Introduction
Cancer represents a major public health issue
worldwide and constitutes an enormous burden on society (1,2).
Breast cancer (BC) is the most commonly diagnosed cancer and the
leading cause of cancer-related deaths among women (3). Although current therapies have proven
effective in reducing primary tumors, the 5-year survival rate of
BC is still poor mainly due to its intensive tendency for invasion
and metastasis (4). Thus, further
study is needed to identify and evaluate biomarkers for prognostic
prediction as well as to develop novel strategies for the
prevention and control of BC.
Lysophosphatidic acid (LPA) receptors are specific G
protein-coupled receptors that bind with lysophosphatidic acid to
mediate a variety of biological processes such as cell
proliferation, migration, invasion and differentiation (5). At least six LPA receptors (LPA1-6)
have been identified (6). Notably,
LPAs have been correlated with the invasive and metastatic
behaviors of several malignant cancers including BC (7–9). LPA2
is associated with a more malignant phenotype in carcinomas of the
breast (10). The hypoxic
microenvironment of a tumor also serves as an independent predictor
of a poor prognosis in various types of cancer including BC
(11); the effects of such hypoxia
are mediated by the transcription factor hypoxia inducible
factor-1α (HIF-1α) (12).
Consistent with this, HIF-1α is closely associated with the
occurrence and progression of tumors (13–15),
and HIF-1α activation represents a final common event in the
pathogenesis of different types of tumors 12,16–18.
Thus, HIF-1α is considered to be specifically responsible for
hypoxia-mediated progression of cancer (19,20).
However, the association between LPA2 and HIF-1α in BC has not yet
been described.
To address these issues, in the present study, we
first detected the expression of LPA2 and HIF-1α in BC tissue
specimens to assess their association with patient
clinicopathological and survival data. Subsequently, we assessed
the role of LPA2 and HIF-1α in BC in vitro and explored the
effects of LPA2 overexpression or knockdown on HIF-1α expression
and cell proliferation, migration and invasion.
Materials and methods
Tissue specimens
Tissue specimens from 156 patients with BC were
collected from consecutive surgical cases at the Department of
Surgical Oncology, the 3201st Hospital of Shaanxi Province, between
2005 and 2009. The histological type for all cases was breast
ductal cancer. All tissues were pathologically examined. The
patients were all females, ranging between the ages of 26 and 77
years. The clinicopathological data of the patients are documented
in Table II. None of the 156
patients received neoadjuvant or adjuvant chemotherapy before
surgery. The present study was approved by the Protection of Human
Subjects Committee of the 3201st Hospital and complies with the
Declaration of Helsinki.
 | Table II.Association of LPA2 and HIF-1α
expression with clinicopathological data from the BC patients. |
Table II.
Association of LPA2 and HIF-1α
expression with clinicopathological data from the BC patients.
|
|
| LPA2 |
| HIF-1α |
|
|---|
|
|
|
|
|
|
|
|---|
| Variable | N | High | Low | P-value | High | Low | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
|
<50 | 79 | 48 | 31 |
| 40 | 39 |
|
|
≥50 | 77 | 43 | 34 | 0.534 | 43 | 34 | 0.514 |
| Menopausal
status |
|
|
|
|
|
|
|
|
Premenopausal | 63 | 29 | 34 |
| 31 | 32 |
|
|
Postmenopausal | 93 | 62 | 31 | 0.010 | 52 | 41 | 0.410 |
| Tumor site |
|
|
|
|
|
|
|
|
Left | 87 | 51 | 36 |
| 46 | 41 |
|
|
Right | 69 | 40 | 29 | 0.935 | 37 | 32 | 0.926 |
| Tumor diameter
(cm) |
|
|
|
|
|
|
|
| ≤2 | 41 | 19 | 22 |
| 21 | 20 |
|
| >2
to ≤5 | 98 | 63 | 35 |
| 51 | 47 |
|
|
>5 | 17 | 9 | 8 | 0.131 | 11 | 6 | 0.600 |
| ER status |
|
|
|
|
|
|
|
|
Negative | 70 | 42 | 28 |
| 45 | 25 |
|
|
Positive | 86 | 49 | 37 | 0.703 | 38 | 48 | 0.012 |
| PR status |
|
|
|
|
|
|
|
|
Negative | 70 | 40 | 30 |
| 36 | 34 |
|
|
Positive | 86 | 51 | 35 | 0.786 | 47 | 39 | 0.688 |
| Her-2 status |
|
|
|
|
|
|
|
|
Negative | 111 | 66 | 45 |
| 60 | 51 |
|
|
Positive | 45 | 25 | 20 | 0.654 | 23 | 22 | 0.739 |
| Nodal
metastasis |
|
|
|
|
|
|
|
|
Negative | 50 | 22 | 28 |
| 12 | 38 |
|
|
Positive | 106 | 69 | 37 | 0.013 | 71 | 35 | <0.001 |
| Histologic
grade |
|
|
|
|
|
|
|
| 1 | 32 | 21 | 11 |
| 14 | 18 |
|
| 2 | 78 | 40 | 38 |
| 39 | 39 |
|
| 3 | 46 | 30 | 16 | 0.203 | 30 | 16 | 0.126 |
| TNM stage |
|
|
|
|
|
|
|
| I | 23 | 9 | 14 |
| 8 | 15 |
|
| II | 60 | 32 | 28 |
| 28 | 32 |
|
|
III | 48 | 30 | 18 |
| 26 | 22 |
|
| IV | 25 | 20 | 5 | 0.026 | 21 | 4 | 0.003 |
Immunohistochemical (IHC)
staining
Formalin-fixed paraffin-embedded tumor sections
(5-µm thickness) were analyzed by IHC staining using an anti-LPA2
rabbit polyclonal antibody (ab38322), anti-HIF-1α (ab82832) (1:200
dilution; Abcam, Cambridge, MA, USA), and a biotin-conjugated
secondary antibody. The staining was performed following the SP kit
procedure (Golden Bridge International, Beijing, China). As a
control, the primary antibody was replaced by phosphate-buffered
saline (PBS). IHC staining results were independently assessed by
two pathologists in a semi-quantitative manner, and graded in
accordance with an immunoreactive score (IRS): IRS is equal to the
percentage of positive cells (0, negative; 1, <10%; 2, 10–50%;
3, 51–80%; 4, >80%) multiplied by the staining intensity (1,
weak; 2, moderate; 3, strong).
The scores ranged from 0 to 12. A score of 6 or
higher was considered high expression and below 6 was considered
low expression. A score of 0 represented a negative staining
result.
Cell culture
The non-tumorigenic MCF-10A and BC MCF-7 cell lines
were obtained from the Cell Bank of Shanghai (Shanghai, China) and
were cultured in Dulbecco's modified Eagle's medium (HyClone,
Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine
serum (Hzsjq Co. Ltd., Hangzhou, China) at 37°C in a humidified
incubator under an atmosphere of 5% carbon dioxide. All experiments
were performed with cells in the logarithmic phase of growth.
Plasmid and siRNA transfection
The plasmid (pENTR223-LPA2) was obtained from the
DNASU Plasmid Repository (Tempe, AZ, USA) and small interfering RNA
(siRNA) molecules targeting human LPA2 (si-LPA2) were
obtained from Santa Cruz Biotechnology (sc-39926; Santa Cruz, CA,
USA). The MCF-7 cells were grown and transfected with the negative
control (NC) (GenePharma Co., Ltd., Shanghai, China), pENTR223-LPA2
or si-LPA2 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's protocol. MCF-7 cells were cultured
in normal medium as a control group (normal group).
Cell proliferation assessment
Following plasmid and siRNA transfection for 48 h,
the colorimetric
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was used to assess the proliferation of the MCF-7 cells. The
MCF-7 cells (100 µl) were seeded at a density of 4×104
cells/ml in 96-well plates and were cultured for 24, 48 and 72 h.
An aliquot of 10 µl MTT solution in PBS (5 mg/ml) was added to each
well and the plates were incubated for 4 h at 37°C. Then, 150 µl
dimethyl sulfoxide (Sigma, St. Louis, MO, USA) was added to each
well and the plates were shaken for 15 min before reading the
optical density of each well at 570 nm on a microplate reader.
MCF-7 cells were cultured in normal medium as a control group. All
experiments were conducted in triplicate.
Cell migration and invasion
assays
Following plasmid and siRNA transfection for 48 h,
migration and invasion assays were conducted in MCF-7 cells using
Transwell plates with 8-µm pore size membranes (Millipore Inc.,
Billerica, MA, USA) as previously described (21). After incubation for 12 h (for
migration assays) or 24 h (for invasion assays), cells remaining in
the upper side of the filter were removed with cotton swabs. The
cells attached on the lower surface were fixed, stained using
crystal violet and washed with PBS. Cells were counted in 5-high
power fields/membrane. The results are presented as the mean number
of cells that migrated/field/membrane. MCF-7 cells were cultured in
normal medium as a control group. All experiments were conducted in
triplicate.
Western blot analyses
Following plasmid and siRNA transfection of MCF-7
cells for 48 h, the proteins were extracted according to standard
procedures of RIPA lysis buffer kit (Santa Cruz Biotechnology).
Equal amounts of protein samples were separated onto a 10% sodium
dodecyl sulfate-polyacrylamide gel using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were then
transferred onto a polyvinylidene difluoride (PVDF) membrane
(Millipore). The membranes were examined with the following
specific primary antibodies: anti-LPA2 rabbit polyclonal or
anti-HIF-1α rabbit polyclonal (1:1,000 dilution) or anti-β-actin
rabbit polyclonal antibodies (bs-0061R; 1:1,000 dilution) followed
by the conjugated secondary antibody goat-anti-rabbit IgG (1:3,000
dilution) (both from Bioss, Woburn, MA, USA). The reactions were
visualized using an enhanced chemiluminescence (ECL) reagent
(Millipore). The band intensities of the western blotting were
measured by densitometry using Quantity One software (Bio-Rad
Laboratories, Hercules, CA, USA). MCF-7 cells were cultured in
normal medium as a control group (normal group). The protein levels
were normalized to those of β-actin protein level used as a loading
control.
Statistical analysis
The Chi-square test was used to analyze the
association of LPA2 and HIF-1α expression with the
clinicopathological variables. The Spearman's rank correlation
coefficient test was performed to associate LPA2 expression with
HIF-1α expression in tissue specimens. Overall survival was defined
as the time from the date of surgery to the date of the last
follow-up or of patient death. Survival curves were calculated
using the Kaplan-Meier method and compared using the log-rank test.
Multivariate analysis using Cox's proportional hazard model was
used to assess prognostic factors. The Student's t-test or one-way
ANOVA was used to compare normally distributed variables. P<0.05
was considered significant. All analyses were performed using SPSS
software version 18.0 (IBM Corp., New York, NY, USA).
Results
Differential LPA2 and HIF-1α protein
expression is associated with the clinicopathological data of the
patients with BC
We first assessed the protein expression of LPA2 and
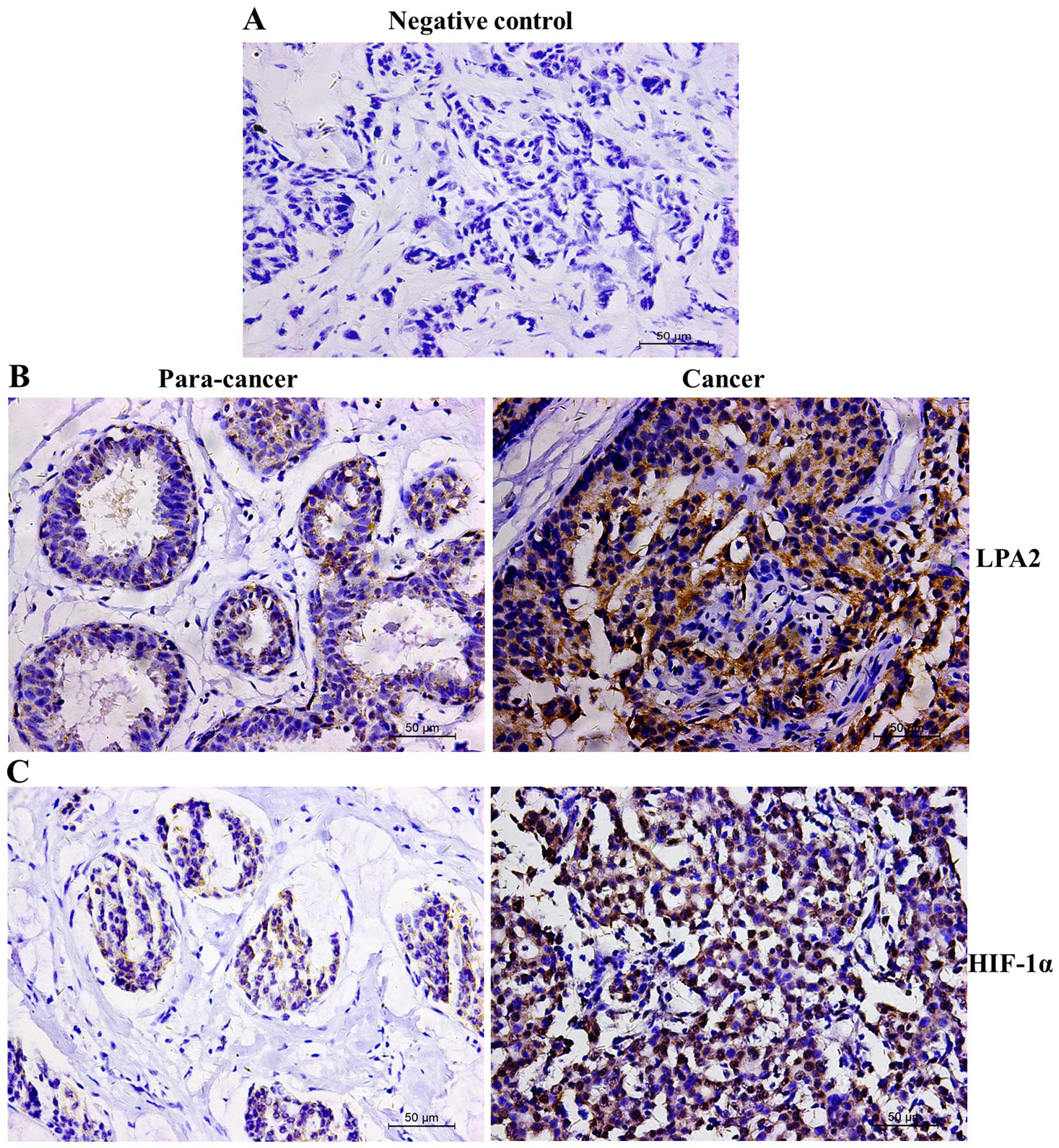
HIF-1α using IHC in 156 BC tissue specimens (Fig. 1A as a negative control) and found
that the LPA2 protein was localized in both the cell membrane and
cytoplasm (Fig. 1B) whereas the
HIF-1α protein was predominantly localized in the nuclei (Fig. 1C). The expression levels of these
two proteins significantly differed between the cancer and
para-cancer normal tissues (Table
I). High expression of the LPA2 protein was significantly
associated with menopausal status, nodal metastasis and TNM stage,
yet not with age, tumor site, tumor diameter, estrogen or
progesterone receptor and Her-2 status, and histologic grade
(Table II). In comparison, HIF-1α
expression was associated with estrogen receptor status, nodal
metastasis and TNM stage, but not with age, menopausal status,
tumor site, tumor diameter, progesterone receptor and Her-2 status
and grade. In addition, LPA2 expression was associated with HIF-1α
expression in the BC tissues. Specifically, Spearman's rank
correlation coefficient test showed that there were 91 cancer cases
expressing a high level of LPA2 vs. 83 cases with HIF-1α expression
(Table III), revealing a positive
association (r=0.562; P<0.001).
 | Table I.Differential expression of LPA2 and
HIF-1α proteins in breast cancer (BC) tissues vs. para-cancer
normal tissues. |
Table I.
Differential expression of LPA2 and
HIF-1α proteins in breast cancer (BC) tissues vs. para-cancer
normal tissues.
|
| LPA2 |
| HIF-1α |
|
|---|
|
|
|
|
|
|
|---|
|
| + | − | P-value | + | − | P-value |
|---|
| Cancer | 110 | 46 |
| 116 | 40 |
|
| Para-carcinoma | 30 | 126 | <0.001 | 33 | 123 | <0.001 |
| Total | 140 | 172 |
| 149 | 163 |
|
 | Table III.Association of LPA2 and HIF-1α
expression. |
Table III.
Association of LPA2 and HIF-1α
expression.
|
| LPA2 |
|
|
|---|
|
|
|
|
|
|---|
|
| High | Low | r | P-value |
|---|
| HIF-1α |
|
|
|
|
|
High | 70 | 13 |
|
|
Low | 21 | 52 | 0.562 | <0.001 |
Association of LPA2 and HIF-1α
expression with the survival of patients with BC
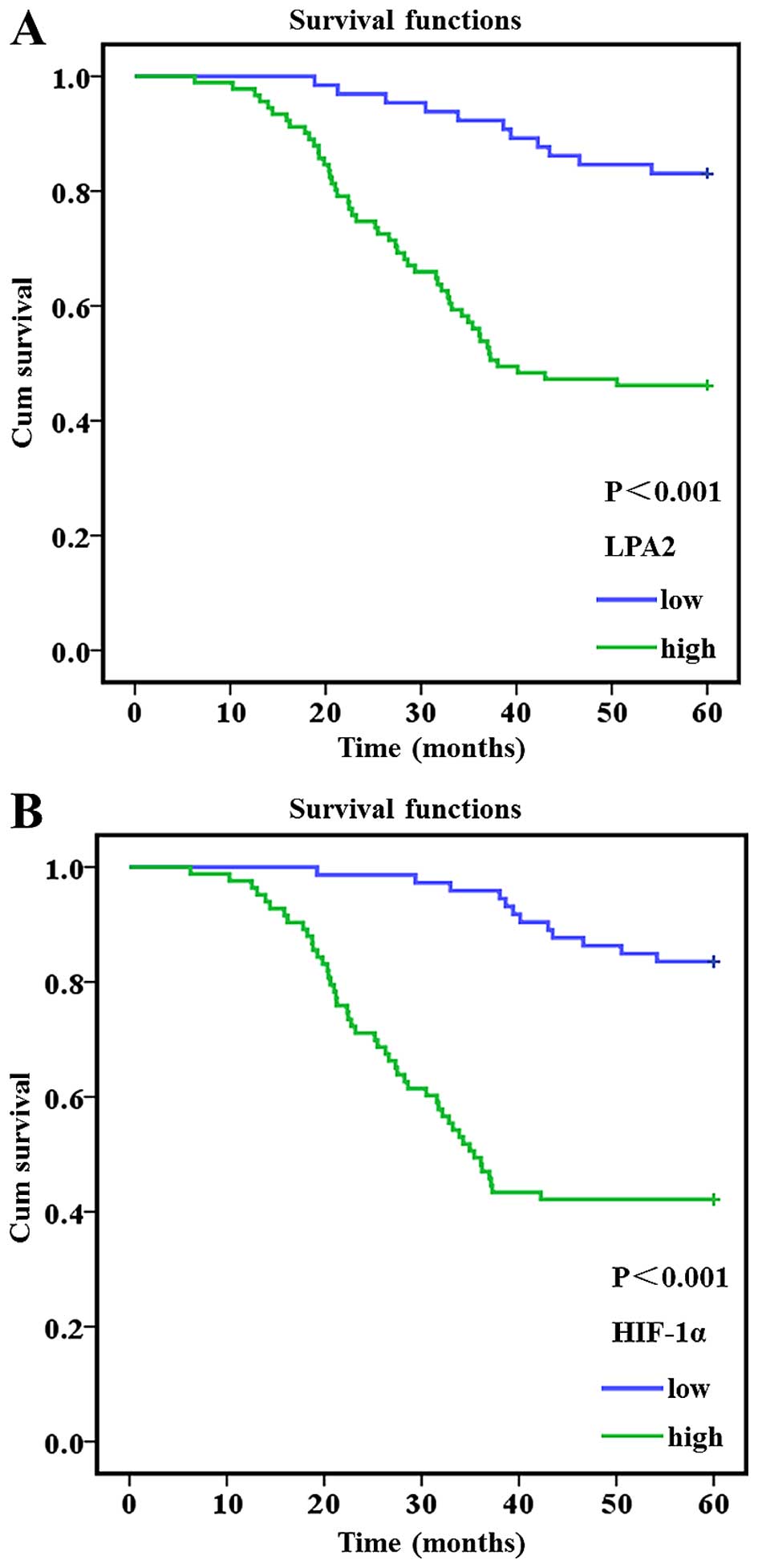
The Kaplan-Meier curves and the log-rank test data
showed that patients with a high LPA2-expressing tumor had a
statistically significantly poorer overall survival compared to
those with a low LPA2-expressing tumor (Fig. 2A and Table IV) of 41 months [95% confidence
interval (CI), 38.053–45.531] vs. 55 months (95% CI, 53.497–58.356;
P<0.001). The same was true for HIF-1α expression (Fig. 2B and Table IV). Furthermore, tumor diameter,
nodal metastasis and TNM stage were all associated with poor
overall patient survival (P<0.05). The multivariate Cox
regression model showed that nodal metastasis [P=0.007, hazard
ratio (HR)=7.516, 95% CI, 1.748–32.327], TNM stage (P<0.001,
HR=2.436, 95% CI, 1.703–3.486), LPA2 expression (P=0.036 HR=2.294,
95% CI, 1.054–4.992) and HIF-1α (P=0.029, HR=2.369, 95% CI,
1.090–5.150) were independent prognostic indicators for the
survival of patients with BC (Table
V).
 | Table IV.Univariate analysis of overall
survival. |
Table IV.
Univariate analysis of overall
survival.
|
|
| Overall
survival |
|
|---|
|
|
|
|
|
|---|
| Variable | N | Median | 95% CI | P-value |
|---|
| LPA2 |
|
|
|
|
|
High | 91 | 41.00±1.908 | 38.053–45.531 |
|
|
Low | 65 | 55.00±1.239 | 53.497–58.356 | <0.001 |
| HIF-1α |
|
|
|
|
|
High | 83 | 39.00±2.017 | 35.839–43.746 |
|
|
Low | 73 | 56.00±0.981 | 54.728–58.575 | <0.001 |
| Age (years) |
|
|
|
|
|
<50 | 79 | 47.00±1.891 | 44.052–51.465 |
|
|
≥50 | 77 | 47.00±1.921 | 43.837–51.368 | 0.920 |
| Menopausal
status |
|
|
|
|
|
Premenopausal | 63 | 49.00±2.128 | 45.431–53.771 |
|
|
Postmenopausal | 93 | 46.00±1.729 | 42.993–49.769 | 0.123 |
| Tumor site |
|
|
|
|
|
Left | 87 | 48.00±1.725 | 44.981–51.742 |
|
|
Right | 69 | 46.00±2.130 | 42.650–50.999 | 0.597 |
| Tumor diameter
(cm) |
|
|
|
|
| ≤2 | 41 | 54.00±1.860 | 51.191–58.480 |
|
| >2
to ≤5 | 98 | 45.00±1.776 | 41.581–48.544 |
|
|
>5 | 17 | 45.00±4.257 | 37.182–53.869 | 0.007 |
| ER status |
|
|
|
|
|
Negative | 70 | 46.00±2.193 | 42.581–51.179 |
|
|
Positive | 86 | 48.00±1.667 | 45.067–51.601 | 0.978 |
| PR status |
|
|
|
|
|
Negative | 70 | 48.00±1.986 | 44.959–52.746 |
|
|
Positive | 86 | 46.00±1.827 | 43.148–50.310 | 0.363 |
| Her-2 status |
|
|
|
|
|
Negative | 111 | 46.00±1.627 | 43.092–49.468 |
|
|
Positive | 45 | 51.00±2.314 | 46.603–55.674 | 0.111 |
| Nodal
metastasis |
|
|
|
|
|
Negative | 50 | 58.00±1.053 | 56.544–60.672 |
|
|
Positive | 106 | 42.00±1.704 | 39.187–45.868 | <0.001 |
| Histologic
grade |
|
|
|
|
| 1 | 32 | 53.00 ±2.569 | 48.704–58.775 |
|
| 2 | 78 | 45.00±2.004 | 41.984–49.840 |
|
| 3 | 46 | 46.00±2.333 | 41.895–51.041 | 0.057 |
| TNM stage |
|
|
|
|
| I | 23 | 58.00±1.157 | 56.550–61.084 |
|
| II | 60 | 53.00±1.869 | 49.397–56.723 |
|
|
III | 48 | 45.00±2.320 | 40.795–49.888 |
|
| IV | 25 | 29.00±2.819 | 23.496–34.547 | <0.001 |
 | Table V.Multivariate Cox proportional hazard
analysis of overall survival. |
Table V.
Multivariate Cox proportional hazard
analysis of overall survival.
|
| Overall
survival |
|
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value |
|---|
| Age (years) | 1.389 | 0.755–2.556 | 0.290 |
| Menopausal
status | 1.281 | 0.651–2.520 | 0.473 |
| Tumor site | 0.962 | 0.555–1.667 | 0.890 |
| Tumor diameter
(cm) | 1.381 | 0.850–2.242 | 0.192 |
| ER status | 0.931 | 0.528–1.642 | 0.805 |
| PR status | 1.288 | 0.753–2.201 | 0.355 |
| Her-2 status | 0.697 | 0.341–1.426 | 0.323 |
| Nodal
metastasis | 7.516 | 1.748–32.327 | 0.007 |
| Grade | 0.803 | 0.535–1.207 | 0.292 |
| TNM stage | 2.436 | 1.703–3.486 | 0.000 |
| LPA2 | 2.294 | 1.054–4.992 | 0.036 |
| HIF-1α | 2.369 | 1.090–5.150 | 0.029 |
LPA2 and HIF-1α are expressed in human
BC cell lines
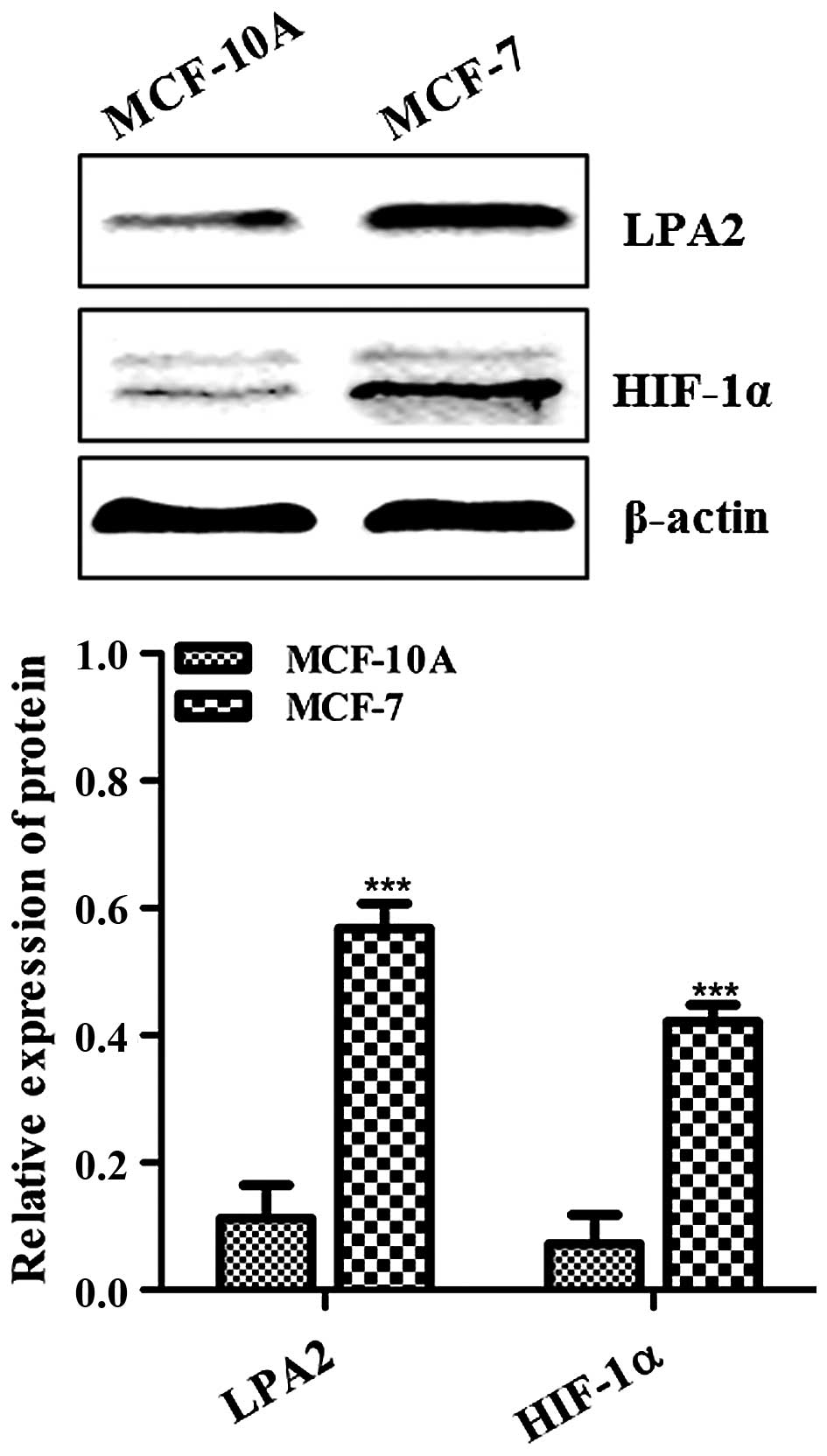
Our ex vivo data demonstrated that LPA2 and
HIF-1α protein expression were positively correlated (r=0.562) and
were associated with a poor overall survival; however, this result
had not previously been reported with regard to both LPA2 and
HIF-1α in BC. Therefore, we further assessed the role of LPA2 and
HIF-1α in a human BC cell line. We first explored the expression
levels of LPA2 and HIF-1α in non-tumorigenic MCF-10A and MCF-7 BC
cells. As shown in Fig. 3, the
levels of both LPA2 and HIF-1α were high in MCF-7 and low in
MCF-10A as assessed by western blot analysis; this difference was
statistically significant (P<0.001).
Effects of LPA2 overexpression in BC
cells
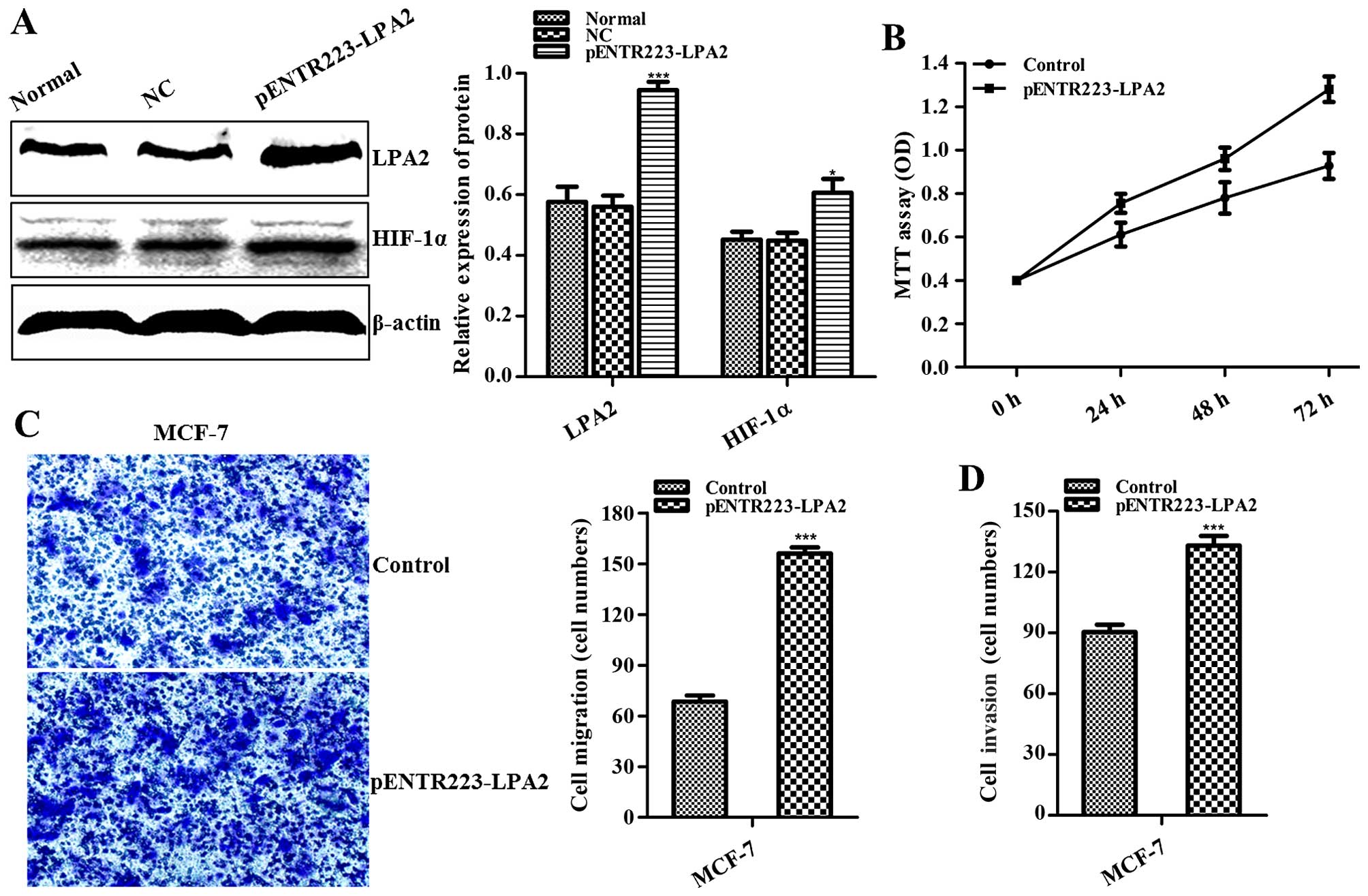
At 48 h following transfection of MCF-7 cells with
the plasmid (pENTR223-LPA2), western blot analysis showed that LPA2
protein was overexpressed in the LPA2-overexpressing cells compared
to that in the normal and NC group (control groups) (P<0.001;
Fig. 4A). Upon LPA2 overexpression,
the level of HIF-1α was elevated in the MCF-7 cells compared to
that in the controls (P<0.05; Fig.
4A). The MTT assay showed that overexpression of LPA2 promoted
the proliferation of MCF-7 cells in a time-dependent manner
(P<0.01 at 72 h; Fig. 4B). To
further analyze the effect of LPA2 overexpression on MCF-7 cells,
cell migration and invasion assays were conducted, demonstrating
that these characteristics of MCF-7 cells were enhanced following
LPA2 overexpression (P<0.001; Fig.
4C and D).
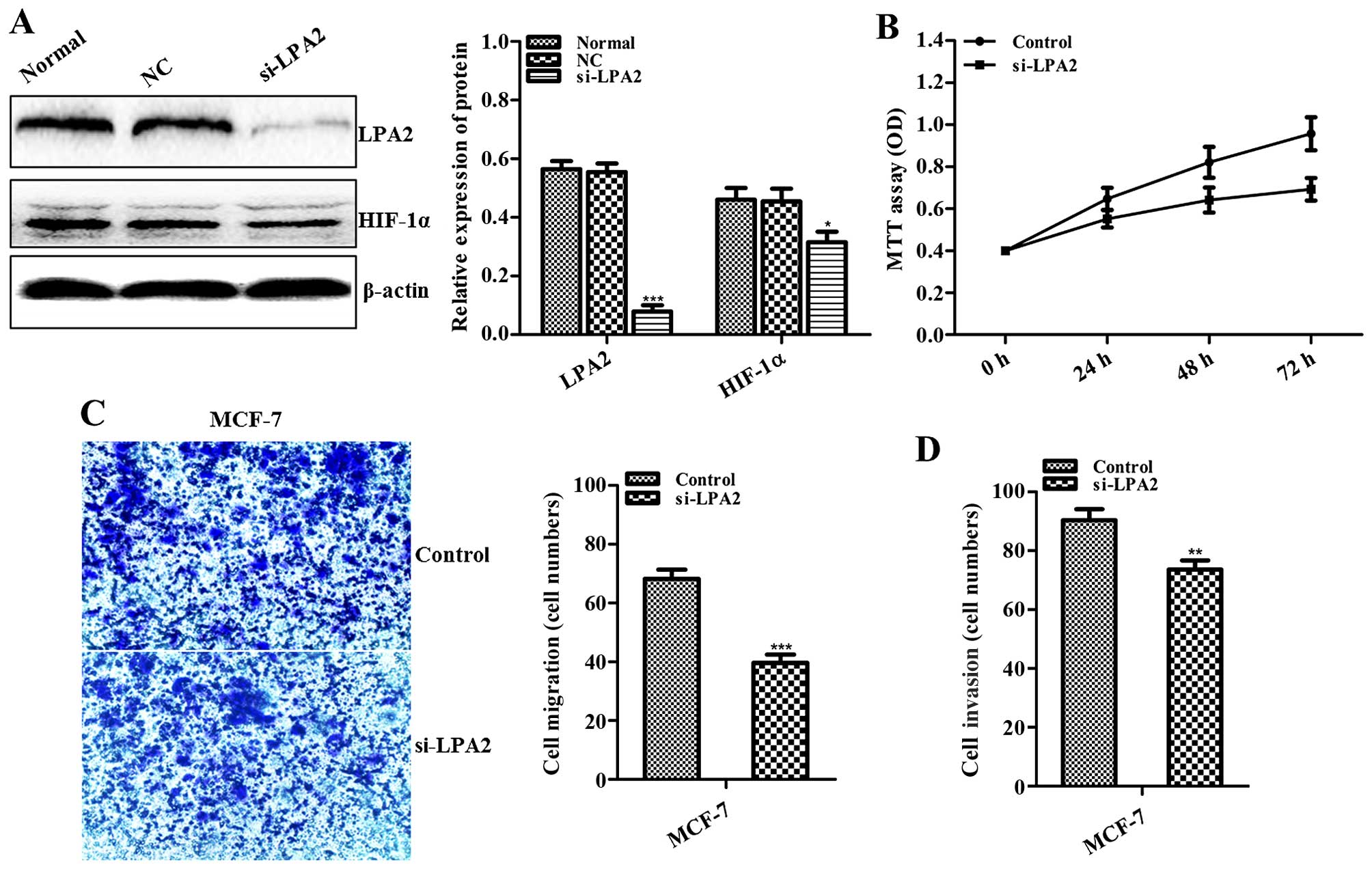
Effects of LPA2 knockdown on BC
cells
The effects of LPA2 knockdown were directly inverse
to those of its upregulation. At 48 h following transfection of
MCF-7 cells with si-LPA2, both LPA2 (P<0.001; Fig. 5A) and HIF-1α (P<0.05; Fig. 5A) expression was downregulated
compared to the controls as assessed by western blot analysis.
Similarly, MCF-7 cell proliferation was inhibited in a
time-dependent manner (P<0.05 at 72 h; Fig. 5B) and their migration and invasion
were reduced following LPA2 downregulation (P<0.001 and
P<0.01; Fig. 5C and D).
Discussion
The invasive and metastatic properties of breast
cancer (BC) play a considerable role in its poor prognosis,
requiring the development of novel strategies to combat its
position as the leading cause of cancer-related deaths among women
worldwide. The results of the present study illustrated the role of
LPA2 in the regulation of HIF-1α expression and BC progression,
suggesting its potential for BC diagnostic and therapeutic
application to help facilitate the prevention and control of this
disease.
LPA receptors are specific G protein-coupled
receptors and multifunctional signaling molecules that control the
proliferation, motility, and differentiation of many cell types
including cancer cells (5,22). Although the expression and functions
of LPA2 have been the subject of only a few studies, increased LPA2
expression has been reported in invasive BC (10,23).
However, the study of the roles of LPA2 in BC has mainly focused on
western populations; in contrast, data related to the effect of
LPA2 on BC in Chinese women or to the overall survival and
prognostic significance of LPA2 in BC are not available. In the
present study, we first analyzed the association of LPA2 expression
in BC tissue specimens with the clinicopathological and survival
data of Chinese women. Our date showed that LPA2 was more highly
expressed in BC tissues than in para-cancer tissues, consistent
with some prior reports from other populations (8,10). We
also found that LPA2 was upregulated more frequently in
postmenopausal than in premenopausal women, suggesting that LPA2
overexpression was more strongly related to the carcinogenesis of
postmenopausal BC, consistent with the study by Kitayama et
al (10). In addition, our data
showed that LPA2 overexpression was significantly associated with
nodal metastasis and later clinical TNM stage and that patients
with high LPA2-expressing tumors had a statistically significant
poorer overall survival compared to those with tumors expressing
low levels of LPA2. These results confirmed the validity of LPA2 as
an independent prognostic indicator for patient survival in BC.
HIF-1α has a close association with tumor occurrence
and progression and its activation represents a final common event
in the pathogenesis of multiple tumor types (12,18,24–27).
Data from the present study showed that BC tumors exhibiting high
HIF-1α expression correlated with statistically significant poorer
overall patient survival compared to those with low levels of
HIF-1α expression, which is consistent with other studies (28). The association between LPA2 and
HIF-1α had not been previously reported; however, the results of
the present study indicated that a high level of LPA2 expression
was associated with a high level of HIF-1α expression in BC
clinical tissue specimens, revealing a positive association
(r=0.562, P<0.001). This suggested the existence of a potential
gene regulatory effect between LPA2 and HIF-1α. In the present
study we aimed to explore whether LPA2 was able to regulate HIF-1α
in BC cells.
To address this issue, we performed in vitro
experiments to assess the effect of LPA2 on HIF-1α expression in
the MCF-7 BC cell line. Consistent with an earlier study, we found
that both LPA2 and HIF-1α exhibited higher expression (MCF-7) than
non-tumorigenic breast (MCF-10A) cells (8,29). In
addition, we found when the level of LPA2 was artificially
increased through exogenous overexpression, the level of HIF-1α was
concurrently elevated compared to MCF-7 controls (P<0.05) and
the proliferation (P<0.01), migration and invasion of MCF-7
cells (P<0.001) was enhanced. Conversely, we also found when the
level of LPA2 was decreased via siRNA knockdown, HIF-1α also
exhibited low expression compared to MCF-7 controls (P<0.05) and
that MCF-7 proliferation (P<0.05), migration (P<0.001) and
invasion (P<0.01) were also inhibited. Together, these data
indicated that LPA2 was able to regulate HIF-1α expression and the
proliferation, migration and invasion of BC cells.
In summary, the present study demonstrated that LPA2
was able to serve as an independent prognostic indicator for
survival in BC and that high LPA2 expression was associated with
poor overall patient survival among Chinese women. LPA2 and HIF-1α
protein expression exhibited a positive association, and LPA2
showed the ability to regulate HIF-1α expression and proliferation,
migration and invasion in BC cells. Thus, our data suggest that
LPA2 may function as a useful prognostic marker of BC and may also
represent a valid therapeutic target for BC management.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81471710).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Long J, Luo GP, Xiao ZW, Liu ZQ, Guo M,
Liu L, Liu C, Xu J, Gao YT, Zheng Y, et al: Cancer statistics:
Current diagnosis and treatment of pancreatic cancer in Shanghai,
China. Cancer Lett. 346:273–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petekkaya I, Ayyildiz V, Kizilarslanoglu
MC, Sahin U, Gezgen G, Roach EC, Karcaaltincaba M and Altundag K:
Prognosis of breast cancer in patients with peritoneal metastasis.
Breast. 21:420–421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gotoh M, Fujiwara Y, Yue J, Liu J, Lee S,
Fells J, Uchiyama A, Murakami-Murofushi K, Kennel S, Wall J, et al:
Controlling cancer through the autotaxin-lysophosphatidic acid
receptor axis. Biochem Soc Trans. 40:31–36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chun J, Hla T, Lynch KR, Spiegel S and
Moolenaar WH: International Union of Basic and Clinical
Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature.
Pharmacol Rev. 62:579–587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sedláková I, Vávrová J, Tošner J and
Hanousek L: Lysophosphatidic acid (LPA) - a perspective marker in
ovarian cancer. Tumour Biol. 32:311–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun K, Cai H, Duan X, Yang Y, Li M, Qu J,
Zhang X and Wang J: Aberrant expression and potential therapeutic
target of lysophosphatidic acid receptor 3 in triple-negative
breast cancers. Clin Exp Med. 15:371–380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun K, Duan X, Cai H, Liu X, Yang Y, Li M,
Zhang X and Wang J: Curcumin inhibits LPA-induced invasion by
attenuating RhoA/ROCK/MMPs pathway in MCF7 breast cancer cells.
Clin Exp Med. 16:37–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kitayama J, Shida D, Sako A, Ishikawa M,
Hama K, Aoki J, Arai H and Nagawa H: Over-expression of
lysophosphatidic acid receptor-2 in human invasive ductal
carcinoma. Breast Cancer Res. 6:R640–R646. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bos R, van der Groep P, Greijer AE,
Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ and van
der Wall E: Levels of hypoxia-inducible factor-1alpha independently
predict prognosis in patients with lymph node negative breast
carcinoma. Cancer. 97:1573–1581. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Span PN and Bussink J: Biology of hypoxia.
Semin Nucl Med. 45:101–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fransén K, Fenech M, Fredrikson M,
Dabrosin C and Söderkvist P: Association between ulcerative growth
and hypoxia inducible factor-1alpha polymorphisms in colorectal
cancer patients. Mol Carcinog. 45:833–840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Putra AC, Tanimoto K, Arifin M and Hiyama
K: Hypoxia-inducible factor-1α polymorphisms are associated with
genetic aberrations in lung cancer. Respirology. 16:796–802. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amelio I and Melino G: The ‘Sharp’ blade
against HIF-mediated metastasis. Cell Cycle. 11:4530–4535. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piccolo S, Enzo E and Montagner M: p63,
Sharp1, and HIFs: Master regulators of metastasis in
triple-negative breast cancer. Cancer Res. 73:4978–4981. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bernaudin M, Tang Y, Reilly M, Petit E and
Sharp FR: Brain genomic response following hypoxia and
re-oxygenation in the neonatal rat. Identification of genes that
may contribute to hypoxia-induced ischemic tolerance. J Biol Chem.
277:39728–39738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Badowska-Kozakiewicz AM, Budzik MP and
Przybylski J: Hypoxia in breast cancer. Pol J Pathol. 66:337–346.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huanna T, Tao Z, Xiangfei W, Longfei A,
Yuanyuan X, Jianhua W, Cuifang Z, Manjing J, Wenjing C, Shaochuan
Q, et al: GALNT14 mediates tumor invasion and migration in breast
cancer cell MCF-7. Mol Carcinog. 54:1159–1171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi JW, Herr DR, Noguchi K, Yung YC, Lee
CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, et al: LPA
receptors: Subtypes and biological actions. Annu Rev Pharmacol
Toxicol. 50:157–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W,
Zhang F, Yu S, Stephens LC, Cui X, Murrow G, et al: Expression of
autotaxin and lysophosphatidic acid receptors increases mammary
tumorigenesis, invasion, and metastases. Cancer Cell. 15:539–550.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)α: Its protein stability and
biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Birner P, Gatterbauer B, Oberhuber G,
Schindl M, Rössler K, Prodinger A, Budka H and Hainfellner JA:
Expression of hypoxia-inducible factor-1 alpha in
oligodendrogliomas: Its impact on prognosis and on neoangiogenesis.
Cancer. 92:165–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu H, Forbes RA and Verma A:
Hypoxia-inducible factor 1 activation by aerobic glycolysis
implicates the Warburg effect in carcinogenesis. J Biol Chem.
277:23111–23115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Montagner M, Enzo E, Forcato M, Zanconato
F, Parenti A, Rampazzo E, Basso G, Leo G, Rosato A, Bicciato S, et
al: SHARP1 suppresses breast cancer metastasis by promoting
degradation of hypoxia-inducible factors. Nature. 487:380–384.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bertout JA, Patel SA and Simon MC: The
impact of O2 availability on human cancer. Nat Rev
Cancer. 8:967–975. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen M, Towers LN and O'Connor KL: LPA2
(EDG4) mediates Rho-dependent chemotaxis with lower efficacy than
LPA1 (EDG2) in breast carcinoma cells. Am J Physiol Cell Physiol.
292:C1927–C1933. 2007. View Article : Google Scholar : PubMed/NCBI
|