Introduction
Colorectal cancer is a common disease worldwide and
a leading cause of cancer-associated mortality (1). Epidemiological studies have previously
indicated that colorectal cancer is associated with metabolic
alteration and changes in energy homeostasis (2,3).
Patients with a higher body mass index (BMI) have an increased risk
of colorectal cancer, and the link between obesity and colorectal
carcinogenesis has been demonstrated by meta-analyses (4–6).
Furthermore, tumor cells are associated with altered metabolites,
as demonstrated in HT29 cells (7).
The differential metabolites between colorectal adenoma and
non-adenoma tissues have been previously identified by gas
chromatography and liquid chromatography time-of-flight mass
spectrometry (8), and the
alteration of metabolite profiles, including acylcarnitine,
glycerophospholipids and arginine, may be used as a biomarker for
cancer (9,10).
Arginine, a semi-essential amino acid, mediates
multiple cellular functions, including polyamine and nitric oxide
(NO) synthesis, in various types of cancer. Arginine
supplementation has been shown to increase the risk of colorectal
carcinogenesis (11). Deprivation
of arginine inhibits DNA synthesis and induces cell apoptosis, and
the breakdown of arginine by arginase results in the inhibition of
tumor growth (12–14). However, supplement-mediated arginine
imbalance causes diverse effects on cancer development. L- and
D-arginines were observed to enhance and inhibit tumor growth,
respectively, in a transplantation animal tumor model (15). In addition, the concentration of
arginine was previously demonstrated to be decreased in the plasma
serum of patients with colon, breast and pancreatic cancer
(16), whereas patients with
colorectal cancer who were provided with L-arginine exhibited
reduced crypt cell hyperproliferation, which is a characteristic of
colorectal cancer development (17–19).
In a previous study, N-hydroxy-L-arginine selectively reduced the
proliferation of breast cancer cells with high arginase activity
(20). These studies indicate that
the homeostasis of arginine in cancer requires further
investigation.
Arginine is a substrate for NO synthase (NOS), and
the production of arginine is catalyzed by the enzymes
argininosuccinate synthase (ASS) and argininosuccinate lyase (ASL).
ASS catalyzes the conversion of citrulline and aspartate into
argininosuccinate, which is then converted to arginine and fumarate
by ASL. ASS is highly expressed in ovarian, gastric and colorectal
cancer tissues compared with normal tissues. By contrast, the
expression of ASS is low in melanoma and hepatocellular carcinoma
(21). Downregulation of ASS is
associated with chemosensitivity, and the combination of arginine
deiminase with chemotherapy enhances the antitumorigenic effect
(22). Cancer cells with
ASS-deficiency are dependent on extracellular arginine and the drug
ADI-PEG has been used against melanoma and hepatocellular carcinoma
to deplete the levels of extracellular arginine (23). The pathway of ASS, ASL and NOS
contributes to NO production, which mediates signal transduction
and acts as an anti-oncogenic target for cancer therapy (24). Decreased NO synthesis has previously
been observed in ASS- and ASL-deficient cancer patients with high
plasma citrulline (25). ASL is
required in the formation of the NO synthesis protein complex, and
functions as a key regulator of NO production (26). ASL has been previously reported to
promote cancer cell growth via cyclin A2, and NO supplementation
rescues cell growth inhibition caused by ASL-targeted short hairpin
RNA (shRNA). The induction of ASL by endoplasmic reticulum (ER)
stress, caused by the accumulation of misfolded proteins, has been
observed in liver and breast cancer (27,28).
Activation of intracellular signal transduction by misfolded
proteins, termed the unfolded protein response (UPR), affects cell
proliferation and is associated with the alteration of amino acid
metabolism (29). Targeting ER
stress components, including PKR-like ER kinase, inositol-requiring
1 and activating transcription factor 6, may be a potential
therapeutic approach in cancer (30,31).
The present study aimed to investigate the function
of ASL in colon cancer and to characterize ASL expression in
response to ER stress by exposing colon cancer cells to
tunicamycin. The results demonstrated that ASL is overexpressed in
colon cancer and that its expression is induced by ER stress.
Inhibition of ASL by shRNA decreased colon cancer cell
proliferation in vitro and in vivo, and decreased ASL
levels were found to be associated with autophagy and NO
levels.
Materials and methods
Reagents, chemicals and
antibodies
The following antibodies were utilized in the
present study: anti-ASL (H00000435-M01) (Abnova Corporation,
Taipei, Taiwan), anti-78 kDa glucose-regulated protein (#61097)
(GRP78; BD Biosciences, Erembodegem, Belgium), anti-β-actin
(MAB1501R) (Chemicon, Pittsburgh, PA, USA), anti-GAPDH (#610979)
(GeneTex, Irvine, CA, USA), anti-cyclin A2 (SC-596), CDK4 (SC-601)
and CDK2 (SC-163) (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), anti-cyclin B1 (#1495-S) and cyclin E1 (#1655-S) (Epitomics,
Burlingame, CA, USA), anti-cyclin D1 (#2926-S) (Cell Signaling
Technology, Inc., Danvers, MA, USA) and anti-microtubule-associated
protein 1 light chain 3β (LC3B) antibody (L7543) (Sigma-Aldrich,
St. Louis, MO, USA). The ASL plasmid was purchased from OriGene
Technologies, Inc. (Rockville, MD, USA).
Cell culture
The human colon cancer cell lines HCT116 and SW480,
and the transfectant cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum,
penicillin and streptomycin, at 37°C in an atmosphere of 5%
CO2.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted from cells using TRIzol
reagent (MDBio, Inc., Taipei, Taiwan) and the cDNA was synthesized
using M-MLV reverse transcriptase (Promega Corporation, Madison,
WI, USA). RT-PCR was performed to detect ASL and GAPDH expression
using Pro Taq polymerase (Protech Technology Enterprise Co.,
Ltd., Taipei, Taiwan) on an ABI thermocycler (Applied Biosystems,
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The PCR
conditions were as follows: initial activation for 5 min at 94°C,
followed by cycles of denaturation for 30 sec at 94°C, annealing
for 30 sec at 52°C (for ASL and GRP78) and extension for 30 sec at
72°C, and an end step for 7 min at 72°C. The primer sequences were
as follows: ASL, TGATGCCCCAGAAGAAA AAC and TTTGCGGACCAGGTAATAGG;
GAPDH, TGA AGGTCGGTGTGAACGGATTTGGC and CATGTAGGC CATGAGGTCC
ACCAC.
Western blot analysis
The protein lysates from cells and clinical cancer
tissues were harvested in radioimmunoprecipitation lysis buffer and
35 µg protein was loaded into SDS-PAGE gels. Proteins were then
transferred to a polyvinylidene difluoride membrane following
electrophoretic separation. The membrane was blocked with 5% milk
and probed with specific antibodies. The detection of protein was
visualized with a UVP BioSpectrum imaging system (UVP, LLC, Upland,
CA, USA).
Tissue samples
The clinical colorectal cancer and adjacent normal
tissues were obtained from the National Cheng Kung University
Hospital (Tainan, Taiwan) with approval of the Institutional Review
Board.
SurvExpress analysis
The overall survival data of the colon cancer
patients with high and low ASL expression were obtained from the
SurvExpress database (bioinformatica.mty.itesm.mx/SurvExpress). The
data were identified from the dataset as ‘Colon Metabase Censoring
Under Revision’ with probe 204608.
RNA interference (RNAi) by shRNA
The ASL-targeting shRNA was obtained from the
National RNAi Core Facility (Academia Sinica, Taipei, Taiwan) and
the target sequence was as follows: CACCTTCAAACTGAACTCCAA.
Colony formation assay
The transfectants were plated in 6-well plates and
incubated at 37°C in a 5% CO2 incubator. The number of
colonies was counted after 10 days.
Anchorage-independent growth
ability
Anchorage-independent growth ability was assessed by
soft agar assay. The lower layer contained 0.6% agar in DMEM, and
5×103 cells were mixed with 0.3% agar and plated in the
upper layer. The number of colonies was quantified after incubation
for 14 days at 37°C in a 5% CO2 incubator.
Tumorigenicity in NOD/SCID mice
Male NOD/SCID mice (8-weeks old) were obtained from
the Animal Center of the National Cheng Kung University. The
protocols were approved by the Animal Welfare Committee at National
Cheng Kung University. Transfectant cells (2×106) were
suspended in 0.2 ml DMEM and subcutaneously injected into the
NOD/SCID mice. Tumor growth was monitored for 35 days.
Monodansylcadaverine (MDC) staining of
autophagy
MDC staining was conducted using a Cayman
autophagy/cytotoxicity dual staining kit (Cayman Chemical Co., Ann
Arbor, MI, USA) according to the manufacturer's instructions.
Images were obtained by fluorescence microscopy.
Intracellular arginine content
The concentration of intracellular arginine was
analyzed by high performance liquid chromatography (HPLC) analysis
using Agilent ZORBAX Eclipse AAA column (cat. no. 993400-902;
Agilent Technologies, Inc., Santa Clara, CA, USA).
MTT assay
The transfectants were plated into 24-well plates at
the number of 2×104. The transfectants were incubated
with MTT working solution (1 mg/ml) at 37°C for 4 h and dimethyl
sulfoxide (DMSO) was used to dissolve the crystals. Absorbance was
detected at a wavelength of 590 nm using a spectrophotometer.
Statistical analysis
All results are presented as the mean ± standard
error of the mean. The statistical significance was determined by
t-test using Prism software (GraphPad, Inc., La Jolla, CA,
USA).
Results
ASL expression is induced by ER stress
and is overexpressed in colorectal cancer tissues
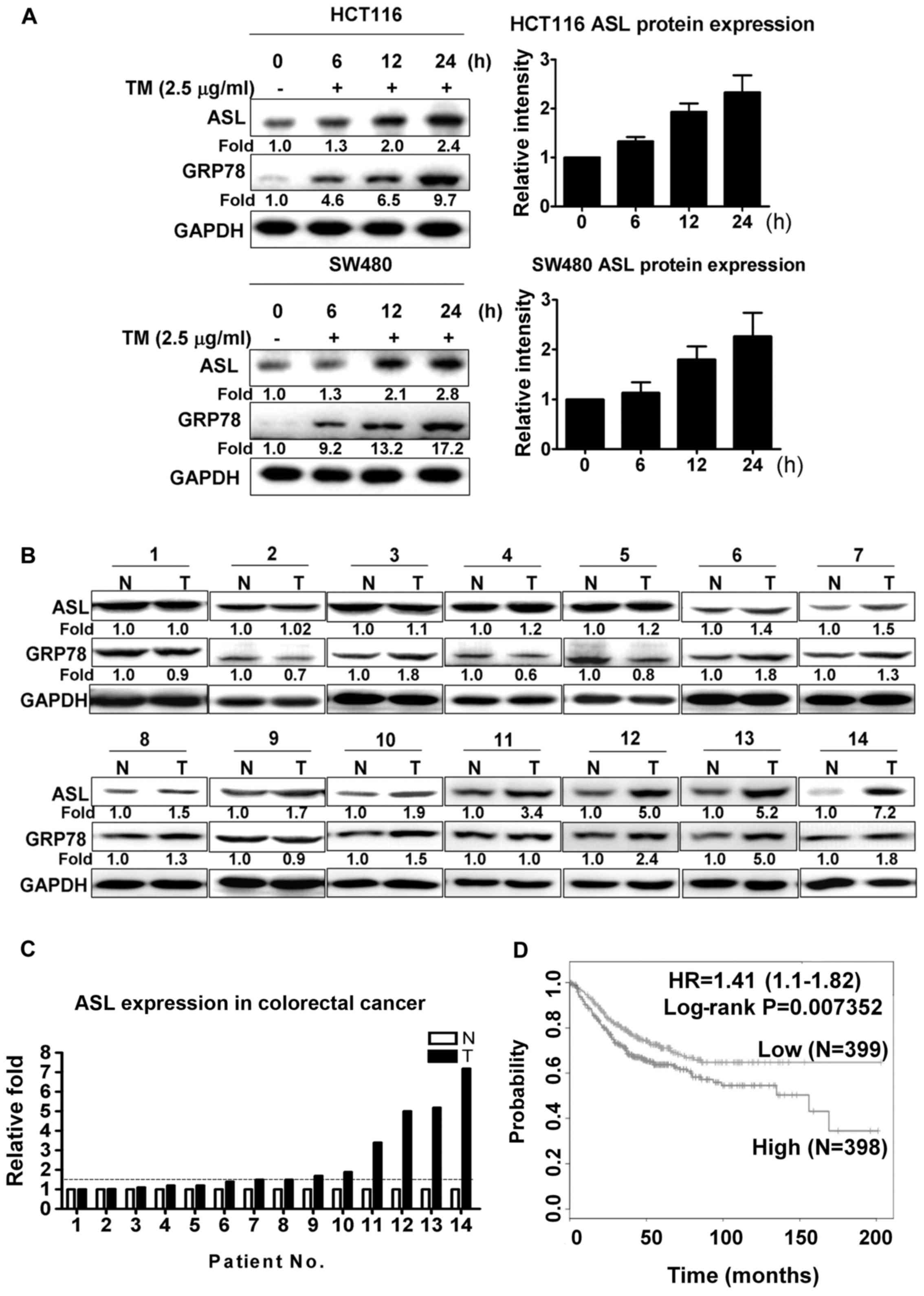
To investigate whether ASL is induced by ER stress,
HCT116 and SW480 colorectal cancer cells were treated with
tunicamycin. As shown by western blot analysis, tunicamycin
significantly increased the ASL expression levels in the HCT116 and
SW480 cells (Fig. 1A). To identify
the association of ASL with the pathological characteristics of
colorectal cancer, ASL protein expression was analyzed in clinical
colon cancer and adjacent normal tissues. Western blot analysis
indicated that the expression level of ASL was significantly
increased in 8 of the 14 paired samples, with the threshold of
increased expression defined as a 1.5-fold change (Fig. 1B and C). To further elucidate the
association of ASL expression with clinical outcome, survival
curves from SurvExpress database were analyzed. Colon cancer
patients with higher ASL expression exhibited reduced survival
rates compared with patients with lower ASL expression (Fig. 1D).
ASL shRNA inhibits cell growth in
colorectal cancer
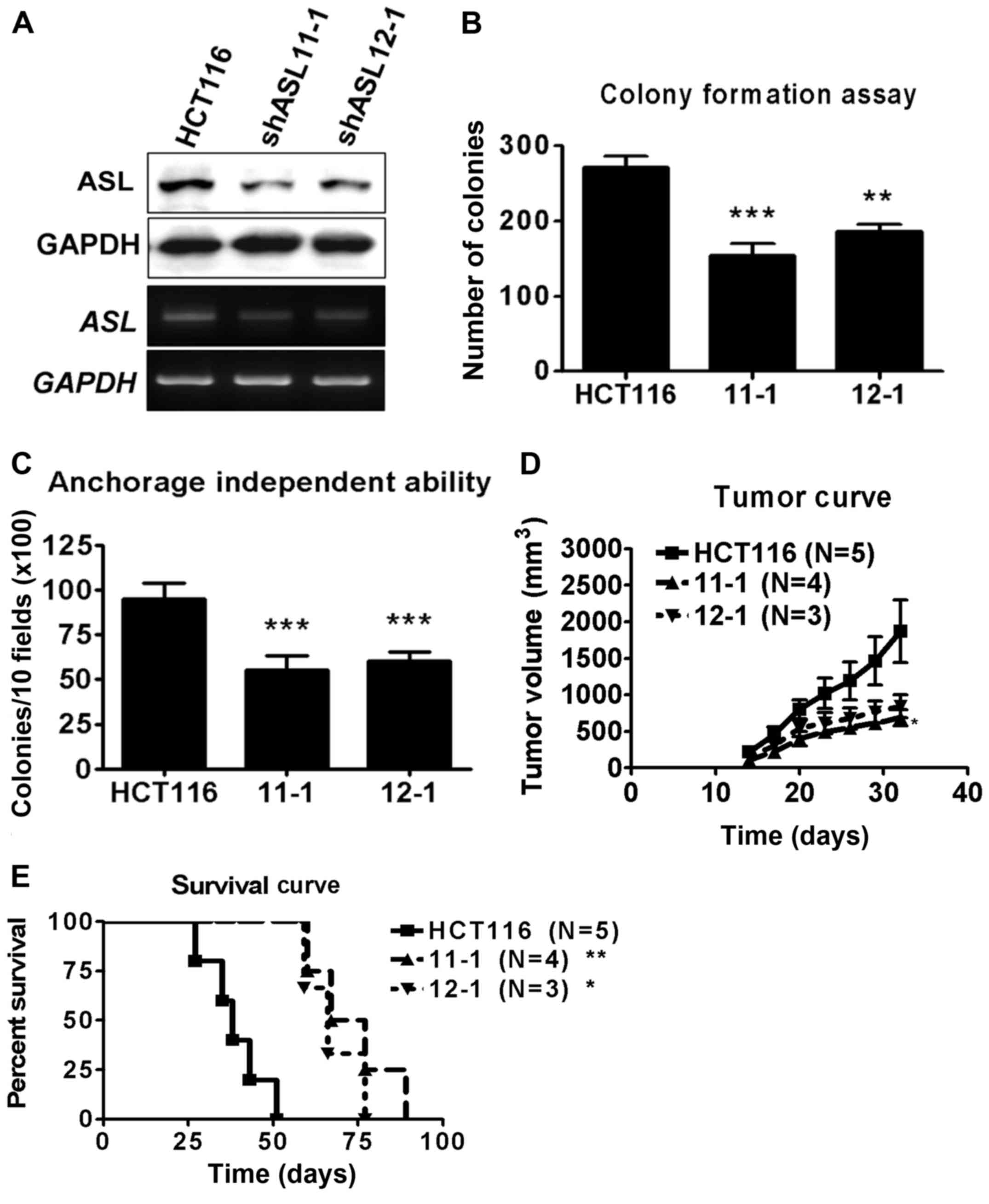
To study the function of ASL in colorectal cancer,
HCT116 cells were transfected with ASL-targeting shRNA, and the
stable transfectant cells were identified by puromycin selection.
The expression level of ASL in the stable transfectants was
examined by western blot and RT-PCR analyses (Fig. 2A). The stable ASL-targeting shRNA
transfectants exhibited decreased anchorage-dependent and
anchorage-independent growth ability compared with the parental
HCT116 cells (Fig. 2B and C). To
determine the effect of ASL on tumorigenic capacity, the
transfectants were subcutaneously injected into NOD/SCID mice.
ASL-targeting shRNA decreased the rate of tumor growth in
vivo (Fig. 2D). In addition,
the NOD/SCID mice transplanted with ASL-knockdown HCT116 cells
demonstrated prolonged survival (Fig.
2E). These data indicate that RNAi of ASL inhibited the
formation of colon cancer.
ASL shRNA decreases cyclin A2
expression in colon cancer
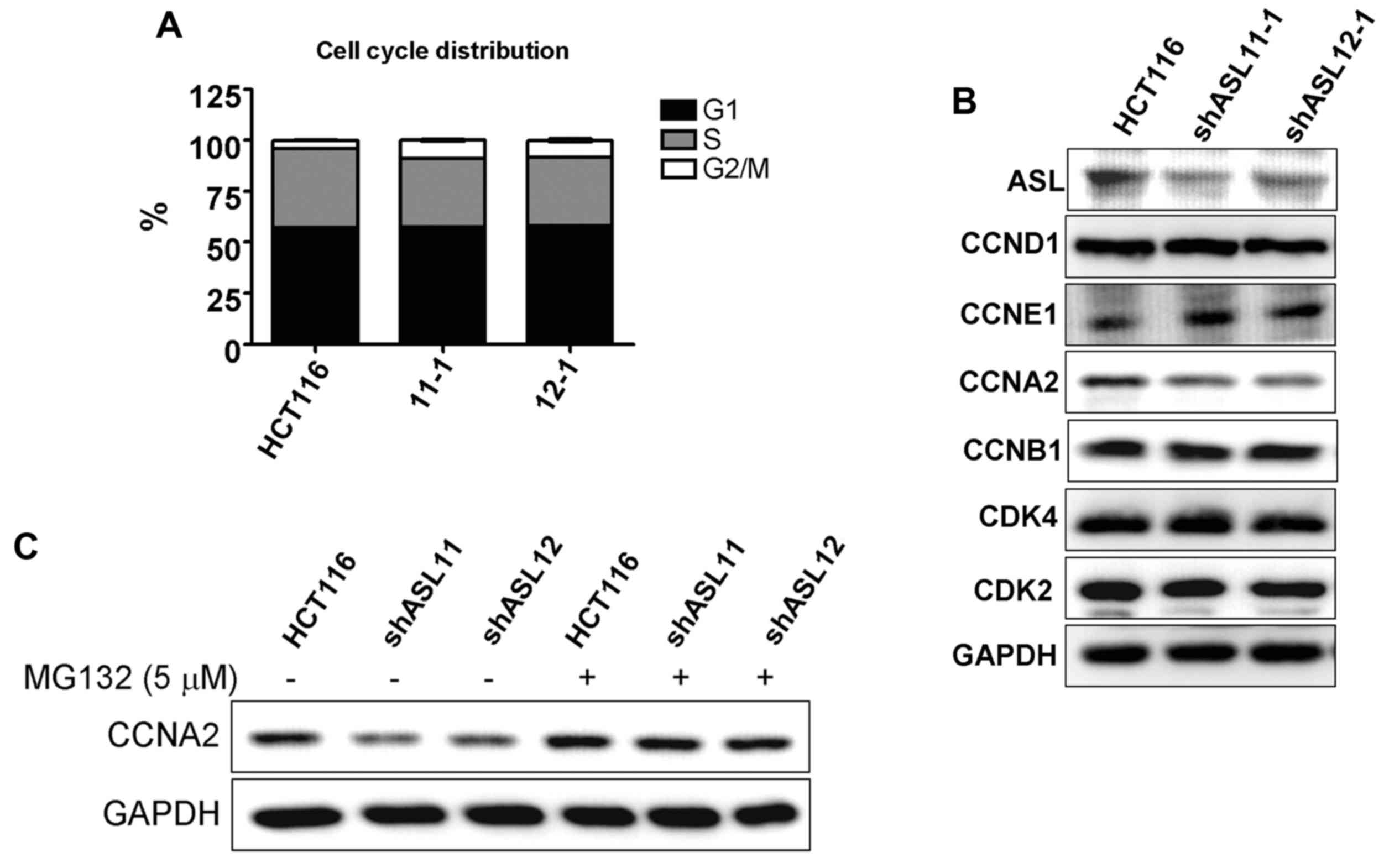
To investigate the mechanism of growth inhibition
induced by ASL-targeted shRNA, cell cycle progression was examined.
Flow cytometric analysis demonstrated that ASL-targeting shRNA
enhanced G2/M phase arrest (Fig.
3A). In addition, western blot analysis demonstrated that
HCT116 transfectants with ASL downregulation exhibited decreased
cyclin A2 expression levels (Fig.
3B). The decrease in cyclin A2 was partially rescued by MG132
proteasome inhibitor treatment, indicating that decreased cyclin A2
may be a result of alterations to degradation (Fig. 3C).
ASL-targeting shRNA induces autophagy
in colon cancer
Autophagy contributes to amino acid maintenance, and
ASL-targeting shRNA has been previously demonstrated to induce
autophagy in liver and breast cancer cells (27,28).
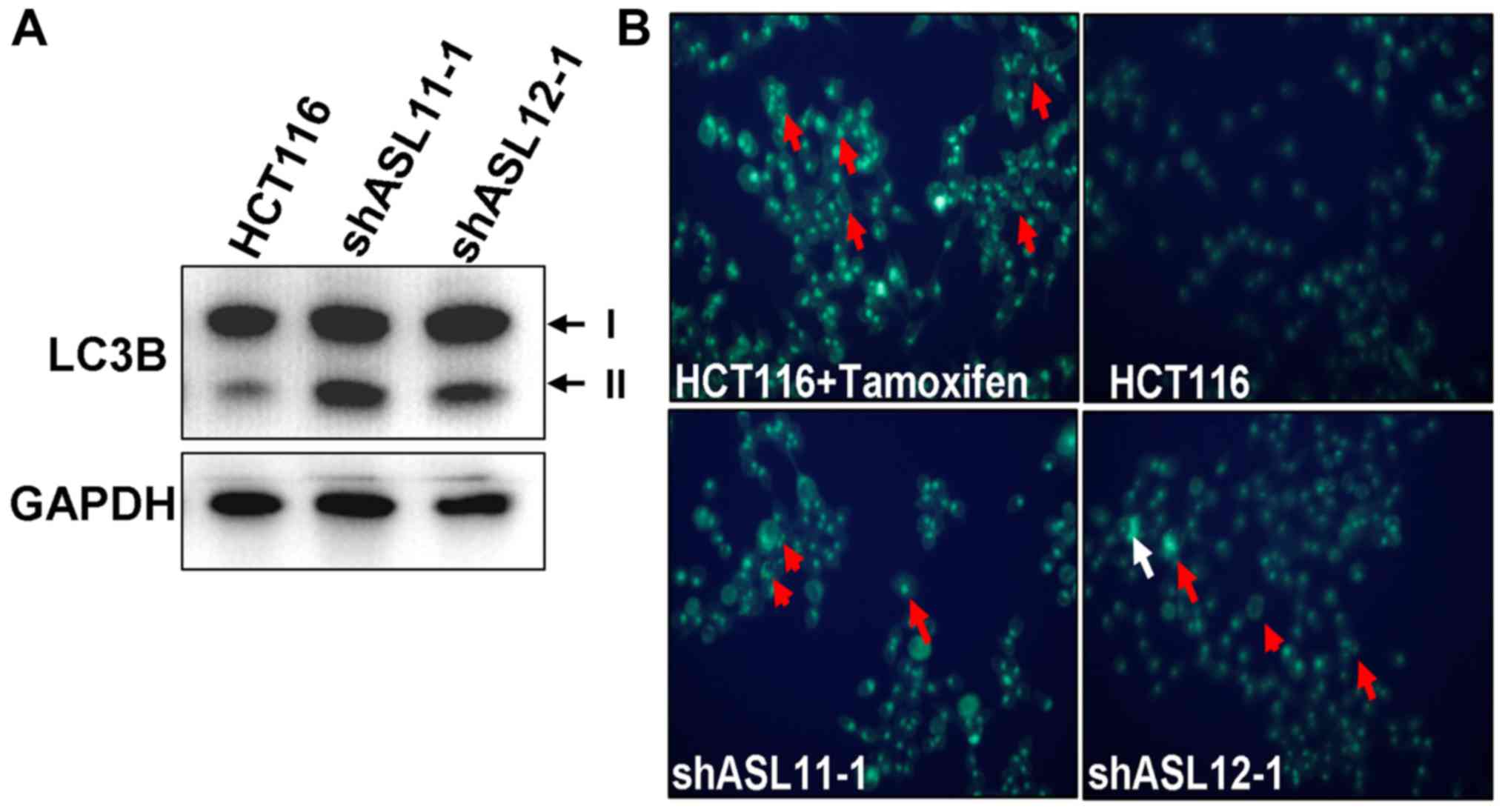
Thus, the present study examined whether ASL-targeting shRNA
induced autophagy in colorectal cancer. Western blot analysis
indicated that ASL-targeting shRNA promoted the conversion of
LC3B-I into LC3B-II (Fig. 4A), and
an MDC staining analysis indicated that the HCT116 ASL-targeting
shRNA transfectants exhibited enhanced autophagosome synthesis
(Fig. 4B). Taken together, these
findings demonstrate that downregulation of ASL is associated with
autophagy in colorectal cancer.
ASL shRNA decreases NO contents in
colon cancer
ASL regulates the regeneration of arginine, which is
a substrate required for NO production. Therefore, the present
study investigated the effect of ASL on arginine and NO production.
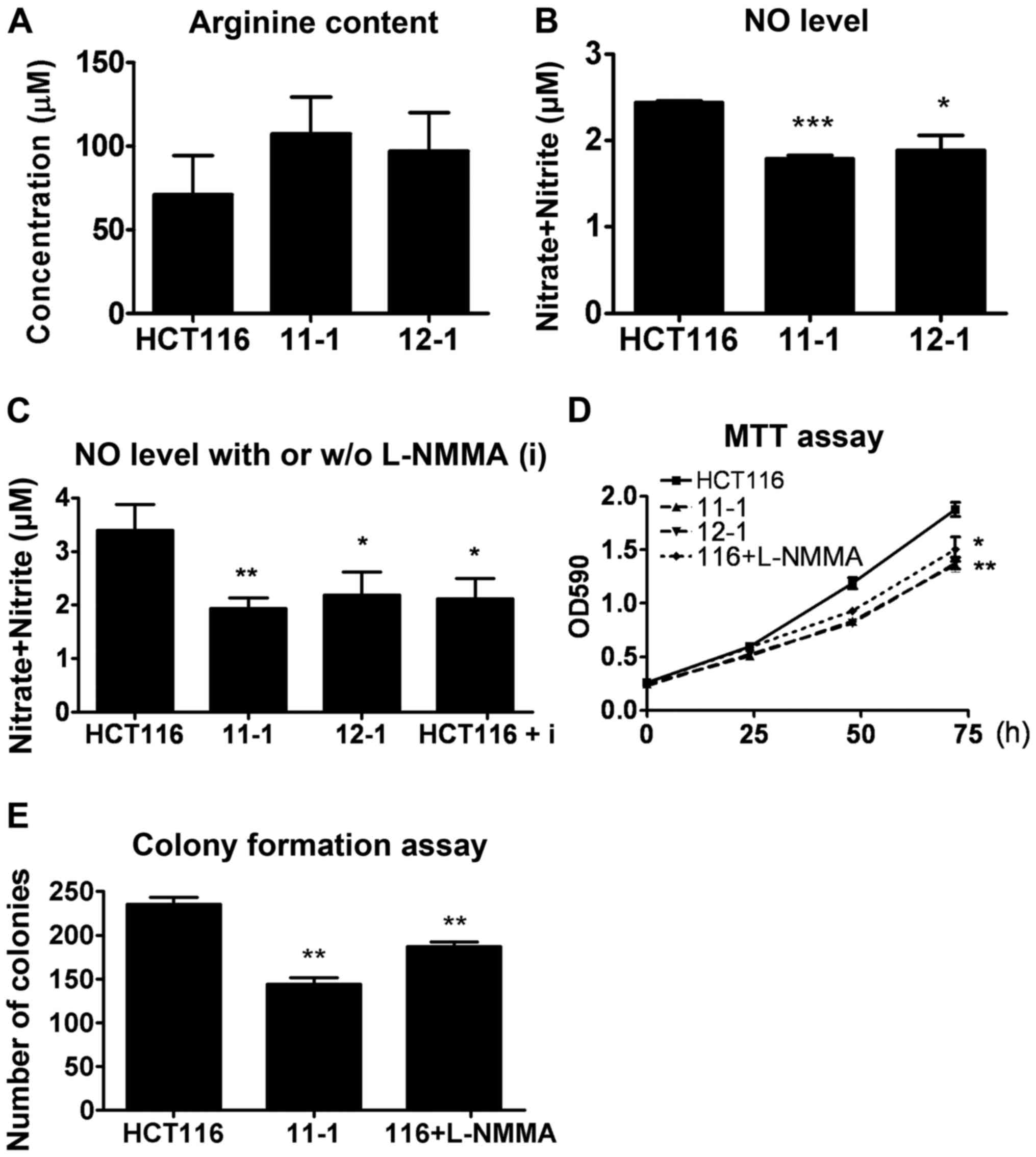
The concentration of arginine in the HCT116 shRNA transfectants was
analyzed by HPLC analysis. This data indicated that ASL-targeting
shRNA did not alter the arginine concentration (Fig. 5A). By contrast, the HCT116
ASL-targeting shRNA transfectants demonstrated decreased NO levels
(Fig. 5B). Furthermore, an
inhibitor of inducible NOS (iNOS), NG-monomethyl-l-arginine
(L-NMMA), significantly reduced NO production, which was similar to
the decrease caused by ASL-targeting shRNA (Fig. 5C). In addition, L-NMMA inhibited the
growth of cancer cells in cell viability and colony formation
assays, suggesting that a reduction in NO mediated by ASL-targeting
shRNA may attenuate tumor growth (Fig.
5D and E).
Discussion
The present study demonstrated that the
argininosuccinate lyase (ASL) expression level was enhanced by ER
stress in colorectal cancer. In addition, the expression of ASL was
increased in colorectal cancer tissues compared with the level
noted in the adjacent normal tissues, and the prognostic analysis
indicated that higher ASL expression was associated with a poor
prognosis for survival. ASL-targeting shRNA inhibited cancer cell
proliferation in vitro and tumor growth in vivo, and
the inhibition of ASL induced cyclin A2 degradation. These data
suggest that the effect of ASL on growth inhibition may be linked
to the degradation of cyclin A2, which results in the G2/M phase
cell cycle arrest. The association between ASL and cyclin A2 in
colorectal cancer is in accordance with previous studies that
demonstrated the effect of ASL on cyclin A2 in liver and breast
cancer (27,28). These findings suggest that ASL may
be a potential therapeutic target in colorectal cancer.
Autophagy is a process of degradation of cellular
components, and is typically activated in response to intracellular
or extracellular stress, including ER stress and starvation
(32). The incidence of colon
cancer is correlated with the intake of various dietary factors
(33), and a previous study
demonstrated the dual effect of autophagy on tumor suppression and
tumor promotion (34). Autophagy
activation is high in colorectal cancer (35), and suppression of autophagy leads to
an antitumor effect following activation of UPR signaling (36). By contrast, autophagy has also been
previously reported to function as a suppressor of cancer
metastasis (37). The present study
demonstrated that ASL-targeting shRNA promoted the conversion of
LC3B-I to LC3B-II and increased autophagosome formation in colon
cancer. Based on the association between ASL and autophagy,
targeting arginine metabolic enzymes and autophagy may be a viable
anticancer treatment approach.
ASL is involved in arginine production, which is
important for protein synthesis and as a substrate for nitric oxide
(NO) production. An increased NO level is observed in various types
of cancer tissue (38). The
induction of NO synthesis by NOS has been previously demonstrated
to promote tumor growth, and it has also been shown that
NO-generating cancer cells increase neovascularization to
facilitate cancer metastasis in vivo (39). However, certain studies have
demonstrated an inhibitory effect of NO in cancer. Transfection of
a melanoma cell line with iNOS cDNA suppressed tumorigenicity and
metastasis (40,41). In addition, the radiation-resistant
HT-29 colon cancer cell line was sensitized to radiation by
treatment with the NO donor DETANONOate, suggesting that NO donors
may have potential as anticancer agents (42). Taken together, these data indicate
that NO exerts a dual protumor and antitumor function during cancer
progression. The present study demonstrated that ASL-knockdown
transfectant cells exhibited decreased NO concentration and reduced
tumorigenic ability compared with the parental cells. This
suggested that inhibition of NO by ASL-targeting shRNA may
contribute to the inhibition of cancer growth. The present study
also demonstrated that the cellular arginine level was not altered
in the ASL-knockdown transfectant cells. A previous study
demonstrated that an increased level of arginine does not alter the
NO concentration; however it does restore the NO inhibition induced
by glutamine in endothelial cells (43). Arginine administration mediates
endothelium-dependent relaxation of blood vessels via NO-dependent
or NO-independent pathways, indicating that arginine has multiple
functions in physiological regulation (44). The underlying mechanism by which ASL
contributes to homeostasis between arginine and NO requires further
investigation.
In conclusion, ASL is overexpressed in colorectal
cancer and the inhibition of ASL decreases tumor growth. Targeting
the metabolic enzyme ASL may be a promising antitumor treatment
strategy.
Acknowledgements
The present study was supported by a grant to M.-D.
Lai, (NSC-100-2325-B-006-008) from the National Science Council
(Taiwan) and (NHRI-EX100-9927B1) from the National Health Research
Institute, Taiwan to establish Centers of Excellence for Cancer
Research in Taiwan, (DOH101-TD-C-111-003) Department of Health,
Executive Yuan (Taiwan).
References
|
1
|
Shike M, Winawer SJ, Greenwald PH, Bloch
A, Hill MJ and Swaroop SV: The WHO Collaborating Centre for the
Prevention of Colorectal Cancer: Primary prevention of colorectal
cancer. Bull World Health Organ. 68:377–385. 1990.PubMed/NCBI
|
|
2
|
Gerber M and Corpet D: Energy balance and
cancers. Eur J Cancer Prev. 8:77–89. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tuominen I, Al-Rabadi L, Stavrakis D,
Karagiannides I, Pothoulakis C and Bugni JM: Diet-induced obesity
promotes colon tumor development in azoxymethane-treated mice. PLoS
One. 8:e609392013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu X, Zhou L, Miao R, Chen W, Zhou Y, Pang
Q, Qu K and Liu C: Association of cancer mortality with
postdiagnosis overweight and obesity using body mass index.
Oncotarget. 7:5023–5029. 2016.PubMed/NCBI
|
|
5
|
Ning Y, Wang L and Giovannucci EL: A
quantitative analysis of body mass index and colorectal cancer:
Findings from 56 observational studies. Obes Rev. 11:19–30. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Renehan AG, Tyson M, Egger M, Heller RF
and Zwahlen M: Body-mass index and incidence of cancer: A
systematic review and meta-analysis of prospective observational
studies. Lancet. 371:569–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galons JP, Fantini J, Vion-Dury J, Cozzone
PJ and Canioni P: Metabolic changes in undifferentiated and
differentiated human colon adenocarcinoma cells studied by
multinuclear magnetic resonance spectroscopy. Biochimie.
71:949–961. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nugent JL, McCoy AN, Addamo CJ, Jia W,
Sandler RS and Keku TO: Altered tissue metabolites correlate with
microbial dysbiosis in colorectal adenomas. J Proteome Res.
13:1921–1929. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kinross JM, Drymousis P, Jiménez B and
Frilling A: Metabonomic profiling: A novel approach in
neuroendocrine neoplasias. Surgery. 154:1185–1193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giskeødegård GF, Hansen AF, Bertilsson H,
Gonzalez SV, Kristiansen KA, Bruheim P, Mjøs SA, Angelsen A, Bathen
TF and Tessem MB: Metabolic markers in blood can separate prostate
cancer from benign prostatic hyperplasia. Br J Cancer.
113:1712–1719. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yerushalmi HF, Besselsen DG, Ignatenko NA,
Blohm-Mangone KA, Padilla-Torres JL, Stringer DE, Guillen JM,
Holubec H, Payne CM and Gerner EW: Role of polyamines in
arginine-dependent colon carcinogenesis in ApcMin/+
mice. Mol Carcinog. 45:764–773. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lamb J and Wheatley DN: Single amino acid
(arginine) deprivation induces G1 arrest associated with inhibition
of cdk4 expression in cultured human diploid fibroblasts. Exp Cell
Res. 255:238–249. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bach SJ and Swaine D: The Effect of
arginase on the retardation of tumour growth. Br J Cancer.
19:379–386. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yeatman TJ, Risley GL and Brunson ME:
Depletion of dietary arginine inhibits growth of metastatic tumor.
Arch Surg. 126:1376–1382. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Szende B, Tyihák E and Trézl L: Role of
arginine and its methylated derivatives in cancer biology and
treatment. Cancer Cell Int. 1:32001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vissers YL, Dejong CH, Luiking YC, Fearon
KC, von Meyenfeldt MF and Deutz NE: Plasma arginine concentrations
are reduced in cancer patients: Evidence for arginine deficiency?
Am J Clin Nutr. 81:1142–1146. 2005.PubMed/NCBI
|
|
17
|
Ma Q, Williamson KE, O'rourke D and
Rowlands BJ: The effects of l-arginine on crypt cell
hyperproliferation in colorectal cancer. J Surg Res. 81:181–188.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma Q, Wang Y, Gao X, Ma Z and Song Z:
L-arginine reduces cell proliferation and ornithine decarboxylase
activity in patients with colorectal adenoma and adenocarcinoma.
Clin Cancer Res. 13:7407–7412. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Motoo Y, Taga K, Su SB, Xie MJ and Sawabu
N: Arginine induces apoptosis and gene expression of
pancreatitis-associated protein (PAP) in rat pancreatic acinar
AR4-2J cells. Pancreas. 20:61–66. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh R, Pervin S, Karimi A, Cederbaum S
and Chaudhuri G: Arginase activity in human breast cancer cell
lines: N(omega)-hydroxy-L-arginine selectively inhibits cell
proliferation and induces apoptosis in MDA-MB-468 cells. Cancer
Res. 60:3305–3312. 2000.PubMed/NCBI
|
|
21
|
Delage B, Fennell DA, Nicholson L, McNeish
I, Lemoine NR, Crook T and Szlosarek PW: Arginine deprivation and
argininosuccinate synthetase expression in the treatment of cancer.
Int J Cancer. 126:2762–2772. 2010.PubMed/NCBI
|
|
22
|
Liu J, Ma J, Wu Z, Li W, Zhang D, Han L,
Wang F, Reindl KM, Wu E and Ma Q: Arginine deiminase augments the
chemosensitivity of argininosuccinate synthetase-deficient
pancreatic cancer cells to gemcitabine via inhibition of NF-κB
signaling. BMC Cancer. 14:6862014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ensor CM, Holtsberg FW, Bomalaski JS and
Clark MA: Pegylated arginine deiminase (ADI-SS PEG20,000
mw) inhibits human melanomas and hepatocellular carcinomas in
vitro and in vivo. Cancer Res. 62:5443–5450. 2002.PubMed/NCBI
|
|
24
|
Huerta S, Chilka S and Bonavida B: Nitric
oxide donors: Novel cancer therapeutics (Review). Int J Oncol.
33:909–927. 2008.PubMed/NCBI
|
|
25
|
Nagasaka H, Tsukahara H, Yorifuji T, Miida
T, Murayama K, Tsuruoka T, Takatani T, Kanazawa M, Kobayashi K,
Okano Y, et al: Evaluation of endogenous nitric oxide synthesis in
congenital urea cycle enzyme defects. Metabolism. 58:278–282. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Erez A, Nagamani SC, Shchelochkov OA,
Premkumar MH, Campeau PM, Chen Y, Garg HK, Li L, Mian A, Bertin TK,
et al: Requirement of argininosuccinate lyase for systemic nitric
oxide production. Nat Med. 17:1619–1626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang HL, Hsu HP, Shieh SC, Chang YS, Chen
WC, Cho CY, Teng CF, Su IJ, Hung WC and Lai MD: Attenuation of
argininosuccinate lyase inhibits cancer growth via cyclin A2 and
nitric oxide. Mol Cancer Ther. 12:2505–2516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang HL, Chen WC, Hsu HP, Cho CY, Hung
YH, Wang CY and Lai MD: Argininosuccinate lyase is a potential
therapeutic target in breast cancer. Oncol Rep. 34:3131–3139.
2015.PubMed/NCBI
|
|
29
|
Harding HP, Zhang Y, Zeng H, Novoa I, Lu
PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al: An
integrated stress response regulates amino acid metabolism and
resistance to oxidative stress. Mol Cell. 11:619–633. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo B and Lee AS: The critical roles of
endoplasmic reticulum chaperones and unfolded protein response in
tumorigenesis and anticancer therapies. Oncogene. 32:805–818. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Alam GN, Ning Y, Visioli F, Dong
Z, Nör JE and Polverini PJ: The unfolded protein response induces
the angiogenic switch in human tumor cells through the PERK/ATF4
pathway. Cancer Res. 72:5396–5406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Willett WC: Diet and cancer: An evolving
picture. JAMA. 293:233–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burada F, Nicoli ER, Ciurea ME, Uscatu DC,
Ioana M and Gheonea DI: Autophagy in colorectal cancer: An
important switch from physiology to pathology. World J Gastrointest
Oncol. 7:271–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sato K, Tsuchihara K, Fujii S, Sugiyama M,
Goya T, Atomi Y, Ueno T, Ochiai A and Esumi H: Autophagy is
activated in colorectal cancer cells and contributes to the
tolerance to nutrient deprivation. Cancer Res. 67:9677–9684. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sakitani K, Hirata Y, Hikiba Y, Hayakawa
Y, Ihara S, Suzuki H, Suzuki N, Serizawa T, Kinoshita H, Sakamoto
K, et al: Inhibition of autophagy exerts anti-colon cancer effects
via apoptosis induced by p53 activation and ER stress. BMC Cancer.
15:7952015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Forbes TA, Hopkins L, Schneider B, Lazarus
L, Leitenberg D, Constant S, Schwartz A, Patierno S and Ceryak S:
Potential role of nitric oxide in chromium-induced lung
carcinogenesis. Cancer Res. 11:(Suppl 8). 54562012. View Article : Google Scholar
|
|
39
|
Jenkins DC, Charles IG, Thomsen LL, Moss
DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC and
Moncada S: Roles of nitric oxide in tumor growth. Proc Natl Acad
Sci USA. 92:4392–4396. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie K, Huang S, Dong Z, Juang SH, Gutman
M, Xie QW, Nathan C and Fidler IJ: Transfection with the inducible
nitric oxide synthase gene suppresses tumorigenicity and abrogates
metastasis by K-1735 murine melanoma cells. J Exp Med.
181:1333–1343. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Juang SH, Xie K, Xu L, Shi Q, Wang Y,
Yoneda J and Fidler IJ: Suppression of tumorigenicity and
metastasis of human renal carcinoma cells by infection with
retroviral vectors harboring the murine inducible nitric oxide
synthase gene. Hum Gene Ther. 9:845–854. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hibbs JB Jr, Taintor RR and Vavrin Z:
Macrophage cytotoxicity: Role for L-arginine deiminase and imino
nitrogen oxidation to nitrite. Science. 235:473–476. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Arnal JF, Münzel T, Venema RC, James NL,
Bai CL, Mitch WE and Harrison DG: Interactions between L-arginine
and L-glutamine change endothelial NO production. An effect
independent of NO synthase substrate availability. J Clin Invest.
95:2565–2572. 1995.
|
|
44
|
Pieper GM: Review of alterations in
endothelial nitric oxide production in diabetes: Protective role of
arginine on endothelial dysfunction. Hypertension. 31:1047–1060.
1998. View Article : Google Scholar : PubMed/NCBI
|