Introduction
Osteosarcoma is the most common type of malignant
bone tumor in children and teenagers (1,2).
Unfortunately, less than 50% of patients living beyond 10 years
results from the response to the current preferred treatment of
preoperative adjuvant chemotherapy followed by surgery (3). Furthermore, no reliable predictors are
applied to guide the choice or the intensity of the chemotherapy
(4). Currently, there is still a
limited role in radiotherapy which should be reserved for
inoperable situations. Doxorubicin, cisplatin, ifosfamide and
high-dose methotrexate with leucovorin rescue are recognized as the
most effective agents against osteosarcoma, but the ideal
combination remains to be defined and needs to further well
investigated (5). Targeting
apoptosis (programmed cell death type I) is a promising approach in
the fight against osteosarcoma (6).
Therefore, the induction of cell apoptosis is one of best
strategies for treating osteosarcoma and multiple cancers (7,8).
Apoptotic function is associated with several
diseases, including cancer genesis, and diabetes, and this process
is considered a critical element of cancer prevention and therapy
(6,9). The three main pathways (death receptor
signal, mitochondrial regulation and endoplasmic reticulum stress)
contribute to apoptotic signaling (10–12).
The death receptor signal is induced by the binding of extrinsic
signals to surface receptors, resulting in activation of caspase-8
followed by the activation of caspase-3/−7 (10,11).
The mitochondrial pathway is regulated by endoplasmic reticulum
(ER) various damage stimuli, which increase reactive oxygen species
(ROS) and subsequent damage DNA. These stresses disrupt
mitochondrial membrane potential (ΔΨm) to cause cells to undergo
apoptosis cascade (11,13). Accumulation of unfolded/misfolded
proteins elicits ER-specific pathway aggregating in ER by excessive
protein traffic (12). The
hallmarks of ER stress are known to stimulate the protein level of
growth arrest- and DNA damage-inducible gene 153 (GADD153),
glucose-regulated protein 78 (GRP78), GRP94 and activating
transcription factor 4 (ATF-4), which can induce intracellular
Ca2+ level to activate calpain and caspase-12 and/or
caspase-4 molecules (13,14). Therefore, induction of apoptosis by
a novel target in these pathways is an attractive approach to fight
the tumor cell system.
The rare earth metals of Lanthanides (Lns) exhibit a
variety of physical or chemical properties and have been applied in
agriculture and medicine for a long time (15,16).
Gadolinium (Gd), a member of Lns family, has multi-biological
effects on organisms (17), and its
compounds have important applications in magnetic resonance imaging
(MRI) as contrast medium and are potential anticancer agents
(18,19). In clinic, chelates of Gd such as
gadobenate dimeglumine (MultiHance) are used as contrast agent in
MRI (20). Gadolinium texaphyrin
complex (MGd) was evaluated in phase III clinical trials for
treating brain metastases of non-small cell lung cancer (21). The Kupffer cell inhibitor gadolinium
chloride (GdCl3) has been demonstrated to inhibit human
hepatoma HepG2 cell proliferation (22,23).
It was also shown that GdCl3 induces HepG2 cell
apoptosis through mitochondria-dependent pathway (22). Hence, the present study investigated
the effects of GdCl3-induced apoptosis and the
underlying mechanisms on human osteosarcoma U-2 OS cells. The
experimental data indicated that GdCl3 triggered U-2 OS
cell apoptosis through death receptor-, mitochondria- and ER
stress-dependent pathways.
Materials and methods
Chemicals and reagents
Gadolinium chloride (GdCl3),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and 4,6-diamidino-2-phenylindole (DAPI) were obtained from
Sigma-Aldrich Co. (St. Louis, MO, USA). McCoys 5A (modified)
medium, fetal bovine serum (FBS), trypsin-EDTA,
penicillin/streptomycin, Fluo-3/AM, 2,7-dichlorodihydrofluorescein
diacetate (H2DCFDA) and 3,3-dihexyloxacarbocyanine
iodide [DiOC6(3)] were
obtained from Thermo Fisher Scientific Inc. (Waltham, MA, USA).
Pan-caspase inhibitor (Z-VAD-FMK), caspase-3 inhibitor
(Z-DEVD-FMK), caspase-8 inhibitor (Z-IETD-FMK) and caspase-9
inhibitor (Z-LEHD-FMK) were purchased from R&D Systems
(Minneapolis, MN, USA). Primary antibodies (anti-Fas/CD95,
anti-FasL, anti-cytochrome c, anti-Apaf-1, anti-GADD153 and
anti-GRP78 and anti-β-actin) and second antibodies [goat
anti-rabbit IgG-horseradish peroxidase (HRP) and goat anti-mouse
IgG-HRP] were obtained from Santa Cruz Biotechnology (Santa Cruz,
CA, USA).
Cell culture
Human osteogenic sarcoma U-2 OS cell line was
obtained from the Bioresource Collection and Research Center (BCRC;
Hsinchu, Taiwan) and cultured in 75-cm2 tissue culture
flasks with McCoys 5A (modified) medium with 10% FBS, 100 Units/ml
penicillin and 100 µg/ml streptomycin at 37°C with 5%
CO2.
Cell viability and morphological
observation
U-2 OS cells (1×104 cells/well) in a
96-well plate were individually pretreated with or without 15 µM
specific caspase inhibitors (Z-VAD-FMK, Z-IETD-FMK, Z-LEHD-FMK and
Z-DEVD-FMK) for 2 h and then exposed to 0, 50, 100, 150 and 200 µM
of GdCl3 for 24 h. After challenge, cell viability was
assessed by an MTT assay, as previously reported by Lu et al
(24). The GdCl3-treated
cells were examined for apoptosis and photographed utilizing a
phase-contrast microscope, as previously described (25).
Apoptosis by TUNEL and DAPI
staining
U-2 OS cells (2×105 cells/well) in
24-well plates were treated with 0, 50, 100, 150 and 200 µM of
GdCl3 for 24 h. Apoptotic DNA breaks were labeled with
the In Situ Cell Death Detection kit, Fluorescein (Roche
Diagnostics GmbH, Mannheim, Germany) according to the vendors
protocol, and the terminal deoxynucleotidyl transferase
(TdT)-mediated dUTP nick-end labeling (TUNEL) positive cells were
monitored using a BD FACSCalibur flow cytometry (BD Biosciences,
San Jose, CA, USA) and BD CellQuest Pro Software (BD Biosciences),
as previously described (26). DAPI
dye was used to countstain condensation nuclei (a characteristic of
apoptosis) in U-2 OS cells with or without 200 µM GdCl3
for 24 h, as detailed by previous studies (27,28).
Assays for caspase-8/−9/−3/−4
activities
U-2 OS cells (5×106 cells per 75T flask)
were exposed to 0, 50, 100, 150 and 200 µM of GdCl3 for
24 h. The cell lysates were harvested and assessed subsequently in
accordance with the manufacturers instruction [caspase-8, caspase-9
and caspase-3 colorimetric assay kits (R&D System Inc.) and
caspase-4 colorimetric assay kit (BioVision Incorporated, Milpitas,
CA, USA)].
Determinations of intracellular
Ca2+, ROS and ΔΨm levels by flow cytometry
U-2 OS cells (2×105 cells/well) were
incubated with or without 50, 100, 150 and 200 µM of
GdCl3 for 6 h. Cells were harvested and individually
stained with 3 µg/ml Fluo-3/AM for cytoplasmic Ca2+, ROS
indicator (5 µM H2DCFDA) and ΔΨm probe [4 nM
DiOC6(3)] at 37°C for 30
min. The mean fluorescence intensity (MFI) was quantified by BD
CellQuest Pro software (BD Biosciences) before analysis by flow
cytometry, as previously described (13,26).
Western blot analysis
U-2 OS cells (5×106 cells/75T flask) were
treated in the presence and absence of 0, 50, 100, 150 and 200 µM
of GdCl3 for 24 h. Cell lysates were collected, and
protein was fractionated on SDS-polyacrylamide gel electrophoresis
(PAGE) after being mixed with protein loading dye and boiled, as
previously described (14,29). The membrane (iBlot Transfer Stack,
PVDF regular; Thermo Fisher Scientific Inc.) was probed with
antibodies against Fas, FasL, cytochrome c, Apaf-1, GADD153
and GRP78, respectively. All blots were normalized to β-actin
signal, and each signal was quantified with NIH ImageJ program for
Windows (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical calculations of the data for comparisons
of two mean values were obtained using Students t-test. Statistical
significance was set at P<0.05. All data were expressed as mean
± standard deviation (SD) of three independent experiments.
Results
GdCl3 reduces cell
viability of human osteosarcoma U-2 OS cells
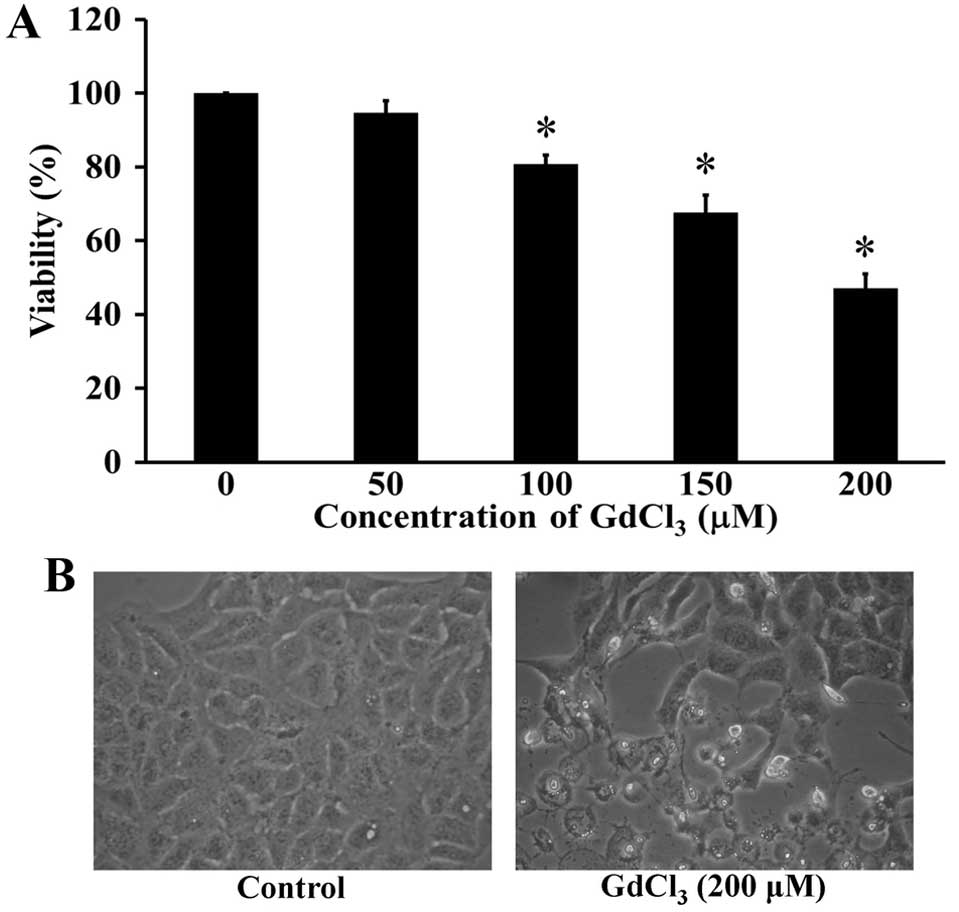
Cells were treated with GdCl3 at the
different concentrations from 0 to 200 µM for 24 h. The number of
viable cells was assessed by the MTT method. The cell viability was
markedly decreased in GdCl3-treated U-2 OS cells in
concentration-dependent manner (Fig.
1A). The inhibitory concentration 50% (IC50) for U-2
OS cells was 198.26±1.69 µM. GdCl3 at 200 µM led to the
morphological changes and apoptotic cell shrinkage in U-2 OS cells.
Also, GdCl3-treated cells were detached from the
surface, and some cell debris was observed, whereas the untreated
control cells were well spread (Fig.
1B). These findings suggested that GdCl3 possessed
cytotoxic effect on human osteosarcoma U-2 OS cells.
GdCl3 provokes U-2 OS cell
apoptosis
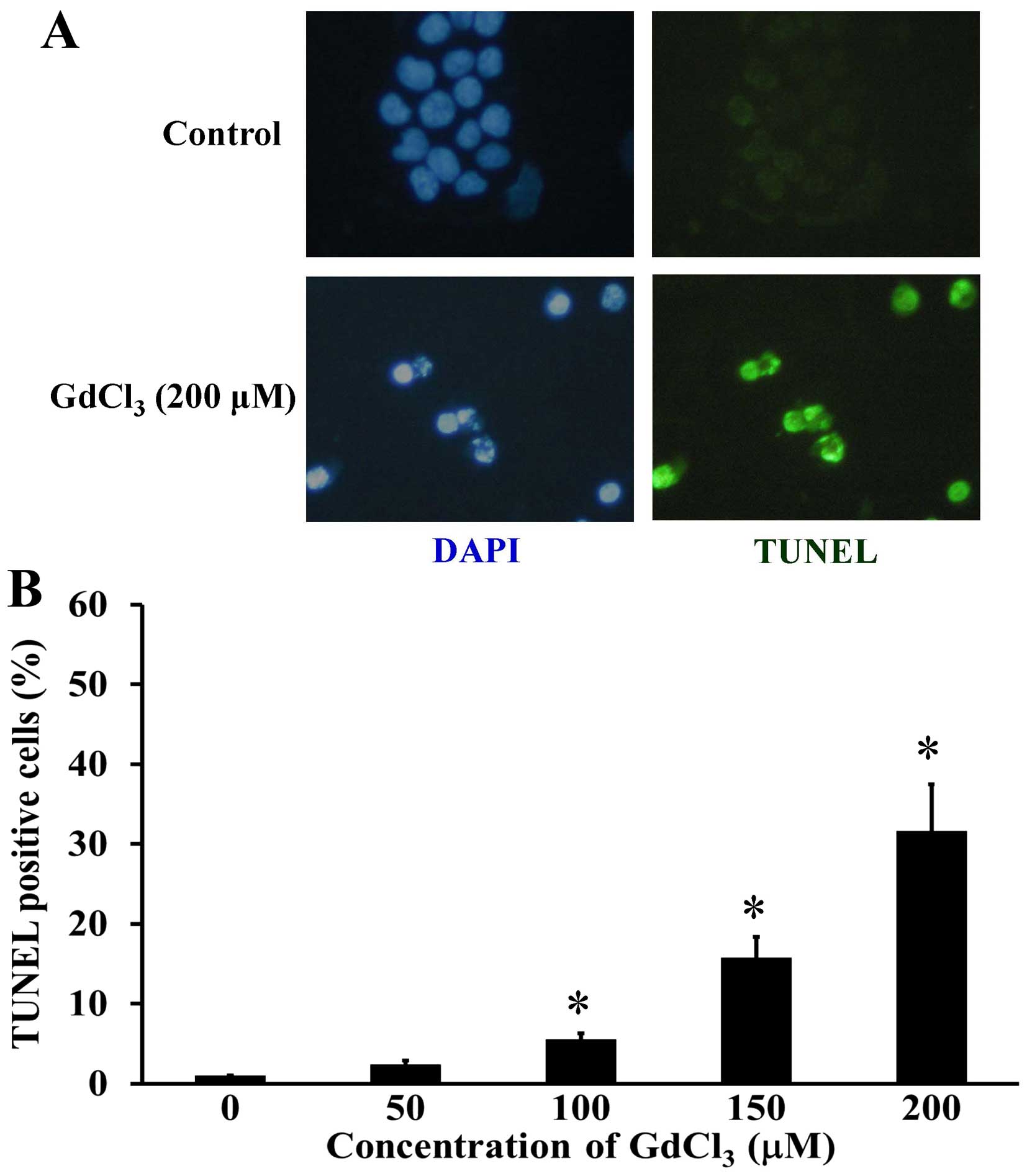
To explore whether GdCl3 induces U-2 OS
cell death through an apoptotic mechanism, TUNEL assay was used for
detecting DNA fragmentation in apoptotic cells. GdCl3
markedly stimulated cell apoptosis (TUNEL positive cells) in
comparison with control cells (Fig.
2A), and this effect was concentration-dependent (Fig. 2B). Furthermore, apoptotic DNA
condensation by DAPI stain was shown in GdCl3-treated
U-2 OS cells (Fig. 2A). Our results
indicated that GdCl3 provoked apoptotic cell death in
U-2 OS cells.
GdCl3 stimulates the
activities of caspase-3, caspase-8, and caspase-9 in U-2 OS
cells
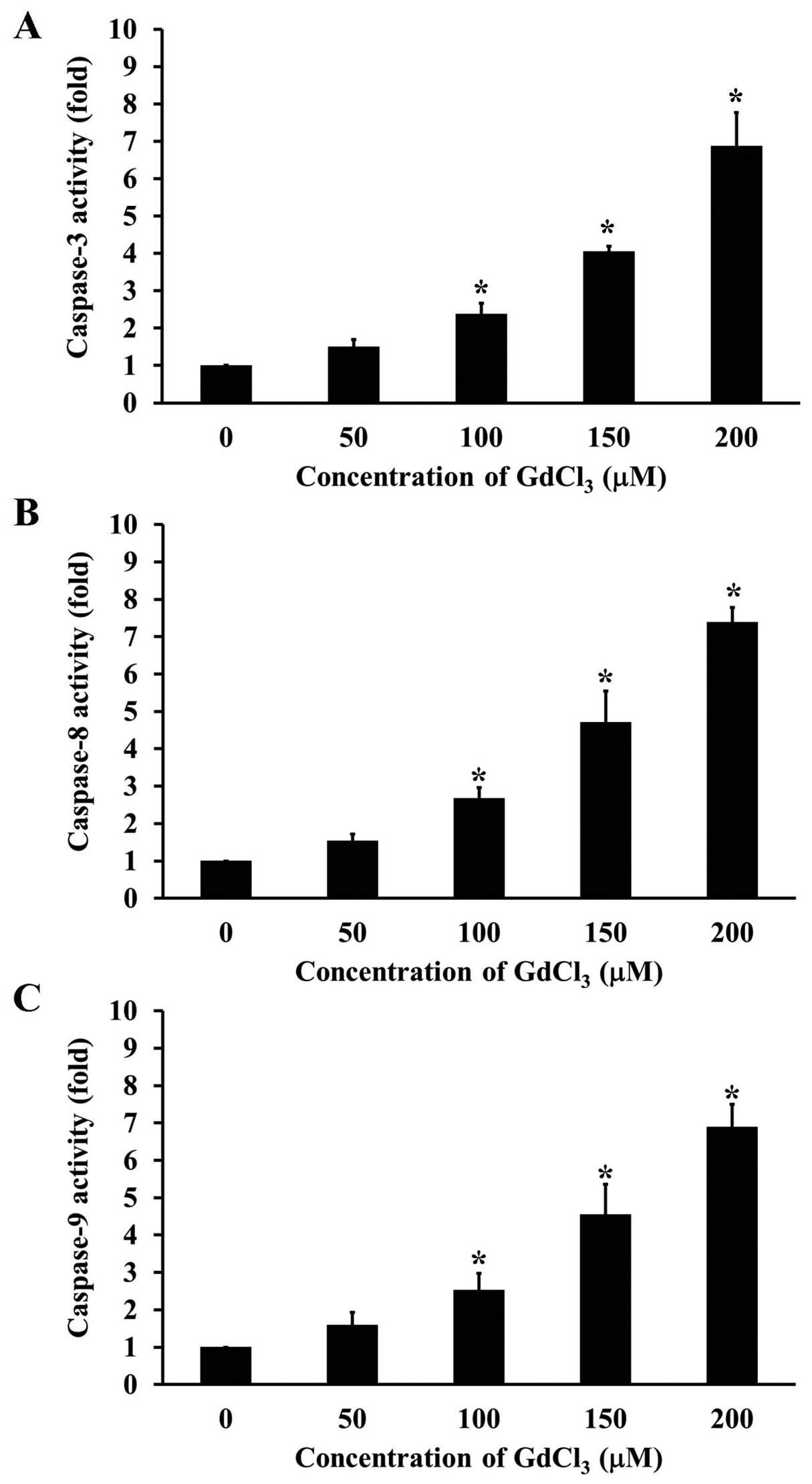
To clarify the mechanism of GdCl3-induced
apoptosis, we further individually investigated the activities of
caspase-3, caspase-8, and caspase-9 in U-2 OS cells prior to
GdCl3 challenge. The activities of caspase-3 (Fig. 3A), caspase-8 (Fig. 3B) and caspase-9 (Fig. 3C) were increased after
GdCl3 exposure in a concentration-dependent manner.
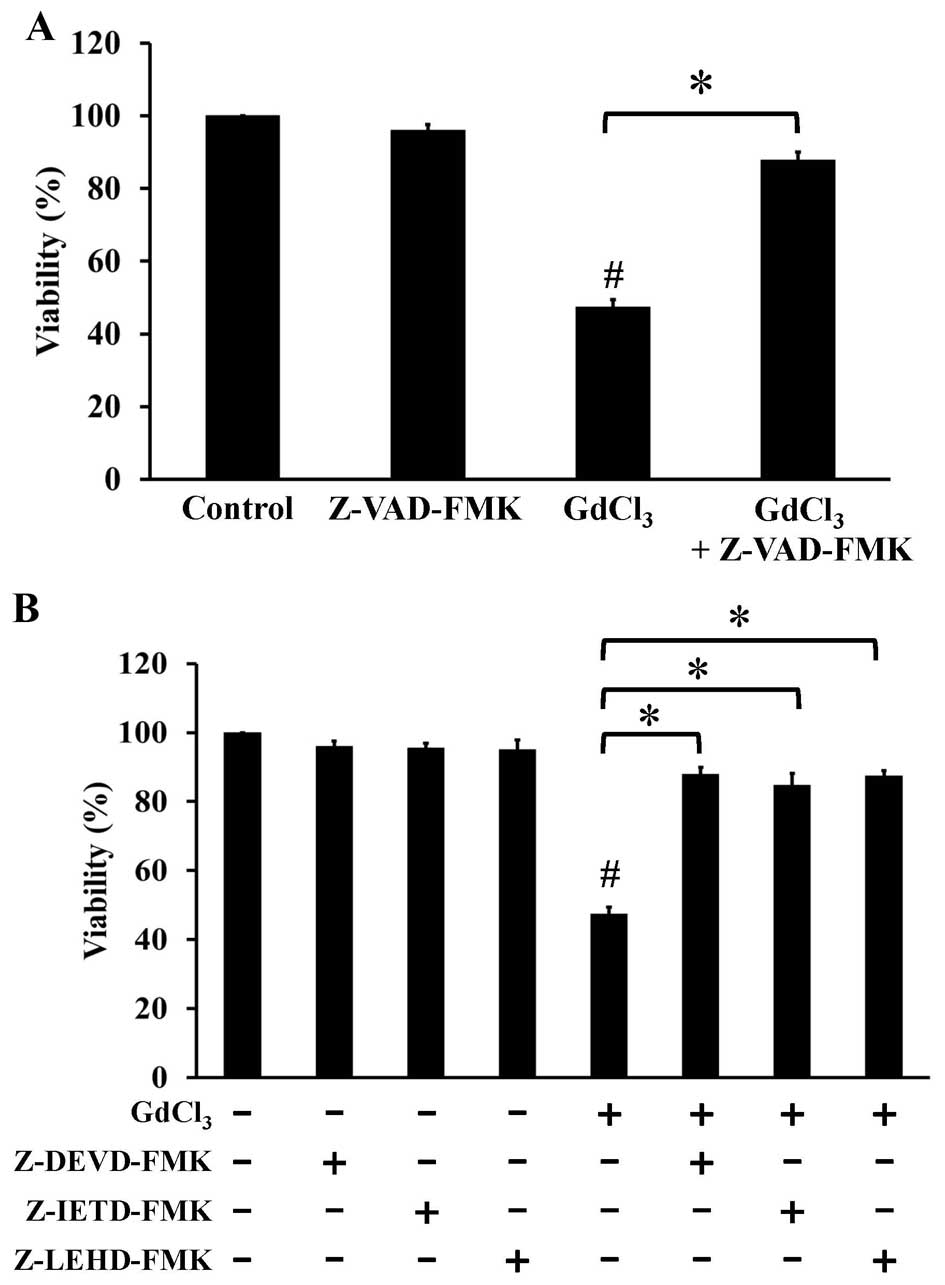
Pretreatment of cells with pan-caspase inhibitor (Z-VAD-FMK)
(Fig. 4A), specific inhibitors to
caspase-3, caspase-8, and caspase-9 (Fig. 4B) significantly prevented the
GdCl3-induced cell apoptosis. Our results suggest that
the caspase cascade-dependent pathway is important in
GdCl3-induced apoptotic death in U-2 OS cells.
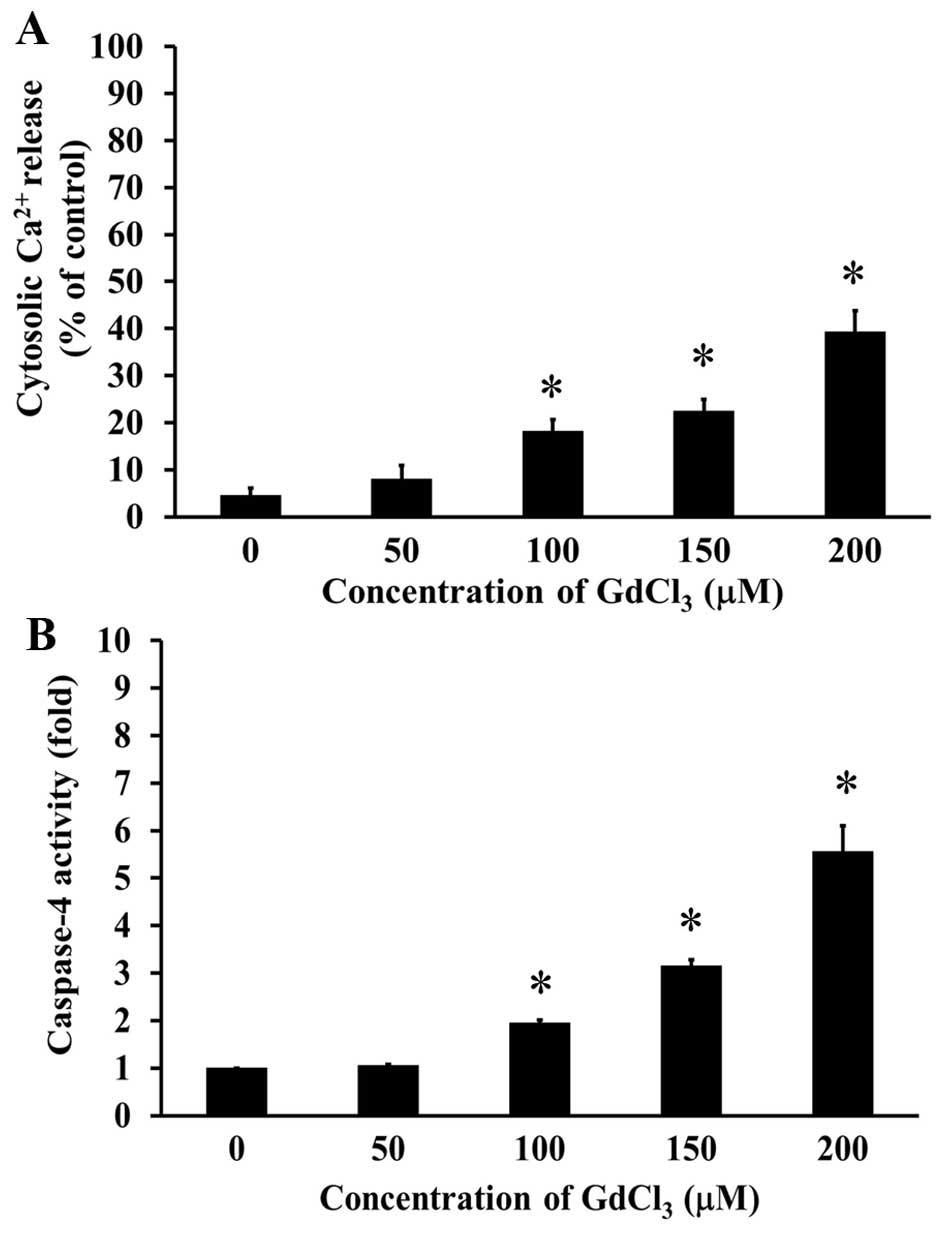
GdCl3 increases cytoplasmic
Ca2+ level and stimulated the activity of caspase-4 in
U-2 OS cells
To further elucidate the possible signaling of
GdCl3-induced apoptosis in U-2 OS cells, we determined
intracellular Ca2+ levels and caspase-4 activity by flow
cytometric analysis and caspase activity assay, respectively. Cells
were treated with 50, 100, 150 and 200 µM of GdCl3 for
24 h, and our data demonstrated that GdCl3 significantly
increased intracellular Ca2+ levels (Fig. 5A). In addition, caspase-4 activity
was concentration-dependently increased in U-2 OS cells after
GdCl3 treatment (Fig.
5B). These results showed that GdCl3 induced
apoptotic response through cytoplasmic Ca2+ level and ER
stress-mediated pathway.
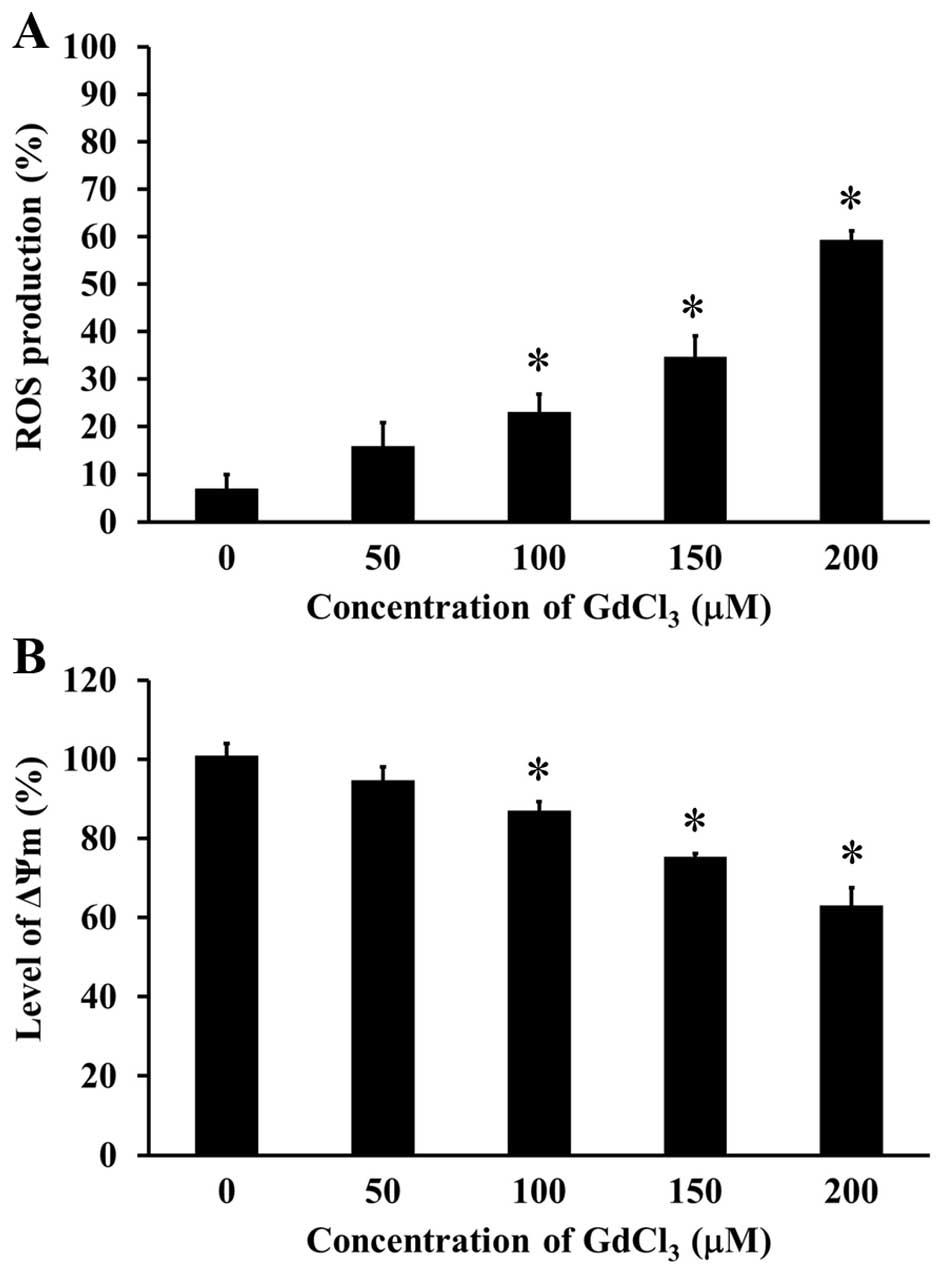
GdCl3 stimulates the ROS
production and loss of ΔΨm level in U-2 OS cells
To examine the effect of GdCl3 on ROS
production and ΔΨm levels in U-2 OS cells, the specific
fluorescence probes were individually used to detect the levels of
ROS and ΔΨm. GdCl3 concentration-dependently increased
intracellular ROS level in U-2 OS cells (Fig. 6A). GdCl3 disrupted ΔΨm
level in U-2 OS cells in a concentration-dependent manner (Fig. 6B). These results suggested that
GdCl3 triggered apoptosis in U-2 OS cells by way of the
ROS and mitochondria-dependent signaling.
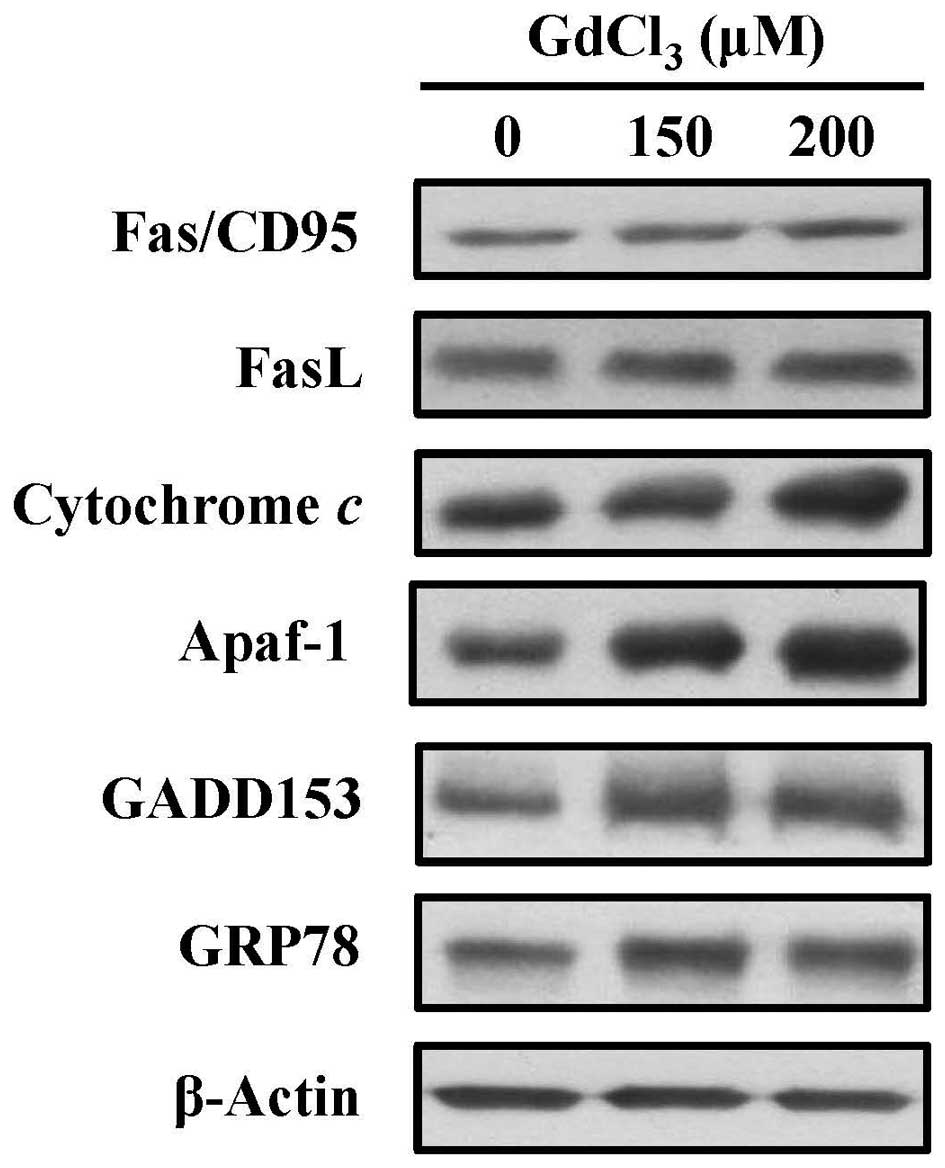
GdCl3 alters the levels of
apoptosis-related proteins in U-2 OS cells
In more detail, in the molecular mechanisms of
apoptotic pathway, we investigated these related protein levels by
western blotting. The levels of Fas, FasL, cytochrome c,
Apaf-1, GADD153 and GRP78 were increased in
GdCl3-treated cells (Fig.
7). These results suggested that GdCl3-induced
apoptotic action might be involved in death receptor-mediated
signaling, mitochondria-dependent pathway and ER stress in U-2 OS
cells.
Discussion
Lanthanide (Ln) compounds have been shown to possess
contrary effects on anticancer activities such as promotion of cell
cycle progression and cell growth by lower concentration treatment
but induction of apoptosis and suppression of cell proliferation at
higher dosing (16,18,19).
Our results indicated that high concentration of GdCl3
caused apoptotic U-2 OS cell death (Fig. 1). This is first to report that the
MRI contrast agent GdCl3 can be successfully applied to
induce apoptosis of human osteosarcoma cells. Moreover, an increase
of TUNEL positive cells (Fig. 2),
activation of caspase-3, caspase-8, caspase-9 (Fig. 3) and caspase-4 (Fig. 5B) was found to suggest that
GdCl3 caused apoptosis of U-2 OS through multiple
pathways. It is well know that Gd3+, like other Ln ions,
caused different effects depending on the type of target cells
(30,31). In NIH 3T3 cells, GdCl3 at
the concentrations of <100 µM exerts proliferation-promoting
effect and activation of extracellular signal-regulated kinase
(ERK) and phosphoinositide 3-kinase (PI3K) signaling pathways
(31). Gadolinium provokes cell
apoptosis through mitochondrial pathway and oxidative stress in
L-02 normal liver cells (32).
Ye et al (22) reported that GdCl3 induces
HepG2 cell apoptosis through mitochondrial and death
receptor-dependent pathways. Similarly, our results also
demonstrated that GdCl3 induced apoptosis through death
receptor and mitochondria-dependent pathways in U-2 OS cells. In
the present study, we found that GdCl3 increased the ROS
production and decreased the levels of ΔΨm (Fig. 6), and western blot analysis showed
that GdCl3 treatment resulted in an increase of
Fas/CD95, FasL, cytochrome c and Apaf-1 protein expression
in U-2 OS cells (Fig. 7). Thus, we
suggest that GdCl3-induced cell death may be mediated
via extrinsic and intrinsic apoptotic pathway in U-2 OS cells.
Calcium (Ca2+) regulates cell
proliferation, apoptosis or differentiation and plays a pivotal
role in carcinogenesis and progression (33). Xia et al (34) has indicated that gadolinium-induced
oxidative stress causes ER stress in rat cortical neurons. Feng
et al (35) also showed that
gadolinium triggers unfolded protein responses (UPRs) in primary
cultured rat cortical astrocytes through increasing an influx of
extracellular Ca2+ level. However, there is no available
report regarding GdCl3-induced ER stress in U-2 OS
cells. In the present study, we investigated the
GdCl3-induced ER stress in apoptotic mechanism of U-2 OS
cells. Our findings demonstrated that the increased levels of
GADD153 and GRP78 were followed by releasing Ca2+ from
ER and activating caspase-4 activity (Fig. 5), finally leading to ER stress and
cell apoptosis. Our results revealed that the activation of ER
stress signaling contribute to GdCl3-induced apoptosis
in U-2 OS cells.
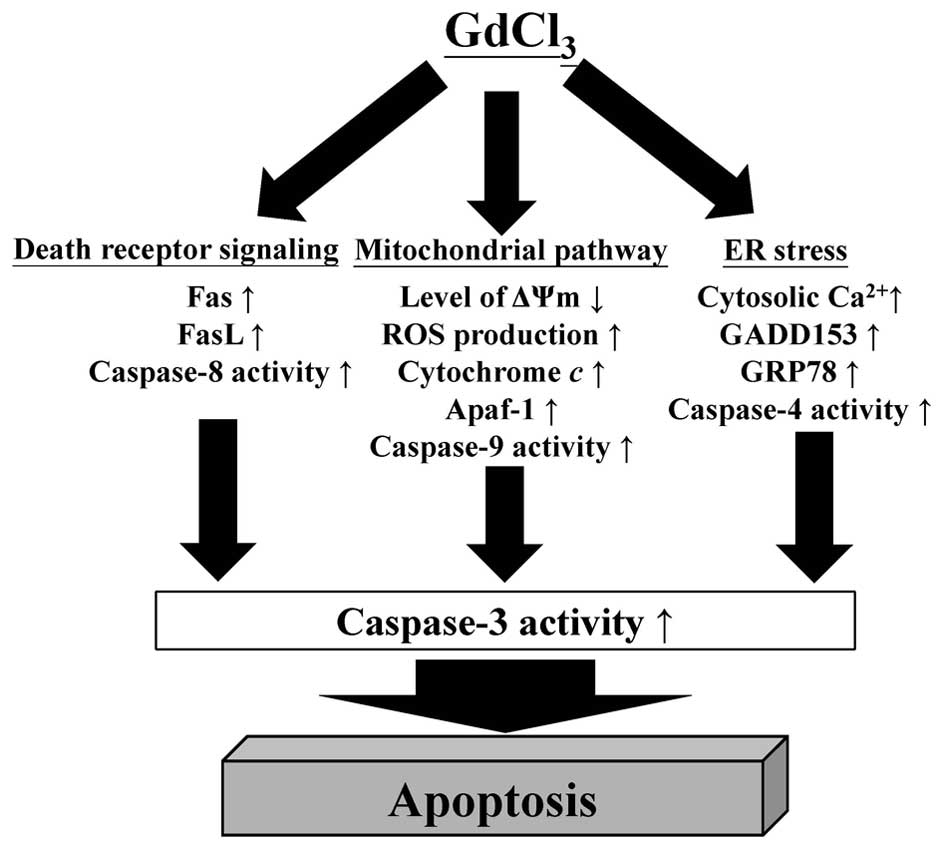
In conclusion, we found that GdCl3
exhibits direct anticancer activity and triggers suppression of
tumor cell proliferation in human osteosarcoma cells.
GdCl3 provoked U-2 OS cell apoptosis by the way of the
death receptor, mitochondria-dependent and ER stress pathways. The
proposed pathway of GdCl3-induced apoptosis of human
osteosarcoma U-2 OS cells is showing in Fig. 8. Taken together, our findings
provide important possible molecular mechanism against osteosarcoma
of GdCl3, showing that GdClv may be a promising
anti-osteosarcoma drug.
Acknowledgements
The present study was financially supported by a
research grant (no. SKH-TMU-104-01) from the Shin-Kong Wu Ho-Su
Memorial Hospital, Taipei, Taiwan.
References
|
1
|
Duong LM and Richardson LC: Descriptive
epidemiology of malignant primary osteosarcoma using
population-based registries, United States, 1999–2008. J Registry
Manag. 40:59–64. 2013.PubMed/NCBI
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Savage SA and Mirabello L: Using
epidemiology and genomics to understand osteosarcoma etiology.
Sarcoma. 2011:5481512011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salas S, Jézéquel P, Campion L, Deville
JL, Chibon F, Bartoli C, Gentet JC, Charbonnel C, Gouraud W,
Voutsinos-Porche B, et al: Molecular characterization of the
response to chemotherapy in conventional osteosarcomas: Predictive
value of HSD17B10 and IFITM2. Int J Cancer. 125:851–860. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Venkatramani R, Murray J, Helman L, Meyer
W, Hicks MJ, Krance R, Lau C, Jo E and Chintagumpala M: Risk-based
therapy for localized osteosarcoma. Pediatr Blood Cancer.
63:412–417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Padma VV: An overview of targeted cancer
therapy. Biomedicine (Taipei). 5:192015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alfranca A, Martinez-Cruzado L, Tornin J,
Abarrategi A, Amaral T, de Alava E, Menendez P, Garcia-Castro J and
Rodriguez R: Bone microenvironment signals in osteosarcoma
development. Cell Mol Life Sci. 72:3097–3113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bian DL, Wang XM, Huang K, Zhai QX, Yu GB
and Wu CH: Expression and regulatory effects of microRNA-182 in
osteosarcoma cells: A pilot study. Oncol Lett. 11:3040–3048.
2016.PubMed/NCBI
|
|
9
|
Ling H, Vincent K, Pichler M, Fodde R,
Berindan-Neagoe I, Slack FJ and Calin GA: Junk DNA and the long
non-coding RNA twist in cancer genetics. Oncogene. 34:5003–5011.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lee TH and Chung JG: Cell death caused by quinazolinone HMJ-38
challenge in oral carcinoma CAL 27 cells: Dissections of
endoplasmic reticulum stress, mitochondrial dysfunction and tumor
xenografts. Biochim Biophys Acta. 1840:2310–2320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee CF, Yang JS, Tsai FJ, Chiang NN, Lu
CC, Huang YS, Chen C and Chen FA: Kaempferol induces
ATM/p53-mediated death receptor and mitochondrial apoptosis in
human umbilical vein endothelial cells. Int J Oncol. 48:2007–2014.
2016.PubMed/NCBI
|
|
15
|
Wang G, Peng Q and Li Y: Lanthanide-doped
nanocrystals: Synthesis, optical-magnetic properties, and
applications. Acc Chem Res. 44:322–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pang X, Li D and Peng A: Application of
rare-earth elements in the agriculture of China and its
environmental behavior in soil. Environ Sci Pollut Res Int.
9:143–148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Long XH, Yang PY, Liu Q, Yao J, Wang Y, He
GH, Hong GY and Ni JZ: Metabolomic profiles delineate potential
roles for gadolinium chloride in the proliferation or inhibition of
HeLa cells. Biometals. 24:663–677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou Z and Lu ZR: Gadolinium-based
contrast agents for magnetic resonance cancer imaging. Wiley
Interdiscip Rev Nanomed Nanobiotechnol. 5:1–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strijkers GJ, Mulder WJ, van Tilborg GA
and Nicolay K: MRI contrast agents: Current status and future
perspectives. Anticancer Agents Med Chem. 7:291–305. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pintaske J, Martirosian P, Graf H, Erb G,
Lodemann KP, Claussen CD and Schick F: Relaxivity of gadopentetate
dimeglumine (Magnevist), gadobutrol (Gadovist), and gadobenate
dimeglumine (MultiHance) in human blood plasma at 0.2, 1.5, and 3
tesla. Invest Radiol. 41:213–221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edelman MJ, Otterson G, Leach J, Malpass
T, Salgia R, Jones D, Mody TD and Govindan R: Multicenter phase II
trial of Motexafin gadolinium and pemetrexed for second-line
treatment in patients with non-small cell lung cancer. J Thorac
Oncol. 6:786–789. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye L, Shi Z, Liu H, Yang X and Wang K:
GdCl3 induced Hep G2 cell death through mitochondrial
and external death pathways without significant elevation of ROS
generation. Biol Trace Elem Res. 151:148–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rose ML, Bradford BU, Germolec DR, Lin M,
Tsukamoto H and Thurman RG: Gadolinium chloride-induced hepatocyte
proliferation is prevented by antibodies to tumor necrosis factor
alpha. Toxicol Appl Pharmacol. 170:39–45. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu CC, Yang SH, Hsia SM, Wu CH and Yen GC:
Inhibitory effects of Phyllanthus emblica L. on hepatic steatosis
and liver fibrosis in vitro. J Funct Foods. 20:20–30. 2016.
View Article : Google Scholar
|
|
25
|
Hui K, Yang Y, Shi K, Luo H, Duan J, An J,
Wu P, Ci Y, Shi L and Xu C: The p38 MAPK-regulated PKD1/CREB/Bcl-2
pathway contributes to selenite-induced colorectal cancer cell
apoptosis in vitro and in vivo. Cancer Lett. 354:189–199. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiang JH, Yang JS, Lu CC, Hour MJ, Chang
SJ, Lee TH and Chung JG: Newly synthesized quinazolinone HMJ-38
suppresses angiogenetic responses and triggers human umbilical vein
endothelial cell apoptosis through p53-modulated Fas/death receptor
signaling. Toxicol Appl Pharmacol. 269:150–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lin JJ, Huang WW, Tsuzuki M, Lee TH and Chung JG: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiang JH, Yang JS, Lu CC, Hour MJ, Liu
KC, Lin JH, Lee TH and Chung JG: Effect of DNA damage response by
quinazolinone analogue HMJ-38 on human umbilical vein endothelial
cells: Evidence for γH2A.X and DNA-PK-dependent pathway. Hum Exp
Toxicol. 33:590–601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wolfbeis OS: An overview of nanoparticles
commonly used in fluorescent bioimaging. Chem Soc Rev.
44:4743–4768. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen L, Yang A, Yao P, Sun X, Chen C, Mo
C, Shi L, Chen Y and Liu Q: Gadolinium promoted proliferation in
mouse embryo fibroblast NIH3T3 cells through Rac and PI3K/Akt
signaling pathways. Biometals. 27:753–762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye LH, Shi Z, Liu HX, Yang XD and Wang K:
Gadolinium induced apoptosis of human embryo liver L02 cell line by
ROS-mediated AIF pathway. J Rare Earths. 29:178–184. 2011.
View Article : Google Scholar
|
|
33
|
Resende RR, Andrade LM, Oliveira AG,
Guimarães ES, Guatimosim S and Leite MF: Nucleoplasmic calcium
signaling and cell proliferation: Calcium signaling in the nucleus.
Cell Commun Signal. 11:142013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xia Q, Feng X, Huang H, Du L, Yang X and
Wang K: Gadolinium-induced oxidative stress triggers endoplasmic
reticulum stress in rat cortical neurons. J Neurochem. 117:38–47.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng XD, Xia Q, Yuan L, Huang HF, Yang XD
and Wang K: Gadolinium triggers unfolded protein responses (UPRs)
in primary cultured rat cortical astrocytes via promotion of an
influx of extracellular Ca2+. Cell Biol Toxicol.
27:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|