Introduction
Breast cancer is the most common cause of cancer
mortality throughout the world. Taking America as an example,
~231,840 new cases of breast cancer were estimated, among which,
40,290 breast cancer deaths are expected to occur among American
females in 2015 (1).
The spreading and metastases formation of breast
cancer are the vital procedure in the whole process of malignant
disease, which is the main cause of cancer-related deaths. In
addition, locally advanced metastases of breast cancer can be
detected on lung, bone, liver and brain, developing into an
incurable disease with adverse prognosis (2).
Furthermore, tumor metastasis to distant tissues is
highly dependent on intravascular microenvironment (3), in which, platelets notably contribute
to the metastatic process.
The basic physiological function of platelets is
haemostasis and thrombosis. However, pro-metastatic activity of
platelets have aroused wide concern (4). When platelets interacted with tumor
cells in cancer patients, a collusive and detrimental pathological
process occurs (5).
Platelets are activated by tumor cells during
circulation, this interaction is defined as the switch of
hypercoagulable state, which is recognized as tumor cell-induced
platelet aggregation (TCIPA) (6).
Based on the above phenomenon, abnormalities of platelets, once
activated by tumors occur in the patients with malignancy, and
thrombocytosis is diagnosed frequently. Furthermore, platelet
physiological function is transferred to the pathological condition
by degrees. The process is associated with coagulation formation
and cancer metastasis (7). On the
contrary, following activation, abnormal platelets are recruited to
the tumors and their microenvironment. After that, tumor is
escorted by the platelets to each essential part of tumor
hematogenous metastasis (8). By
virtue of platelet abnormalities, thrombi forms within a blood
vessel, then, thrombosis is developed, which is a hallmark of most
cancer metastasis.
Sequentially, in the first stage of metastasis,
platelets facilitate tumor invasion to adjacent blood vessels.
Then, in the circulation, platelets are enriched in the vicinity of
tumor cells, forming a tumor-platelet (T-P) clot, which shield
tumor cells escaping from the immune response of T-cell and
surveillance of NK cells, and prevent damage of shear stress
resulting from blood flow. Next, the clot, consisting of platelet
and tumor, is arrested at a distant metastatic organ. Eventually,
metastatic colonization adapt to the foreign microenvironment for
survival and development after extravasation (9).
During the progress of TCIPA, two types of
mechanisms on (T-P) interaction have been recognized. The first,
direct physical binding of platelet receptors and corresponding
tumor-derived ligands leads to the activation of platelet and the
enhancement of platelet adhesion and aggregation. The binding is
not only a simple cell to cell cohesion, but also a kind of
high-affinity specific interaction. The tissue factor (TF),
glycoprotein VI (GPVI) and glycoprotein Ibα (GPIbα) expressed on
tumor cells are respectively bound to the peroxisome activation
receptor (PAR) and platelet integrin αIIbβ3. Notably, the selectin
P on the surface of the platelets is tethered to selectin P ligand
(SELPLG) protein on tumor cells (10). Secondly, countless soluble factors
secreted from activated platelets and tumor cells indirectly
remodel a suitable and common microenvironment, in which, the
ability of tumor cells to survive and metastasize is strengthen.
For example, vascular endothelial growth factor (VEGF),
transforming growth factor-β1 (TGF-β1) and platelet-derived growth
factor (PDGF) affects the function of cancer-related inflammation,
angiogenesis, tumor growth and metastasis (10). Leading by above analysis, TCIPA was
the critical point of cancer progress, shown to interrelate closely
with metastasis. Thus, TCIPA-targeted therapy should be a feasible
strategy of cancer treatment such as aspirin (ASA).
In traditional medicine, certain drugs have been
discovered that affect anti-aggregation, such as Caulis
Spatholobi. Caulis Spatholobi, the vine stem of
Spatholobus suberectus Dunn., have been used widely as a
kind of drug for invigorating blood circulation and eliminating
stasis. However, there is no report on the efficacy of 80% ethanol
extracts of Caulis Spatholobi (SET) blocking TCIPA, and
eventually inhibiting tumor metastasis. Overall, we identified the
optimal treatment dose and time of SET, discuss the anti-TCIPA and
anti-metastasis efficacy of SET in vitro and in vivo,
and revealed the preliminary mechanism of SET for inhibiting TCIPA.
Thus, our results showed that SET could effectively contribute to
TCIPA as a potential target for anti-metastatic therapy.
Materials and methods
Cell culture
The murine mammary carcinoma cell stably expressing
the firefly luciferin gene (4T1-luc) and non-labeled 4T1 cells were
obtained from Dr Ganlin Zhang, Beijing Hospital of Traditional
Chinese Medicine (Beijing, China). The cell line was cultured in
RPMI-1640 medium (Gibco, Grand Island, NY, USA) and supplemented
with 10% fetal bovine serum (HyClone, Logan, UT, USA), 100 mg/ml
streptomycin and 100 U/ml penicillin at 37°C in a humidified
atmosphere with 5% CO2.
Reagents
SET was extracted by the Dalian Institute of
Chemical Physics, Chinese Academy of Sciences (Dalian, China). ASA
was purchased from the National Institutes for Food and Drug
Control (Beijing, China).
Extraction and preparation of
drug
Two kilograms of Caulis Spatholobi were
decocted thrice with anhydrous ethanol. The liquids were merged and
concentrated to constant weight on a rotary vacuum evaporator.
Further purification of the extracts (20 g) was performed by
HPD-100 macroporous adsorbent column chromatography eluted with
different concentrations of alcohol. Respectively, the fractions of
20, 40, 60, 80 and 100% alcohol were concentrated to constant
weight on a rotary vacuum evaporator and crushed into powder before
experiments. The extracts were named SE1, SE2, SE3, SET, and SE4,
respectively.
In vitro cytotoxicity of the Caulis
Spatholobi extracts
The screening of cytotoxicity was assessed by MTT
(Amresco, USA) assay as previously described (11). Briefly, the 4T1 cells were plated
into a 96-well cell culture cluster at 5×103 per well.
Then, the cells were treated with SE1, SE2, SE3, SET, and SE4 in
different concentration, respectively, for 24, 48, 72 and 96 h.
Another experiment was performed using SET as treatment, which was
identified by the above preliminary test. The method was conducted
as mentioned above, however, the time of drug treatment was
adjusted to 3, 6, 12 and 24 h. At mentioned time points, MTT was
added to each well till the final concentration of 0.5 mg/ml, and
the cells were incubated at 37°C for 4 h. A total of 150 µl of
dimethyl sulfoxide (DMSO) was replaced in each well instead of
medium with MTT. With fully mixing, the absorbances of solution
were determined in 570 nm performed a microplate reader (Molecular
Devices, USA). The inhibitory rate of 4T1 cells was calculated as
follows. Inhibitory rate = (1 - mean experimental absorbance/mean
negative control absorbance) × 100%. Half maximal inhibitory
concentration was calculated as previously described (12).
Collection of blood and preparation of
platelet
Fresh blood was obtained as previously described
(13). In brief, blood was obtained
by retro-orbital venous plexus sampling, which was collected into
an Eppendorf tube mixed with acid citrate dextrose solution (38 mM
citric acid/75 mM trisodium citrate/100 mM dextrose). The blood was
centrifuged at 280 × g for 8 min at room temperature, supernatant
containing plasma and buffy coat were transferred to a new tube,
centrifuged at 280 × g for 4 min. Then, platelet-rich plasma (PRP)
was collected into a fresh tube and prepared for study.
ADP-stimulated platelet
aggregation
The different doses of SET were designed as low,
medium, high (20, 40 and 80 µg/ml) groups and ASA (300 µM) as
positive control group compared with negative control. The
following study in vitro was based on identical methods. The
platelet aggregation was measured by whole blood aggregometers
(Chrono-Log, Havertown, PA, USA) (14). Briefly, 500 µl of whole blood with
equivalent volume of normal saline (NS) was placed in aggregometer,
incubated for 10 min at 37°C with stirring at 1,200 rpm. The
samples were treated by SET as inhibitors. ADP (10 µM) was added to
the mixture as activator for platelet aggregation. Finally, the
rates of aggregation were recorded through a curve. To determine
the optimal dose, the different concentrations of SET were used to
inhibit the platelet aggregation induced by ADP.
Tumor cell-induced platelet
aggregation
As described by the previous method, PRP was used
within 2 h after centrifugation. Next, carboxyfluorescein
diacetate-succinimidyl ester (CFDA-SE; Beyotime, Shanghai, China),
a sort of cell fluorescent label, was incubated with PRP at a final
concentration of 5 µM for 10 min. The labeled platelets were washed
by Tyrode's solution twice, then, resuspended with fresh Tyrode's
solution. The next section of test was performed after at least 1
h. The TCIPA was detected by confocal microscopy and flow cytometry
assay as previously described (15). For the microscopic observation of
TCIPA, briefly, 1×105 cells/ml 4T1 cells, pre-treated by
SET and ASA, were plateted in the confocal dish until the
logarithmic phase. Fluorescently labeled platelets were placed into
the dish and incubated with tumor cells for 30 min at 37°C. The
non-aggregated platelets were removed. T-P cell aggregation was
visualized by confocal microscopy (Olympus, Tokyo, Japan). For flow
cytometry assay, 1×105 pre-treated 4T1 cells were
incubated with 200 µl of platelet preloaded with CFDA-SE for 10
min. A final concentration of 2% paraformaldehyde was added as a
fixative stored at 4°C, prepared to flow cytometry assay.
In vivo metastatic seeding assay
For lung seeding and metastasis analysis, the mouse
model of pulmonary metastasis was established by vein tail
injection as previously described (16). Briefly, 2×104 4T1-luc
cells in 100 µl of NS were injected into 8-week-old female Balb/c
mice by vein tail. Simultaneously, mice were administrated with
different doses of SET (2.2, 4.4 and 8.8 mg/kg). ASA (22.5 µmol/kg)
was used as positive control drug compared with model group (tumor
only). Imaging assay was conducted after injection of tumor cells
and daily therapy. Mice were anesthetized by isoflurane gas, then
visualized by using small animal in vivo imaging system
(Kodak, USA) 10 min after injection of luciferin. Until the mice of
model group died, mice were sacrificed and the lungs were collected
into 4% paraformaldehyde for observation and hematoxylin and eosin
(H&E) staining (17) using a
microscope (Olympus). Survival curve was employed to evaluate
overall survival. The study was approved by the Ethics Committee of
Beijing Administration Rule of Laboratory Animal, Beijing,
China.
Sample preparation of TCIPA for
zymography
The method of cell plating and drug treatment was
measured as mentioned above. In the logarithmic phase, the 4T1
cells cultured in FBS-free medium were incubated with 200 µl PRP,
or not, for 10 min. Then, the reaction was terminated and samples
were collected by centrifugation at 900 × g for 10 min. Supernatant
was stored at −80°C until the activities of Matrix
metalloproteinase (MMP)-2 and −9 were detected by zymography.
Zymography assay
Gelatin zymography was used to detect the activity
of MMP-2 and −9 in the platelet supernatants as previously
described (18). In brief, samples
of TCIPA were separated by 10% SDS-PAGE with copolymerized gelatin
(0.2%; Sigma Chemical Co., St. Louis, MO, USA) with a substrate for
gelatinolytic proteases. After electrophoresis, the gels were
washed with 2.5% Triton X-100 for 20 min repeated three times, and
then incubated for 48 h at 37°C in incubating buffer (25 mM Tris
HCl, 0.9% NaCl, 5 mM CaCl2 and 0.05% Na3N, pH
7.5). The conditioned medium of 4T1 cells was used as control.
After 72 h reaction, gels were fixed and stained in 0.1% Coomassie
blue R-250 (Sigma Chemical Co.), 40% methanol and 10% acetic acid
for 1 h and then discolored in 8% acetic acid with 4% methanol. The
gelatinolytic activities were presented by separate bands.
RT-PCR assay
The methods of cell plating and drug treatment were
measured as indicated above. Total RNA was isolated by TRIzol
reagent (Life Technologies, Grand Island, NY, USA). The detailed
procedure of RT-PCR was measured by RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA)
(19). According to the kit
instruction, 1 µg total RNA was performed to the following reverse
transcriptase in a final volume of 20 µl with AMV reverse
transcriptase. Subsequently, several specific primers were used to
amplify cDNAs of selectin P (SELP) forward,
5′-CGAGCCCAACAACAAGAA-3′ and reverse, 5′-GGGTAGCAGGAGCAGGTAT-3′;
selectin P ligand (SELPLG) forward, 5′-CTGTCACTGAGGCAGAGTCG-3′ and
reverse, 5′-TGAGCAGCCACGGTGTTG-3′; GPVI forward,
5′-CCTTCCATCTTACCCACA-3′ and reverse, 5′-CTGCTGAAAGCCAAGTTAT-3′;
integrin α2b (ITGA2B) forward, 5′-TGACAGGGAGCAGGAAGA-3′ and
reverse, 5′-GCTGGGCGTGGATAGTTT-3′. GAPDH (20) forward, 5′-GTGGATATTGTTGCCATCA-3′ and
reverse, 5′-ACTCATACAGCACCTCAG-3′ was compared with each experiment
as internal control. The PCR products were analyzed by agarose gel
electrophoresis and ethidium bromide staining.
Statistical analysis
The values of data were presented as means ±
standard deviation. Statistical significance was determined by
one-way analysis of variance followed by LSD post hoc test using
SPSS Statistics 17.0. P<0.05 was considered to be statistically
significant, *P<0.05, **P<0.01, ***P<0.001.
Results
Screening and dose identification of
SET in 4T1 cells
Taking breast cancer as cell model, we screened the
proliferation inhibitory effect of SET in 4T1 cells by MTT assay.
The proliferation of 4T1 cells was inhibited by SE1, SE2, SE3, SET,
SE4 in a time- and dose-dependent manner, respectively. Taking the
50% inhibition rate as the cytotoxic criteria, to avoid the
non-selective cytotoxicity, various doses of tested drugs and
prolonged treatment times were designed and the cell viability was
evaluated (Fig. 1A-E). The result
showed little cytotoxicity for the tested extracts with low doses
when they are treated for <48 h. In contrast, in long-term
treatment (>48 h), the non-selective cytotoxicity can be clearly
detected in all of the high dose extracts (>400 µg/ml). Based on
preliminary analysis, in order to determine a drug that interacts
with 4T1 cells with low non-selective cytotoxicity, SET was
selected for more detailed testing. The morphological analysis
revealed that the SET treatment was time- and dose-dependent
(Fig. 1F). Such influence of SET
was further quantified by MTT assay. As shown in Fig. 1G after 6-h treatment, cell
inhibition rate approached or exceeded 50%, which implied that
<6 h of treatment is optimal for efficacy study. Moreover,
IC50 values calculated at 3, 6, 12 and 24 h were
2929.153, 1309.211, 132.26 and 26.458 µg/ml (Fig. 1H), respectively. Thus, the safe and
effective concentrate interval (<400 µg/ml) and treatment time
(6 h) of SET were determined for further research.
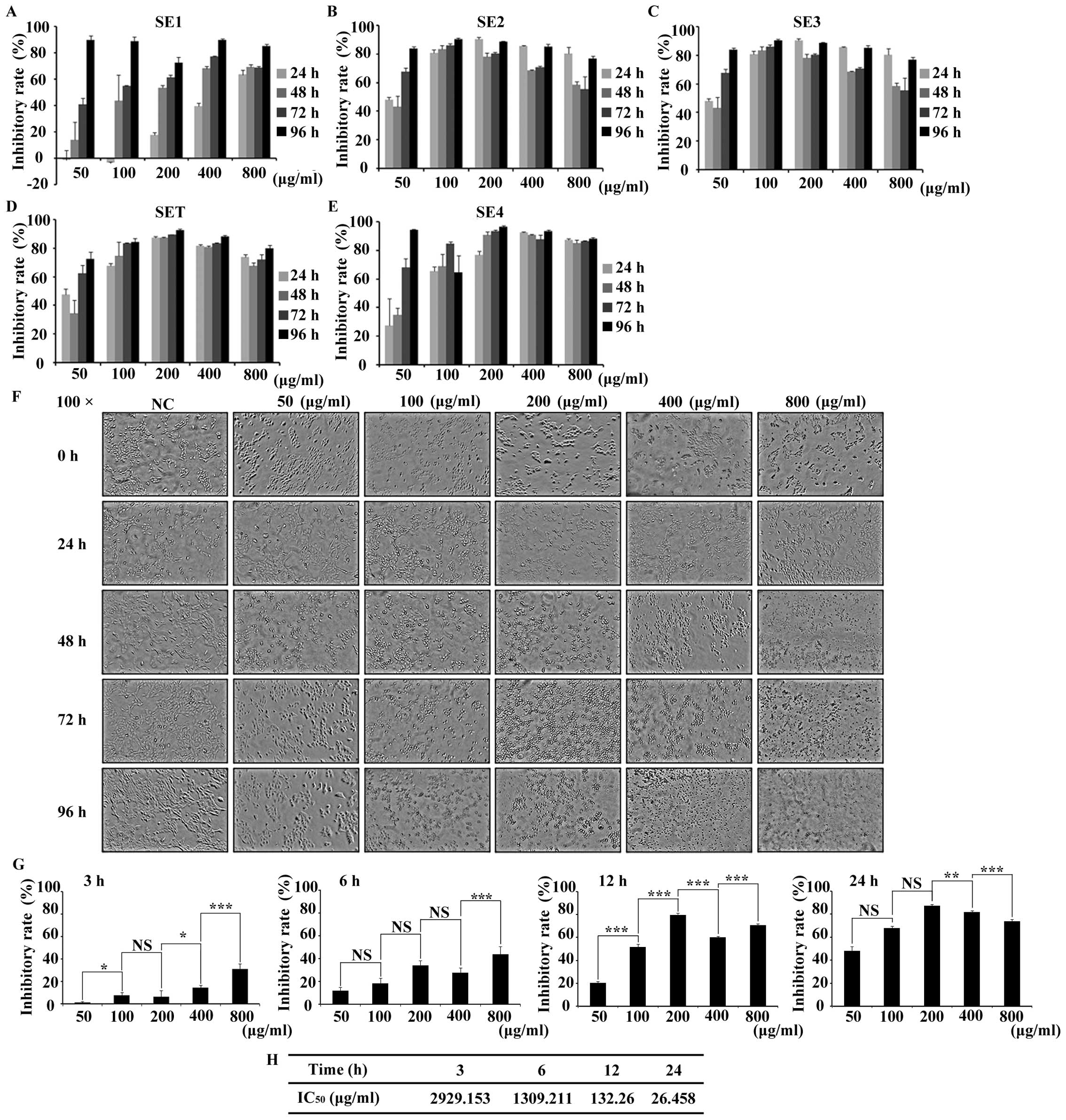 | Figure 1.The screening of cytotoxicity of 80%
ethanol extracts of Caulis Spatholobi (SET) in the 4T1 cell
line. (A-E) Different ethanol extract of Caulis Spatholobi
inhibited the 4T1 cell proliferation. The cell viability was
greatly suppressed by (A) SE1, (B) SE2, (C) SE3, (D) SET and (E)
SE4 treatment for >48 h and 100 µg/ml in a dose- and
time-dependent manner. (F) The morphological observation (×100) of
SET-treated 4T1 cells for 3, 6, 12 and 24 h. Within 6 h, SET had
little non-selective cytotoxicity in cell proliferation except at
the dose of 800 µg/ml. (G) The statistics of inhibition rate of
SET, which was consistent with earlier results (F). (H)
IC50 value of each time point could be estimated and it
reduced sharply with the time extension. Data are presented as
means ± 95% CI, *P<0.05, **P<0.01, ***P<0.001, N=3,
one-way ANOVA test and means ± standard error of triplicates. NS,
no significant difference. |
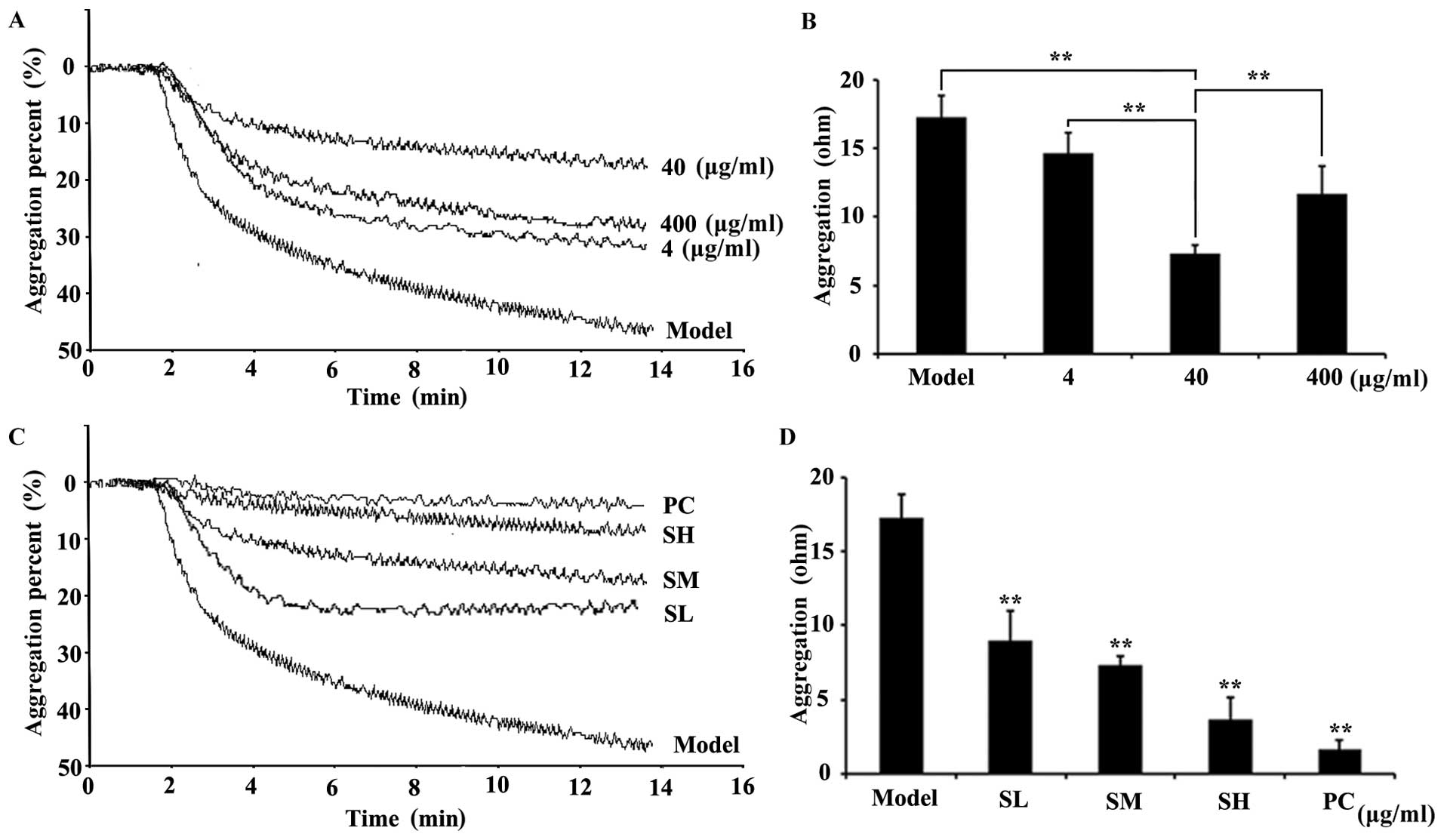
Effects of SET on the suppression of
platelet aggregation induced by ADP
Inspired by the traditional anticoagulation efficacy
of Caulis Spatholobi, by using the active extract (Fig. 1), we further questioned whether our
newly prepared extract (SET) affects the inhibition of platelet
aggregation induced by ADP. The platelet aggregation assay results
clearly showed that, in the model group, the platelet aggregation
could be obviously induced in response to ADP stimulation. In
contrast, the SET treatment, with 4, 40 and 400 µg/ml doses, could
largely reverse such changes, as represented by the decrease of
resistance values (Ohm). This result suggested that the coagulation
cascade was significantly blocked by SET. Notably, the
anticoagulation activity of SET exhibits in a typical
dose-dependent manner. SET at 40 µg/ml exerted the strongest
efficacy (Fig. 2A and B). Taking
these data into account, the 40 µg/ml dose interval was chosen for
further efficacy study. A more detailed dose-dependent efficacy
study was also performed. The treatment of SET, with 20, 40 and 80
µg/ml doses, significantly inhibited platelet aggregation
dose-dependently. Compared with the model group, the SET treated
groups showed an obviously decrease of platelets aggregation
(Fig. 2C and D). Consequently, SET
was able to inhibit the activation induced by ADP. However, the
role of SET in the interaction between tumor and platelet and the
inhibitory effects on the TCIPA are still unknown.
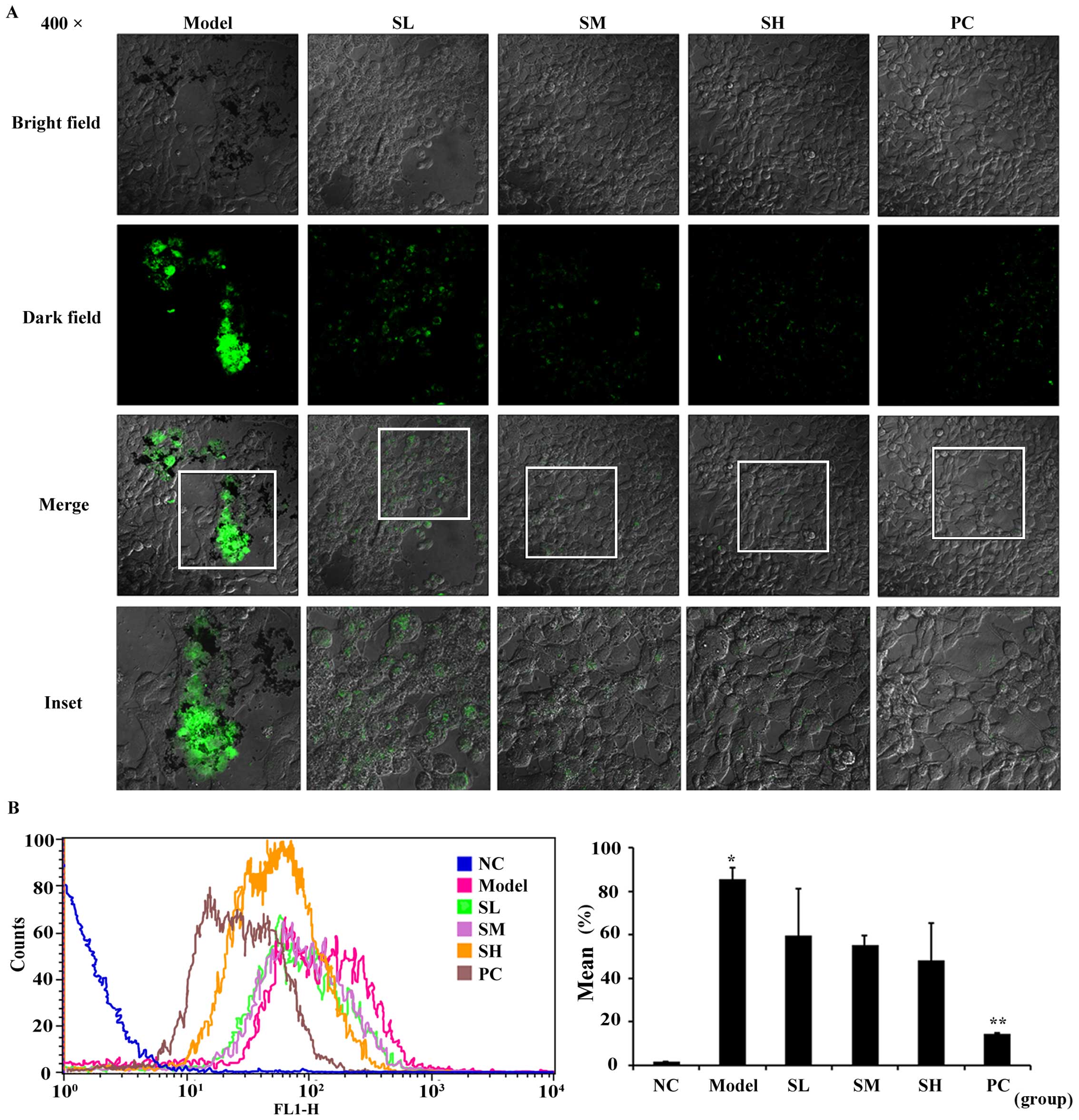
Effects of SET on the inhibition of
TCIPA
To further reveal the efficacy of SET in
vitro, we focused on TCIPA, during which SET exerted the
effectiveness on the adhesion of platelets to 4T1 cells. In this
study, 4T1 cells were incubated with platelets stained with
fluorescent probe CFDA-SE. In the presence or absences of SET,
non-aggregated platelets were totally washed and removed. Then, the
stained platelets were observed in the vicinity of tumor cells by
using a confocal microscope (Fig.
3A). The result showed that the quantity and intensity of green
fluorescence in model group was much clearer. In drug-treated
groups, fluorescence intensity was markedly decreased, which
represents the reduced level of TCIPA. Additionally, such effect
was further quantified via flow cytometry analysis. In the absence
of SET, TCIPA could be clearly detected as measured by the elevated
positive fluorescent ratio. On the contrary, in the presence of
SET, the fluorescent positive rate was decreased obviously
(Fig. 3B). Therefore, SET was able
to efficiently inhibit the platelet aggregation induced by tumor
cells. Anti-TCIPA therapy may be a potential drug target for SET
that regulates the process of tumor metastasis in the blood
circulation.
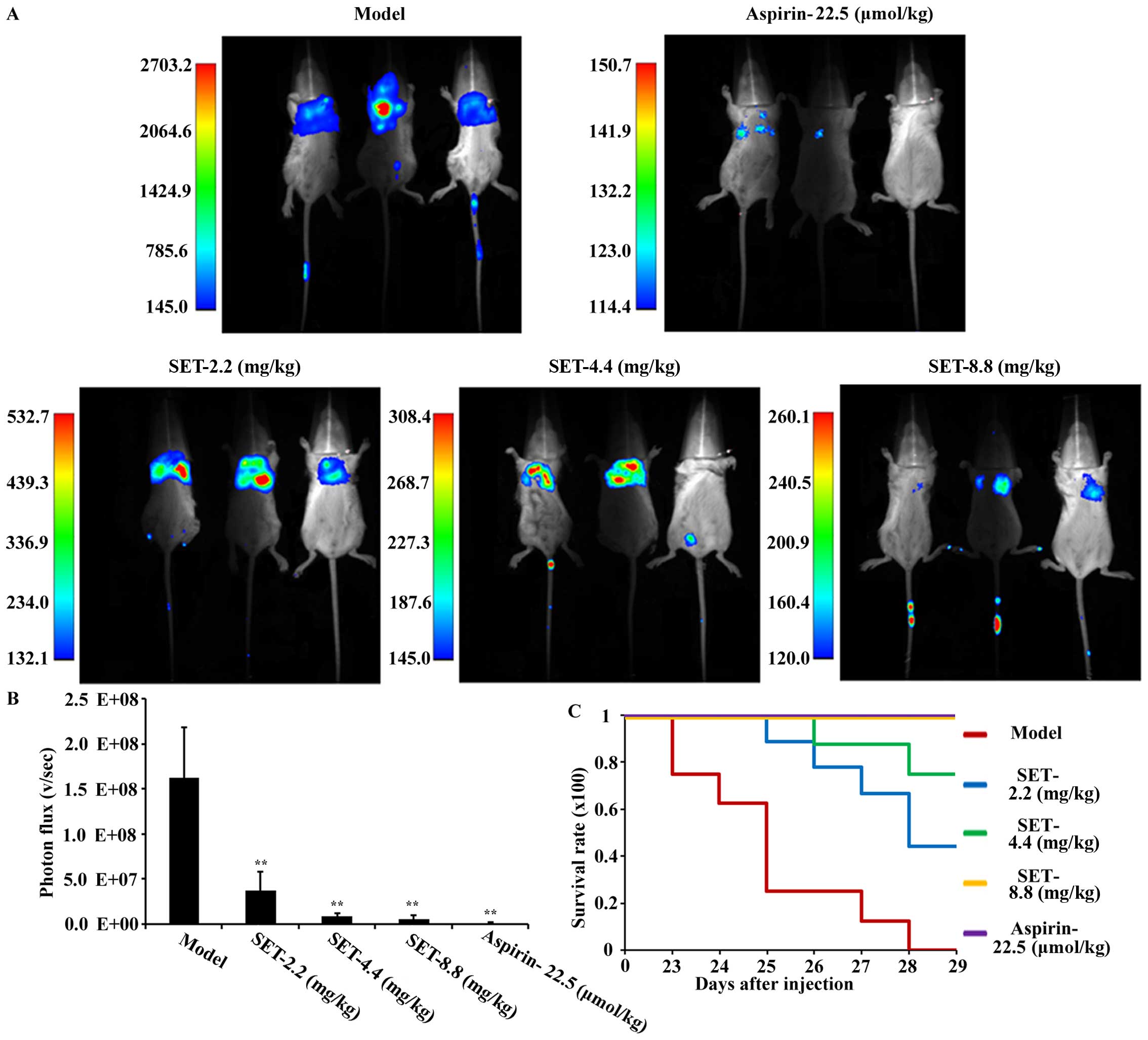
In vivo anti-metastatic efficacy of
SET in mouse tail vein injection model
To confirm the efficacy of SET in vivo,
excluding the effect of SET on the tumor in situ, we
selected the tail vein injection model for detecting and analyzing
the intensity of lung metastasis of breast cancer. Briefly, for
experiments, drug administration was initiated 2 days before
modeling with low-, medium- and high-dose of SET, once daily. The
ASA was also used for positive control. Metastatic process was
measured by small animal in vivo imaging system. The
bioluminescence imaging on the lung was first observed on the 21st
day after tumor cell injection in the modeling group. As shown in
Fig. 4A, mice treated with SET were
visualized and showed less metastases compared to modeling mice.
For quantitative analysis, the photon statistics measurements of
coherent fields are shown in Fig.
4B. They are negatively influenced in a significant
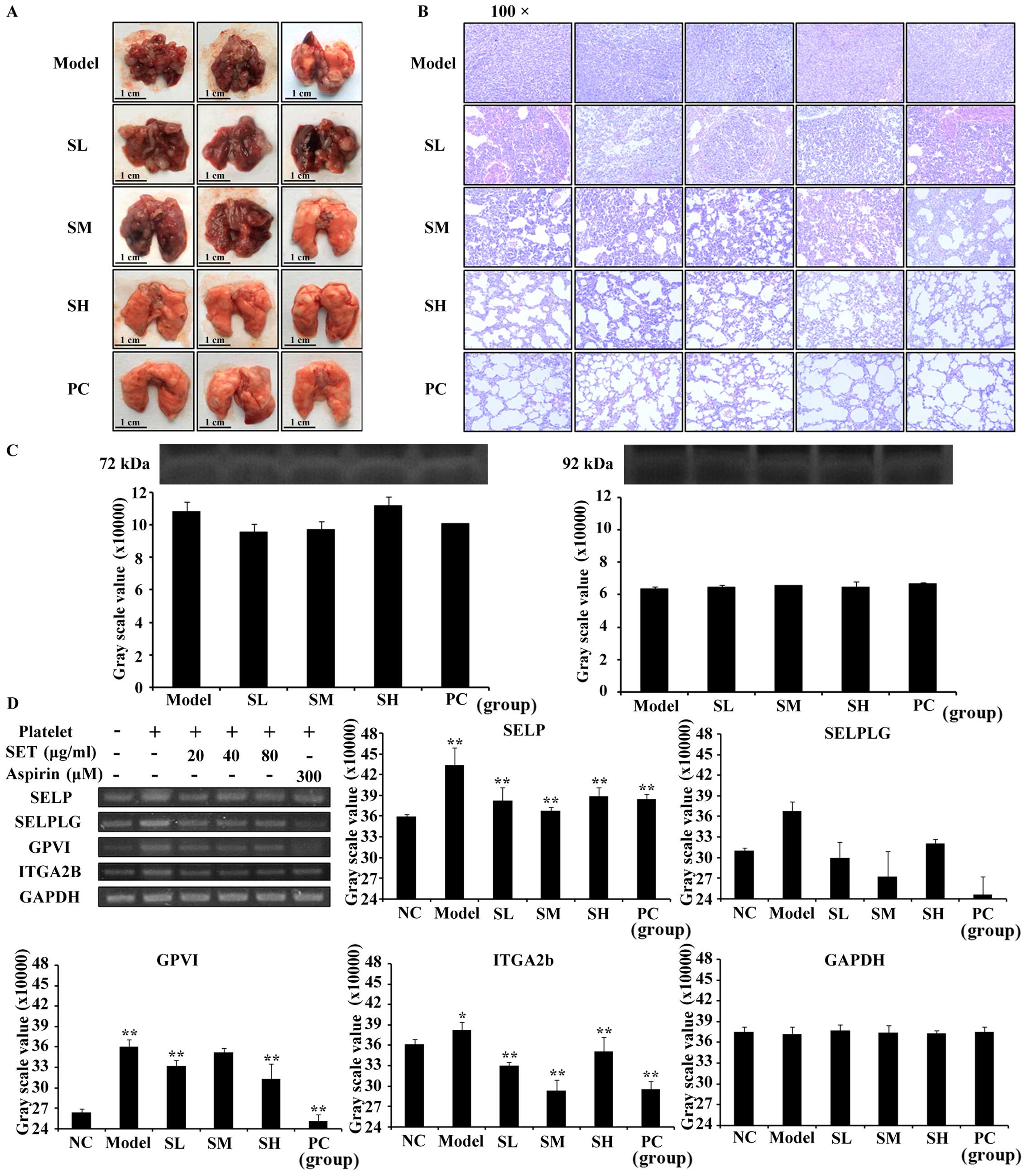
dose-dependent manner. To further analyze the intensity of
metastasis in the lung, we performed general observation and
H&E staining assay (Fig. 5A and
B). Consistently, both of the results confirmed the obvious
inhibition of lung metastasis in SET-treated mice. Moreover, the
mice in the modeling group showned a poor survival rate compared
with SET-treated mice (Fig. 4C).
Thus, an obvious anti-metastasis efficacy of SET was evaluated.
Consequently, SET has the possibility to be an anti-metastatic drug
by targeting TCIPA in breast cancer.
MMP-2 and −9 released during
TCIPA
In recent studies, MMP-2 and −9 were proven to play
an important role in the association with platelet and tumor cell
(21). Leading by this, zymography
assay was measured to detect whether MMP-2 and −9 were released or
not during TCIPA. As shown in Fig.
5C, we found that MMP-2 and −9 were released during TCIPA.
However, the gray scale value of zymographic bands in each group
had no statistical difference which indicated that SET failed to
regulate the secretion of MMP-2 or −9 during T-P interaction.
Consequently, the efficacy of SET on the inhibition of TCIPA was
MMP-independent.
To investigate the exact mechanism of SET for
blocking the association between platelets and tumor cells, we
focused the efficacy of SET in the regulation of adhesive
interaction between platelets and tumor cells. As commonly
recognized, the key adhesive molecules such as SELP, SELPLG, GPVI,
ITGA2B involved in tumor metastasis played a crucial role in TCIPA
(22). Therefore, we detected the
expression of above molecules as measured by RT-PCR assay. As
expected, SET significantly inhibited SELP, GPVI, and ITGA2B
expression compared with model group (Fig. 5D). While through analysis of RNA
bands and measurement of the intensity of gray scale value, the
expression of SELPLG could not be regulated by SET efficiently. We
then identified that the expression of SELP, GPVI, and ITGA2B were
suppressed by SET during TCIPA. Consequently, the mechanism of SET
to inhibit TCIPA and reduce metastasis may relate with regulating
the cell adhesion molecules and then suppressing adhesion of tumor
cells and platelets.
Discussion
Hypercoagulable state is recognized as a
pathological process whose main feature is an imbalance between
coagulation and anticoagulation. The over-activated coagulation or
the excessive suppression of anticoagulation both could contribute
to the abnormality.
In 1865, it was found for the first time that cancer
patients had abnormality of blood coagulation, and were at high
risk for developing venous thrombosis (23). Substantial research proved that
blood viscosity in patients with advanced tumor kept at a high
level, and was characterized by increasing of fibrinogen,
coagulation factor, especially platelets. The increase of blood
viscosity in circulation is affected by two synergetic sides. On
the one hand, the coagulation system can be pathologically
strengthened by certain tumor cells, resulting in blood
hypercoagulability (24). On the
other hand, tumor cell-induced hypercoagulable state provides an
adaptive internal environment for facilitating the tumor cell
escape from immune attack and shear stress accelerating
hematogenous metastasis (25).
In 1973, Gasic et al first proposed the
interaction between platelet and tumor cell through the in
vitro culture of tumor cell-induced platelet activation
(26). A platelet, as a vital
factor for hypercoagulable state, promote metastasis and aggravate
the malignant process (27).
Accordingly, braking the collusion between the platelet and the
tumor cell, tumor cell lose its shelter, then metastasis will be
suppressed effectively. Fortunately, anticoagulant medication has
been identified effective on certain cancers.
Taking ASA as an example, since 1990s, ASA has been
used to be an antithrombotic therapy as a platelet inhibitor,
treating and protecting from cancer-related thrombosis. Definitely,
initial study shows that daily ASA reduces the long-term risk of
several cancers and decreases the probability of distant metastasis
(28). It is confirmable that TCIPA
inhibition of treatment is able to have a favorable function on
cancer progress, especially on metastasis (29). A meta-analysis evaluated that daily
low-dose ASA as the anti-platelet therapy decreased the risk of
adenocarcinomas with distant metastasis (30).
Although ASA was one of effective drugs on the
inhibition of TCIPA, the deficiencies of clinical medication
appeared gradually. For instance, emergence of drug resistance and
reduction of drug susceptibility were severe with ASA as a single
drug in metastatic treatment (31).
In order to overcome this, new drugs are essential for
anti-platelet therapy.
We focused on the novel extract from Caulis
Spatholobi. As a traditional anticoagulant drug, Caulis
Spatholobi has been used for improving hypercoagulable state
and inhibiting the thrombosis in Chinese medicine. Initially,
irregular menstruation, dysmenorrhea and rheumatic arthralgia were
the typical scope of treatment for Caulis Spatholobi
(32). In addition, various drug
efficacies of Caulis Spatholobi have been found and
determined including the antiviral, improvement of hematological
system and antitumor activities (33,34).
In our study, through MTT assay and ADP-induced
platelet aggregation test, we determined the optimized time and
dosage of drug treatment. After our efforts, we had discovered the
efficacy of anti-platelet aggregation. By using confocal
microscope, and flow cytometry analysis, the pathological
activation of TCIPA could be observed clearly. SET was able to
dissociate the aggregation by blocking the interaction of platelets
and tumor cells. Moreover, the anti-metastasis efficacy of SET was
also measured by mouse tail vein injection model in vivo.
The metastasis was efficiently blocked by SET through suppressing
the process of TCIPA. Molecularly, zymography assay proved that the
anti-TCIPA efficacy of SET was regulated MMP-2 and
−9-independently. In the presence of SET, the expressions of some
adhesive molecules were downregulated, which indicates the
preliminary molecular target of SET in TCIPA. Above all, by such
mechanism, SET successfully dissociates the T-P complex and as a
result, attenuates vascular transportation and dissemination of
metastatic breast cancer cells.
Future work should pay more attention to two aspects
of the study. Firstly, the material basis and the active chemical
compound analysis of SET remain unclear. Therefore, further
detailed separation, extraction, purification and identification
studies are certainly necessary. Secondly, the anti-TCIPA efficacy
of SET is a complicated pharmacological process, in which, the
exact pathway and mechanism of SET remain to be further revealed.
Overall, our study provided convincing evidence as well as
practical approach for clinical chemotherapy, especially metastatic
therapy, targeting TCIPA in the future.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81303272).
Glossary
Abbreviations
Abbreviations:
|
TCIPA
|
tumor cell-induced platelet
aggregation
|
|
SET
|
80% ethanol extracts of Caulis
Spatholobi
|
|
ASA
|
aspirin
|
|
MMP
|
matrix metalloproteinase
|
|
SELP
|
selectin P
|
|
SELPLG
|
selectin P ligand
|
|
GPVI
|
glycoprotein VI
|
|
ITGA2B
|
integrin α2b
|
References
|
1
|
DeSantis CE, Fedewa SA, Sauer A Goding,
Kramer JL, Smith RA and Jemal A: Breast cancer statistics, 2015:
Convergence of incidence rates between black and white women. CA
Cancer J Clin. 66:31–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baselga J, Cortés J, Kim S-B, Im SA, Hegg
R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al:
CLEOPATRA Study Group: Pertuzumab plus trastuzumab plus docetaxel
for metastatic breast cancer. N Engl J Med. 366:109–119. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wood SL, Pernemalm M, Crosbie PA and
Whetton AD: The role of the tumor-microenvironment in lung
cancer-metastasis and its relationship to potential therapeutic
targets. Cancer Treat Rev. 40:558–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson SA: The Circulating Platelet.
Elsevier; New York, NY: 2012, View Article : Google Scholar
|
|
5
|
Byzova TV, Kerr ΒΑ, Feng W and McCabe P:
Platelets and the tumor cell microenvironment. Blood.
122:SCI-32-SCI-32. 2013.
|
|
6
|
Riedl J, Pabinger I and Ay C: Platelets in
cancer and thrombosis. Hamostaseologie. 34:54–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li N: Platelets in cancer metastasis: To
help the ‘villain’ to do evil. Int J Cancer. 138:2078–2087. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goubran HA, Burnouf T, Radosevic M and
El-Ekiaby M: The platelet-cancer loop. Eur J Intern Med.
24:393–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goubran HA, Stakiw J, Radosevic M and
Burnouf T: Platelet-cancer interactions. Semin Thromb Hemost.
40:296–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stockert JC, Blázquez-Castro A, Cañete M,
Horobin RW and Villanueva A: MTT assay for cell viability:
Intracellular localization of the formazan product is in lipid
droplets. Acta Histochem. 114:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bentz J, O'Connor MP, Bednarczyk D,
Coleman J, Lee C, Palm J, Pak YA, Perloff ES, Reyner E, Balimane P,
et al: Variability in P-glycoprotein inhibitory potency
(IC50) using various in vitro experimental systems:
Implications for universal digoxin drug-drug interaction risk
assessment decision criteria. Drug Metab Dispos. 41:1347–1366.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frenette PS, Johnson RC, Hynes RO and
Wagner DD: Platelets roll on stimulated endothelium in vivo: An
interaction mediated by endothelial P-selectin. Proc Natl Acad Sci
USA. 92:7450–7454. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Angiolillo DJ, Fernández-Ortiz A, Bernardo
E, Ramírez C, Sabaté M, Bañuelos C, Hernández-Antolín R, Escaned J,
Moreno R, Alfonso F, et al: High clopidogrel loading dose during
coronary stenting: Effects on drug response and interindividual
variability. Eur Heart J. 25:1903–1910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shou L-M, Zhang Q-Y, Li W, Xie X, Chen K,
Lian L, Li ZY, Gong FR, Dai KS, Mao YX, et al: Cantharidin and
norcantharidin inhibit the ability of MCF-7 cells to adhere to
platelets via protein kinase C pathway-dependent downregulation of
α2 integrin. Oncol Rep. 30:1059–1066. 2013.PubMed/NCBI
|
|
16
|
Labelle M, Begum S and Hynes RO: Platelets
guide the formation of early metastatic niches. Proc Natl Acad Sci
USA. 111:E3053–E3061. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang WC, Shyh-Chang N, Yang H, Rai A,
Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, et al: Glycine
decarboxylase activity drives non-small cell lung cancer
tumor-initiating cells and tumorigenesis. Cell. 148:259–272. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Medina C, Harmon S, Inkielewicz I,
Santos-Martinez MJ, Jones M, Cantwell P, Bazou D, Ledwidge M,
Radomski MW and Gilmer JF: Differential inhibition of tumour
cell-induced platelet aggregation by the nicotinate aspirin prodrug
(ST0702) and aspirin. Br J Pharmacol. 166:938–949. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Dongen JJ, Macintyre EA, Gabert JA,
Delabesse E, Rossi V, Saglio G, Gottardi E, Rambaldi A, Dotti G,
Griesinger F, et al: Standardized RT-PCR analysis of fusion gene
transcripts from chromosome aberrations in acute leukemia for
detection of minimal residual disease. Report of the BIOMED-1
Concerted Action: Investigation of minimal residual disease in
acute leukemia. Leukemia. 13:1901–1928. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu T, Tang H, Lang Y, Liu M and Li X:
MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by
targeting prohibitin. Cancer Lett. 273:233–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li M, Xing S, Zhang H, Shang S, Li X, Ren
B, Li G, Chang X, Li Y and Li W: A matrix metalloproteinase
inhibitor enhances anti-cytotoxic T lymphocyte antigen-4 antibody
immunotherapy in breast cancer by reprogramming the tumor
microenvironment. Oncol Rep. 35:1329–1339. 2016.PubMed/NCBI
|
|
22
|
Gay LJ and Felding-Habermann B:
Contribution of platelets to tumour metastasis. Nat Rev Cancer.
11:123–134. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sørensen HT, Mellemkjaer L, Steffensen FH,
Olsen JH and Nielsen GL: The risk of a diagnosis of cancer after
primary deep venous thrombosis or pulmonary embolism. N Engl J Med.
338:1169–1173. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Donati MB, Gambacorti-Passerini C, Casali
B, Falanga A, Vannotti P, Fossati G, Semeraro N and Gordon SG:
Cancer procoagulant in human tumor cells: Evidence from melanoma
patients. Cancer Res. 46:6471–6474. 1986.PubMed/NCBI
|
|
25
|
Kannagi R: Carbohydrate-mediated cell
adhesion involved in hematogenous metastasis of cancer. Glycoconj
J. 14:577–584. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gasic GJ, Gasic TB, Galanti N, Johnson T
and Murphy S: Platelet-tumor-cell interactions in mice. The role of
platelets in the spread of malignant disease. Int J Cancer.
11:704–718. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Falanga A: Thrombophilia in cancer. Semin
Thromb Hemost. 31:104–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rothwell PM, Wilson M, Price JF, Belch JF,
Meade TW and Mehta Z: Effect of daily aspirin on risk of cancer
metastasis: A study of incident cancers during randomised
controlled trials. Lancet. 379:1591–1601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mitrugno A, Williams D, Kerrigan SW and
Moran N: A novel and essential role for FcγRIIa in cancer
cell-induced platelet activation. Blood. 123:249–260. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patrignani P, Filabozzi P and Patrono C:
Selective cumulative inhibition of platelet thromboxane production
by low-dose aspirin in healthy subjects. J Clin Invest.
69:1366–1372. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hankey GJ and Eikelboom JW: Aspirin
resistance. Lancet. 367:606–617. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wagner H, Bauer R, Melchart D, Xiao P-G
and Staudinger A: Caulis Spatholobi-JixuetengChromatographic
Fingerprint Analysis of Herbal Medicines Volume III. 3. 1st.
Springer; Switzerland: pp. 235–242. 2014
|
|
33
|
Yan LG, Ruan JS, Zhang L, Fan FT, Zhang F,
Wang AY, Zheng SZ, Zeng L, Li WL and Lu Y: Effect of aqueous
extracts of several kinds of herbs on human platelet aggregation
and expression of P-selectin in vitro. Chin J Integr Med.
21:286–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu B, Liu J, Chen J, Zhu D, Zhou H and
Wang X: A study on anticancer activity of Caulis Spatholobi extract
on human osteosarcoma Saos-2 cells. Afr J Tradit Complement Altern
Medicines. 10:256–260. 2013. View Article : Google Scholar
|