miR-892b in 3 different bladder cancer cell
lines, EJ, T-24 and 5637, and one normal human urothelial cell line
(HUC). Quantitative real-time-PCR showed that the basal levels of
miR-892b were significantly lower in all the 3 bladder cancer cells
than in HUC cells (Fig. 1A). EJ and
T24 cells exhibited the least expression of the 3 cancer cells;
therefore, we selected EJ and T24 cells for further experiments. We
then investigated the effect of miR-892b on the proliferation of EJ
and T24 cells, and the cells were transfected with Lipofectamine
2000 (control), miR-892b mimic (miR-892b), and inhibitor of
miR-892b (inhibitor) at concentrations from 10 to 100 nM.
Introducing miR-892b mimic into EJ cells significantly inhibited
proliferation in a dose-dependent manner (Fig. 1B). In contrast, neither control nor
inhibitor showed any meaningful decrease in the growth of the
cells. Images of the transfected cells supported the result
(Fig. 1C). Similar results were
obtained from T24 cells (Fig. 1B and
C). Subsequent experiments were performed with a miR-892b
concentration of 30 nM (~IC50) for EJ cells and 50 nM
(~IC50) for T24 cells, respectively.
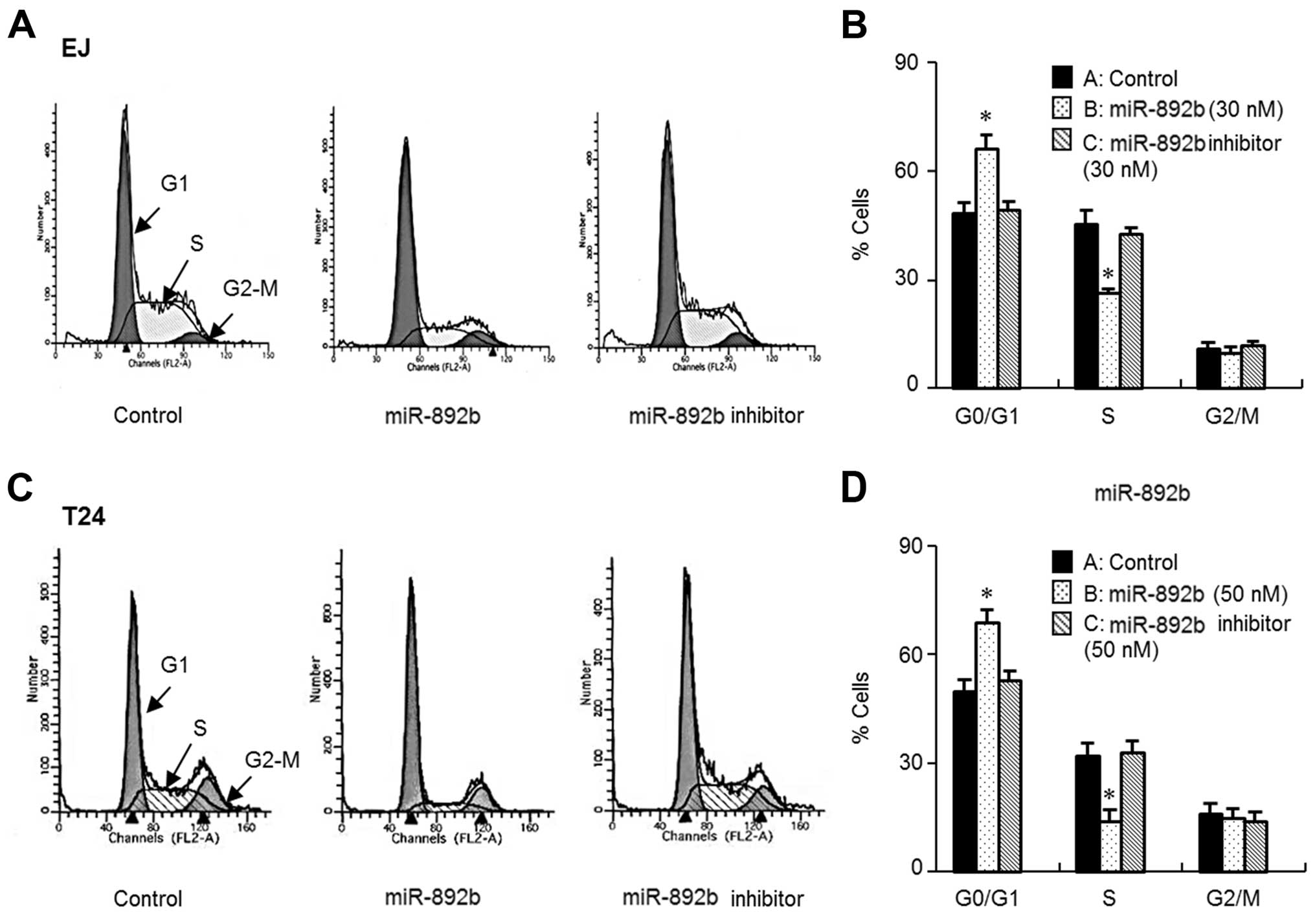
miR-892b causes G1 phase cell cycle
arrest in bladder cancer cells
To elucidate the cause of miR-892b-mediated growth
inhibition of bladder cancer cells, we investigated the cell cycle
phase distribution of miR-892b-transfected cells through flow
cytometric analysis. As demonstrated by the DNA histograms of the
cell cycle, introduction of miR-892b resulted in accumulation in
the G1 phase (Fig. 2A–D). In
agreement with that result, G1 phase accumulation was followed by a
corresponding decrease in the S phase population (Fig. 2A–D). Based on the results of flow
cytometry, we searched the microRNA database (miRanda) to find
possible targets of miR-892b in terms of cell cycle regulators,
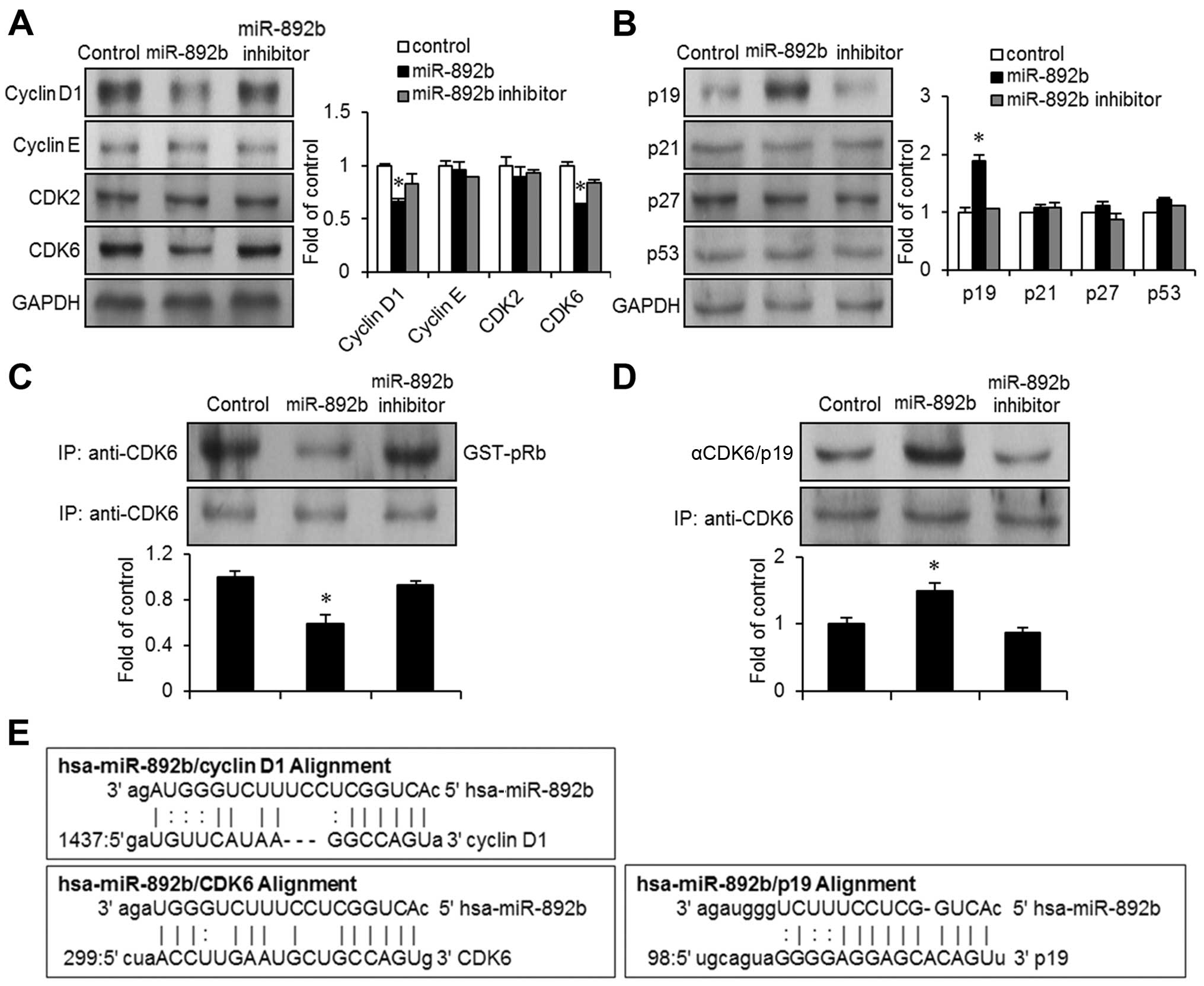
including cyclins, CDKs and CDK inhibitors. We identified cyclin
D1, CDK6 and p19ARF as possible causes of G1 phase cell
cycle arrest in bladder cancer EJ cells. First, we investigated
changes in protein expression levels of the G1 phase cell cycle
regulators in miR-892b transfectants using immunoblots. In line
with bioinformatics analysis, the expression of cyclin D1 and CDK6
were significantly reduced in miR-892b transfectants (Fig. 3A). However, the expression levels of
cyclin E and CDK2 were not significantly altered. Progression of
the cell cycle often requires activation of CDKs, which form a
complex with corresponding cyclins. CDK6 couples with cyclin D1 and
phosphorylates Rb (18). We
investigated the kinase activity of CDK6 in miR-892b transfectants
with immunoprecipitation assays. In accordance with the immunoblot
results (Fig. 3A), the kinase
activity of CDK6 was reduced by ~50% in miR-892b transfectants
compared to the control or the miR-892b inhibitor (Fig. 3C). This strongly suggests that
miR-892b targets cyclin D1-associated growth signals in bladder
cancer cells.
miR-892b leads to the upregulation of
p19ARF, resulting in growth inhibition of bladder cancer
cells
Based on the finding that miR-892b-induced G1 phase
cell cycle arrest in bladder cancer EJ cells, we investigated the
effect of miR-892b on protein levels of CDK inhibitors associated
with G1 to S phase cell cycle progression. Immunoblot analysis
demonstrated upregulation of p19ARF in
miR-892b-transfected EJ cells (Fig.
3B). However, expression levels of p21WAF1/CIP1,
p27KIP1 and p53 were unchanged (Fig. 3B). Upregulation of p19ARF
by miR-892b was confirmed via immunoprecipitation experiments with
CDK6 followed by immunoblotting with p19ARF or CDK6
(control). p19ARF/CDK6 complex formation was increased
by ~50% in miR-20b-transfected cells compared with control cells
(Fig. 3D). Taken together, these
results demonstrate that p19ARF is a critical effector
in miR-892b-mediated G1 phase cell cycle arrest of bladder cancer
EJ cells.
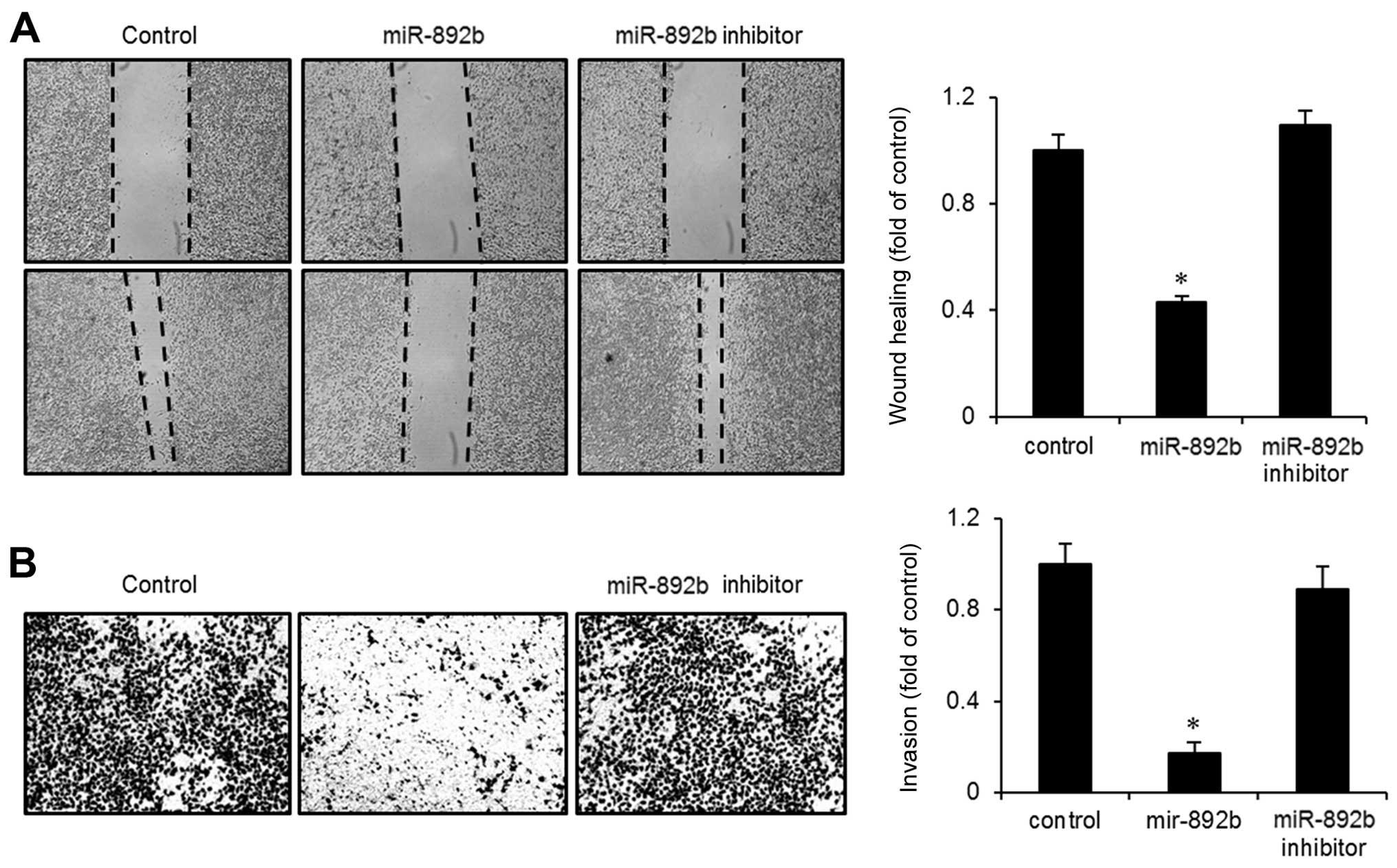
miR-892b reduces wound-healing migration
and invasiveness of bladder cancer cells
During the progression of bladder cancer,
transformed cells frequently acquire the ability to invade other
tissues. We investigated the effect of miR-892b on the migration
and invasion of bladder cancer cells through wound-healing and
Boyden chamber invasion assays. As shown in Fig. 4A, miR-892b transfectants exhibited
slower recovery in a wounded area than did control or miR-892b
inhibitor-tranfected cells. Wound-closure rate was ~60% lower in
the miR-892b transfectants than in the control. Similarly, Boyden
chamber invasion assays showed that invasiveness into the
Matrigel-coated chamber was reduced >50% compared to the control
or the inhibitor (Fig. 4B). Taken
together, these results suggest that miR-892b has tumor-suppressive
effects that regulate cellular motility via migration and
invasion.
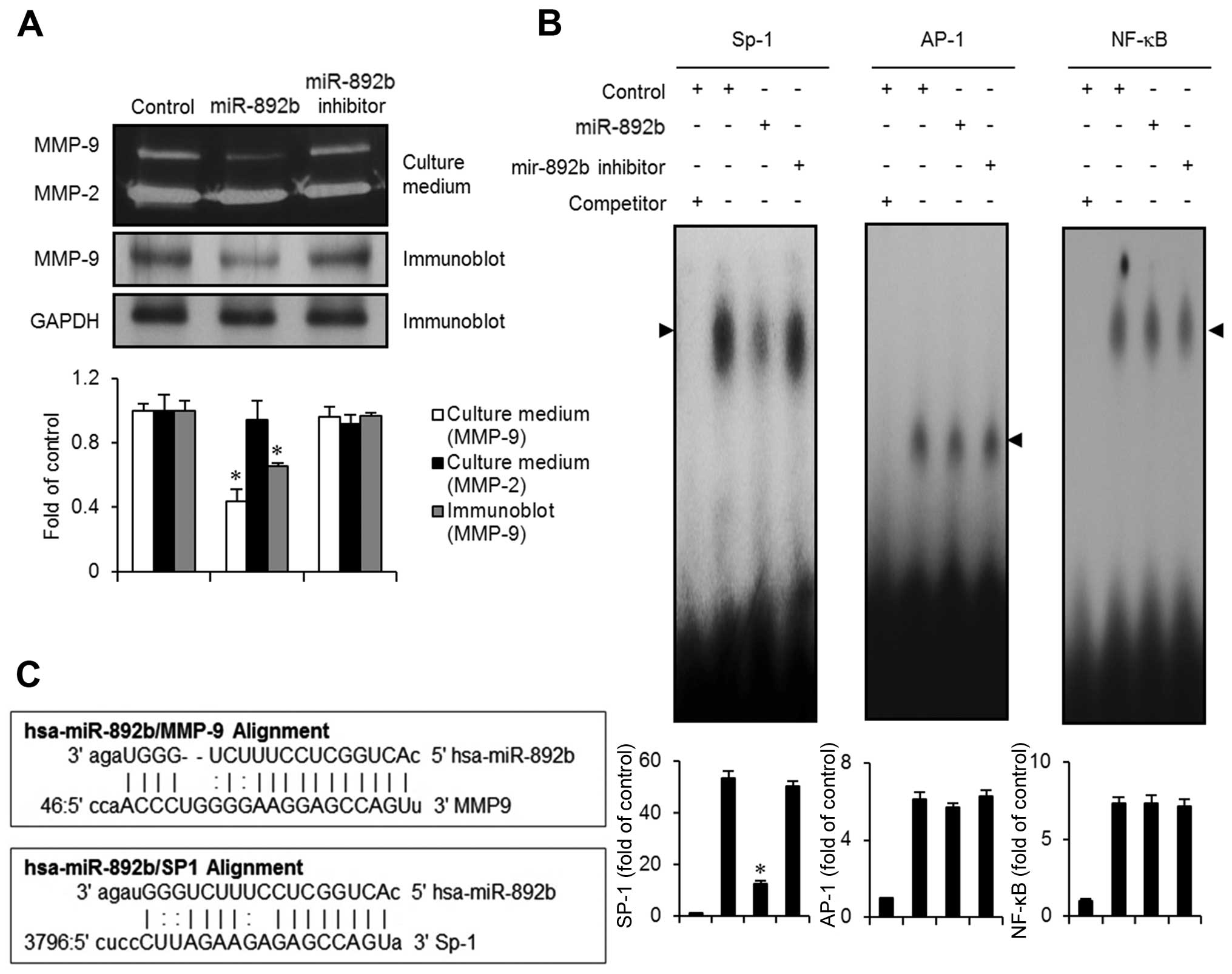
miR-892b downregulates MMP-9 expression
via activation of the transcription factor SP-1
In order to identify the molecular targets
responsible for the inhibition of invasion and migration caused by
miR-892b, we used miRanda to search for microRNA targets and found
MMP-9 as a candidate (Fig. 5C).
Since MMP-2, -3, -7 and -9 were previously reported to be key
molecules associated with aggressiveness and poor prognosis in
bladder cancer (19,20), we examined the protein expression
levels of MMP-2 and MMP-9 in miR-892b-transfected cells using
gelatin zymography assays. As shown in Fig. 5A, expression of MMP-9 was
significantly reduced in the growth medium obtained from
miR-892b-transfected cells. However, no significant changes in
MMP-9 levels were observed in the medium from the control or the
miR-892b inhibitor cells. Immunoblot analysis of the miR-892b
transfectants also showed a reduction in MMP-9 expression (Fig. 5A). miRanda bioinformatics analysis
for microRNA targets identified Sp-1 as one of the targets of
miR-892b. Thus, using EMSA assays, we investigated whether
transcription factors including Sp-1, AP-1 and NF-κB are modulated
by miR-892b transfection in EJ cancer cells. Nuclear extracts from
miR-892b transfectants exhibited a reduction in activity of the
Sp-1 binding motif (Fig. 5B).
However, no specific binding activity of AP-1 or NF-κB was observed
in the transfectants (Fig. 5B).
Taken together, these results suggest that the Sp-1 transcription
factor is involved in the suppression of MMP-9-mediated migration
and invasion of bladder cancer EJ cells induced by miR-892b.
Discussion
Bladder cancer is one of the most common types of
cancers worldwide, causing annually ~150,000 deaths (21). Standard therapeutic options to date
for advanced bladder cancer consist of cisplatin-based
combinatorial chemotherapies, although new therapeutic approaches
are being explored. Earlier studies by others demonstrated that
microRNAs regulate cellular functions such as cellular
proliferation, differentiation and cell death (7,22).
miR-892b was previously found to be a modulator of MCL1 in
colorectal cancer, sensitizing cells to a BCL inhibitor, ABT-263
(navitoclax) (23). However, the
cellular function of miR-892b and its cellular signaling networks
in cancer states have not been reported.
Based on predicted-target bioinformatics analysis of
miR-892b, we found that miR-892b regulates signaling molecules
associated with cellular processes including proliferation,
migration and invasion of bladder cancer cells. Upon initial
evaluation, the basal expression of miR-892b was shown to be
significantly lower in all bladder cancer cell lines tested than in
normal human urothelial cells, which indicated that miR-892b may be
associated with transformation of bladder cells. As speculated,
transfection of a miR-892b mimic to bladder cancer cells resulted
in growth arrest. Transfection of a miR-892b inhibitor did not
cause any alteration in the growth of bladder cancer cells. These
results suggest that, in bladder cancers, a cellular mechanism
exists to downregulate miR-892b, which limits its tumor suppressive
function. In addition, we found that miR-892b participates in the
progression of the cell cycle from G1 to S phase in bladder cancer
cells. Among known G1 phase cell cycle regulators, we examined the
expression level of cyclin D1, cyclin E, CDK2 and CDK6. Cyclin D
and E are key regulators of the cell cycle and are associated with
the initiation and progression of bladder cancer (24,25).
We found that cyclin D1, but not cyclin E, was downregulated in
miR-892b-transfected EJ bladder cancer cells. Further immune
complex kinase assays showed that the activity of CDK6, but not
that of CDK2, was reduced by miR-892b transfection. Notably, Zhao
et al reported that targeting CDK6 in bladder cancer cells
by transfecting miR-29c reduced the growth and invasiveness of
bladder cancer cells (26). In
agreement with that result, we found that introduction of miR-892b
to bladder cancer cells significantly inhibited proliferation by
lowering protein levels of cyclin D1 and CDK6. The activity of CDKs
is tightly controlled by CDK inhibitors such as
p16INK4A, p19ARF, p21WAF1/CIP1 and
p27KIP1. Mutations in CDKs are frequently observed in
certain types of cancer, as in the case of p16INK4A in
melanoma (27). However, signature
mutations or mutation hot spots in CDKs for bladder cancers are
relatively understudied. Previously, the correlation of
p21WAF1/CIP1, p27KIP1 and p53 levels were
examined in 51 patient-derived tumor specimens with overall
survival rate. They concluded that downregulation of
p27KIP1 and overexpression of cyclin D1 and D3 predicted
survival of bladder cancer patients (28). Another recent study showed that
miR-451 inhibits the proliferation of esophageal carcinoma cells by
targeting p19ARF (29).
In the present study, p19ARF was upregulated in miR-892b
transfectants. However, p21WAF1/CIP1 and
p27KIP1 levels remained unchanged. These results
demonstrated for the first time that miR-892b impedes proliferation
of bladder cancer cells through G1 phase cell cycle arrest by
inducing the p19ARF/cyclin D1/CDK6 cascade pathway.
Managing invasiveness of bladder cancer is one of
the most difficult clinical challenges. The 5-year survival rate
for patients with stage 4 bladder cancer is ~10% for both men and
women, which is attributable to invasiveness or metastasis
(1). In the present study, we found
that miR-892b inhibits the invasion and migration of bladder cancer
cells through wound recovery and Boyden chamber invasion assays.
During disease progression, bladder cancer cells penetrate
surrounding muscle tissues through the generation of proteolytic
enzymes such as matrix methalloproteinases (MMPs) (30). Previous studies demonstrated that
MMPs are closely correlated with poor prognosis in bladder cancer
patients (19,20,31).
Similarly, we previously identified that MMP-9, but not MMP-2, was
associated with invasion of bladder cancer cells (14,17,33).
The activity of MMP-9 in bladder cancer cells is tightly regulated
by transcription factors, including Sp-1, AP-1 and NF-κB (17,32,33).
In our bioinformatic analysis, Sp-1 was identified as a binding
candidate for miR-892b. Utilizing EMSA assays, we verified that
Sp-1 was the critical transcription factor regulated by miR-892b.
However, neither AP-1 nor NF-κB was regulated by mir-892b. Our data
suggest that Sp-1 is essential for miR-892b-mediated suppression of
MMP-9 expression leading to the reduction in the migration and
invasion of bladder cancer cells.
In conclusion, we demonstrated that aberrant
expression of miR-892b is associated with progression of bladder
cancer cells. Re-introduction of a miR-892b mimic significantly
inhibits the proliferation and migration of bladder cancer cells,
which suggests that miR-892b may be a novel target for treatment of
bladder cancer.
Acknowledgments
The present study was supported by the National
Research Foundation of Korea (NRF) grant by the government of Korea
(MSIP) (no. 2014007036). This study was also supported by the
Functional Districts of the Science Belt support program, Ministry
of Science, ICT and Future Planning (2015K000284).
References
|
1
|
American Cancer Society: Cancer Facts
& Figures 2015. American Cancer Society, Inc.; Atlanta, GA:
2015
|
|
2
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lindgren D, Liedberg F, Andersson A,
Chebil G, Gudjonsson S, Borg A, Månsson W, Fioretos T and Höglund
M: Molecular characterization of early-stage bladder carcinomas by
expression profiles, FGFR3 mutation status, and loss of 9q.
Oncogene. 25:2685–2696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Habuchi T, Marberger M, Droller MJ,
Hemstreet GP III, Grossman HB, Schalken JA, Schmitz-Dräger BJ,
Murphy WM, Bono AV, Goebell P, et al: Prognostic markers for
bladder cancer: International Consensus Panel on bladder tumor
markers. Urology. 66(Suppl 1): S64–S74. 2005. View Article : Google Scholar
|
|
5
|
Kamai T, Takagi K, Asami H, Ito Y, Oshima
H and Yoshida KI: Decreasing of p27Kip1 and cyclin E
protein levels is associated with progression from superficial into
invasive bladder cancer. Br J Cancer. 84:1242–1251. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liukkonen T, Rajala P, Raitanen M, Rintala
E, Kaasinen E and Lipponen P; The Finnbladder Group: Prognostic
value of MIB-1 score, p53, EGFr, mitotic index and papillary status
in primary superficial (Stage pTa/T1) bladder cancer: A prospective
comparative study. Eur Urol. 36:393–400. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Z, Liu K, Wang Y, Xu Z, Meng J and Gu
S: Upregulation of microRNA-96 and its oncogenic functions by
targeting CDKN1A in bladder cancer. Cancer Cell Int. 15:1072015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lei M, Xie W, Sun E, Sun Y, Tian D, Liu C,
Han R, Li N, Liu M, Han R, et al: microRNA-21 regulates cell
proliferation and migration and cross talk with PTEN and p53 in
bladder cancer. DNA Cell Biol. 34:626–632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiu Y, Liu Z, Xia S, Jin C, Yin H, Zhao W
and Wu Q: MicroRNA-137 upregulation increases bladder cancer cell
proliferation and invasion by targeting PAQR3. PLoS One.
9:e1097342014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng Y, Kang Y, He Y, Liu J, Liang B, Yang
P and Yu Z: microRNA-99a acts as a tumor suppressor and is
down-regulated in bladder cancer. BMC Urol. 14:502014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu D, Zhou Y, Pan H, Zhou J, Fan Y and Qu
P: microRNA-99a inhibiting cell proliferation, migration and
invasion by targeting fibroblast growth factor receptor 3 in
bladder cancer. Oncol Lett. 7:1219–1224. 2014.PubMed/NCBI
|
|
13
|
Zhang J, Wang S, Han F, Li J, Yu L, Zhou
P, Chen Z, Xue S, Dai C and Li Q: MicroRNA-542-3p suppresses
cellular proliferation of bladder cancer cells through
post-transcriptionally regulating survivin. Gene. 579:146–152.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park SL, Cho TM, Won SY, Song JH, Noh DH,
Kim WJ and Moon SK: MicroRNA-20b inhibits the proliferation,
migration and invasion of bladder cancer EJ cells via the targeting
of cell cycle regulation and Sp-1-mediated MMP-2 expression. Oncol
Rep. 34:1605–1612. 2015.PubMed/NCBI
|
|
15
|
Yun SJ, Jeong P, Kim WT, Kim TH, Lee YS,
Song PH, Choi YH, Kim IY, Moon SK and Kim WJ: Cell-free microRNAs
in urine as diagnostic and prognostic biomarkers of bladder cancer.
Int J Oncol. 41:1871–1878. 2012.PubMed/NCBI
|
|
16
|
Moon SK, Jung SY, Choi YH, Lee YC,
Patterson C and Kim CH: PDTC, metal chelating compound, induces G1
phase cell cycle arrest in vascular smooth muscle cells through
inducing p21Cip1 expression: Involvement of p38 mitogen
activated protein kinase. J Cell Physiol. 198:310–323. 2004.
View Article : Google Scholar
|
|
17
|
Lee SJ, Cho SC, Lee EJ, Kim S, Lee SB, Lim
JH, Choi YH, Kim WJ and Moon SK: Interleukin-20 promotes migration
of bladder cancer cells through extracellular signal-regulated
kinase (ERK)-mediated MMP-9 protein expression leading to nuclear
factor (NF-κB) activation by inducing the up-regulation of
p21WAF1 protein expression. J Biol Chem. 288:5539–5552.
2013. View Article : Google Scholar
|
|
18
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase
progression. Genes Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reis ST, Leite KR, Piovesan LF,
Pontes-Junior J, Viana NI, Abe DK, Crippa A, Moura CM, Adonias SP,
Srougi M, et al: Increased expression of MMP-9 and IL-8 are
correlated with poor prognosis of Bladder Cancer. BMC Urol.
12:182012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seiler R, Thalmann GN and Fleischmann A:
MMP-2 and MMP-9 in lymph-node-positive bladder cancer. J Clin
Pathol. 64:1078–1082. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grimson A, Farh KK, Johnston WK,
Garrett-Engele P, Lim LP and Bartel DP: MicroRNA targeting
specificity in mammals: Determinants beyond seed pairing. Mol Cell.
27:91–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lam LT, Lu X, Zhang H, Lesniewski R,
Rosenberg S and Semizarov D: A microRNA screen to identify
modulators of sensitivity to BCL2 inhibitor ABT-263 (navitoclax).
Mol Cancer Ther. 9:2943–2950. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eissa S, Ahmed MI, Said H, Zaghlool A and
El-Ahmady O: Cell cycle regulators in bladder cancer: Relationship
to schistosomiasis. IUBMB Life. 56:557–564. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shan G and Tang T: Expression of cyclin D1
and cyclin E in urothelial bladder carcinoma detected in tissue
chips using a quantum dot immunofluorescence technique. Oncol Lett.
10:1271–1276. 2015.PubMed/NCBI
|
|
26
|
Zhao X, Li J, Huang S, Wan X, Luo H and Wu
D: MiRNA-29c regulates cell growth and invasion by targeting CDK6
in bladder cancer. Am J Transl Res. 7:1382–1389. 2015.PubMed/NCBI
|
|
27
|
FitzGerald MG, Harkin DP, Silva-Arrieta S,
MacDonald DJ, Lucchina LC, Unsal H, O'Neill E, Koh J, Finkelstein
DM, Isselbacher KJ, et al: Prevalence of germ-line mutations in
p16, p19ARF, and CDK4 in familial melanoma: Analysis of a
clinic-based population. Proc Natl Acad Sci USA. 93:8541–8545.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lopez-Beltran A, Luque RJ,
Alvarez-Kindelan J, Quintero A, Merlo F, Carrasco JC, Requena MJ
and Montironi R: Prognostic factors in stage T1 grade 3 bladder
cancer survival: The role of G1-S modulators (p53, p21Waf1,
p27kip1, Cyclin D1, and Cyclin D3) and proliferation index
(ki67-MIB1). Eur Urol. 45:606–612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zang WQ, Yang X, Wang T, Wang YY, Du YW,
Chen XN, Li M and Zhao GQ: MiR-451 inhibits proliferation of
esophageal carcinoma cell line EC9706 by targeting CDKN2D and
MAP3K1. World J Gastroenterol. 21:5867–5876. 2015.PubMed/NCBI
|
|
30
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ramón de Fata F, Ferruelo A, Andrés G,
Gimbernat H, Sánchez-Chapado M and Angulo JC: The role of matrix
metalloproteinase MMP-9 and TIMP-2 tissue inhibitor of
metalloproteinases as serum markers of bladder cancer. Actas Urol
Esp. 37:480–488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mian BM, Dinney CP, Bermejo CE, Sweeney P,
Tellez C, Yang XD, Gudas JM, McConkey DJ and Bar-Eli M: Fully human
anti-interleukin 8 antibody inhibits tumor growth in orthotopic
bladder cancer xenografts via down-regulation of matrix
metal-loproteases and nuclear factor-kappaB. Clin Cancer Res.
9:3167–3175. 2003.PubMed/NCBI
|
|
33
|
Lee EJ, Lee SJ, Kim S, Cho SC, Choi YH,
Kim WJ and Moon SK: Interleukin-5 enhances the migration and
invasion of bladder cancer cells via ERK1/2-mediated
MMP-9/NF-κB/AP-1 pathway: Involvement of the p21WAF1 expression.
Cell Signal. 25:2025–2038. 2013. View Article : Google Scholar : PubMed/NCBI
|