Introduction
Trifluridine/tipiracil (TFTD) (formerly used with
the code name TAS-102) is a novel antitumor therapeutic agent
(1,2). It comprises a mixture of two distinct
chemicals, trifluridine and tipiracil (TPI), at a molar ratio of
1:0.5. Trifluridine is an analog of thymidine that exhibits two
distinct mechanisms of antitumor action. It inhibits the enzyme
thymidylate synthase (3) and it
intercalates with DNA (4). TPI
enhances the bioavailability of trifluridine by the inhibition of
the in vivo degradation of the latter compound by the enzyme
thymidine phosphorylase. Consequently, TPI can produce a more
durable and sustainable response to trifluridine (5).
The antitumor effects of TFTD on colon cancer
xenograft models resistant to 5-fluorouracil (5-FU) involve notably
the incorporation of trifluridine in DNA (6). The primary cytotoxic mechanism of TFTD
at twice-daily oral dosing (the dose of administration used
clinically) is thought to cause DNA incorporation of trifluridine
(7). The effect of TFTD on
metastatic colorectal cancer in patients who were resistant to,
and/or intolerant of, standard chemotherapies was recently
evaluated in a randomized phase II clinical trial (8). The overall survival (OS) period of the
patients who received TFTD with the best supportive care was
significantly longer than the OS period of patients who received
the corresponding placebo with the best supportive care (8). Furthermore, TFTD significantly
prolonged the OS period (median OS, 7.1 months; 95% CI, 6.5–7.8
months vs. median OS, 5.3 months; 95% CI, 4.6–6 months for placebo)
and progression-free survival (PFS) in patients with metastatic
colorectal cancer refractory to standard chemotherapies, as
demonstrated by an international multi-center randomized
double-blind phase III clinical study (RECOURSE study) (9). In addition, the study indicated that
TFTD exhibited a favorable safety profile. These results led to the
regulatory approval of the drug in the USA and recently, in
Europe.
Tumor angiogenesis is a complex process that
represents a perturbed balance of highly regulated proangiogenic
and antiangiogenic mechanisms (10). Vascular endothelial growth factor
(VEGF) is considered to be one of the most important factors
involved in tumor angiogenesis (11). Bevacizumab is a monoclonal antibody
that blocks angiogenesis by binding to VEGF-A (a ligand for VEGFR1
and VEGFR2). It was the first antiangiogenic agent approved for
cancer therapy. The major challenges to the success of
antiangiogenic therapy include the associated toxicity risks, the
limitation of efficacy through the possible development of
resistance, and the induction or promotion of metastatic
progression (12,13). Nintedanib is an oral triple
angiokinase inhibitor that simultaneously inhibits VEGFs,
platelet-derived growth factor receptors, and fibroblast growth
factor receptor signaling pathways (14). Nintedanib has demonstrated
significant activity against several tumor types in preclinical
studies. An alternating regimen of nintedanib (250 mg, twice daily)
and then afatinib (50 mg, once daily) was evaluated in patients
with advanced pretreated colorectal cancer in a phase II clinical
study (15). The median PFS was 1.9
months and the median OS was 5.5 months. Nintedanib in combination
with mFOLFOX6 showed efficacy as a first-line therapy in a phase II
clinical study that included patients with metastatic colorectal
cancer (16). Furthermore,
nintedanib with mFOLFOX6 exhibited a favorable safety profile
(16,17). A double-blind, randomized, phase III
study of nintedanib vs. placebo in refractory colorectal cancer is
currently ongoing (NCT02149108).
TFTD in combination with irinotecan hydrochloride
(18), oxaliplatin (19), bevacizumab, cetuximab, or
panitumumab (20) exhibited
superior in vivo activity against human colorectal cancer,
including 5-FU-resistant tumors, compared with any of these drugs
alone, as demonstrated by previous studies. In the present study,
the effects of TFTD in combination with nintedanib against human
colorectal tumor xenografts in a nude mouse model were evaluated.
The present study provides additional evidence for the therapeutic
options for human colorectal cancer.
Materials and methods
Reagents
Trifluridine and TPI were obtained from Taiho
Pharmaceutical Co., Ltd. (Tokyo, Japan). Nintedanib was purchased
from Medchem Express (Monmouth Junction, NJ, USA). Hydroxypropyl
methylcellulose (HPMC) was obtained from Shin-Etsu Chemical Co.,
Ltd. (Tokyo, Japan).
Cancer cell lines
The human colon cancer cell line HT-29 was purchased
from the American Type Culture Collection (ATCC, Rockville, MD,
USA). The human colorectal carcinoma DLD-1 and HCT116 cells were
purchased from Dainippon Pharma (Osaka, Japan). The 5-FU-resistant
cell line DLD-1/5-FU was established using a long-term culture in
the presence of 5-FU in vitro (21). These cell lines were cultured in
RPMI-1640 (HT-29, DLD-1 and DLD-1/5-FU) or Dulbecco's modified
Eagle's cell culture media (DMEM) (HCT116) supplemented with 10%
fetal bovine serum (FBS) at 37°C in a humidified atmosphere of 5%
CO2 in air. HT-29 cells possess a wild-type kras
status, whereas DLD-1 and HCT116 cells a mutant.
Animals
Five-week-old male nude mice (BALB/c nu/nu)
were purchased from Clea Japan (Tokyo, Japan) and were housed under
specific pathogen-free conditions, with food and water provided
ad libitum. The procedures of all animal studies were
performed according to the protocol and guidelines of the
Institutional Animal Care and Use Committee of Taiho Pharmaceutical
Co. Ltd. Ethical approval was obtained prior to execution of the
animal experimentation.
Cytotoxicity assay and evaluation of
the combination effect in vitro
The drug cytotoxicity was measured with the crystal
violet assay (22). The cells
(2,000–4,000) were cultured in a 96-well microplate with 100 µl
medium per well for 24 h. Trifluridine and nintedanib were
dissolved at the concentrations of 10 mM in dimethyl sulfoxide and
the corresponding solutions were prepared using the culture medium
under aseptic conditions. A total of 100 µl of the drug solution
(trifluridine: 0.18–10 µM; nintedanib: 0.18–10 µM) were added into
the culture medium. Following incubation of the plates for 72 h,
the culture medium was removed and the cells were fixed with 4%
glutaraldehyde for 30 min. The fixed cells were stained with 0.1%
crystal violet for 2 min and washed and dissolved in 0.05 M
NaH2PO4/50% ethanol. The absorbance was
measured at a wavelength of 540 nm using a microplate reader
(Spectra MAX 190; Molecular Devices, Tokyo, Japan).
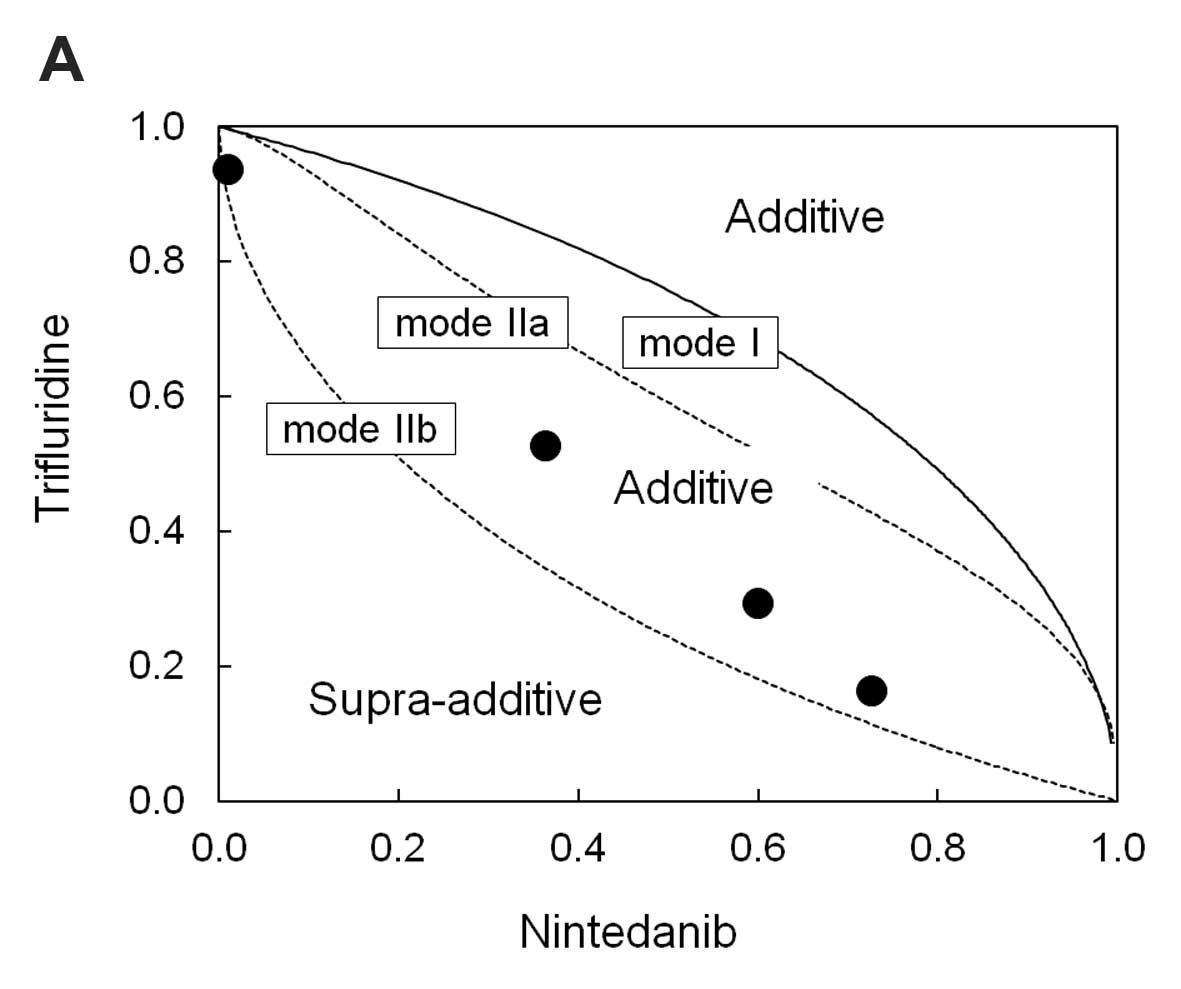
The cytotoxic effects of the trifluridine and
nintedanib combination were analyzed using the isobologram method
(23). A total of 3 isoeffect
curves (modes I, IIa, and IIb), based on the growth inhibition
curves of trifluridine alone and nintedanib alone, were drawn. The
total area enclosed by the three curves represented an ‘envelope of
additivity’. The combination of drug treatment was considered to
show a supra-additive (synergistic) interaction, when the
experimentally observed IC50 values were included in the
left side of the envelope, whereas when the IC50 values
were included in the envelope, the combination was considered as
additive. The combination was considered to be sub-additive, when
the IC50 values were included on the right side of the
envelope and were within the dotted line square. Finally, when the
IC50 values fell outside the square, the combination was
considered to be protective.
In vivo antitumor activity. The cancer cell
lines (DLD-1, DLD-1/5-FU, HT-29, or HCT116) were transplanted
subcutaneously into the dorsal region of each nude mouse at a
density of 4×106 cells/mouse. Following 1 week of cell
growth, the animals were grouped so as to possess a uniform mean
and a standard deviation of the tumor volume (calculated using the
equation below). Each group consisted of 6 mice at day 0.
TFTD was prepared by mixing trifluridine and TPI at
a molar ratio of 1:0.5 in 0.5% HPMC solution. The dose of TFTD was
expressed on the basis of the trifluridine content. TFTD was
administered orally from day 1 to 14, twice a day at 6-h intervals
at the reported effective dose (150 mg/kg/day) (6). Nintedanib was administered orally from
day 1 to 14, twice a day at 6-h intervals at the reported effective
dose (40 mg/kg/day) (14,24). The vehicle solution that consisted
of 0.5% HPMC solution was administered at 10 ml/kg to the control
mouse group, following the same administration schedules as for the
test drugs.
The tumor diameters were measured twice a week, and
the tumor volume (V) was estimated as: V = 0.5 × length ×
width2. The relative tumor volume (RTV) was calculated
using the following formula: RTV = (tumor volume on the measured
day)/(tumor volume on day 0). The tumor growth inhibition ratio
(TGI, %) was calculated using the following formula: TGI (%) = [1 -
(RTV of the treated group)/(RTV of the control group)] × 100 (%).
The antitumor effect of the drugs, based on the RTV measurements,
was evaluated 24 h after the final drug administration (day
15).
The change in the body weight (BWC) was used for the
determination of the toxicity caused by the drug treatments. BWC
was calculated using the following formula: BWC (%) = [(body weight
on the last day) - (body weight on day 0)]/(body weight on day 0) ×
100 (%). Toxicity was defined as a BWC indicating a weight loss of
>20%, or toxic death. The experimental endpoint was defined as
the day on which the average tumor volume in the average body
weight within each group reached more than 10%.
Extraction and quantification of
trifluridine incorporated into tumor DNA
TFTD monotherapy and TFTD combined with nintedanib
were administered from day 1 to 14 to the nude mice bearing HT-29
and HCT116 xenografts. On day 15 the mice were sacrificed, the
tumor diameters corresponding to each mouse were measured and the
tumors were stored in liquid nitrogen. The genomic DNA of the HT-29
and HCT116 tumor cells was extracted and purified using a Getpure
DNA kit (Dojindo Molecular Technology, Kumamoto, Japan) following
the manufacturer's instructions. The purified DNA was completely
digested by DNase I and alkaline phosphatase enzymes to the
deoxyribonucleoside level (including trifluridine) according to
previously described methods (25,26).
The samples were then prepared for LC/MS/MS analysis as follows. An
aliquot that consisted of water (10 µl), 1 M hydrochloric acid (10
µl) and internal standard (20 µl) was added to a 100-µl aliquot of
sample. The mixture was extracted with 1 ml of methyl t-butyl ether
followed by centrifugation (15,000 × g, 5°C, 5 min). The
supernatant was dried under nitrogen at 40°C and the residue was
reconstituted with 0.1 ml of mobile phase that consisted of 0.1%
acetic acid/acetonitrile (75/25, v/v). A 5-µl aliquot of the
reconstituted sample was injected into an API 4000 LC/MS/MS system
(AB Sciex, Foster City, CA, USA).
Statistical analysis
The differences in the mean RTV between the treated
and control groups on day 15 were assessed using the Aspin-Welch
two-sided t-test (27). The
combinatorial antitumor effect of TFTD and nintedanib was analyzed
using the Aspin-Welch two-tailed t-test. The statistical
significance was determined at P<0.05 and the P-values were
calculated using EXSUS, ver. 8.1 (CAC Exicare Corp., Osaka, Japan).
The differences in the trifluridine-mediated DNA incorporation
between the trifluridine and nintedanib-treated group and the
trifluridine group were assessed using the Student's one-sided
t-test.
Results
Combination effect of trifluridine and
nintedanib on colorectal cancer cell lines in vitro
The isobologram plots were drawn using three
isoeffect curves (mode I, mode IIa, and mode IIb) based on the 72-h
growth inhibition curves for DLD-1, HT-29, and HCT116 cells
(Fig. 1A-C) with trifluridine or
nintedanib alone. Based on available dose-response curves, we
analyzed the combined effect of the two drugs at the points of
IC50. The IC50 values for trifluridine in
DLD-1, HT-29, and HCT116 cells were 4.3×10−6,
3.8×10−6, and 1.8×10−6 M respectively,
whereas the corresponding IC50 values for nintedanib
were 3.4×10−6, 1.4×10−6 and
2.5×10−6 M, respectively. In the DLD-1 and HT-29 cells,
a 72-h exposure to the combination treatment resulted in an
additive effect (Fig. 1A and B). In
the HCT116 cells the aforementioned combination treatment resulted
in a sub-additive effect (Fig.
1C).
Antitumor efficacy of TFTD/nintedanib
combination therapy in vivo
The in vivo efficacy of TFTD monotherapy,
nintedanib monotherapy, and TFTD and nintedanib combination in
human colorectal cancer xenograft models was evaluated.
Nude mice bearing DLD-1 tumors were treated with 150
mg/kg TFTD, 40 mg/kg nintedanib, or a combination of TFTD and
nintedanib for 14 consecutive days. On day 15, TFTD monotherapy and
nintedanib monotherapy resulted in a significant reduction in tumor
growth in vivo (P<0.01) (Fig.
2A). In addition, the combination therapy exhibited greater
antitumor activity than both monotherapies.
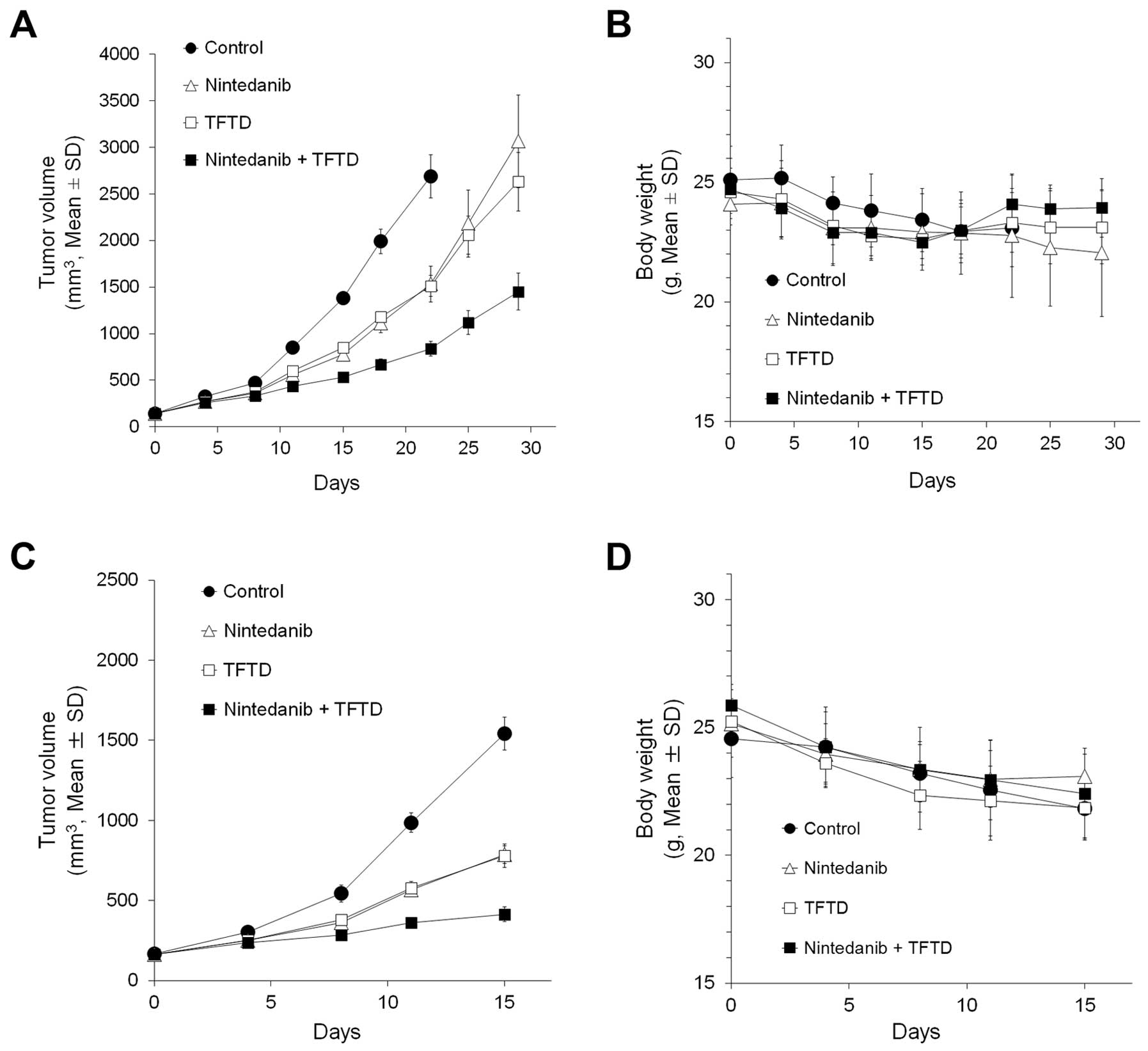 | Figure 2.DLD-1 (A and B), DLD-1/5-FU (C and
D), HT-29 (E and F), and HCT116 (G and H) cancer cells were
transplanted subcutaneously into the dorsal region of nude mice.
Tumor volume change in human colorectal tumors (A, C, E and G) and
body weight change in tumor-bearing nude mice (B, D, F and H). The
mice were treated with vehicle (0.5% HPMC, 10 ml/kg, orally twice
daily from days 1 to 14, ●), nintedanib (40 mg/kg, orally twice
daily from days 1 to 14, △), TFTD (150 mg/kg, orally twice daily
from days 1 to 14, ◻), or a combination of TFTD and nintedanib, ◼).
The values indicate the mean ± SD (n=6). The tumor volume and body
weight were measured twice weekly. |
The efficacy of the aforementioned treatments was
evaluated in nude mice bearing tumors that were derived from
5-FU-resistant human colorectal cancer cells, DLD-1/5-FU (Fig. 2C). TFTD monotherapy and nintedanib
monotherapy resulted in a significant reduction in tumor growth
in vivo (P<0.01). The antitumor efficacy of both
monotherapies was similar between the 5-FU-resistant DLD-1 cells
and the parent DLD-1 cells. This indicated that no cross-resistance
had occurred between DLD-1/5-FU and either of the monotherapies.
The TFTD/nintedanib combination therapy exhibited greater antitumor
activity against DLD-1/5-FU compared with the antitumor activity
exhibited by both monotherapies. Thus, the combination therapy
showed a similar antitumor effect against the DLD-1/5-FU (tumor
growth inhibition rate 72.8%) and the DLD-1 (tumor growth
inhibition rate 61.5%) tumors (data not shown).
The efficacy of the above treatments was further
evaluated in the HT-29 (Fig. 2E)
and HCT116 (Fig. 2G) xenograft
models. TFTD and nintedanib monotherapies both significantly
suppressed tumor growth when compared with control (P<0.01). The
combination therapy significantly suppressed tumor growth when
compared to each monotherapy (P<0.01).
Fig. 3 summarizes
the antitumor effects of the administered therapies as evaluated by
the mean RTV at day 15. The antitumor activity of the
TFTD/nintedanib combination therapy, for all human colorectal
cancer xenografts, was significantly greater than that of either
monotherapy (P<0.01).
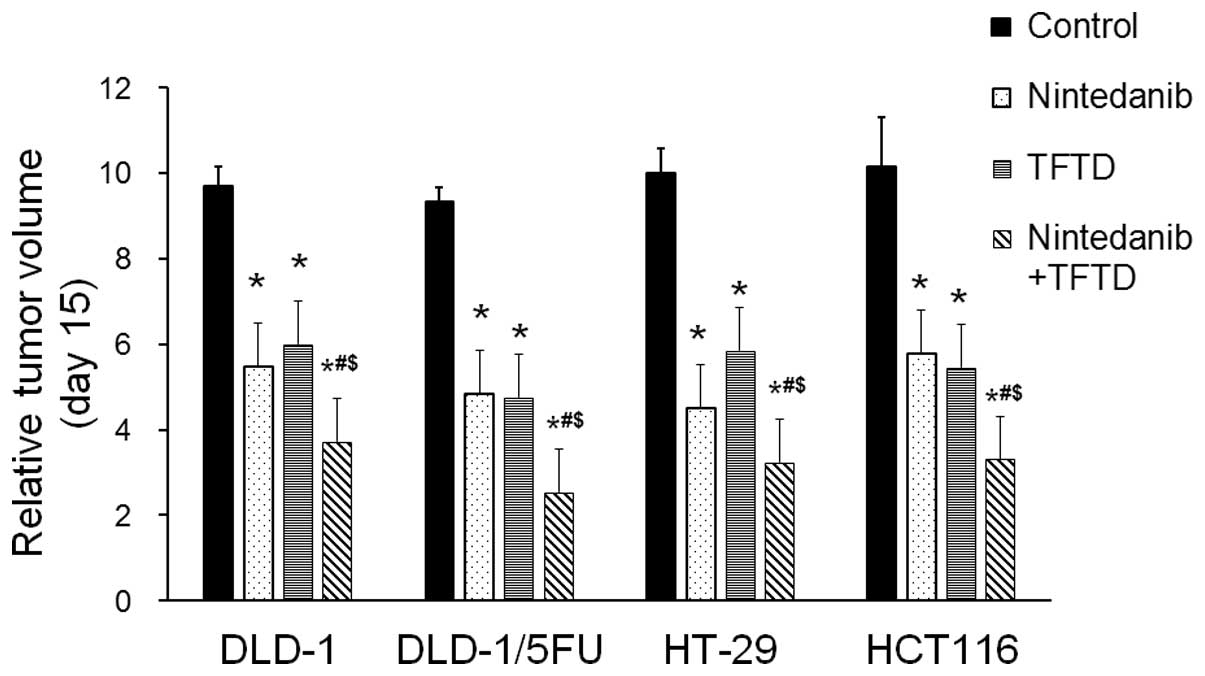 | Figure 3.The relative tumor volume exhibited
in the human colorectal DLD-1, DLD-1/5-FU, HT-29 and HCT116 tumors,
following administration of the drug treatment. The mice were
administered with control (0.5% HPMC, 10 ml/kg, orally twice daily
from days 1 to 14), nintedanib (40 mg/kg, orally twice daily from
days 1 to 14), TFTD (150 mg/kg, orally twice daily from days 1 to
14), or a combination of TFTD and nintedanib (150 mg/kg and 40
mg/kg, respectively. Both therapies were administered orally twice
daily from days 1 to 14). The tumor volumes were measured at 24 h
after the final administration of the therapies (day 15). The
values indicate the mean ± SD (n=6). *P<0.01 vs. the control
group. #P<0.01 vs. the nintedanib monotherapy group.
$P<0.01 vs. the TFTD monotherapy group. |
The tumor volume and body weight of the mice were
monitored following the evaluation of the antitumor effects caused
by the administration of the compounds. The tumor volume of the
drug-free control DLD-1 group exceeded 10% of the body weight loss
of each animal on day 22 (data not shown). All mice in the control
group were immediately euthanized because the tumor burden had
exceeded a human endpoint (the experimental endpoint described in
Materials and methods). The evaluation and monitoring was not
carried out beyond day 15 for any of the drug treatments of DLD-1
(Fig. 2A) and/or the control or
drug treatments of the HT-29, DLD-1/5-FU and HCT116 tumor
xenografts (Fig. 2C-H) since it was
anticipated that, in these cases, the tumor burden would reach the
experimental endpoint.
In the present study no severe adverse events were
noted for all TFTD-treated xenograft models, including a greater
than 20% reduction in the body weight, diarrhea, or death, due to
toxicity. Thus, any potential toxic effects of TFTD were
well-tolerated. Notably, the superior antitumor efficacy of the
TFTD/nintedanib combination was not associated with any significant
increase in body weight loss (Table
I).
 | Table I.BWC in mice implanted with human
colorectal DLD-1, DLD-1/5-FU, HT-29, and HCT116 tumor cells after
treatment with TFTD and nintedanib. |
Table I.
BWC in mice implanted with human
colorectal DLD-1, DLD-1/5-FU, HT-29, and HCT116 tumor cells after
treatment with TFTD and nintedanib.
|
|
| Tumors |
|---|
|
|
|
|
|---|
| Groups | Dose(mg/kg) | DLD-1 | DLD-1/5-FU | HT-29 | HCT116 |
|---|
| Control |
| -6.7±3.6 | -11.1±2.2 | -6.0±3.2 | -7.5±5.8 |
|
| Nintedanib | 40 |
-4.9±4.8ns |
-8.0±4.8ns |
-4.8±4.0ns |
-5.2±6.8ns |
| TFTD | 150 |
-7.8±6.2ns |
-13.5±3.5ns |
-15.4±5.8ns |
-17.3±5.2ns |
| Combination | 40+150 |
-9.0±2.4ns |
-13.5±4.4ns |
-14.4±3.8ns |
-16.3±5.1ns |
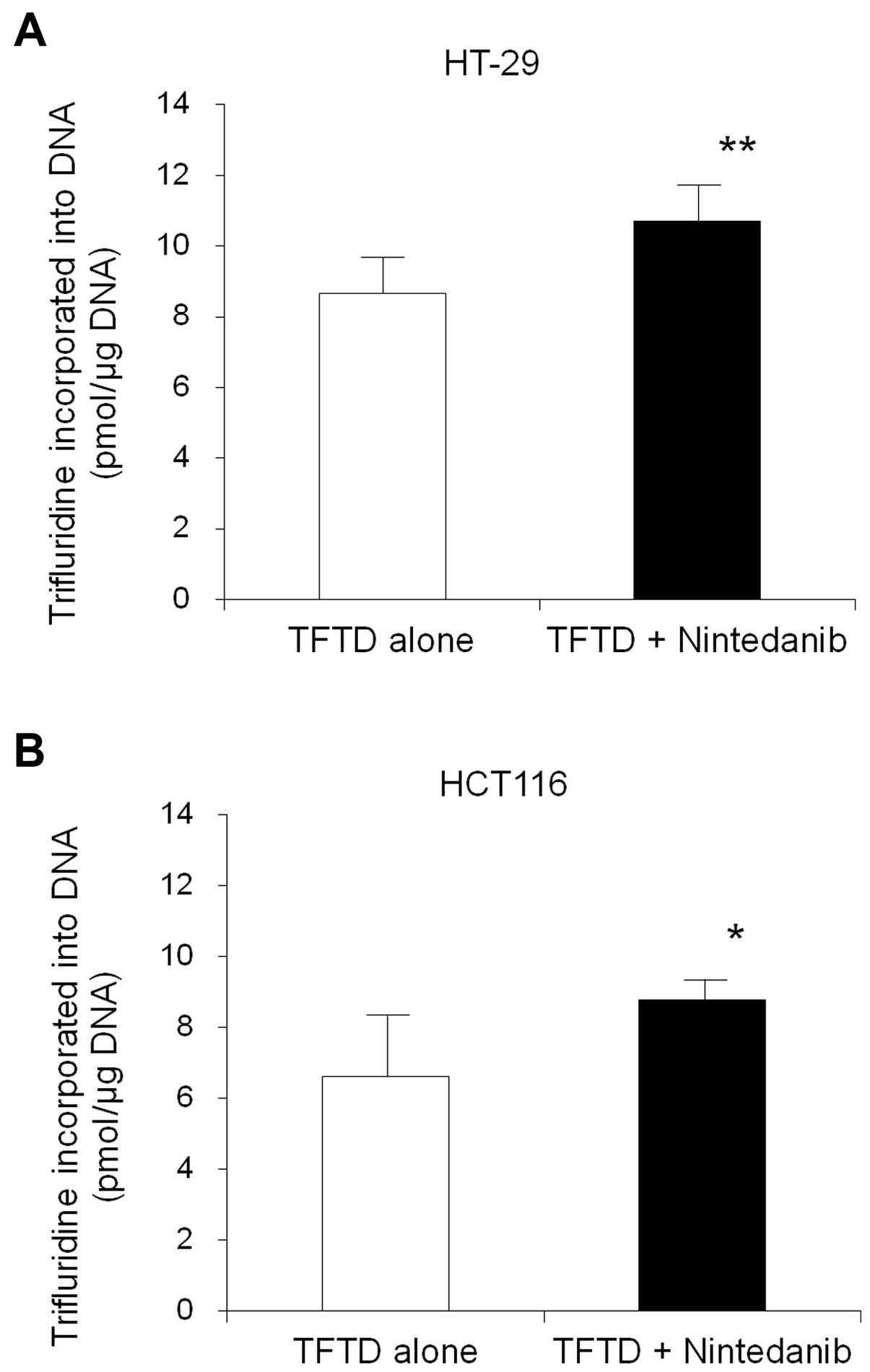
Trifluridine-mediated DNA incorporation in HT-29 and
HCT116 tumors after TFTD/nintedanib combination therapy. The amount
of trifluridine that was incorporated into the DNA of HT-29 and
HCT116 tumors that had been exposed to both treatments, was
assessed, in order to provide insight in the mechanism underlying
the efficacy noted by the TFTD/nintedanib combination therapy,
compared with the TFTD monotherapy. The incorporation of
trifluridine following treatment with TFTD alone for 14 consecutive
days was measured as 8.7±1.0 and 6.6±1.7 (pmol/µg DNA) in the HT-29
and HCT116 tumors, respectively. The corresponding values for the
TFTD/nintedanib treatment was 10.7±1.0 and 8.8±0.6 (pmol/µg DNA),
respectively (Fig. 4). These values
were significantly higher than those of the TFTD monotherapy
group.
Discussion
The present study evaluated i) the antitumor effects
of trifluridine, the antineoplastic agent of TFTD
(trifluridine/tipiracil mixture), in combination with nintedanib on
human colorectal tumors in vitro and ii) the antitumor
effects of TFTD in combination with nintedanib on human colorectal
tumors in vivo. The combination of trifluridine and
nintedanib exerted an additive effect on the growth inhibition of
DLD-1 and HT-29 cells, and a sub-additive effect on the growth
inhibition of HCT116 cells in vitro. The exact cause of the
sub-additive effect noted in the HCT116 cells is unknown. However,
the TFTD/nintedanib combination therapy was superior to the drug
monotherapies. In addition, the TFTD/nintedanib combination therapy
suppressed the growth of the HT-29, HCT116, DLD-1 and DLD-1/5-FU
cells, in nude mice. This suppression was significantly greater
than the effects of the monotherapies. Notably, this antitumor
activity occurred in the absence of any increased toxicity.
A total of three of the colorectal cancer cell lines
used in the present study carry a KRAS mutation (HT-29 DLD-1 and
HCT116). In the present study, the trifluridine and nintedanib
monotherapies and the TFTD/nintedanib combination therapy indicated
a similar anticancer activity in vitro and in vivo,
irrespective of the KRAS status of the colorectal cancer
cell lines (Figs. 1A-C and 2A, E and G). TFTD has been shown to
improve overall survival in a clinical setting regardless of
KRAS tumor status that is consistent with these results
(8,9), Consequently, the combination therapy
with TFTD and nintedanib may also be useful in the clinical
treatment of colorectal tumors irrespective of the KRAS
mutation status.
DLD-1/5-FU is a 5-FU-resistant clone of the DLD-1
cell line. It was developed by repeated 5-day exposures of
stepwise-increasing concentrations of 5-FU in vitro
(21). The mechanism of tumor cell
resistance to 5-FU is thought to involve reduced incorporation of
5-FU into RNA. In a study conducted by our group (6), TFTD indicated a dose-dependent effect
against DLD-1/5-FU and parent DLD-1 tumors in vivo, whereas
the efficacy of the drug administration between the two cell lines
was similar (tumor growth inhibition rate 73.2% at 150 mg/kg/day
for DLD-1/5-FU vs. 73.4% at 150 mg/kg/day for DLD-1). This result
is consistent with the findings noted in the present study.
Trifluridine exhibits higher resistance to the
enzyme DNA glycosylase than 5-FU (28) and its incorporation into DNA induces
instability of the DNA (29). In
the present study, we showed that TFTD/nintedanib combination
therapy was more effective than TFTD and/or nintedanib monotherapy
in the DLD-1/5-FU cancer xenografts and that no cross-resistance
occurred in the DLD-1/5-FU xenografts following administration of
the drug therapy. As a result, the combination therapy with TFTD
and nintedanib may be considered a promising option for the
patients suffering from cancer that is refractory to 5-FU-based
therapy.
The incorporation of trifluridine in the DNA in
HT-29 and HCT116 tumors following treatment with TFTD combined with
nintedanib for 14 consecutive days was higher than that observed
for TFTD monotherapy. In a previous study (20), the combination of TFTD with
bevacizumab was shown to increase the antitumor efficacy and the
levels of the phosphorylated trifluridine in SW48 and HCT116
tumors. Jain proposed that blood vessels in tumors are structurally
and functionally abnormal (30). An
imbalance of proangiogenic and antiangiogenic factors produces
irregular and leaky blood vessels. These blood vessels are poorly
structured and hyper-permeable. They cause increased interstitial
fluid pressure (hydrostatic and colloid osmotic pressures) in most
tumors (31). This process can
potentially limit the accumulation of trifluridine in tumors.
Nintedanib and bevacizumab inhibit angiogenesis by the competitive
inhibition/antagonism of VEGF and the modulation of the tumor
vasculature. The latter improves tumor blood supply and increases
trifluridine accumulation and phosphorylation in tumors.
Furthermore, this hypothesis is in agreement with the results
demonstrated in the present study, as the combination of
trifluridine and nintedanib indicated a similar antitumor efficacy
between HT-29 and HCT116 xenografts in vivo, irrespective of
the mode of drug action.
In the present study nintedanib monotherapy and
nintedanib with TFTD combination therapy exhibited potent antitumor
activity against HT-29 colorectal tumor cells. This antitumor
potency was comparable to the effects noted on DLD-1 cells
(Fig. 2A and E). It has been
demonstrated that HT-29 cells display intrinsically higher HIF-VEGF
signaling intensity and hypoxia tolerance than DLD-1 cells
(32). Nintedanib, unlike
bevacizumab, may attenuate the survival signaling that usually
protects these cells from hypoxia-mediated cell death. Hypoxia is
thought to play an important role in the malignant progression of
colorectal cancer (33,34). Nintedanib has shown an increased
cytotoxicity for bevacizumab-resistant HT-29 cells under hypoxia
(32). Similarly, TFTD/nintedanib
combination therapy may exert potent antitumor activity against
advanced colorectal tumors.
In the present study the combination of TFTD and
nintedanib exhibited superior antitumor efficacy than the TFTD or
nintedanib monotherapies. The preclinical findings presented in the
study indicate that the combination therapy with TFTD and
nintedanib is a promising treatment option for a range of
colorectal cancers. A clinical study evaluating the combined TFTD
and nintedanib therapy is ongoing (no. UMIN000017114) and it is
expected that the outcome of the trial will be highly
informative.
Glossary
Abbreviations
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
HIF
|
hypoxia inducible factor
|
|
BWC
|
body weight change
|
|
OS
|
overall survival
|
|
LC-MS/MS
|
liquid chromatography-tandem mass
spectrometry
|
|
PFS
|
progression-free survival
|
|
RTV
|
relative tumor volume
|
|
TGI
|
tumor growth inhibition
|
|
TFTD
|
trifluridine and tipiracil
|
|
TPI
|
tipiracil
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Heidelberger C, Birnie GD, Boohar J and
Wentland D: Fluorinated pyrimidines. XX. Inhibition of the
nucleoside phosphorylase cleavage of 5-fluoro-2′-deoxyuridine by
5-trifluoromethyl-2′-deoxyuridine. Biochim Biophys Acta.
76:315–318. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gottschling H and Heidelberger C:
Fluorinated pyrimidines. XIX some biological effects of
5-trifluoromthyluracil and 5-trifluoromethyl-2′-deoxyuridine of
Escherichia coli and bacteriophage T4G. J Mol Biol. 7:541–560.
1963. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reyes P and Heidelberger C: Fluorinated
pyrimidines. XXVI. Mammalian thymidylate synthetase: Its mechanism
of action and inhibition by fluorinated nucleotides. Mol Pharmacol.
1:14–30. 1965.PubMed/NCBI
|
|
4
|
Fujiwara Y, Oki T and Heidelberger C:
Fluorinated pyrimidines. XXXVII. Effects of
5-trifluoromethyl-2′-deoxyuridine on the synthesis of
deoxyribonucleic acid of mammalian cells in culture. Mol Pharmacol.
6:273–280. 1970.PubMed/NCBI
|
|
5
|
Fukushima M, Suzuki N, Emura T, Yano S,
Kazuno H, Tada Y, Yamada Y and Asao T: Structure and activity of
specific inhibitors of thymidine phosphorylase to potentiate the
function of antitumor 2′-deoxyribonucleosides. Biochem Pharmacol.
59:1227–1236. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Emura T, Suzuki N, Yamaguchi M, Ohshimo H
and Fukushima M: A novel combination antimetabolite, TAS-102,
exhibits antitumor activity in FU-resistant human cancer cells
through a mechanism involving FTD incorporation in DNA. Int J
Oncol. 25:571–578. 2004.PubMed/NCBI
|
|
7
|
Lenz HJ, Stintzing S and Loupakis F:
TAS-102, a novel antitumor agent: A review of the mechanism of
action. Cancer Treat Rev. 41:777–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshino T, Mizunuma N, Yamazaki K, Nishina
T, Komatsu Y, Baba H, Tsuji A, Yamaguchi K, Muro K, Sugimoto N, et
al: TAS-102 monotherapy for pretreated metastatic colorectal
cancer: A double-blind, randomised, placebo-controlled phase 2
trial. Lancet Oncol. 13:993–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: RECOURSE Study Group: Randomized trial of
TAS-102 for refractory metastatic colorectal cancer. N Engl J Med.
372:1909–1919. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al-Husein B, Abdalla M, Trepte M, Deremer
DL and Somanath PR: Antiangiogenic therapy for cancer: An update.
Pharmacotherapy. 32:1095–1111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moreira IS, Fernandes PA and Ramos MJ:
Vascular endothelial growth factor (VEGF) inhibition - a critical
review. Anticancer Agents Med Chem. 7:223–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rini BI, Michaelson MD, Rosenberg JE,
Bukowski RM, Sosman JA, Stadler WM, Hutson TE, Margolin K, Harmon
CS, DePrimo SE, et al: Antitumor activity and biomarker analysis of
sunitinib in patients with bevacizumab-refractory metastatic renal
cell carcinoma. J Clin Oncol. 26:3743–3748. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason
GA, Christensen JG and Kerbel RS: Accelerated metastasis after
short-term treatment with a potent inhibitor of tumor angiogenesis.
Cancer Cell. 15:232–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hilberg F, Roth GJ, Krssak M, Kautschitsch
S, Sommergruber W, Tontsch-Grunt U, Garin-Chesa P, Bader G, Zoephel
A, Quant J, et al: BIBF 1120: Triple angiokinase inhibitor with
sustained receptor blockade and good antitumor efficacy. Cancer
Res. 68:4774–4782. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bouche O, Maindrault-Goebel F, Ducreux M,
Lledo G, Andre T, Stopfer P, Amellal N, Merger M and De Gramont A:
Phase II trial of weekly alternating sequential BIBF 1120 and
afatinib for advanced colorectal cancer. Anticancer Res.
31:2271–2281. 2011.PubMed/NCBI
|
|
16
|
Van Cutsem E, Prenen H, D'Haens G,
Bennouna J, Carrato A, Ducreux M, Bouché O, Sobrero A, Latini L,
Staines H, et al: A phase I/II, open-label, randomised study of
nintedanib plus mFOLFOX6 versus bevacizumab plus mFOLFOX6 in
first-line metastatic colorectal cancer patients. Ann Oncol.
26:2085–2091. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Awasthi N and Schwarz RE: Profile of
nintedanib in the treatment of solid tumors: The evidence to date.
Onco Targets Ther. 8:3691–3701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nukatsuka M, Nakagawa F, Saito H, Sakata
M, Uchida J and Takechi T: Efficacy of combination chemotherapy
using a novel oral chemotherapeutic agent, TAS-102, with irinotecan
hydrochloride on human colorectal and gastric cancer xenografts.
Anticancer Res. 35:1437–1445. 2015.PubMed/NCBI
|
|
19
|
Nukatsuka M, Nakagawa F and Takechi T:
Efficacy of combination chemotherapy using a novel oral
chemotherapeutic agent, TAS-102, with oxaliplatin on human
colorectal and gastric cancer xenografts. Anticancer Res.
35:4605–4615. 2015.PubMed/NCBI
|
|
20
|
Tsukihara H, Nakagawa F, Sakamoto K,
Ishida K, Tanaka N, Okabe H, Uchida J, Matsuo K and Takechi T:
Efficacy of combination chemotherapy using a novel oral
chemotherapeutic agent, TAS-102, together with bevacizumab,
cetuximab, or panitumumab on human colorectal cancer xenografts.
Oncol Rep. 33:2135–2142. 2015.PubMed/NCBI
|
|
21
|
Murakami Y, Kazuno H, Emura T, Tsujimoto
H, Suzuki N and Fukushima M: Different mechanisms of acquired
resistance to fluorinated pyrimidines in human colorectal cancer
cells. Int J Oncol. 17:277–283. 2000.PubMed/NCBI
|
|
22
|
Saotome K, Morita H and Umeda M:
Cytotoxicity test with simplified crystal violet staining method
using microtitre plates and its application to injection drugs.
Toxicol In Vitro. 3:317–321. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chou TC and Talalay P: Generalized
equations for the analysis of inhibitions of Michaelis-Menten and
higher-order kinetic systems with two or more mutually exclusive
and nonexclusive inhibitors. Eur J Biochem. 115:207–216. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kudo K, Arao T, Tanaka K, Nagai T, Furuta
K, Sakai K, Kaneda H, Matsumoto K, Tamura D, Aomatsu K, et al:
Antitumor activity of BIBF 1120, a triple angiokinase inhibitor,
and use of VEGFR2+pTyr+ peripheral blood
leukocytes as a pharmacodynamic biomarker in vivo. Clin Cancer Res.
17:1373–1381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki N, Nakagawa F, Nukatsuka M and
Fukushima M: Trifluorothymidine exhibits potent antitumor activity
via the induction of DNA double-strand breaks. Exp Ther Med.
2:393–397. 2011.PubMed/NCBI
|
|
26
|
Tanaka N, Sakamoto K, Okabe H, Fujioka A,
Yamamura K, Nakagawa F, Nagase H, Yokogawa T, Oguchi K, Ishida K,
et al: Repeated oral dosing of TAS-102 confers high trifluridine
incorporation into DNA and sustained antitumor activity in mouse
models. Oncol Rep. 32:2319–2326. 2014.PubMed/NCBI
|
|
27
|
Bauer P, Röhmel J, Maurer W and Hothorn L:
Testing strategies in multi-dose experiments including active
control. Stat Med. 17:2133–2146. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki N, Emura T and Fukushima M: Mode of
action of trifluorothymidine (TFT) against DNA replication and
repair enzymes. Int J Oncol. 39:263–270. 2011.PubMed/NCBI
|
|
29
|
Markley JC, Chirakul P, Sologub D and
Sigurdsson ST: Incorporation of 2′-deoxy-5-(trifluoromethyl)uridine
and 5-cyano-2′-deoxyuridine into DNA. Bioorg Med Chem Lett.
11:2453–2455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: A new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heldin CH, Rubin K, Pietras K and Ostman
A: High interstitial fluid pressure - an obstacle in cancer
therapy. Nat Rev Cancer. 4:806–813. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mésange P, Poindessous V, Sabbah M,
Escargueil AE, de Gramont A and Larsen AK: Intrinsic bevacizumab
resistance is associated with prolonged activation of autocrine
VEGF signaling and hypoxia tolerance in colorectal cancer cells and
can be overcome by nintedanib, a small molecule angiokinase
inhibitor. Oncotarget. 5:4709–4721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ioannou M, Paraskeva E, Baxevanidou K,
Simos G, Papamichali R, Papacharalambous C, Samara M and Koukoulis
G: HIF-1α in colorectal carcinoma: Review of the literature. J
BUON. 20:680–689. 2015.PubMed/NCBI
|
|
34
|
Shimomura M, Hinoi T, Kuroda S, Adachi T,
Kawaguchi Y, Sasada T, Takakura Y, Egi H, Okajima M, Tashiro H, et
al: Overexpression of hypoxia inducible factor-1 alpha is an
independent risk factor for recurrence after curative resection of
colorectal liver metastases. Ann Surg Oncol. 20:(Suppl 3).
S527–S536. 2013. View Article : Google Scholar : PubMed/NCBI
|