Introduction
According to the World Health Organization,
colorectal cancer (CRC) is one of the most lethal diseases
worldwide (1). The
tumor-node-metastasis (TMN) classification is based on the standard
staging process, which helps understand the histopathological
features of CRC and is an important factor in deciding its
prognosis. The invasive growth types have also been evaluated as
valuable prognostic factors (2,3). In
patients with CRC, the invasive growth type influences local
recurrence, liver metastasis and disease-free survival (2–5).
In contrast, the association between CRC and
myofibroblasts in the tumor microenvironment has only recently
attracted considerable attention. Although the myofibroblast is
known as a principal cellular component in the granulation tissue
of healing wounds, cancer stromal cells containing myofibroblasts
construct the extracellular matrix (ECM) (6,7). The
ECM not only plays a role in one of the scaffolding structures but
also influences cancer proliferation, and activities of invasion
and metastasis (6,8). In patients with CRC, there is a
correlation between the concentration of tumor stroma and patient
cumulative survival (9). The
myofibroblasts in the stroma of CRC also serve an important
function in promoting the desmoplastic stromal reaction and
influence tumor invasion, microvessel density around the invasive
lesion and metastatic carcinomas (10–12).
Moreover, myofibroblast activation in tumor metastatic lymph nodes
influences the microenvironment supporting CRC metastasis (13).
In both the invasion and metastasis of CRC, three
histological layers of the colorectum, the submucosa (SM), the
muscularis propria (MP) and the subserosa (SS), may play an
important role in the mechanical and physiological protection
against the invasive growth of CRC. MP is composed exclusively of
smooth muscle bundles and comprises tight connective tissue,
whereas SM and SS are composed mainly of loose connective tissue
(14,15). However, it is unclear how
myofibroblasts are distributed around the CRC invasive border of
these three layers and whether there is an association between the
invasive growth type of CRC and the distribution of myofibroblasts
around the invasive lesions.
In the present study, we focused on the association
between the invasive growth type of CRC and myofibroblast
distribution in SM, MP and SS layers, separately. Furthermore, we
investigated the association between the density of myofibroblasts
and the clinicopathological factors such as lymph node metastasis
and venous invasion, supported by immunohistochemical staining and
imaging analysis.
Materials and methods
Patients and tissues
One hundred and fifty patients with advanced
invasive CRC, defined as adenocarcinoma, which had invaded the
subserosal layer of the colorectal wall (pT3), underwent surgical
resection from January 2008 to December 2009 at Hirosaki University
Hospital. All patients were not treated with neoadjuvant
chemotherapy and did not have synchronous multiple CRCs. We used
surgically resected specimens that were fixed with 10% formalin,
then embedded in paraffin and stained with hematoxylin and eosin
(H&E) for pathological evaluation. We confirmed whether or not
they had liver metastasis by referring to medical records from when
the 150 patients underwent operative resection.
Pathological analysis
We divided the 150 cases into two invasive growth
types. For the purposes of the present study, we described these
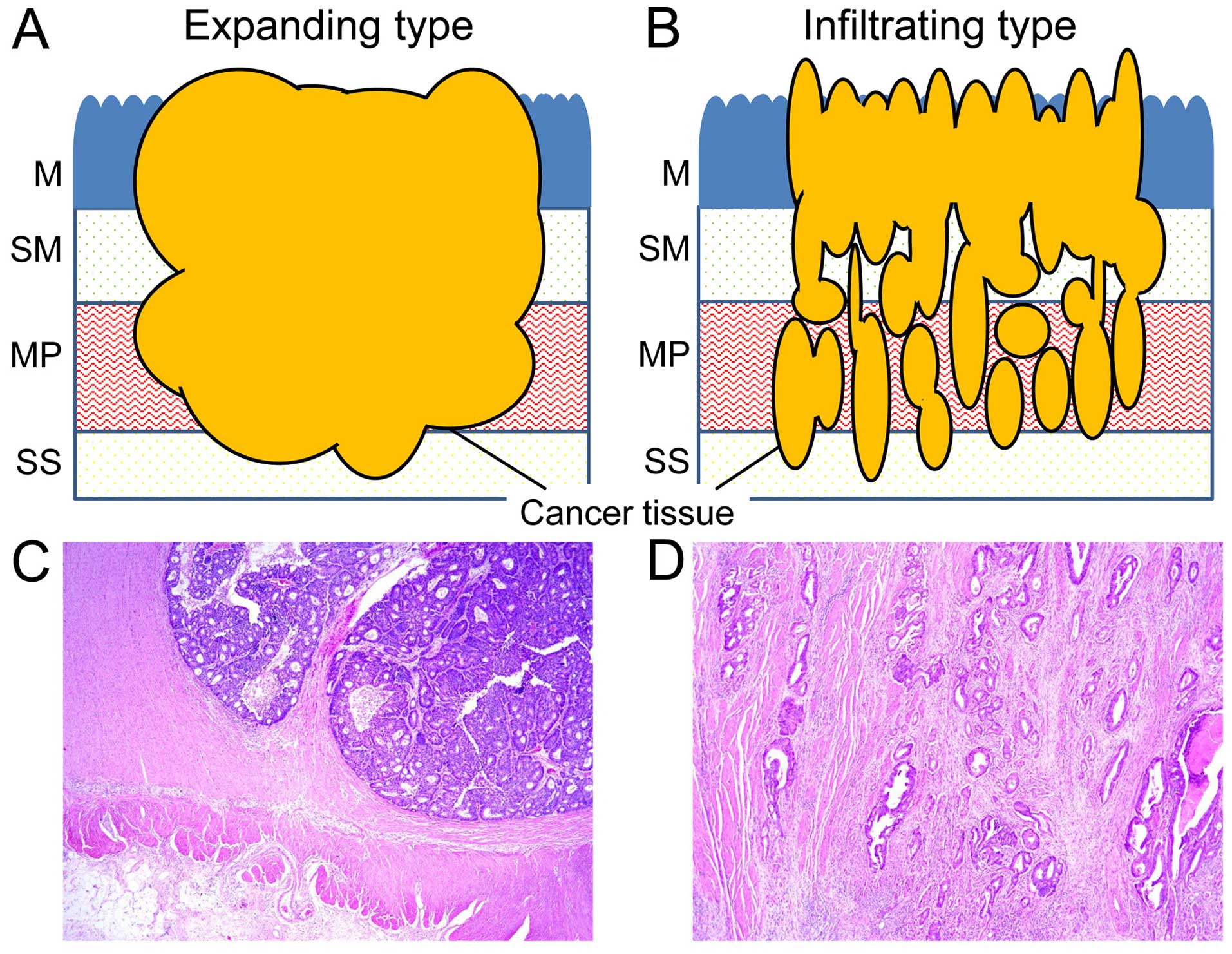
invasive growth types as the expanding and infiltrating types. The
expanding and infiltrating types are shown in Fig. 1A and B. A representative case of the
expanding (Fig. 1C) and
infiltrating type (Fig. 1D) with
adenocarcinoma lesion of the MP (H&E staining) are shown. The
expanding type was defined as the overall expansion growth type of
adenocarcinoma, with a clear invasive margin. The infiltrating type
was defined as a widespread streaming form of adenocarcinoma. These
two novel defined types are different from the INF classification
since the INF classification specifically denotes lesions with
borderline tumor invasive properties (2). Degrees of lymphatic and venous vessel
invasion were classified as 0, no invasion; 1, mild invasion; 2,
moderate invasion and 3, severe invasion. Several histological
features were assessed: the location of the primary adenocarcinoma,
the dominant histological type and lymph node metastasis. These
parameters referred to the Japanese Classification of Colorectal
Carcinoma (16).
Immunohistochemistry
We selected the paraffin-embedded specimen which
recognized three colorectal wall layers (SM, MP and SS), and the
specimen also had the invasive lesion of the adenocarcninoma
diagnosed by H&E staining. The selected paraffin embedded
specimen was a representative block of each case, and we used 4-µm
serial sections for immunohistochemistry. The sections were mounted
on saline-coated glass slides. The antibodies used included
α-smooth muscle actin (α-SMA) (1:100; clone 1A4) and desmin (1:100;
clone D-33) (both from Dako, Glostrup, Denmark). Immunostaining for
α-SMA and desmin was performed using the standard
avidin-biotin-peroxidase complex method with an automated
immunostainer (BenchMark XT; Ventana Medical System, Tucson, AZ,
USA). The signature characteristic of the myofibroblast was the
α-SMA-positive and desmin-negative pattern, whereas that of smooth
muscle was the α-SMA- and desmin-positive pattern.
Imaging analysis
We used imaging analysis to investigate
myofibroblast density. Each invasive growth type had an invasive
lesion of the three colorectal walls, SM, MP and SS. To obtain all
images, we used an Olympus microscope BX50 with U PlanApo objective
lens (magnification, ×4), DP control software and a digital camera
DP-70 (all from Olympus, Tokyo, Japan). We applied ImageJ software
(NIH, Bethesda, MD, USA) to view and analyze our obtained images
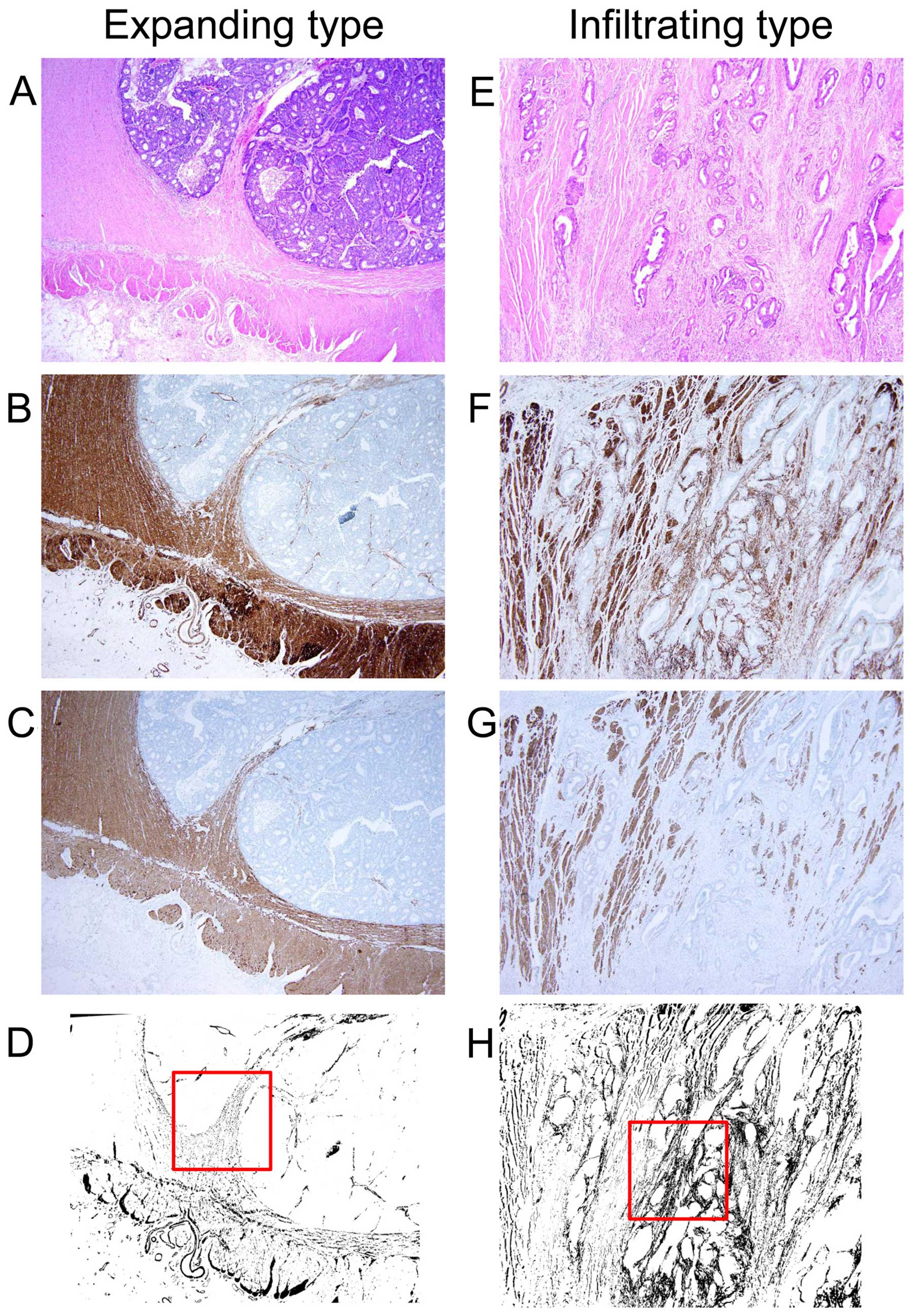
(17). The images of both α-SMA and
desmin staining were binarized. We conducted a subtraction image
pasting the binarized image of desmin onto the binarized images of
α-SMA using the subtraction mode in ImageJ software. The
subtraction images were shown as the value of α-SMA minus that of
desmin and we could interpret the subtraction image as the
existence of myofibroblasts in the representative section of each
case. The representative images of the expanding and infiltrating
types which invaded the MP layer are shown in Fig. 2A-H, respectively. From all 150
cases, we obtained the subtraction image of the three histological
layers (SM, MP and SS) and measured the myofibroblast density of
the 1×1 mm2 area in the invasive border of each layer.
We selected a hotspot myofibroblast density area from each invasive
lesion (Fig. 2D and H).
Statistical analysis
All values are presented as the means ± standard
error of the mean (SEM). A Chi-square test for non-continuous
variables was performed, whereas the Mann-Whitney and Welch t-tests
were used for continuous variables in comparing each parameter.
Differences were considered to indicate a statistically significant
result, when the P-value was <0.05. Statistical analysis was
performed with R (http://www.r-project.org) and Microsoft Excel software
(Microsoft Corporation, Redmond, WA, USA).
Results
Difference in clinicopathological
characteristics between the expanding and infiltrating types of
CRC
We classified the growth type of 150 CRC resections
into either the expanding (67 cases) or the infiltrating type (83
cases) according to our definition (Fig. 1). The clinicopathological
characteristics are summarized in Table
I. With respect to age, gender, location and histological type,
there was no significant difference between the expanding and
infiltrating types. Twenty-seven patients (40.3%) with the
expanding type of cancer had lymph node metastasis and 55 patients
(66.3%) with the infiltrating type of cancer had lymph node
metastasis. The percentage of low or high lymphatic invasion with
the expanding type was 85.1 and 14.9%, respectively. In contrast,
the percentage of low and high lymphatic invasion which existed in
the infiltrating type was 42.1 and 57.9%, respectively. The
percentage of low and high venous invasion with the expanding type
was 80.6 and 19.4%, respectively, while the percentage of low and
high venous invasion which existed in the infiltrating type was
62.7 and 37.3%, respectively. Three patients with the expanding
type had liver metastasis, while 6 patients with the infiltrating
type had liver metastasis. With respect to lymph node metastasis,
lymphatic invasion and venous invasion, there were significant
differences between the expanding and infiltrating types
(P<0.003).
 | Table I.Differences in the clinicopathological
characteristics between the expanding and the infiltrating
types. |
Table I.
Differences in the clinicopathological
characteristics between the expanding and the infiltrating
types.
| Variables | Expanding n=67 | Infiltrating
n=83 | P-value |
|---|
| Age, years median
(range) | 65.6 (26–87) | 66.6 (39–93) | 0.655 |
| Gender, n |
|
| 0.314 |
| Male | 41 | 44 |
|
|
Female | 26 | 39 |
|
| Location, n |
|
| 0.353 |
|
Cecum | 4 | 2 |
|
|
Ascending | 13 | 14 |
|
|
Transverse | 10 | 4 |
|
|
Descending | 3 | 4 |
|
|
Sigmoid | 14 | 21 |
|
|
Rectal | 23 | 38 |
|
| Histological type,
n |
|
| 0.105 |
| Well
and mod | 60 | 79 |
|
|
Por | 4 | 3 |
|
|
Muc | 3 | 1 |
|
| Lymph node
metastasis, n (%) |
|
| 0.001 |
| pN
(−) | 40 (59.7) | 28 (33.7) |
| pN
(+) | 27 (40.3) | 55 (66.3) |
| Lymphatic invasion,
n (%) |
|
| <0.0001 |
| Low
(ly0 or ly1) | 57 (85.1) | 35 (42.1) |
|
| High
(ly2 or ly3) | 10 (14.9) | 48 (57.9) |
|
| Venous invasion, n
(%) |
|
| 0.003 |
| Low (v0
or v1) | 54 (80.6) | 52 (62.7) |
|
| High
(v2 or v3) | 13 (19.4) | 31 (37.3) |
|
| Liver
metastasis(+), n | 3 | 6 |
|
Myofibroblast distribution in the
invasive lesion at each colorectal wall stratified by expanding
type vs. infiltrating type
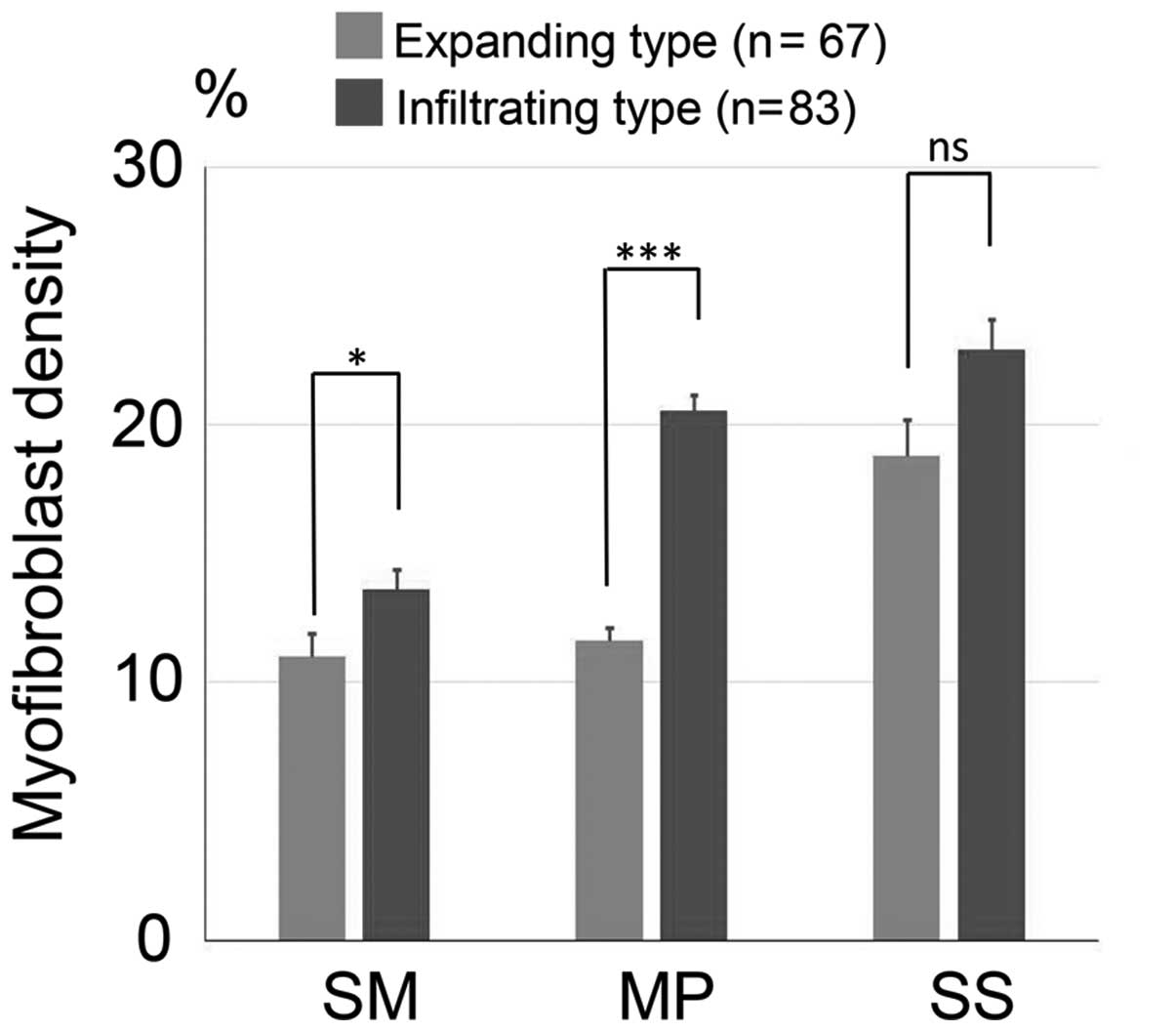
We measured the myofibroblast density around the
invasive front of each layer (SM, MP and SS) for the expanding and
infiltrating types (Fig. 3). In 67
cases of the expanding type, the mean myofibroblast density for
each layer of the invasive legion was 11.03±0.88% (SM), 11.62±0.50%
(MP) and 19.24±1.34% (SS). In contrast, in 83 cases of the
infiltrating type, the mean myofibroblast density was 13.60±0.79%
(SM), 20.52±0.62% (MP) and 22.40±1.07% (SS). Significantly more
myofibroblasts were located around the invasive lesion of the SM
(P=0.018) and the MP (P<0.001) in the infiltrating type than in
the expanding type.
Association between the myofibroblast
distribution, the invasive growth types and lymph node
metastasis
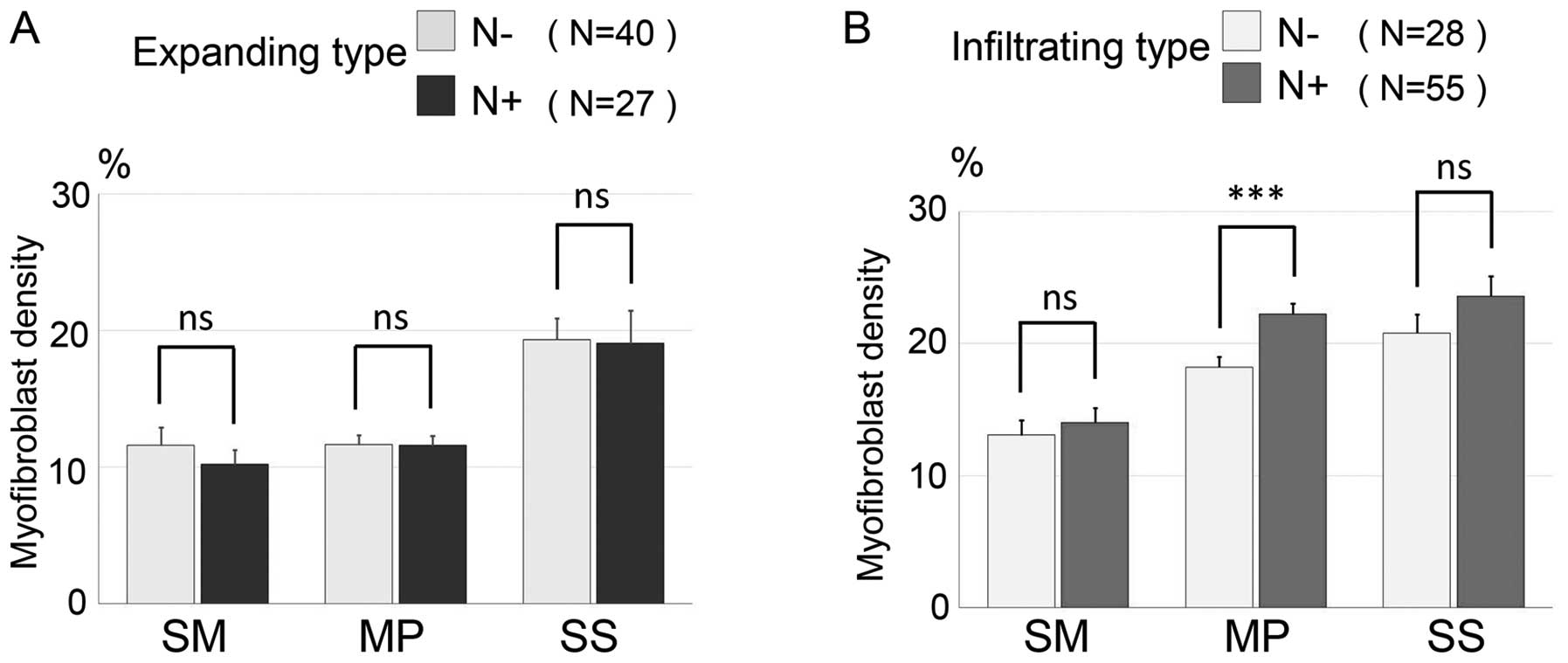
We stratified 67 cases of the expanding type and 83
cases of the infiltrating type into lymph node metastasis-negative
and a -positive groups. Then, we investigated myofibroblast
distribution of the three invasive layers (Fig. 4A and B). In the expanding type, the
mean myofibroblast densities of the three layers within the lymph
node metastasis-negative group (n=40) were 11.58±1.31% (SM),
11.64±0.69% (MP) and 19.35±1.56% (SS), while the mean myofibroblast
densities in the lymph node metastasis-positive group (n=27) were
10.25±1.04% (SM), 11.59±0.71% (MP) and 19.06±2.42% (SS). In the
expanding type, there was no significant difference in
myofibroblast densities of each colorectal wall layer between the
lymph node metastasis-positive and the -negative groups. In the
infiltrating type, the mean myofibroblast densities of the three
layers within the lymph node-negative group (n=28) were 12.93±1.33%
(SM), 17.55±0.66% (MP) and 20.54±1.56% (SS), while the mean
myofibroblast densities in the lymph node-positive group (n=55)
were 13.94±0.99% (SM), 22.04±0.80% (MP) and 23.34±1.38% (SS). In
the infiltrating type, the lymph node-positive group had a higher
myofibroblast density for each layer than the lymph node-negative
group. Furthermore there was a significant difference between the
lymph node metastasis-positive and metastasis-negative groups
relating to the myofibroblast density of the MP layer
(P<0.001).
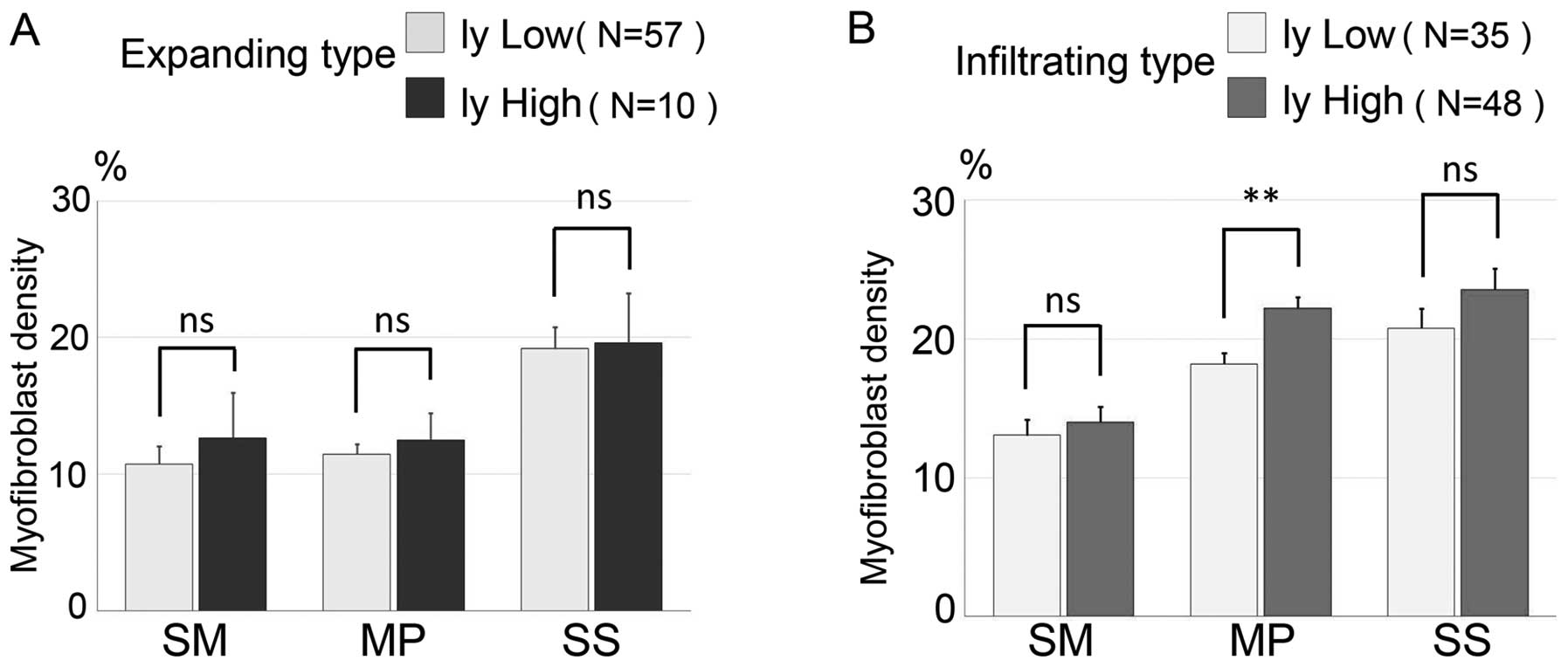
Association between the distribution
of myofibroblast density, invasive growth types and lymphatic
vessel invasion
To investigate the association between the
distribution of myofibroblasts and the degree of lymphatic vessel
invasion, we stratified the 67 cases of the expanding type and 83
cases of the infiltrating type into either low lymphatic vessel
invasion or high lymphatic vessel invasion groups. We analyzed the
distribution of myofibroblasts around the three colorectal wall
layers (Fig. 5A and B). In the
expanding type, the mean myofibroblast densities in each of the
three layers within the low lymphatic vessel invasion group (n=57)
were 10.74±0.87% (SM), 11.47±0.48% (MP) and 19.18±1.45% (SS), and
the mean myofibroblast densities in the high lymphatic vessel
invasion group (n=10) were 12.66±3.30% (SM), 12.48±1.98% (MP) and
19.58±3.64% (SS). There was no significant difference between the
low and high groups in the expanding type. In the infiltrating
type, the mean myofibroblast densities for each of the three layers
in the low lymphatic vessel invasion group (n=35) were 13.07±1.10%
(SM), 18.27±0.80% (MP) and 20.79±1.39% (SS), and the mean
myofibroblast densities in the high lymphatic vessel invasion group
(n=48) were 13.39±1.11% (SM), 22.22±0.80% (MP) and 23.57±1.52%
(SS). In the infiltrating type, there was a significant difference
in myofibroblast density of the MP layer between the low and high
lymphatic vessel invasion group (P=0.007).
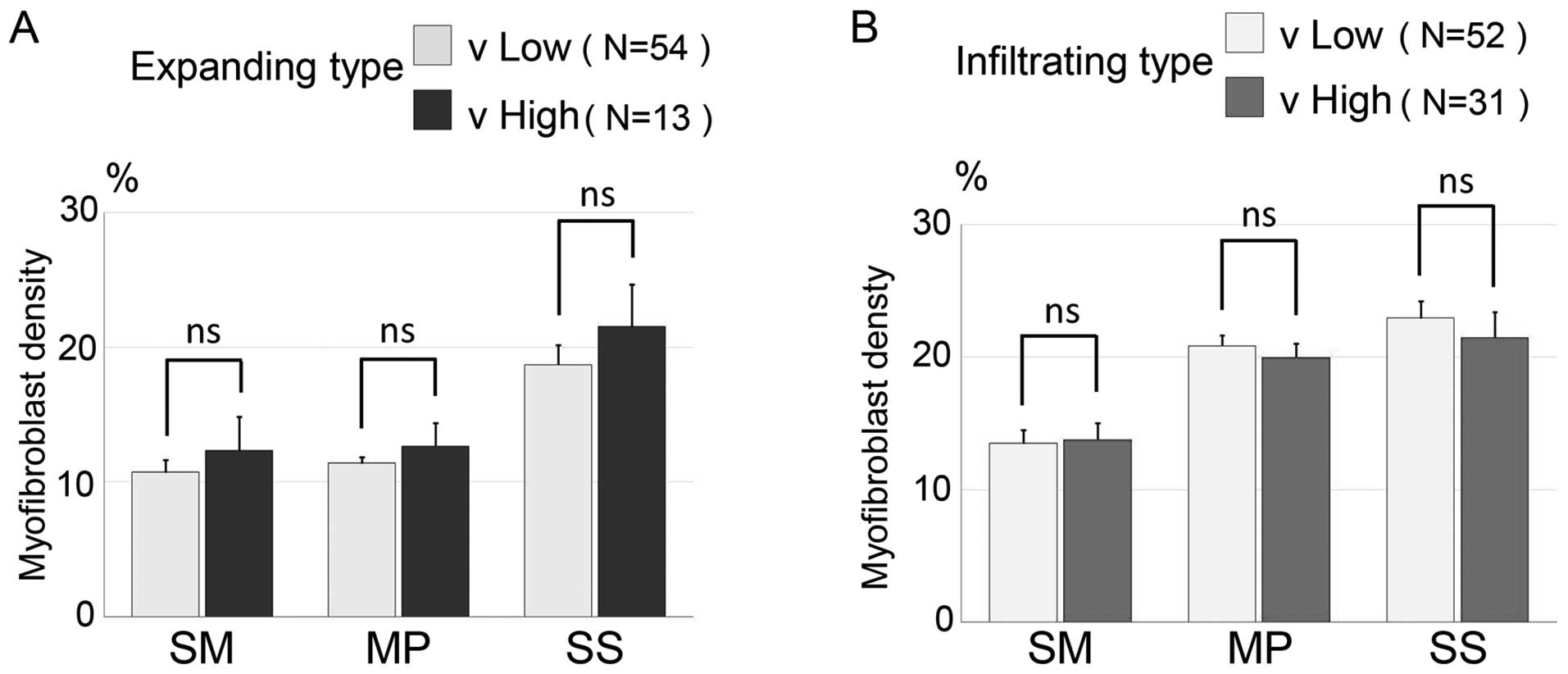
Association between the distribution
of myofibroblast density, invasive growth types and venous vessel
invasion
To investigate the association between the
distribution of myofibroblasts and the degree of venous vessel
invasion, we stratified the 67 cases of the expanding type and the
83 cases of the infiltrating type into a low venous vessel invasion
and a high venous vessel invasion group and analyzed the
distribution of myofibroblasts around each of the three colorectal
walls (Fig. 6A and B). In the
expanding type, the mean myofibroblast densities of each layer in
the low venous invasion group (n=54) were 10.72±0.92% (SM),
11.37±0.48% (MP) and 18.69±1.48% (SS), while the mean myofibroblast
densities in the high venous vessel invasion group (n=13) were
12.31±2.49% (SM), 12.65±1.69% (MP) and 21.51±3.15% (SS). There was
no significant difference between the low venous vessel and high
venous vessel invasion groups within the expanding type. In the
infiltrating type, the mean myofibroblast densities of each layers
in the low venous invasion group (n=52) were 13.77±0.92% (SM),
20.85±0.78% (MP) and 22.95±1.27% (SS) and the mean myofibroblast
densities in the high venous vessel invasion group (n=31) were
13.77±1.26% (SM), 19.98±1.02% (MP) and 21.47±1.90% (SS). There was
no significant difference between the low and high venous vessel
invasion groups within the infiltrating type.
Discussion
In the present study, we found that the infiltrating
type of colorectal cancer (CRC) was aggressive, as evidenced by
lymph node metastasis and by lymphatic and venous invasion, and was
more progressive than the expanding type. Previous studies
identified that the invasive growth type correlates strongly with
liver metastasis and is one of the most important factors that
determines prognosis for patients with CRC (3,4,18,19).
Moreover, the infiltrating type carries a high risk of liver
metastasis and a worse prognosis compared to the expanding type
(4,20). Although the definition of growth
type is different between our research and previous studies, we
suspect that the present study reflects prior investigations.
The present study revealed that the quantity of
myofibroblasts which were located around the CRC invasive lesion
was significantly different between the infiltrating and expanding
types. Myofibroblasts are considered to be a type of
cancer-associated fibroblasts (CAFs) and to be involved in the
desmoplastic reaction (21). CAFs
actively associate with neoplastic cells and form a myofibroblastic
microenvironment that promotes cancer growth, angiogenesis and
survival, which supports malignancy (22). CAFs are not present peritumorally as
individual cells, but they combine to fully deploy a desmoplastic
program which is associated with CRC malignancy, and they have an
important role in the prognosis of CRC patients (21–23).
Therefore, we suggest that the infiltrating type induces a high
number of myofibroblasts which give rise to a very strong
desmoplastic reaction via interaction of CAFs with malignant
potential.
We found that as the invasion of the CRC became
deeper, the number of myofibroblasts increased around the invasive
lesions, and the invasive growth type of CRC had a significantly
higher density of myofibroblasts than the expanding type. It is
possible that the large quantity of myofibroblasts altered the
adhesive and migratory properties of CRC cells, which consequently
aided CRC invasion into the deeper layers of the SM, MP and SS.
Myofibroblasts promote CRC invasion and metastasis as they
proliferate around the invasive lesion and alter the adhesive and
migratory properties of CRC cells (12,24).
The relationship between the adhesion molecule (for example
E-cadherin) and the malignant potential of CRC is debated (25,26).
However, one study showed that myofibroblasts co-cultured with CRC
cells may be involved in the invasiveness of CRC, even when
E-cadherin expression prevents tumor cell invasiveness in
vitro (27). In contrast, in
previous studies, myofibroblasts at the deep border of CRC may
reduce the invasive activity of CRC cells, and entrap tumor cells
since myofibroblasts have been shown to be involved in the wound
repair process, and desmoplastic reactions are known to be involved
in local remodeling in the healing of wounds (14,28).
However, such myofibroblasts are thought to arise from resident
fibroblasts, which are transiently present (14). We suggest that this type of
myofibroblast is different from the myofibroblasts located near the
CRC invasive lesion. Therefore, the present study predicts that
myofibroblasts located next to the invasive lesion accelerate
invasive growth by altering the adhesion and migratory system of
CRC, comparable to a cellular foothold. Moreover, the infiltrating
type may be particularly relevant relating to this effect compared
to the expanding type.
Our results showed that the myofibroblast density of
MP was high in both the lymph node metastasis-positive and the high
lymphatic vessel invasion groups, particularly in the infiltrating
type. The lymphatic vessels exist in three colorectal wall levels,
the SM, MP and SS despite the differences regarding histological
structure. The distribution of lymphatic vessels in normal colonic
tissue tends to increase in frequency with depth throughout the
wall (29). We suggest that
myofibroblasts invade through the connective tissue of the MP and
are strongly associated with lymphatic invasion of CRC in the MP
compared with the SM and SS, which have loose connective tissue.
The functions of α-SMA-positive myofibroblasts may be associated
with the promotion of the ECM of tumor cells and lymphogenesis of
the metastatic microenvironment in oral tongue squamous cell
carcinoma (30). With respect to
CRC, proliferation of myofibroblasts in the peritumoral areas was
predicted to play an important role in lymphangiogenesis, and was
also associated with lymph node metastasis (12). Moreover, the finding that
CRC-invading MP may result in a greater ability to induce
angiogenesis in adjacent normal tissue has been reported by other
studies (31). The present study
predicted that myofibroblasts participate in both lymphangiogenesis
and lymphatic vessel invasion of the MP layer, which affects lymph
node metastasis of the infiltrating type compared with the
expanding type.
In conclusion, we revealed that the infiltrating
type of CRC has a greater malignant potential than the expanding
type through myofibroblast contribution. Furthermore, we have shown
that myofibroblasts present in the MP play a more important role in
the malignant potential of the infiltrating type than that of the
expanding type.
Acknowledgements
The present study was supported by Grants-in-Aid for
Science from the Ministry of Education, Culture, Sports, Science
and Technology in Japan, and a grant for Hirosaki University
Institutional Research.
Glossary
Abbreviations
Abbreviations:
References
|
1
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: World Health Organization Classification of Tumours of
the Digestive system. 3. 4th. IARC; Lyon: pp. 132–146. 2010
|
|
2
|
Hase H, Mochizuki H, Utsunomiya K, Iwamoto
K, Kuranaga K, Watanabe C and Tamakuma S: A study on prognostic
value of infiltrative growth (INF) in patients with colorectal
cancer. J Jpn Soc Coloproctol. 49:463–467. 1996. View Article : Google Scholar
|
|
3
|
Pinheiro RS, Herman P, Lupinacci RM, Lai
Q, Mello ES, Coelho FF, Perini MV, Pugliese V, Andraus W,
Cecconello I, et al: Tumor growth pattern as predictor of
colorectal liver metastasis recurrence. Am J Surg. 207:493–498.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajaganeshan R, Prasad R, Guillou PJ,
Chalmers CR, Scott N, Sarkar R, Poston G and Jayne DG: The
influence of invasive growth pattern and microvessel density on
prognosis in colorectal cancer and colorectal liver metastases. Br
J Cancer. 96:1112–1117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujita S, Shimoda T, Yoshimura K, Yamamoto
S, Akasu T and Moriya Y: Prospective evaluation of prognostic
factors in patients with colorectal cancer undergoing curative
resection. J Surg Oncol. 84:127–131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bissell MJ and Radisky D: Putting tumours
in context. Nat Rev Cancer. 1:46–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seemayer TA, Schürch W and Lagacé R:
Myofibroblasts in human pathology. Hum Pathol. 12:491–492. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JH, Richards CH, McMillan DC, Horgan
PG and Roxburgh CS: The relationship between tumour stroma
percentage, the tumour microenvironment and survival in patients
with primary operable colorectal cancer. Ann Oncol. 25:644–651.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okamoto Y, Fujimori T, Ohkura Y, Sugai T,
Arai T, Watanabe G, Wada R, Ueno H, Togashi K, Yao T, et al:
Histological assessment of intra- and inter-institutional
reliabilities in detection of desmoplastic reaction in biopsy
specimens of early colorectal carcinomas. Pathol Int. 63:539–545.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsujino T, Seshimo I, Yamamoto H, Ngan CY,
Ezumi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N and Monden M:
Stromal myofibroblasts predict disease recurrence for colorectal
cancer. Clin Cancer Res. 13:2082–2090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang P, Hong JW, Ubukata H, Liu G, Katano
M, Motohashi G, Kasuga T, Watanabe Y, Nakada I and Tabuchi T:
Myofibroblasts correlate with lymphatic microvessel density and
lymph node metastasis in early-stage invasive colorectal carcinoma.
Anticancer Res. 25:2705–2712. 2005.PubMed/NCBI
|
|
13
|
Yeung TM, Buskens C, Wang LM, Mortensen NJ
and Bodmer WF: Myofibroblast activation in colorectal cancer lymph
node metastases. Br J Cancer. 108:2106–2115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakayama H, Enzan H, Miyazaki E, Naruse K,
Kiyoku H and Hiroi M: The role of myofibroblasts at the tumor
border of invasive colorectal adenocarcinomas. Jpn J Clin Oncol.
28:615–620. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ueno H, Hase K, Hashiguchi Y, Ishiguro M,
Kajiwara Y, Shimazaki H and Mochizuki H: Growth pattern in the
muscular layer reflects the biological behaviour of colorectal
cancer. Colorectal Dis. 11:951–959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Japanese Society for Cancer of the Colon
and Rectum, . Japanese Classification of Colorectal Carcinoma. 2nd
English. Kanehara & Co., Ltd; Tokyo: 2009
|
|
17
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jass JR, Love SB and Northover JM: A new
prognostic classification of rectal cancer. Lancet. 1:1303–1306.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jass JR, Ajioka Y, Allen JP, Chan YF,
Cohen RJ, Nixon JM, Radojkovic M, Restall AP, Stables SR and Zwi
LJ: Assessment of invasive growth pattern and lymphocytic
infiltration in colorectal cancer. Histopathology. 28:543–548.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morikawa T, Kuchiba A, Qian ZR,
Mino-Kenudson M, Hornick JL, Yamauchi M, Imamura Y, Liao X,
Nishihara R, Meyerhardt JA, et al: Prognostic significance and
molecular associations of tumor growth pattern in colorectal
cancer. Ann Surg Oncol. 19:1944–1953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karagiannis GS, Petraki C, Prassas I,
Saraon P, Musrap N, Dimitromanolakis A and Diamandis EP: Proteomic
signatures of the desmoplastic invasion front reveal collagen type
XII as a marker of myofibroblastic differentiation during
colorectal cancer metastasis. Oncotarget. 3:267–285. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karagiannis GS, Poutahidis T, Erdman SE,
Kirsch R, Riddell RH and Diamandis EP: Cancer-associated
fibroblasts drive the progression of metastasis through both
paracrine and mechanical pressure on cancer tissue. Mol Cancer Res.
10:1403–1418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ueno H, Shinto E, Shimazaki H, Kajiwara Y,
Sueyama T, Yamamoto J and Hase K: Histologic categorization of
desmoplastic reaction: Its relevance to the colorectal cancer
microenvironment and prognosis. Ann Surg Oncol. 22:1504–1512. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin M, Pujuguet P and Martin F: Role of
stromal myofibroblasts infiltrating colon cancer in tumor invasion.
Pathol Res Pract. 192:712–717. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kitadai Y, Ellis LM, Tucker SL, Greene GF,
Bucana CD, Cleary KR, Takahashi Y, Tahara E and Fidler IJ:
Multiparametric in situ mRNA hybridization analysis to predict
disease recurrence in patients with colon carcinoma. Am J Pathol.
149:1541–1551. 1996.PubMed/NCBI
|
|
26
|
Gofuku J, Shiozaki H, Tsujinaka T, Inoue
M, Tamura S, Doki Y, Matsui S, Tsukita S, Kikkawa N and Monden M:
Expression of E-cadherin and alpha-catenin in patients with
colorectal carcinoma. Correlation with cancer invasion and
metastasis. Am J Clin Pathol. 111:29–37. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dimanche-Boitrel MT, Vakaet L Jr, Pujuguet
P, Chauffert B, Martin MS, Hammann A, Van Roy F, Mareel M and
Martin F: In vivo and in vitro invasiveness of a rat colon-cancer
cell line maintaining E-cadherin expression: An enhancing role of
tumor-associated myofibroblasts. Int J Cancer. 56:512–521. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hewitt RE, Powe DG, Carter GI and Turner
DR: Desmoplasia and its relevance to colorectal tumour invasion.
Int J Cancer. 53:62–69. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duff SE, Jeziorska M, Kumar S, Haboubi N,
Sherlock D, O'Dwyer ST and Jayson GC: Lymphatic vessel density,
microvessel density and lymphangiogenic growth factor expression in
colorectal cancer. Colorectal Dis. 9:793–800. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ding L, Zhang Z, Shang D, Cheng J, Yuan H,
Wu Y, Song X and Jiang H: α-Smooth muscle actin-positive
myofibroblasts, in association with epithelial-mesenchymal
transition and lymphogenesis, is a critical prognostic parameter in
patients with oral tongue squamous cell carcinoma. J Oral Pathol
Med. 43:335–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fox SH, Whalen GF, Sanders MM, Burleson
JA, Jennings K, Kurtzman S and Kreutzer D: Angiogenesis in normal
tissue adjacent to colon cancer. J Surg Oncol. 69:230–234. 1998.
View Article : Google Scholar : PubMed/NCBI
|