Introduction
Breast cancer has become one of the most common
malignant tumors, and its incidence rate has rapidly grown in
recent years. There is evidence that diabetes can increase the risk
of breast cancer and is related with its prognosis. Clinically,
these two diseases emerge simultaneously (1). Metformin is a biguanide drug that is
widely used in patients with type 2 diabetes (2). Recently, a large number of
epidemiological data showed that metformin can reduce the incidence
of breast cancer and improve the prognosis (3).
MicroRNAs (miRNAs) are small, non-coding RNAs
(4–6). Mature miRNAs are single-stranded,
containing 19–25 nt. miRNAs post-transcriptionally repress gene
expression by recognizing complementary target sites in the
3′-untranslated region (3′-UTR) of target mRNAs. Increasing
evidence supports an important role for miRNAs in the processes of
proliferation, apoptosis, invasion/metastasis and angiogenesis
(7,8).
MicroRNA-27a (miR-27a) is located on chromosome 19
and is highly expressed in breast, gastric, pancreatic and colon
cancer as an oncogenic miRNA (9–11).
miR-27a regulates cell growth and differentiation and can mediate
drug resistance in a dose-dependent manner (9,12).
Furthermore, miR-27a promotes tumor cell metastasis by inducing
epithelial-mesenchymal transition. In breast cancer cells, miR-27a
participates in cell apoptosis, cell cycle checkpoints and
metabolism (10,13,14).
AMP-activated protein kinase (AMPK), which functions
as a cellular energy sensor, occurs as a heterotrimer composed of
α1, α2, β1, β2, γ1 and γ2 subunits. The α-subunit serves as the
catalytic subunit, whereas the β- and γ-subunits serve regulatory
functions (15,16). AMPKα1 is widely expressed in cells,
and the AMPKα2 subunit is only expressed in hepatocytes, skeletal
muscle and cardiac muscle cells. Current studies on metabolism have
focused on the role of AMPKα2. Jørgensen et al found that
the uptake of glucose in skeletal muscle cells was increased after
activation of AMPK by AICAR, and the AMPKα2 subunit plays an
important role in this process, while the AMPKα1 subunit was not
activated (17). Musi et al
found that metformin activated AMPK in the liver cells of mice,
which caused a decrease in hepatic glucose output and further
confirmed that this involved AMPKα2 172 thr phosphorus
acid increase (18). However, there
are few studies on cancer regarding AMPKα2.
Recently, Fox et al found that AMPKα2 was
widely suppressed in breast cancer tissues and MCF-7 cells
(19). Kim et al
demonstrated that AMPKα2 was uniquely suppressed in tumors compared
with normal samples (20). However,
the mechanism explaining how AMPKα2 mRNA expression is
downregulated remains unknown.
Therefore, the present study aimed to explain this
phenomenon from the relationship between miRNAs and their target
genes and to predict how AMPKα2 is a downstream target gene of
miR-27a, thus, exploring a new mechanism for metformin in the
treatment of breast cancer regarding miRNAs.
Materials and methods
Cell lines and cell culture
The MCF-7 cell line was obtained from the Tumor Cell
Bank at the Chinese Academy of Medical Sciences. Cells were
cultured in RPMI-1640 supplemented with 10% fetal bovine serum
(FBS), 100 U/ml penicillin and 100 µg/ml streptomycin in humidified
air at 37°C with 5% CO2. The media were replaced every
1–2 days.
MTT assay
MCF-7 cells were seeded in 96-well plates at 4,000
cells/well in 200 µl cell culture medium and incubated at 37°C for
24 h. All cells were divided into 3 groups: i) blank wells
containing medium only; ii) untreated control cells; and iii) test
cells treated with different concentrations of metformin. After
incubation for 48 h, the cells were incubated with 20 µl MTT (final
concentration of 0.5 mg/ml) at 37°C for 4 h. The medium was
removed, and the precipitated formazan was dissolved in 150 µl
dimethyl sulfoxide (DMSO). After shaking for 15 min, the absorbance
at 490 nm was detected. This procedure was repeated at 24, 48 and
72 h after transfection. MTT was carried out 3 times.
Cell transfection
In brief, specific concentrations of mimics or
inhibitors of miR-27a (GenePharma, Shanghai, China) were
transfected into cell cultures using the transfection reagent
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) to activate or
inactivate miR-27a activity, respectively. Negative controls were
used for both reactions (see the RNA oligo sequences in Table I). Six hours after transfection, the
transfection solution was replaced with complete culture medium.
For RNA extraction and protein isolation, cells were treated for 48
h, and were then harvested. miRNA transfection efficiencies were
determined by real-time PCR.
 | Table I.RNA oligo sequences. |
Table I.
RNA oligo sequences.
| Name | RNA oligo sequence
(5′-3′) |
|---|
| hsa-miR-27a
mimics |
UUCACAGUGGCUAAGUUCCGC |
| MicroRNA mimic
NC |
UUGUACUACACAAAAGUACUG |
| hsa-miR-27a
inhibitors |
GCGGAACUUAGCCACUGUGAA |
| MicroRNA inhibitor
NC |
CAGUACUUUUGUGUAGUACAA |
MTT assay after transfection
Cells were plated in 96-well plates at
104/well and transfected with miR-27a mimics, miR-27a
inhibitors and their corresponding negative control. After
transfection, the cells were cultured for 48 h. The effects of
miR-27a mimics and miR-27a inhibitors on cell growth and viability
were determined by the MTT assay as described above.
Quantitative RT-PCR
In the present study, we validated the expression
levels of miR-27a using tail real-time PCR. The reverse
transcription and PCR primers used are listed in Table II. Briefly, RNA was extracted using
an miRNA isolation kit (Invitrogen). Then, 1 µg RNA was reverse
transcribed into cDNA using the RT primer. Subsequently, miRNAs
were PCR amplified on a real-time PCR system using specific forward
primers and a universal reverse primer. The qRT-PCR consisted of 40
cycles (95°C for 5 sec and 60°C for 20 sec) after an initial
denaturation step (95°C for 10 sec). The expression levels were
normalized against human small nuclear U6 RNA and measured by the
comparative Ct (ΔΔCt) method. Relative fold-changes were
calculated by the equation 2−ΔΔCt.
 | Table II.Reverse transcription and PCR
primers. |
Table II.
Reverse transcription and PCR
primers.
| Gene name |
| RNA oligo sequence
(5′-3′) |
|---|
| U6 | F |
GCTTCGGCAGCACATATACTAAAAT |
| miR-27a | F |
TTCACAGTGGCTAAGTTCCGC |
| AMPKα2 | F |
GCCAAGAAGCAAATGAGAATG |
|
| R |
GACACAACGCAAACTCCTGA |
| GAPDH | F |
ACCACAGTCCATGCCATCAC |
|
| R |
TCCACCACCCTGTTGCTGTA |
For mRNA expression analysis, total cellular RNA was
isolated and reverse transcribed to cDNA. Real-time PCR
amplification was performed using SYBR® Premix Ex Taq™
(RR041A; Takara). The PCR reaction contained 1 µl RT product, 10 µl
2X SYBR Premix Ex Taq, 1 µl forward primer and 1 µl reverse primer
(5 µmol/l each), and nuclease-free water in a final volume of 20
µl. Standard PCR samples were analyzed using a Bio-Rad iQ5 thermal
cycler. Melting curves were generated for each real-time RT-PCR to
verify the specific amplification products and primer dimers of
each PCR reaction. All quantitative PCR reactions were performed in
triplicate. The primers are listed in Table II. The expression levels of the
target genes were normalized to GAPDH. The qRT-PCR was carried out
at least 3 times.
Western blot analysis
Cells were lysed with RIPA buffer. The cytosolic
extracts were prepared by centrifuging the lysates twice at 12,000
rpm for 10 min at 4°C. The protein concentration in each lysate was
measured by the bicinchoninic acid assay (BCA). Equal amounts of
protein were separated by SDS-PAGE. After electrophoresis, proteins
were transferred onto a nitrocellulose membrane and blocked in 5%
BSA in Tris-buffered saline and Tween-20 (TBST) at room temperature
for 2 h, then, the membrane was incubated with a rabbit anti-AMPKα2
monoclonal antibody (1:1,000; #2757; CST) or the polyvinylidene
fluoride (PVDF) membrane was incubated with a rabbit anti-caspase-3
polyclonal antibody (1:1,000; #25546-1-AP; Proteintech) overnight
at 4°C. After thorough rinsing with TBST, the membranes were
incubated with peroxidase-conjugated secondary antibody (1:10,000;
#E030120; HRP; Sanjiang) for 1 h. After rinsing, chemiluminescent
detection was performed using an enhanced chemiluminescence (ECL)
western blot detection kit followed by exposure and development of
the X-ray film. Western blot results were analyzed by densitometry.
The western blotting was carried out at least 3 times.
Luciferase reporter assay for
targeting the AMPKα2 3′-UTR
For the luciferase reporter experiments, a human
AMPKα2 3′-UTR segment of 207 base pairs was amplified from rat cDNA
by PCR and inserted into the pMIR-REPORT luciferase vector with a
cytomegalovirus promoter using the SacI and HindIII
sites immediately downstream from the stop codon of luciferase. The
following sets of primers were used to generate specific fragments:
for rat AMPKα2 3′-UTR, forward primer
5′-GGACTAGTAAATGTTGGCTGAAGCTG-3′ and reverse primer
5′-CCCAAGCTTTGTTGCATTCAAAATCCACT-3′. Nucleotide substitutions were
introduced by PCR mutagenesis to yield mutated binding sites. The
pMIR-AMPKα2 3′-UTR, miR-27a mimics, and pMIR-REPORT β-galactosidase
control plasmid were then co-transfected into MCF-7 cells for 24 h.
The luciferase activity was measured following the manufacturer's
instructions. Experiments were performed in triplicate.
Statistical analysis
Each experiment had three or more replicates. All
data were expressed as the mean ± standard deviation (SD).
Statistical analyses were performed with a Student's t-test or
analysis of variance (ANOVA), and a P<0.05 was considered to
indicate a statistically significant result (*P<0.05,
**P<0.01 and ***P<0.001 are indicated in the figures).
Results
Metformin inhibits the growth of MCF-7
cells and promotes apoptosis
In order to investigate the effects of metformin on
the proliferation and apoptosis of the human breast cancer cell
line MCF-7, MCF-7 cells were cultured in vitro with final
concentrations of 1, 2, 5, 10 and 20 mmol/l of metformin and
incubated for 48 h. The MTT method was used to detect cell optical
density (OD) for increasing concentrations of metformin. The
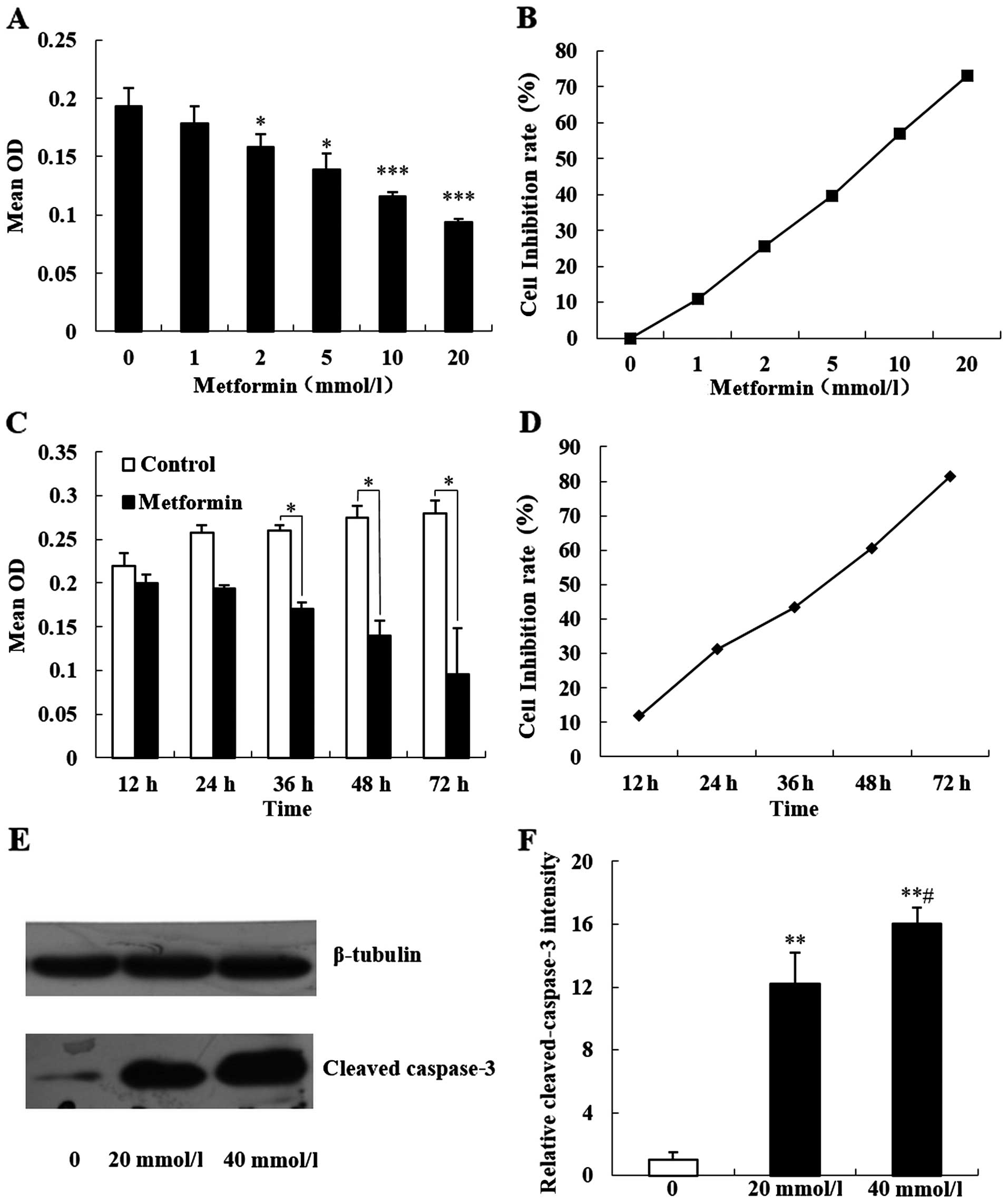
average OD value was gradually decreased, while the cell
proliferation inhibition rate was significantly increased (Fig. 1A and B; P<0.05). Then, a final
concentration of 20 mmol/l metformin was used to act on MCF-7 cells
for 12, 24, 36, 48 and 72 h, respectively. MTT results showed that
increasing metformin intervention times had an average OD value
that was gradually decreased, and the cell proliferation inhibition
rate was significantly increased (Fig.
1C and D; P<0.05).
Caspase-3 is a key member of the caspase family of
apoptosis execution and is activated in the early stage of
apoptosis. Activated caspase-3 is decomposed into two large
subunits (17 kDa) and two small subunits (13 kDa). We treated MCF-7
cells with 20 and 40 mmol/l metformin to react for 48 h. Western
blot results showed that the expression of 17 kDa caspase-3 in the
human breast cancer MCF-7 cells was significantly increased
(Fig. 1E and F; P<0.05).
Metformin decreases the expression of
miR-27a and upregulates AMPKα2 expression in MCF-7 cells
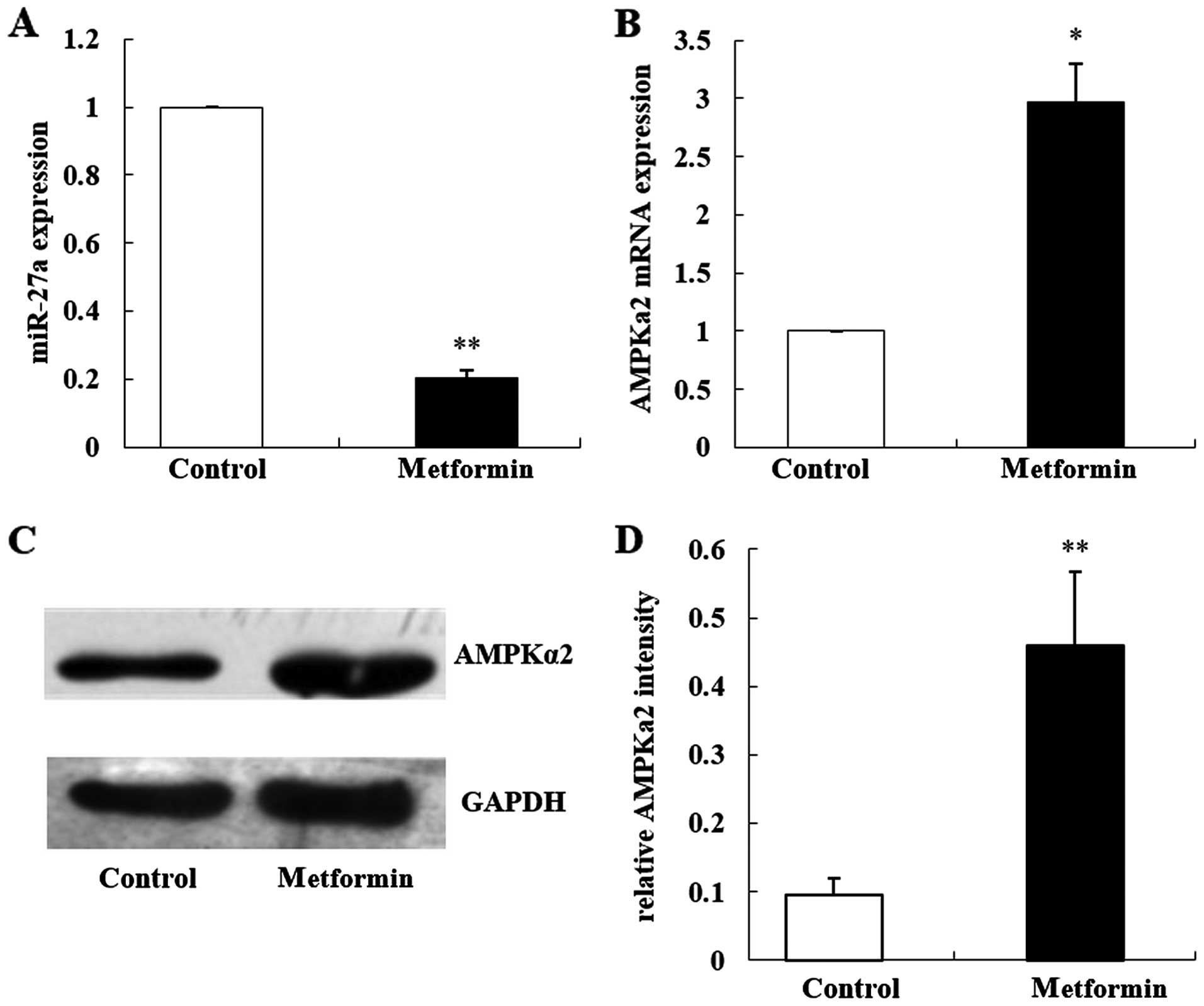
Our previous study found that the primary culture
from liver tissues of C57BL/6 mice had the AMPK-specific activator
AICAR, and the expression of the miR-27 family was significantly
inhibited (21). Metformin was
found to play a major role in breast cancer through the AMPK
signaling pathway. In the present study, we treated MCF-7 cells
with 20 mmol/l metformin for 48 h. qRT-PCR results showed that
after metformin intervention, the expression of miR-27a was
significantly decreased by ~80% when compared with the control
group (Fig. 2A; t=−5.37,
P=0.006).
Fox et al found that the expression of AMPKα2
in breast cancer tissues and MCF-7 cells was inhibited, and AMPKα2
is a tumor-suppressor (19). In
order to investigate the effects of metformin on the expression of
AMPKα2, we observed the effects of metformin on the expression of
AMPKα2 together with miR-27a using qRT-PCR and western blot
analyses. The results showed that the expression of AMPKα2 mRNA was
3.3-fold higher when compared with that noted in the control group
(Fig. 2B; P<0.05), and the
expression of the AMPKα2 protein was 4-fold higher when compared
with that noted in the control group (Fig. 2C and D; P<0.01).
miR-27a inhibits apoptosis in MCF-7
cells and promotes proliferation
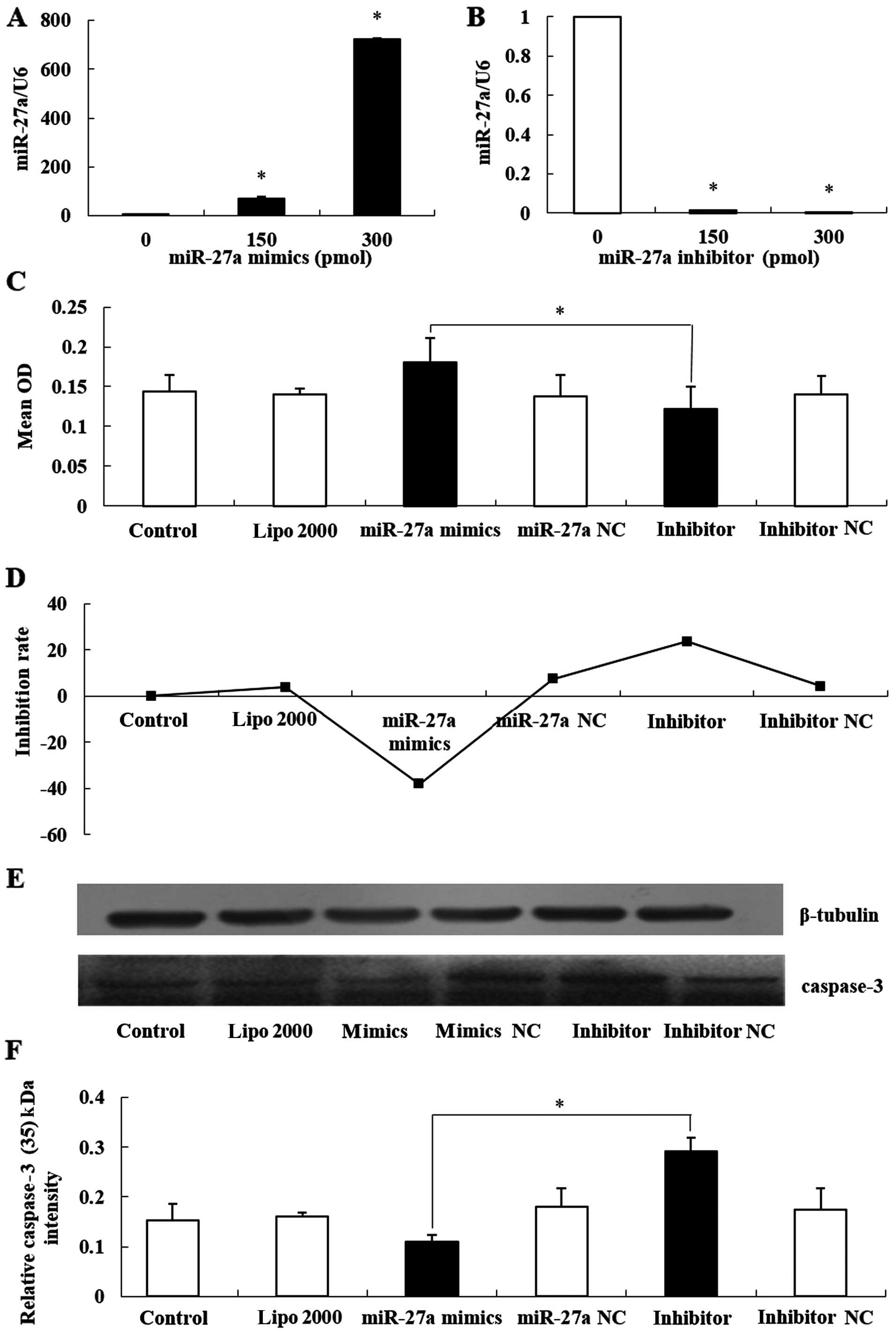
In order to investigate the role of miR-27a in
inhibiting proliferation and promoting apoptosis in MCF-7 cells,
miR-27a mimics and miR-27a inhibitors were transfected into MCF-7
cells. qRT-PCR assays showed that the expression of miR-27a was
increased nearly 1,000-fold when 300 pmol miR-27a mimics were
transfected into the cells, and the expression of miR-27a was
almost completely inhibited by 300 pmol of the miR-27a inhibitor
(Fig. 3A and B; P<0.05).
Furthermore, we used 300 pmol miR-27a mimics and
miR-27a inhibitors to transfect MCF-7 cells, and the OD values were
determined by the MTT method. The groups with overexpressed miR-27a
had the highest OD values and the groups transfected with the
inhibitors had the lowest OD values. Therefore, we concluded that
overexpression of miR-27a promotes human breast cancer cell MCF-7
proliferation and silencing miR-27a expression inhibits MCF-7 cell
proliferation (Fig. 3C and D;
P<0.05). In addition, we used western blot analyses to detect
the expression of caspase-3 (17 kDa) in each group. The results
showed that the expression of caspase-3 (17 kDa) in the miR-27a
overexpression group was significantly decreased, and in the
inhibitor group was significantly increased (Fig. 3E and F; P<0.05).
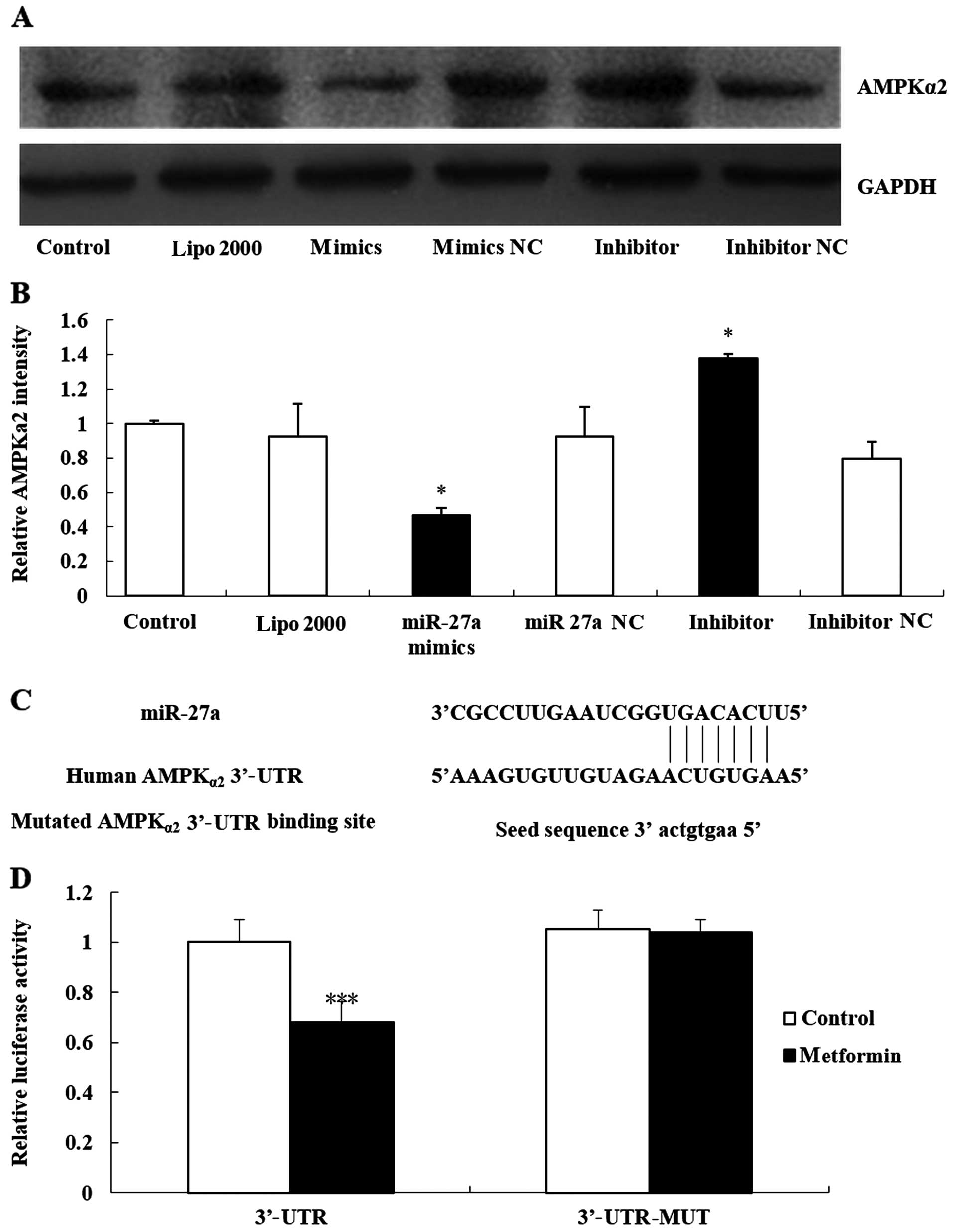
miR-27a targets AMPKα2 expression
The biological function of microRNAs is to control
target genes. In a previous study, we found that metformin
significantly inhibited the expression of AMPKα2 and increased the
expression of miR-27a. Whether AMPKα2 is the target gene of miR-27a
remained unclear. Therefore, we first used western blot analyses to
detect the expression of the AMPKα2 protein in MCF-7 cells by
transfecting 300 pmol miR-27a mimics and miR-27a inhibitors. The
results showed that the expression of AMPKα2 proteins in the
miR-27a overexpression group was significantly decreased, and the
expression of AMPKα2 in the miR-27a inhibitor group was
significantly increased (Fig. 4A and
B; P<0.05).
Furthermore, we used luciferase assays to confirm
that the effects of AMPKα2 were truly mediated by direct binding of
miR-27a to the 3′-UTR of the AMPKα2 gene. These assays were
conducted in MCF-7 cells. Luciferase reporter plasmids were
prepared and co-transfected with miR-27a mimics, causing
overexpression of miR-27a. This overexpression of miR-27a
significantly decreased AMPKα2 3′-UTR luciferase activity (F=0.021,
P=0.001). However, no such suppression was observed when we
performed similar experiments using a mutated 3′-UTR (Fig. 4C and D). All the data confirmed that
the binding of miR-27a to the 3′-UTR of the AMPKα2 gene was
required to produce these effects.
Discussion
In the present study, we described another new form
of regulation for metformin on breast cancer in regards to miRNAs.
We showed that miR-27a mediates the antiproliferative effects of
metformin in MCF-7 cells through its target gene, AMPKα2.
Recent pilot studies carried out using population
registries raise the possibility that metformin may reduce cancer
risk and/or improve cancer prognosis. One study showed an
unexpectedly lower risk of cancer diagnosis among diabetic patients
taking metformin compared with a control group of diabetics using
other treatments (22); another
study showed lower cancer-specific mortality among subjects with
diabetes using metformin compared with diabetics using other
treatments (23). Zhou et al
indicated that metformin does not directly activate AMPK (24). Shaw et al showed that
metformin activated AMPK and reduced blood glucose, which depended
on LKBl (25). LKB1 was previously
described as a tumor-suppressor gene with relevance to epithelial
neoplasia (26). The mammalian
target of rapamycin (mTOR) is an integrated signal for the
regulation of cell growth factors and nutrient metabolism (27). The activation of AMPK mediated by
metformin was found to inhibit mTOR signaling and the effects of
metformin on tumor cells was through activation of AMPK, acting on
mTOR and activating tumor suppressor gene 2 (TSC2), therefore
playing an antitumor role (28).
Zakikhani et al found that metformin can inhibit the growth
of MCF-7 cells by activating the AMPK pathway in vitro, and
when the concentration of metformin increased, the activity of AMPK
increased gradually, and the inhibition of mTOR became more obvious
(29). The present study also drew
the conclusion that with the gradual increase in metformin
concentration and the exposure time, the proportion of apoptosis in
MCF-7 cells was also increased. In the present study, 20 mmol/l
metformin activated the cleavage of caspase-3 and 40 mmol/l
metformin augmented this tendency.
Furthermore, in vivo and in vitro
studies indicated that metformin inhibited the growth of tumor
cells in the G0/G1 phase, and inhibited the expression of cyclin D1
in MCF-7 cells, decreasing the growth activity of the cells
(30). In addition, studies by
Hirsch et al confirmed that metformin effectively killed
breast cancer stem cells (31).
Cufí et al and other studies showed that metformin affected
TGF induced by EMT and played a role in tumorigenesis (32).
The research on miRNAs has attracted great attention
in recent years. miRNAs play roles in almost all aspects of cancer
biology, such as proliferation, apoptosis, invasion/metastasis and
angiogenesis. miRNAs post-transcriptionally repress gene expression
by recognizing complementary target sites in the 3′-untranslated
region (3′-UTR) of target mRNAs. Approximately 50% of human miRNA
genes were confirmed to be located in a mutated region that was
associated with tumors. This suggests that the abnormal expression
of miRNAs may be closely related to the formation of tumors and
other diseases (33). In recent
years, abnormal changes in miRNA expression have been closely
associated with the occurrence of many malignant tumors, such as
acute lymphoblastic leukemia (34),
adenocarcinoma (9), pancreatic
(35), breast (36) and liver cancer (37). Therefore, in this experiment we
aimed to verify whether metformin can alter the expression of
miRNAs and promote apoptosis in cancer cells.
In the present study, we confirmed that the
expression of miR-27a after metformin intervention was
significantly lower compared with the control group. Therefore, we
can see that metformin inhibited the expression of miR-27a in MCF-7
cells; however, the specific mechanism needs to be further
studied.
Data revealed a significant and widespread reduction
in AMPKα2 expression in mammary epithelial carcinoma, regardless of
tumor grade, lymph node status or patient age. Fox et al
conducted a series of experiments to verify if re-expressing the
AMPKα2 isoform could affect tumorigenic properties. The results
showed that AMPKα2 through cyclin D1 arrested the cell cycle
through the mTOR pathway to reduce protein synthesis, and also
acted directly on P53, leading to apoptosis. Lee et al
indicated that the loss of AMPKα2 activity caused the instability
of P53 and led to the formation of liver cancer cells (38). The mechanism of the downregulation
of AMPKα2 is unknown and warrants further investigation. However,
data from the present study demonstrated that AMPKα2 was suppressed
at the mRNA level, suggesting that RNA degradation control
mechanisms may play a significant role. Therefore, this experiment
tried to explain this phenomenon from the relationship between
miRNAs and their target genes.
The results of the present study confirmed that
after a certain concentration of metformin intervention, the
expression of miR-27a was significantly downregulated, and the
expression of AMPKα2 mRNA was significantly higher than that of the
control group (P<0.05). Western blot data also confirmed that
AMPKα2 protein expression was also increased. In order to
understand the relationship between miR-27a and AMPKα2, the miR-27a
RNA oligos were used to transfect MCF-7 cells. We found that the
expression of AMPKα2 was significantly decreased after transfection
with miR-27a mimics in a concentration-dependent manner, and
western blot experiments confirmed this point. The MTT assay also
confirmed the same results, showing that the group transfection
with miR-27a mimics had mean OD values significantly higher than
that of the others, and the proliferation inhibition rate was
significantly decreased.
These results indicated that AMPKα2 may be a
downstream target gene of miR-27a. However, a clear relationship
between miR-27a and AMPKα2 was demonstrated through luciferase
reporter assays targeting AMPKα2-UTR, which verified the results of
the experiment. Overexpression of miR-27a significantly suppressed
AMPKα2 3′-UTR luciferase activity. However, the repression was not
observed when we performed the same experiments using mutated
3′-UTR.
Finally, it can be concluded that metformin reduced
the expression of miR-27a in a specific way, and miR-27a combined
with the AMPKα2 3′-UTR, meaning that metformin could increase the
expression of AMPKα2. Accordingly, the expression of P53 may
increase, which activated caspase-3, resulting in the apoptosis of
MCF-7 cells. This may be a novel mechanism for inhibiting breast
cancer cell growth by metformin.
There are many other details which need to be
elucidated through experiments. It must be determined whether
metformin inhibits miR-27a expression in breast cancer cell lines
other than MCF-7. Analysis of a panel of breast cancer cell lines
for miR-27a and AMPKα2 expression levels must be carried out to
determine if they are inversely correlated, Similarly, its clinical
relevance should be determined, i.e. by performing a meta-analysis
study using breast cancer patient data (it would be interesting to
analyze miR-27a expression in metformin-treated tumors vs.
untreated tumors). It also must be determined whether miR-27a
targets AMPKα2 by inducing RNA destabilization or translational
repression. This requires further investigation. As miRNAs target
multiple genes simultaneously, in order to prove that miR-27a
mediates the antiproliferation effects of metformin in MCF-7
through AMPKα2 targeting, we must perform AMPKα2 rescue experiments
(i.e. overepxression of AMPKα2 in the presence of miR-27a or AMPKα2
knockdown in the presence of miR-27a inhibitor).
Our conclusions were rooted in the laboratory-based
study, and the dose of metformin we used based on the references we
had read. Actually, the dose we used was 3 orders of magnitude
higher than the dose used clinically. We chose cells, namely
tissue, as our subject, and long-time use of metformin could cause
drug accumulation in tissues, leading to higher concentration in
tissue than in serum. Furthermore, Cooper et al showed that
a clinically relevant dose of metformin for 11 days could cause
increased [18F]FDG, suggesting longer incubations with
low dose of metformin could cause similar effects as higher dose
over shorter time periods (39).
Thus, our design was reasonable.
The importance of this experiment lies in that it
implies a new mechanism of how metformin activates AMPK, and in the
future whether we can conduct a new drug concerning miR-27a
antagonist, may be vital for tumor patients.
miRNA-based therapy has gradually advanced. miRNA
analogs or miRNA expression vectors have been successfully used in
the expression of miRNAs in vitro. miR-145 and 5-FU have
been combined to treat breast cancer, and it was found that it can
play a role in cancer therapy (40). Research has shown that the
combination of 5-FU and miR-21 can effectively inhibit the growth
of breast cancer cells. Therefore, we believe that miRNA-based
treatment programs may be applied for the treatment of human cancer
and achieve satisfactory efficacy.
References
|
1
|
Noto H, Goto A, Tsujimoto T and Noda M:
Cancer risk in diabetic patients treated with metformin: A
systematic review and meta-analysis. PLoS One. 7:e334112012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Witters LA: The blooming of the French
lilac. J Clin Invest. 108:1105–1107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bodmer M, Meier C, Krähenbühl S, Jick SS
and Meier CR: Long-term metformin use is associated with decreased
risk of breast cancer. Diabetes Care. 33:1304–1308. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chuang JC and Jones PA: Epigenetics and
microRNAs. Pediatr Res. 61:24R–29R. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Latronico MV, Catalucci D and Condorelli
G: MicroRNA and cardiac pathologies. Physiol Genomics. 34:239–242.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu J, Valencia-Sanchez MA, Hannon GJ and
Parker R: MicroRNA-dependent localization of targeted mRNAs to
mammalian P-bodies. Nat Cell Biol. 7:719–723. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu T, Tang H, Lang Y, Liu M and Li X:
MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by
targeting prohibitin. Cancer Lett. 273:233–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mertens-Talcott SU, Chintharlapalli S, Li
X and Safe S: The oncogenic microRNA-27a targets genes that
regulate specificity protein transcription factors and the
G2-M checkpoint in MDA-MB-231 breast cancer cells.
Cancer Res. 67:11001–11011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Tang S, Le SY, Lu R, Rader JS,
Meyers C and Zheng ZM: Aberrant expression of oncogenic and
tumor-suppressive microRNAs in cervical cancer is required for
cancer cell growth. PLoS One. 3:e25572008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lerner M, Lundgren J, Akhoondi S, Jahn A,
Ng HF, Moqadam F Akbari, Vrielink JA Oude, Agami R, Den Boer ML,
Grandér D, et al: MiRNA-27a controls FBW7/hCDC4-dependent cyclin E
degradation and cell cycle progression. Cell Cycle. 10:2172–2183.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mertens-Talcott SU, Noratto GD, Li X,
Angel-Morales G, Bertoldi MC and Safe S: Betulinic acid decreases
ER-negative breast cancer cell growth in vitro and in vivo: role of
Sp transcription factors and microRNA-27a:ZBTB10. Mol Carcinog.
52:591–602. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Banerjee N, Talcott S, Safe S and
Mertens-Talcott SU: Cytotoxicity of pomegranate polyphenolics in
breast cancer cells in vitro and vivo: Potential role of miRNA-27a
and miRNA-155 in cell survival and inflammation. Breast Cancer Res
Treat. 136:21–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hardie DG: AMP-activated protein kinase:
An energy sensor that regulates all aspects of cell function. Genes
Dev. 25:1895–1908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hardie DG, Carling D and Gamblin SJ:
AMP-activated protein kinase: Also regulated by ADP? Trends Biochem
Sci. 36:470–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jørgensen SB, Viollet B, Andreelli F,
Frøsig C, Birk JB, Schjerling P, Vaulont S, Richter EA and
Wojtaszewski JF: Knockout of the α2 but not
α1 5-AMP-activated protein kinase isoform abolishes
5-aminoimidazole-4-carboxamide-1-β-4-ribofuranosidebut not
contraction-induced glucose uptake in skeletal muscle. J Biol Chem.
279:1070–1079. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Musi N, Hirshman MF, Nygren J, Svanfeldt
M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O,
Efendic S, et al: Metformin increases AMP-activated protein kinase
activity in skeletal muscle of subjects with type 2 diabetes.
Diabetes. 51:2074–2081. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fox MM, Phoenix KN, Kopsiaftis SG and
Claffey KP: AMP-activated protein kinase α 2 isoform suppression in
primary breast cancer alters AMPK growth control and apoptotic
signaling. Genes Cancer. 4:3–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim YH, Liang H, Liu X, Lee JS, Cho JY,
Cheong JH, Kim H, Li M, Downey TJ, Dyer MD, et al: AMPKα modulation
in cancer progression: Multilayer integrative analysis of the whole
transcriptome in Asian gastric cancer. Cancer Res. 72:2512–2521.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Liu W, Ying H, Zhao W and Zhang H:
Analysis of microRNA expression profile induced by AICAR in mouse
hepatocytes. Gene. 512:364–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Evans JM, Donnelly LA, Emslie-Smith AM,
Alessi DR and Morris AD: Metformin and reduced risk of cancer in
diabetic patients. BMJ. 330:1304–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bowker SL, Majumdar SR, Veugelers P and
Johnson JA: Increased cancer-related mortality for patients with
type 2 diabetes who use sulfonylureas or insulin: Response to
Farooki and Schneider. Diabetes Care. 29:1990–1991. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou G, Myers R, Li Y, Chen Y, Shen X,
Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al: Role of
AMP-activated protein kinase in mechanism of metformin action. J
Clin Invest. 108:1167–1174. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shaw RJ, Lamia KA, Vasquez D, Koo SH,
Bardeesy N, Depinho RA, Montminy M and Cantley LC: The kinase LKB1
mediates glucose homeostasis in liver and therapeutic effects of
metformin. Science. 310:1642–1646. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hemminki A, Avizienyte E, Roth S, Loukola
A, Aaltonen LA, Järvinen H and de la Chapelle A: A serine/threonine
kinase gene defective in Peutz-Jeghers syndrome. Duodecim.
114:667–668. 1998.(In Finnish). PubMed/NCBI
|
|
27
|
Markman B, Atzori F, Pérez-García J,
Tabernero J and Baselga J: Status of PI3K inhibition and biomarker
development in cancer therapeutics. Ann Oncol. 21:683–691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bell DS: Successful utilization of
aliskiren, a direct renin inhibitor in Bartter syndrome. South Med
J. 102:413–415. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zakikhani M, Dowling R, Fantus IG,
Sonenberg N and Pollak M: Metformin is an AMP kinase-dependent
growth inhibitor for breast cancer cells. Cancer Res.
66:10269–10273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ben Sahra I, Laurent K, Loubat A,
Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le
Marchand-Brustel Y and Bost F: The antidiabetic drug metformin
exerts an antitumoral effect in vitro and in vivo through a
decrease of cyclin D1 level. Oncogene. 27:3576–3586. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hirsch HA, Iliopoulos D, Tsichlis PN and
Struhl K: Metformin selectively targets cancer stem cells, and acts
together with chemotherapy to block tumor growth and prolong
remission. Cancer Res. 69:7507–7511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cufí S, Vazquez-Martin A,
Oliveras-Ferraros C, Martin-Castillo B, Joven J and Menendez JA:
Metformin against TGFβ-induced epithelial-to-mesenchymal transition
(EMT): From cancer stem cells to aging-associated fibrosis. Cell
Cycle. 9:4461–4468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim KB, Lotan R, Yue P, Sporn MB, Suh N,
Gribble GW, Honda T, Wu GS, Hong WK and Sun SY: Identification of a
novel synthetic triterpenoid,
methyl-2-cyano-3,12-dioxooleana-1,9-dien-28-oate, that potently
induces caspase-mediated apoptosis in human lung cancer cells. Mol
Cancer Ther. 1:177–184. 2002.PubMed/NCBI
|
|
35
|
Ma Y, Yu S, Zhao W, Lu Z and Chen J:
miR-27a regulates the growth, colony formation and migration of
pancreatic cancer cells by targeting Sprouty2. Cancer Lett.
298:150–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hassan MQ, Gordon JA, Beloti MM, Croce CM,
van Wijnen AJ, Stein JL, Stein GS and Lian JB: A network connecting
Runx2, SATB2, and the miR-23a~27a~24-2 cluster regulates the
osteoblast differentiation program. Proc Natl Acad Sci USA.
107:19879–19884. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dobreva G, Chahrour M, Dautzenberg M,
Chirivella L, Kanzler B, Fariñas I, Karsenty G and Grosschedl R:
SATB2 is a multifunctional determinant of craniofacial patterning
and osteoblast differentiation. Cell. 125:971–986. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee CW, Wong LL, Tse EY, Liu HF, Leong VY,
Lee JM, Hardie DG, Ng IO and Ching YP: AMPK promotes p53
acetylation via phosphorylation and inactivation of SIRT1 in liver
cancer cells. Cancer Res. 72:4394–4404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cooper AC, Fleming IN, Phyu SM and Smith
TA: Changes in [18F]Fluoro-2-deoxy-D-glucose incorporation induced
by doxorubicin and anti-HER antibodies by breast cancer cells
modulated by co-treatment with metformin and its effects on
intracellular signalling. J Cancer Res Clin Oncol. 141:1523–1532.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim SJ, Oh JS, Shin JY, Lee KD, Sung KW,
Nam SJ and Chun KH: Development of microRNA-145 for therapeutic
application in breast cancer. J Control Release. 155:427–434. 2011.
View Article : Google Scholar : PubMed/NCBI
|