Introduction
Oral squamous cell carcinoma (OSCC) is the most
common head and neck neoplasm affecting ~274,000 individuals
worldwide (1). The 5-year survival
rate of patients with OSCC is only 53% (1–3). The
development of OSCC has been shown to be associated with oral
habits including betel quid chewing, as well as tobacco and/or
alcohol consumption, which lead to continuous generation of
reactive oxygen species (ROS) (4–6).
Excessive ROS generation due to imbalances in the redox status
mediates DNA damage and/or lipid peroxidation, which are thought to
subsequently promote the development of OSCC (7). A recent study showed that oxidative
stress markers, 8-hydroxy-2′-deoxyguanosin and malondialdehyde,
were higher in the saliva of patients with OSCC than levels in the
saliva of healthy normal subjects, while the antioxidant vitamins C
and E were lower (8). Thus,
accumulating evidence has implicated a role for ROS generation in
the pathogenesis of OSCC.
Recently, the NADPH oxidase (Nox/Duox) family, a
family of enzymes that generate ROS, has been shown to play an
important role in cancer development and progression (9,10). To
date, the roles of Nox1, Nox2, Nox4 and
Nox5 in cancer have been implicated, while those of
Duox1, Duox2 and Nox3 in carcinogenesis are
not well-reported (9). Upregulated
Nox1 expression and subsequent ROS generation was found to
promote cancer cell growth, as well as escape from cancer cell
death through the redox-dependent activation of p38MAPK and AKT
signaling pathways (11). Given the
experimental evidence that the Nox family plays a pivotal role in
cancer cell development, it would be interesting to examine the
involvement of Nox/Duox family members in the pathogenesis
of OSCC.
In the present study, we found that Nox1 and
Nox4 mRNAs were highly expressed in a significant subset of
OSCC cell lines. Knockdown of Nox1 significantly induced
apoptosis, and enhanced the cisplatin-induced cytotoxic effect in
OSCC cells. Additionally, we report the underlying molecular
mechanism responsible for this signaling.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) and
penicillin-streptomycin were purchased from Wako Pure Chemical
Industries, Ltd. (Osaka, Japan). Fetal bovine serum (FBS) was
obtained from Nichirei Biosciences, Inc. (Tokyo, Japan).
Diphenyleneiodonium (DPI), 2′,7′-dichlorofluorescein diacetate
(DCFH-DA), N-acetylcysteine (NAC), MTT and ammonium
pyrrolidine dithiocarbamate (PDTC) were obtained from Sigma-Aldrich
(St. Louis, MO, USA). AKT inhibitor, perifosine, was obtained from
Cayman Chemical Co. (Ann Arbor, MI, USA). Propidium iodide (PI) was
obtained from Merck Millipore (Billerica, MA, USA). Annexin V-FITC
was obtained from MBL (Nagoya, Japan). Rabbit anti-NOX1 and
anti-NOX4 antibodies were obtained from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Anti-AKT, anti-phosphorylated-AKT (Ser473),
anti-β-actin and HRP-conjugated anti-rabbit IgG were purchased from
Cell Signaling Technologies Inc. (Beverly, MA, USA).
Cell culture
Five OSCC cell lines (HSC-2, HSC-3, HSC-4, SAS and
OSC-19) were obtained from the Japanese Collection of Research
Bioresources Cell Bank. HSC-2, HSC-3 and HSC-4 cell lines were
established from individual patients with OSCC (12). The cell lines were maintained in
DMEM supplemented with 10% heat-inactivated FBS and
penicillin-streptomycin at 37°C in a 5% CO2 humidified
atmosphere. The cells were detached from 90-mm dishes using trypsin
and seeded in either 96- or 6-well plates for experimental
purposes.
Cell viability MTT assay
The OSCC cells were seeded in 96-well plates
(5×103 cells/well) and incubated for 24 h at 37°C. Then,
the cells were incubated with medium containing the indicated
concentrations of DPI, PDTC and NAC. After 72 h of incubation, MTT
solution was added into each well. Following 4 h of incubation,
lysis buffer (10% SDS in 0.01 M of hydrogen chloride) was added and
incubated overnight. Finally, the absorbance at 550 nm was measured
using a SpectraMax M5 spectrophotometer (Molecular Devices,
Sunnyvale, CA, USA).
Annexin V assay
Apoptosis was evaluated using Annexin V
(AxV)-FITC/PI double staining-based FACS analysis as previously
described (13). Briefly, OSCC
cells were seeded in 6-well plates (1×105 cells/well)
and incubated for 24 h at 37°C. Then, the cells were incubated with
the indicated concentrations of DPI followed by incubation in
AxV-FITC and PI (10 µg/ml) at room temperature for 15 min.
Finally, fluorescence intensities were determined by FACS using a
FACSCanto II (BD, Franklin Lakes, NJ, USA).
ROS assay
ROS generation was detected using DCFH-DA as
previously described (14).
Briefly, HSC-2 and HSC-3 cells were incubated with DPI (5 or 10
µmol/l). The cells were further incubated with the DCFH-DA
fluorescent probe in the presence of DPI for 0.5 h. After 48 h
incubation, the cells were examined using FACSCanto II, which
analyzed 10,000 events (determined by forward and side scatter).
Data are presented as mean fluorescence intensity of triplicate
determinations ± SE.
RT-PCR analysis
The OSCC cells (1×105 cells/well) were
seeded in 6-well plates. Following incubation for 48 h, total RNA
was extracted from the cells using NucleoSpin® RNA
(Takara Bio, Inc., Shiga, Japan), and 2 µg total RNA was
reverse-transcribed with High-Capacity cDNA Reverse Transcription
kit (Life Technologies, Inc., Tokyo, Japan). mRNA expression levels
of seven Nox/Duox family members (Nox1, Nox2,
Nox3, Nox4, Nox5, Duox1 and
Duox2) were examined using Veriti® Thermal Cycler
(Applied Biosystems, Carlsbad, CA, USA) with specific primer sets
as listed in Table I. mRNA
expression of GAPDH was used as an internal control.
 | Table IPrimer sets for RT-PCR analyses. |
Table I
Primer sets for RT-PCR analyses.
| Gene | | Sequence
information | Size (bp) |
|---|
| Nox1 | Sense |
5′-GGAGCAGGAATTGGGGTCAC | 236 |
| Antisense |
5′-TTGCTGTCCCATCCGGTGAG | |
| TaqMan ID | Hs00246598_m1 | 98 |
| Nox2 | Sense |
5′-GGAGTTTCAAGATGCGTGGAAACTA | 550 |
| Antisense |
5′-GCCAGACTCAGAGTTGGAGATGCT | |
| Nox3 | Sense |
5′-GGATCGGAGTCACTCCCTTCGCTG | 458 |
| Antisense |
5′-ATGAACACCTCTGGGGTCAGCTGA | |
| Nox4 | Sense |
5′-CTCAGCGGAATCAATCAGCTGTG | 286 |
| Antisense |
5′-AGAGGAACACGACAATCAGCCTTAG | |
| TaqMan ID | Hs00418356_m1 | 109 |
| Nox5 | Sense |
5′-ATCAAGCGGCCCCCTTTTTTTCAC | 239 |
| Antisense |
5′-CTCATTGTCACACTCCTCGACAGC | |
| Duox1 | Sense |
5′-TTCACGCAGCTCTGTGTCAA | 97 |
| Antisense |
5′-AGGGACAGATCATATCCTGGCT | |
| Duox2 | Sense |
5′-ACGCAGCTCTGTGTCAAAGGT | 91 |
| Antisense |
5′-TGATGAACGAGACTCGACAGC | |
| GAPDH | Sense |
5′-GAGTCAACGGATTTGGTCGT | 185 |
| Antisense |
5′-GACAAGCTTCCCGTTCTCAG | |
| TaqMan ID | Hs99999905_m1 | 122 |
Quantitative RT-PCR (qRT-PCR)
analysis
HSC-2 and HSC-3 cells (1×105 cells/well)
were seeded in 6-well plates and incubated for 24 h. The following
day, real-time qRT-PCR analysis was performed using the
StepOnePlus™ Real-Time PCR System (Applied Biosystems). qRT-PCR
analysis using TaqMan probes was performed according to the
manufacturer's instructions. Gene specific TaqMan probes used in
this study are listed in Table
1.
Western Blot analysis
HSC-2 and HSC-3 cells (2×105 cells/well)
were incubated with DPI as described above. The cells were washed
with ice-cold PBS and lysed in loading buffer [125 mmol/l Tris (pH
6.8), 4% SDS, 10% β-mercaptoethanol, 20% glycerol and 0.02%
bromophenol blue]. Western blot analysis was performed as
previously described (15). The
relative protein levels were calculated after normalization to an
internal control β-actin.
RNA interference
HSC-2 and HSC-3 cells (1×105 cells/well)
were plated in 6-well plates. On the following day, the cells were
transfected using Lipofectamine RNAi/MAX (Life Technologies Inc.)
to deliver the indicated concentrations of Nox1 or
Nox4 siRNA (Santa Cruz Biotechnology, Inc.) according to the
manufacturer's protocol. siGENOME RISC-Free siRNA (Dharmacon-Thermo
Fisher Scientific, Tokyo, Japan) was used as a negative
control.
Statistical analysis
At least three independent experiments and three
replications per experiment were performed. The results are
expressed as the mean ± SE. Statistical significance between groups
was determined using Student's t-tests. Statistical analyses were
performed using SPSS 23.0 (SPSS, Inc., Chicago, IL, USA).
Statistical significance was defined as P<0.05.
Results
ROS scavengers inhibit cellular growth in
a panel of OSCC cell lines
To investigate the involvement of ROS in OSCC cell
growth, we first examined whether a Nox inhibitor, DPI, as well as
ROS scavengers, including PDTC and NAC, affect the cell survival in
five OSCC cell lines, HSC-2, HSC-3, HSC-4, SAS and OSC-19. The MTT
assay revealed that the cell viability was dose-dependently
suppressed by treatment with DPI (Fig.
1A), PDTC (Fig. 1B) or NAC
(Fig. 1C). The IC50
values ranged from 0.07 µM (SAS cells) to 1.59 µM
(OSC-19 cells) for DPI, 40 µM (HSC-4 cells) to >100
µM (SAS cells) for PDTC and 2.53 mM (HSC-2 cells) to 14.0 mM
(HSC-4 cells) for NAC among the OSCC cell lines tested (Table II). These results suggest that
scavenging of ROS may suppress cell growth in OSCC cells.
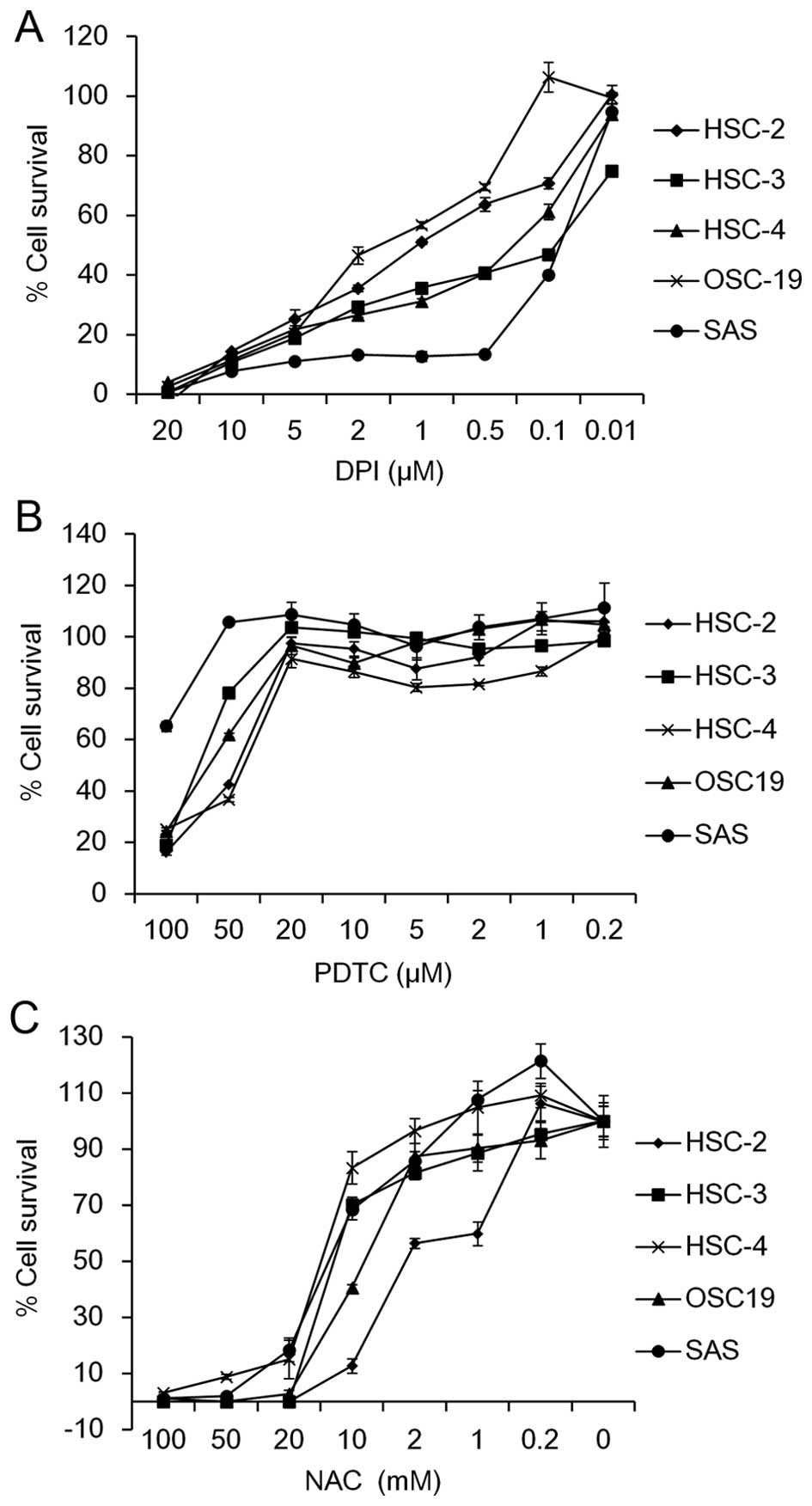 | Figure 1Effect of DPI, PDTC and NAC on cell
survival of OSCC cells. Five OSCC cell lines (HSC-2, HSC-3, HSC-4,
OSC19 and SAS) were seeded into 96-well plates (2.5×103
cells/well). On the following day, the cells were treated with the
indicated concentrations of (A) DPI (20, 10, 5, 2, 1, 0.5, 0.1 and
0.01 µM), (B) PDTC (100, 50, 20, 10, 5, 2, 1 and 0.2
µM) and (C) NAC (100, 50, 20, 10, 2, 1 and 0.2 µM)
for 72 h. The percentage of cell survival of the five OSCC cell
lines was measured by MTT assay. Data are expressed relative to the
mean optical density (550 nm) in the untreated cells, which was
arbitrarily defined as 100%. Data are represented as the mean ± SE
(n=3). |
 | Table IIIC50 values of the OSCC
cell lines treated with DPI, PDTC or NAC. |
Table II
IC50 values of the OSCC
cell lines treated with DPI, PDTC or NAC.
| Cell lines | DPI
IC50 (μM) | PDTC
IC50 (μM) | NAC
IC50 (mM) |
|---|
| HSC2 | 1.05 |
44.1 | 2.53 |
| HSC3 | 0.08 |
69.5 | 12.20 |
| HSC4 | 0.24 |
40.0 | 14.00 |
| OSC19 | 1.59 |
62.1 | 7.24 |
| SAS | 0.07 | >100.0 | 12.90 |
Expression levels of the Nox/Duox family
in OSCC cell lines
Since DPI reduced the cell viability in OSCC cells,
we assumed that the Nox/Duox family plays an important role
in cell survival. To clarify this issue, we first examined
Nox/Duox mRNAs in five OSCC cell lines using RT-PCR
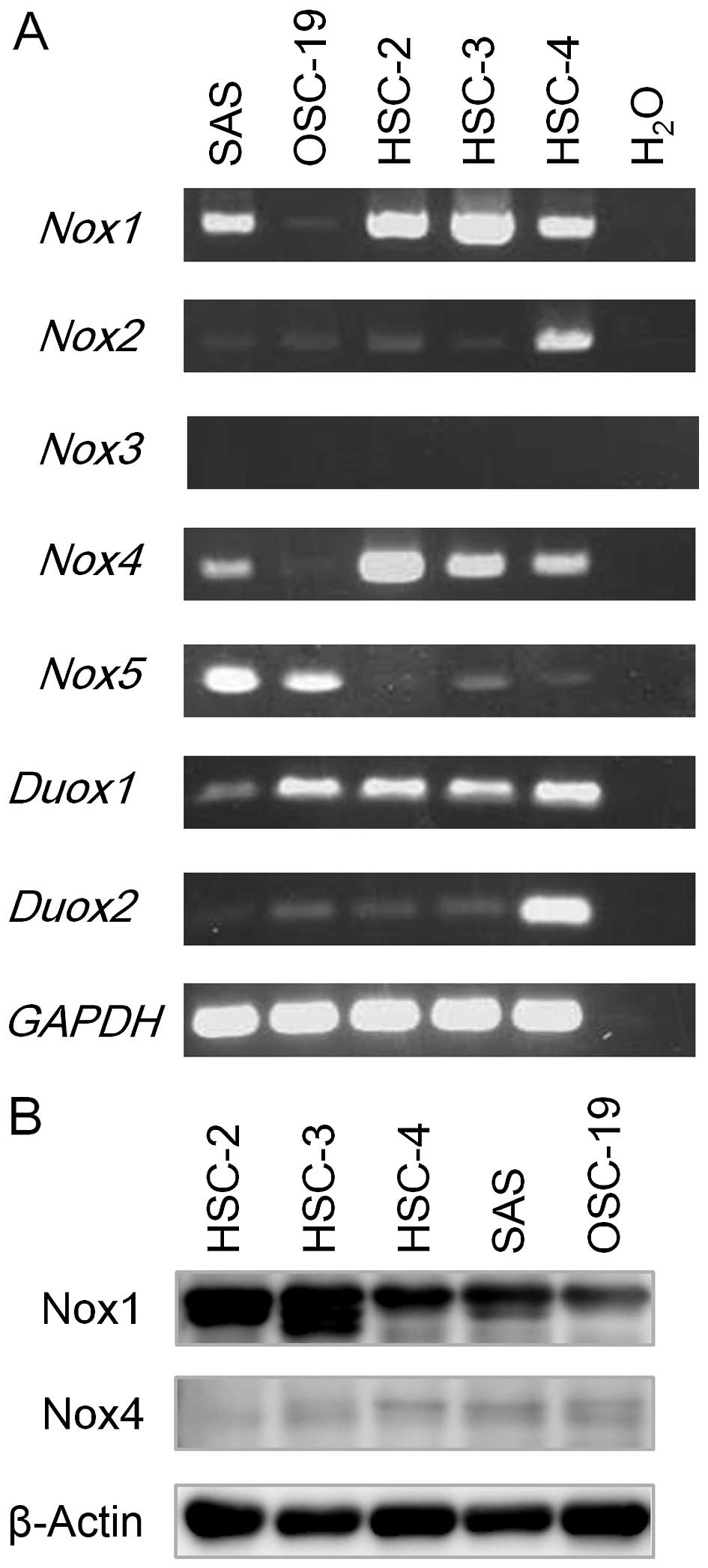
analysis. As shown in Fig. 2A,
while Nox1 and Nox4 mRNA expression was readily
detected in four cell lines, except for OSC-19, Nox2 and
Nox3 mRNA expression was primarily detected only in HSC-4
cells. Nox5 mRNA expression was detected in both SAS and
OSC-19 cells (Fig. 2A).
Additionally, Duox1 mRNA expression was detectable in all of
the cell lines examined, while that of Duox2 was readily
detected in the HSC-4 cells but diminished in the OSC-19, HSC-2 and
HSC-3 cells (Fig. 2A). These
results are summarized in Table
III. We also examined protein expression of Nox1 and Nox4; Nox1
protein expression was elevated in the HSC-2 and HSC-3 cells, while
Nox4 was slightly expressed in the five OSCC cell lines examined
(Fig. 2B).
 | Table IIIRT-PCR analysis of mRNA expression of
the Nox/Duox family. |
Table III
RT-PCR analysis of mRNA expression of
the Nox/Duox family.
| Nox family
| Duox family
|
|---|
| Cell lines | Nox1 | Nox2 | Nox3 | Nox4 | Nox5 | Duox1 | Duox2 |
|---|
| SAS | + | − | − | + | ++ | − | − |
| OSC-19 | − | − | − | − | + | + | + |
| HSC-2 | + | − | − | ++ | − | + | + |
| HSC-3 | ++ | − | − | + | − | + | + |
| HSC-4 | + | + | − | + | − | + | ++ |
Nox inhibitor DPI suppresses ROS
generation and induces apoptosis
To investigate the involvement of the Nox/Duox
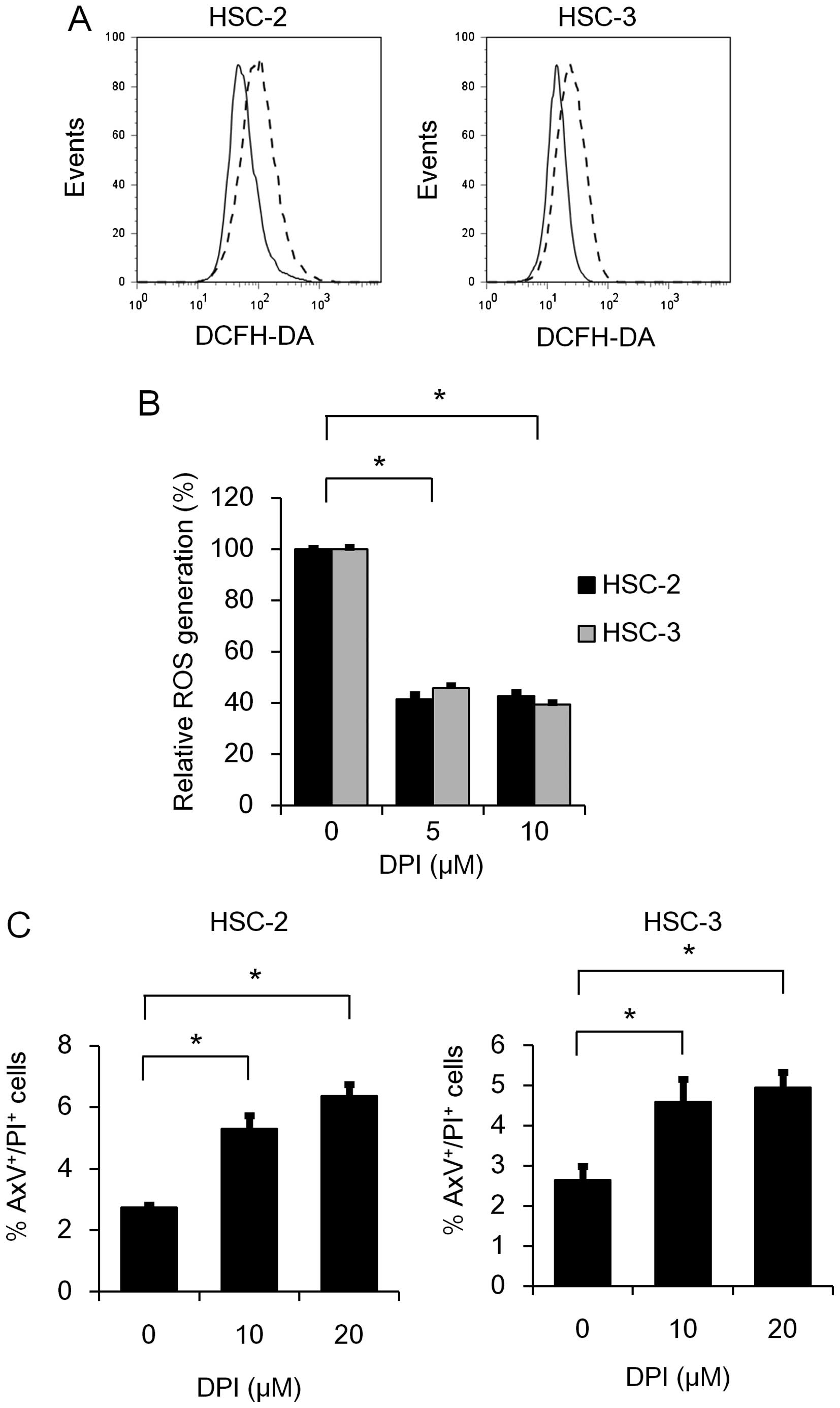
family in ROS generation, we further examined the effect of DPI on
ROS generation in HSC-2 and HSC-3 cells. As expected, RO generation
was significantly suppressed by DPI treatment (Fig. 3A and B). This result prompted us to
further examine the effect of DPI on the induction of apoptosis in
HSC-2 and HSC-3 cells. As shown in Fig.
3C, the percentages of AxV+/PI+ cells was
significantly increased 48 h after DPI treatment in both the HSC-2
and HSC-3 cell lines, suggesting that the Nox family may play a
role in the anti-apoptotic effect as well as ROS generation.
Knockdown of Nox1, but not Nox4,
suppresses cell survival
Since Nox1 and Nox4 mRNA expression
was readily detectable in the HSC-2 and HSC-3 cells, we next
examined the effect of Nox1 or Nox4 knockdown on cell
viability using MTT assay. We verified that transfection of
Nox1 or Nox4 siRNAs significantly reduced their
endogenous mRNA levels (65–70% in Nox1 or 50–55% in
Nox4), respectively, in the cells compared to those
transfected with control siRNA (Fig. 4A
and B). Knockdown of Nox1, but not Nox4,
significantly suppressed the cell viability in both the HSC-2 and
HSC-3 cell lines (Fig. 4C). In
addition, the percentages of AxV+/PI+ cells
were significantly increased after the transfection of Nox1
siRNAs (Fig. 4D), strongly
suggesting that Nox1 contributes to anti-apoptosis in OSCC
cells. Unexpectedly, under Nox1 knockdown, no significant
reduction in intracellular ROS was detected (Fig. 4E), suggesting that Nox1 may
contribute to cell survival through a mechanism other than ROS
generation.
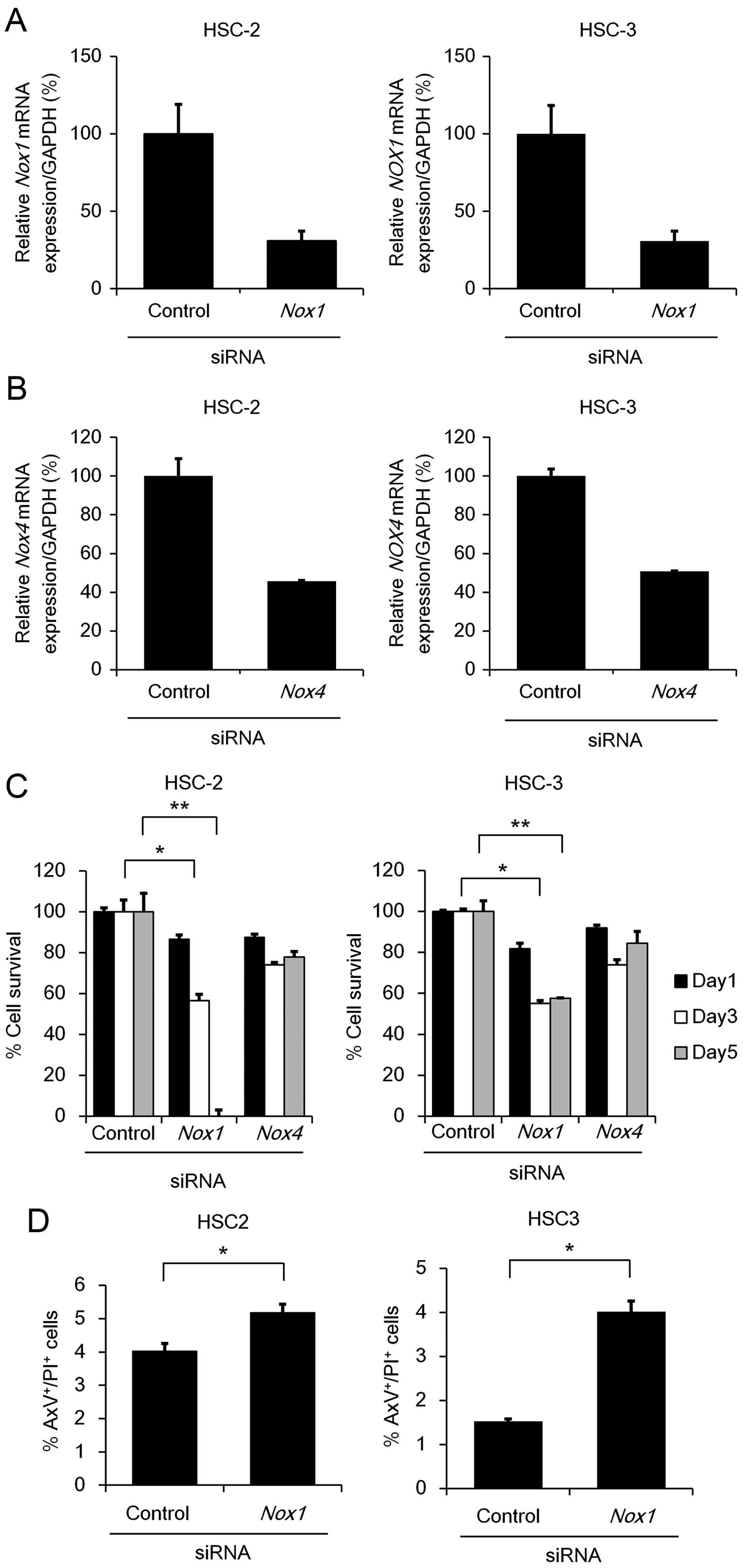 | Figure 4Cell survival and apoptosis under
Nox1 or Nox4 knockdown. (A and B) HSC-2 and HSC-3
cells (1×105 cell/well) were transfected with 50 nM of
siRNA specific to Nox1, Nox4 or a non-specific
control siRNA. After 48 h, total RNA was extracted from the cells
and mRNA expression levels of Nox1 (A) or Nox4 (B)
were analyzed using qRT-PCR with specific TaqMan probes. The
relative gene expression levels are shown after normalization to
GAPDH mRNA expression. Data are expressed relative to the
mRNA levels found in the cells transfected with control siRNA,
which was arbitrarily defined as 100% (n=3). (C) HSC-2 and HSC-3
cells (2.5×103 cells/well) were transfected with 50 nM
of siRNA specific to Nox1, Nox4 or a non-specific
control siRNA. After 72 h, MTT assay was performed as described in
Fig. 1A. Data are expressed as the
mean ± SE (n=3). (D) Apoptosis under Nox1 or Nox4
knockdown. HSC-2 and HSC-3 cells were seeded and transfected as
described in (A). After 48 h, the cells were stained with AxV-FITC
and PI. Data are expressed as the mean ± SE (n=3).
*P<0.05; **P<0.005, significant
difference. (E) The effect of Nox1 or Nox4 knockdown
on intracellular ROS generation. HSC-2 and HSC-3 cells were seeded
and transfected as described in (A). After 48 h, the cells were
labeled with DCFH-DA (5 µM) and alterations in the
intracellular ROS generation were measured by FACS analysis.
Representative results of flow cytometic analyses in HSC-2 (left
panel) and HSC-3 (right panel) cells are shown. Bar graphs show the
percentage of ROS generation relative to the mean fluorescence
intensity of the untreated cells, which was arbitrarily defined as
100% (n=3). Vertical line, no staining; gray or black line, control
or Nox1 siRNA (50 nM), respectively. |
Nox1 knockdown, as well as perifosine
treatment, prevents phosphorylation of AKT
AKT (protein kinase B) is a regulator of cell
survival in response to growth factors. AKT is activated through
its phosphorylation, and this kinase inhibits apoptosis-inducing
proteins, thereby promoting cell survival. Thus, we investigated
phosphorylated AKT levels in OSCC cell lines using western blot
analysis. As shown in Fig. 5A,
phosphorylated forms of AKT were readily detected in the HSC-2,
HSC-3 and HSC-4 cells (Fig. 5A). We
therefore examined the effect of a specific AKT inhibitor
perifosine on cell survival using MTT assay. As shown in Fig. 5B, perifosine treatment
dose-dependently reduced cell viability in the OSCC cell lines
(Fig. 5B), suggesting that AKT
plays a pivotal role in OSCC cell survival. Since the Nox family
has been shown to enhance phosphorylation of AKT (9), we then examined the effect of
Nox1 knockdown on AKT phosphorylation using western blot
analysis. As shown in Fig. 5C,
Nox1 knockdown significantly reduced the phosphorylation
level of AKT. These results suggest that Nox1 contributes to cell
survival through activation of the AKT signaling pathway.
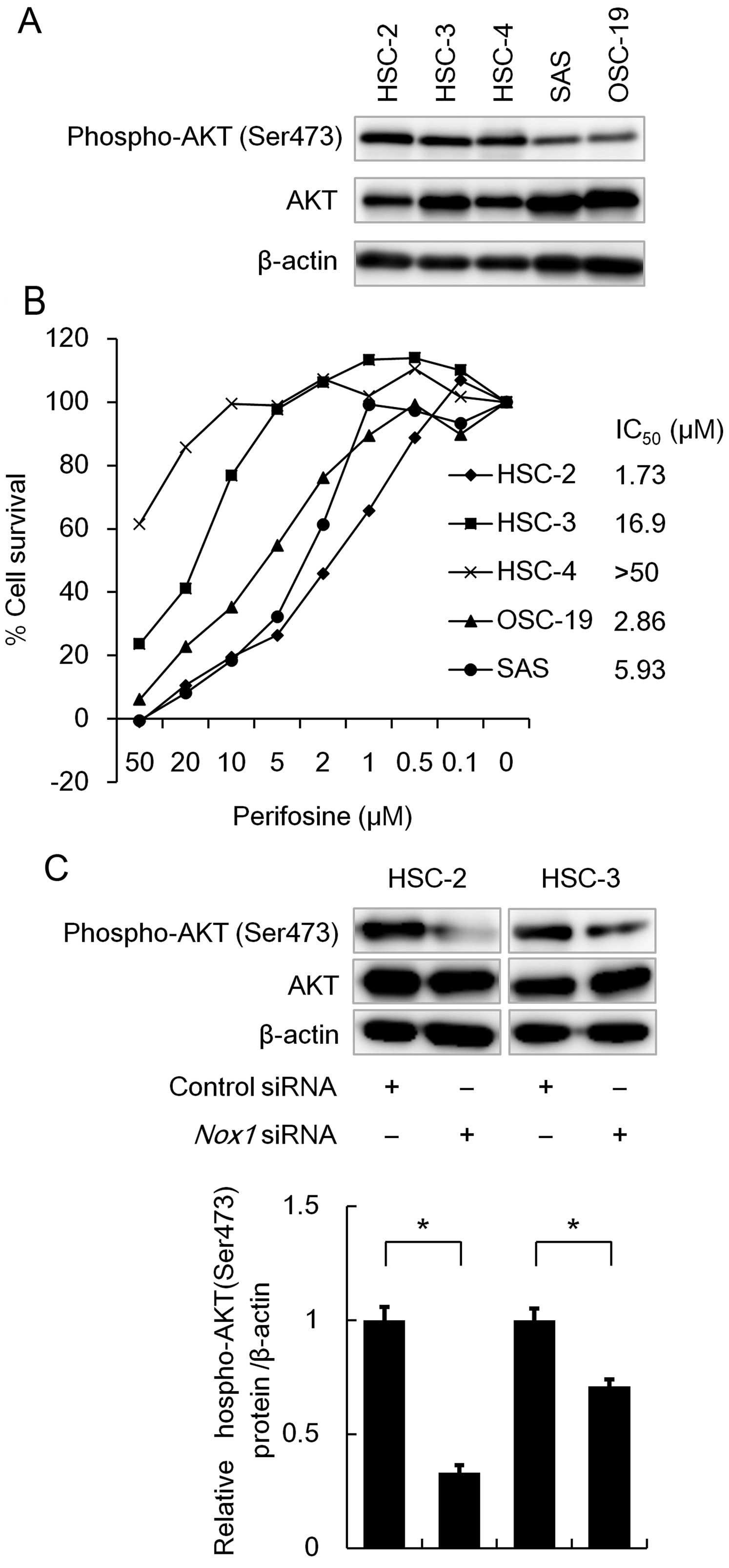 | Figure 5Effect of Nox1 knockdown on the
phosphorylation level of AKT. (A) Phosphorylation levels of AKT in
OSCC cell lines. OSCC cell lines were seeded in a 6-well plate
(1×105 cells/well) and incubated for 48 h. A total of
2.5 µg of cell lysate was subjected to western blot analysis
to detect AKT or phosphorylated AKT, while 1 µg was
subjected to detect β-actin protein. (B) The effect of AKT
inhibitor, perifosine, on cell viability in OSCC cell lines. OSCC
cell lines were seeded in a 96-well plate (2.5×103
cells/well). On the following day, the cells were treated with the
indicated concentrations (50, 20, 10, 5, 2, 1, 0.5 and 0.1
µM) of perifosine for 72 h. The percentage of cell survival
of five OSCC cell lines was measured by MTT assay. Data are
expressed relative to the mean optic density (550 nm) found in the
untreated cells, which was arbitrarily defined as 100%. Data are
expressed as the mean ± SE (n=3). The IC50 values of
perifosine were determined for each cell line using MTT assay. (C)
HSC-2 and HSC-3 cells (1×105 cells/well) were
transfected with 50 nM of siRNA specific to Nox1 or control siRNA.
After incubation for 48 h, the cells were lysed and the cell
lysates were subjected to western blot analysis, as described in
(A). *P<0.05, a significant difference (n=3). The
data are represented as the mean ± SE of three separate
experiments. |
Nox1 knockdown and cisplatin treatment
act cooperatively to suppress cell survival and induce
apoptosis
Cisplatin is one of the most frequently used
anticancer drugs for OSCC treatment. Therefore, we sought to
examine whether inhibition of AKT activity or Nox1 knockdown
influences the cytotoxic effect of cisplatin. As shown in Fig. 6A, the perifosine and cisplatin
combination treatment significantly suppressed cell viability in
both te HSC-2 and HSC-3 cell lines, compared to the cisplatin
monotherapy. Similarly, combined perifosinecisplatin treatment
decreased cell viability in the SAS and OSC-19 cells (data not
shown). Notably, the Nox1 knockdown and cisplatin
combination treatment significantly suppressed cell viability,
compared to the cisplatin monotherapy (Fig. 6B). This novel finding prompted us to
examine the effect of Nox1 knockdown and cisplatin
combination treatment on induction of apoptosis. As expected, the
treatment significantly induced apoptosis (Fig. 6C), indicating that Nox1 knockdown
enhances the cytotoxic effect of cisplatin in OSCC cells.
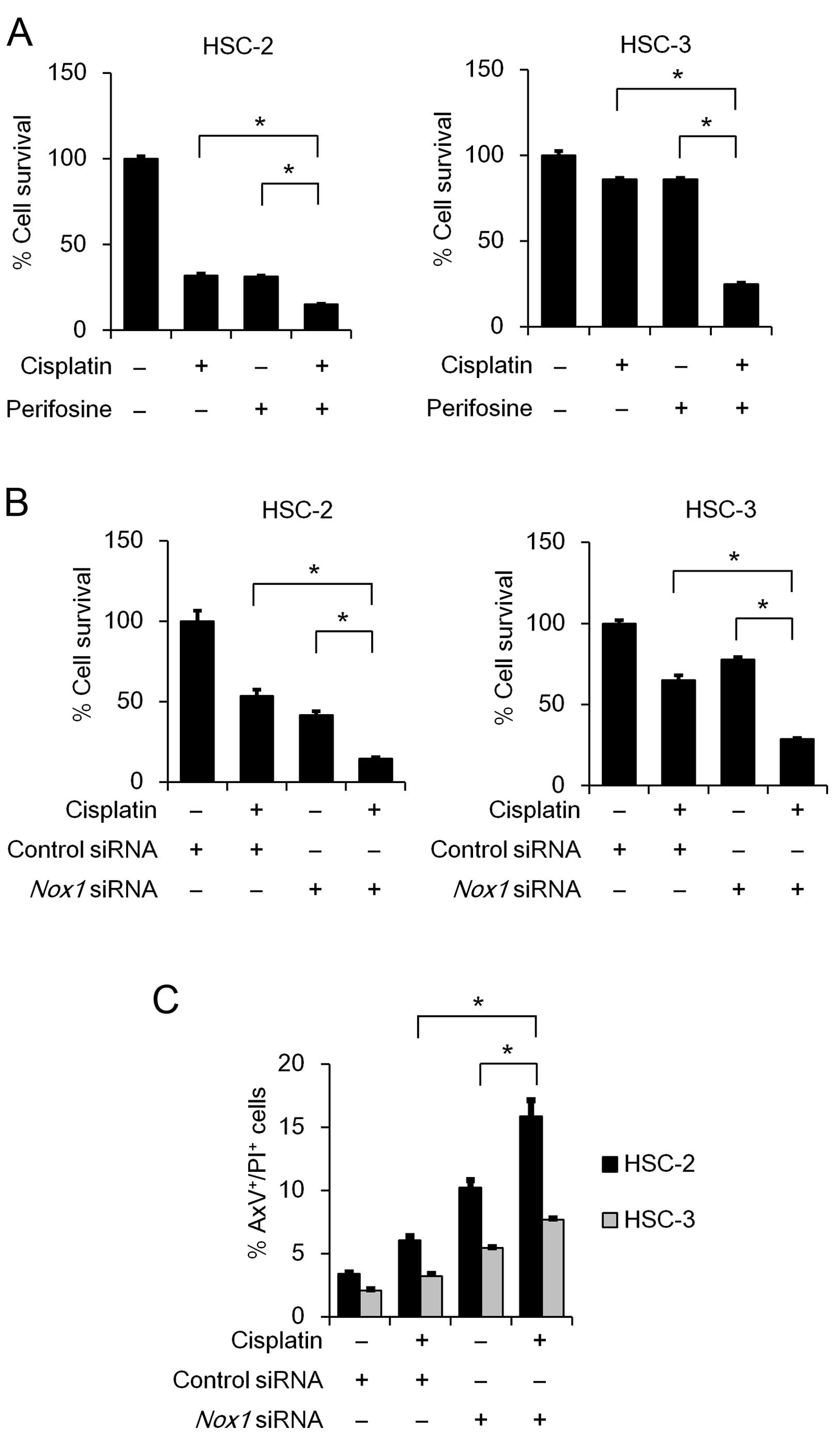 | Figure 6Combinatorial effect of cisplatin
with either perifosine treatment or Nox1 knockdown on cell
viability and apoptosis. (A) HSC-2 and HSC-3 cells were seeded in a
96-well plate (2.5×103 cells/well). On the following
day, the cells were treated with cisplatin (HSC-2, 5 µM;
HSC-3, 2.5 µM) and/or perifosine (HSC-2, 2 µM; HSC-3,
10 µM) for 72 h. (B and C) HSC-2 and HSC-3 cells were
transfected with 50 nM of Nox1 siRNA or control siRNA in the
presence or absence of cisplatin (HSC-2, 5 µM; HSC-3, 2.5
µM). (B) After incubation for 72 h, MTT analysis of the
growth rate was performed as described in Fig 1A. Data are presented relative to the
mean optical density (550 nm) in the untreated cells, which was
arbitrarily defined as 100%. Data are expressed as means ± SE
(n=3). (C) After incubation for 48 h, the cells were stained with
AxV-FITC and PI. Bar graphs showing the percentage of apoptosis
(AxV+/PI+ cells) are presented. Data are
represented as the mean ± SE (n=3). *P<0.05,
significant difference. |
Discussion
Nox/Duox family members are regarded as major
sources of ROS generation, which plays an important role in cancer
development (9,16). It has been reported that inhibition
of Nox activity induces apoptosis in a number of cancerous cells
including pancreatic cancer, mesothelioma and sarcoma (17–19).
In the present study, we demonstrated that knockdown of Nox1
but not Nox4 significantly reduced the cell viability in a
subset of OSCC cell lines. We also found that Nox1 knockdown
decreased phosphorylated AKT levels accompanied by induction of
apoptosis. Our observation that the specific AKT inhibitor
perifosine reduced the cell viability suggested that Nox1-mediated
AKT phosphorylation contributes to cell survival in OSCC cells.
The Nox/Duox family consists of seven members. Our
RT-PCR analysis revealed that Nox1 and Nox4 mRNAs
were highly expressed in a significant subset of OSCC cell lines.
Knockdown of Nox1, but not Nox4, significantly
suppressed the cell viability by 50% in OSCC cells. Additionally,
Nox1 knockdown significantly induced the apoptosis of OSCC
cells. Notably, Nox1 knockdown markedly enhanced
cisplatin-induced apoptosis. This novel finding raises the
possibility that Nox1 is a potential therapeutic target for a
significant subset of oral cancer cells. We unexpectedly observed
that Nox1 knockdown had almost no suppressive effect on ROS
generation. It may be possible that Nox1 exerts anti-apoptotic
effects through a mechanism other than ROS generation. Puca et
al reported that Nox1 inhibits acetylation of p53 (K382), which
is a target of SIRT1 deacetylase, and impaired p53 pro-apoptotic
transcriptional activity (20).
Thus, it may be interesting to investigate the role of p53 in
Nox1-mediated anti-apoptotic signaling in OSCC cells.
It was previously reported that Nox4-mediated
induction of pro-inflammatory cytokines after epidermal growth
factor receptor inhibition (21)
and erlotinib-induced cytotoxicity is associated with hydrogen
peroxide production thorough Nox4 signaling in head and neck
squamous cell carcinoma (HNSCC) cells (22). We recently reported that Nox4
knockdown induced apoptosis in mesothelioma (19). However, in the present study,
Nox4 knockdown did not induce apoptosis in OSCC cells. Thus,
it would also be interesting to pursue the mechanism underlying the
discrepancy in the effect of Nox4 knockdown between OSCC and
mesothelioma cells.
AKT, a regulator of cell survival, is activated
through its phosphorylation and inhibits apoptosis-inducing
proteins, thereby preventing cell death and prolonging cell
survival. Recent studies have also implicated the use of a
selective inhibitor for phosphatidylinositol 3-kinases (PI3K)/AKT
as treatment for patients with non-small cell lung cancer,
hematologic malignancies and HNSCC (23–25).
We observed that Nox1 knockdown attenuated the
phosphorylation level of AKT, raising the possibility that
inhibition of Nox1 activity may suppress cell survival by
inhibiting the AKT signaling pathway. Kozaki et al reported
that a missense mutation of PIK3CA, which encodes the
110-kDa catalytic subunit of PI3K, was detected in OSCC cell lines,
including HSC-2 and HSC-3, as well as OSCC patients tumors
(26). In the present study,
Nox1 knockdown significantly attenuated the phosphorylation
of AKT and reduced cell viability in the OSCC cells. This suggested
that inhibition of Nox1 potentially suppresses activation of AKT,
thereby promoting apoptosis in OSCC cells harboring a mutation of
the PIK3CA gene.
In conclusion, we demonstrated that Nox1 likely
contributes to cell survival through its anti-apoptotic effects in
a significant subset of OSCC cells. Based on our experimental data,
we hypothesized that Nox1 contributes to cell survival through the
AKT signaling pathway without initiating ROS generation. It would
be of particular interest to investigate the molecular mechanism by
which Nox1 mediates the AKT signaling pathway in OSCC cells.
Although we only performed in vitro experiments using a
subset of OSCC cell lines in the present study, our novel finding
that Nox1 knockdown enhanced cisplatin-induced cytotoxic
effects provides an attractive option for the combined therapy of
Nox1 knockdown and cisplatin chemotherapy in OSCC treatment.
Additional studies such as in vivo experiments and/or Nox1
expression status in patients with OSCC are warranted to further
understand the molecular mechanisms underlying the pathogenesis
related to cell survival in OSCC and the associated clinical
applications.
Abbreviations:
|
AxV
|
Annexin V
|
|
CDDP
|
cisplatin
|
|
DCFH-DA
|
2′,7′-dichlorodihydrofluorescein
diacetate
|
|
DPI
|
diphenyleneiodonium
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
NAC
|
N-acetylcysteine
|
|
Nox
|
NADPH oxidase
|
|
OSCC
|
oral squamous cell carcinoma
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
PI
|
propidium iodide
|
|
PIK3CA
|
phosphatidylinositol-4,5-bisphosphate
3-kinase, catalytic subunit α
|
|
PDTC
|
pyrrolidine dithiocarbamate
|
|
ROS
|
reactive oxygen species
|
Acknowledgments
The present study was partly supported by a grant
from the Strategic Research Foundation Grant-aided Project for
Private Universities from the Ministry of Education, Culture,
Sports, Science and Technology, Japan (MEXT) (S1101027 to S. K., H.
K. and Y. H.).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sklenicka S, Gardiner S, Dierks EJ, Potter
BE and Bell RB: Survival analysis and risk factors for recurrence
in oral squamous cell carcinoma: Does surgical salvage affect
outcome? J Oral Maxillofac Surg. 68:1270–1275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dissanayaka WL, Pitiyage G, Kumarasiri PV,
Liyanage RL, Dias KD and Tilakaratne WM: Clinical and
histopathologic parameters in survival of oral squamous cell
carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 113:518–525.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen YK, Huang HC, Lin LM and Lin CC:
Primary oral squamous cell carcinoma: An analysis of 703 cases in
southern Taiwan. Oral Oncol. 35:173–179. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fioretti F, Bosetti C, Tavani A,
Franceschi S and La Vecchia C: Risk factors for oral and pharyngeal
cancer in never smokers. Oral Oncol. 35:375–378. 1999. View Article : Google Scholar
|
|
6
|
Altieri A, Bosetti C, Gallus S, Franceschi
S, Dal Maso L, Talamini R, Levi F, Negri E, Rodriguez T and La
Vecchia C: Wine, beer and spirits and risk of oral and pharyngeal
cancer: A case-control study from Italy and Switzerland. Oral
Oncol. 40:904–909. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kolanjiappan K, Ramachandran CR and
Manoharan S: Biochemical changes in tumor tissues of oral cancer
patients. Clin Biochem. 36:61–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaur J, Politis C and Jacobs R: Salivary
8-hydroxy-2-deoxyguanosine, malondialdehyde, vitamin C, and vitamin
E in oral pre-cancer and cancer: diagnostic value and free radical
mechanism of action. Clin Oral Investig. 20:315–319. 2016.
View Article : Google Scholar
|
|
9
|
Block K and Gorin Y: Aiding and abetting
roles of NOX oxidases in cellular transformation. Nat Rev Cancer.
12:627–637. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weyemi U, Redon CE, Parekh PR, Dupuy C and
Bonner WM: NADPH oxidases NOXs and DUOXs as putative targets for
cancer therapy. Anticancer Agents Med Chem. 13:502–514. 2013.
|
|
11
|
Sancho P and Fabregat I: NADPH oxidase
NOX1 controls autocrine growth of liver tumor cells through
up-regulation of the epidermal growth factor receptor pathway. J
Biol Chem. 285:24815–24824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Momose F, Araida T, Negishi A, Ichijo H,
Shioda S and Sasaki S: Variant sublines with different metastatic
potentials selected in nude mice from human oral squamous cell
carcinomas. J Oral Pathol Med. 18:391–395. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakaoka T, Ota A, Ono T, Karnan S, Konishi
H, Furuhashi A, Ohmura Y, Yamada Y, Nakaoka T, Ota A, Ono T, Karnan
S, Konishi H, Furuhashi A, Ohmura Y, Yamada Y, Hosokawa Y and
Kazaoka Y: Combined arsenic trioxide-cisplatin treatment enhances
apoptosis in oral squamous cell carcinoma cells. Cell Oncol.
37:119–129. 2014. View Article : Google Scholar
|
|
14
|
Hossain E, Ota A, Karnan S, Damdindorj L,
Takahashi M, Konishi Y, Konishi H and Hosokawa Y: Arsenic augments
the uptake of oxidized LDL by upregulating the expression of
lectin-like oxidized LDL receptor in mouse aortic endothelial
cells. Toxicol Appl Pharmacol. 273:651–658. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takahashi M, Ota A, Karnan S, Hossain E,
Konishi Y, Damdindorj L, Konishi H, Yokochi T, Nitta M and Hosokawa
Y: Arsenic trioxide prevents nitric oxide production in
lipopolysac-charide -stimulated RAW 264.7 by inhibiting a
TRIF-dependent pathway. Cancer Sci. 104:165–170. 2013. View Article : Google Scholar
|
|
16
|
Ralph SJ, Rodríguez-Enríquez S, Neuzil J,
Saavedra E and Moreno-Sánchez R: The causes of cancer revisited:
'mitochondrial malignancy' and ROS-induced oncogenic transformation
- why mitochondria are targets for cancer therapy. Mol Aspects Med.
31:145–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mochizuki T, Furuta S, Mitsushita J, Shang
WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayama J, Konagai A,
et al: Inhibition of NADPH oxidase 4 activates apoptosis via the
AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic
cancer PANC-1 cells. Oncogene. 25:3699–3707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanaka M, Miura Y, Numanami H, Karnan S,
Ota A, Konishi H, Hosokawa Y and Hanyuda M: Inhibition of NADPH
oxidase 4 induces apoptosis in malignant mesothelioma: Role of
reactive oxygen species. Oncol Rep. 34:1726–1732. 2015.PubMed/NCBI
|
|
19
|
Zhang B, Liu Z and Hu X: Inhibiting cancer
metastasis via targeting NAPDH oxidase 4. Biochem Pharmacol.
86:253–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Puca R, Nardinocchi L, Starace G, Rechavi
G, Sacchi A, Givol D and D'Orazi G: Nox1 is involved in p53
deacetylation and suppression of its transcriptional activity and
apoptosis. Free Radic Biol Med. 48:1338–1346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fletcher EV, Love-Homan L, Sobhakumari A,
Feddersen CR, Koch AT, Goel A and Simons AL: EGFR inhibition
induces proinflammatory cytokines via NOX4 in HNSCC. Mol Cancer
Res. 11:1574–1584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Orcutt KP, Parsons AD, Sibenaller ZA,
Scarbrough PM, Zhu Y, Sobhakumari A, Wilke WW, Kalen AL, Goswami P,
Miller FJ Jr, et al: Erlotinib-mediated inhibition of EGFR
signaling induces metabolic oxidative stress through NOX4. Cancer
Res. 71:3932–3940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fumarola C, Bonelli MA, Petronini PG and
Alfieri RR: Targeting PI3K/AKT/mTOR pathway in non small cell lung
cancer. Biochem Pharmacol. 90:197–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barrett D, Brown VI, Grupp SA and Teachey
DT: Targeting the PI3K/AKT/mTOR signaling axis in children with
hematologic malignancies. Paediatr Drugs. 14:299–316.
2012.PubMed/NCBI
|
|
25
|
Simpson DR, Mell LK and Cohen EE:
Targeting the PI3K/AKT/mTOR pathway in squamous cell carcinoma of
the head and neck. Oral Oncol. 51:291–298. 2015. View Article : Google Scholar
|
|
26
|
Kozaki K, Imoto I, Pimkhaokham A, Hasegawa
S, Tsuda H, Omura K and Inazawa J: PIK3CA mutation is an oncogenic
aberration at advanced stages of oral squamous cell carcinoma.
Cancer Sci. 97:1351–1358. 2006. View Article : Google Scholar : PubMed/NCBI
|