Introduction
Oncogene activation plays a critical role in the
development of human malignancies. Chromosome 20q13.2 shows
frequent aberrations in various cancers, and an increased copy
number in this region has been observed in breast cancer (1). The results from cytological studies
have identified several putative oncogenes that reside within a
~2-Mb region associated with recurrent amplifications at 20q13.2 in
12% of primary breast tumors (2,3). In
addition, high-level amplification of 20q13.2 significantly
associates with the histological grade of breast cancers and
shorter disease-free survival (4).
Mapping of the 20q13 amplicon found in breast cancers by
high-resolution analysis of comparative genomic-hybridization
arrays identified CYP24A1 as the driver for 20q13
amplification, suggesting that CYP24A1 is a potential
oncogene (1,5).
The vitamin D 24-hydroxylase CYP24A1 functions
specifically in the metabolic inactivation of
1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3), the
hormonally active form of vitamin D. The
1α,25(OH)2D3 form of vitamin D serves as a
ligand for the vitamin D receptor and maintains tissue homeostasis
by regulating the expression of genes affecting cell proliferation,
differentiation, and apoptosis (6).
Vitamin D bioavailability, which is regulated by a coordinated
balance between 1α,25(OH)2D3 biosynthesis and
catabolism, determines cellular responses to vitamin D. CYP24A1
expression restricts the access of
1α,25(OH)2D3 to the transcriptional machinery
by converting 1α,25(OH)2D3 to rapidly
excreted inactive derivatives, which limits vitamin D signaling
within cells. Accumulating evidence has shown that a wide spectrum
of vitamin D metabolites, such as
1α,24,25-(OH)3D3 and
24-oxo-1α,25-(OH)2D3, are detectable in
different types of tumor cells, and CYP24A1 expression is elevated
in various cancers and correlates both with dedifferentiation and
poor prognosis (7–9).
The relationship between cellular vitamin D
bioavailability and tumorigenesis is compelling, and the existing
knowledge regarding dysregulated CYP24A1 expression supports
its candidacy as a putative oncogene. Indeed, previous data
revealed that the knockdown of CYP24A1 in MDA-MB-231 cells
by antisense oligonucleotides resulted in reduced proliferation and
inhibited colony formation (10).
However, the underlying mechanism whereby vitamin D depletion
through cellular metabolic processes contributes to increased
susceptibility to carcinogenic insults remains to be defined. Here,
we investigated whether the manipulation of CYP24A1
expression modulates the tumorigenicity of breast carcinoma cells.
The data suggest that CYP24A1-mediated vitamin D metabolism
promotes cell survival and breast cancer growth.
Materials and methods
High-performance liquid chromatography
(HPLC)
To examine the metabolic activity of CYP24A1 in the
event of CYP24A1 manipulation, normal phase HPLC analysis
was performed using 3H-labelled
1α,25(OH)2D3 on an Alliance 2695 separation
module, equipped with a 996 photodiode array detector (Waters,
Milford, MA, USA), as described elsewhere in detail (11).
Cell culture and transfection
The breast cancer cell lines MCF7 and MDA-MB-231
were obtained from a local distributor (Summit Pharmaceuticals
International, Tokyo, Japan) of the American Type Culture
Collection (Manassas, VA, USA). MCF7 cells are estrogen receptor
(ER)-positive cells, and MDA-MB-231 cells are ER-negative cells.
All cells were maintained in Dulbecco's modified Eagle's medium
(Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine
serum (FBS) (Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin,
and 100 µg/ml streptomycin (Sigma).
Cells were transfected with different types of
CYP24A1-specific small-hairpin RNA (shRNA)-expressing
lentivirus plasmids (Sigma) using FuGENE6 (Roche, Basel,
Switzerland) to generate stable transfectants. Transfected clones
were selected in 0.6 µg/ml puromycin (Sigma). Drug-resistant
clones were picked after more than 14 days of selection and
screened for CYP24A1 expression. We obtained the following
MDA-MB-231 cell transfectants: CYP24A1 shRNA #1296 (harboring shRNA
clone NM_000782.2-1296s1c1) and CYP24A1 shRNA #1016 and CYP24A1
shRNA #1016S (harboring shRNA clone NM_000782.2-1016s1c1).
CYP24A1-shRNA #1016S is a transfectant originating from a single
clone that showed efficient suppression of constitutive
CYP24A1. A negative-control shRNA was used as a control.
Cells were also transfected with CYP24A1-specific
small-interfering RNAs (siRNAs; Santa Cruz Biotechnology, Santa
Cruz, CA, USA), or a plasmid containing the full-length
CYP24A1 cDNA (OriGene, Rockville, MD, USA).
Semiquantitative reverse
transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted using the TRIzol reagent
(Invitrogen), and subsequent RT-PCR was performed using the
Superscript II Reverse Transcriptase kit (Invitrogen). Samples were
incubated at 42°C for 50 min, after which the reactions were
terminated by incubation at 70°C for 15 min. Then, the cDNA was
incubated with 0.5 U of Taq DNA polymerase (Takara, Shiga, Japan)
and appropriate primers to amplify the genes of interest. The
cycling conditions were as follows: 20–40 cycles of 30 sec at 96°C,
30 sec at 58°C, and 1 min at 72°C, followed by a final elongation
time of 7 min at 72°C. The sequences of the PCR primers used are
available upon request. The expression of each gene of interest was
analyzed using cycling parameters that were optimized previously
for detection linearity, allowing for semiquantitative analysis of
signal intensities. PCR experiments were repeated in 3 independent
experiments to ensure that the quantified expression was
reproducible. Densitometric analyses of gel bands were performed
using ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Microarray analysis
Gene-expression changes caused by CYP24A1
suppression were examined by microarray analysis. Total RNA was
analyzed using the Agilent Whole Human Genome Oligo Microarray
(4×44K; Agilent Technologies, Santa Clara, CA, USA).
Gene-expression analysis was outsourced to Bio Matrix Research
(Chiba, Japan). Briefly, total RNA (100 ng) was converted into
cDNA, and complimentary RNA (cRNA) was synthetized by in
vitro transcription and subsequently labeled with cyanine 3-CTP
and cyanine 5-CTP, using the Low Input Quick Amp Labeling kit.
Labeled cRNA was hybridized with a microarray chip for 17 h using
the Gene Expression Hybridization kit, and slides were scanned
using an Agilent Microarray Scanner and Feature Extraction Software
9.5, with default settings. Raw data were normalized using the
Quantile algorithm of the Gene Spring Software package, version
11.0 (all from Agilent).
Western blot analysis
Aliquots of whole cell lysates (20 µg) were
separated on 12% sodium dodecyl sulfate-polyacrylamide gels and
electroblotted onto nitrocellulose membranes. Membranes were then
immunoblotted with antibodies against CYP24A1 (sc-32166; 1:50,
Santa Cruz Biotechnology), the cleaved form of caspase-3
(sc-22171-R; 1:75, Santa Cruz Biotechnology), Pdlim2 (SAB1407872;
1:100, Sigma), and β-actin (A5316; 1:1,000, Sigma). The membranes
were incubated with appropriate peroxidase-labeled secondary
antibodies (1:2,000, Dako, Glostrup, Denmark), and bands were
visualized using enhanced chemiluminescence (GE Healthcare,
Buckingham, UK).
Apoptosis and anoikis induction
Oxidative stress-induced apoptosis was stimulated by
incubating cells in 0-100 µM H2O2 for
24 h. Anoikis was induced by adding MDA-MB-231 cells to
agarose-coated dishes to avoid cell attachment in the presence or
absence of 100 nM 1α,25(OH)2D3 (Sigma).
Deoxynucleotidyl transferase-mediated
nick end labeling (TUNEL) and cell-proliferation assays
Apoptosis was assessed in cells cultured on
collagen-coated glass coverslips by performing TUNEL assays. In
addition, tumor tissues developed in mice were fixed in 70%
methanol and studied in TUNEL assays. Apoptotic cells were
visualized using an In Situ Cell Death Detection kit (11684817910;
M7240, Roche). The procedure was also performed without terminal
deoxynucleotidyl transferase, as a negative control. To examine the
cell-proliferation rate, cells were manually counted every 24 h up
through day 7 after an equal number of cells had been plated. In
addition, cellular DNA synthesis was assessed by
immunohistochemical labeling of Ki-67 (MIB-1 clone; 1:100, Dako).
The cells of interest were counted under a light microscope under
low magnification (×100) in 10 randomly selected fields in each
section. The results were confirmed in triplicate independent
analyses.
Colony-forming assays
Soft agar assays were performed in 6-cm dishes to
assess colony formation in 3 dimensions. Cells (2.5×103)
were uniformly suspended in 6 ml of 0.33% agarose gel with growth
medium supplemented with 5% FBS and then overlaid onto the base
layer of 1% agarose gel. Plates were incubated for at least 3
weeks, and cell clusters approximately >50 µm in diameter
were defined as positive. Colonies developing on cell culture
dishes in soft agar suspensions were counted using phase-contrast
microscopy under low magnification (×100) in 10 separate random
fields in each plate. For quantitative analysis, the number of
colonies formed by control cells was defined as 100%.
Tumor growth in vivo
We injected cells of parental MDA-MB-231 and its
transfectants (2×105 cells in 50 µl/mouse) into
the mammary fat pads of 6 or 7-week-old female athymic nude mice
(Charles River Japan, Yokohama, Japan). Animals were euthanized
when skin ulceration of the primary tumor occurred. Tumor volumes
were calculated in 2 dimensions in a time-dependent manner, after
the inoculation of cells in independent duplicate experiments. The
volume (V) of the primary tumors was calculated by the following
equation: V = (π/6) × (L × W2), where L is the length
representing the largest tumor diameter and W is the perpendicular
width of tumors. We examined primary tumors macroscopically for the
formation of tumors, and then the tumors were subjected to
histological evaluations. The maintenance and handling of animals
were conducted using protocols approved by the Animal Care
Committee of Kochi University School of Medicine.
Statistical analysis
Unless otherwise specified, all data are expressed
as means ± standard deviations from at least 3 independent
experiments, each performed in triplicate wells. Statistical
differences were analyzed using Student's t-test. A P-value of
<0.05 was considered statistically significant.
Results
Establishment of CYP24A1-silenced
cells
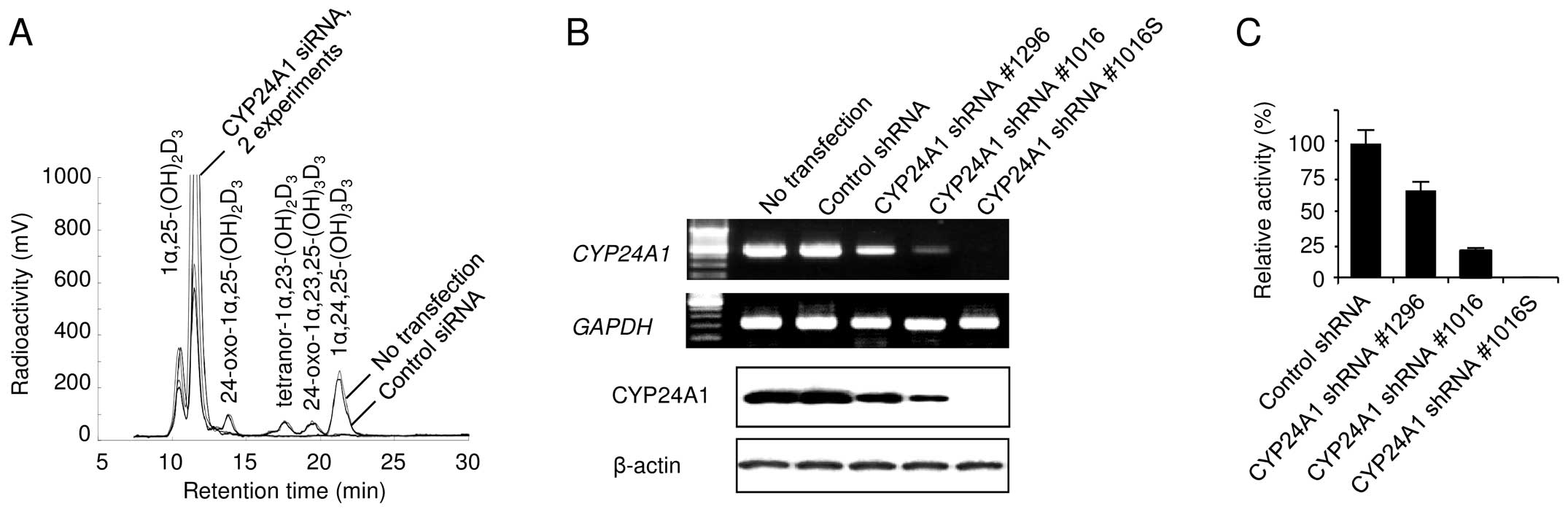
We transfected MDA-MB-231 cells with siRNAs against
CYP24A1 to examine the suppressive effect of CYP24A1
silencing on vitamin D metabolism by HPLC analysis (Fig. 1A). We also transfected different
types of CYP24A1-specific shRNAs into MDA-MB-231 cells to
establish several different stable transfectants with various
CYP24A1 expression levels. Although our preliminary observations
revealed that the phenotypes of at least 3 independent clones were
similar, the cell populations were mixed to establish a stably
transfected cell line to avoid possible clonal variation within the
clonal transfectants. Finally, we obtained pools of transfectants,
designated as CYP24A1 shRNA #1296 and CYP24A1 shRNA #1016. To
select for clones with more specific phenotypes, we generated a
clonal transfectant designated as CYP24A1 shRNA #1016S, which was
isolated following independent transfections and subsequent
screening for low CYP24A1 expression. CYP24A1
expression was lower in the shRNA transfectants, compared to the
constitutive CYP24A1 expression observed in control cells
(Fig. 1B). CYP24A1
expression was significantly lower in CYP24A1 shRNA #1016 cells
than in CYP24A1 shRNA #1296 cells. A striking effect of
CYP24A1-specific shRNA was observed in CYP24A1 shRNA #1016S
cells, which showed no detectable CYP24A1 protein expression. In
addition, the CYP24A1 expression level was closely correlated with
the metabolism of 1α,25(OH)2D3 in each
transfectant (Fig. 1C).
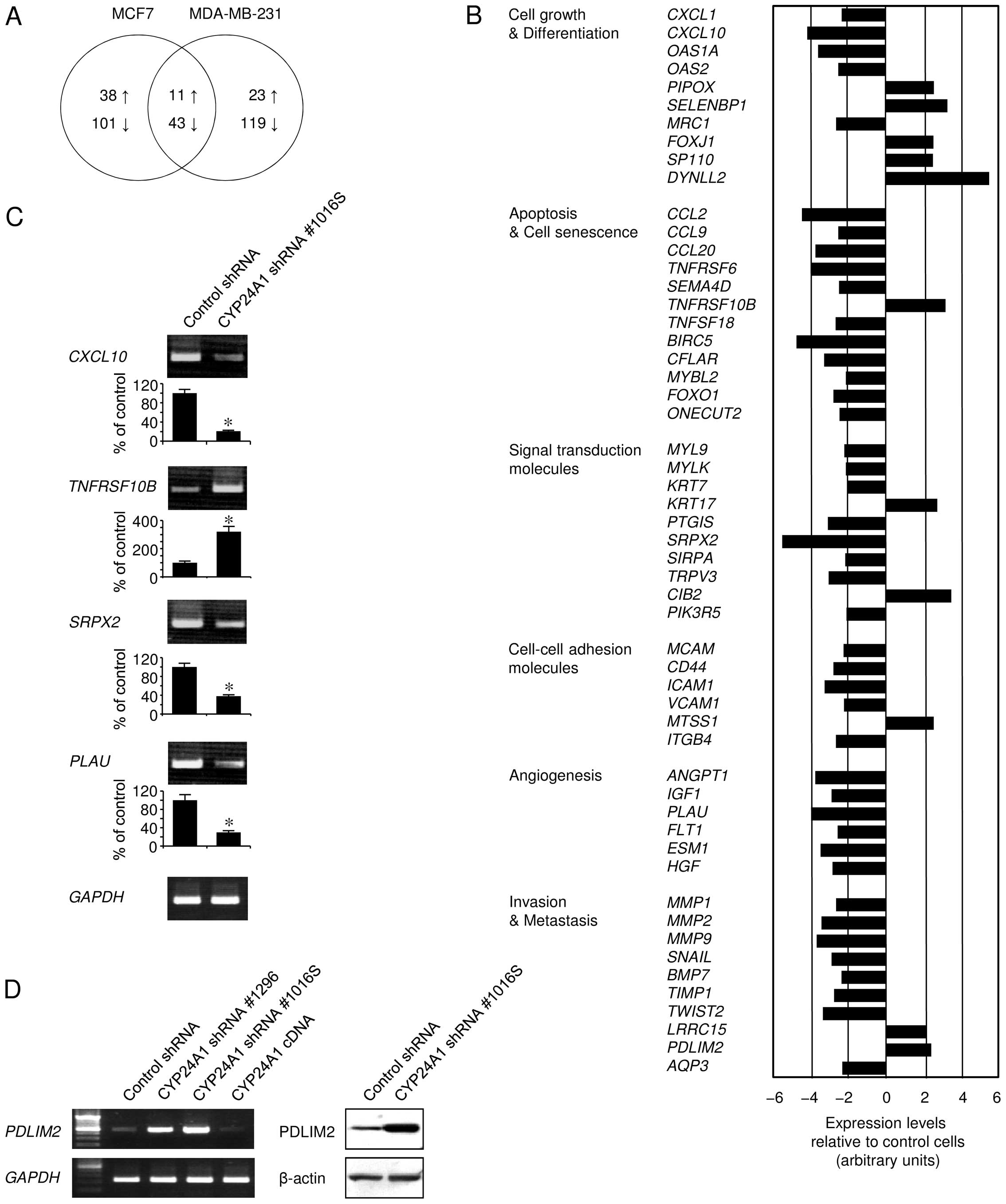
CYP24A1 suppression alters the
gene-expression profile
We examined alterations in the gene-expression
profile following CYP24A1 suppression, using microarray
analysis to test the possibility that mRNAs induced or suppressed
by CYP24A1 silencing were responsible for the tumor
pathology (Fig. 2). Breast tumors
expressing hormone receptors, such as ER, generally display a less
aggressive phenotype and are associated with prolonged disease-free
and overall survival. To exclude the possibility that the hormone
receptor status might affect the tumor pathology, we examined
gene-expression alterations following shRNA-mediated stable
CYP24A1 silencing, both in ER-positive MCF7 cells and
ER-negative MDA-MB-231 cells, although these cell lines show
different oncogenic behaviors. We found that the changes in
gene-expression patterns between these cell types were different,
but at least shared some similarities (Fig. 2A). CYP24A1 silencing affected
the expression of an extensive range of oncogenic pathway members,
including oncogenes, tumor-suppressor genes, and components of the
apoptosis machinery (Fig. 2B and
C). We also observed altered signaling in pathways mediating
cell growth and differentiation, as well as with a variety of
signal-transduction molecules. As evidenced by the altered
expression of cell-adhesion molecules, CYP24A1 suppression
may affect the tumor microenvironment and tumor cell-interstitial
cell interactions. Signaling regulating angiogenesis was also
modulated by silencing CYP24A1 expression. Further, our data
provided evidence of the possible association of CYP24A1
downregulation with gene families involved in tumor cell invasion
and metastasis, such as different types of matrix
metalloproteinases. PDLIM2 expression was also associated
with CYP24A1 expression (Fig.
2D), which is consistent with previous data showing that
PDLIM2 expression is driven by vitamin D (12). The alteration of gene expression
observed suggested that many of the genes involved in
cancer-related pathways are modulated to favor cell survival when
CYP24A1 is constitutively expressed.
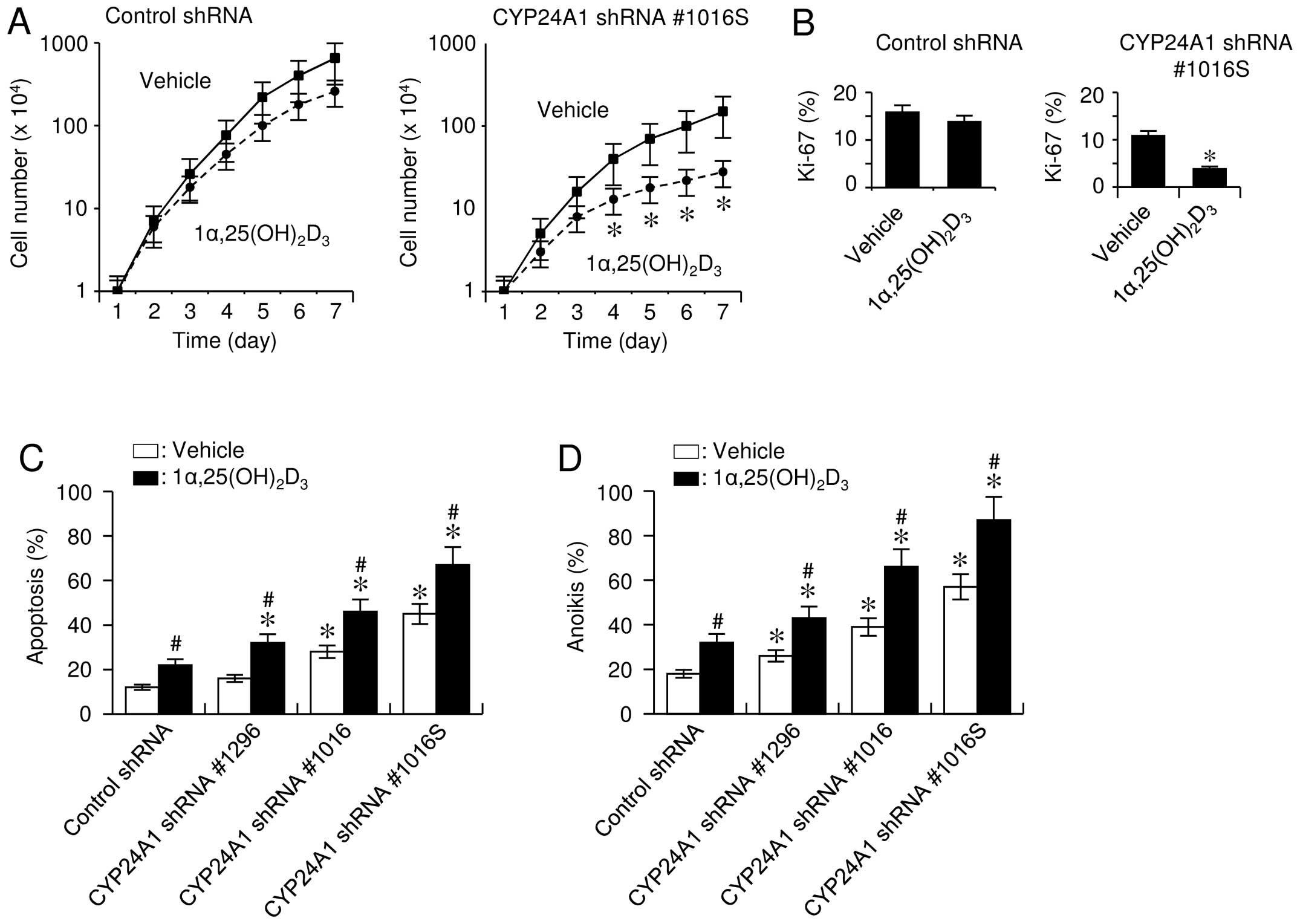
CYP24A1 suppression inhibits cell growth
and provokes apoptosis
To investigate the effect of CYP24A1
signaling on cell proliferation, cell numbers were analyzed in a
time-dependent manner. Manual cell counting revealed an
appreciable, but not significant reduction in cell growth,
regardless of the presence of 1α,25(OH)2D3.
However, CYP24A1-silenced cells that were exposed to
1α,25(OH)2D3 exhibited significantly
decreased cell growth (Fig. 3A) and
DNA-synthesis rates (Fig. 3B).
We also examined the effect of altered
CYP24A1 expression on apoptosis because
1α,25(OH)2D3 is known to exert pro-apoptotic
effects in cells (6). When cells
were incubated under oxidative stress caused by
H2O2, TUNEL assay results consistently
revealed that 1α,25(OH)2D3 treatment enhanced
the sensitivity of cells to H2O2-induced cell
death, and CYP24A1 suppression conferred a significant
increase in sensitivity to apoptosis (Fig. 3C). The CYP24A1 expression
level inversely correlated with the apoptosis-sensitizing effect
caused by 1α,25(OH)2D3, suggesting that the
catabolic activity of the enzyme was important for apoptosis
sensitivity. In addition, cells with downregulated CYP24A1
expression gained increased sensitivity to anoikis, and
1α,25(OH)2D3 enhanced this effect (Fig. 3D).
Although we observed that the basal levels of cell
growth and apoptosis differed between the control- and
CYP24A1-shRNA transfectants, there was evidence that normal
FBS contained active concentrations of various growth and
differentiation factors, including
1α,25(OH)2D3, which can modulate the
transcriptional machinery driven by these biologically relevant
factors (13). This finding was
also explained by the observation that basal levels of cell growth
and apoptosis were unchanged following CYP24A1 suppression
in media supplemented with charcoal-dextran-treated FBS (data not
shown). These data indicated that the expression level of
CYP24A1 contributes to determine the sensitivity of cells to
apoptosis.
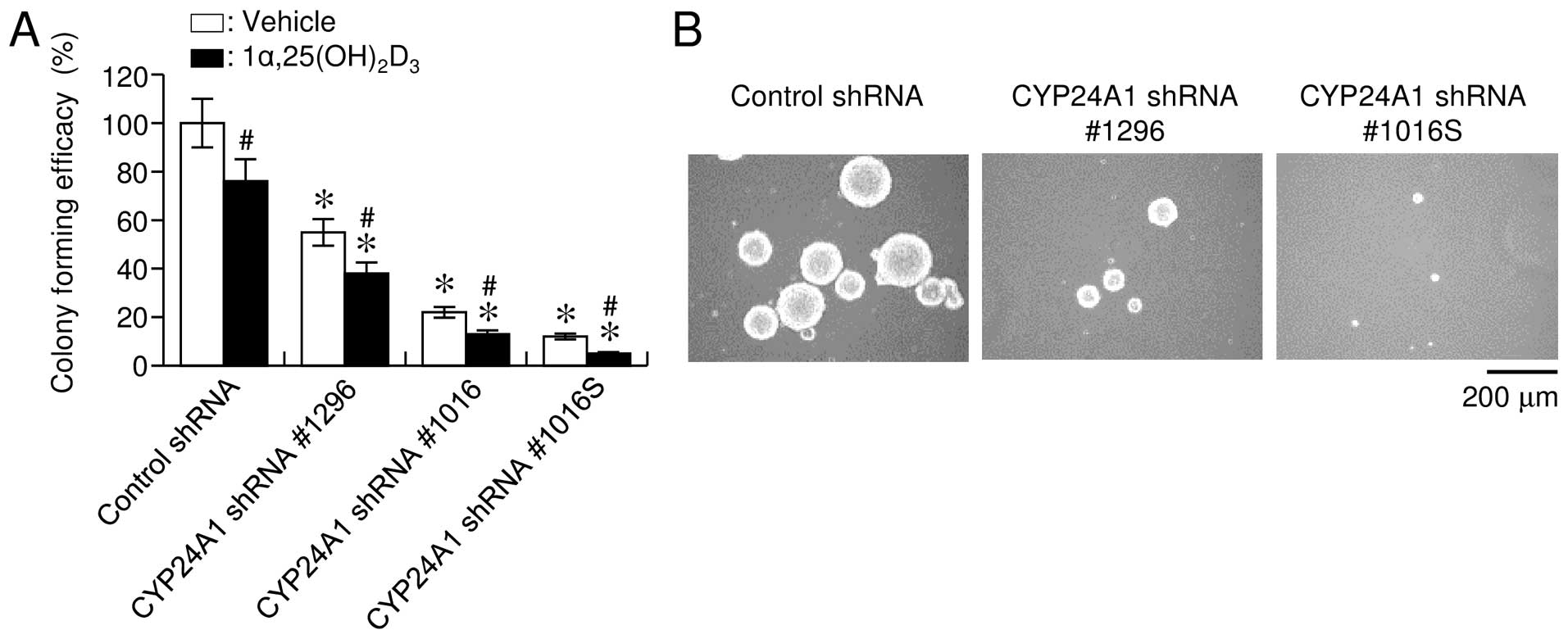
CYP24A1 suppression inhibits tumorigenic
potency in vitro
Consistent with the apoptosis-sensitizing effect of
CYP24A1 inhibition, CYP24A1 downregulation
significantly suppressed colony formation in soft agar, as
evidenced by a decreased number and size of colonies (Fig. 4A and B). Treatment with
1α,25(OH)2D3 inhibited anchorage-independent
growth, and this effect was more evident following CYP24A1
suppression (Fig. 4A). Our
observations suggested that the expression level of CYP24A1
was significantly associated with the colony-formation capability
of cells, supporting that CYP24A1 expression had a
stimulatory oncogenic effect on breast carcinoma cells.
CYP24A1 suppression abrogates
tumorigenicity in vivo
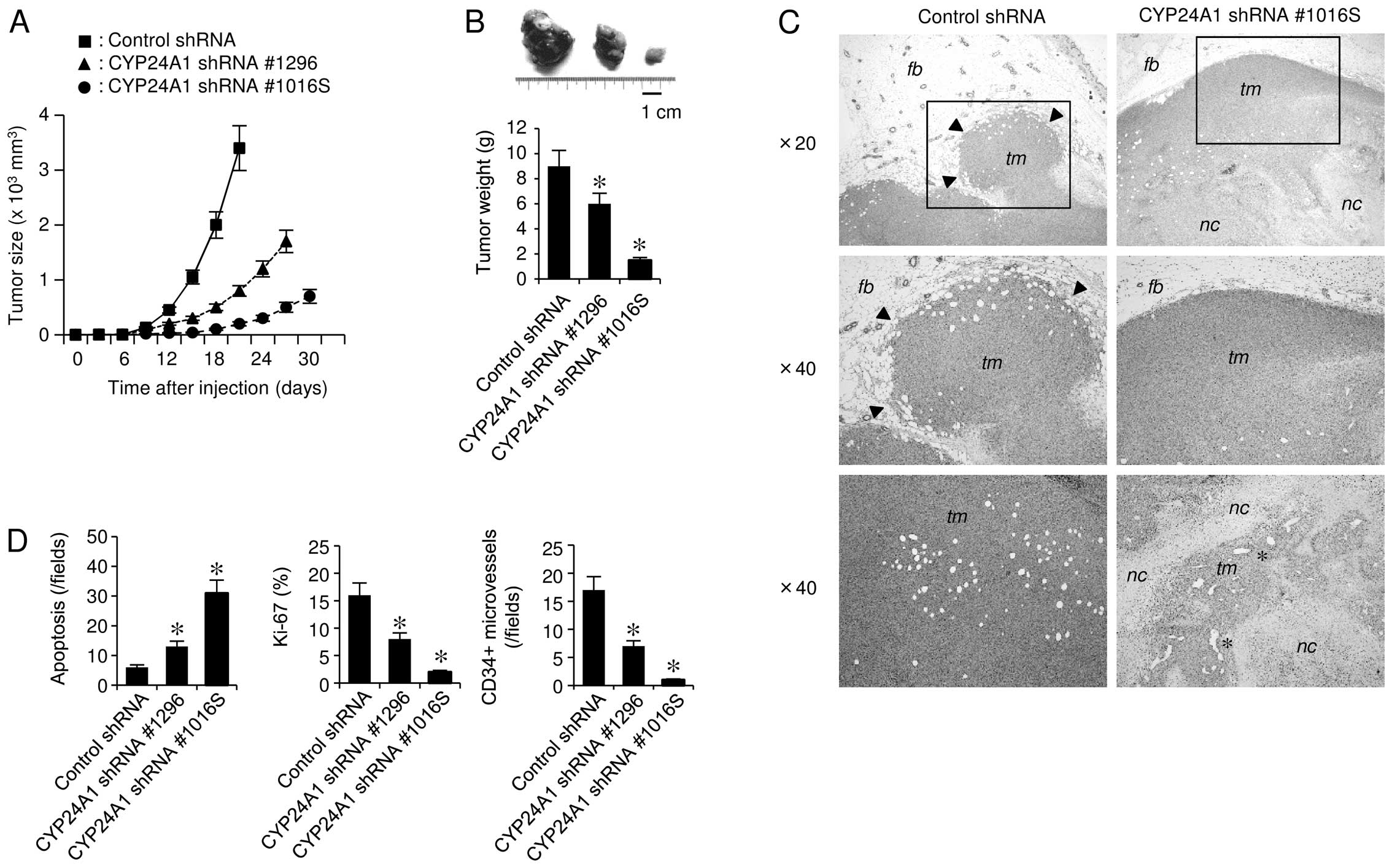
To address whether CYP24A1 affects
tumorigenicity in vivo, we employed an
orthotopic-transplantation mouse model. CYP24A1 suppression
inhibited tumorigenicity in vivo, showing a markedly
decelerated rate of tumor growth (Fig.
5A and B). In addition, the cells with lower metabolic activity
showed greater retardation of tumor formation in mammary fat pads.
CYP24A1-expressing tumors showed high-grade malignant
characteristics, such as invasive growth into the surrounding
tissue (Fig. 5C). However,
CYP24A1-suppressed tumors did not show invasiveness, and the tumor
tissues underwent massive necrosis, which was accompanied by
increased apoptosis and reduced mitosis (Fig. 5D). These findings are partially
explained by the ability of CYP24A1 suppression to cause
upregulation of the anti-invasion marker PDLIM2 (Fig. 2D). Histological examinations also
revealed a marked decrease of CD34-positive microvascular
components in tumors developed from CYP24A1-silenced cells
(Fig. 5D). Consistently, cancer
cells with CYP24A1 downregulation showed significantly lower
expression levels of pro-angiogenic genes, such as angiopoietin
(Fig. 2B). This observation was in
good agreement with evidence that vitamin D can inhibit
angiogenesis (6).
Discussion
Much attention has been paid to the roles of
1α,25(OH)2D3 in many cell types because of
its pleiotropic anticancer effects. Here, we unveiled a mechanistic
link between CYP24A1-mediated intracellular vitamin D metabolism
and enhanced tumorigenicity. Our observations are consistent with
accumulating evidence, demonstrating that active vitamin D
metabolism occurred in different types of cancer cells and that
elevated CYP24A1 expression was detectable in various malignancies
(7–9). CYP24A1 expression affected the
expression of a wide variety of signaling molecules associated with
the carcinogenic events, modulating the gene-expression profile in
tumor cells to potentially confer unique cell-survival properties
to the affected cells. These findings are supported by
epidemiological data showing that vitamin D deficiency could
increase the risk for cancer (14,15).
The data indicated that CYP24A1-mediated vitamin D insufficiency
contributed the development of breast neoplasia, implicating
CYP24A1 as a candidate oncogene.
Our unpublished data revealed that CYP24A1
silencing conferred targeted cells with increased susceptibility to
several differently acting apoptogens in various cell types. As a
result, CYP24A1 downregulation suppressed colony formation
in vitro in several breast carcinoma cell lines, regardless
of expression of the ER. In addition, we found that CYP24A1
overexpression could transform NIH/3T3 fibroblasts, causing them to
show enhanced focus formation in confluent cultures, as also
observed following overexpression of the KRAS oncogene in
these cells. However, CYP24A1 overexpression in
non-transformed breast epithelial MCF-10A cells did not cause
anchorage-independent growth in soft agar (data not shown). These
results indicated that the vitamin D metabolic activity promoted by
CYP24A1 stimulated oncogenesis in breast carcinoma cells.
Unexpectedly, we did not observe elevated CYP24A1
expression in primary breast carcinomas by immunohistochemistry,
using different types of tissue microarrays and archived
formalin-fixed, paraffin-embedded tissue specimens, whereas CYP24A1
expression was shown to be elevated in breast neoplasia (16). In addition, we found no significant
increase in the CYP24A1 copy number by fluorescent in
situ hybridization, although high copy-number gain was reported
to be a key determinant of CYP24A1 overexpression (17). We cannot explain these discrepant
observations; however, it is clear that several commercially
available CYP24A1 antibodies and CYP24A1 probes did not
function properly with tissue specimens under our reaction
conditions. Alternatively, the possibility remains that breast
cancers do not have increased CYP24A1 expression.
Informatics data revealed the presence of 2 vitamin
D-responsive elements (VDREs) located immediately upstream of the
CYP24A1 gene. Data from promoter studies have also
demonstrated that multiple VDRE sites and potential Sp1 sites are
synergistically involved in the regulation of CYP24A1
expression (18). As a corollary,
transcriptional complexity should be noted in understanding the
regulatory mechanism of CYP24A1 overexpression during
carcinogenesis (19). Therefore,
future studies are warranted to better understand the regulatory
mechanism of CYP24A1 in neoplasia of the breast.
Our present findings showed that many genes involved
in tumorigenesis were modulated to favor cell survival when CYP24A1
was constitutively expressed. Based on the gain-of-function effect
of CYP24A1 on carcinogenesis, recapitulation of CYP24A1 expression
in cancer cells would provide a selective growth advantage to these
cells, thereby enabling evasion of normal apoptotic mechanisms.
Therefore, strategies that decrease the activity of CYP24A1 may be
an important means of increasing the sensitivity of cancer cells to
pro-apoptotic therapies.
Acknowledgments
This study was supported in part by a grant from the
Grants-in-Aid for Scientific Research program from the Japan
Society for the Promotion of Science.
References
|
1
|
Albertson DG, Ylstra B, Segraves R,
Collins C, Dairkee SH, Kowbel D, Kuo WL, Gray JW and Pinkel D:
Quantitative mapping of amplicon structure by array CGH identifies
CYP24 as a candidate oncogene. Nat Genet. 25:144–146. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kallioniemi A, Kallioniemi OP, Piper J,
Tanner M, Stokke T, Chen L, Smith HS, Pinkel D, Gray JW and Waldman
FM: Detection and mapping of amplified DNA sequences in breast
cancer by comparative genomic hybridization. Proc Natl Acad Sci
USA. 91:2156–2160. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hodgson JG, Chin K, Collins C and Gray JW:
Genome amplification of chromosome 20 in breast cancer. Breast
Cancer Res Treat. 78:337–345. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanner MM, Tirkkonen M, Kallioniemi A,
Holli K, Collins C, Kowbel D, Gray JW, Kallioniemi OP and Isola J:
Amplification of chromosomal region 20q13 in invasive breast
cancer: Prognostic implications. Clin Cancer Res. 1:1455–1461.
1995.PubMed/NCBI
|
|
5
|
Weiss MM, Snijders AM, Kuipers EJ, Ylstra
B, Pinkel D, Meuwissen SG, van Diest PJ, Albertson DG and Meijer
GA: Determination of amplicon boundaries at 20q13.2 in tissue
samples of human gastric adenocarcinomas by high-resolution
microarray comparative genomic hybridization. J Pathol.
200:320–326. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deeb KK, Trump DL and Johnson CS: Vitamin
D signalling pathways in cancer: Potential for anticancer
therapeutics. Nat Rev Cancer. 7:684–700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mimori K, Tanaka Y, Yoshinaga K, Masuda T,
Yamashita K, Okamoto M, Inoue H and Mori M: Clinical significance
of the overexpression of the candidate oncogene CYP24 in esophageal
cancer. Ann Oncol. 15:236–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parise RA, Egorin MJ, Kanterewicz B, Taimi
M, Petkovich M, Lew AM, Chuang SS, Nichols M, El-Hefnawy T and
Hershberger PA: CYP24, the enzyme that catabolizes the
antiproliferative agent vitamin D, is increased in lung cancer. Int
J Cancer. 119:1819–1828. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tannour-Louet M, Lewis SK, Louet JF,
Stewart J, Addai JB, Sahin A, Vangapandu HV, Lewis AL, Dittmar K,
Pautler RG, et al: Increased expression of CYP24A1 correlates with
advanced stages of prostate cancer and can cause resistance to
vitamin D3-based therapies. FASEB J. 28:364–372. 2014.
View Article : Google Scholar
|
|
10
|
Townsend K, Banwell CM, Guy M, Colston KW,
Mansi JL, Stewart PM, Campbell MJ and Hewison M: Autocrine
metabolism of vitamin D in normal and malignant breast tissue. Clin
Cancer Res. 11:3579–3586. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masuda S, Kaufmann M, Byford V, Gao M,
St-Arnaud R, Arabian A, Makin HL, Knutson JC, Strugnell S and Jones
G: Insights into Vitamin D metabolism using cyp24 over-expression
and knockout systems in conjunction with liquid chromatography/mass
spectrometry (LC/MS). J Steroid Biochem Mol Biol. 89–90:149–153.
2004. View Article : Google Scholar
|
|
12
|
Vanoirbeek E, Eelen G, Verlinden L,
Carmeliet G, Mathieu C, Bouillon R, O'Connor R, Xiao G and Verstuyf
A: PDLIM2 expression is driven by vitamin D and is involved in the
proadhesion, and anti-migration and -invasion activity of vitamin
D. Oncogene. 33:1904–1911. 2014. View Article : Google Scholar
|
|
13
|
Cao Z, West C, Norton-Wenzel CS and Rej R,
Davis FB, Davis PJ and Rej R: Effects of resin or charcoal
treatment on fetal bovine serum and bovine calf serum. Endocr Res.
34:101–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schwartz GG and Blot WJ: Vitamin D status
and cancer incidence and mortality: Something new under the sun. J
Natl Cancer Inst. 98:428–430. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spina CS, Tangpricha V, Uskokovic M,
Adorinic L, Maehr H and Holick MF: Vitamin D and cancer. Anticancer
Res. 26A:2515–2524. 2006.
|
|
16
|
Lopes N, Sousa B, Martins D, Gomes M,
Vieira D, Veronese LA, Milanezi F, Paredes J, Costa JL and Schmitt
F: Alterations in Vitamin D signalling and metabolic pathways in
breast cancer progression: A study of VDR, CYP27B1 and CYP24A1
expression in benign and malignant breast lesions. BMC Cancer.
10:4832010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Höbaus J, Hummel DM, Thiem U, Fetahu IS,
Aggarwal A, Müllauer L, Heller G, Egger G, Mesteri I,
Baumgartner-Parzer S, et al: Increased copy-number and not DNA
hypomethylation causes overexpression of the candidate
proto-oncogene CYP24A1 in colorectal cancer. Int J Cancer.
133:1380–1388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tashiro K, Ishii C and Ryoji M: Role of
distal upstream sequence in vitamin D-induced expression of human
CYP24 gene. Biochem Biophys Res Commun. 358:259–265. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pike JW and Meyer MB: Regulation of mouse
Cyp24a1 expression via promoter-proximal and downstream-distal
enhancers highlights new concepts of 1,25-dihydroxyvitamin D(3)
action. Arch Biochem Biophys. 523:2–8. 2012. View Article : Google Scholar
|