Introduction
Breast cancer is the most common malignancy in
females worldwide (1). Breast
cancer develops from mammary epithelial cells and interacts with
the surrounding stromal tissues which are essential for malignant
transformation and progression of the disease (2). It is estimated that there were 1.7
million breast cancer cases diagnosed with 521,900 mortalities
worldwide in 2012 (3). Rapid
progress in medical treatment has markedly improved the prognosis
and early diagnosis, however, breast cancer is still the second
leading cause of cancer-related deaths among female patients. The
main reasons are late diagnosis, limited therapeutic options and
the metastasis (4,5). To date, surgery remains the most
effective treatment which often has negative physiological and
psychological effects on patients (6). Therefore, it is important to develop
molecular therapeutic targets against the tumorigenesis,
progression and metastasis of breast cancer, which have been proved
to be beneficial for patients with breast cancer. However, the
molecular mechanisms underlying breast cancer development and
metastasis are not fully understood. Therefore, discovery of new
therapeutic targets and development of novel drugs for breast
cancer may increase the therapeutic options and improve the quality
life and survival of these patients.
Tumor necrosis factor-α-induced protein 8-like-2
(TNFAIP8L2 or TIPE2) is a member of the TNFAIP8 family, and is
essential for maintaining immune homeostasis (7,8).
Previous studies have demonstrated that TIPE2 is associated with
caspase-8 and inhibits the activation of activator protein (AP)-1
and NF-κB. TIPE2-deficient cells showed hyper-responsiveness to
Toll-like receptor and T cell receptor activation (9). TIPE2 was found to be involved in
inflammatory and immunological diseases, such as hepatitis and
systemic lupus erythematosus (10,11).
Downregulation of TIPE2 in humans is associated with autoimmune
diseases and TIPE2 knockout in mice resulted in systemic
inflammation (8,10). Research has demonstrated that TIPE2
inhibits inducible nitric oxide synthase and nitric oxide
production to suppress inflammation by switching arginine
metabolism from nitric oxide synthase to arginase (12). The TIPE family, such as TNFAIP8 and
TIPE1, plays important roles in most human cancers (13–15).
Previous studies have also shown that TIPE2 expression is reduced
or absent in various human cancers, such as gastric and lung
cancer, and hepatocellular carcinoma (16–18).
TIPE2 is not detected or weakly expressed in many human cancer cell
lines (19). Therefore, TIPE2 may
play an important role in cancer development and progression.
In the present study, we examined the expression of
TIPE2 in breast cancer and their adjacent normal tissues. We also
established a breast cancer cell line overexpressing TIPE2 using a
lentivirus system to investigate the effects of TIPE2 on the
tumorigenesis, cell growth, apoptosis, migration and invasion of
breast cancer in vitro and in vivo. Furthermore, we
elucidated its underlying mechanisms in breast cancer development
and metastasis.
Materials and methods
Cell culture
Human embryonic kidney 293T and human breast cancer
MDA-MB-231 cell lines were purchased from the Cell Bank of the
Chinese Scientific Academy (Shanghai, China). 293T cells were
cultured in Dulbeccos modified Eagles medium (DMEM) with 10% fetal
bovine serum (FBS). MDA-MB-231 cells were cultured in L-15 medium
with 10% FBS.
Immunohistochemistry
Breast cancer tissue microarrays were purchased from
Outdo Biotech (Shanghai, China). Tissue microarrays consisted of
2-mm cores with cancer and adjacent normal tissues from patients
with invasive ductal carcinoma of the breast (98 cancer cases). The
patient ages ranged from 36 to 74 years. Tissue microarrays were
stained with rabbit anti-TIPE2 (1:100; Sigma, St. Louis, MO, USA)
and HRP-conjugated anti-rabbit IgG secondary antibodies (1:1,000;
MultiSciences Biotech, Hangzhou, China). DAB peroxidase substrate
kit (MultiSciences Biotech) was then used to produce the enzymatic
reaction. All slides were scored by evaluating the staining
intensity and the percentage of positive cells as follows: staining
intensity: 0, no staining; 1, weak staining; 2, moderate staining;
and 3, strong staining. The percentage of positive cells was as
follows: 0, <1%; 1, 1–33%; 2, 34–66%; and 3, 67–100%. The
staining intensity and the percentage of positive cells for each
slide were combined to produce a final grade of TIPE2 expression
score: 0, total score=0; 1+, total score=1–2; 2+, total score=3–4;
and 3+, total score=5–6.
Plasmid and stable cell
construction
The tipe2 gene was amplified from cDNA generated
from human peripheral blood mononuclear cells (PBMCs). The specific
primers used for the tipe2 gene were as follows: sense,
5′-GGATCCATGGAGTCCTTCAGCTCAAAGAG-3′ and antisense,
5′-GGATCCTCAGAGCTTCCCTTCGTCTAGC-3′, product size, 555 bp. Then, the
sequence of tipe2 was subcloned into the lentiviral pRRL vector.
The pRRL vector and lentivirus packaging plasmids were packaged in
293T cells according to the protocol (20). The TIPE2-expressing lentivirus was
produced by 293T cells. In brief, 293T cells were seeded in
10-cm-diameter dishes 24 h prior to transfection, and the culture
DMEM was changed 2–4 h prior to transfection. For the transfection
of one dish, DNA plasmids were added to ddH2O to a final
volume of 450 µl; 50 µl of 2.5 M CaCl2 was added to the
plasmid mixture and gently mixed well, and then dropwise 500 µl of
2X HEPES-buffered saline was added, and finally the precipitate was
added to the cultures. The medium was replaced after 12–24 h, the
conditioned medium containing lentivirus was collected after 48 h
and filtered through 0.22-µm pore-size acetate filters. Then, the
MDA-MB-231 cells were infected with the
TIPE2-expressing-lentivirus. Empty lentiviral vector was used as a
control. The transfected cells expressed GFP protein as the
lentivirus included the GFP encoding sequence. In addition, the
MDA-MB-231 cell line stably expressing TIPE2 or the vector control
was established by sorting GFP-positive cells using a BD FACS flow
cytometer (BD Biosciences, San Jose, CA, USA) and confirmed by
fluorescence microscopy.
Intracellular staining
The rabbit anti-TIPE2 (1:100) and the PE goat
anti-rabbit IgG secondary antibodies (1:100; MultiSciences Biotech)
were used for detecting TIPE2 expression in the transfected
MDA-MB-231 cells. The cells were collected and fixed with 4%
formaldehyde solution, and then stained with the anti-TIPE2
antibody. Flow cytometric analyses were performed using a BD
FACSCalibur flow cytometer (BD Biosciences) and analyzed using
FlowJo software (Tree Star, Ashland, OR, USA).
Cell proliferation and cell apoptosis
assays
Cell proliferation was determined using Cell
Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) according to the
manufacturer's instructions. MDA-MB-231/vector and MDA-MB-231/TIPE2
cells (3,000/well) were plated in a 96-well plate and subjected to
CCK-8 assay after 48 h. CCK-8 solution was added to each well and
incubated for 4 h. The optical density (OD) value was detected at a
wavelength of 450 nm on a microplate reader. For cell apoptosis
assay, MDA-MB-231/vector and MDA-MB-231/TIPE2 cells
(3×105/well) were seeded into a 6-well plate and
incubated for 48 h. Then, the cells were collected and stained with
Annexin V and 7-AAD (BD Biosciences), and were analyzed by flow
cytometry. The data were analyzed by FlowJo software.
Migration and invasion assays
The migration and invasion abilities of the
MDA-MB-231/vector and MDA-MB-231/TIPE2 cells were assessed by
Transwell assay and Transwell smeared with Matrigel assay (BD
Biosciences), respectively. For both assays, MDA-MB-231/vector and
MDA-MB-231/TIPE2 cells were cultured in serum-free medium in the
upper chamber, wherein 1×105 cells were planted to
migrate or invade through a filter (8 µm) toward 10%
serum-containing medium in the bottom chamber of a 24-well plate
for 16 h in the migration assay and for 24 h in the invasion assay,
respectively. The migrating and invading cells that passed through
the membrane were stained with 0.1% crystal violet and counted
under a microscope.
Western blot analysis
Cells were lysed in the presence of protease and
phosphatase inhibitors (Thremo Scientific Pierce, Rockford, IL,
USA) and the total protein concentration was determined. Protein
was separated by SDS-PAGE and transferred to polyvinylidene
difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The
membranes were blocked with 5% milk in Tris-buffered saline and
Tween-20 (TBST), and then incubated with primary antibodies
overnight. The following primary antibodies (Cell Signaling
Technology, Inc., Danvers, MA, USA) were used: anti-AKT (1:1,000)
and phospho-AKT (1:1,000), anti-p38 (1:1,000) and phospho-p38
(1:1,000) and anti-GAPDH (1:3,000), followed by secondary
antibodies (1:3,000; goat anti-rabbit IgG or goat anti-mouse IgG;
Cell Signaling Technology, Inc.) conjugated with horseradish
peroxidase for 1 h at room temperature. After washing,
immunoreactive bands were visualized with enhanced
chemiluminescence (ECL) using an ECL commercial kit (Thermo
Scientific Pierce).
Experimental animals
Specific pathogen-free female BALB/c nude mice
(6–8-weeks old) were purchased from the Shanghai Laboratory Animal
Center (Shanghai, China). All mice were housed in specific
pathogen-free facilities and in accordance with the National Animal
Care and Use Committee. All animal studies were approved by the
Institutional Laboratory Animal Care and Use Committee at Fudan
University.
Tumor xenograft model
MDA-MB-231/vector and MDA-MB-231/TIPE2 cells
(1×106 cells in 100 µl PBS) were subcutaneously injected
into the right hind legs of female nude mice. After two weeks of
injection, the tumor sizes were measured every three or four days
using a caliper, and the tumor volume (V) was calculated according
to the standard formula: V = 1/2 × length × width2. When
the tumor volume reached ~1,000 mm3 (35 days post
injection of tumor cells), the mice were sacrificed and the tumors
were removed and photographed.
Statistical method
Statistical analysis was performed using GraphPad
Prism 5 software (GraphPad, San Diego, CA, USA). The data are
presented as mean ± standard deviation (SD). The data of the groups
were compared with Student's t-test (unpaired, two-tailed). For all
analysis, a p-value <0.05 was considered to indicate a
statistically significant difference.
Results
Expression of TIPE2 is reduced in
human breast cancer
Previous studies have shown that TIPE2 is
significantly correlated with various types of human cancers, such
as gastric and lung cancer, and hepatocellular carcinoma (16–18).
However, the expression of TIPE2 in breast cancer is still unclear.
To determine the expression of TIPE2 in breast cancer, we examined
the levels of TIPE2 expression in breast cancer and adjacent normal
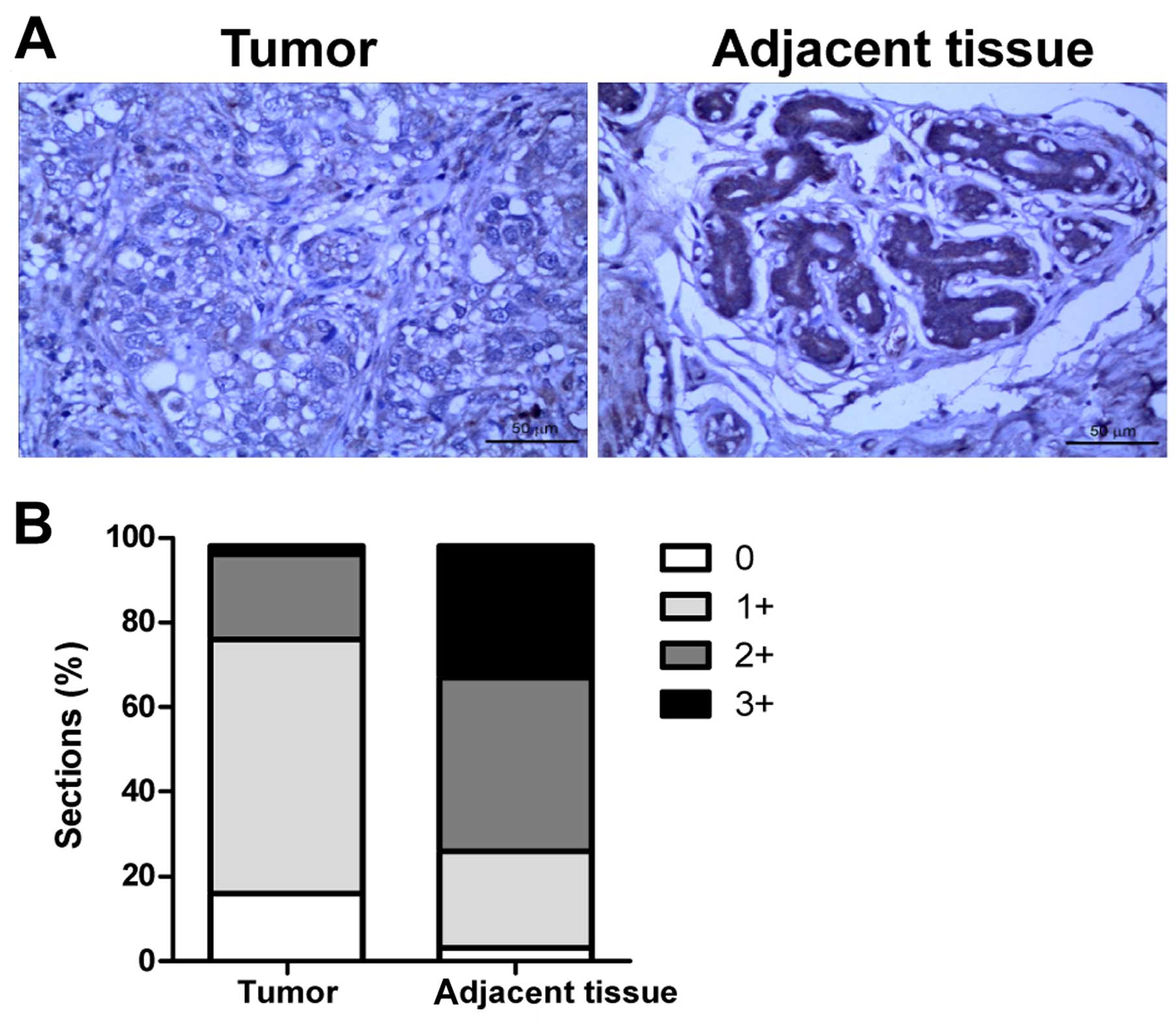
tissues by immunohistochemistry. As shown in Fig. 1, the intensity and proportion of
TIPE2-positive stained cells were markedly downregulated in the
breast cancer tissues compared to the high levels of TIPE2
expression in the samples of adjacent normal tissues (Fig. 1A and B). The data indicate that
TIPE2 may play an important role in breast carcinogenesis.
TIPE2 attenuates cell proliferation
and promotes cell apoptosis
To investigate the function of TIPE2 in breast
cancer, we transfected breast cancer cell line MDA-MB-231 with a
lentivirus expressing TIPE2 to establish cells stably expressing
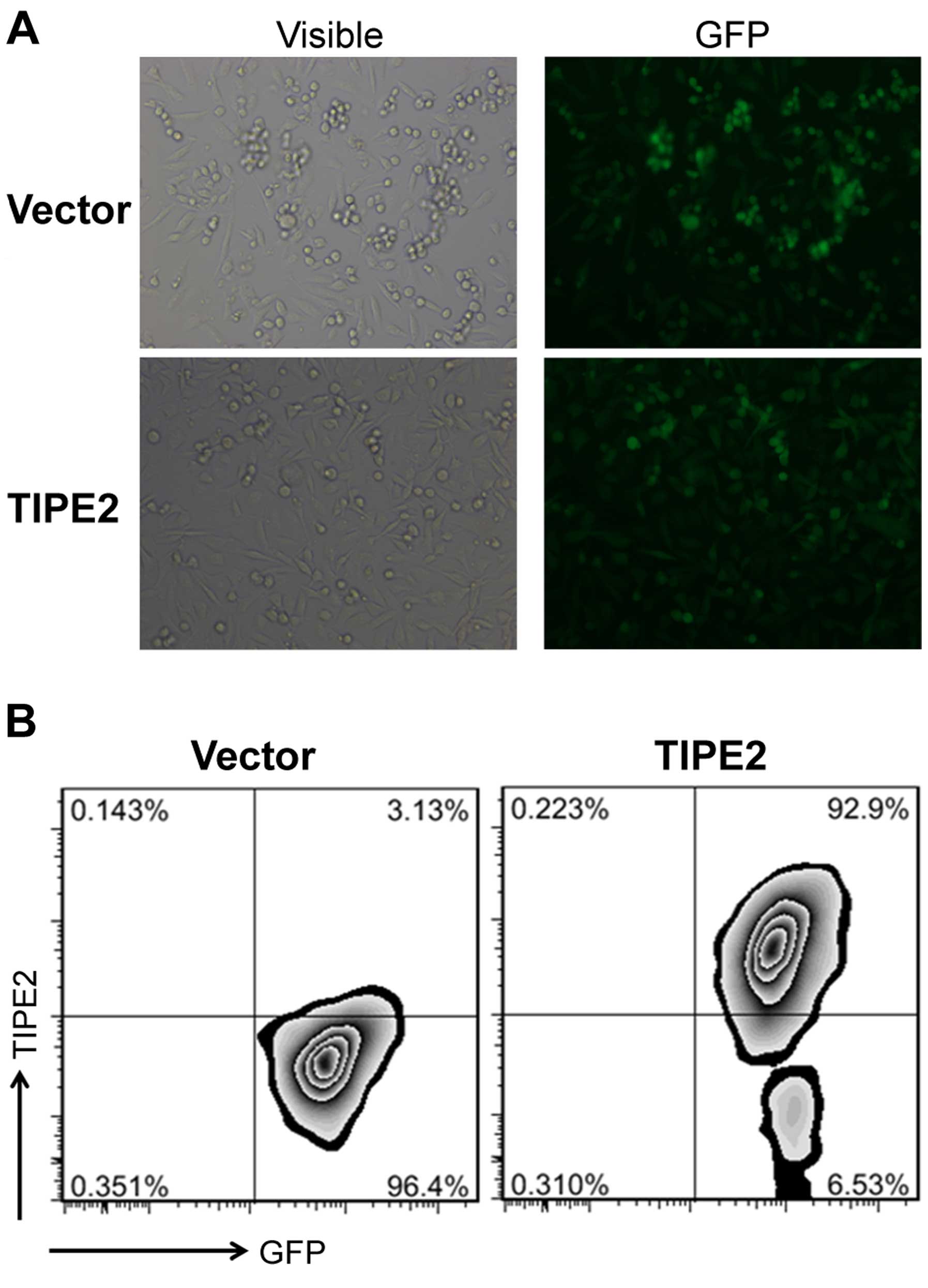
TIPE2 or a vector control by sorting GFP-positive cells. The
expression of GFP in the transfected cells was determined by
fluorescence microscopy (Fig. 2A).
We found that TIPE2 was overexpressed in the transfected
MDA-MB-231/TIPE2 cells compared to the vector control via
intracellular staining by flow cytometry (Fig. 2B). To assess the effects of TIPE2
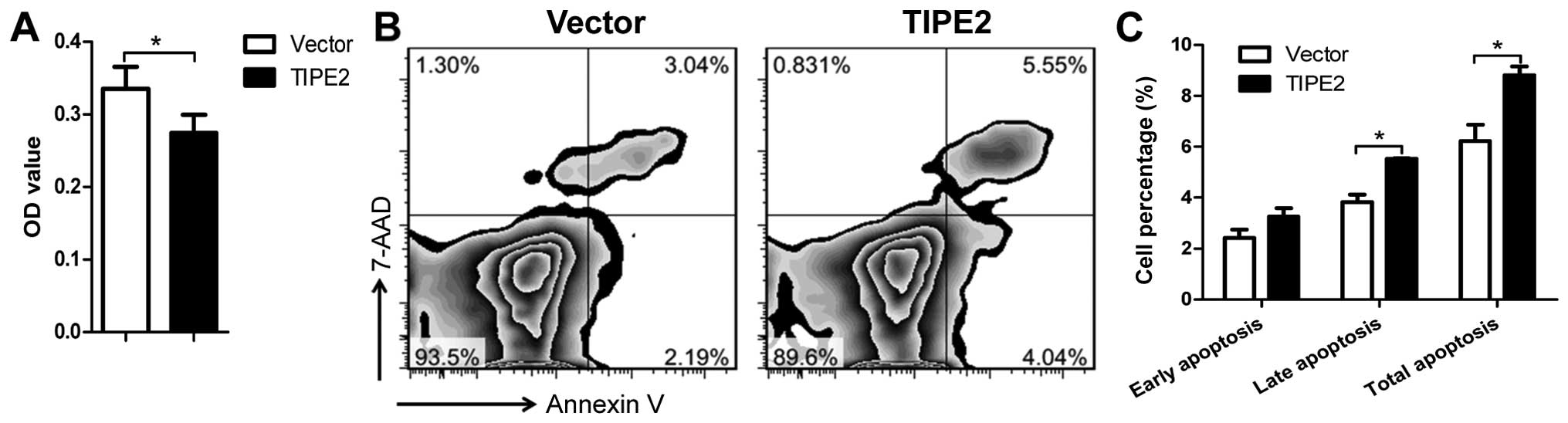
expression on tumor cell proliferation and apoptosis in
vitro, we examined cell growth inhibition and apoptosis of the
MDA-MB-231/vector and MDA-MB-231/TIPE2 cells. The cell
proliferation was significantly decreased by TIPE2 expression as
determined by CCK-8 assay (Fig.
3A). The rate of apoptosis in the cells expressing TIPE2
(5.23%) was significantly increased compared to that of the vector
control cells (9.59%) (Fig. 3B and
C). These results suggest that TIPE2 markedly inhibited the
cell proliferation and promoted apoptosis in the cancer cells in
vitro.
TIPE2 suppresses the migration and
invasion of breast cancer cells
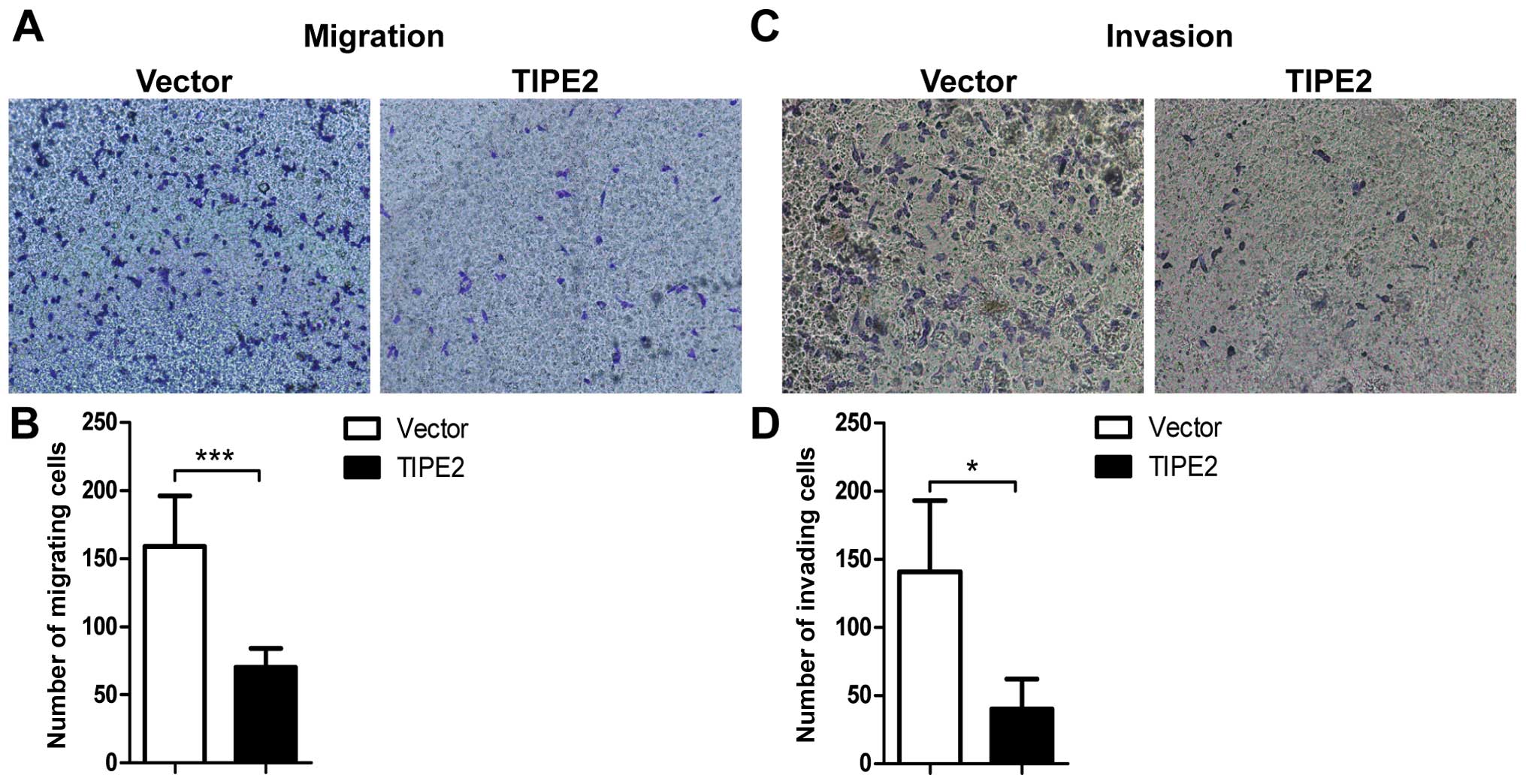
To explore the effect of TIPE2 on breast cancer cell
metastasis in vitro, we further investigated the role of
TIPE2 in breast cancer cell migration and invasion by Transwell
assays. Overexpression of TIPE2 significantly reduced the migrating
cell number of the MDA-MB-231 cells compared to that of the vector
control (Fig. 4A and B). TIPE2 also
attenuated the number of cells invading through Matrigel (Fig. 4C and D). Therefore, the results
indicated that TIPE2 effectively suppressed the ability of
migration and invasion of the breast cancer cells.
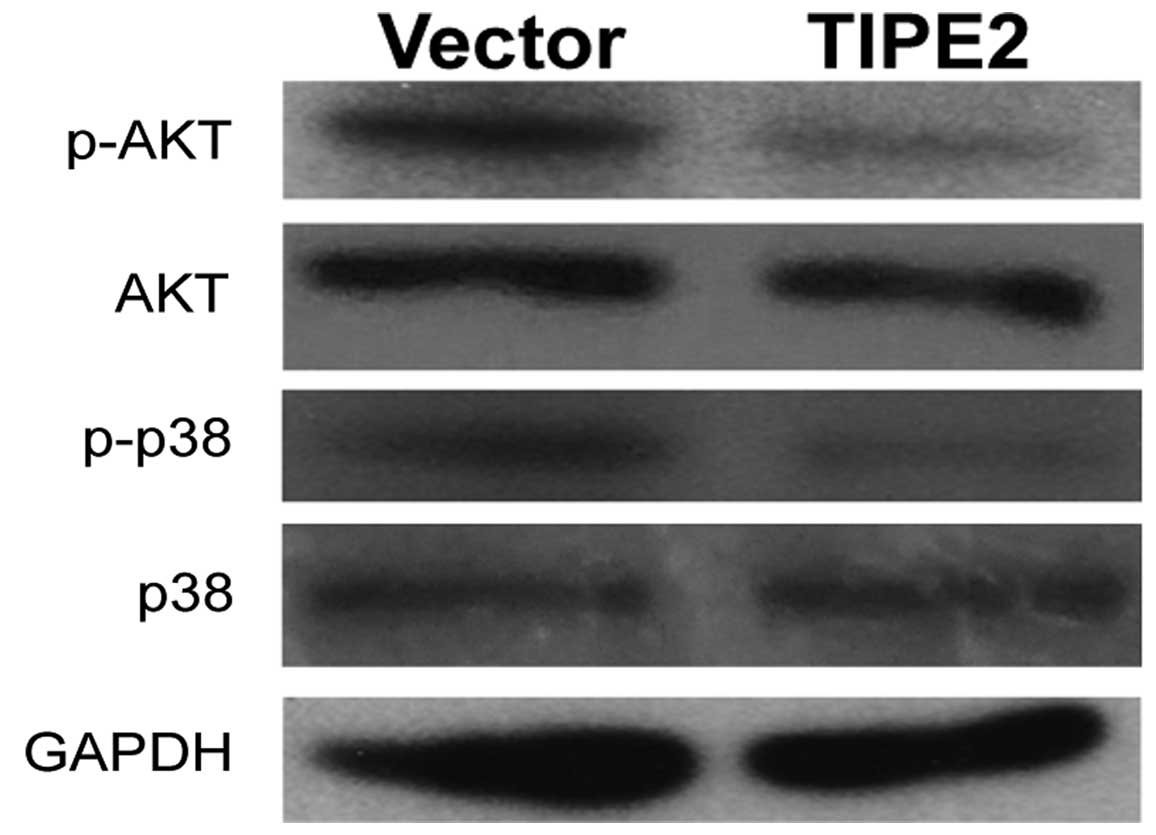
Overexpression of TIPE2 inhibits
PI3K/AKT and p38/MAPK signaling pathways
The PI3K/AKT and p38/MAPK signaling pathways are
crucial to the control of cell proliferation, apoptosis and
metastasis. To identify the signal transduction pathways involved
in the effects of the overexpression of TIPE2 on the breast cancer
cells, we examined the expression levels and activation status of
AKT and p38. The expression of AKT, p-AKT, p38 and p-p38 in the
MDA-MB-231/vector and MDA-MB-231/TIPE2 cells was detected by
western blot analysis. TIPE2 overexpression in the MDA-MB-231 cells
significantly downregulated the phosphorylation of AKT and p38
(Fig. 5). The data suggest that
suppression of the development of the breast cancer cells by TIPE2
was possibly via inhibition of the AKT and p38 signaling
pathways.
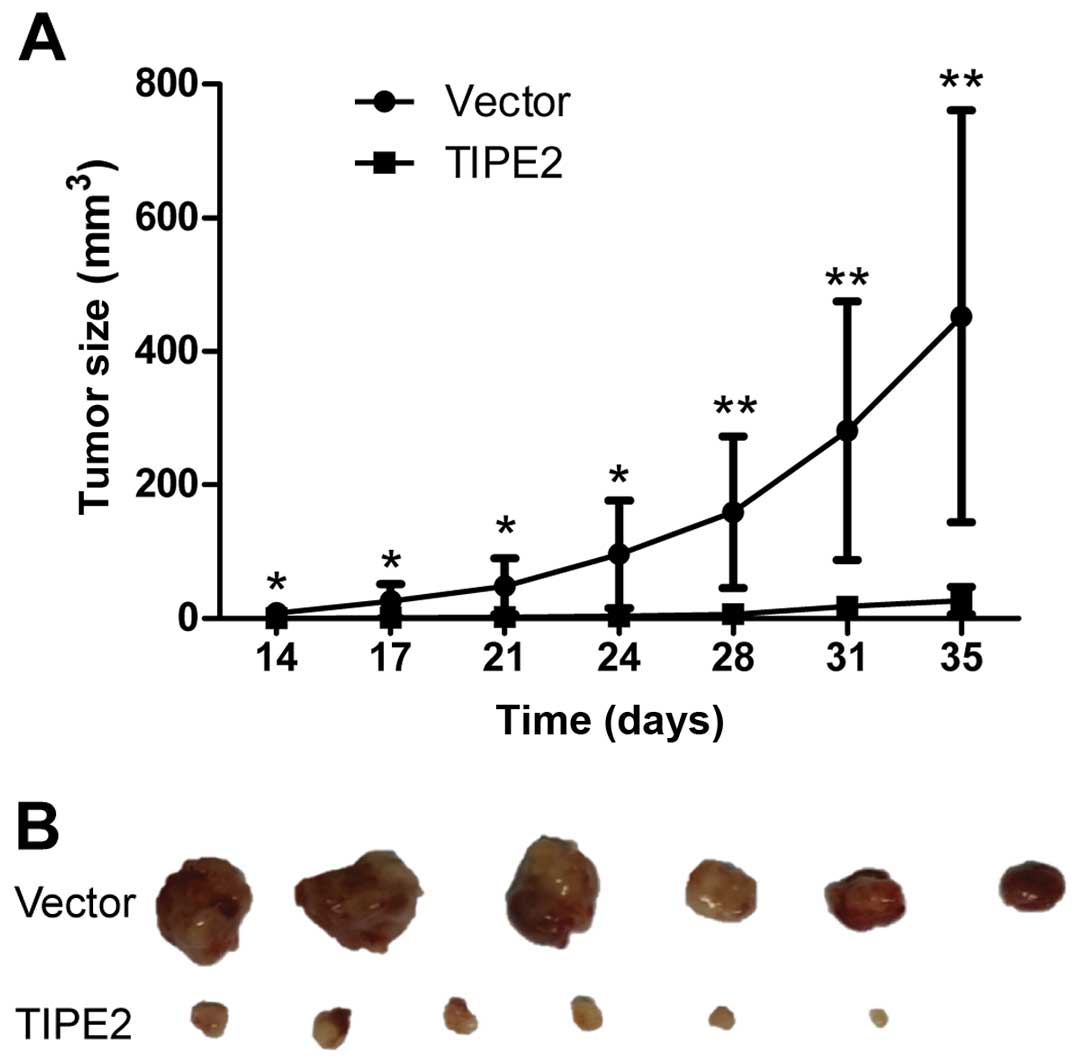
TIPE2 inhibits the tumorigenesis of
breast cancer in vivo
To investigate the effect of TIPE2 on the
tumorigenesis of breast cancer in vivo, we established
subcutaneous MDA-MB-231/vector and MDA-MB-231/TIPE2 tumor
xenografts in a nude mouse model by cell injection. The cells began
to form measurable tumors on day 14 after inoculation. The tumor
volume of the TIPE2 group was significantly less than that of the
vector control group during the progression of the breast cancer
(Fig. 6A). Mice were sacrificed ~35
days after tumor cell inoculation due to the large tumor volume in
the control group mice. The tumors were removed from the mice and
photographed. The tumor size in the vector control group was
significantly larger than that in the TIPE2 group (Fig. 6B). The results demonstrated that
TIPE2 significantly suppressed the tumorigenesis and progression of
breast cancer in vivo.
Discussion
Previous studies have shown that the expression of
TIPE2 is involved in the carcinogenesis of human cancers (16–18).
TIPE2 was found to inhibit the proliferation of gastric cancer
cells by upregulating the expression of p27, and suppressed the
metastasis of gastric cancer via negatively regulating β-catenin
signaling by inhibiting AKT and activating GSK3β (18,21).
TIPE2 downregulation was found to be associated with the poor
prognosis of non-small cell lung cancer, and overexpression of
TIPE2 inhibited cell proliferation and invasion by suppressing
Bcl-xL and N-cadherin expression (17). TIPE2 was reported as an inhibitor of
the oncogenic Ras and could promote cell death via competitively
binding to Ras-interacting domain of RGL, thereby inhibiting
RGL-induced activation of Ral GTPase and AKT. Overexpression of
TIPE2 in Ras 3T3 cells significantly delayed tumor onset in
vivo (16). In hepatocellular
carcinoma, overexpression of TIPE2 led to hepatocellular carcinoma
cell death and inhibited Ras-induced tumorigenesis (16). TIPE2 also inhibited the metastasis
of hepatocellular carcinoma via suppressing Ral and Rac1 GTPases
and reducing F-actin polymerization and expression of matrix
metallopeptidase 9 (MMP9) and urokinase plasminogen activator (uPA)
(22). These studies indicate that
TIPE2 may exert tumor-suppressive effects against cancer
development.
However, the expression of TIPE2 and its regulatory
mechanisms in breast cancer are still unclear. In the present
study, TIPE2 expression was found to be downregulated in breast
cancer tissues compared to that in their adjacent normal tissues.
Thus, we speculated that TIPE2 may affect the development of breast
cancer. Then, we established a TIPE2-overexpressing breast cancer
cell line by lentivirus system. We found that TIPE2 overexpression
inhibited the proliferation and promoted the apoptosis of the
cancer cells. Furthermore, TIPE2 also suppressed the migration and
invasion of the breast cancer cells in vitro. Additionally,
overexpression of TIPE2 attenuated the tumorigenesis and growth of
breast cancer in vivo. These results indicate that TIPE2
plays an important role in the regulation of the tumorigenesis,
growth and metastasis of breast cancer.
To investigate the effect of TIPE2 on the signaling
pathways in breast cancer cells, we detected the expression of
several common signaling molecules in the cells overexpressing
TIPE2. We found that the level of phosphorylated AKT was
downregulated by TIPE2. The PI3K/AKT signaling pathway plays an
important role in tumorigenesis and AKT activation regulates
critical cancer cell physiology, including cell survival,
proliferation, metastasis, apoptosis and metabolism (23). The PI3K/AKT signaling pathway is
involved in breast cancer pathogenesis, and it may be a factor for
drug resistance to systemic treatments of breast cancer (24). We also found that TIPE2 decreased
the expression of phosphorylated p38. It has been reported that p38
contributes to the proliferation and metastasis of breast cancer,
and inhibition of p38 activation can suppress the growth and
metastasis of breast cancer cells (25–27).
Therefore, TIPE2 may suppress the tumorigenesis, growth and
metastasis of breast cancer by inhibiting AKT and p38
phosphorylation.
Taken together, we demonstrated that TIPE2 was
weakly expressed in human breast cancer compared to that observed
in adjacent normal tissues. Overexpression of TIPE2 significantly
suppressed the growth and metastasis of breast cancer possibly via
inhibition of the activation of ATK and p38. Therefore, TIPE2 may
become a potential therapeutic target for breast cancer
therapy.
Acknowledgements
The present study was supported by project funding
from Shanghai City (15DZ2291700).
References
|
1
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bissell MJ and Hines WC: Why don't we get
more cancer? A proposed role of the microenvironment in restraining
cancer progression. Nat Med. 17:320–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Filipova A, Seifrtova M, Mokry J, Dvorak
J, Rezacova M, Filip S and Diaz-Garcia D: Breast cancer and cancer
stem cells: A mini-review. Tumori. 100:363–369. 2014.PubMed/NCBI
|
|
6
|
Edge SB: Quality measurement in breast
cancer. J Surg Oncol. 110:509–517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lou Y and Liu S: The TIPE (TNFAIP8) family
in inflammation, immunity, and cancer. Mol Immunol. 49:4–7. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun H, Gong S, Carmody RJ, Hilliard A, Li
L, Sun J, Kong L, Xu L, Hilliard B, Hu S, et al: TIPE2, a negative
regulator of innate and adaptive immunity that maintains immune
homeostasis. Cell. 133:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freundt EC, Bidere N and Lenardo MJ: A
different TIPE of immune homeostasis. Cell. 133:401–402. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li D, Song L, Fan Y, Li X, Li Y, Chen J,
Zhu F, Guo C, Shi Y and Zhang L: Down-regulation of TIPE2 mRNA
expression in peripheral blood mononuclear cells from patients with
systemic lupus erythematosus. Clin Immunol. 133:422–427. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang LY, Fan YC, Zhao J, Gao S, Sun FK,
Han J, Yang Y and Wang K: Elevated expression of tumour necrosis
factor-α-induced protein 8 (TNFAIP8)-like 2 mRNA in peripheral
blood mononuclear cells is associated with disease progression of
acute-on-chronic hepatitis B liver failure. J Viral Hepat.
21:64–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lou Y, Zhang G, Geng M, Zhang W, Cui J and
Liu S: TIPE2 negatively regulates inflammation by switching
arginine metabolism from nitric oxide synthase to arginase. PLoS
One. 9:e965082014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui J, Zhang G, Hao C, Wang Y, Lou Y,
Zhang W, Wang J and Liu S: The expression of TIPE1 in murine
tissues and human cell lines. Mol Immunol. 48:1548–1555. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong QZ, Zhao Y, Liu Y, Wang Y, Zhang PX,
Jiang GY, Dong XJ, Cui QZ and Wang EH: Overexpression of SCC-S2
correlates with lymph node metastasis and poor prognosis in
patients with non-small-cell lung cancer. Cancer Sci.
101:1562–1569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar D, Gokhale P, Broustas C,
Chakravarty D, Ahmad I and Kasid U: Expression of SCC-S2, an
antiapoptotic molecule, correlates with enhanced proliferation and
tumorigenicity of MDA-MB 435 cells. Oncogene. 23:612–616. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gus-Brautbar Y, Johnson D, Zhang L, Sun H,
Wang P, Zhang S, Zhang L and Chen YH: The anti-inflammatory TIPE2
is an inhibitor of the oncogenic Ras. Mol Cell. 45:610–618. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Li X, Liu G, Sun R, Wang L, Wang J
and Wang H: Down-regulated TIPE2 is associated with poor prognosis
and promotes cell proliferation in non-small cell lung cancer.
Biochem Biophys Res Commun. 457:43–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Q, Zhao M, Dong T, Zhou C, Peng Y,
Zhou X, Fan B, Ma W, Han M and Liu S: Tumor necrosis
factor-α-induced protein-8 like-2 (TIPE2) upregulates p27 to
decrease gastic cancer cell proliferation. J Cell Biochem.
116:1121–1129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang G, Hao C, Lou Y, Xi W, Wang X, Wang
Y, Qu Z, Guo C, Chen Y and Zhang Y: Tissue-specific expression of
TIPE2 provides insights into its function. Mol Immunol.
47:2435–2442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dull T, Zufferey R, Kelly M, Mandel RJ,
Nguyen M, Trono D and Naldini L: A third-generation lentivirus
vector with a conditional packaging system. J Virol. 72:8463–8471.
1998.PubMed/NCBI
|
|
21
|
Wu J, Zhang H, Xu C, Xu H, Zhou X, Xie Y
and Tao M: TIPE2 functions as a metastasis suppressor via
negatively regulating β-catenin through activating GSK3β in gastric
cancer. Int J Oncol. 48:199–206. 2016.PubMed/NCBI
|
|
22
|
Cao X, Zhang L, Shi Y, Sun Y, Dai S, Guo
C, Zhu F, Wang Q, Wang J, Wang X, et al: Human tumor necrosis
factor (TNF)- alpha-induced protein 8-like 2 suppresses
hepatocellular carcinoma metastasis through inhibiting Rac1. Mol
Cancer. 12:1492013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lauring J, Park BH and Wolff AC: The
phosphoinositide-3-kinase-Akt-mTOR pathway as a therapeutic target
in breast cancer. J Natl Compr Canc Netw. 11:670–678.
2013.PubMed/NCBI
|
|
24
|
Yang SX, Polley E and Lipkowitz S: New
insights on PI3K/AKT pathway alterations and clinical outcomes in
breast cancer. Cancer Treat Rev. 45:87–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen L, Mayer JA, Krisko TI, Speers CW,
Wang T, Hilsenbeck SG and Brown PH: Inhibition of the p38 kinase
suppresses the proliferation of human ER-negative breast cancer
cells. Cancer Res. 69:8853–8861. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim MS, Lee EJ, Kim HR and Moon A: p38
kinase is a key signaling molecule for H-Ras-induced cell motility
and invasive phenotype in human breast epithelial cells. Cancer
Res. 63:5454–5461. 2003.PubMed/NCBI
|
|
27
|
Liu C, Wang S, Zhu S, Wang H, Gu J, Gui Z,
Jing J, Hou X and Shao Y: MAP3K1-targeting therapeutic artificial
miRNA suppresses the growth and invasion of breast cancer in vivo
and in vitro. Springerplus. 5:112016. View Article : Google Scholar : PubMed/NCBI
|