Introduction
Gap junctions (GJs) are intercellular channels which
allow the passage of small molecules to diffuse (secondary
messengers, metabolites and ions) between the cytoplasm of adjacent
cells. GJ channels are comprised of membrane-integrated proteins
called connexins. Twenty-one members of the connexin protein family
have been reported in the human and 20 in the mouse. Two hexameric
oligomers formed by connexins constitute a single GJ channel
(1–3). Gap junction intercellular
communication (GJIC) plays a vital role in physiological and
pathological processes, including cell growth, differentiation,
homeostasis and inflammatory responses (4–7).
Moreover, GJIC enhances chemotherapeutic drug-induced apoptosis,
such as oxaliplatin (8,9), vinblastine (10) and doxorubicin (11). Inhibition of GJIC (channel closure)
is related to kinase-modulated connexin phosphorylation, and Src
and PKC are involved in connexin 43 (Cx43) phosphorylation (p-Cx43)
(12).
Platinum-based drugs are the most widely used
chemotherapeutic agents in cancer treatment. Oxaliplatin (OHP), a
third-generation platinum-based compound, is approved for use in
colon, non-small cell lung cancer (NSCLC) and pancreatic cancer
(13,14). Unfortunately, neurotoxicity
(15,16) and drug resistance (17–20)
limit the clinical use of oxaliplatin. Hence, it is urgent to
enhance the anticancer effect of oxaliplatin and decrease
off-target toxicity.
Gefitinib, a selective epidermal growth factor
receptor (EGFR) tyrosine kinase inhibitor, was first approved for
clinical use for the treatment of NSCLC (21). However, patients who develop an
acquired resistance to gefitinib may subsequently become refractory
(22–24) and skin toxic effects occur (25). Dose-escalation of gefitinib does not
improve progression-free survival and overall survival (26). Furthermore, activation of EGFR may
increase Src and PKC-modulated Cx43 phosphorylation, consequently
inhibiting GJIC (12).
Recent studies have reported that gefitinib may
enhance the anticancer effect of oxaliplatin in metastatic
colorectal cancer (27) and NSCLC
(28). Nevertheless, the potential
mechanisms of the enhanced antitumor efficacy remain to be
elucidated. Our previous study showed that gefitinib induced
apoptosis via the mitochondrial pathway in I-10 testicular cancer
cells (29). In the present study,
we aimed to investigate the apoptosis induced by oxaliplatin
combined with gefitinib, and to reveal the potential mechanisms of
GJIC modulated by gefitinib in I-10 cells.
Materials and methods
Cell lines and cell culture
The testicular cancer cell line (I-10) was purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). The cells were maintained in Ham's F-12 nutrient mixture
(F-12) with 2.5% fetal bovine serum (FBS), 15% horse serum (all
from Gibco, Grand Island, NY, USA), 100 U/ml penicillin and 100
U/ml streptomycin in a humidified incubator supplemented with 5%
CO2 at 37°C.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cells (5×104) were seeded in 96-well
plates and cultured for 24 h (70–80% confluency). Cells were
subjected to a range of oxaliplatin (Sigma-Aldrich, St. Louis, MO,
USA) concentration (0, 5, 10, 20, 40, 80, 160 and 320 µM) for 24 h.
Where indicated, 1 µM gefitinib (Sigma-Aldrich) was added 24 h
before oxaliplatin stimuli. Oxaliplatin was freshly dissolved in
phosphate-buffered solution (PBS) at stock solutions. Gefitinib
stock solutions were prepared in dimethyl sulfoxide (DMSO)
(Sigma-Aldrich). Cells exposure to oxaliplatin and gefitinib were
performed in the dark. The cells were treated with fresh medium
with 20 µl of MTT (5 mg/ml) (Sigma-Aldrich) solution for 4 h. Then,
the medium was removed and the formed dark blue formazan was
dissolved in 150 µl DMSO. The absorbance [optical density, (OD)] at
570 nm was measured by a microplate reader (Bio-Rad Laboratories,
Hercules, CA, USA).
Flow cytometry
Cells (1×105) were seeded in 12-well
plates and cultured for 24 h (70–80% confluency). The cells were
pretreated with 1 µM gefitinib for 24 h and then exposed to 40 µM
oxaliplatin for 8 h. The samples were collected, rinsed twice with
cold PBS, suspended in binding buffer and incubated with 5 µl
Annexin V-FITC and 5 µl propidium iodide (PI) (FITC/Annexin V
apoptosis detection kit; BD Biosciences, New York, NY, USA) at room
temperature in the dark for 15 min. The samples were immediately
analyzed by Auccri C6 flow cytometer (BD Biosciences). Early-stage
apoptotic cells were shown in the lower right quadrant (Annexin
V+/PI−).
Hoechst 33258 staining assay
Cells (1×105) were seeded in 12-well
plates and cultured for 24 h (70–80% confluency). The cells were
pretreated with 1 µM gefitinib for 24 h and then exposed to 40 µM
oxaliplatin for 16 h. Then, the medium was removed and the cells
were fixed with 4% paraformaldehyde solution for 30 min. Fixed
cells were washed twice with PBS and stained with 500 µl Hoechst
33258 (10 µg/ml) (Sigma-Aldrich) for 30 min in the dark. The
late-stage apoptotic cells (shrunken nuclei, chromatin condensation
and apoptotic body) were photographed with fluorescence microscopy
(Olympus IX73; Olympus, Tokyo, Japan) and counted at a
magnification of ×200.
Western blot analysis
Cells were washed three times with cold PBS and
harvested using lysis buffer (Beyotime, Shanghai, China). Cell
lysate was centrifuged and protein concentration was determined
using the BCA method. Protein samples were analyzed by 10% sodium
dodecyl sulfate-polyacrylamide electrophoresis (SDS-PAGE) gel and
electrophoretically transferred onto polyvinylidene difluoride
(PVDF) membranes (0.45 µm; Millipore, Billerica, MA, USA) followed
by immunoblotting. Rabbit antibodies against Bcl-2 (1:1,000)
(12789-1-AP), Bax (1:1,000) (50599-2-Ig), Src (1:1,000)
(11097-1-AP), PKC (1:1,000) (12919-1-AP) (ProteinTech, Chicago, IL,
USA), caspase-3 (1:1,000) (ab90437), caspase-9 (1:200) (ab25758),
Cx43 (1:8,000) (ab11370), p-Cx43 (1:1,000) (ab30559) (Abcam,
Cambridge, MA, USA), and mouse antibody against GAPDH (1:5,000)
(60004-1-lg) (ProteinTech) were used. Immunopositive bands were
visualized by Immobilon Western™ Chemiluminescent HRP Substrate
(Millipore) and quantified with ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
Immunofluorescence assay
Cells (1×105) plated in 12-well plates
were cultured for 24 h to reach 70–80% confluency, and were treated
with 1 µM gefitinib for 24 h; 8 µM PP2 for 8 h; 10 µM GF109203X for
10 h. The cells were fixed in 4% paraformaldehyde for 15 min. Fixed
cells were washed twice with PBS and incubated with 5% bovine serum
albumin for 2 h. Cells were incubated with rabbit antibody against
Cx43 (1:1,000) at 4°C overnight and with FITC-anti-rabbit IgG
(1:100) (Sigma-Aldrich) at 37°C for 2 h in the dark. Then, the
cells were stained with 5 µg/ml 4′,6-diamidino-2-phenylindole
(DAPI) for 5 min in the dark. The cells were captured using
fluorescence microscopy (Olympus) at a magnification of ×200.
‘Parachute’ dye-coupling assay
Functional GJIC was performed as previously
described (30). Cells
(1×105) plated in 12-well dishes were cultured for 24 h
to reach 70–80% confluency, and were treated with gefitinib, PP2 or
GF109203X as mentioned above. Donor cells from one well were
incubated with 10 µg/ml calcein AM and 5 µg/ml CM-DiI (ProteinTech)
in growth medium at 37°C for 30 min. Calcein-AM is intracellularly
converted into the GJ-permeable dye calcein and CM-DiI is a
membrane dye that does not spread to coupled cells. Then, the donor
cells were trypsinized and seeded onto the receiver cells at a
1:200 donor/receiver ratio. Cells were allowed to attach to the
monolayer of the receiver cells and form GJs for 4 h at 37°C and
photographed with fluorescence microscopy (Olympus). The average
number of receiver cells containing dye per donor cell was counted
at a magnification of ×200 as a measure of the degree of GJIC.
Statistical analysis
All of the experiments had a minimum of three
determinations. Data are presented as mean and standard deviation
(SD). Statistical analyses between two groups were performed by
Student's t-test using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered statistically significant.
Results
Gefitinib enhances oxaliplatin-induced
apoptosis
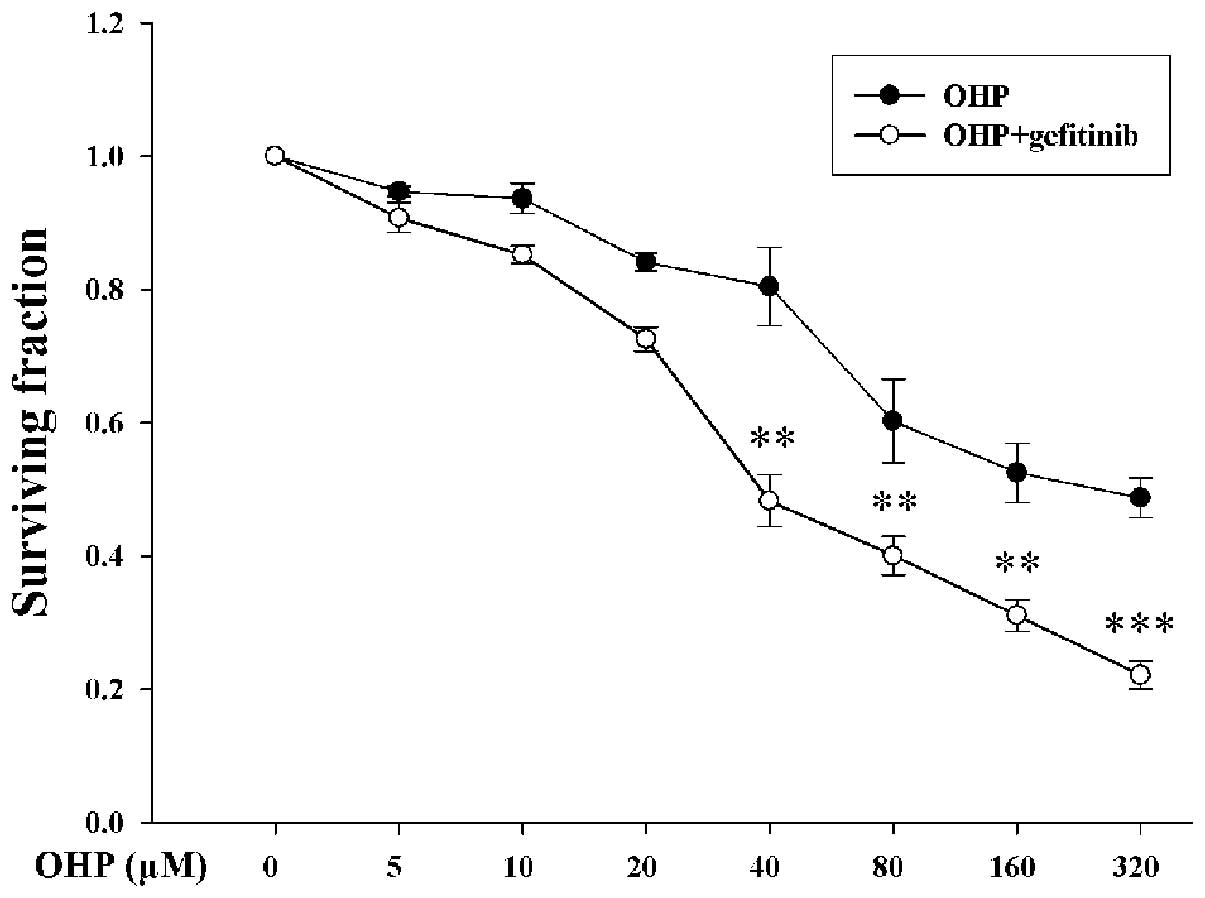
Cell survival was assessed by MTT assay. I-10 cells
were pretreated with or without 1 µM gefitinib (no toxicity on I-10
cells) for 24 h, followed by exposure to 0–320 µM oxaliplatin for
24 h. After treatment with 40, 80, 160 and 320 µM oxaliplatin
combined with and without gefitinib, cell surviving fractions were
0.48±0.04, 0.40±0.29, 0.31±0.02, 0.22±0.02 and 0.80±0.06,
0.60±0.06, 0.53±0.04, 0.49±0.03, respectively (Fig. 1). Cell survival was significantly
decreased in the presence of gefitinib (P<0.05).
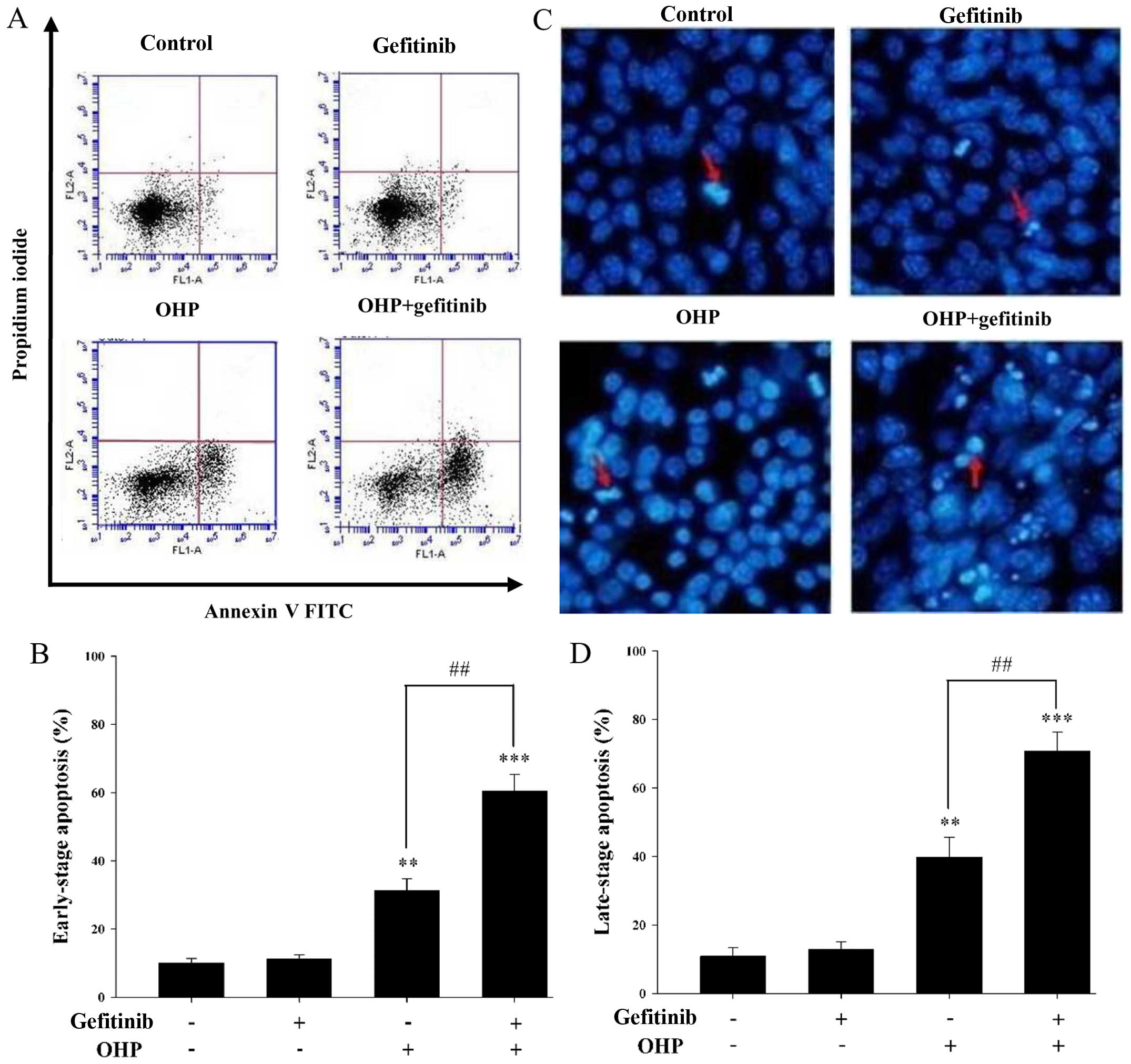
Chemotherapeutic drugs show antitumor effects by
increasing the apoptosis of cancer cells which include early-stage
and late-stage apoptosis. Annexin V/PI staining assay was used to
assess the early-stage apoptosis. As shown in Fig. 2A, early-stage apoptosis was induced
following treatment with 40 µM oxaliplatin for 8 h with 1 µM
gefitinib pretreatment for 24 h. As shown in Fig. 2B, the rate of early-stage apoptosis
was 60.47±4.80% in the presence of gefitinib and 31.23±3.47% in the
absence of gefitinib. As shown in Fig.
2C, late-stage apoptosis was induced by 40 µM oxaliplatin for
16 h with 1 µM gefitinib pretreatment for 24 h as assessed by
Hoechst 33258 staining assay. As shown in Fig. 2D, the ratio of late-stage apoptosis
was 70.69±5.58% in the presence of gefitinib and 39.80±5.81% in the
absence of gefitinib. Both the early- and late-stage apoptosis
rates were increased by ~40% when cells were pretreated with
gefitinib (P<0.05).
Obviously, these results demonstrated that
oxaliplatin-induced apoptosis was enhanced by gefitinib in the I-10
cells.
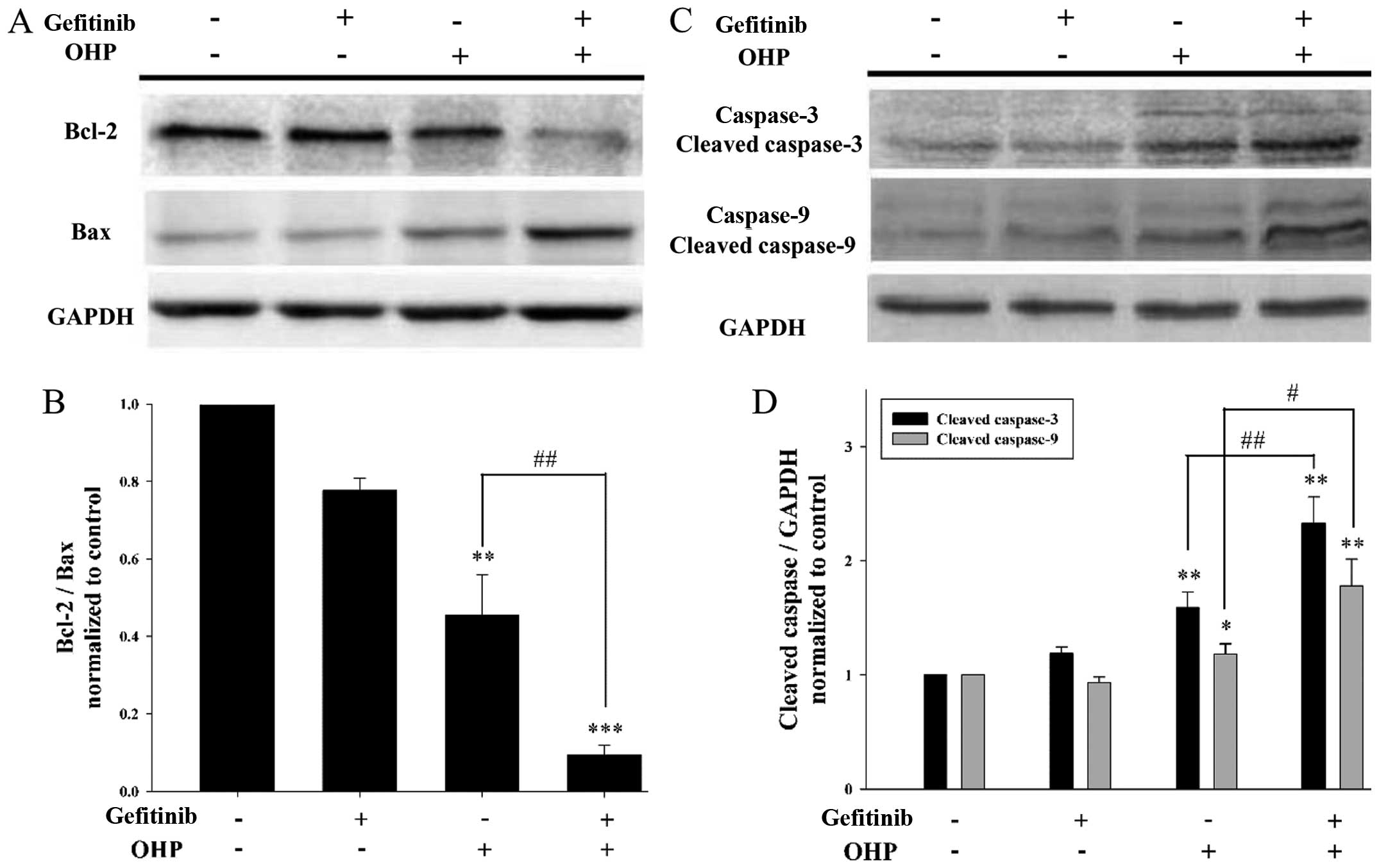
Gefitinib decreases the ratio of
Bcl-2/Bax and increases the cleavage of caspase-3 and −9 during
oxaliplatin-induced apoptosis
Bcl-2, Bax and caspase-3 and −9 are vital
participators in the mitochondrial apoptosis pathway, which is
involved in apoptosis induced by platinum-based drugs. Therefore,
western blotting was used to assess the expression levels of Bcl-2,
Bax and caspase-3 and −9 after I-10 cells were exposed to 40 µM
oxaliplatin for 12 h with 1 µM gefitinib pretreatment for 24 h
(Fig. 3). As shown in Fig. 3A, the expression of Bcl-2 (protects
against apoptosis) was decreased in the presence of gefitinib, and
contrarily the expression of Bax (promotes apoptosis) was
increased. Thus, the ratio of Bcl-2/Bax was significantly decreased
by gefitinib (P<0.05) (Fig. 3B).
In addition, as shown in Fig. 3C and
D, the cleavage of caspase-3 and −9 was greatly increased in
the presence of gefitinib (P<0.05). These results suggest that
gefitinib modulates apoptosis-related proteins (Bcl-2, Bax and
caspase-3 and −9) during oxaliplatin-induced apoptosis in I-10
cells.
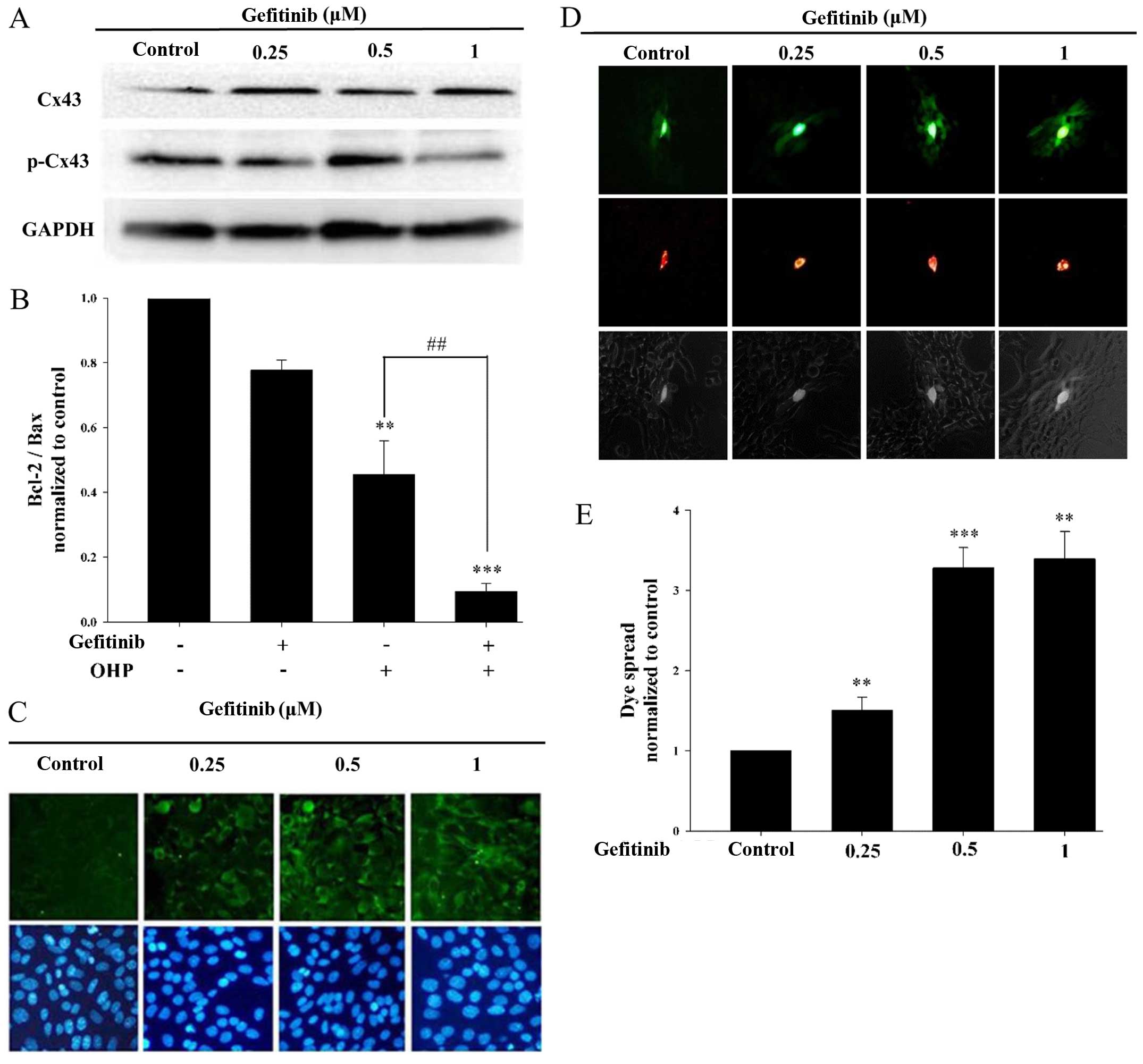
Gefitinib inhibits Cx43
phosphorylation and enhances GJIC
Cx43 is the most abundant connexin in testicular
tissue (31). Our previous study
demonstrated that GJ channels composed of Cx43 decreased the ratio
of Bcl-2/Bax and increased the cleavage of caspase-3 and −9 during
oxaliplatin-induced apoptosis in I-10 cells (8). To determine whether gefitinib could
affect the function of GJIC in I-10 cells, western blotting was
used to assess the expression levels of Cx43 and p-Cx43 (Fig. 4A). As shown in Fig. 4B, gefitinib increased the expression
of Cx43 (functional GJ), and contrarily decreased the expression of
p-Cx43 (non-effective GJ). Moreover, Cx43 protein expression on the
membrane was assessed by immunofluorescence assay. As shown in
Fig. 4C, gefitinib increased the
expression of Cx43 on the membrane, which was consistent with the
western blotting results. Parachute assay was used to measure the
effect of gefitinib on GJIC (Fig.
4D). As shown in Fig. 4E,
gefitinib markedly increased the degree of dye spread which
represents GJIC function. These results indicate that the function
of GJIC in I-10 cells was enhanced by the inhibition of Cx43
phosphorylation by gefitinib.
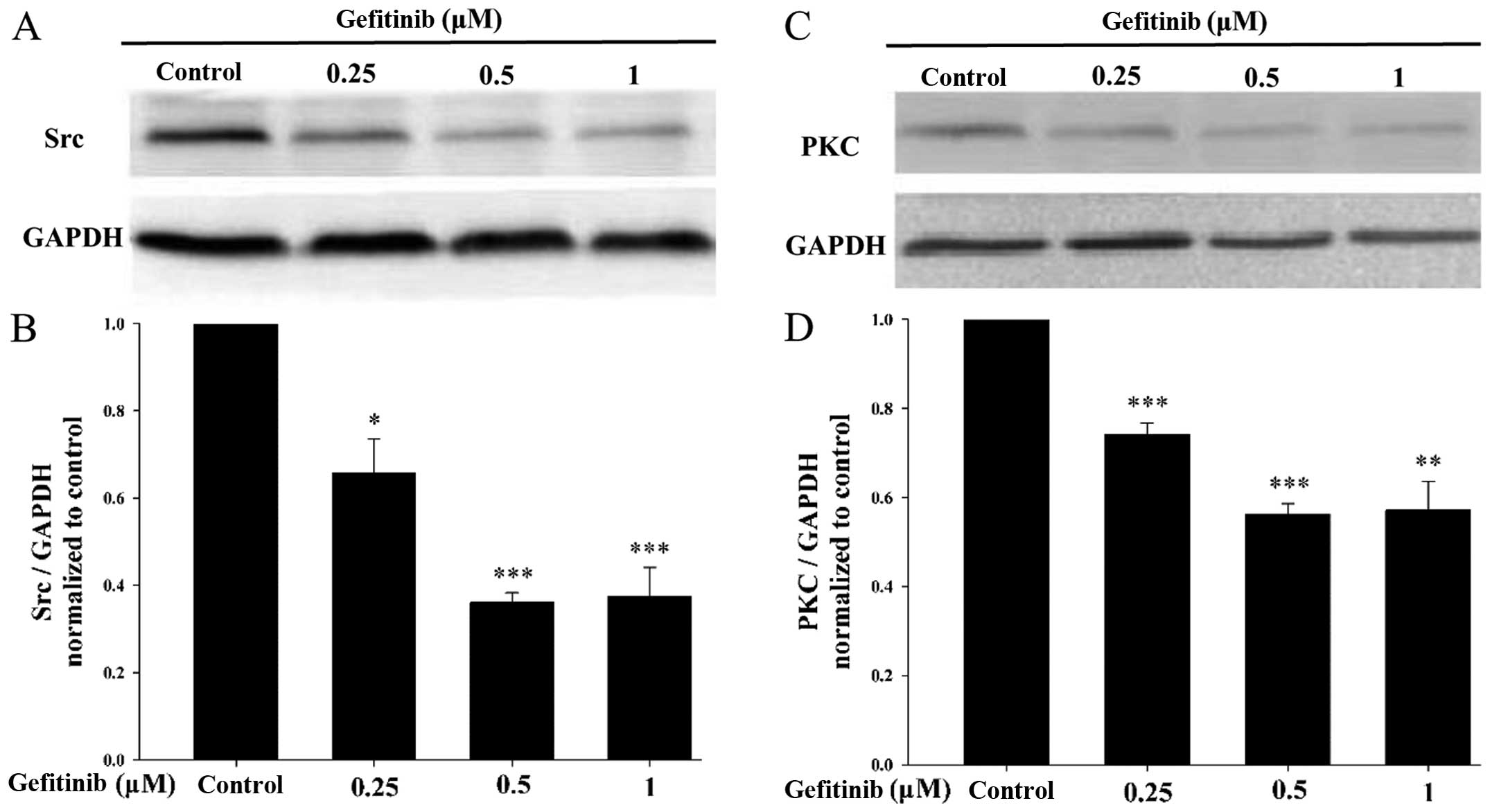
Gefitinib reduces the expression
levels of Src and PKC
Src and PKC inhibit GJIC by increasing Cx43
phosphorylation (12). We aimed to
asertain whether there is a relationship between GJIC function
enhanced by gefitinib and Src and PKC. Western blotting was used to
detect the effects of gefitinib on Src and PKC expression levels.
As shown in Fig. 5, the expression
levels of Src and PKC were obviously decreased by gefitinib.
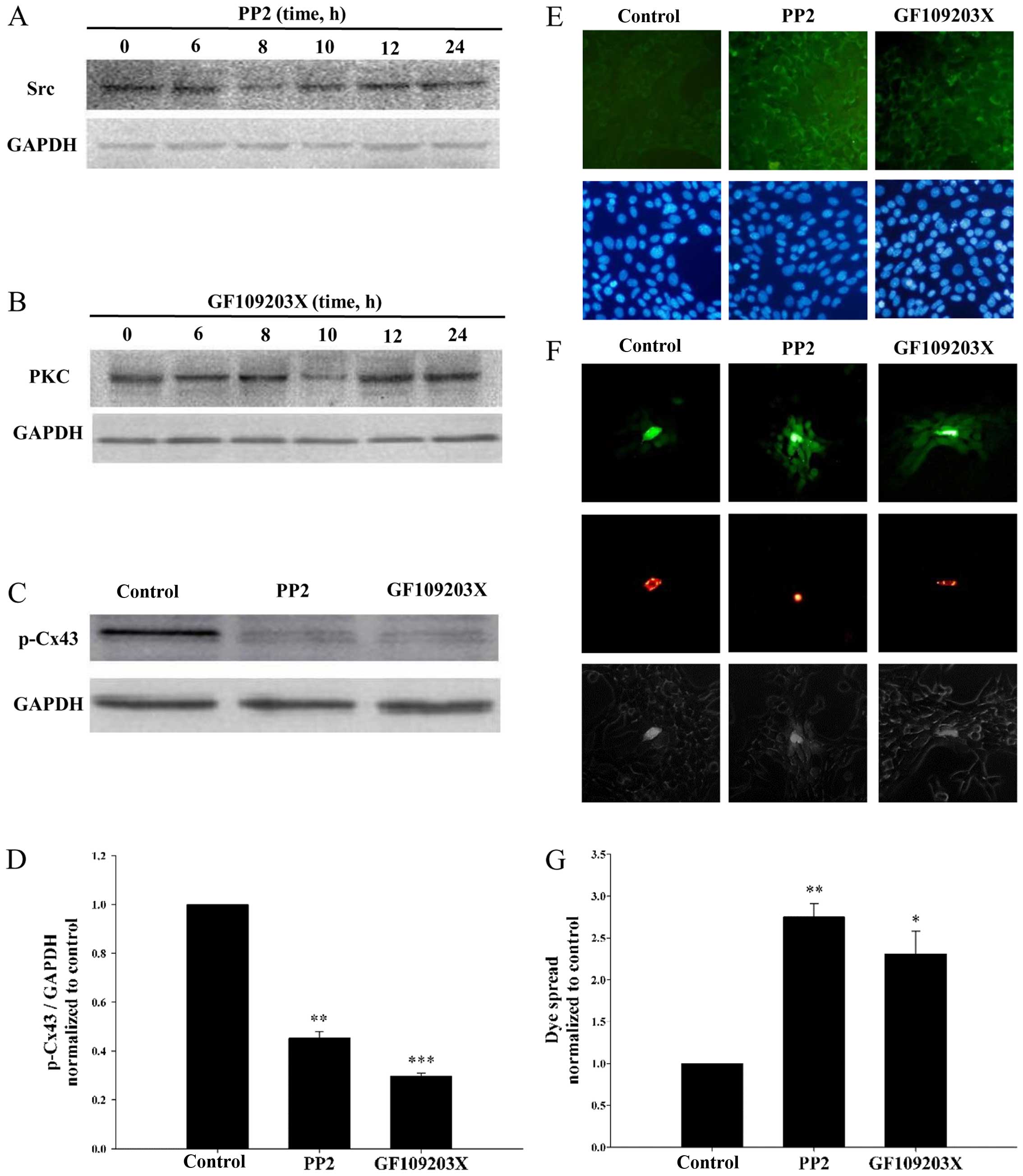
To further verify the relationship between GJIC and
the expression levels of Src and PKC, PP2 (Src inhibitor) and
GF109203X (PKC inhibitor) were used to treat the I-10 cells. Due to
the time restriction concerning PP2 and GF109203X, cells were
treated with 8 µM PP2 and 10 µM GF109203X for 0, 6, 8, 10, 12 and
24 h. As shown in Fig. 6A and B,
the optimum time points for the inhibition of Src and PKC were 8
and 10 h. Thus, I-10 cells were incubated with PP2 for 8 h;
GF109203X for 10 h in the next experiments. Then, the expression of
p-Cx43 was detected by western blotting (Fig. 6C). PP2 and GF109203X significantly
decreased p-Cx43 expression (Fig.
6D). In contrast, the expression of Cx43 on the membrane was
largely increased by PP2 and GF109203X (Fig. 6E). Moreover, the parachute assay was
used to detect the effects of PP2 and GF109203X on GJIC (Fig. 6F). As shown in Fig. 6G, PP2 and GF109203X significantly
enhanced GJIC. These results further determined that gefitinib
enhanced GJIC (Fig. 4) by
inhibiting Src- and PKC-induced Cx43 phosphorylation.
Discussion
The results of the present study showed for the
first time that gefitinib can enhance oxaliplatin-induced apoptosis
in I-10 testicular cancer cells as assessed by cell survival,
apoptosis, Bcl-2/Bax (apoptosis regulator) and activation of
caspase-3 and −9. Moreover, we demonstrated that gefitinib enhanced
gap junction intercellular communication (GJIC) by inhibiting the
expression levels of Src and PKC which act to increase Cx43
phosphorylation and decrease GJIC. Therefore, the synergistic
influence of gefitinib on oxaliplatin anti-neoplastic effect is
attributable to the upregulation of gap junction (GJ) channels
composed of Cx43.
Oxaliplatin is an essential chemotherapeutic drug in
cancer treatment. The primary mechanism of oxaliplatin-induced
apoptosis is that reactive platinum molecules react with DNA to
form inter-strand and intra-strand DNA adducts that arrest
transcription, DNA replication and repair. As DNA is the essential
genetic material in organisms, DNA-damage results in apoptosis
(13,32,33).
Additionally, oxaliplatin-induced apoptosis is influenced by a
variety of factors, such as p53, autophagy, Bcl-2 family and
receptor interacting protein kinase 1 (RIP1). Shi et al
(34) reported that
oxaliplatin-induced colorectal cancer cell apoptosis was enhanced
by apoptosis stimulated protein of p53-2 (ASPP2) via inhibition of
autophagy. Timme et al (35)
showed that increased protein levels of Mcl-1 and/or Bcl-xL as well
as reduction in Bax and Bak activation attenuated
oxaliplatin-induced apoptosis in human colon cancer cells. Shan
et al (36) indicated that
downregulation of RIP1 promoted oxaliplatin-induced apoptosis in
tongue squamous cell carcinoma.
Recent studies have reported that gefitinib, one of
the EGFR tyrosine kinase (EGFR-TK) inhibitors, shows an antitumor
effect in bladder cancer (37) and
glioblastoma (38). Gefitinib
causes the malfunction of EGFR-TK which leads to obstruction of
mitogen-activated protein kinase (MAPK) and the
phosphatidylinositol-3-kinase (PI3K)/AKT pathways. As a result,
cancer cell proliferation is decreased while apoptosis is increased
(39,40). In the present study, we showed that
oxaliplatin-induced apoptosis is enhanced by gefitinib in I-10
cells. Our previous studies demonstrated that the mitochondrial
pathway is involved in oxaliplatin-induced apoptosis (8). To explore the mechanisms, we first
determined the expression of apoptosis-related proteins. The
Bcl-2/Bax signaling pathway and caspase-3 and −9 are crucial
regulators of apoptosis. Long et al (41) reported that the expression of
Bcl-2/Bax is involved in diabetes mellitus-induced testicular
damages. Bcl-2, an anti-apoptotic protein in the Bcl-2 protein
family, prevents cell apoptosis by interfering with the
caspase-3-dependent proteolytic cascade (42). In contrast, Bax is a pro-apoptotic
factor which binds to and antagonizes the Bcl-2 protein. In the
present study, we showed that gefitinib significantly decreased the
ratio of Bcl-2/Bax during oxaliplatin-induced apoptosis in I-10
cells. Caspase protein is downstream of Bcl-2/Bax and caspase
activation acts as the most important executor for chemotherapeutic
drug-induced apoptosis. In the present study, the cleavage of
caspase-3 and −9 was increased by gefitinib during
oxaliplatin-induced apoptosis in I-10 cells. These results suggest
that the oxaliplatin-induced apoptosis enhanced by gefitinib is
associated with downregulation of Bcl-2/Bax and activation of
caspase-3 and −9.
The cytotoxicity of chemotherapy and radiotherapy is
enhanced by GJIC in a variety of tumor cells (43–46).
Toxic drug metabolites and apoptotic signals can propagate through
GJ channels, so that the toxic effects are amplified. This is
called the ‘bystander’ effect. The candidate signals include the
following second messengers such as 1,4,5-trisphosphate
(IP3) and Ca2+. Eugenin and Berman (47) showed that IP3 and
intracellular Ca2+ released from HIV-infected astrocytes
transmitted through GJs resulted in bystander apoptosis of
neighboring uninfected astrocytes. Decrock et al (48) reported that the cytochrome
c-induced apoptosis was markedly dependent on the GJ
channels permeable to IP3.
Cx43 is the most abundant connexin in testicular
tissue. Its life cycle (trafficking, assembly, gating,
internalization and degradation) is related to phosphorylation and
phosphorylation event leading to the downregulation of GJIC
(12). The activation of EGFR
contributes to Src activation, which mediates direct
phosphorylation of Cx43 (49–52).
Furthermore, PKC activated directly by Src mediates the
phosphorylation of Cx43, causing decreased GJ assembly and reduced
half-life of Cx43 (51,53). As a result, GJIC is inhibited
(channel closure) by Src and PKC. In the present study, we showed
that gefitinib inhibited Cx43 phosphorylation and enhanced the
function of GJIC in I-10 cells. Meanwhile, the expression levels of
Src and PKC were decreased by gefitinib. Moreover, PP2 (Src
inhibitor) and GF109203X (PKC inhibitor) also enhanced the function
of GJIC. Therefore, we attribute the enhanced GJIC to the decreased
Src and PKC expression levels induced by gefitinib.
In summary, our results clearly demonstrated that
gefitinib enhanced oxaliplatin-induced apoptosis. In addition,
gefitinib downregulated the ratio of Bcl-2/Bax and increased
cleavage of caspase-3 and −9 during oxaliplatin-induced apoptosis.
We attributed these effects to the enhanced GJIC induced by
gefitinib through inhibition of the expression levels of Src and
PKC. These findings offer a new therapeutic strategy for testicular
cancer chemotherapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (81402930), the Anhui
Provincial Natural Science Foundation of the Institution of Higher
Education (no. KJ2015A180), the Anhui Province Outstanding Youth
Elite Support Program of the Institution of Higher Education (no.
gxyqZD2016158), and the Bengbu Medical College Natural Science
Foundation (no. BYKY1407ZD).
References
|
1
|
Goodenough DA, Goliger JA and Paul DL:
Connexins, connexons, and intercellular communication. Annu Rev
Biochem. 65:475–502. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nielsen MS, Axelsen LN, Sorgen PL, Verma
V, Delmar M and Holstein-Rathlou NH: Gap junctions. Compr Physiol.
2:1981–2035. 2012.PubMed/NCBI
|
|
3
|
Su V and Lau AF: Connexins: Mechanisms
regulating protein levels and intercellular communication. FEBS
Lett. 588:1212–1220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vinken M, Vanhaecke T, Papeleu P, Snykers
S, Henkens T and Rogiers V: Connexins and their channels in cell
growth and cell death. Cell Signal. 18:592–600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stains JP and Civitelli R: Connexins in
the skeleton. Semin Cell Dev Biol. 50:31–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Plotkin LI and Stains JP: Connexins and
pannexins in the skeleton: Gap junctions, hemichannels and more.
Cell Mol Life Sci. 72:2853–2867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kidder GM and Cyr DG: Roles of connexins
in testis development and spermatogenesis. Semin Cell Dev Biol.
50:22–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tong X, Han X, Yu B, Yu M, Jiang G, Ji J
and Dong S: Role of gap junction intercellular communication in
testicular leydig cell apoptosis induced by oxaliplatin via the
mitochondrial pathway. Oncol Rep. 33:207–214. 2015.PubMed/NCBI
|
|
9
|
Yu BB, Dong SY, Yu ML, Jiang GJ, Ji J and
Tong XH: Total flavonoids of Litsea coreana enhance the
cytotoxicity of oxaliplatin by increasing gap junction
intercellular communication. Biol Pharm Bull. 37:1315–1322. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takano Y, Iwata H, Yano Y, Miyazawa M,
Virgona N, Sato H, Ueno K and Yano T: Up-regulation of connexin 32
gene by 5-aza-2-deoxycytidine enhances vinblastine-induced
cytotoxicity in human renal carcinoma cells via the activation of
JNK signalling. Biochem Pharmacol. 80:463–470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang RP, Hossain MZ, Huang R, Gano J, Fan
Y and Boynton AL: Connexin 43 (cx43) enhances chemotherapy-induced
apoptosis in human glioblastoma cells. Int J Cancer. 92:130–138.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thévenin AF, Kowal TJ, Fong JT, Kells RM,
Fisher CG and Falk MM: Proteins and mechanisms regulating
gap-junction assembly, internalization, and degradation.
Physiology. 28:93–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wheate NJ, Walker S, Craig GE and Oun R:
The status of platinum anticancer drugs in the clinic and in
clinical trials. Dalton Trans. 39:8113–8127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marmiroli P, Cavaletti G, Carozzi V, Riva
B, Lim D and Genazzani AA: Calcium-related neurotoxicity of
oxaliplatin: Understanding the mechanisms to drive therapy. Curr
Med Chem. 22:3682–3694. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Cesare Mannelli L, Tenci B, Zanardelli
M, Failli P and Ghelardini C: α7 Nicotinic receptor promotes the
neuroprotective functions of astrocytes against oxaliplatin
neurotoxicity. Neural Plast. 2015:3969082015.PubMed/NCBI
|
|
17
|
Moutinho C, Martinez-Cardús A, Santos C,
Navarro-Pérez V, Martínez-Balibrea E, Musulen E, Carmona FJ,
Sartore-Bianchi A, Cassingena A, Siena S, et al: Epigenetic
inactivation of the BRCA1 interactor SRBC and resistance to
oxaliplatin in colorectal cancer. J Natl Cancer Inst.
106:djt3222014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martinez-Balibrea E, Martínez-Cardús A,
Ginés A, de Porras V Ruiz, Moutinho C, Layos L, Manzano JL, Bugés
C, Bystrup S, Esteller M, et al: Tumor-related molecular mechanisms
of oxaliplatin resistance. Mol Cancer Ther. 14:1767–1776. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin X, Stenvang J, Rasmussen MH, Zhu S,
Jensen NF, Tarpgaard LS, Yang G, Belling K, Andersen CL, Li J, et
al: The potential role of Alu Y in the development of resistance to
SN38 (Irinotecan) or oxaliplatin in colorectal cancer. BMC
Genomics. 16:4042015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ginés A, Bystrup S, de Porras V Ruiz,
Guardia C, Musulén E, Martínez-Cardús A, Manzano JL, Layos L, Abad
A and Martínez-Balibrea E: PKM2 subcellular localization is
involved in oxaliplatin resistance acquisition in HT29 human
colorectal cancer cell lines. PLoS One. 10:e01238302015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukuoka M, Yano S, Giaccone G, Tamura T,
Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S,
Rischin D, et al: Multi-institutional randomized phase II trial of
gefitinib for previously treated patients with advanced
non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin
Oncol. 21:2237–2246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jackman D, Pao W, Riely GJ, Engelman JA,
Kris MG, Jänne PA, Lynch T, Johnson BE and Miller VA: Clinical
definition of acquired resistance to epidermal growth factor
receptor tyrosine kinase inhibitors in non-small-cell lung cancer.
J Clin Oncol. 28:357–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Yao K, Shi C, Jiang Y, Liu K,
Zhao S, Chen H, Reddy K, Zhang C, Chang X, et al: 244-MPT overcomes
gefitinib resistance in non-small cell lung cancer cells.
Oncotarget. 6:44274–44288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu W, Ning J, Li C, Hu J, Meng Q, Lu H
and Cai L: Overexpression of Sphk2 is associated with gefitinib
resistance in non-small cell lung cancer. Tumour Biol.
37:6331–6336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen KL, Lin CC, Cho YT, Yang CW, Sheen
YS, Tsai HE and Chu CY: Comparison of skin toxic effects associated
with gefitinib, erlotinib, or afatinib treatment for non-small cell
lung cancer. JAMA Dermatol. 152:340–342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xue C, Hong S, Li N, Feng W, Jia J, Peng
J, Lin D, Cao X, Wang S, Zhang W, et al: Randomized, multicenter
study of gefitinib dose-escalation in advanced non-small-cell lung
cancer patients achieved stable disease after one-month gefitinib
treatment. Sci Rep. 5:106482015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fisher GA, Kuo T, Ramsey M, Schwartz E,
Rouse RV, Cho CD, Halsey J and Sikic BI: A phase II study of
gefitinib, 5-fluorouracil, leucovorin, and oxaliplatin in
previously untreated patients with metastatic colorectal cancer.
Clin Cancer Res. 14:7074–7079. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li N, Ou W, Ye X, Sun HB, Zhang L, Fang Q,
Zhang SL, Wang BX and Wang SY: Pemetrexed-carboplatin adjuvant
chemotherapy with or without gefitinib in resected stage IIIA-N2
non-small cell lung cancer harbouring EGFR mutations: A randomized,
phase II study. Ann Surg Oncol. 21:2091–2096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji J, Tong XH, Zhang XY, Gao Q, Li BB and
Wu XX: Gefitinib inhibits the growth and induces the apoptosis of
mouse I-10 Leydig testicular cancer cells in vitro. Zhonghua Nan Ke
Xue. 21:797–802. 2015.(In Chinese). PubMed/NCBI
|
|
30
|
Goldberg GS, Bechberger JF and Naus CC: A
pre-loading method of evaluating gap junctional communication by
fluorescent dye transfer. Biotechniques. 18:490–497.
1995.PubMed/NCBI
|
|
31
|
Chevallier D, Carette D, Segretain D,
Gilleron J and Pointis G: Connexin 43 a check-point component of
cell proliferation implicated in a wide range of human testis
diseases. Cell Mol Life Sci. 70:1207–1220. 2013.PubMed/NCBI
|
|
32
|
Todd RC and Lippard SJ: Inhibition of
transcription by platinum antitumor compounds. Metallomics.
1:280–291. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hato SV, Khong A, de Vries IJ and
Lesterhuis WJ: Molecular pathways: The immunogenic effects of
platinum-based chemotherapeutics. Clin Cancer Res. 20:2831–2837.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi Y, Han Y, Xie F, Wang A, Feng X, Li N,
Guo H and Chen D: ASPP2 enhances oxaliplatin (L-OHP)-induced
colorectal cancer cell apoptosis in a p53-independent manner by
inhibiting cell autophagy. J Cell Mol Med. 19:535–543. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Timme CR, Gruidl M and Yeatman TJ:
Gamma-secretase inhibition attenuates oxaliplatin-induced apoptosis
through increased Mcl-1 and/or Bcl-xL in human colon cancer cells.
Apoptosis. 18:1163–1174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shan B, Ma F, Wang M and Xu X:
Down-regulating receptor interacting protein kinase 1 (RIP1)
promotes oxaliplatin-induced Tca8113 cell apoptosis. Med Sci Monit.
21:3089–3094. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mansure JJ, Nassim R, Chevalier S,
Szymanski K, Rocha J, Aldousari S and Kassouf W: A novel mechanism
of PPAR gamma induction via EGFR signalling constitutes rational
for combination therapy in bladder cancer. PLoS One. 8:e559972013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li J, Zhu S, Kozono D, Ng K, Futalan D,
Shen Y, Akers JC, Steed T, Kushwaha D, Schlabach M, et al:
Genome-wide shRNA screen revealed integrated mitogenic signaling
between dopamine receptor D2 (DRD2) and epidermal growth factor
receptor (EGFR) in glioblastoma. Oncotarget. 5:882–893. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Herbst RS, Fukuoka M and Baselga J:
Gefitinib - a novel targeted approach to treating cancer. Nat Rev
Cancer. 4:956–965. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang GC, Yu CT, Tsai CH, Tsai JR, Chen
JC, Wu CC, Wu WJ and Hsu SL: An epidermal growth factor inhibitor,
Gefitinib, induces apoptosis through a p53-dependent upregulation
of pro-apoptotic molecules and downregulation of anti-apoptotic
molecules in human lung adenocarcinoma A549 cells. Eur J Pharmacol.
600:37–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Long L, Wang J, Lu X, Xu Y, Zheng S, Luo C
and Li Y: Protective effects of scutellarin on type II diabetes
mellitus-induced testicular damages related to reactive oxygen
species/Bcl-2/Bax and reactive oxygen
species/microcirculation/staving pathway in diabetic rat. J
Diabetes Res. 2015:2525302015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Swanton E, Savory P, Cosulich S, Clarke P
and Woodman P: Bcl-2 regulates a caspase-3/caspase-2 apoptotic
cascade in cytosolic extracts. Oncogene. 18:1781–1787. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mesnil M, Piccoli C, Tiraby G, Willecke K
and Yamasaki H: Bystander killing of cancer cells by herpes simplex
virus thymidine kinase gene is mediated by connexins. Proc Natl
Acad Sci USA. 93:1831–1835. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vrionis FD, Wu JK, Qi P, Waltzman M,
Cherington V and Spray DC: The bystander effect exerted by tumor
cells expressing the herpes simplex virus thymidine kinase (HSVtk)
gene is dependent on connexin expression and cell communication via
gap junctions. Gene Ther. 4:577–585. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jensen R and Glazer PM:
Cell-interdependent cisplatin killing by Ku/DNA-dependent protein
kinase signaling transduced through gap junctions. Proc Natl Acad
Sci USA. 101:6134–6139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He B, Tong X, Wang L, Wang Q, Ye H, Liu B,
Hong X, Tao L and Harris AL: Tramadol and flurbiprofen depress the
cytotoxicity of cisplatin via their effects on gap junctions. Clin
Cancer Res. 15:5803–5810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Eugenin EA and Berman JW: Cytochrome c
dysregulation induced by HIV infection of astrocytes results in
bystander apoptosis of uninfected astrocytes by an IP3
and calcium-dependent mechanism. J Neurochem. 127:644–651. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Decrock E, Krysko DV, Vinken M, Kaczmarek
A, Crispino G, Bol M, Wang N, De Bock M, De Vuyst E, Naus CC, et
al: Transfer of IP3 through gap junctions is critical,
but not sufficient, for the spread of apoptosis. Cell Death Differ.
19:947–957. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Postma FR, Hengeveld T, Alblas J, Giepmans
BN, Zondag GC, Jalink K and Moolenaar WH: Acute loss of cell-cell
communication caused by G protein-coupled receptors: A critical
role for c-Src. J Cell Biol. 140:1199–1209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sorgen PL, Duffy HS, Sahoo P, Coombs W,
Delmar M and Spray DC: Structural changes in the carboxyl terminus
of the gap junction protein connexin43 indicates signaling between
binding domains for c-Src and zonula occludens-1. J Biol Chem.
279:54695–54701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pahujaa M, Anikin M and Goldberg GS:
Phosphorylation of connexin43 induced by Src: Regulation of gap
junctional communication between transformed cells. Exp Cell Res.
313:4083–4090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Johnstone SR, Billaud M, Lohman AW, Taddeo
EP and Isakson BE: Posttranslational modifications in connexins and
pannexins. J Membr Biol. 245:319–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lampe PD, TenBroek EM, Burt JM, Kurata WE,
Johnson RG and Lau AF: Phosphorylation of connexin43 on serine368
by protein kinase C regulates gap junctional communication. J Cell
Biol. 149:1503–1512. 2000. View Article : Google Scholar : PubMed/NCBI
|