Introduction
Prostate cancer (PCa), which is a
heterogeneous-multifocal disease, ranks the most common cancer in
men (1). The PCa incidence is
growing, particularly in developed countries. One in six males in
America will suffer from PCa in their lifetime, and every year
there are >900,000 newly diagnosed PCa cases in the world
(2). Genetic and environmental
factors have been reported to be involved in the control of
carcinogenesis and progression of PCa (3).
Over the past decades, recurrence of PCa, which
often demonstrates chemotherapy-resistant and
androgen-independence, has drawn increasing attention. Great
efforts have been made and considerable progress has been achieved
to understand the molecular mechanism of the disease including
epithelial-mesenchymal transition (EMT) (4), multidrug resistance gene expression
(5), the mutation or amplification
of androgen receptor and cancer stem cells (CSCs) or CSC-like cells
(6). CSC model was first verified
in acute myeloid leukemia (AML) in 1997 (7,8). This
model supposed that cancers possessed hierarchical organization as
the normal tissues did to a large degree and a small subset of
tumor cells which were characterized by remarkable ability to
generate new tumors constituted CSCs. Subsequently, CSCs have been
identified in numerous human malignancies, including PCa, liver
cancer, pancreatic cancer, brain cancer and breast cancer (9–13).
Consequently, it is important to identify the novel markers of
CSCs, as more effective therapies might be available for patients
with cancers.
As small non-coding RNAs consisting of 18–22
nucleotides, microRNAs (miRNAs) serve as important regulators in
post-transcriptional regulation of target genes and mRNA silence by
binding to the 3′-untranslated region (3'UTR), leading to
inhibition or degradation of targeted mRNAs (14). Some miRNAs have been confirmed to be
involved in numerous biological processes (15,16).
miRNAs have been shown to be involved in the control of recurrence
of PCa (17), and have also been
reported to be involved in regulating characteristics of CSCs
(18). It has been previously shown
that miR-574 is substantially downregulated in CSCs (19), and REL is believed to be a
significant regulator of cancer cell proliferation (20). In this study, we confirmed the
regulatory relationship between miR-574 and REL and verified that
miR-574/REL signaling pathway is involved in the control of
recurrence of PCa by modulating the expression of REL.
Materials and methods
Study population and sample
collection
In this study, we collected PCa samples with
recurrence (n=24) and without recurrence (n=24) from Dongying
People's Hospital of Shandong. Patients with a prostate-specific
antigen (PSA) elevation of >0.2 ng/ml after initially receiving
radical prostatectomy (RP) or radiotherapy with curative intent is
defined as biochemical recurrence. The study protocol was approved
by the Ethics Committee of Dongying People's Hospital of Shandong.
Written informed consents were obtained from all patients prior to
the study.
Western blot analysis
Proteins were extracted from the cells using 1X
Radioimmunoprecipitation Assay (RIPA) Lysis buffer (Upstate
Biotechnology, Lake Placid, NY, USA) and the protein level was
determined using protein assay reagents according to standard
protocols (Bio-Rad Laboratories, Hercules, CA, USA). Western blot
analysis was performed to assess protein expression. Briefly, 25 µg
of total protein was loaded on Life Technologies NuPAGE®
4–12% Bis-Tris gel (Thermo Fisher Scientific) and electrophoresed.
After transfering to a pure nitrocellulose membrane (Bio-Rad
Laboratories), we blocked the membranes with Odyssey®
blocking buffer (LI-COR Biosciences, Lincoln, NE, USA). The
membranes were then incubated in primary antibodies buffer (Odyssey
blocking buffer, 0.1% Tween-20®) overnight at 4°C. The
primary antibodies, anti-REL and anti-actin, were purchased from
Cell Signaling Technology, Inc. (Beverly, MA, USA). The following
day, membranes were washed four times for 5 min in Tris-buffered
saline and Tween-20 (TBST). Subsequently, membranes were incubated
in secondary antibodies IRDye® 680LT goat anti-mouse lgG
or anti-rabbit lgG (Cell Signaling Technology, Inc.) plus Odyssey
blocking buffer and 0.1% Tween-20 at 1:20,000 dilution for 1 h.
RNA isolation and real-time PCR
We extracted total RNA from PC-3 cells or tissue
samples using High Pure Isolation kit in accordance with the
manufacturer's instructions (Roche Life Science, West Sussex, UK).
The miRNA Q-PCR detection kit (GeneCopoeia) was employed to
quantify miR-574 level according to the manufacturer's
instructions. Briefly, the protocol was conducted for 35 cycles at
95°C for 5 min, 95°C for 10 sec, and 55°C for 10 sec. In total, 50
cycles were performed. The PCR amplification for the quantification
of the miR-574 or REL and U6 was performed using TaqMan miRNA
Reverse Transcription kit (Applied Biosystems, Foster City, CA,
USA) according to the manufacturer's instructions. Primer sets for
miR-574 or REL were designed using Primer3 software version 1.0
(Whitehead Institute for Biomedical Research, Cambridge, MA, USA).
All the reactions were performed in triplicate and data were
expressed as 2−ΔΔCt.
Luciferase assay
The 3'UTR segment of miR-574 and REL siRNA was
amplified and subcloned into the pmirGLO luciferase reporter vector
(Promega). The corresponding mutant constructs were generated by
mutating the seed regions of the miR-574 or REL siRNA binding
sites. The cells (3.5×104) were seeded in triplicate in
24-well plates and cotransfected with wild-type (Wt)/mutant (Mt)
3'UTR vectors and miR-574 mimics or scramble control using
Lipofectamine 2000. After 48 h of transfection, the cells were
measured for luciferase activity on the Dual-Luciferase Reporter
Assay System (Promega) according to the manufacturer's
instructions. The firefly luciferase activities were normalized to
Renilla luciferase activity. All experiments were repeated three
times.
Cell proliferation assay
The viability was determined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The cells were seeded at a density of 2×103
cells/well in 96-well culture plates and incubated for 24 h at 37°C
prior to transfection. The following day, cells were transfected
with miR-574 or REL siRNA. After 48 h, 20 ml of MTT solution (5
mg/ml in PBS) was added to each well. Samples were further
incubated for 4 h. The absorbance was read on a
SPECTRAmax® microplate spectrophotometer (Molecular
Devices, LLC, Sunnyvale, CA, USA) at a wavelength of 490 nm.
Experiments were carried out in triplicates.
CSC culture and transfection
Tumorsphere (prostatosphere) was cultured as
described previously (18).
Resulting tumorspheres were maintained at least 2 weeks with medium
being changed at a 3-day interval. These prostatosphere cultures
contained mainly cells with stemness markers and were considered as
CSCs. miR-574 or REL siRNA mimics and scramble control mimics
(GenePharma, Suzhou, China) were transfected in CSCs at a
concentration of 50 nM with Lipofectamine 2000 reagent
(Invitrogen).
Apoptosis analysis
PC-3 cells were seeded in 6-well plates
(3.5×105 cells/well) and transfected with mimics or
inhibitors of miR-574 or NC as a control. Twenty-four hours later,
50 nmol/l of paclitaxel was added in media. After 48 h of
incubation, cells were harvested and washed with cold PBS, stained
with 5 µl Annexin V-FITC and 10 µl propidium iodide (PI) (20
µg/ml). The mixture was incubated at room temperature in the dark
for 15 min. Cell apoptosis was analyzed on the FACScan flow
cytometer (Becton Dickinson, USA). Each experiment with triplicate
samples was repeated three times.
Statistical analysis
The target genes of specific miRNAs were predicted
using two prediction algorithms, TargetScan (http://www.targetscan.org/) and miRDB (http://mirdb.org/cgi-bin/search.cgi). The t-test
(two groups) or one-way ANOVA (three groups or more) was used for
assessing the statistical significance of each differential
expression analysis result. All statistical analysis was performed
using SPSS 20.0 (IBM, Inc., Chicago, IL, USA). P<0.05 was
considered significant.
Results
REL is the virtual target of
miR-574-5p
miR-574-5p has been reported involved with many
diseases such as colorectal cancer, liver metastasis and lung
cancer. In order to understand the role of miR-574-5p in PCa
recurrence, we used online miRNA target prediction tools to search
the regulatory gene of miR-574-5p, and consequently identified
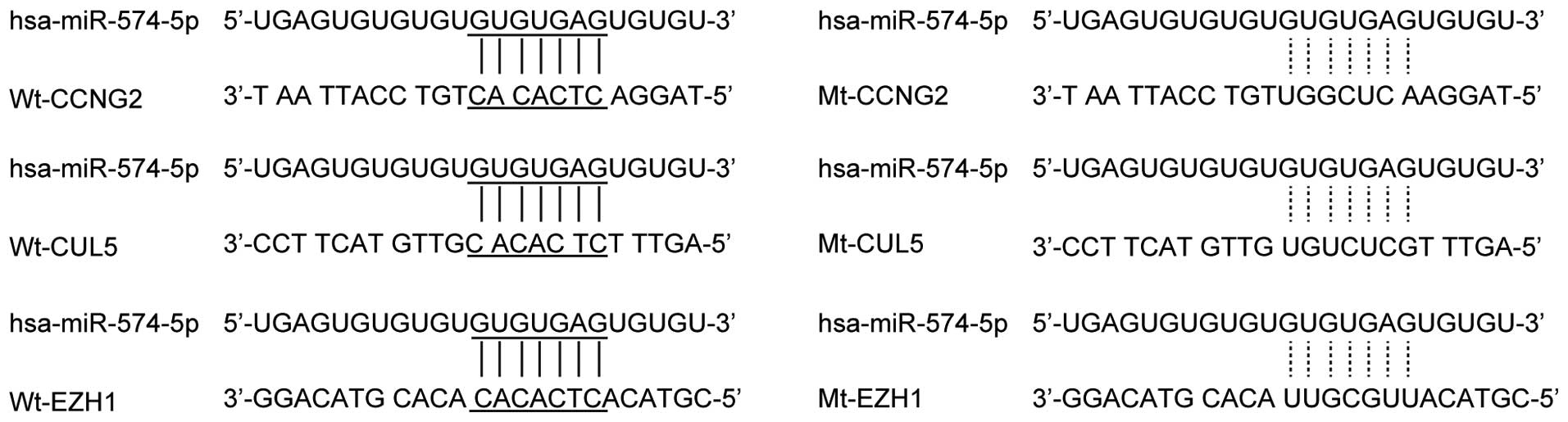
CCNG2, CUL5, EZH1 and REL as the candidate target genes of
miR-574-5p in prostate CSCs with the ‘seed sequence’ in the 3'UTR
(Fig. 1). Furthermore, to validate
the regulatory relationship among miR-574-5p and CCNG2, CUL5, EZH1
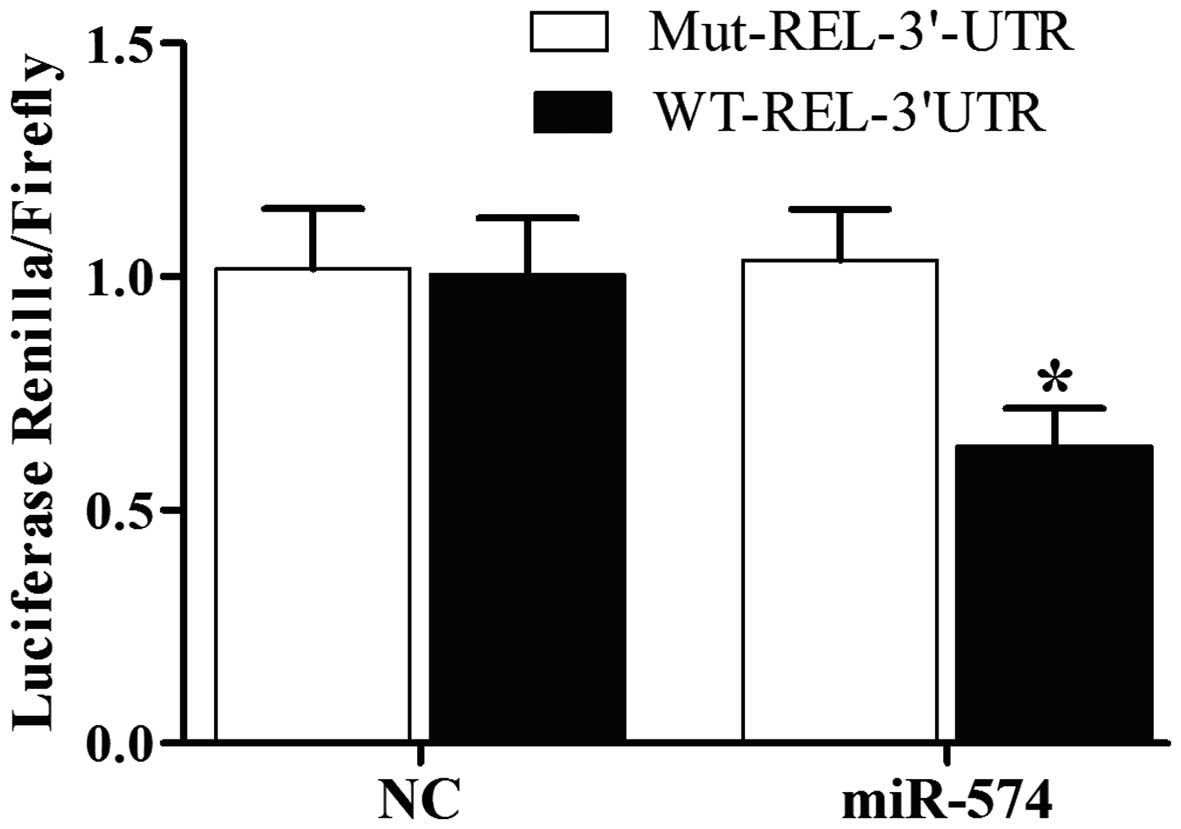
and REL, we also conducted luciferase activity reporter assay in
prostate CSCs, we can see the luciferase activity from the cells
cotransfected with miR-574-5p and wild-type REL 3'UTR decreased
significantly (Fig. 2), while cells
cotransfected with miR-574-5p and CCNG2, CUL5, EZH1 3'UTR were
comparable with scramble control (Fig.
2). The results confirmed that REL was a validated target of
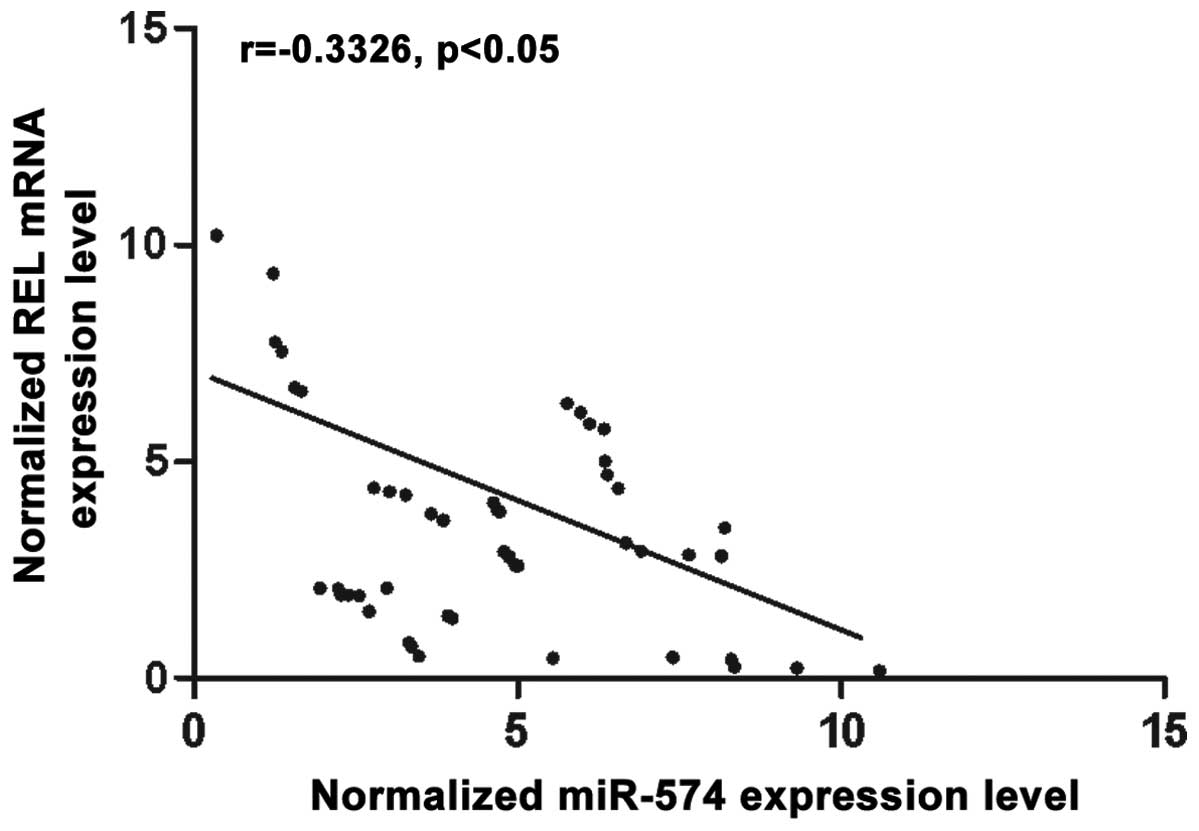
miR-574-5p in prostate CSCs. To further investigate the modulatory
relationship between miR-574-5p and REL, we then analyzed the
correlation between the expression level of miR-574-5p and REL mRNA
among the tissues (n=48), they showed negative regulatory
relationship (Fig. 3).
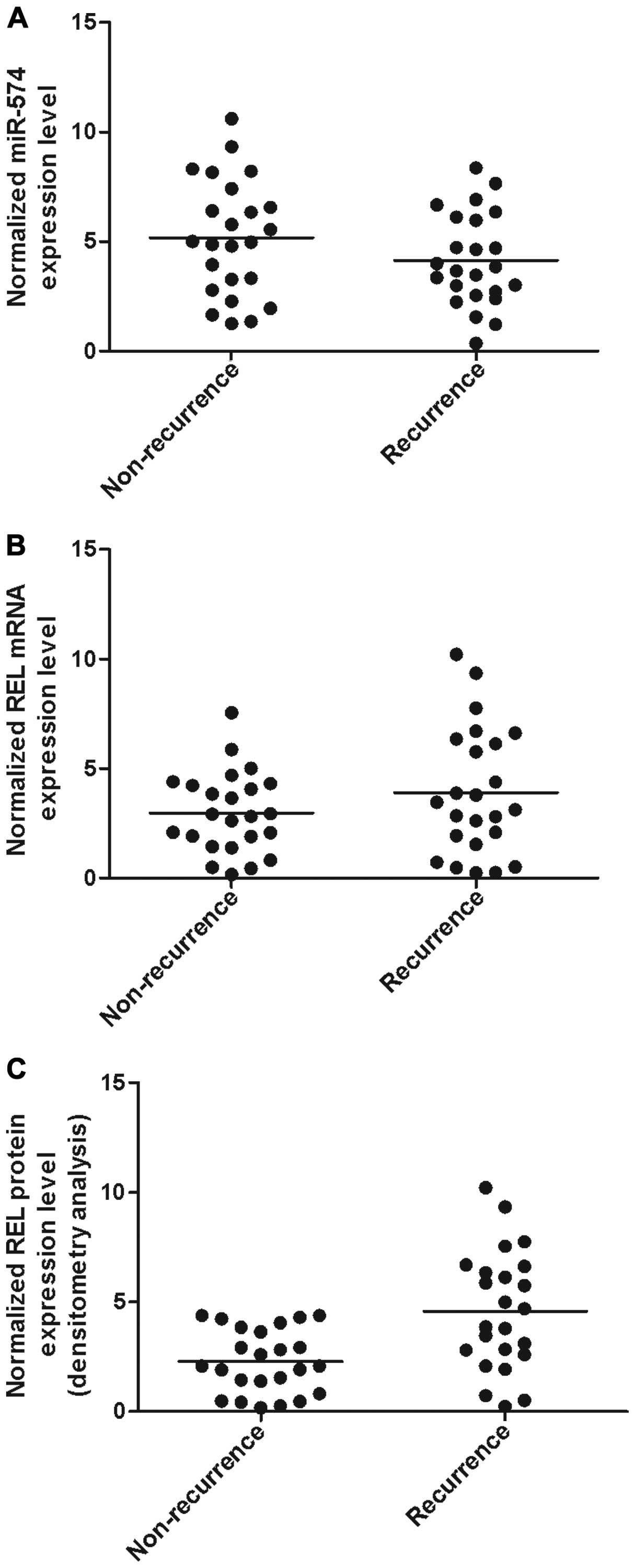
Determination of expression patterns
of miR-574 and REL in tissues with different groups
The tissues of two different groups (recurrence,
n=24; non-recurrence, n=24) were used to further explore the impact
on the interaction between miR-574 and REL 3'UTR. Using real-time
PCR, we found that the expression of miR-574 decreased in
recurrence groups (Fig. 4A)
compared with non-recurrence group while the expression of REL mRNA
(Fig. 4B) increased in the
recurrence group compared with non-recurrence group; the expression
of REL protein (Fig. C) was measured by densitometry analysis and
we found it increased in the recurrence group compared with the
normal group. To further validate the hypothesis of the negative
regulatory relationship between miR-574-5p and REL, we investigated
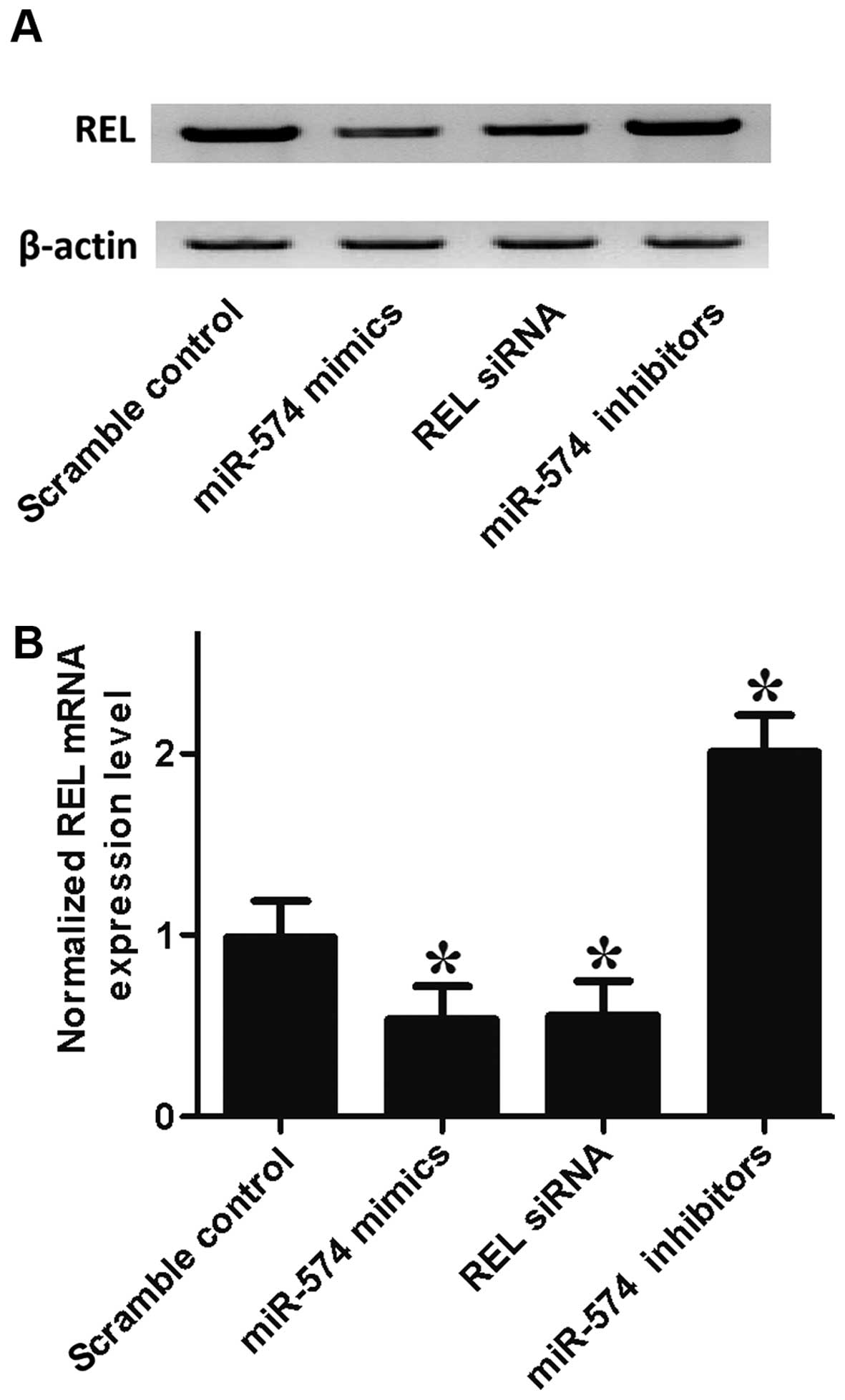
the mRNA/protein expression level of REL of prostate CSCs, by
transfection with the prostate CSCs with scramble control, miR-574
mimics, REL siRNA and miR-574 inhibitors. As shown in Fig. 5, the REL protein (upper panel) and
mRNA expression level (lower panel) of prostate CSCs treated with
miR-574 mimics and REL siRNA were apparently lower than the
scramble control, while cells treated miR-574 inhibitors were
higher than the scramble control, validating the negative
regulatory relationship between miR-574 and REL.
miR-574 and REL interfere with the
viability in prostate CSCs
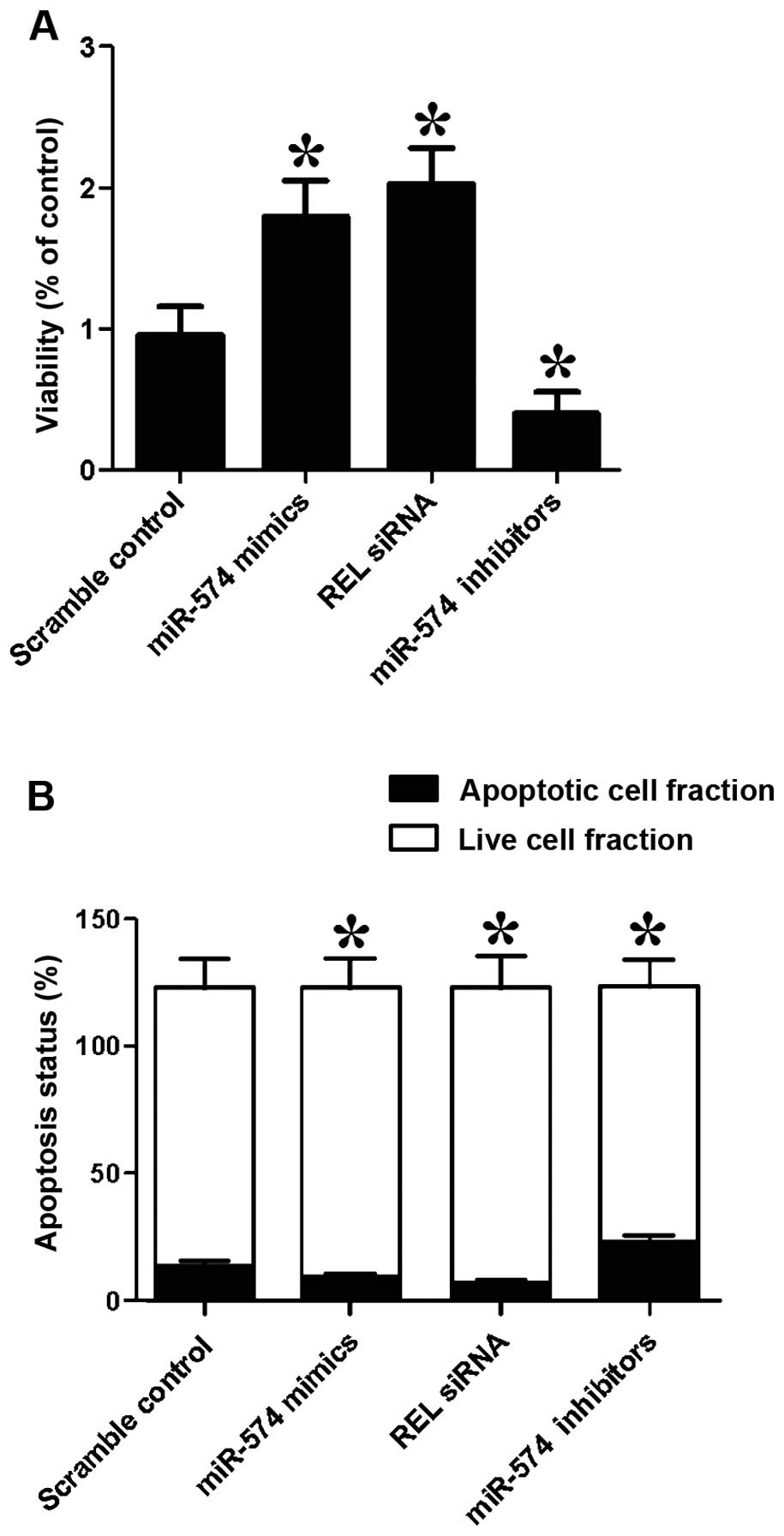
We also investigated the relative viability of
prostate CSCs when transfected with scramble control, miR-574
mimics, REL siRNA and miR-574 inhibitors. Cells transfected with
miR-574 inhibitors showed evident downregulated viability (Fig. 6A) when compared with the scramble
controls, while cells transfected with miR-574 mimics and REL siRNA
showed comparably lower viability, indicating miR-574 positively
interfered with the viability of prostate CSCs, while REL
negatively interfered with the viability of prostate CSCs.
miR-574 and REL interfere with
apoptosis in prostate CSCs
We then investigated the relative apoptosis of
prostate CSCs when transfected with scramble control, miR-574
mimics, REL siRNA and miR-574 inhibitors. When transfected with
miR-574 mimics and REL siRNA, the number of surviving cells were
more and the number of apoptotic cells were less than the scramble
controls, while cells transfected with miR-574 inhibitors showed
comparably less survival cells and more apoptotic cells. The
results indicated that miR-574 inhibited apoptosis and REL
accelerated apoptosis.
Discussion
CSCs have been proven to be present in numerous
malignancies and it is believed that they are related to cancer
recurrence, metastasis and resistance to chemo/radiotherapy
(21). CSCs in PCa ranking the most
common cancer in men worldwide have been identified (22). Several features of PCa CSCs such as
metastatic potential, functional characteristics, gene expression
profiles and molecular signatures have been reported (23). Most data on CSCs were achieved from
PCa cell lines, mainly from animal models and metastasis where the
main bias was generated, leading to clinical objection of the
findings. Some molecular markers for CSC including α2β1 integrin
and CD40, CD44, and CD133 were identified by the above research
(24). In this study, we found that
he expression of miR-574 decreased in recurrence groups (Fig. 4A) compared with non-recurrence group
while the expression of REL mRNA (Fig.
4B) increased in recurrence group compared with non-recurrence
group; the expression of REL protein (Fig. 4C) was measured by densitometry
analysis and we found that it increased in recurrence group
compared with normal group.
Previous functional study indicated that
overexpression of miR-574 led to inhibition of the invasion,
migration, proliferation ability of gastric cancer cells (25). Downregulation of miR-574 in gastric
cancer might be related to progression and development (25). Moreover, recent research considered
that miR-574 served as tumor suppressor in bladder cancer (26). miRNAs exert a biological function by
inducing target mRNA degradation, consequently, each miRNA can
inhibit the production of a number of proteins (27). It is known that miR-574 suppressed
the invasion, migration, proliferation ability and induced
apoptosis of BC cells by targeting mRNAs of mesoderm development
candidate 1 (MESDC1) directly (26). However, it remains unclear whether
miR-574-3p exhibits the suppressive effect by targeting CUL2
directly in gastric cancer cells, which need further research to be
proven. In this study, we conducted luciferase activity reporter
assay in PCa cells, and found that the luciferase activity from the
cells cotransfected with miR-574-5p and wild-type REL 3'UTR
decreased significantly (Fig. 2),
and then we analyzed the correlation between the expression level
of miR-574-5p and REL mRNA in the tissues (n=48), the results
showed negative regulatory relationship (Fig. 3). The results confirmed that REL was
a validated target of miR-574-5p in prostate CSCs.
Currently, PCa can be diagnosed at the early stage,
however, it remains hard to predict if it stays dormant or develops
into a metastatic, advanced disease, which might result from the
absence of clinically proven molecular markers predicting the
progression of PCa. Numerous candidate proteins and genes are under
investigation, however, few of them were established by
multivariate analyses (28). It has
been shown that REL, a factor of the NF-κB transcription factor
family, served as an independent factor to predict the biochemical
recurrence (29). Moreover, high
levels of nuclear REL was detected in lymph node metastases by
staining as well as in patients who suffered bone metastases
(30). Therefore, it appears that
REL participates in PCa progression while how other NF-κB family
members function remains largely unknown. Encoded by the REL gene,
REL is a unique member of NF-κB family. REL has a predominant
expression in myeloid and lymphoid tissues, which might be result
from unique regulators for activation of REL. IκBα, inhibitor of
NF-κB, preferentially inhibits p50/p65 dimers, whereas p65/REL is
controlled by IκBε and the protease activities of MALT1 and the
non-redundant regulator IκBβ induces activation of REL (31–35).
Nuclear localization of REL was impaired by MALT1 inhibitors which
also exhibited selective activity against ABC-DLBCL in vivo
(36). Additionally, it has been
found that activation of 50/c-REL and degradation of IκBα in
B-cells (37) were related to a
novel signal pathway which was dependent on IκB kinase (IKK) and
independent of proteasome. The stimuli for this pathway was
different from that of the non-canonical NF-κB pathway. But there
is little knowledge on the upstream stimuli for activation of
NF-κB, for example signaling differentially regulatingREL,
mitogen-activated protein kinases (MAPK), Toll-like receptors
(TLR), tumor-necrosis factor (TNF) receptors, T-cell receptors
(TCR), B-cell receptors (BCR) and other NF-κB subunits (38). In this study, we found that the
cells transfected with miR-574 inhibitors showed evidently
downregulated viability (Fig. 6A)
when compared with the scramble controls, while cells transfected
with miR-574 mimics and REL siRNA showed comparably lower
viability, indicating miR-574 positively interfered with the
viability of prostate CSCs, while REL negatively interfered with
the viability of prostate CSCs. Furthermore, we investigated the
relative apoptosis of prostate CSCs when transfected with scramble
control, miR-574 mimics, REL siRNA and miR-574 inhibitors. When
transfected with miR-574 mimics and REL siRNA, the number of
survival cells were more and the number of apoptotic cells were
less than the scramble controls, while cells transfected with
miR-574 inhibitors showed comparably less survival cells and more
apoptotic cells. The results indicated miR-574 inhibited apoptosis
and REL accelerated apoptosis.
Taken together, the findings indicated that REL is a
direct target of miR-574 in prostate CSCs, and miR-574 might be a
novel prognostic and therapeutic target in the management of PCa
recurrence.
References
|
1
|
Yamamoto S, Kawakami S, Yonese J, Fujii Y,
Urakami S, Masuda H, Numao N, Ishikawa Y, Kohno A and Fukui I:
Long-term oncological outcome and risk stratification in men with
high-risk prostate cancer treated with radical prostatectomy. Jpn J
Clin Oncol. 42:541–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Utomo NB, Mochtar CA and Umbas R: Primary
hormonal treatment in localized and locally advanced prostate
cancer: Effectiveness and survival predictive factors. Acta Med
Indones. 44:10–15. 2012.PubMed/NCBI
|
|
4
|
Gravdal K, Halvorsen OJ, Haukaas SA and
Akslen LA: A switch from E-cadherin to N-cadherin expression
indicates epithelial to mesenchymal transition and is of strong and
independent importance for the progress of prostate cancer. Clin
Cancer Res. 13:7003–7011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Theyer G, Schirmböck M, Thalhammer T,
Sherwood ER, Baumgartner G and Hamilton G: Role of the
MDR-1-encoded multiple drug resistance phenotype in prostate cancer
cell lines. J Urol. 150:1544–1547. 1993.PubMed/NCBI
|
|
6
|
Linja MJ, Savinainen KJ, Saramäki OR,
Tammela TL, Vessella RL and Visakorpi T: Amplification and
overexpression of androgen receptor gene in hormone-refractory
prostate cancer. Cancer Res. 61:3550–3555. 2001.PubMed/NCBI
|
|
7
|
Mackillop WJ, Ciampi A, Till JE and Buick
RN: A stem cell model of human tumor growth: Implications for tumor
cell clonogenic assays. J Natl Cancer Inst. 70:9–16.
1983.PubMed/NCBI
|
|
8
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai
P, Chu PW, Lam CT, Poon RT and Fan ST: Significance of
CD90+ cancer stem cells in human liver cancer. Cancer
Cell. 13:153–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schratt GM, Tuebing F, Nigh EA, Kane CG,
Sabatini ME, Kiebler M and Greenberg ME: A brain-specific microRNA
regulates dendritic spine development. Nature. 439:283–289. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mehler MF and Mattick JS: Non-coding RNAs
in the nervous system. J Physiol. 575:333–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amankwah EK, Anegbe E, Park H, Pow-Sang J,
Hakam A and Park JY: miR-21, miR-221 and miR-222 expression and
prostate cancer recurrence among obese and non-obese cases. Asian J
Androl. 15:226–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rane JK, Scaravilli M, Ylipää A, Pellacani
D, Mann VM, Simms MS, Nykter M, Collins AT, Visakorpi T and
Maitland NJ: MicroRNA expression profile of primary prostate cancer
stem cells as a source of biomarkers and therapeutic targets. Eur
Urol. 67:7–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lessard L, Bégin LR, Gleave ME, Mes-Masson
AM and Saad F: Nuclear localisation of nuclear factor-kappaB
transcription factors in prostate cancer: An immunohistochemical
study. Br J Cancer. 93:1019–1023. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagler C, Zänker KS and Dittmar T: Cell
fusion, drug resistance and recurrence CSCs. Adv Exp Med Biol.
714:173–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tu SM and Lin SH: Prostate cancer stem
cells. Clin Genitourin Cancer. 10:69–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tirino V, Desiderio V, Paino F, De Rosa A,
Papaccio F, La Noce M, Laino L, De Francesco F and Papaccio G:
Cancer stem cells in solid tumors: An overview and new approaches
for their isolation and characterization. FASEB J. 27:13–24. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Z, Filho MS and Nör JE: The biology
of head and neck cancer stem cells. Oral Oncol. 48:1–9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su Y, Ni Z, Wang G, Cui J, Wei C, Wang J,
Yang Q, Xu Y and Li F: Aberrant expression of microRNAs in gastric
cancer and biological significance of miR-574-3p. Int
Immunopharmacol. 13:468–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tatarano S, Chiyomaru T, Kawakami K,
Enokida H, Yoshino H, Hidaka H, Nohata N, Yamasaki T, Gotanda T,
Tachiwada T, et al: Novel oncogenic function of mesoderm
development candidate 1 and its regulation by miR-574-3p in bladder
cancer cell lines. Int J Oncol. 40:951–959. 2012.PubMed/NCBI
|
|
27
|
Chekulaeva M and Filipowicz W: Mechanisms
of miRNA-mediated post-transcriptional regulation in animal cells.
Curr Opin Cell Biol. 21:452–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tricoli JV, Schoenfeldt M and Conley BA:
Detection of prostate cancer and predicting progression: Current
and future diagnostic markers. Clin Cancer Res. 10:3943–3953. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ross JS, Kallakury BV, Sheehan CE, Fisher
HA, Kaufman RP Jr, Kaur P, Gray K and Stringer B: Expression of
nuclear factor-kappa B and I kappa B alpha proteins in prostatic
adenocarcinomas: Correlation of nuclear factor-kappa B
immunoreactivity with disease recurrence. Clin Cancer Res.
10:2466–2472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ismail HA, Lessard L, Mes-Masson AM and
Saad F: Expression of NF-kappaB in prostate cancer lymph node
metastases. Prostate. 58:308–313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gilmore TD and Gerondakis S: The c-Rel
transcription factor in development and disease. Genes Cancer.
2:695–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clark JM, Aleksiyadis K, Martin A, McNamee
K, Tharmalingam T, Williams RO, Mémet S and Cope AP: Inhibitor of
kappa B epsilon (IκBε) is a non-redundant regulator of
c-Rel-dependent gene expression in murine T and B cells. PLoS One.
6:e245042011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alves BN, Tsui R, Almaden J, Shokhirev MN,
Davis-Turak J, Fujimoto J, Birnbaum H, Ponomarenko J and Hoffmann
A: IκBε is a key regulator of B cell expansion by providing
negative feedback on cRel and RelA in a stimulus-specific manner. J
Immunol. 192:3121–3132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Refaat A, Zhou Y, Suzuki S, Takasaki I,
Koizumi K, Yamaoka S, Tabuchi Y, Saiki I and Sakurai H: Distinct
roles of transforming growth factor-beta-activated kinase 1
(TAK1)-c-Rel and interferon regulatory factor 4 (IRF4) pathways in
human T cell lymphotropic virus 1-transformed T helper 17 cells
producing interleukin-9. J Biol Chem. 286:21092–21099. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thome M, Charton JE, Pelzer C and
Hailfinger S: Antigen receptor signaling to NF-kappaB via CARMA1,
BCL10, and MALT1. Cold Spring Harb Perspect Biol. 2:a0030042010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fontan L, Yang C, Kabaleeswaran V, Volpon
L, Osborne MJ, Beltran E, Garcia M, Cerchietti L, Shaknovich R,
Yang SN, et al: MALT1 small molecule inhibitors specifically
suppress ABC-DLBCL in vitro and in vivo. Cancer Cell. 22:812–824.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
O'Connor S, Shumway SD, Amanna IJ, Hayes
CE and Miyamoto S: Regulation of constitutive p50/c-Rel activity
via proteasome inhibitor-resistant IkappaBalpha degradation in B
cells. Mol Cell Biol. 24:4895–4908. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Q and Verma IM: NF-kappaB regulation in
the immune system. Nat Rev Immunol. 2:725–734. 2002. View Article : Google Scholar : PubMed/NCBI
|