Introduction
Mitochondrial serine hydroxylmethyltransferase
(SHMT) is a key enzyme in the serine/glycine pathway. This pathway
involves the conversion of serine to glycine by catalyzing the
transfer of a β-carbon from serine to tetrahydrofolate (THF) and
generating 5,10-methylene-THF and glycine as byproducts. Two
SHMT genes have been identified in the human genome,
SHMT1 and SHMT2. SHMT1 encodes the cytoplasmic
isozyme involved in de novo synthesis of thymidylate
(1). In contrast, SHMT2
encodes the mitochondrial isozyme that participates in the
synthesis of mitochondrial thymidine monophosphate (dTMP) (1,2).
SHMT1 and SHMT2 have important roles in human
biochemical pathways, including the folate cycle, homocysteine
metabolism and nuclear de novo thymidylate biosynthesis
(3). Studies have shown that
SHMT1 and SHMT2 expression is upregulated in cancer.
Specifically, SHMT2 expression is significantly increased in
cancers involving the breast, lung, ovary, prostate and skin
(4–7). Moreover, elevated expression of
SHMT2 has been found to be associated with poor prognosis in
human cancers (8).
Worldwide, breast cancer remains a major cause of
female deaths (9). Breast cancer
can be broadly classified into four major molecular subtypes,
depending on the specific genetic profile (i.e., luminal A, luminal
B, triple-negative/basal-like and HER2 status) (10,11).
Each subtype has unique clinical, histopathological and prognostic
characteristics (3). Luminal A and
luminal B breast cancer have high expression of estrogen receptor
(ER+). HER2-positive and basal-like/triple-negative
breast cancers (TNBCs) (12) are
ER-negative (ER−) and are associated with a poor
prognosis (13). Recent studies
suggest that the 5-year survival rate in patients with ER-negative
breast cancer is 30%, compared with a 90% survival rate for luminal
A patients (14). The
classification of molecular subtypes was used for therapeutic
protocol selection and also for prediction of cancer metastases and
post-relapse survival (15).
Numerous gene signatures have been developed to
predict survival of breast cancer patients. Examples of these
predictors include PI3K signature (16), 21-gene recurrence score (17) and core serum response signature
(CSR) (18). The HER2-derived
prognostic predictor (19) and
7-gene immune response module (20)
have been proposed as means to identify patients with ER-negative
breast cancer. However, these methods are costly and lack specific
targets. Developing more accurate and economical gene signatures
for therapeutic purposes may provide significant benefit to the
medical community.
The objective of the present study was to evaluate
the prognostic and therapeutic value of SHMT2 as a potential
biomarker for breast cancer cases. We compared its performance with
other currently available biomarkers and gene signatures. Five
independent breast cancer microarray data-sets were analyzed using
individual and pooled approaches. We found that SHMT2 had a
prognostic value in a specific subgroup of breast cancer patients.
The prognostic power of SHMT2 mRNA was comparable to other
gene signatures and biomarkers, most notably for patients in the
ER-negative breast cancer subgroup. We also found that SHMT2
had a potential predictive role in stage II breast cancer
treatment.
Materials and methods
Breast cancer tissue samples
We used a retrospective population-based outcome
strategy to analyze 128 breast cancer cases (ZJU set). All patients
underwent modified radical mastectomy at Zhejiang University (ZJU)
Hospital (Hangzhou, China) from January 2002 to December 2006. The
protocol for the use of human tissues was reviewed and approved by
the Institutional Review Board (IRB). All patients provided written
informed consent for the tissue samples to be used for scientific
research. Patients who did not provide informed consent or who had
multiple cancers were excluded from the study. The period of
progression-free survival (PFS) was defined as the time from the
date of the surgery to the date of tumor recurrence (local relapse
or metastasis). Overall survival (OS) time was the time from the
date of the surgery to the date the patient was last examined. Only
breast cancer-related deaths were included as the end of the
survival period. All ZJU set participants were followed up twice
per year, until 30 September 2010. Pathoclinical and demographic
data were collected by reviewing hospital records. The results for
the characteristics of all 128 assessable breast cancer patients
are presented in Table I.
 | Table IDemographic distribution of SHMT2 in
breast cancer (ZJU set) patients. |
Table I
Demographic distribution of SHMT2 in
breast cancer (ZJU set) patients.
| SHMT2
| P-valuesb |
|---|
| High (%a) | Low (%) |
|---|
| Age (years) | | | 0.5442 |
| <50 | 49 (76.6) | 15 (23.4) | |
| ≥50 | 46 (71.9) | 18 (28.1) | |
| Histological
type | | | 0.0582 |
| Ductal
carcinoma | 79 (78.2) | 22 (21.8) | |
| Lobular
carcinoma | 9 (50.0) | 5 (50.0) | |
| Other | 6 (75.0) | 2 (25.0) | |
| pT stagec | | | 0.0203 |
| T0–1 | 21 (60.0) | 14 (40.0) | |
| T2–4 | 71 (80.1) | 17 (19.9) | |
| pN stagec | | | 0.0175 |
| 0 | 39 (63.9) | 22 (36.1) | |
| 1–3 | 42 (80.8) | 10 (19.2) | |
| >3 | 14 (93.3) | 1 (6.7) | |
| Grade | | | 0.0067 |
| 1, Well | 8 (66.7) | 4 (33.3) | |
| 2, Mod | 27 (60.0) | 18 (40.0) | |
| 3, Poor | 47 (87.0) | 7 (13.0) | |
| ER | | | 0.3225 |
| Negative | 36 (78.3) | 10 (21.7) | |
| Positive | 44 (69.8) | 19 (30.2) | |
| PR | | | 0.1004 |
| Negative | 43 (81.1) | 10 (18.9) | |
| Positive | 37 (67.3) | 18 (32.7) | |
| HER2 | | | 0.2964 |
| Negative | 66 (73.3) | 24 (26.7) | |
| Positive | 20 (75.4) | 4 (24.6) | |
| MKI67 | | | 0.0179 |
| Negative | 26 (61.0) | 16 (39.0) | |
| Positive | 56 (82.3) | 12 (17.7) | |
| Molecular
subtype | | | 0.5496 |
| Luminal A | 20 (75.0) | 12 (25.0) | |
| Luminal
B/HER2− | 20 (80.0) | 5 (20.0) | |
| Luminal
B/HER2+ | 6 (75.0) | 2 (25.0) | |
| Basal-like
TNBC | 15 (83.3) | 3 (16.7) | |
| HER2-positive | 10 (83.3) | 2 (16.7) | |
| CD44/CD24
status | | | 0.4380 |
|
CD44+/CD24− | 37 (71.1) | 15 (28.9) | |
| Other | 40 (74.0) | 10 (26.0) | |
Quantitative immunohistochemistry (IHC)
assays
Hematoxylin and eosin staining results for all
tissue blocks were reviewed by a pathologist. Then, the tissue
samples were re-arranged and assembled into multiple tissue arrays
(MTA). Each MTA was stored at room temperature. Protein levels of
genes were stained in tissue sections from the MTAs, based on
standardized deparaffinization and staining protocol (21,22).
For quality control, negative and positive samples were also
included in each IHC staining set.
A commercially available anti-SHMT2 antibody
(ab88664; Abcam) was used for the IHC procedure. SHMT2 specificity
was confirmed using peptide blocking assay. The IHC condition for
SHMT2 was optimized based on serial dilution results. Antibodies
against ER (clone, SP1), PR (clone, PgR636), HER2 (clone, A0485)
and Ki-67 (clone, MIB-1) were purchased from the Dako Company. The
TP53 antibody (clone, DO-7) was purchased from the Vector
Laboratories.
The cut-off values for ER, PR, HER2 and Ki-67
positivity were based on standard, published protocols (23,24).
Each sample was assigned to one of four intrinsic subtypes (i.e.,
luminal A, luminal B, HER2-positive and basal-like/TNBC, subtype),
based on the IHC staining results (11). For the breast cancers, a
CD44+/CD24−/low result indicated the presence
of tumor stem/progenitor cells.
Double-blinding was performed to maintain quality
control of IHC scoring and prevent observer and system bias. Two
observers independently evaluated each IHC staining result.
Discrepancies were peer reviewed by the two readers and an
additional pathologist; missing samples were left blank. Most of
the SHMT2 staining was visible in the cytoplasm and formed
sand-like particles, but some heterogeneous staining was present in
a few of the samples. SHMT2 is a mitochondrial protein. The
immunoreactivity in the mitochondria was scored according to
percentage and intensity of the staining. Stain intensity was
scored as 0 (negative), 1 (weak), 2 (moderate) or 3 (strong). The
morphologic pattern of IHC staining in each cell varied due to
heterogeneity in cancer cells. In our scoring system, only
specimens with at least 10% of the cells with positive staining
were taken into consideration. The highest staining scores were
tallied as the final score. Protein expression level consisted of
four subgroups. These subgroups were negative (−), weakly positive
(+), positive (++) and strongly positive (+++) (Fig. 2A). In the Cox analysis, (−) and (+)
were grouped as SHMT2-low, and (++) and (+++) were grouped
as SHMT2-high.
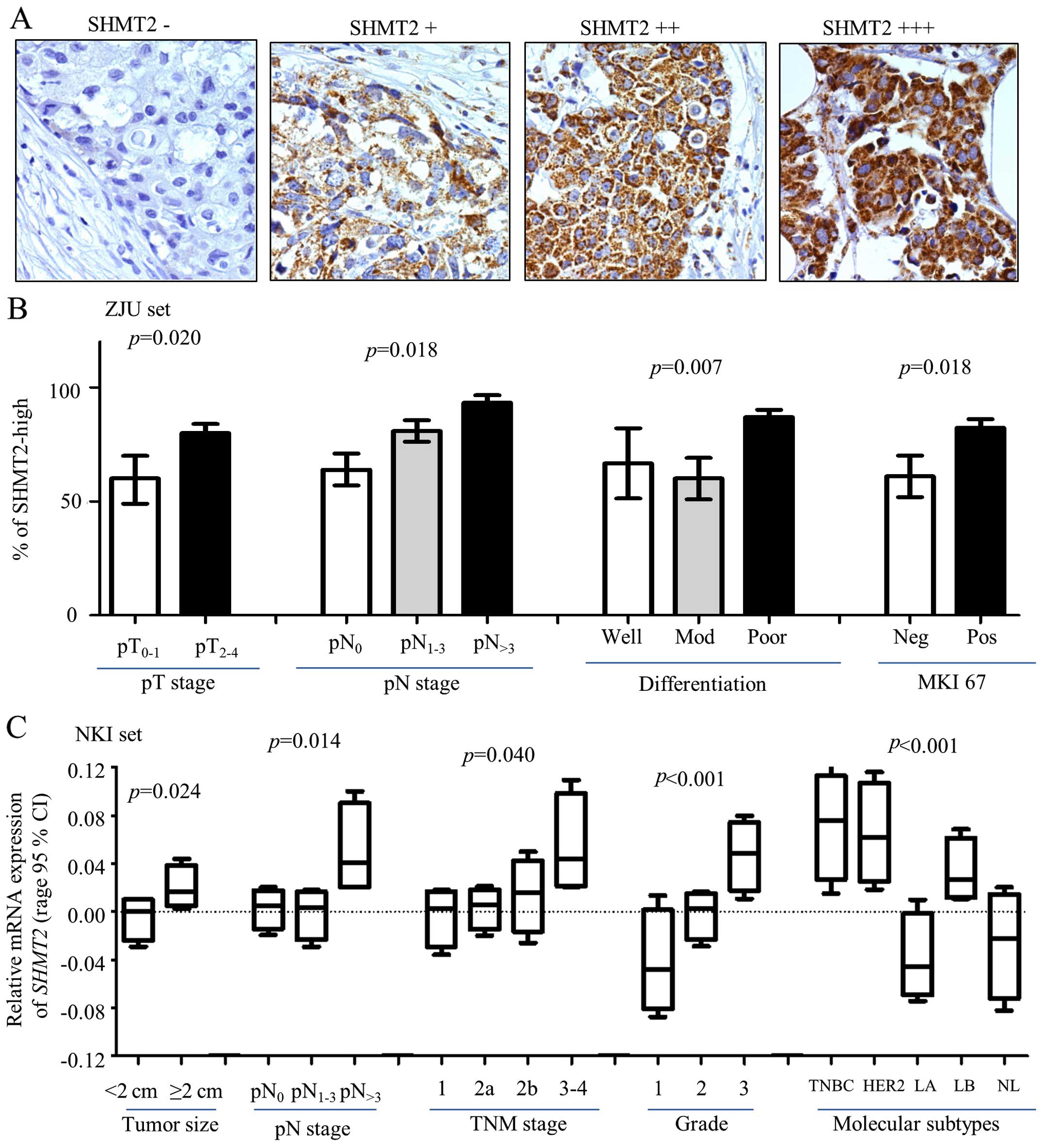 | Figure 2Clinical relevance of SHMT2 in breast
cancer. Protein expression levels of SHMT2 and other biomarkers
were determined using immunohistochemistry (IHC) staining. (A)
Representative IHC images stained with SHMT2 (magnification, ×200).
(B) SHMT2 protein expression levels associated with pathological
tumor and lymph node stage (pT and pN stages), poor differentiation
and MKI-67 in ZJU set. Here, pT is pathological tumor stage; and
the number represent tumor stage. pN is pathological lymph node
stage; the number indicates how many lymph nodes involved. pN>3
is >3 lymph nodes were involved. Mod, moderate differentiation;
Neg, negative; Pos, positive. (C) ANOVA analysis result for SHMT2
mRNA expression levels and tumor size, pN and TNM stages, Elson
grade, and intrinsic molecular subtypes of breast cancer. TNBC,
basal-like breast cancer; HER2, Her2-positive, LA, luminal A; LB,
luminal B; NL, normal breast-like. The p-values represent overall
ANOVA analysis results. |
Public gene mRNA expression microarray
datasets
Published data from human breast tissue gene
expression array results are available from NIH/GEO or www.ebi.ac.uk/arrayexpress (4). Five independent breast cancer
microarray datasets were chosen for the present study: Pawitan
(GSE1456) (25), Ivshina (GSE4922)
(26), Wang (GSE2034) (27), Desmedt (GSE7390) (28) and NKI (29) datasets (Table II). Detailed information concerning
the downloaded datasets is presented in Table II. Three microarray SHMT2
fragments (214096_s_at, 214095_at and 214437_at) were used in
Affymetrix U133 arrays. Since these three fragments yielded
consistent results, we used the 214065_at signal to represent the
expression levels of SHMT2 in the Pawitan, Ivshina, Wang and
Desmedt microarray datasets (using Affymetrix chips). In the NKI
set (using Agilent chip), one probe for SHMT2 was found and
was analyzed.
 | Table IIResults of the overall review of the
ZJU and worldwide microarray datasets. |
Table II
Results of the overall review of the
ZJU and worldwide microarray datasets.
| Dataset | ZJU | Ivshina | Wang | Pawitan | Desmedt | NKIb |
|---|
| No. of
patients | 223 | 249 | 286 | 159 | 198 | 295 |
| Assessible
casesa | 128 | 213 | 253 | 159 | 158 | 295 |
| Date of study | 1999–2006 | 1987–1989 | 1980–1995 | 1994–1996 | 1980–1998 | 1984–1995 |
| Microarray | N/A | Affimetrix | Affimetrix | Affimetrix | Affimetrix | Agilent |
| | HG-U133 | HG-U133 | HG-U133 | HG-U133 | 25K Chip |
| Accession no. | N/A | GSE4922 | GSE2034 | GSE1456 | GSE7390 | N/A |
| SHMT2
fragments | N/A | | | | | |
| 214096_s_at | N/A | Y | Y | Y | Y | N/A |
| 214095_at | N/A | Y | Y | Y | Y | N/A |
| 214437_at | N/A | Y | Y | Y | Y | N/A |
| Age at
diagnosis | Y | Y | N/A | N/A | Y | Y |
| Histological
type | Y | N/A | N/A | N/A | Y | Y |
| Elson grade | Y | Y | N/A | Y | Y | Y |
| Tumor size | Y | Y | N/A | N/A | Y | Y |
| Lymph node | Y | Y | Y | N/A | Y | Y |
| Metastasis | Y | N/A | Y | N/A | Y | Y |
| TNM stage | Y | Y | N/A | N/A | Y | Y |
| ER status | Y | Y | Y | Y | Y | Y |
| PR status | Y | N/A | N/A | N/A | N/A | Y |
| HER2 status | Y | N/A | N/A | Y | N/A | Y |
| MKI67 status | Y | N/A | N/A | N/A | N/A | Y |
| P53 mutation | Y | Y | N/A | N/A | N/A | N |
| Molecular
subtype | Y | N/A | N/A | Y | N/A | Y |
| Chemotherapy | Y | N/A | N/A | N/A | N/A | Y |
| Radiotherapy | Y | N/A | N/A | N/A | N/A | Y |
| Hormone
therapy | Y | N/A | N/A | N/A | N/A | Y |
| OS monthsc (range) | 8.9–104.9 | N/A | N/A | 2.2–101.9 | 4.9–303.6 | 1.0–220.1 |
| PFS monthsd (range) | 2.5–104.9 | 0–153.0 | 2.0–171.0 | 2.2–101.9 | 4–231.4 | 1.0–220.1 |
In the pooled analysis, we normalized the mRNA
expression levels for the datasets. We re-stratified patients into
four categories (Q1, Q2, Q3 and
Q4), based on the percentile of gene expression
(Fig. 3, legend). Stratified
SHMT2 expression level datasets were then pooled into a new
dataset for further analysis.
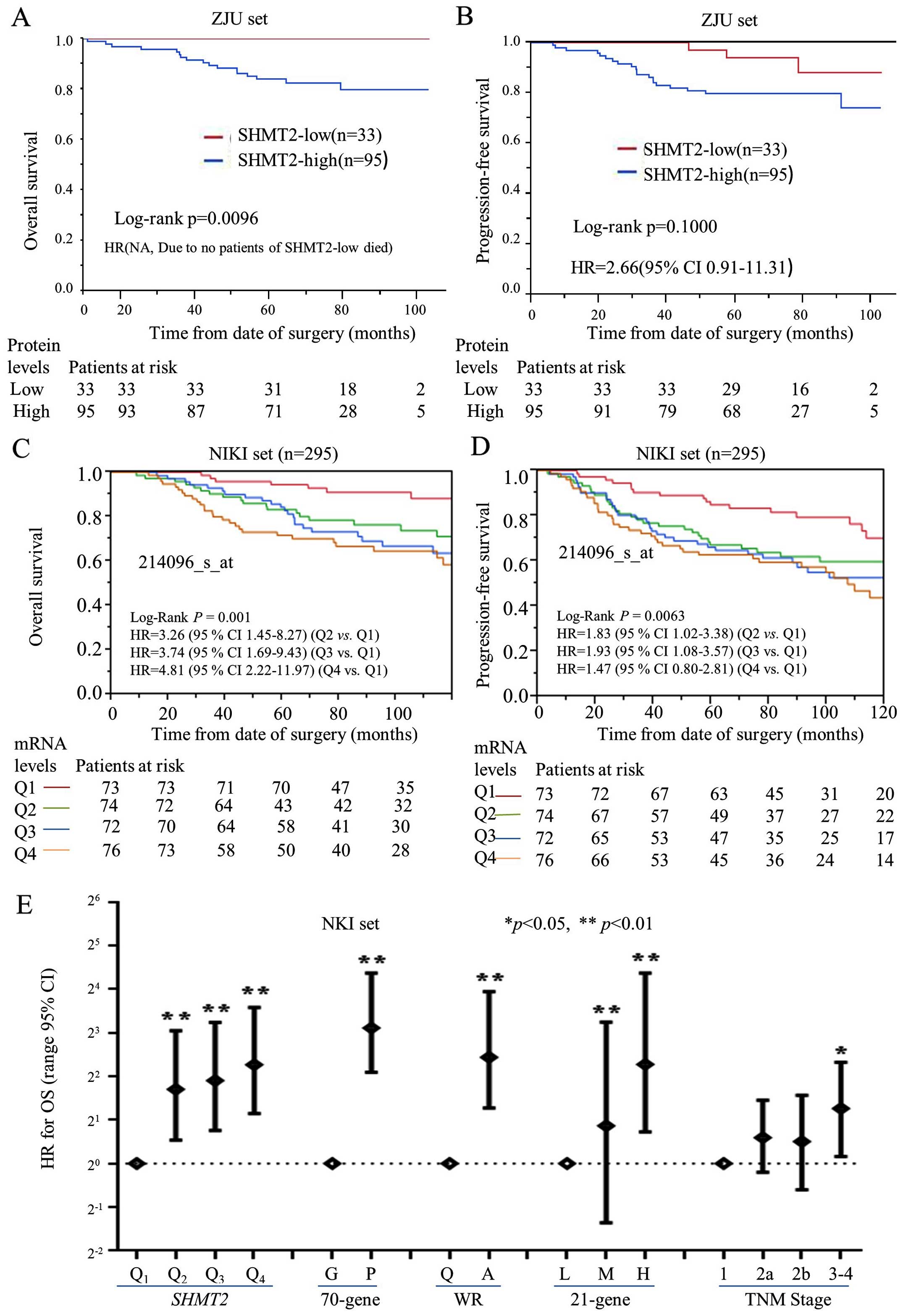 | Figure 3The prognostic significance and
performance of SHMT2 in breast cancer patients. Kaplan-Meier
analysis was performed to validate the prognostic significance of
SHMT2. For protein levels of SHMT2 (ZJU set), the analysis was
stratified as SHMT2-low (− and +) and SHMT2-high (++ and +++). For
the mRNA levels of SHMT2 (NKI set), breast cancer patients were
stratified into four subgroups based on their SHMT2 expression
levels. Q0 was the 0 to the 25th percentile;
Q1 was the 25th percentile to the median; Q2
was the median to the 75th percentile; and Q3 was the
75th percentile to the maximum. The Q0, Q1,
Q2, and Q3 subgroups represent SHMT2
mRNA levels, from low to high. The stratification method is
described in Materials and methods. (A) The protein levels of SHMT2
and OS. (B) The protein levels of SHMT2 and PFS. (C) The mRNA
levels of SHMT2 and OS. (D) The mRNA levels of SHMT2 and
PFS. (E) The Cox analyses for SHMT2, the 70-gene signature,
wound-response gene signature, 21-gene recurrence score, and TNM
stage, NKI set; *p<0.05, **p<0.01. |
Gene set enrichment analysis (GSEA)
GSEA analysis software v2.0.14x (JAVA version) was
downloaded from the Broad Institute Gene Set Enrichment Analysis
website (www.broad.mit.edu/gsea). The standard protocol was
based on previous published study protocols (30). The Pawitan set (159 cases) format
was modified and converted into a GSEA dataset. The gene sets were
downloaded from the Board Institute website/Molecular Signature
Database (MSig DB). The number of permutations was set to 1,000,
and the phenotype label was based on SHMT2 (214065_at)
expression levels. The ranked-list metric was generated and
calculated using a Pearson model.
Data management and statistical
methods
The worldwide gzene expression database was
downloaded, converted, constructed and managed using MS-Excel. The
JMP 10.0 (SAS Institute Inc., Cary, NC, USA) application was used
to perform the statistical analysis. Categorical variables were
compared using χ2 analysis, Fisher's exact or the
binomial tests of proportions. Kaplan-Meier analysis and Cox hazard
proportional hazard models were used for outcome analysis. Data
from patients with distant metastatic breast cancer that was not
completely resected were excluded from the PFS analysis.
Multivariate Cox analysis was used to adjust for covariate effects,
and a stratified data analysis was used to reduce the effects of
confounding on the estimated hazard ratios (HRs). Missing data were
coded and excluded from the analysis.
Results
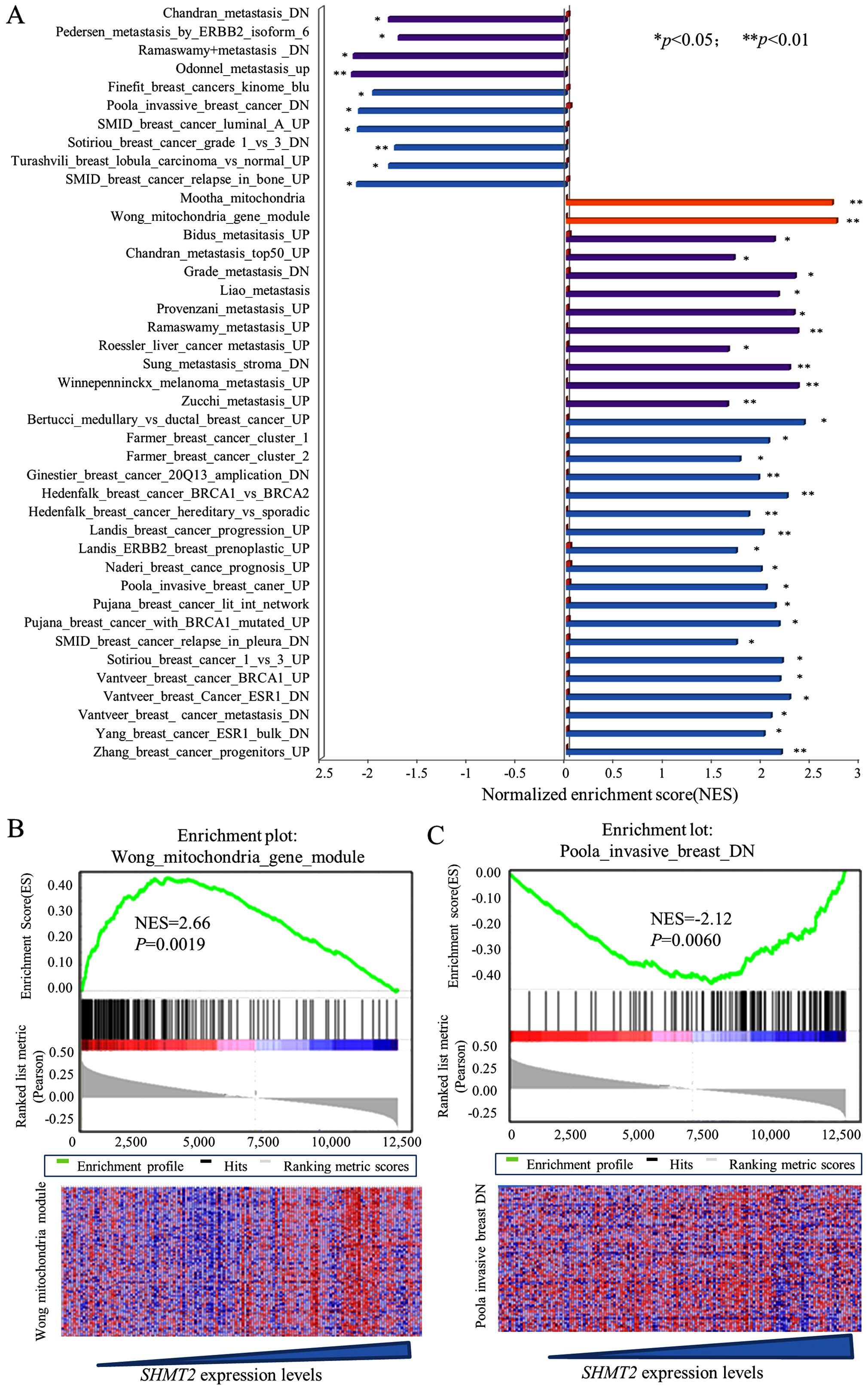
SHMT2 enriches cancer invasion-related
gene signatures in breast cancers
To explore the clinical relevance and biological
effects of SHMT2, we performed a GSEA on a downloaded
microarray dataset of 159 breast cancer cases (GSE1456) and on
other downloaded gene array datasets. Since the between-set results
were similar, representative results from the Pawitan set analysis
are presented. The results for the SHMT2 enriched gene
signatures are presented in Fig. 1A
along with their statistical significance. Representative results
are presented in Fig. 1B and C. The
results revealed that SHMT2 was positively associated with
at least two mitochondrial gene sets (Mootha and Wong gene
modules). This finding reflected the compatibility and location of
SHMT2 genes in the mitochondria. The overall GSEA results
(Fig. 1A) revealed that
SHMT2 was positively and significantly associated with
metastasis, cancer invasion and poor survivability related gene
sets. Taken together, these results suggested that high expression
of SHMT2 may be associated with aggressiveness of breast
cancer.
The expression of SHMT2 correlates with
the aggressiveness of the breast cancer
The associations between SHMT2 protein expression
levels and clinical characteristics of breast cancer were analyzed
in the ZJU set. IHC staining of SHMT2 scoring are presented in
Fig. 2A. The IHC staining results
indicated that SHMT2 was primarily observed in the cytoplasm with
mitochondrial location, and that some particle spread was present.
To increase the study's statistical power, we have re-stratified
subjects into SHMT2-low (− and +) and SHMT2-high (++ and +++)
categories. The results of our statistical analysis indicated that
increased SHMT2 protein levels were associated with larger tumor
size (p=0.0203), positive lymph nodes (p=0.0175), poor
differentiation (p=0.0067) and MKI67-positive results (p=0.0179)
(Fig. 2B and Table I). The SHMT2 protein expression in
various molecular subtypes of breast cancer varied, but not
significantly. The results for the percentages of SHMT2-high varied
in molecular subtypes. There were 75.0% for the luminal A cases,
80.0% for the luminal B/HER2(−) cases, 75.0% for the of luminal
B/Her2(+) cases, 83.3% for the basal-like TNBC cases and 83.3% for
the HER2(+), subtype cases (Table
I). The overall IHC results were consistent with the results
yielded from NKI set (miRNA expression data) (Fig. 2C). The ANOVA results indicated that
the SHMT2 mRNA expression level was significantly related to
pT, pN and TNM stages, and Elson grade. It is also higher in the
TNBC, Her2-positive and luminal B subtype. Patients with these
molecular subtypes had poor outcomes. Similar results were also
revealed for the Ivshina, Desmedt, Pawitan and Wong datasets (data
not shown). Overall, clinical relevance analysis validated GSEA
results, which suggested that mitochondrial SHMT2 may be an
indicator of breast cancer aggressiveness.
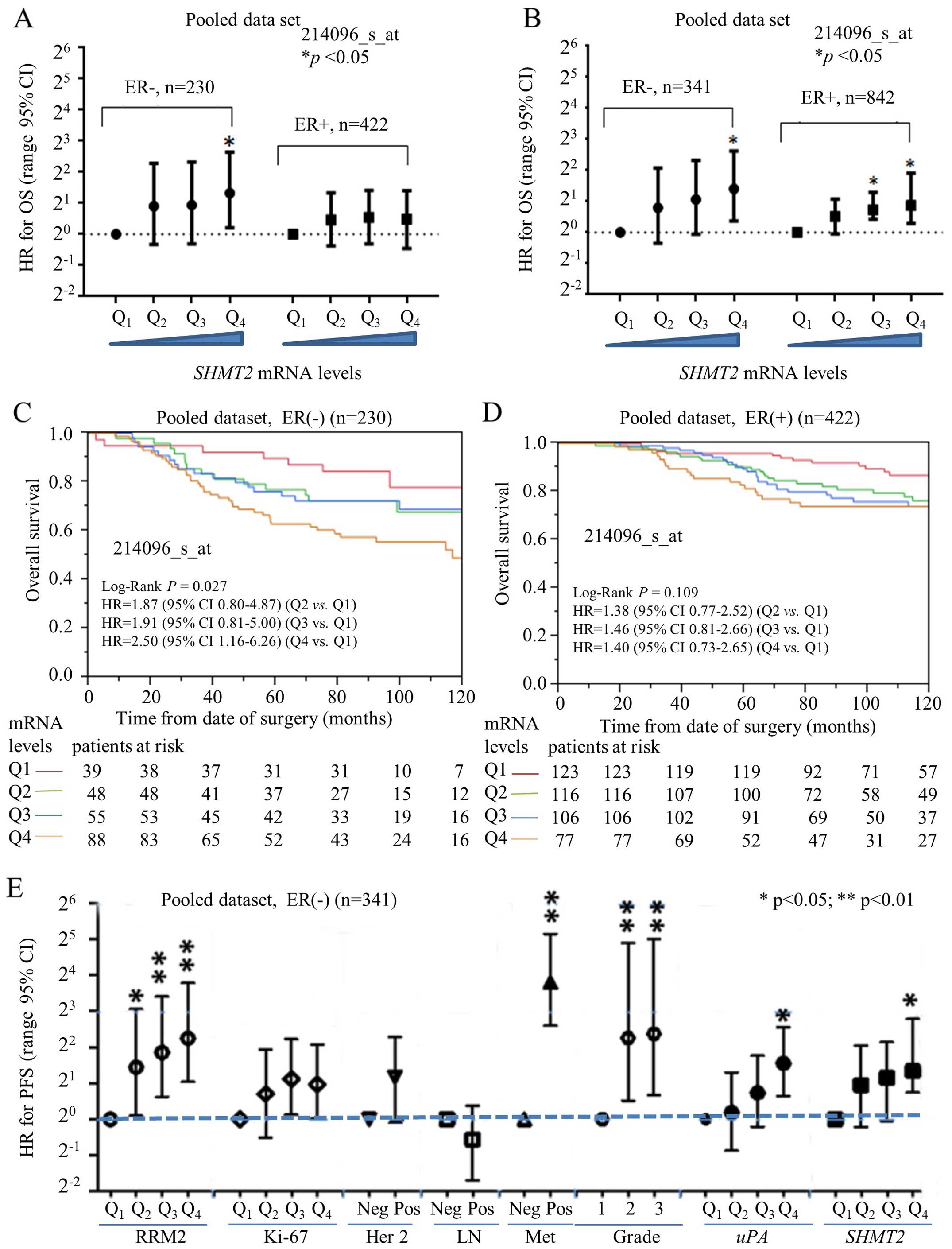
Prognostic validation of SHMT2
The prognostic significance of SHMT2 protein was
also evaluated, based on the ZJU set. The Kaplan-Meier analysis of
overall survival (OS) and progression-free survival (PFS) (Fig. 3A and B) revealed that high protein
expression levels of SHMT2 negatively affected OS. The multivariate
Cox proportional hazard analysis also indicated that high levels of
SHMT2 protein were significantly and negatively associated with PFS
in breast cancer patients. The HR for SHMT2 was 2.66 (95%
CI, 0.91–11.31), after adjusting for ER status, Elson histological
stage and adjuvant chemotherapy (Fig.
3B). The HR for OS could not be calculated since no patient
with SHMT2-low expression died from breast cancer.
The prognostic value of SHMT2 mRNA was
validated using univariate and multivariate Cox analyses, based on
five worldwide microarray datasets. We re-categorized SHMT2
scores into four subgroups (Q1, Q2,
Q3 and Q4) according to the mRNA expression
levels of SHMT2. The lowest expression subgroup,
Q1, was set as the baseline for calculation of the
HRs.
OS and PFS analyses revealed that the mRNA
expression levels of SHMT2 were significantly associated
with poor survival in a dose-dependent manner in the Pawitan, Wang,
Ivshina and NKI sets, but not in the Desmedt set (Table III). We found that there was a
statistically significant trend between SHMT2 mRNA
expression levels and the HRs for OS and PFS for all of the sets,
except the Desmedt set. The pooled analysis results revealed that
the adjusted HRs for PFS were 1.31 in Q2 (95% CI,
0.91–1.89), 1.63 in Q3 (95% CI, 1.15–2.34) and 1.75 in
Q4 (95% CI, 1.21–2.56). The Kaplan-Meier analysis
further revealed that SHMT2 mRNA levels correlated with the
PFS and OS of patients in NIKI sets (Fig. 3C and D). The Cox and Kaplan-Meier
results also indicated that as SHMT2 mRNA levels increased
the likelihood of a poor outcome increased. SHMT2 expression
increased the probability of poor survival among breast cancer
patients in a dose-dependent manner.
 | Table IIIUnivariate and multivariate analyses
for SHMT2 and survival microarray datasets. |
Table III
Univariate and multivariate analyses
for SHMT2 and survival microarray datasets.
| Dataset | Overall survival
| Progression-free
survival
|
|---|
HR
(95% CI) | Adjusted
HR
(95% CI)a | HR
(95% CI) | Adjusted
HR
(95% CI)a |
|---|
| Desmedt |
| Q1 | Reference | Reference | Reference | Reference |
| Q2 | 0.73
(0.33–1.57) | 0.70
(0.31–1.51) | 1.13
(0.63–2.05) | 1.08
(0.59–2.07) |
| Q3 | 0.80
(0.36–1.70) | 0.69
(0.30–1.53) | 1.16
(0.64–2.11) | 1.30
(0.70–2.40) |
| Q4 | 1.52
(0.78–3.03) | 1.21
(0.57–2.59) | 1.48
(0.83–2.66) | 1.60
(0.86–2.99) |
| Pawitan |
| Q1 | Reference | Reference | Reference | Reference |
| Q2 | 3.06
(1.04–11.05)b | 2.51
(0.81–9.32) | 3.79
(1.16–16.94)b | 3.90
(1.19–17.44)b |
| Q3 | 2.82
(0.94–10.31) | 2.07
(0.64–7.88) | 3.06
(0.88–13.96) | 2.25
(0.61–10.56) |
| Q4 | 4.38
(1.58–15.36)c | 2.78
(0.96–10.12) | 8.26
(2.81–35.20)c | 5.29
(1.73–23.03)c |
| Wang |
| Q1 | N/A | N/A | Reference | Reference |
| Q2 | N/A | N/A | 1.27
(0.73–2.22) | 1.28
(0.74–2.24) |
| Q3 | N/A | N/A | 0.91
(0.51–1.62) | 0.93
(0.52–1.67) |
| Q4 | N/A | N/A | 1.81
(1.08–3.09)b | 1.91
(1.11–3.33)b |
| Ivshina |
| Q1 | N/A | N/A | Reference | Reference |
| Q2 | N/A | N/A | 1.31
(0.65–2.76) | 1.37
(0.67–2.97) |
| Q3 | N/A | N/A | 2.20
(1.14–4.51)b | 2.11
(1.07–4.46)b |
| Q4 | N/A | N/A | 2.72
(1.43–5.53)c | 2.49
(1.23–5.38)b |
| NKI |
| Q1 | Reference | Reference | Reference | Reference |
| Q2 | 3.26
(1.45–8.27)c | 3.18
(1.42–8.07)c | 2.03
(1.13–3.75)b | 2.04
(1.14–3.77)b |
| Q3 | 3.74
(1.69–9.43)c | 2.75
(1.21–7.04)b | 2.32
(1.31–4.27)c | 1.91
(1.06–3.58)b |
| Q4 | 4.81
(2.22–11.97)c | 2.79
(1.24–7.11)c | 2.64
(1.51–4.87)c | 1.94
(1.07–3.63)b |
| Pooled |
| Q1 | Reference | Reference | Reference | Reference |
| Q2 | 1.71
(1.07–2.80)b | 1.28
(0.75–2.22) | 1.46
(1.10–1.97)c | 1.31
(0.91–1.89) |
| Q3 | 1.85
(1.15–3.02)b | 1.30
(0.76–2.26) | 1.55
(1.17–2.08)c | 1.63
(1.15–2.34)c |
| Q4 | 2.82
(1.81–4.51)c | 1.47
(0.86–2.56) | 2.26
(1.72–3.00)c | 1.75
(1.21–2.56)c |
The prognostic performance of SHMT2 mRNA
level was also compared with current prognostic gene signatures
[70-genes (31), wound-response
genes (16), the 21 gene recurrence
score (17)] and TNM staging in the
NKI dataset. The Cox analyses revealed that the HR was positively
correlated with increased SHMT2 mRNA levels (Fig. 3E). The prognostic performance of
SHMT2 was similar to the performance of the 70-genes,
wound-response genes and the 21 gene recurrence score. Compared to
TNM staging, both the SHMT2 mRNA levels and the gene
signatures were more efficient prognostic indicators.
Prognostic performance of SHMT2 in the
ER-positive vs
ER-negative subgroups. ER-negative breast cancers
(e.g., HER2-positive and TNBC subtypes) have typically a poorer
prognosis compared to ER-positive breast cancers (e.g., luminal A
and B subtypes) (13). Currently,
only a few biomarkers [e.g., uPA (17)] are available to predict the outcome
and survival for ER-negative breast cancer patients. Our previous
study revealed that ribonucleotide reductase small subunit M2
(RRM2) is a potential prognostic biomarker for ER-negative breast
cancers (22). In the present
study, we used a stratified approach to compare the prognostic
performance of SHMT2 in ER-positive vs. ER-negative breast
cancer subtypes. Multivariate Cox analysis indicated that the HR
value for OS steadily increased and significantly as levels of
SHMT2 increased in the ER-negative subgroup (Fig. 4A). For PFS, however, SHMT2
was predictive for poor survival in both the ER-negative and
ER-positive cancers (Fig. 4B).
Kaplan-Meier analysis revealed SHMT2 had better prognostic
value in the ER-positive and ER-negative subgroups. The results of
the OS analysis indicated that SHMT2 significantly affected
the survival of patients in the ER-negative (log-rank p=0.027)
(Fig. 4C), but not in the
ER-positive (log-rank p=0.109), breast cancer subgroups (Fig. 4D). Therefore, the overall prognostic
performance of SHMT2 was better in the patients with
ER-negative breast cancer.
The prognostic value of SHMT2 in ER-negative
breast cancer patients was further compared with other markers
(e.g., HER-2 status, lymph node involvement, distant organ
metastasis, Elson histological stage, RRM2 and uPA). The analysis
of the pooled dataset revealed that the HR for SHMT2
steadily increased with increased SHMT2 mRNA expression
levels for OS (data not shown) and for PFS (Fig. 4E). The prognostic performance of
SHMT2 was comparable to uPA and was better than Ki-67, HER-2
and lymph node involvement. However, SHMT2 was less
efficient than RRM2 and distant metastasis.
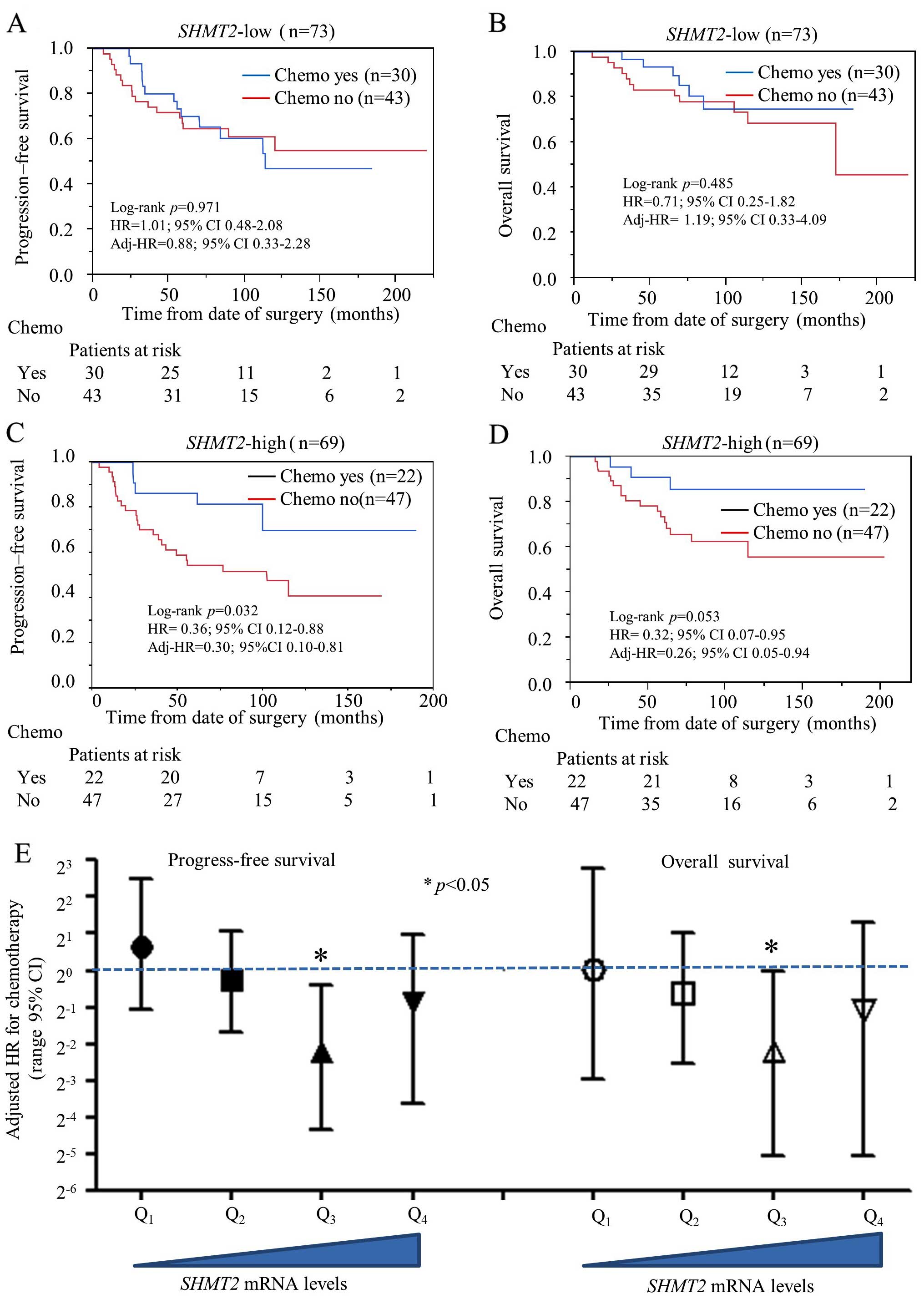
Predictive capability of SHMT2 for
chemotherapy selection in stage IIa breast cancer
Since SHMT2 has a genetic role in cancer
development, we were also interested in exploring its potential
role as a predictive biomarker for chemotherapy selection. We
performed a stratified analysis to compare chemotherapy efficacy
between the SHMT2-low and SHMT2-high breast cancer
patients. The NKI set was used for the analysis since it was the
only dataset with chemotherapy information. Only data from stage
IIa patients were selected for analysis to avoid TNM
staging-related confounding effects. Other potential confounding
factors (hormone therapy, Elson grade and intrinsic molecular
subtype) were also added to adjust the HR. Our results indicate
that chemotherapy did not reduce the relative risk of OS and
relapse in the SHMT2-low subgroup (Fig. 5A and B). However, chemotherapy did
significantly reduce probability of relapse (adjusted HR=0.30; 95%
CI, 0.10–0.81) and death (adjusted HR=0.26; 95% CI, 0.05–0.94) in
the SHMT2-high subgroups (Fig.
5C and D). Multivariate Cox analysis revealed that the relative
risk of chemotherapy decreased significantly as SHMT2 mRNA
expression increased (Fig. 5E).
Overall, our findings suggest SHMT2 may be a valid
predictive biomarker for chemotherapy selection and efficacy.
Discussion
Combining multiple-tissue arrays with worldwide
public microarray databases provides researchers the ability to
discover novel biomarkers. The present study revealed that
SHMT2 protein and mRNA levels were significantly associated
with tumor grade, size, relapse, metastasis and positive lymph
nodes (Table I and Fig. 2). The GSEA results provided further
support for these findings (Fig.
1). We found that higher SHMT2 expression was
significantly correlated with poor overall survival (OS) (log-rank
p=0.0096), but not with progression-free survival (PFS) (Fig. 3A and B). This difference was due to
the limited number of cases (128 cases) recorded in the ZJU set.
Prognostic validation results indicated that SHMT2
overexpression was significantly associated with poor survival for
breast cancer patients in a dose-dependent manner in four of five
independent breast cancer microarray datasets. SHMT2 did not
significantly affect the survival of breast cancer patients in the
Desmedt set, most likely due to the small sample size of 158 cases.
Nevertheless, our analysis of the Desmedt set indicated that there
was an increasing trend in HR values as mRNA levels increased. We
further assessed the prognostic performance of SHMT2 mRNA
and compared it with multiple gene signatures, including 70-genes,
wound-response genes and the 21 gene recurrence score. Our findings
suggested that SHMT2 not only correlated with poor prognosis
in different breast cancer subgroups, but it also had prognostic
power similar to several known multiple gene signatures.
Only a limited number of biomarkers are widely
available for clinical application for ER-negative breast cancer
patients. In view of the precision medicine era and current focus
on outcome based therapy, it is likely that more breast cancer
subtypes may be classified in the future. Identification of novel
therapeutic targets and prognostic biomarkers may be critical for
clinical and drug discovery purposes for breast cancer. Our
findings suggest that SHMT2 levels were significantly
associated with poor OS and PFS in patients with ER-negative and
ER-positive breast cancer, but particularly in ER-negative cases
(Fig. 4). In the ER-negative breast
cancers, the prognostic value of SHMT2 proved greater than
Ki-67, HER-2 and lymph node involvement, but poorer than RRM2 and
distant metastasis (Fig. 4E).
SHMT2 may be a valid prognostic biomarker for ER-negative
breast cancer. The reasons why SHMT2 had greater prognostic
efficiency in ER-negative breast cancer remain unknown. We seek to
investigate the mechanisms involved in future studies.
Alterations in cellular metabolism have recently
been recognized as a hallmark feature of cancer growth (32). Glycine metabolism is a key step in
cancer cell proliferation. Study results also link the folate cycle
to various enzymes that regulate DNA synthesis and DNA methylation
(33,34). The folate cycle pathway may
contribute to tumor homeostasis by providing essential precursors
to sustain cancer cell growth and affect methylation capacities
(35). SHMT2 has been
implicated as a critical factor in serine/glycine metabolism in
several cancer cell types, including breast cancer (36). This phenomenon ultimately affects
patient mortality (37). Whether
indeed, SHMT2 is a cancer driver gene, it may serve as a
promising target for anticancer agents. Recent developments in our
understanding of serine/glycine metabolic pathways provide novel
translational opportunities for drug development, dietary
intervention and biomarker discovery (38). Even though the SHMT2
localization in mitochondria makes it a difficult target for
inhibition, we believe that further drug discovery efforts are
warranted.
SHMT2 inhibitors are not commercially
available for cancer therapy. However, agents such as methotrexate
and fluorouracil, which inhibit downstream targets of SHMT2,
are currently being used (38).
These compounds have been used in combinations with other agents to
treat breast cancer. Examples of these combinations include CMF
(cytoxan, methotrexate and fluorouracil), FAC (fluorouracil,
adriamycin and cytoxan) and CAF (cytoxan, adriamycin and
fluorouracil). We performed a preliminary stratified analysis of
stage IIa subgroup breast cancers (NKI set) and found that
chemotherapy significantly reduced the relapse of breast cancer in
SHMT2-high subgroup (HR=0.36; 95% 0.12–0.87), but not in
SHMT2-low subgroup (HR=1.01; 95% 0.48–2.08), patients
(Fig. 5). While reasons why
SHMT2 had greater prognostic efficiency for ER-negative
breast cancer compared to ER-positive breast cancer cases remain
unknown, we intend to investigate the mechanisms involved in this
difference in future studies.
We recognize that the small sample size of ZJU set
was a limitation to the present study. We recommend further studies
to validate our findings on prognostic value of SHMT2 in ER
negative patients and chemotherapy selection. While GSEA analysis
was performed in the present study, we did not perform laboratory
experiments or animal studies to explore the exact mechanism of the
SHMT2 effect on prognosis or chemotherapy response.
Taken together, our findings confirm the
SHMT2 significance as a prognostic biomarker for breast
cancer, particularly in ER-negative subgroup patients.
Acknowledgments
The present study was supported by the National
Natural Science foundation of China (nos. 81171546 and 81301654),
the Zhejiang Medical Research Platform for Important Programs (no.
2014ZDA018), the Committee of Hangzhou Science and Technology (no.
20140633B11), and the Foundation of Education Department of
Zhejiang Province (no. Y201328265).
Abbreviations:
|
SHMT2
|
serine hydroxymethyltransferase 2
(mitochondrial)
|
|
THF
|
tetrahydrofolate
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
MKI-67
|
marker of proliferation Ki-67
|
|
TNBC
|
triple-negative breast cancer, or
basal-like breast cancer
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
IHC
|
immunohistochemistry
|
|
GSEA
|
gene set enrichment analysis
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
HR
|
proportional hazard ratio
|
|
95% CI
|
95% confidence interval
|
|
RRM2
|
human ribonucleotide reductase small
subunit M2
|
|
IRM
|
immune response module
|
|
HDPP
|
HER2-derived prognostic predictor
|
|
pT stage
|
pathological tumor stage
|
|
pN stage
|
pathological lymph node stage
|
References
|
1
|
Anderson DD and Stover PJ: SHMT1 and SHMT2
are functionally redundant in nuclear de novo thymidylate
biosynthesis. PLoS One. 4:e58392009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hebbring SJ, Chai Y, Ji Y, Abo RP, Jenkins
GD, Fridley B, Zhang J, Eckloff BW, Wieben ED and Weinshilboum RM:
Serine hydroxymethyltransferase 1 and 2: Gene sequence variation
and functional genomic characterization. J Neurochem. 120:881–890.
2012.PubMed/NCBI
|
|
3
|
Kim SK, Jung WH and Koo JS: Differential
expression of enzymes associated with serine/glycine metabolism in
different breast cancer subtypes. PLoS One. 9:e1010042014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee GY, Haverty PM, Li L, Kljavin NM,
Bourgon R, Lee J, Stern H, Modrusan Z, Seshagiri S, Zhang Z, et al:
Comparative oncogenomics identifies PSMB4 and SHMT2 as potential
cancer driver genes. Cancer Res. 74:3114–3126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu XY and Lu L: Vitamin B6 deficiency,
genome instability and cancer. Asian Pac J Cancer Prev.
13:5333–5338. 2012. View Article : Google Scholar
|
|
6
|
Leivonen SK, Rokka A, Ostling P, Kohonen
P, Corthals GL, Kallioniemi O and Perälä M: Identification of
miR-193b targets in breast cancer cells and systems biological
analysis of their functional impact. Mol Cell Proteomics.
10:M110.0053222011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Antonov A, Agostini M, Morello M, Minieri
M, Melino G and Amelio I: Bioinformatics analysis of the serine and
glycine pathway in cancer cells. Oncotarget. 5:11004–11013. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye J, Fan J, Venneti S, Wan YW, Pawel BR,
Zhang J, Finley LW, Lu C, Lindsten T, Cross JR, et al: Serine
catabolism regulates mitochondrial redox control during hypoxia.
Cancer Discov. 4:1406–1417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma FJ, Liu ZB, Hu X, Ling H, Li S, Wu J
and Shao ZM: Prognostic value of myeloid differentiation primary
response 88 and Toll-like receptor 4 in breast cancer patients.
PLoS One. 9:e1116392014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sørlie T, Wang Y, Xiao C, Johnsen H, Naume
B, Samaha RR and Børresen-Dale AL: Distinct molecular mechanisms
underlying clinically relevant subtypes of breast cancer: Gene
expression analyses across three different platforms. BMC Genomics.
7:1272006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ; Panel members: Strategies for
subtypes - dealing with the diversity of breast cancer: Highlights
of the St. Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reis-Filho JS and Tutt AN: Triple negative
tumours: A critical review. Histopathology. 52:108–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carey LA, Perou CM, Livasy CA, Dressler
LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S,
et al: Race, breast cancer subtypes, and survival in the Carolina
Breast Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martinez-Chacin RC, Keniry M and Dearth
RK: Analysis of high fat diet induced genes during mammary gland
development: Identifying role players in poor prognosis of breast
cancer. BMC Res Notes. 7:5432014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tobin NP, Harrell JC, Lövrot J, Egyhazi
Brage S, Frostvik-Stolt M, Fernö M, Perou CM, Bergh J, Hatschek T
and Lindström LS; TEX Trialists Group: The molecular subtype and
tumor characteristics of breast cancer metastases significantly
influence patient post-relapse survival. Ann Oncol. Oct 31–2014.
View Article : Google Scholar
|
|
16
|
Saal LH, Johansson P, Holm K,
Gruvberger-Saal SK, She QB, Maurer M, Koujak S, Ferrando AA,
Malmström P, Memeo L, et al: Poor prognosis in carcinoma is
associated with a gene expression signature of aberrant PTEN tumor
suppressor pathway activity. Proc Natl Acad Sci USA. 104:7564–7569.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paik S, Shak S, Tang G, Kim C, Baker J,
Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al: A
multigene assay to predict recurrence of tamoxifen-treated,
node-negative breast cancer. N Engl J Med. 351:2817–2826. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang HY, Nuyten DS, Sneddon JB, Hastie T,
Tibshirani R, Sørlie T, Dai H, He YD, van't Veer LJ, Bartelink H,
et al: Robustness, scalability, and integration of a wound-response
gene expression signature in predicting breast cancer survival.
Proc Natl Acad Sci USA. 102:3738–3743. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Staaf J, Ringnér M, Vallon-Christersson J,
Jönsson G, Bendahl PO, Holm K, Arason A, Gunnarsson H, Hegardt C,
Agnarsson BA, et al: Identification of subtypes in human epidermal
growth factor receptor 2 - positive breast cancer reveals a gene
signature prognostic of outcome. J Clin Oncol. 28:1813–1820. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Teschendorff AE, Miremadi A, Pinder SE,
Ellis IO and Caldas C: An immune response gene expression module
identifies a good prognosis subtype in estrogen receptor negative
breast cancer. Genome Biol. 8:R1572007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Zhang H, Lai L, Wang X, Loera S,
Xue L, He H, Zhang K, Hu S, Huang Y, et al: Ribonucleotide
reductase small subunit M2 serves as a prognostic biomarker and
predicts poor survival of colorectal cancers. Clin Sci.
124:567–578. 2013. View Article : Google Scholar :
|
|
22
|
Zhang H, Liu X, Warden CD, Huang Y, Loera
S, Xue L, Zhang S, Chu P, Zheng S and Yen Y: Prognostic and
therapeutic significance of ribonucleotide reductase small subunit
M2 in estrogen-negative breast cancers. BMC Cancer. 14:6642014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harris L, Fritsche H, Mennel R, Norton L,
Ravdin P, Taube S, Somerfield MR, Hayes DF and Bast RC Jr; American
Society of Clinical Oncology: American Society of Clinical Oncology
2007 update of recommendations for the use of tumor markers in
breast cancer. J Clin Oncol. 25:5287–5312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deyarmin B, Kane JL, Valente AL, van Laar
R, Gallagher C, Shriver CD and Ellsworth RE: Effect of ASCO/CAP
guidelines for determining ER status on molecular subtype. Ann Surg
Oncol. 20:87–93. 2013. View Article : Google Scholar
|
|
25
|
Smeds J, Miller LD, Bjöhle J, Hall P,
Klaar S, Liu ET, Pawitan Y, Ploner A and Bergh J: Gene profile and
response to treatment. Ann Oncol. 16(Suppl 2): ii195–ii202. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ivshina AV, George J, Senko O, Mow B,
Putti TC, Smeds J, Lindahl T, Pawitan Y, Hall P, Nordgren H, et al:
Genetic reclassification of histologic grade delineates new
clinical subtypes of breast cancer. Cancer Res. 66:10292–10301.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM,
Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME and
Yu J: Gene-expression profiles to predict distant metastasis of
lymph-node-negative primary breast cancer. Lancet. 365:671–679.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Desmedt C, Piette F, Loi S, Wang Y,
Lallemand F, Haibe-Kains B, Viale G, Delorenzi M, Zhang Y,
d'Assignies MS, et al: Strong time dependence of the 76-gene
prognostic signature for node-negative breast cancer patients in
the TRANSBIG multicenter independent validation series. Clin Cancer
Res. 13:3207–3214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van de Vijver MJ, He YD, van't Veer LJ,
Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C,
Marton MJ, et al: A gene-expression signature as a predictor of
survival in breast cancer. N Engl J Med. 347:1999–2009. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES, et al: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van't Veer LJ, Dai H, van de Vijver MJ, He
YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ,
Witteveen AT, et al: Gene expression profiling predicts clinical
outcome of breast cancer. Nature. 415:530–536. 2002. View Article : Google Scholar
|
|
32
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zwart SR, Jessup JM, Ji J and Smith SM:
Saturation diving alters folate status and biomarkers of DNA damage
and repair. PLoS One. 7:e310582012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang TC, Song YS, Wang H, Zhang J, Yu SF,
Gu YE, Chen T, Wang Y, Shen HQ and Jia G: Oxidative DNA damage and
global DNA hypomethylation are related to folate deficiency in
chromate manufacturing workers. J Hazard Mater. 213–214:440–446.
2012. View Article : Google Scholar
|
|
35
|
Garrow TA, Brenner AA, Whitehead VM, Chen
XN, Duncan RG, Korenberg JR and Shane B: Cloning of human cDNAs
encoding mitochondrial and cytosolic serine
hydroxymethyltransferases and chromosomal localization. J Biol
Chem. 268:11910–11916. 1993.PubMed/NCBI
|
|
36
|
Jain M, Nilsson R, Sharma S, Madhusudhan
N, Kitami T, Souza AL, Kafri R, Kirschner MW, Clish CB and Mootha
VK: Metabolite profiling identifies a key role for glycine in rapid
cancer cell proliferation. Science. 336:1040–1044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Paone A, Marani M, Fiascarelli A, Rinaldo
S, Giardina G, Contestabile R, Paiardini A and Cutruzzolà F: SHMT1
knockdown induces apoptosis in lung cancer cells by causing uracil
misincorporation. Cell Death Dis. 5:e15252014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Amelio I, Cutruzzolá F, Antonov A,
Agostini M and Melino G: Serine and glycine metabolism in cancer.
Trends Biochem Sci. 39:191–198. 2014. View Article : Google Scholar : PubMed/NCBI
|