Introduction
Thyroid cancer has increased rapidly in recent
decades. Anaplastic thyroid carcinoma (ATC) is the most aggressive
type in thyroid malignancies with extra thyroidal invasion, distant
metastasis, and resistance to conventional therapies. Accordingly,
90% of patients with ATC die within 6 months of diagnosis,
accounting up to 14–39% of all thyroid cancerrelated deaths
annually (1). ATC is derived from
pre-existing or coexisting papillary or follicular thyroid cancer
cells through the sequential accumulation of genetic mutations
during the proliferation of mature thyroid follicular cells
(2). In many cases, patients with
ATCs also harbor a pre-existing or coexisting well-differentiated
thyroid cancer of follicular origin. These events are accompanied
by a dedifferentiation process that occurs as the cancer cells
acquire the neoplastic phenotype, with a marked epithelial to
mesenchymal transition (3).
Attempts to identify biological markers that would
facilitate targeted therapy have led to the realization that
thyroid cancer might originate from cancer stem cells (CSCs).
Though self-renewal and differentiation capacity are hallmark
traits of normal stem cells, CSCs are found to also possess the
high proliferative capacity and phenotypic plasticity (4). These similarities have given rise to
the hypothesis that the CSCs are a subpopulation of cancer cells
possessing tumor-initiating capability (5–7). In
light of several pivotal studies, the existence of ‘stem-like’
cells or CSCs has been demonstrated in various types of solid
cancer including ATC (8–15). Further studies have shown that CSCs
possess typical characteristics of normal thyroid stem cells and
are thought to give rise to all ATC cells.
Existing evidence suggests that the present
treatments of ATC may not be effective at targeting ATC-CSCs
(15,16). Whether CSCs simply are tumor cells
at undifferentiated stage remains undetermined, one key molecular
characteristic of CSCs is their capability of extensive
proliferation and spherical clone formation in suspension culture
conditions (17). To better
understand the mechanisms underlying the involvement of CSCs in
ATC, vigorous effort has been devoted to the isolation of putative
CSCs. Tumor sphere-forming assays have been widely applied to
identify potential CSC populations (18). CSCs can also be isolated using flow
cytometry according to the expression of specific cell surface
markers, such as CD133 and CD44 (19,20).
Both methods, however, are technically challenging, laborious and
time-consuming with varying degrees of success. In this regard,
this study has established a new experimental strategy to reliably
isolate and enrich CSC population in ATC cells.
MicroRNAs (miRNAs) are a class of endogenous
noncoding small RNAs playing important roles in tumor formation
(21–23). Recent evidence suggests that many
miRNAs are functionally linked to the carcinogenesis of ATC due to
their roles in regulating the target genes involved in different
cell signaling pathways (24–26).
By using the isolated CSCs as a model, we screened the miRNA
expression profiles and identified miRNA-148a and its target INO80
as important regulators of the stem cells characteristics of
ATC-CSCs. Furthermore, our functional analysis provided evidence
demonstrating that the inversed expression of microRNA-148a and
INO80 is critical for CSCs to maintain their proliferative and
tumor-forming capacity.
Materials and methods
Cell culture
The tumor tissues were obtained from 15 female
patients (pathological and clinical features are shown in Table I) with biopsy-diagnosed anaplastic
thyroid carcinoma. These patients had received chemotherapy
followed by modified radical thyroidectomy. Written informed
consent was obtained from each individual, which was approved by
the Biomedical Research Ethics Committee of Affiliated Zhongshan
Hospital of Fudan University (no. 201102). All samples were
received in the laboratory within 20 min of surgery and immediately
mechanically disaggregated, then digested with 0.25% trypsin-EDTA
for 15 min. Disaggregated tumor cells were cultured in RPMI-1640
supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island,
NY, USA), 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml
streptomycin (all from Cambrex, Walkersville, MD, USA). At 80%
confluence, cells were treated with 40 µmol/l cisplatin (Sigma) and
10 µmol/l paclitaxel (Sigma) for 24 h. Drug-resistant cells were
maintained and subcultured with 0.25% trypsin-EDTA for 1–2 min at
37°C. The selected cancer cells were then cultured under stem cell
conditions by resuspension in serum-free DMEM/F12 supplemented with
5 µg/ml insulin (Sigma-Aldrich, St. Louis, MO, USA), 10 ng/ml human
recombinant epidermal growth factor, 10 ng/ml basic fibroblast
growth factor (both from Invitrogen, WA, USA), 12 ng/ml leukemia
inhibitory factor (Gibco), and 10 ng/ml noggin. Cells grown under
these conditions formed non-adherent mammospheres after
enzymatically dissociated with 0.25% trypsin-EDTA for 1–2 min at
37°C every three days.
 | Table I.Baseline characteristics for all
patients (n=15). |
Table I.
Baseline characteristics for all
patients (n=15).
|
Characteristics | No. of
patients |
|---|
| Age, years |
|
|
<65 | 10 |
|
≥65 | 5 |
| Gender |
|
|
Female | 15 |
| Charlson-Deyo
comorbidity score |
|
| 0 | 10 |
| 1 | 4 |
| ≥2 | 1 |
| Tumor size |
|
|
<6 | 7 |
| ≥6 | 8 |
| Metastasis |
|
|
Yes | 6 |
| No | 9 |
| WBC count |
|
|
<1×1010 | 9 |
|
≥1×1010 | 6 |
| Chemotherapy |
|
|
Yes | 15 |
| Surgical
margins |
|
|
Negative | 11 |
|
Positive | 4 |
| Surgery |
|
| Radical
thyroidectomy | 15 |
Animals
NOD-SCID nude mice, 4 weeks of age, were purchased
from Shanghai Laboratory Animal Co. Mice were kept under
pathogen-free conditions according to the standard guidelines of
the Institutional Animal Care and Use Committee.
Chemoresistant tumor model
Chemoresistant tumor models established in this
study were approved by the Biomedical Research Ethics Committee of
Affiliated Zhongshan Hospital of Fudan University (no. 201113).
Subcutaneous xenografts were established with mammospheres obtained
as above. Indicated dissociated cell numbers from mammospheres were
injected subcutaneously into the mammary fat pads of female
BALB/c-nu/nu mice. Cisplatin treatments started once the size of
the xenograft reached 5 mm in diameter. Mice were randomly assigned
to four groups (each n=3), treated with cisplatin intraperitoneally
at 0, 1, 2 or 4 mg/kg every 4 days for 4 weeks. The tumor volume
was calculated as follows, tumor volume (mm3) = L ×
W2 × 0.4. Mice were sacrificed by cervical dislocation.
Tumor xenografts were harvested, weighed and underwent
immunohistochemical (IHC) analysis. The presence of tumors was
confirmed by necropsy.
Lentiviral vector production
Oligonucleotides encoding miR148a pre-miRNA was
synthesized according to previously published sequences and cloned
in the lentiviral vector (lenti-mir). ShRNA target INO80 were
cloned into PLKO.1 lentiviral vector. Lentiviral vectors were
produced by transient transfection of 293T cells. Briefly, the
transfection mixture consisted of 20 µg lentiviral vectors, 10 µg
pSPAX and 5 µg pMD2.G (kindly provided by Didier Trono). The volume
of mixture was adjusted to 250 µl with H2O. Then 250 µl
of 0.5 M CaCl2 was added to the solution. Subsequently
500 µl of 2X HeBS (0.28 M NaCl, 0.05 M HEPES, 1.5 M
Na2HPO4) was added. The final solution was
left to incubate for 30 min. The culture dishes were placed into a
37°C humidified incubator with 5% CO2. Fourteen hours
later the medium was aspirated and 10 ml of fresh DMEM-10% FBS,
prewarmed to 37°C, was gently added. Cultures were further
incubated for 28 h, after which virus was collected. Concentrated
supernatants were titrated with serial dilutions of vector stocks
on 1×105 HeLa cells followed by fluorescence-activated
cytometric analysis (Beckton-Dickinson Immunocytometry Systems).
Titer of lenti-EGFP vectors were calculated to be between
0.1–1×109 TU/ml according to the formula:
1×105 HeLa cell × % EGFP-positive cell × 1,000/µl virus
(data not shown).
Quantitative reverse transcription PCR
and mRNA expression analyses
Total RNA from samples was harvested using TRIzol
(Invitrogen) and miRNeasy mini kit (Qiagen) according to the
manufacturer's instructions. RNA quantity was measured using the
NanoDrop 1000. The samples were then labeled using the miRCURY™
Hy3™/Hy5™ Power labeling kit and hybridized on the miRCURY™ LNA
Array (v.18.0). Replicated miRNAs were averaged and miRNAs with
intensities ≥30 in all samples were chosen for calculating the
normalization factor. Expressed data were normalized using the
median normalization.
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen) and treated with DNase I (Promega), according
to the manufacturer's instructions. Then the cDNA was synthesized
using SuperScript II reverse transcriptase (Invitrogen). For
quantitative gene expression analyses, cDNA was subjected to PCR
amplification using Sybr Green I (Invitrogen) and the ABI PRISM
7700 system (Applied Biosystems, Foster City, CA, USA) for
real-time detection of PCR products. The housekeeping gene 18S RNA
served as the internal reference and was amplified parallel to the
gene of interest for every template, in a separate reaction tube.
Mean mRNA ratios, representing gene expression relative to the
b-Gus reference gene, were calculated as the means of five
individual mRNA ratios assessed in five independently performed PCR
reactions. The primer sequences of all genes for PCR are as
follows: 18S, 5′-CGTTGATTAAGTCCCTGCCCTT-3′;
5′-TCAAGTTCGACCGTCTTCTCA-3′. CD133, 5′-TGGATGCAGAACTTGACAA-3′;
5′-ATACCTGCTACGACAGTCGTG-3′. SOX-2, 5′-CCTGGCAGCTGGAAGACAAAT-3′;
5′-TCCTCCAAATGCAATCACAG-3′. OCT-4, 5′-GGCCCGAAAGAGAAAGCG-3′;
5′-ACCCAGCAGCCTCAAAATCCTC-3′. Nanog, 5′-GGGCCTGAAGAAAACTATCCA-3′;
5′-TGCTATTCTTCGGCCAGTT-3′. CD44, 5′-AGATCAGTCACAGACCTGC-3′;
5′-AAACTGCAAGAATCAAAGCC-3′. CD24, 5′-TGCTCCTACCCACGCAGATT-3′;
5′-GGCCAACCCAGAGTTGGAA-3′. CD90, 5′-TCAGGAAATGGCTTTTCC-3′;
5′-TCCTCAATGAGATGCCATAAGT-3′. BMI-1, 5′-CCACCTGATGTGTGTGCTTTG-3′;
5′-TTCAGTAGTGGTCTGGTCTTGT-3′. c-MYC, 5′-AATAGAGCTGCTTCGCCTAGA-3′;
5′-GAGGTGGTTCATACTGAGCAAG-3′. onfFN, 5′-ACGTGCCTGGGCAACGGAG-3′;
5′-ACTTCTGGTCGGCATCATA-3′. ALDH1; 5′-CAGACGGGCTGTATGAGTATCT-3′;
5′-ATGGAGGTTGCCAGACGAATC-3′.
Relative mRNA levels were calculated based on the Ct
values, corrected for 18S RNA expression, according to the method
2−ΔΔCt. All experiments were done in triplicate.
Cell growth assay
To establish growth curves 1×105 cells
were cultivated in a 6-cm dish and grown for the indicated times.
Cell number was subsequently determined with the CASY1 DT cell
counter (Schaerfe Systems). For the determination of mitochondrial
activity 1×103 cells were seeded in a 96-well plate and
allowed to grow for 7 days. The activity of the mitochondrial
dehydrogenases was determined using WST (Roche Diagnostics)
according to the manufacturer's instructions. The results for
growth kinetics and metabolic activity were derived from three
independent cultures.
Transwell migration assay
In vitro chemotaxis was assayed using the HTS
Transwell-96 system from Corning (Corning, NY, USA). Cells were
serum starved overnight in serum-free medium. 100 µl cells diluted
to 75×104/ml in migration buffer (DMEM with 5% BSA) were
added in the upper wells, chemoattractants diluted in a serum-free
medium were placed to the lower wells. Cells were allowed to
migrate for 48 h. Then migrated cells were detached, stained with
CyQuant dye and counted using a fluorescence plate reader. All
experiments were performed in triplicate.
Flow cytometry sorting
Flow cytometric cell sorting was performed using an
Epics Altra flow cytometer (Beckman Coulter, Fullerton, CA, USA).
The antibodies used for cell sorting were anti-CD44-FITC and
anti-CD24-PE (Pharmingen, Franklin Lakes, NJ, USA). The cells were
sorted twice, purity of which was verified by flow cytometry.
Immunohistochemistry
Fixed cells or cryosections (pretreated with 3%
hydrogen peroxide in methanol for 5 min to quench endogenous
peroxidase activity) were washed in phosphate-buffered saline (PBS)
and then blocked for 1 h in washing buffer containing 5% normal
goat serum (Sigma). Then the sections were sequentially incubated
with mouse antibodies against PCNA and Ki67 antigen (BD
Biosciences), biotinylated goat anti-mouse IgG and streptavidin
conjugated to horseradish peroxidase. Diaminobenzidine (Dako Corp.,
Carpinteria, CA, USA) was used for color development. Nuclei were
lightly counter stained with hematoxylin. Negative control slides
were stained with isotype mouse immunoglobulin in place of primary
antibodies.
Western blotting
Total protein was isolated from primary thyroid
cancer, primary thyroid cancer-derived tumor stem cell or cells
transfected with lenti-let-7a in cell lysis buffer (20 mM Tris, pH
8.0, 100 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40). Protein
concentration was measured using the Bio-Rad protein assay kit. The
membrane was first probed with antibodies against Ras (Applied
Biomaterials) and HMGA2 (Sigma). After being washed extensively
three times in TBS-T, samples were incubated in a horseradish
peroxidase-conjugated second antibody (1:5,000) at room temperature
for an hour, and the blot was developed with ECL reagents (Amersham
Biosciences). Western blotting was conducted using standard
procedures, using anti-Tg (Sigma), anti-cyclin NIS (Cell Signaling
Technology, USA), anti-TP (Cell Signaling Technology), anti-onfFN
(Sigma), anti-E-cadherin (Cell Signaling Technology),
anti-fibronectin (Sigma), anti-vimentin (Cell Signaling
Technology), and anti-INO80 (Sigma). To control for equal sample
loading in each group, immunoblotting was performed using
antibodies against the cytosolic marker GAPDH (1:5,000, Sigma). The
western blotting experiment was repeated at least three times.
Statistical analysis
Data collected from each (experimental and control)
group were expressed as the mean ± SEM. One-way analysis of
variance and unpaired Student's t-test were performed to analyze
the differences between groups using GraphPad Prism 5 program. A
P-value <0.01 was denoted as statistically significant between
experimental and control groups.
Results
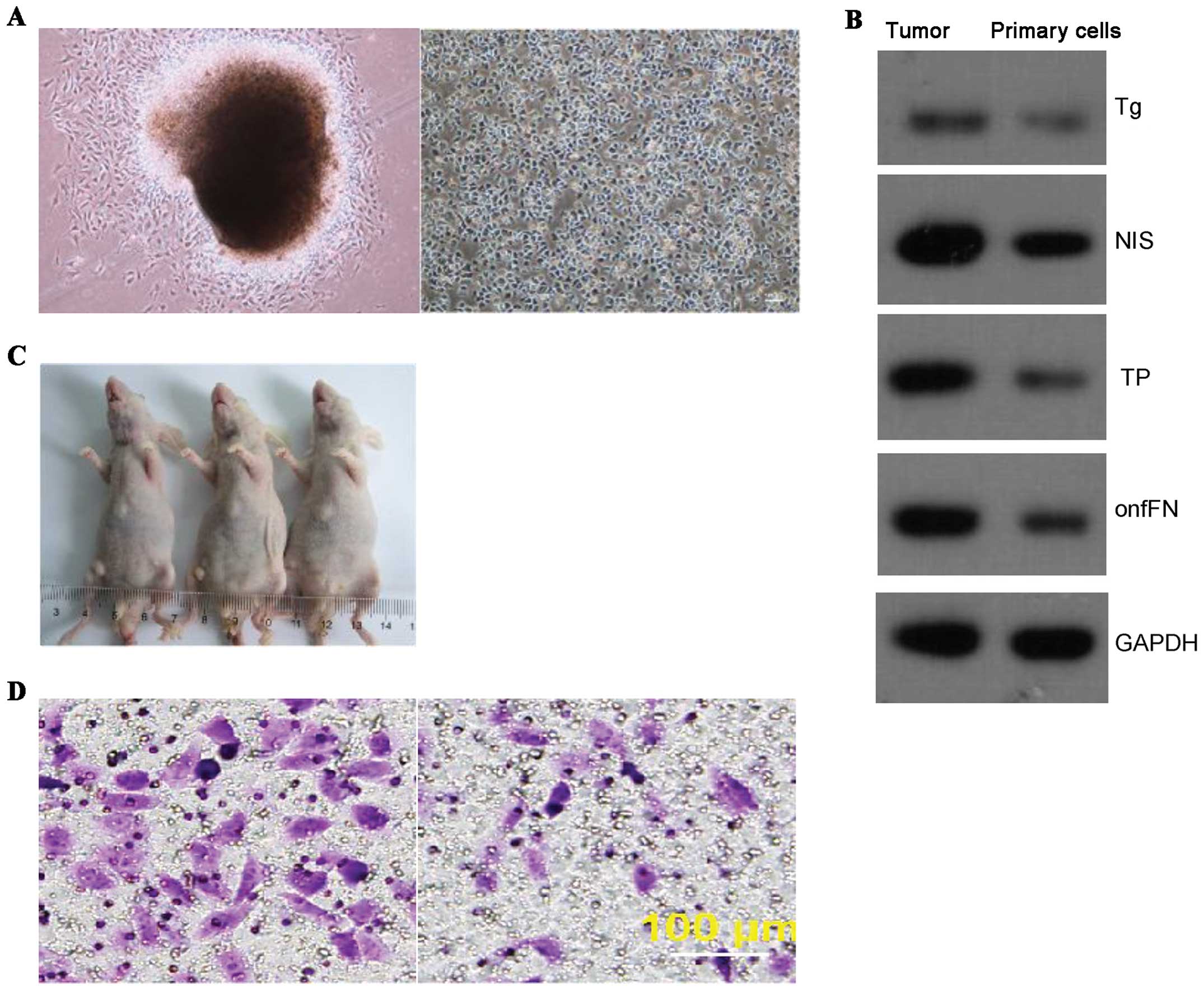
Patient-derived thyroid cancer cells
in culture retain characteristics of cancer cells
Primary ATC cells were separated and cultured as
described in Materials and methods. Cells from isolated clones were
able to maintain original morphology after numerous passages in
culture. Tumor formation of 1×106 p20 cells were
injected into each nude mouse (Fig.
1A). Western blot analysis revealed notable expression of the
thyroid cancer specific proteins such as thyroglobulin (Tg),
thyroperoxidase (TPO), sodium/iodide symporter (NIS), and oncofetal
fibronectin (onfFN) in passage-20 (p20) cells (Fig. 1B). More importantly, the p20 cells
retained the capacity of tumor formation when subcutaneously
inoculated in nude mice (Fig. 1C).
These cells also showed similar characteristics of invasion
(Fig. 1D, left) and migration
(Fig. 1D, right) to those observed
typically in thyroid cancer cells.
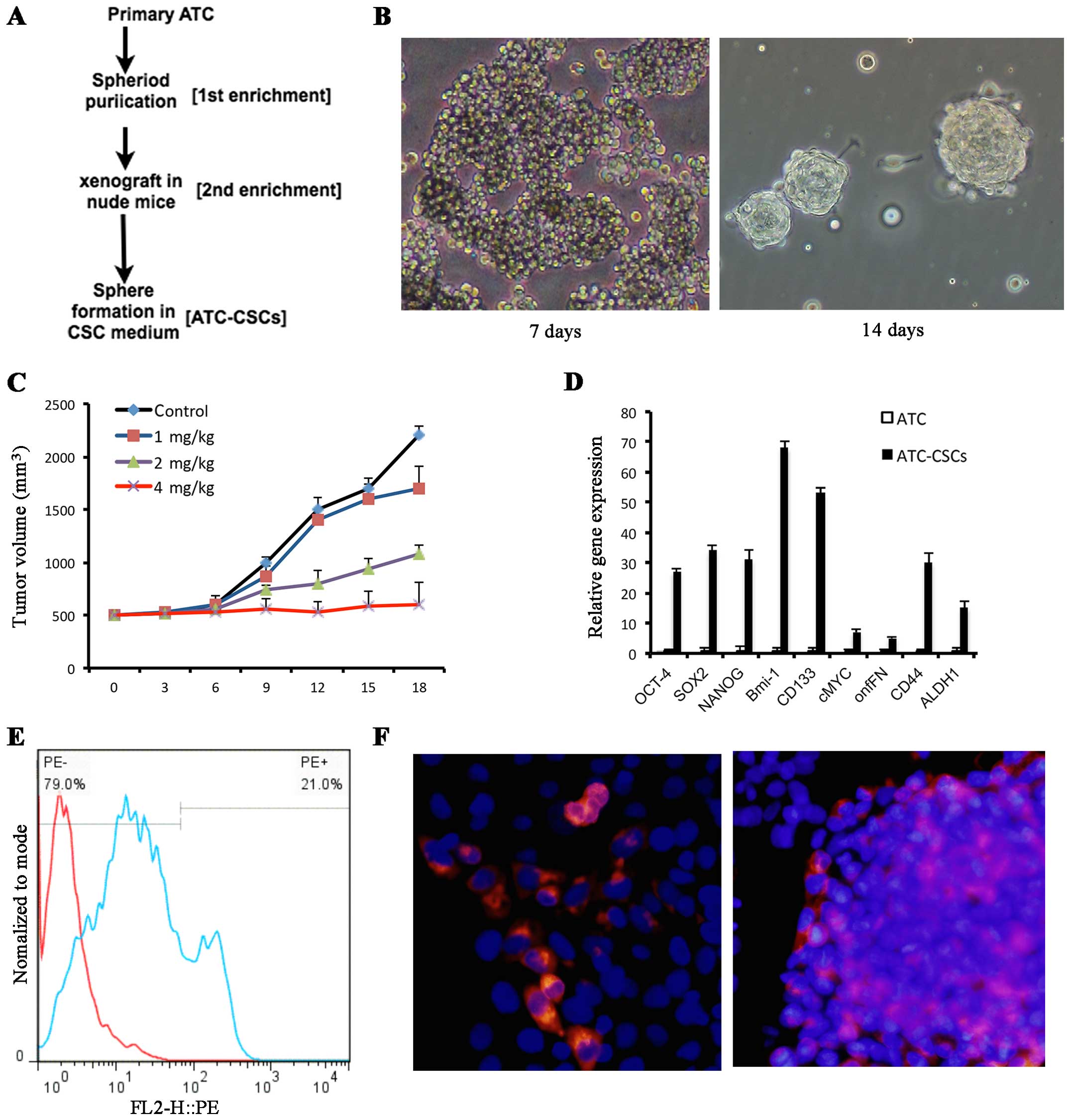
Cisplatin treatment enriches ATC-CSCs
in a xenograft model
The workflow of ATC-CSC enrichment and isolation is
illustrated in Fig. 2A. When grown
in a suspension culture system with stem cell specific media and
polyhema-precoated dishes, a subpopulation of the p20 cells
displayed CSC-like properties as they aggregated and gradually
formed spheres after 7–14 days (Fig.
2B). To determine whether this CSC population could be enriched
upon chemotherapy, cells collected from the formed spheres were
implanted in nude mice to give rise to subcutaneous tumor
formation. When the size of tumors reached 5 mm in diameter, the
mice were injected intraperitoneally with various doses of
cisplatin As shown in Fig. 2C,
cisplatin treatment resulted in a dose-dependent tumor inhibition
among the xenografts. When given at 4 mg/kg, cisplatin was able to
completely abolish the tumor growth over an 18-day period (Fig. 2C). Subsequently, the tumor cells
were collected from mice receiving 4 mg/kg cisplatin. The CSC
population was expanded based upon the unique capability of its
cells to grow and form spheres in the CSC conditional medium. The
expression of stem cell marker genes, including OCT-4, SOX2, NANOG,
BMI-1, CD133, CD44, C-MYC, onfFN and ALDH1, was found to be
consistently upregulated in cells from these spheres when compared
with the p20 primary cells (Fig.
2D). In addition, both flow cytometry (Fig. 2E) and immunocytochemistry (Fig. 2F) analyses demonstrated that these
sphere cells expressed considerably higher levels of the stem cell
surface marker CD133. Taking together; these results indicate a
potent positive effect of cisplatin in enriching the ATC-CTC
population.
Upregulated miR-148a attenuates stem
cell features in ATC-CSCs
Next, we performed microarray analysis to identify
specific miRNAs whose expression may be differentially regulated in
ATC-CSCs vs. primary ATCs. Among 17 miRNAs that showed decreased
expression in CSCs, miR-148a exhibited the greatest reduction in
ATC-CSCs with a ~150-fold decrease in expression (Table II).
 | Table II.Expression of miR-148a is drastically
reduced in ATC-CSCs.a |
Table II.
Expression of miR-148a is drastically
reduced in ATC-CSCs.a
| Transcript ID
(array design) | logFC | FC |
|---|
| hsa-miR-148a | −7.23 | −149.60 |
| hsa-miR-192-5p | −5.79 |
−55.37 |
| hsa-miR-5572 | −5.67 |
−50.80 |
| hsa-miR-3653 | −5.66 |
−50.62 |
|
hsa-miR-3613-5p | −5.55 |
−46.85 |
|
hsa-miR-6754-5p | −5.13 |
−35.13 |
| hsa-miR-185-3p | −4.77 |
−27.26 |
| hsa-miR-660-3p | −4.76 |
−27.13 |
|
hsa-miR-199b-5p | −4.64 |
−24.99 |
|
hsa-miR-6726-5p | −4.48 |
−22.30 |
| hsa-miR-1299 | −4.47 |
−22.15 |
| hsa-miR-124-3p | −4.37 |
−20.68 |
|
hsa-miR-7152-3p | −4.35 |
−20.42 |
|
hsa-miR-92a-1-5p | −4.11 |
−17.22 |
| hsa-miR-1290 | −4.02 |
−16.17 |
| hsa-miR-5093 | −3.96 |
−15.59 |
| hsa-miR-543 | −3.95 |
−15.46 |
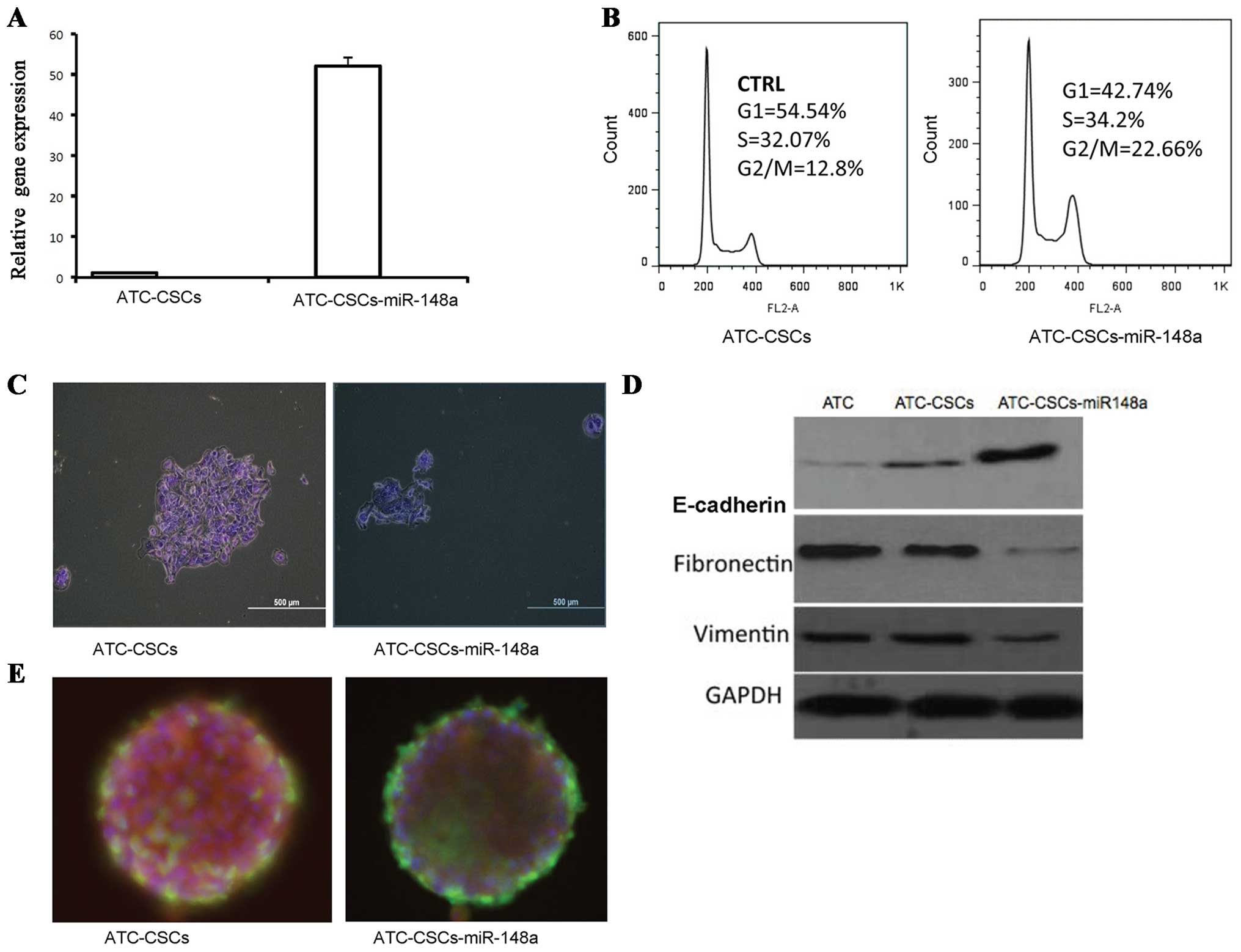
To define the role of miR-148a in ATC-CSCs, we
generated a recombinant lentivirus encoding miR-148a. Infection of
ATC-CTCs with this virus resulted in a near 50-fold increase in
miR-148a expression (Fig. 3A).
Interestingly, miR-148a overexpression induced a significant G2/M
arrest in ATC-CSCs as revealed by propidium iodide staining
(Fig. 3B). Overexpressed miR-148a
also significantly inhibited the cell proliferation of ATC-CSCs in
adherent culture condition (Fig.
3C). To assess the EMT status of the miR-148a-overexpressing
cells, we analyzed expression of the EMT-related proteins including
E-cadherin, fibronectin and vimentin. As expected, E-cadherin, an
epithelial marker downregulated during EMT, was expressed at higher
levels in ATC-CSCs than in ATCs. Overexpression of miR148a further
increased E-cadherin in ATC-CSCs (Fig.
3D). On the other hand, expression of fibronectin and vimentin,
two EMT-associated makers, was significantly suppressed in
miR-148a-overexpressing CSCs (Fig.
3D). Moreover, immunofluorescence analysis revealed a decreased
staining for stem cell marker OCT-4 in miR148-overexpressing cells
along with an increased staining for TG, a marker for
differentiated cells (Fig. 3E).
Collectively, these results suggest that miR148a may act to impede
stemness in ATC-CSCs via downregulating EMT.
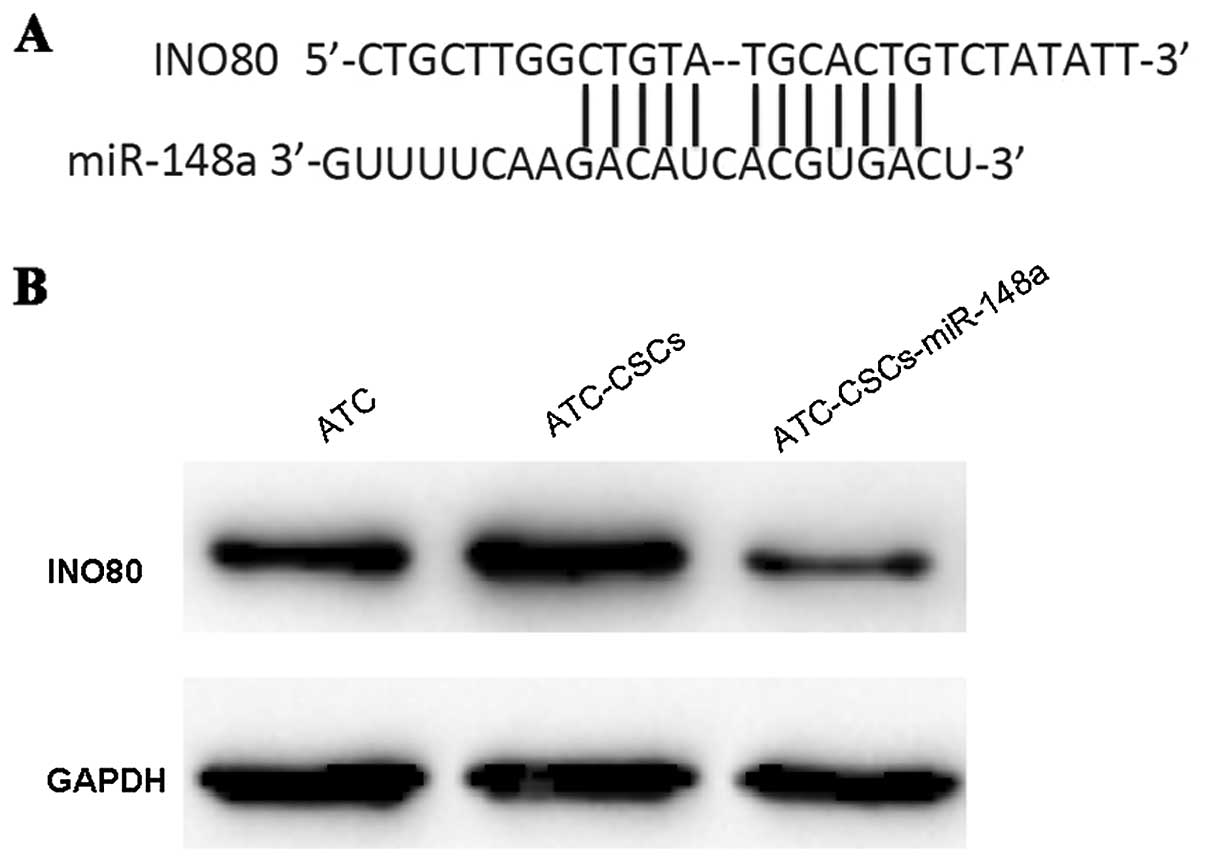
miR-148a targets INO80 in
ATC-CSCs
To understand the mechanisms underlying the role of
miR-148a, we searched for predicted targets of miR-148a using the
Target Scan software. INO80, a chromatin remodeler important for
endothelial stem cell (ESC) self-renewal, was subsequently
identified as a potential target with high confidence score.
Sequence analysis further showed that miR-148a could directly
target the 3'UTR region of INO80 transcript (Fig. 4A). Overexpression of miR-148a was
sufficient to induce a significant reduction of INO80 expression in
ATC-CSCs (Fig. 4B), confirming the
direct regulation of INO80 by miR-148a in living cells.
INO80 is required for CSC gene
expression and tumor formation
To determine whether INO80 regulates self-renewal of
CSCs and the expression of stem cell-specific genes, we performed
knockdown experiments using a lentivirus encoding a shRNA
specifically targeting INO80 (shINO80). The tumor formation of
5×105 of ATC-CSCs cells, ATC-CSCs-shINO80 were injected
into nude mice, respectively, and were measured 2 weeks later. As
shown in Fig. 5A, infection with
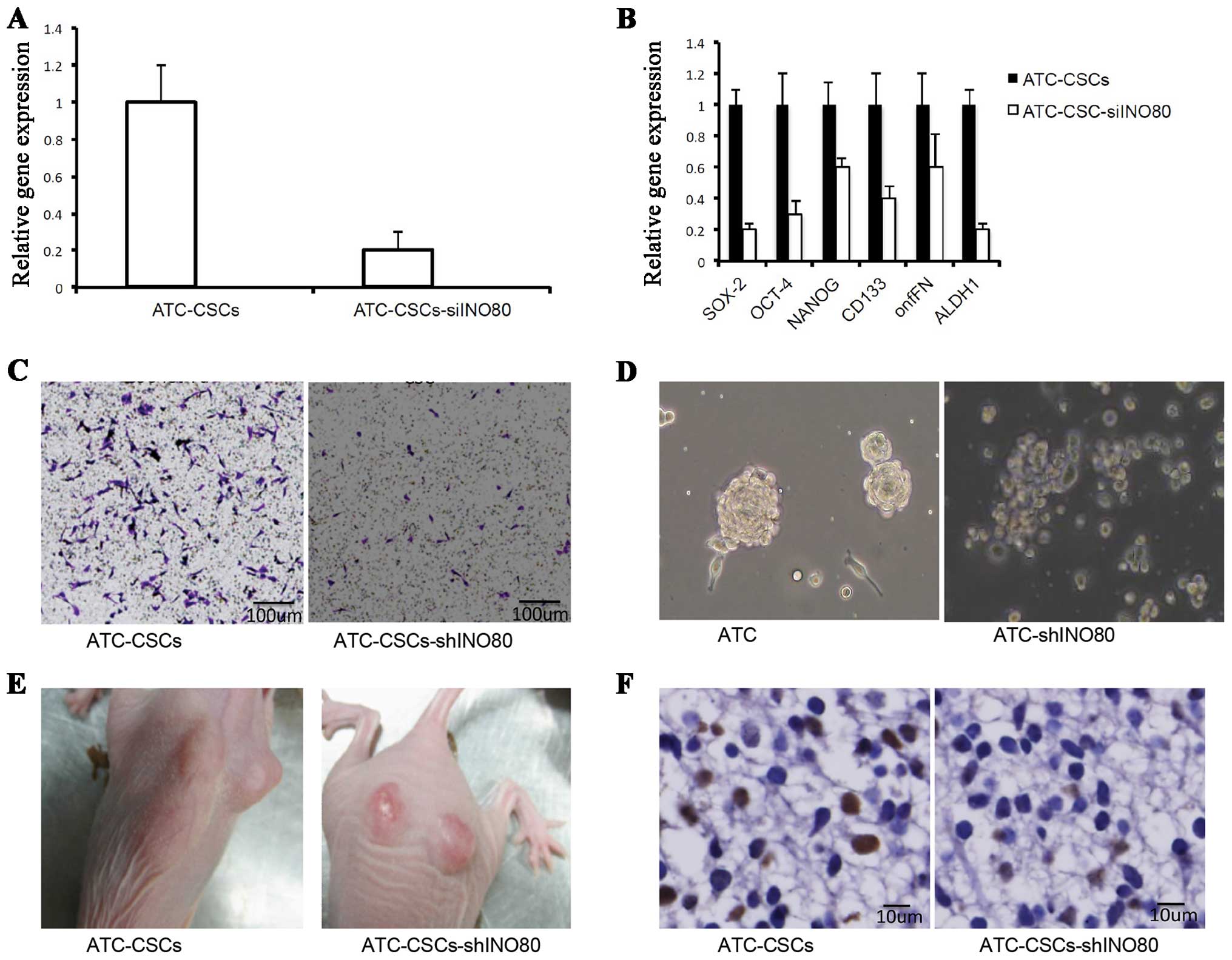
such virus significantly suppressed INO80 expression in CSCs.
Importantly, INO80 knockdown led to the downregulation of various
stem cell-specific factors, including OCT-4, SOX2, NANOG, CD133,
onfFN and ALDH1 (Fig. 5B),
suggesting that INO80 positively regulates the stem cell
characteristics. Transwell assays demonstrated that the migration
of ATC-CSCs was also inhibited when transduced with the shINO80
virus (Fig. 5C).
To evaluate the effect of shINO80 on sphere-forming
potentials, we cultured cells infected with or without shINO80
virus in a non-adhesive culture system. As shown in Fig. 5D, the cells unexposed to shINO80
virus were able to form spheres, whereas those infected with
shINO80 virus (ATC-CSCs-shINO80) failed to do the same. Upon
inoculation into nude mice, ATC-CSCs-shINO80 form tumors that were
significantly smaller in size than those originated from control
ATC-CSCs (Fig. 5E). Additionally, a
significantly reduced staining for cell proliferation marker PCNA
was observed in tumors from ATC-CSCs-shINO80 (Fig. 5F). Overall, these results suggest
that the ability of ATC-CSCs to form spheres in vitro and
tumors in vivo are both repressed upon INO80 knockdown.
Roles of miR-148a and INO80 in tumor
formation in vivo
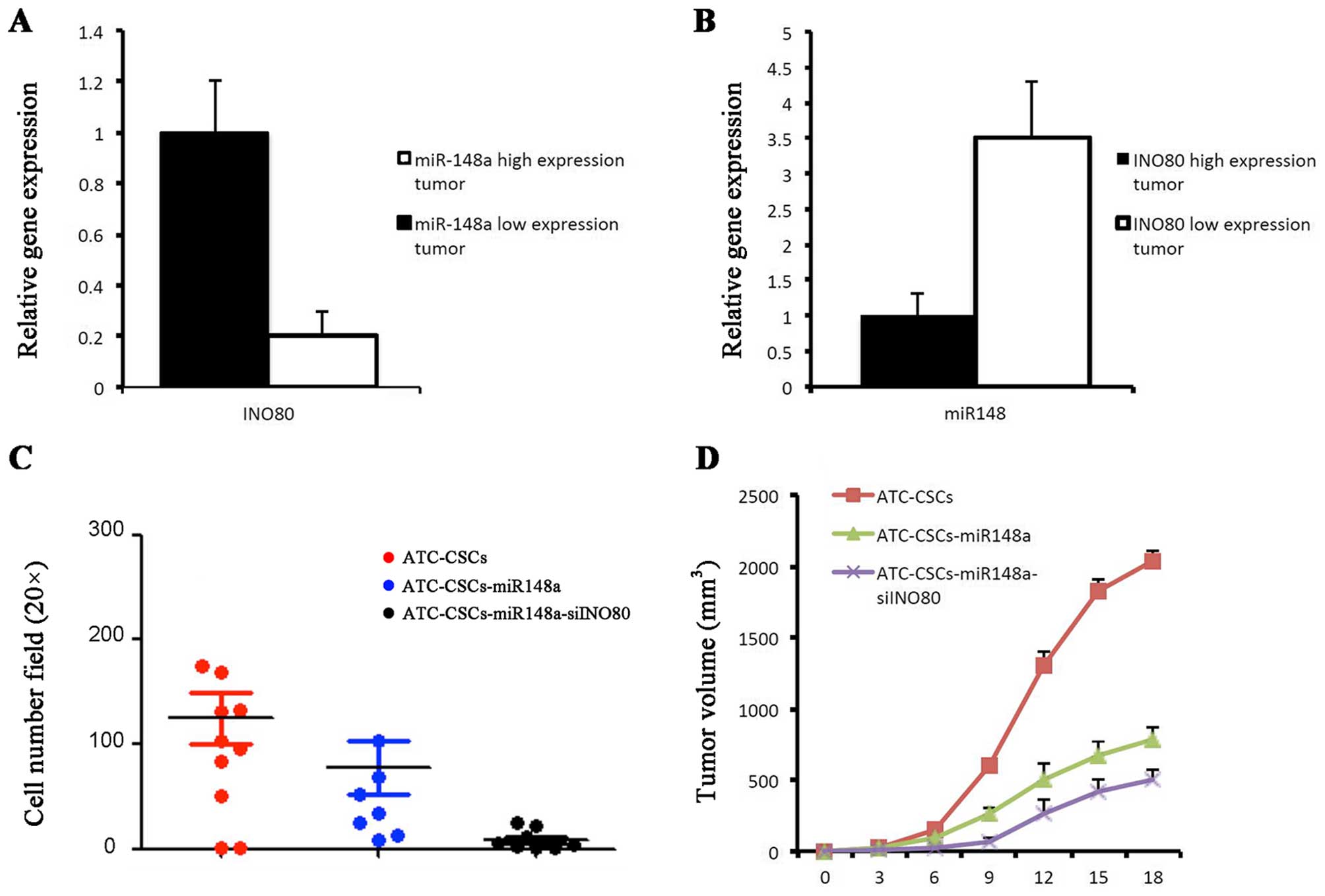
Using qPCR assay, we found an inverse relationship
between the expression of miR-148 and INO80 in primary ATC samples.
Tumors in which miR-148a was downregulated exhibited increased
expression of INO80, and vice versa (Fig. 6A and B). To explore miR-148a and
INO80 as potential therapeutic targets in the treatment of ATC, we
established ATC-CSC clones stably expressing either miR-148a alone
or miR-148a with shINO80. In Tanswell assays miR-148a and shINO80
showed synergistic effects in inhibiting cell migration (Fig. 6C). In the nude mouse xenograft
model, ATC-CSCs overexpressing miR-148a alone gave rise to tumors
that were markedly smaller than those derived from control
ATC-CSCs. Coexpression of shINO80 with miR-148a was able to further
reduce the tumor volume (Fig. 6D).
Taken together, these results suggest a possibility of preventing
CSC-initiated tumor formation through combining approaches that
upregulate miR-148a and downregulate INO80.
Discussion
In this study, we have successfully purified and
enriched a CSC population from primary ATC samples. The derived
ATC-CSCs were capable of forming spheres in culture and tumors
in vivo with high expression of stem cell markers. In our
data, thyroid cancer specific proteins Tg, TPO, NIS, and onfFN were
expressed in passage-20 cells. Moreover, we obtained compelling
evidence that downregulation of miRNA-148 expression is a key
feature of these ATC-CSCs. In line with this notion is the finding
that forced expression of miRNA-148a induced cell cycle arrest,
inhibited proliferation and diminished the overall stemness of
ATC-CSCs.
Increasing evidence has pointed to important roles
for miR-148a in various forms of tumors such as hepatocellular
carcinoma, gastric cancer, pancreatic cancer and colorectal
carcinoma (27–30). In particular, miR-148a was recently
found to be associated with the growth, invasion and metastasis of
tumor cells. To our knowledge, however, this study represents the
first to demonstrate that the expression of miR-148a is profoundly
lower in ATC-CSCs than in differentiated ATC cells. Although the
detailed mechanisms underlying the role of miRNA-148a remain
incompletely defined, our data indicate that downregulation of
INO80 at least partially accounts for the effects of miRNA-148a
overexpression in ATC-CSCs.
The INO80 protein complex was first identified in
Saccharomyces cerevisiae for its capability of regulating chromatin
structure (31,32). Previous RNAi screens have identified
the INO80 complex as a novel self-renewal factor (33,34).
INO80 belongs to the INO80 subfamily and is known to participate in
a variety of nuclear transactions, including transcriptional
regulation, DNA repair and DNA replication (35–37). A
recent study provided evidence implicating an essential role of
INO80 in the organization of the pluripotency network and the
regulation of the pluripotent state (38). This study contributes to the
existing knowledge by demonstrating that the expression of INO80 is
upregulated in ATC-CSCs, and knockdown of INO80 can significantly
decrease the expression of stem cell marker genes.
Several lines of evidence are supportive of INO80
being a direct target of miRNA-148a. Lastly, similar to that
observed with miR-148a overexpression, both proliferative and
sphere-forming abilities of ATC-CSCs was repressed in response to
INO80 knockdown. Data from our functional analysis further
demonstrated that the inversed expression of miR-148a and INO80 is
critical for ATC-CSCs to maintain their stem cell identity.
In conclusion, in our experiment, we found the
expression of INO80 was downregulated upon miRNA-148
overexpression, and knockdown of INO80 can attenuate stem
cell-specific properties. We suspect INO80 as a target gene of
miR-148a. We will perform additional experiments to prove it. This
study offers critical insight into a novel mechanism for the
regulation of proliferation and differentiation of thyroid cancer
stem cells. Specifically, we provide evidence that miR-148a
inhibits the stem cell characteristics of ATC-CSCs via
downregulating the expression of INO80. Our findings highlight a
potential therapeutic application for targeting INO80 through
enhancing miRNA-148a in the treatment of anaplastic thyroid
carcinoma. The results that miRNA-148a overexpression along with
INO80 knockdown in ATC-CSCs inhibit the volume gain of derived
tumors are in strong support of this possibility.
Acknowledgements
This study was supported by Zhongshan Hospital,
Fudan University (no. 81070717).
References
|
1
|
Shaha AR: Implications of prognostic
factors and risk groups in the management of differentiated thyroid
cancer. Laryngoscope. 114:393–402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kondo T, Ezzat S and Asa SL: Pathogenetic
mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer.
6:292–306. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Derwahl M: Linking stem cells to thyroid
cancer. J Clin Endocrinol Metab. 96:610–613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Organista-Nava J, Gómez-Gómez Y and
Gariglio P: Embryonic stem cell-specific signature in cervical
cancer. Tumour Biol. 35:1727–1738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim CF and Dirks PB: Cancer and stem cell
biology: How tightly intertwined? Cell Stem Cell. 3:147–150. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y and Armstrong SA: Cancer:
Inappropriate expression of stem cell programs? Cell Stem Cell.
2:297–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gu W, Yeo E, McMillan N and Yu C:
Silencing oncogene expression in cervical cancer stem-like cells
inhibits their cell growth and self-renewal ability. Cancer Gene
Ther. 18:897–905. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schatton T, Murphy GF, Frank NY, Yamaura
K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM,
Weishaupt C, et al: Identification of cells initiating human
melanomas. Nature. 451:345–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Friedman S, Lu M, Schultz A, Thomas D and
Lin RY: CD133+ anaplastic thyroid cancer cells initiate
tumors in immunodeficient mice and are regulated by thyrotropin.
PLoS One. 4:e53952009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zito G, Richiusa P, Bommarito A, Carissimi
E, Russo L, Coppola A, Zerilli M, Rodolico V, Criscimanna A, Amato
M, et al: In vitro identification and characterization of CD133
(pos) cancer stem-like cells in anaplastic thyroid carcinoma cell
lines. PLoS One. 3:e35442008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Todaro M, Iovino F, Eterno V, Cammareri P,
Gambara G, Espina V, Gulotta G, Dieli F, Giordano S, De Maria R, et
al: Tumorigenic and metastatic activity of human thyroid cancer
stem cells. Cancer Res. 70:8874–8885. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kreso A and Dick JE: Evolution of the
cancer stem cell model. Cell Stem Cell. 14:275–291. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang ZJ and Wechsler-Reya RJ: Hit ‘em
where they live: Targeting the cancer stem cell niche. Cancer Cell.
11:3–5. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Q, Liu H, Xiong H, Liu Z, Wang LE,
Qian J, Muddasani R, Lu V, Tan D, Ajani JA, et al: Polymorphisms at
the microRNA binding-site of the stem cell marker gene CD133 modify
susceptibility to and survival of gastric cancer. Mol Carcinog.
54:449–458. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei XD, He J, Gao JX, Wang JY, Ma BJ and
Chen J: Investigation on biological characteristics of
CD133+ cancer stem cells in human laryngeal carcinoma
Hep-2 cell line. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
48:578–583. 2013.(In Chinese). PubMed/NCBI
|
|
21
|
Fan X, Chen X, Deng W, Zhong G, Cai Q and
Lin T: Up-regulated microRNA-143 in cancer stem cells
differentiation promotes prostate cancer cells metastasis by
modulating FNDC3B expression. BMC Cancer. 13:612013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bu P, Chen KY, Chen JH, Wang L, Walters J,
Shin YJ, Goerger JP, Sun J, Witherspoon M, Rakhilin N, et al: A
microRNA miR-34a-regulated bimodal switch targets Notch in colon
cancer stem cells. Cell Stem Cell. 12:602–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Luo N, Luo Y, Peng Z, Zhang T and
Li S: microRNA-150 inhibits human CD133-positive liver cancer stem
cells through negative regulation of the transcription factor
c-Myb. Int J Oncol. 40:747–756. 2012.PubMed/NCBI
|
|
24
|
Cheng Q, Zhang X, Xu X and Lu X: MiR-618
inhibits anaplastic thyroid cancer by repressing XIAP in one ATC
cell line. Ann Endocrinol (Paris). 75:187–193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiong Y, Zhang L and Kebebew E: MiR-20a is
upregulated in anaplastic thyroid cancer and targets LIMK1. PLoS
One. 9:e961032014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Yang WQ, Zhu H, Qian YY, Zhou L,
Ren YJ, Ren XC, Zhang L, Liu XP, Liu CG, et al: Regulation of
autophagy by miR-30d impacts sensitivity of anaplastic thyroid
carcinoma to cisplatin. Biochem Pharmacol. 87:562–570. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan L, Huang S, He R, Rong M, Dang Y and
Chen G: Decreased expression and clinical significance of miR-148a
in hepatocellular carcinoma tissues. Eur J Med Res. 19:682014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia J, Guo X, Yan J and Deng K: The role
of miR-148a in gastric cancer. J Cancer Res Clin Oncol.
140:1451–1456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liffers ST, Munding JB, Vogt M, Kuhlmann
JD, Verdoodt B, Nambiar S, Maghnouj A, Mirmohammadsadegh A, Hahn SA
and Tannapfel A: MicroRNA-148a is down-regulated in human
pancreatic ductal adenocarcinomas and regulates cell survival by
targeting CDC25B. Lab Invest. 91:1472–1479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang H, Li Y, Huang Q, Ren X, Hu H, Sheng
H and Lai M: MiR-148a promotes apoptosis by targeting Bcl-2 in
colorectal cancer. Cell Death Differ. 18:1702–1710. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen X, Mizuguchi G, Hamiche A and Wu C: A
chromatin remodelling complex involved in transcription and DNA
processing. Nature. 406:541–544. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Papamichos-Chronakis M, Watanabe S, Rando
OJ and Peterson CL: Global regulation of H2A.Z localization by the
INO80 chromatin-remodeling enzyme is essential for genome
integrity. Cell. 144:200–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chia NY, Chan YS, Feng B, Lu X, Orlov YL,
Moreau D, Kumar P, Yang L, Jiang J, Lau MS, et al: A genome-wide
RNAi screen reveals determinants of human embryonic stem cell
identity. Nature. 468:316–320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu G, Kim J, Xu Q, Leng Y, Orkin SH and
Elledge SJ: A genome-wide RNAi screen identifies a new
transcriptional module required for self-renewal. Genes Dev.
23:837–848. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Conaway RC and Conaway JW: The INO80
chromatin remodeling complex in transcription, replication and
repair. Trends Biochem Sci. 34:71–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morrison AJ and Shen X: Chromatin
remodelling beyond transcription: The INO80 and SWR1 complexes. Nat
Rev Mol Cell Biol. 10:373–384. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Watanabe S and Peterson CL: The INO80
family of chromatin-remodeling enzymes: Regulators of histone
variant dynamics. Cold Spring Harb Symp Quant Biol. 75:35–42. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Du Y, Ward JM, Shimbo T, Lackford
B, Zheng X, Miao YL, Zhou B, Han L, Fargo DC, et al: INO80
facilitates pluripotency gene activation in embryonic stem cell
self-renewal, reprogramming, and blastocyst development. Cell Stem
Cell. 14:575–591. 2014. View Article : Google Scholar : PubMed/NCBI
|