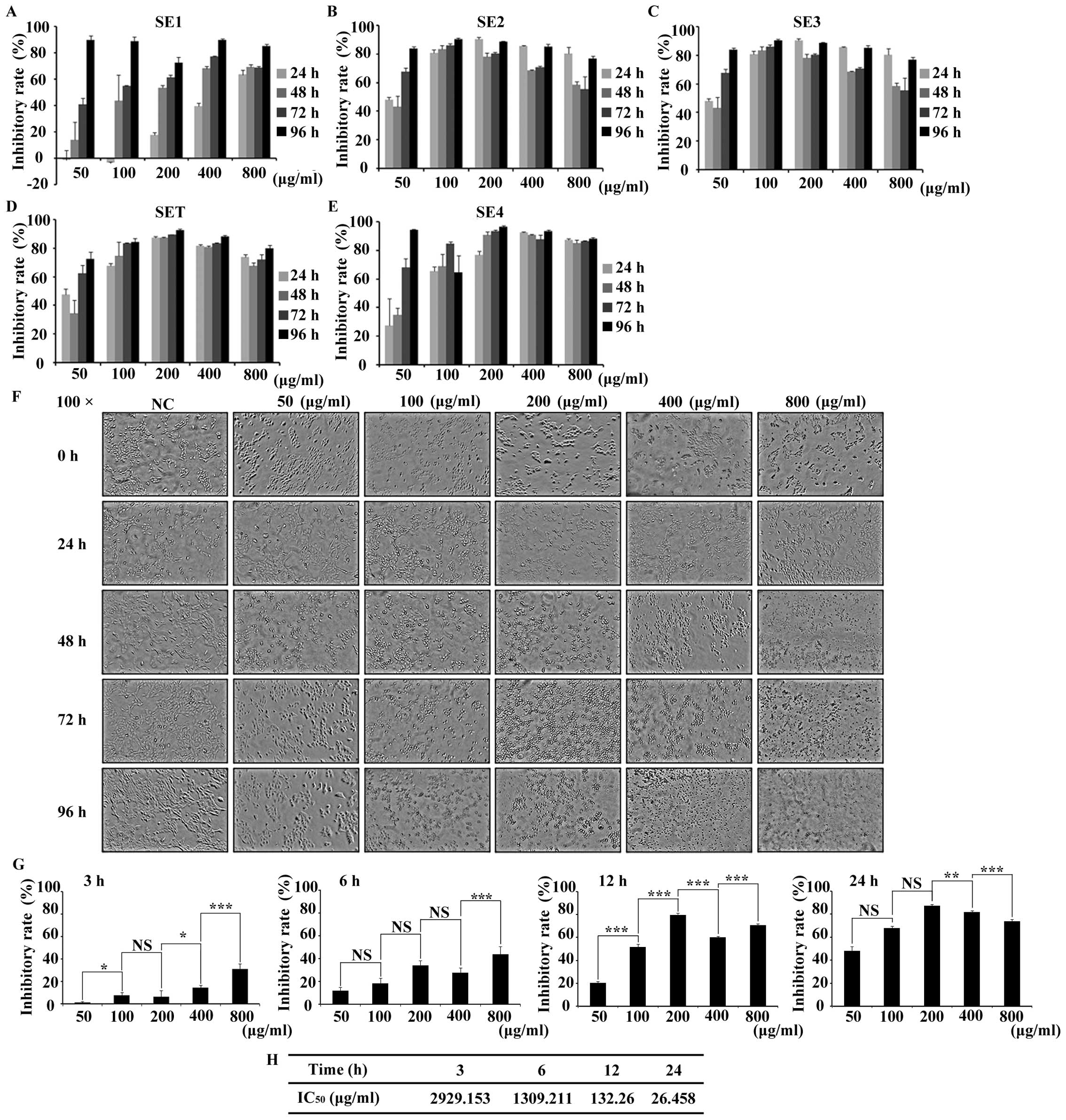
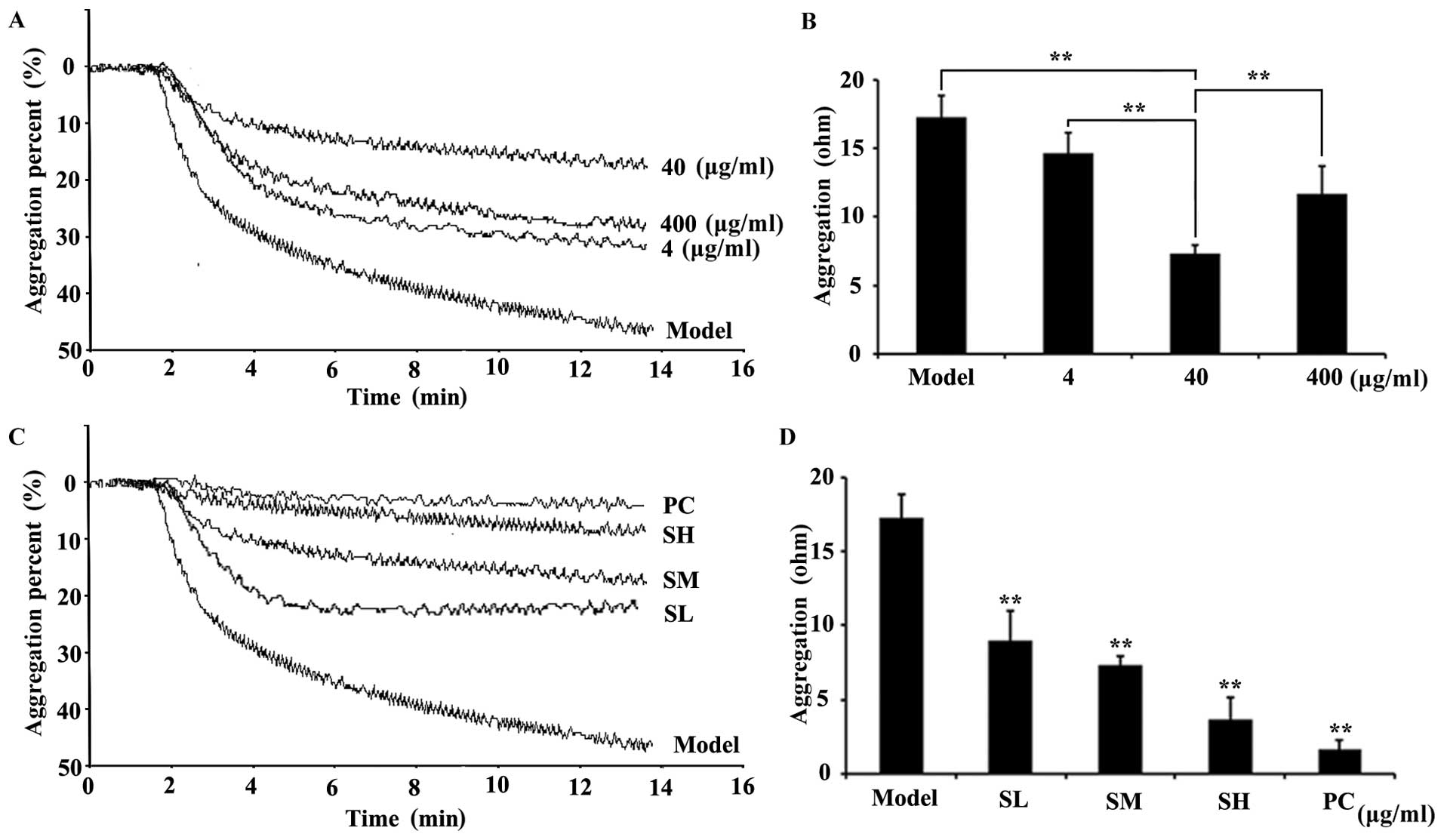
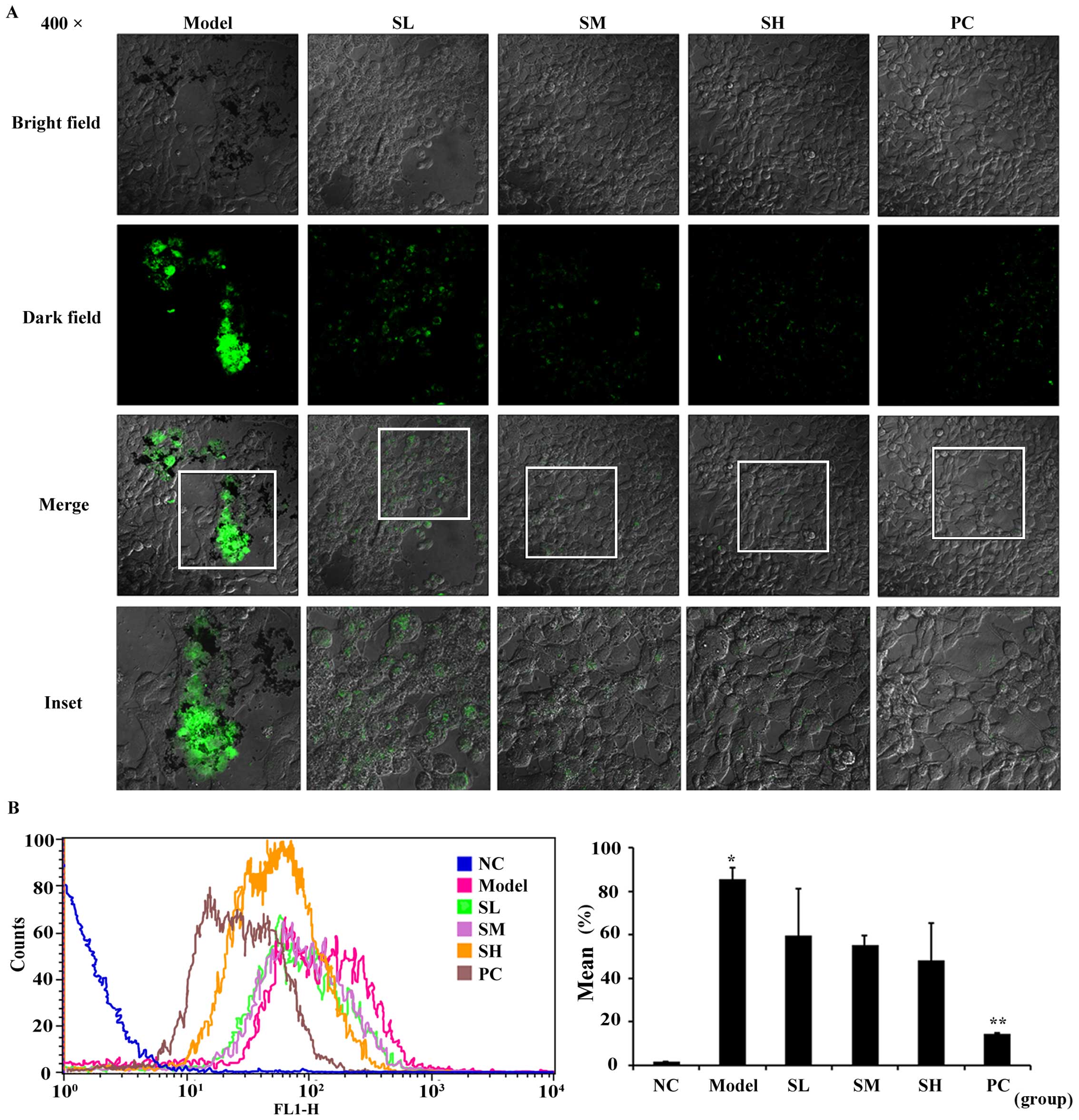
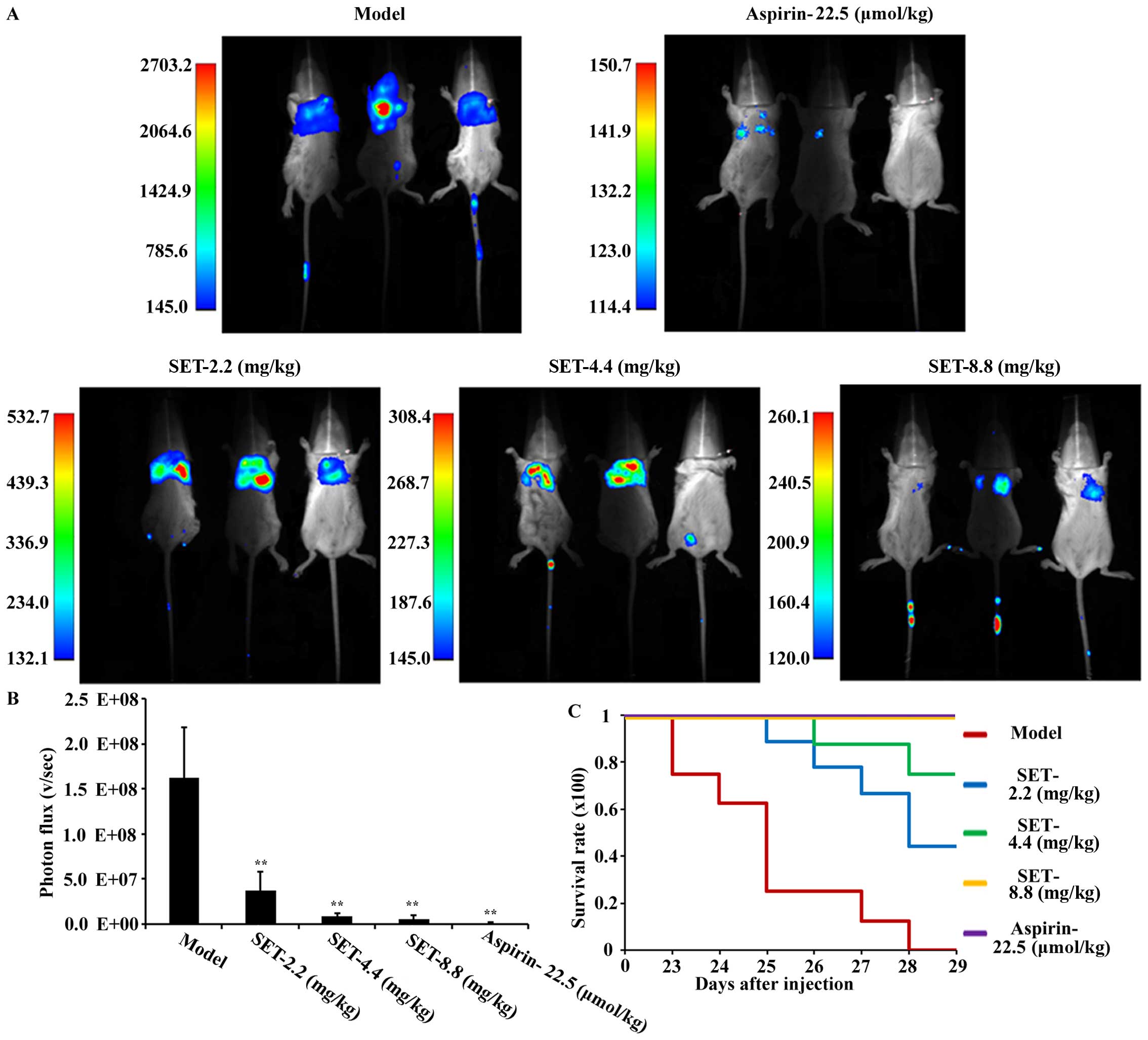
|
1
|
DeSantis CE, Fedewa SA, Sauer A Goding,
Kramer JL, Smith RA and Jemal A: Breast cancer statistics, 2015:
Convergence of incidence rates between black and white women. CA
Cancer J Clin. 66:31–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baselga J, Cortés J, Kim S-B, Im SA, Hegg
R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al:
CLEOPATRA Study Group: Pertuzumab plus trastuzumab plus docetaxel
for metastatic breast cancer. N Engl J Med. 366:109–119. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wood SL, Pernemalm M, Crosbie PA and
Whetton AD: The role of the tumor-microenvironment in lung
cancer-metastasis and its relationship to potential therapeutic
targets. Cancer Treat Rev. 40:558–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson SA: The Circulating Platelet.
Elsevier; New York, NY: 2012, View Article : Google Scholar
|
|
5
|
Byzova TV, Kerr ΒΑ, Feng W and McCabe P:
Platelets and the tumor cell microenvironment. Blood.
122:SCI-32-SCI-32. 2013.
|
|
6
|
Riedl J, Pabinger I and Ay C: Platelets in
cancer and thrombosis. Hamostaseologie. 34:54–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li N: Platelets in cancer metastasis: To
help the ‘villain’ to do evil. Int J Cancer. 138:2078–2087. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goubran HA, Burnouf T, Radosevic M and
El-Ekiaby M: The platelet-cancer loop. Eur J Intern Med.
24:393–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goubran HA, Stakiw J, Radosevic M and
Burnouf T: Platelet-cancer interactions. Semin Thromb Hemost.
40:296–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stockert JC, Blázquez-Castro A, Cañete M,
Horobin RW and Villanueva A: MTT assay for cell viability:
Intracellular localization of the formazan product is in lipid
droplets. Acta Histochem. 114:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bentz J, O'Connor MP, Bednarczyk D,
Coleman J, Lee C, Palm J, Pak YA, Perloff ES, Reyner E, Balimane P,
et al: Variability in P-glycoprotein inhibitory potency
(IC50) using various in vitro experimental systems:
Implications for universal digoxin drug-drug interaction risk
assessment decision criteria. Drug Metab Dispos. 41:1347–1366.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frenette PS, Johnson RC, Hynes RO and
Wagner DD: Platelets roll on stimulated endothelium in vivo: An
interaction mediated by endothelial P-selectin. Proc Natl Acad Sci
USA. 92:7450–7454. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Angiolillo DJ, Fernández-Ortiz A, Bernardo
E, Ramírez C, Sabaté M, Bañuelos C, Hernández-Antolín R, Escaned J,
Moreno R, Alfonso F, et al: High clopidogrel loading dose during
coronary stenting: Effects on drug response and interindividual
variability. Eur Heart J. 25:1903–1910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shou L-M, Zhang Q-Y, Li W, Xie X, Chen K,
Lian L, Li ZY, Gong FR, Dai KS, Mao YX, et al: Cantharidin and
norcantharidin inhibit the ability of MCF-7 cells to adhere to
platelets via protein kinase C pathway-dependent downregulation of
α2 integrin. Oncol Rep. 30:1059–1066. 2013.PubMed/NCBI
|
|
16
|
Labelle M, Begum S and Hynes RO: Platelets
guide the formation of early metastatic niches. Proc Natl Acad Sci
USA. 111:E3053–E3061. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang WC, Shyh-Chang N, Yang H, Rai A,
Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, et al: Glycine
decarboxylase activity drives non-small cell lung cancer
tumor-initiating cells and tumorigenesis. Cell. 148:259–272. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Medina C, Harmon S, Inkielewicz I,
Santos-Martinez MJ, Jones M, Cantwell P, Bazou D, Ledwidge M,
Radomski MW and Gilmer JF: Differential inhibition of tumour
cell-induced platelet aggregation by the nicotinate aspirin prodrug
(ST0702) and aspirin. Br J Pharmacol. 166:938–949. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Dongen JJ, Macintyre EA, Gabert JA,
Delabesse E, Rossi V, Saglio G, Gottardi E, Rambaldi A, Dotti G,
Griesinger F, et al: Standardized RT-PCR analysis of fusion gene
transcripts from chromosome aberrations in acute leukemia for
detection of minimal residual disease. Report of the BIOMED-1
Concerted Action: Investigation of minimal residual disease in
acute leukemia. Leukemia. 13:1901–1928. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu T, Tang H, Lang Y, Liu M and Li X:
MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by
targeting prohibitin. Cancer Lett. 273:233–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li M, Xing S, Zhang H, Shang S, Li X, Ren
B, Li G, Chang X, Li Y and Li W: A matrix metalloproteinase
inhibitor enhances anti-cytotoxic T lymphocyte antigen-4 antibody
immunotherapy in breast cancer by reprogramming the tumor
microenvironment. Oncol Rep. 35:1329–1339. 2016.PubMed/NCBI
|
|
22
|
Gay LJ and Felding-Habermann B:
Contribution of platelets to tumour metastasis. Nat Rev Cancer.
11:123–134. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sørensen HT, Mellemkjaer L, Steffensen FH,
Olsen JH and Nielsen GL: The risk of a diagnosis of cancer after
primary deep venous thrombosis or pulmonary embolism. N Engl J Med.
338:1169–1173. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Donati MB, Gambacorti-Passerini C, Casali
B, Falanga A, Vannotti P, Fossati G, Semeraro N and Gordon SG:
Cancer procoagulant in human tumor cells: Evidence from melanoma
patients. Cancer Res. 46:6471–6474. 1986.PubMed/NCBI
|
|
25
|
Kannagi R: Carbohydrate-mediated cell
adhesion involved in hematogenous metastasis of cancer. Glycoconj
J. 14:577–584. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gasic GJ, Gasic TB, Galanti N, Johnson T
and Murphy S: Platelet-tumor-cell interactions in mice. The role of
platelets in the spread of malignant disease. Int J Cancer.
11:704–718. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Falanga A: Thrombophilia in cancer. Semin
Thromb Hemost. 31:104–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rothwell PM, Wilson M, Price JF, Belch JF,
Meade TW and Mehta Z: Effect of daily aspirin on risk of cancer
metastasis: A study of incident cancers during randomised
controlled trials. Lancet. 379:1591–1601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mitrugno A, Williams D, Kerrigan SW and
Moran N: A novel and essential role for FcγRIIa in cancer
cell-induced platelet activation. Blood. 123:249–260. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patrignani P, Filabozzi P and Patrono C:
Selective cumulative inhibition of platelet thromboxane production
by low-dose aspirin in healthy subjects. J Clin Invest.
69:1366–1372. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hankey GJ and Eikelboom JW: Aspirin
resistance. Lancet. 367:606–617. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wagner H, Bauer R, Melchart D, Xiao P-G
and Staudinger A: Caulis Spatholobi-JixuetengChromatographic
Fingerprint Analysis of Herbal Medicines Volume III. 3. 1st.
Springer; Switzerland: pp. 235–242. 2014
|
|
33
|
Yan LG, Ruan JS, Zhang L, Fan FT, Zhang F,
Wang AY, Zheng SZ, Zeng L, Li WL and Lu Y: Effect of aqueous
extracts of several kinds of herbs on human platelet aggregation
and expression of P-selectin in vitro. Chin J Integr Med.
21:286–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu B, Liu J, Chen J, Zhu D, Zhou H and
Wang X: A study on anticancer activity of Caulis Spatholobi extract
on human osteosarcoma Saos-2 cells. Afr J Tradit Complement Altern
Medicines. 10:256–260. 2013. View Article : Google Scholar
|