Introduction
Laparoscopy has been widely used in colorectal
surgery, and CO2 is commonly used to create laparoscopic
pneumoperitoneum. However, controversies exist regarding the effect
of CO2 pneumoperitoneum on tumor proliferation and
metastasis. It is believed that CO2 pneumoperitoneum
could potentially promote colon cancer cell proliferation or
metastasis under certain conditions (1–3).
However, the intraperitoneal hyperthermic chemoperfusion (IHCP) was
demonstrated to eradicate free tumor cells and micrometastases,
preventing the peritoneal dissemination of tumors, and is commonly
used as adjuvant therapy for open surgeries of gastric, colon and
ovarian cancers (4). However, the
questions that remain open are how to reduce the adverse influence
of CO2 pneumoperitoneum on the therapeutic effect of
colon cancer surgery, and how to utilize IHCP as the combined
therapy. It was speculated that the therapeutic effect may be
improved by combined therapy of hyperthermic CO2
pneumoperitoneum and intraperitoneal 5-fluorouracil (5-FU)
chemotherapy. In the present study, we investigated the combined
effect of hyperthermic CO2 pneumoperitoneum and 5-FU on
the proliferation and invasion of colon cancer in vitro and
in vivo.
Materials and methods
Cell culture and nude mice
Colon cancer cell line SW-480 was procured from
Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China)
and cultured with L-15 medium containing 10% calf serum in an
incubator at 37°C supplemented with 5% CO2, 20%
O2 and 75% N2. The culture medium was changed
every other day, and the cells at logarithmic growth phase were
used for experiments. Nude mice were Balb/C, male, age, 4–6 weeks;
weight, 18–20 g, total 72, purchased from East China Normal
University Minhang Laboratory Animal Center, raised under the
condition of SPF.
Equipment and machine
L-15 culture medium was obtained from Gibco (Thermo
Fisher Scientific, Waltham, MA, USA), calf serum was from Hangzhou
Tianhang Biological Technology Co., Ltd. (Hangzhou, China) and 5-FU
was from Shanghai Xudong Haipu Pharmaceutical Co., Ltd. (Shanghai,
China). The following reagents or kits were used: cell counting
kit-8 (CCK-8) cytotoxicity analysis kit, Annexin V-FITC apoptosis
detection kit (both from Dojindo Laboratories, Kumamoto, Japan),
KGI cell DNA content detection kit and Transwell detection kit
(Corning, Inc., Corning, NY, USA), TRIzol reagent (Invitrogen,
Carlsbad, CA, USA), RNA extraction kit-RNAiso Plus (Takara, Shiga,
Japan), First Strand cDNA synthesis kit (Thermo Fisher Scientific),
and Maxima SYBR-Green/ROX qPCR master mix (Thermo Fisher
Scientific). The first antibodies were procured from Abcam
(Cambridge, MA, USA), including mouse monoclonal antibody against
heat shock protein-70 (HSP-70) or hypoxia-inducible factor-1α
(HIF-1α), and rabbit monoclonal antibody against mitochondrial
membrane potential-9 (MMP-9) or caspase-3. Polymerase chain
reaction (PCR) primers were designed and synthesized by Sangon
Biotech (Shanghai, China). The equipment included the enzyme-linked
immunosorbent assay reader (Thermo Fisher Scientific), flow
cytometry (BD FACSCalibur; BD Biosciences, Flanklin Lakes, NJ,
USA), western blot electrophoresis (Bio-Rad, Hercules, CA, USA),
and fluorescent-PCR machine (Applied Biosystems, Foster City, CA,
USA).
Cell treatment and experimental
grouping
The disposable 2-L urine collection bag was used to
simulate the pneumoperitoneum. A small cut was made on the lateral
part of the urine collection bag through which a balanced plate was
placed inside and the petri dish or a 96-well plate was attached.
Then, the cut was sealed using a sealer. One port of the urine
collection bag was connected to 100% CO2 gas, and the
other port was connected to a pressure meter to monitor the
pressure of CO2 at 12 mmHg. The temperature was set at
43 and 37°C using a cell culture incubator for 2 h. 5-FU was used
at a concentration of 30 µg/ml [IC50 (5)]. The cells were grouped as follows:
control group (ctrl), cells treated with only CO2
pneumoperitoneum at 37°C (group A), cells treated with hyperthermic
CO2 pneumoperitoneum at 43°C (group B), cells treated
with 5-FU only (group C), cells treated with CO2
pneumoperitoneum at 37°C and 5-FU (group D), and cells treated with
hyperthermic CO2 pneumoperitoneum at 43°C and 5-FU
(group E). The cells were placed back into the normal
incubator.
In vivo tumor establishment and
grouping
The in vivo tumor growth and metastasis assay
was approved by the Ethics Committee of the Fifth People's Hospital
of Shanghai. The SW-480 single cell suspension was injected into
cecum subserosal of Balb/c nude mice to establish in situ
colon cancer nude mouse model according to the experimental methods
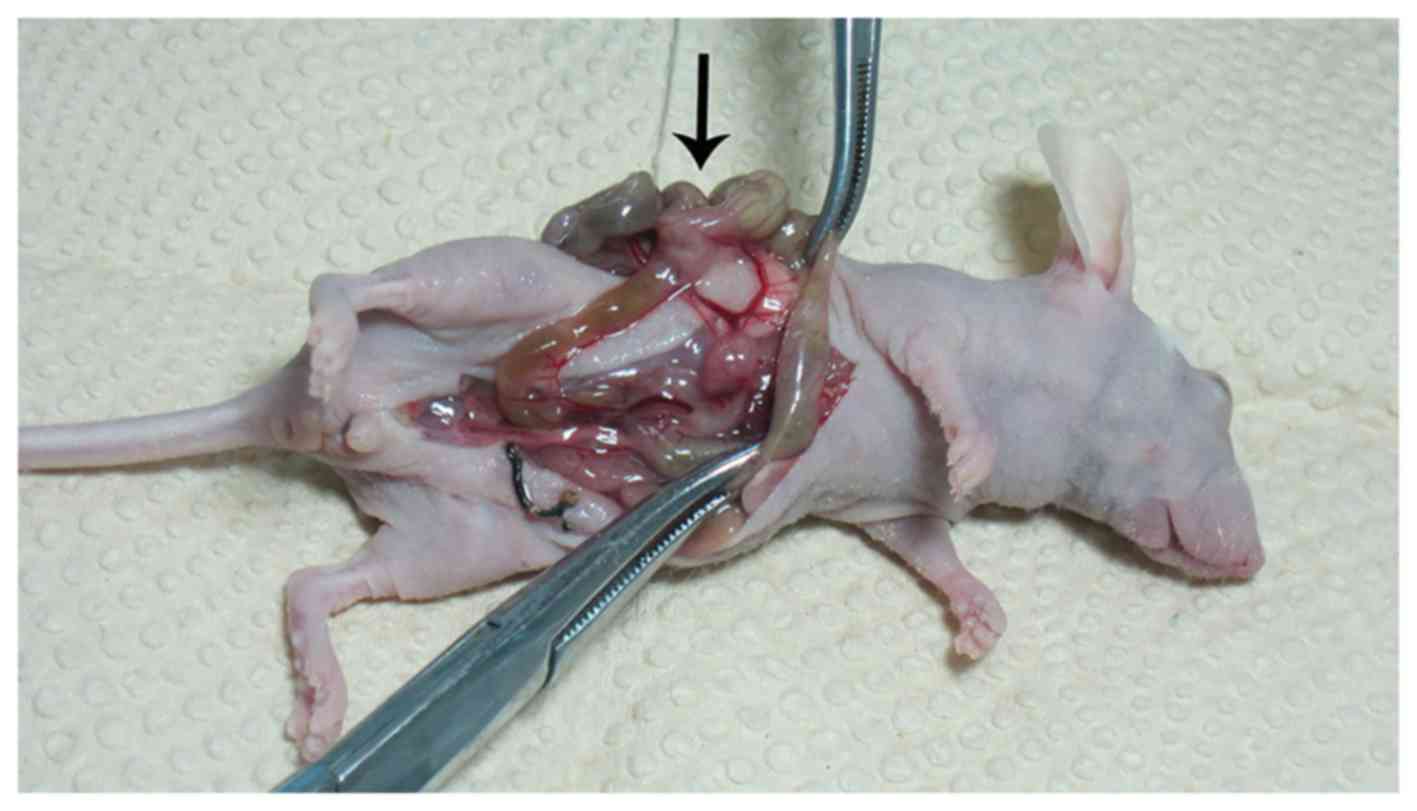
of Zheng et al (6). We used
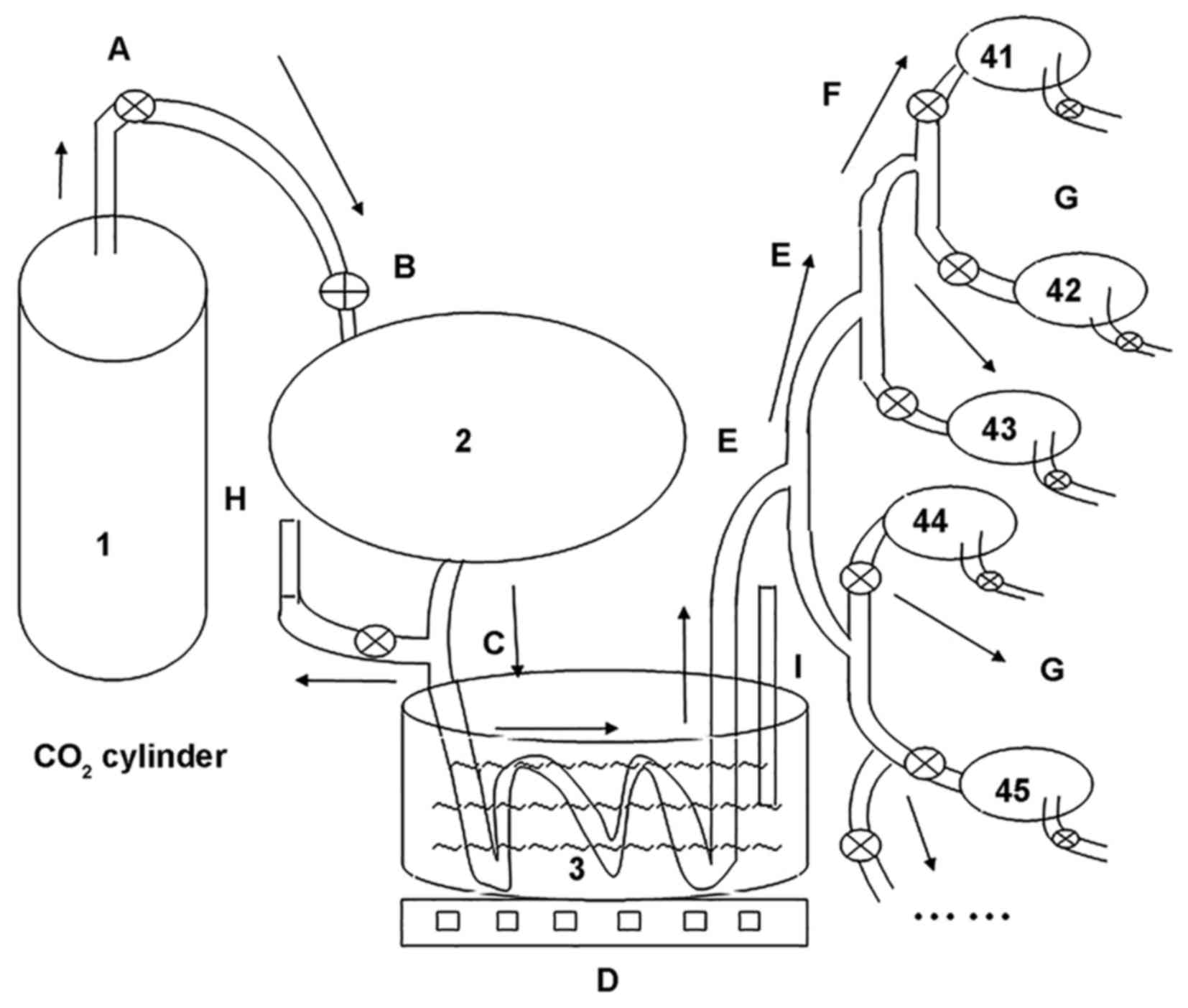
an in-house device for many mice simultaneously to warm in the
CO2 pneumoperitoneum research experiment (Chinese patent
no. ZL201520774334.4) (Fig. 1), we
established different pneumoperitoneum intervention. The model mice
were grouped as follows: control group (ctrl), CO2
pneumoperitoneum at 37°C (group A), hyperthermic CO2
pneumoperitoneum at 43°C (group B), 5-FU (group C), CO2
pneumoperitoneum at 37°C and 5-FU (group D), and hyperthermic
CO2 pneumoperitoneum at 43°C and 5-FU (group E). Each
group had 12 mice.
Proliferation and morphology of
cells
The cells were seeded onto the 6-well plate at a
density of 5×105/well in 2 ml. The cells from each group
were observed under the microscope and photographed at 12, 24, 36,
48, 60 and 72 h, respectively, after treatment. The experiment was
repeated three times.
Cell proliferation inhibition
detection by CCK-8 test
The cells (1×104/well) at logarithmic
growth phase were seeded onto the 96-well plate and cultured for 24
h. The cells of each well were treated separately and continuously
cultured for 12, 24, 36, 48, 60 and 72 h, respectively. CCK-8 (10
µl) was added to each well for 4 h, avoiding light. The optical
density value at 450 nm was measured to determine the number of
viable cells. The triplicate wells were used to calculate the
average, and the cell proliferation inhibition was calculated to
plot the curve of proliferation inhibition.
Cell apoptosis detection by
fluorescence-activated cell sorting analysis
The cells were seeded onto the 6-well plate at a
density of 5×105/well in 2 ml and cultured for 24 h. The
cells of each well were treated separately and collected after
another 12 h of normal culture. The cells were resuspended in 1X
binding buffer after washing with ice-cold phosphate-buffered
saline (PBS) twice and adjusted to the density of
1×106/ml. Cell suspensions (100 µl) were transferred to
a 5-ml tube for fluorescence-activated cell sorting (FACS). Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit 5 µl each, were added into cell suspensions and
incubated for 15 min at room temperature with avoiding light. The
cell mixture was incubated on ice after adding 400 µl of 1X binding
buffer, and FACS analysis was conducted within 1 h.
FITC−/PI− was defined as normal cells,
FITC+/PI− was defined as apoptotic cells at
an early stage, FITC+/PI+ was defined as
apoptotic cells at a late stage, and
FITC−/PI+ was defined as necrotic cells. The
experiment was repeated three times, and the apoptotic index (AI)
and necrotic rate were calculated using the average measurements.
AI = (number of apoptotic cells at a late stage + number of
apoptotic cells at an early stage)/total number of cells; necrotic
rate = number of necrotic cells/total number of cells.
Transwell assay
The cells at the logarithmic growth phase were
seeded onto the 24-well plate at a density of 5×104/well
in 2 ml and cultured for 24 h. The normal culture was continued for
24 h after treatment of each group, and the cells were suspended in
a serum-free medium after washing twice with PBS. Cells (100 µl)
were seeded into the Transwell chamber at a density of
2×105/ml in triplicate format and continuously cultured
for 24 h before removing the matrix gel as well as the cells in the
upper chamber; the Transwell cells were counted under a microscope
using Giemsa staining.
Protein expression detection by
western blot analysis
The cells were continuously cultured for 12 h after
treatment, and 1×107 cells were collected. The RIPA
lysis buffer (150 µl) was added, and the supernatants were used for
total protein determination. The protein concentration ranged
between 1.5 and 2.5 mg/ml. The supernatants were aliquoted into 20
µl X2 and stored at −80°C. Sodium dodecyl sulfate (SDS) (10%)
polyacrylamide separating gel and 6% concentrating gel were
prepared; 80-µg proteins from each group were mixed with 5X SDS
loading buffer and subjected to SDS-polyacrylamide gel
electrophoresis (PAGE) after denaturing at 100°C for 10 min. The
PAGE was conducted until the dye and protein markers migrated to
the desired position. The proteins were then transferred onto a
polyvinylidene fluoride (PVDF) membrane. The first antibody was
incubated with the PVDF membrane overnight after blocking with
skimmed milk solution for 1 h, and the secondary antibody was
incubated for 1 h at room temperature before developing the
enhanced chemiluminescence blot and photographing. The molecular
weight of the targeted protein was estimated using the protein
marker, and β-actin was adopted as internal control to evaluate the
total amount of protein loaded onto each lane. The images were
analyzed using the ImageJ software; the amount of targeted protein
= relative gray scale × area (mm2). The expression level
of HSP-70, caspase-3, HIF-lα and MMP-9 was calculated separately
for comparison.
Fluorescence quantitative PCR
The cells were collected after each treatment, total
RNA was extracted using TRIzol reagent, and cDNA was synthesized
using a First Strand cDNA synthesis kit. The primers were designed
and synthesized by Sangon Biotech as follows: HSP-70 forward,
TACTGTGGACCTG CCAATCG and reverse, TAGCATCATTCCGCTCCTTC; HIF-1α
forward, GCAGCAACGACACAGAAACT and reverse, AGCGGTGGGTAATGGAGAC;
MMP-9, forward, CCAAC TACGACACCGACGAC and reverse, TGGAAGATGAATGG
AAACTGG; caspase-3 forward, AGATGGTTTGAGCCTG AGCA and reverse,
CAGTGCGTATGGAGAAATGG; β-actin forward, GATGCAGAAGGAGATCACTG and
reverse, TAGT CCGCCTAGAAGCATTTG. The specific primers and Maxima
SYBR-Green/ROX qPCR Master were mixed for quantitative PCR (qPCR),
with the reaction conditions as follows: 50°C pretreated for 2 min,
95°C pre-denaturing for 10 min, 95°C denaturing for 15 sec, and
60°C annealing and extension for 60 sec for a total of 40 cycles.
Triplicate wells were used, and β-actin was the internal control.
Quantitation was represented by cycle threshold value (Ct value).
Relative mRNA value = 2−ΔCt (ΔCt = Cttarget
gene-Ctβ-actin).
In vivo tumor growth and metastasis
assay
Logarithmic growth of SW-480 cells were made equal
1×107 cells/l into single cell suspension. The nude
mouse abrosia for 1 day were aerosol anesthesia with B halothane,
disinfected abdominal skin was cut a 0.8 cm incision in the left
lower abdomen, then the cecum, sucked up 0.1 ml cell suspension
with OT needle, injected into cecum subserosal, in situ
colon cancer modelwas established. After 4 weeks, the model mice
bearing a tumor were administered aerosol anesthesia again, and a
different pneumoperitoneum intervention was established. We applied
the in-house device many for many mice to simultaneously warm in
the CO2 pneumoperitoneum experiment and 5-FU
intraperitoneal chemotherapy, 5-FU 25 mg/kg, pneumoperitoneum
duration of 1 h, at pressure of 12 mmHg. The model mice were
sacrificed at 6 weeks. We observed in each group, transplantation
tumor weight and viscera metastasis through celiotomy, tumor
inhibitory rate = (treatment group weight-control group
weight)/control group weight ×100%.
Statistical analysis
The data are presented as mean ± standard deviation
(SD), and the differences between two groups were analyzed using
χ2 test. The differences among groups were analyzed
using one-way analysis of variance test. SPSS 13.0 software (SPSS
Inc., Chicago, IL, USA) was used in the present study and P<0.05
was considered as statistically significant.
Results
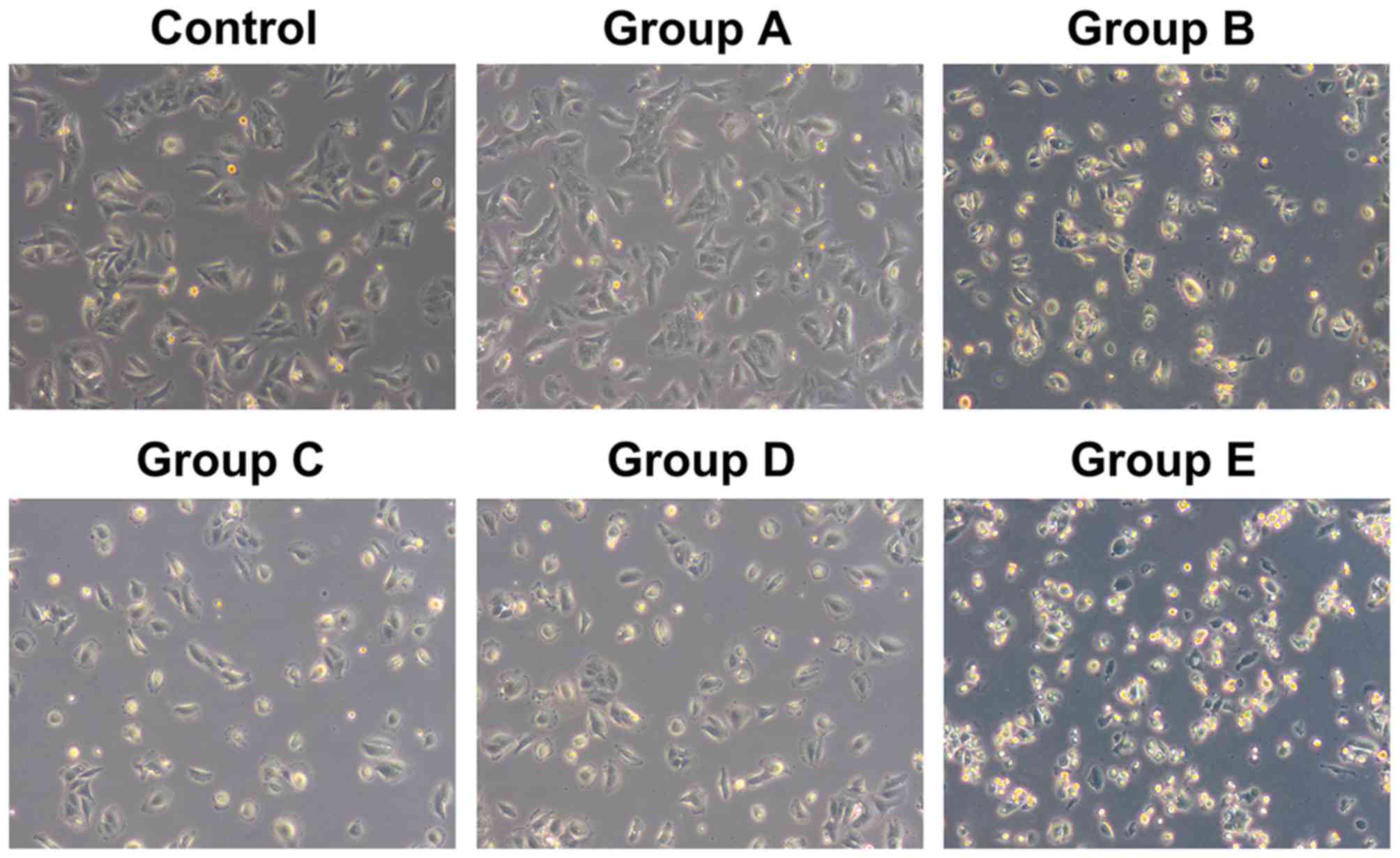
Dynamic observation of the cell
proliferation and morphology under a microscope
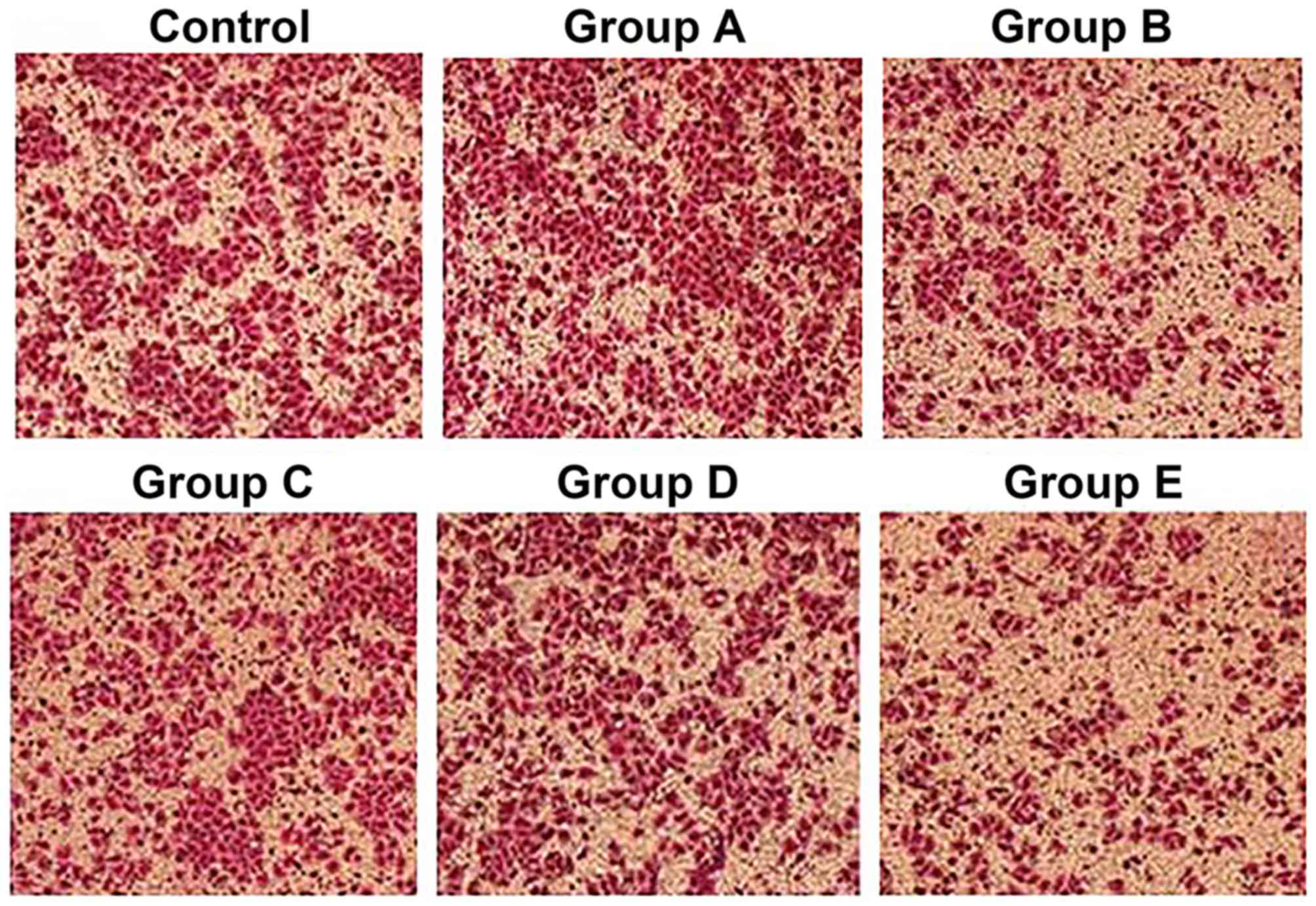
The cells from the control group and group A, with
rod-like, spindle-like, leave-like or branch-like morphology, were
attached to the wall of the petri dish. All cells were in good
condition with a rapid growth rate, and reached 100% confluence
within 3 or 4 days, without obvious dead cells. However, the
majority of cells from groups B and E started to shrink after 12 h
and presented with triangular or round morphology without
parapodium. The refraction of cells increased; they were detached
from the wall of the petri dish and suspended into the culture
medium. The cell death and cell debris could be observed after 24
h. The total cell number was reduced and normal morphology loss was
aggravated with time. The dead cells and cell debris filled the
whole petri dish after 48 h in group E, whereas the cell morphology
was not significantly altered within 48 h in groups C and D. The
shrinkage and detachment of cells from the petri dish were observed
after 48 h, and necrosis and cell debris of small portion of cells
were found after 72 h (Fig. 2).
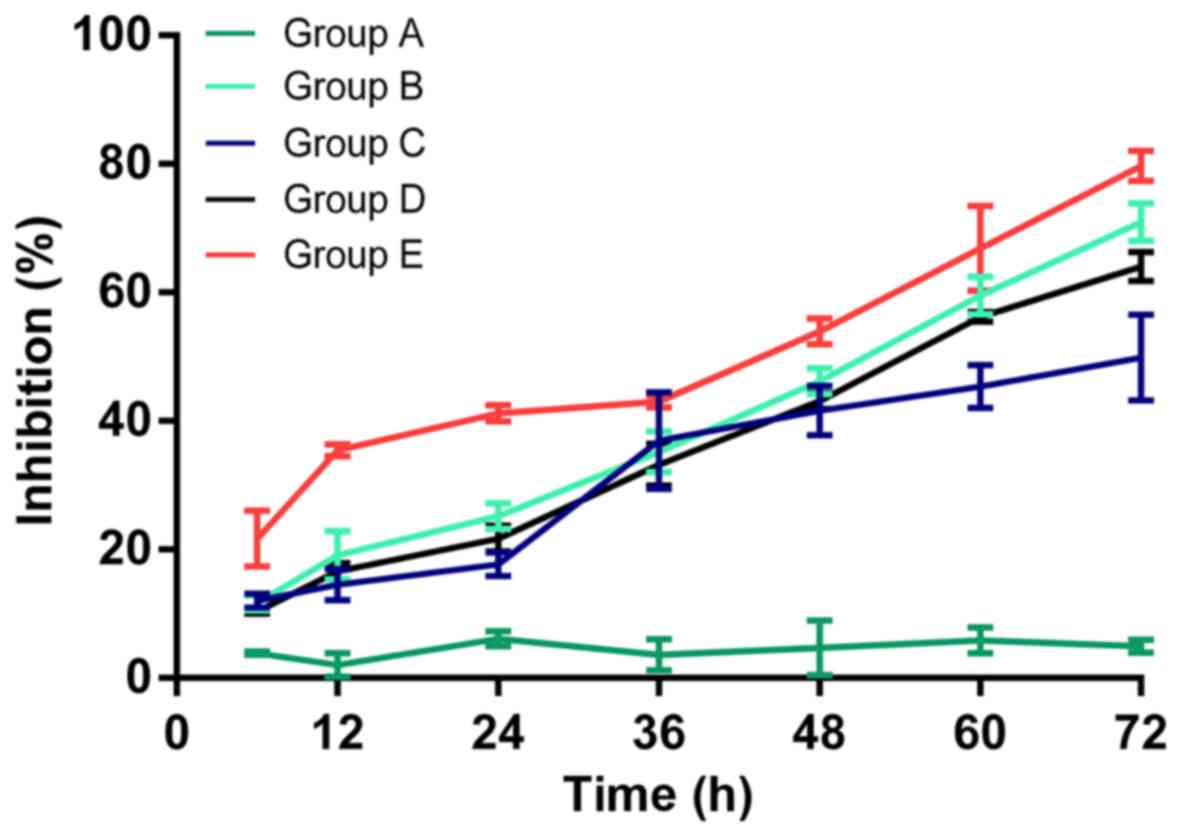
Detection of cell proliferation
inhibition by CCK-8 test
The cell proliferation inhibition of each group
detected by CCK-8 is shown in Fig.
3. The inhibition rate was 2.05±1.80, 6.16±1.16, 4.72±4.23 and
4.96±1.01% in group A; 19.16±3.77, 25.18±2.07, 46.19±2.00 and
71.00±2.97% in group B; 14.52±2.42, 17.67±1.87, 41.60±3.87 and
49.85±6.73% in group C; 16.58±1.26, 21.69±2.03, 43.09±1.31 and
64.01±2.28% in group D; and 35.49±0.93, 41.18±1.24, 53.96±2.02 and
79.68±2.35% in group E, for 12, 24, 48 and 72 h, respectively.
Compared with the control group, no obvious alteration in cell
proliferation was observed in group A, whereas significant cell
proliferation inhibition was observed in groups B, D, D and E
(P<0.05); the strongest inhibition was observed in group E
compared with groups C and D (P<0.05), indicating that
hyperthermic CO2 pneumoperitoneum could reinforce the
inhibitory effect of 5-FU on cell proliferation.
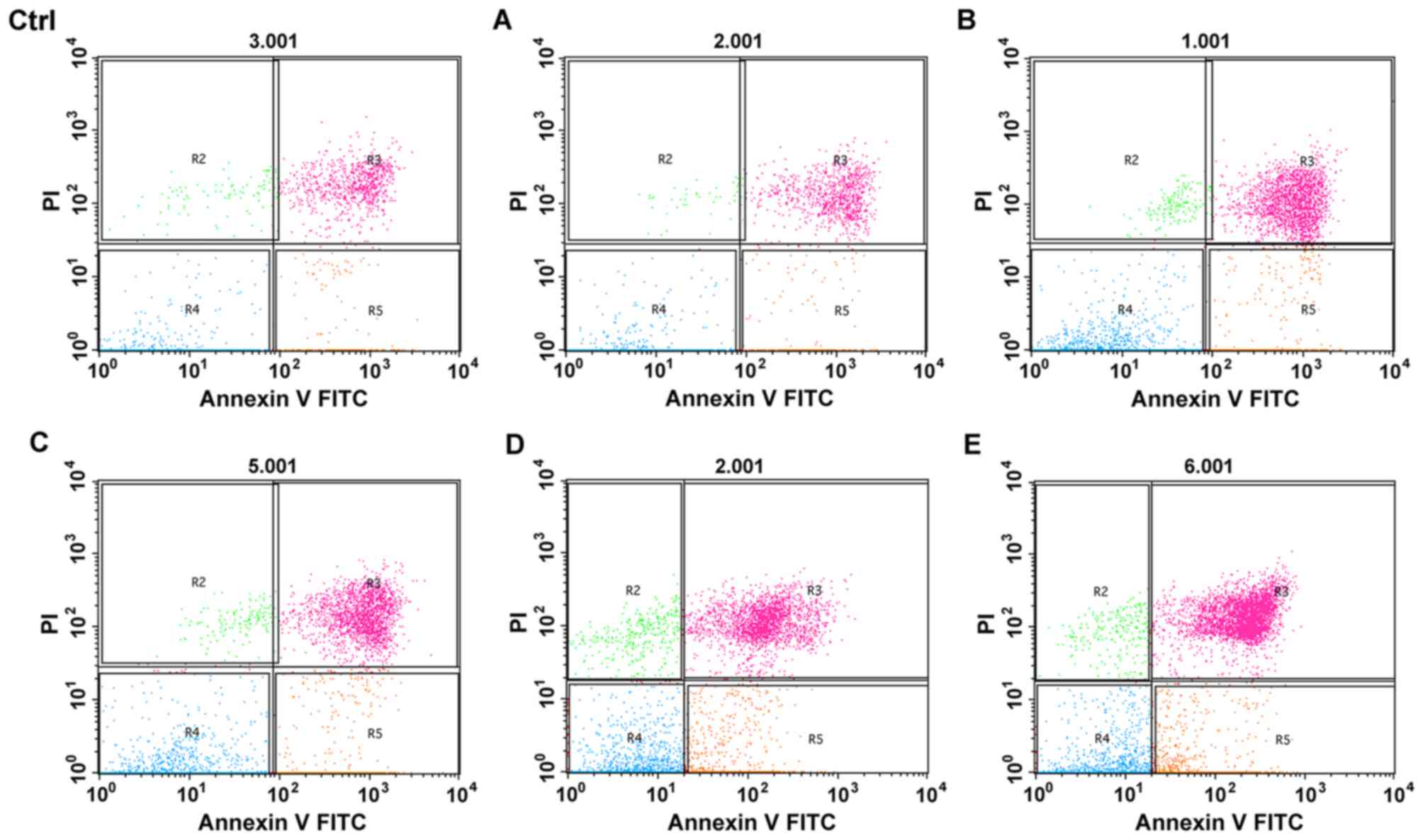
Detection of cell apoptosis by FACS
analysis
The apoptosis of cells after treatment for 12 h was
analyzed using FACS with Annexin V/PI-staining. The rate of
apoptosis was calculated using the following formula: apoptosis
rate (%)=R3+R5 (Fig. 4). The
apoptosis rate was 11.37±0.87, 13.26±0.95, 27.45±1.14, 29.73±0.88,
36.61±0.51 and 65.20±3.11% in the control group and groups A, B, C,
D and E, respectively. No significant difference in the apoptosis
rate was observed between the control group and group A
(P>0.05), while apoptosis significantly increased in groups B,
C, D and E (P<0.05); the most significant apoptosis was observed
in group E (P<0.05), indicating that hyperthermic CO2
pneumoperitoneum could enhance the apoptosis induced by 5-FU.
Effect on cell invasion
The invasion of cells was tested by Transwell assay
(Fig. 5). The Transwell cell number
was 243.7±14.0, 354.2±17.0, 84.5±9.7, 105.7±9.2, 126.6±8.8 and
46.2±7.13 for the control group and groups A, B, C, D and E,
respectively. Compared with the control group, the Transwell cell
number decreased in groups B, C, D and E, but not in group A; the
decrease was most significant in group E (P<0.05), indicating
that hyperthermic CO2 pneumoperitoneum and 5-FU
chemotherapy both have inhibitory effect of cell invasion,
hyperthermic CO2 pneumoperitoneum was able to strengthen
the inhibition of cell invasion induced by 5-FU.
Detection of protein expression by
western blot analysis
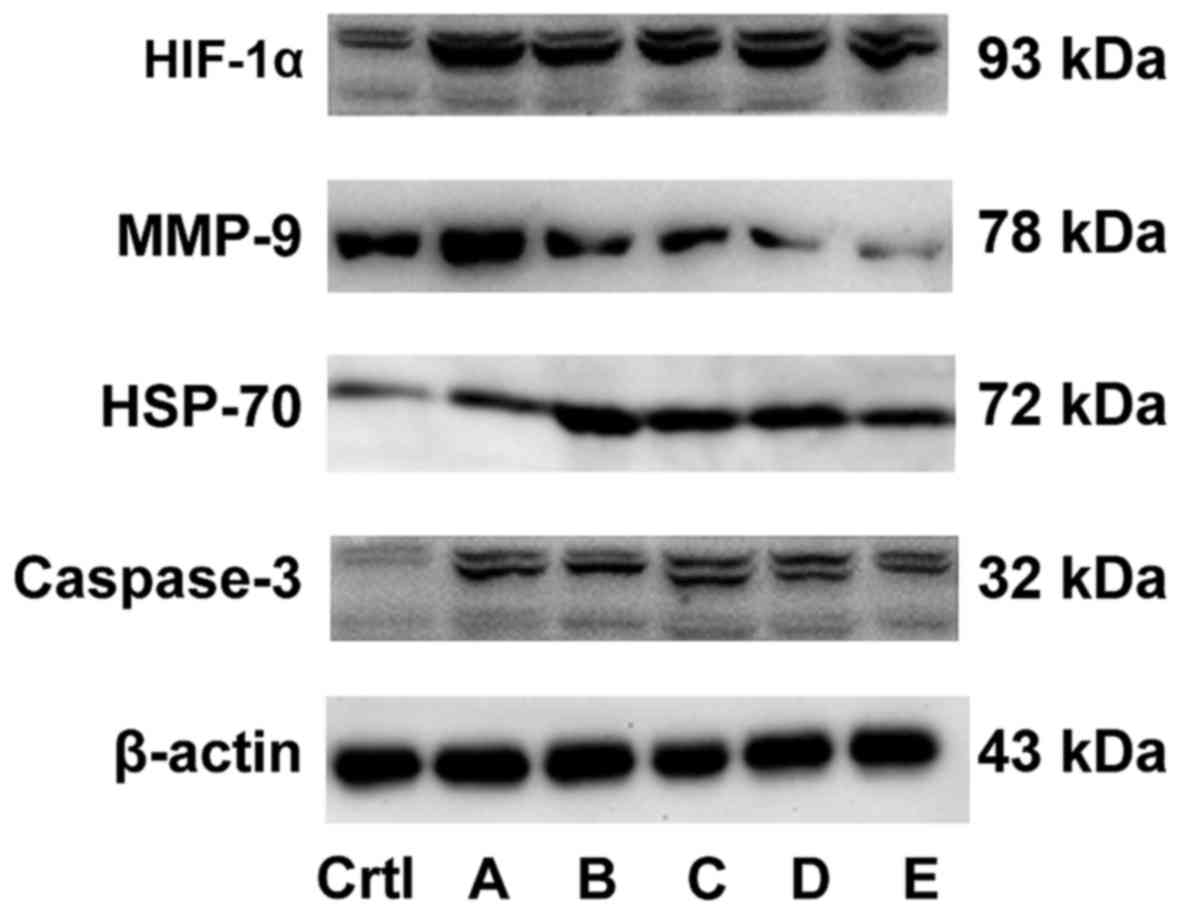
The western blot analysis is shown in Fig. 6 (statistical table.xlsx). Compared
with the control group, the expression level of HSP-70 and
caspase-3 protein remained unchanged and the expression level of
HIF-1α and MMP-9 proteins increased in group A. The expression of
caspase-3, HSP-70 and HIF-1α increased and the expression of MMP-9
protein decreased in groups B and E. The expression of caspase-3
increased, the expression of MMP-9 decreased, and no change in the
expression of HIF-1α and HSP-70 was observed in group C. The
expression of HIF-1α and caspase-3 increased, the expression of
MMP-9 decreased, and no change in the expression of HSP-70 was
observed in group D, indicating that the combined effect of
hyperthermic CO2 pneumoperitoneum and 5-FU could promote
cell apoptosis by upregulating the expression of HIF-1α, HSP-70 and
caspase-3 and inhibit cell invasion by downregulating the
expression of MMP-9.
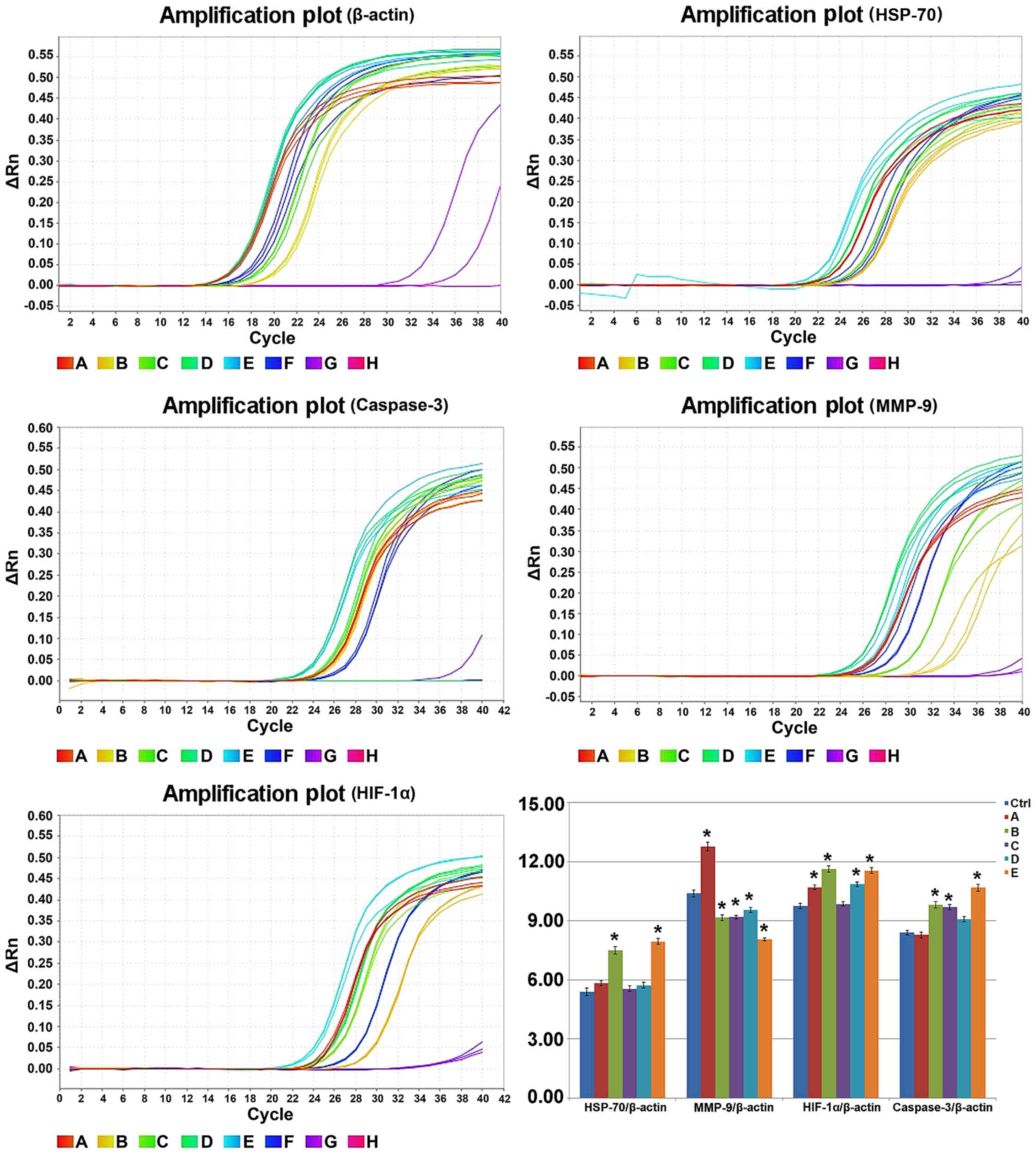
Detection of mRNA level by RT-PCR
The RT-PCR result of each targeted gene 12 h after
treatment is shown in Fig. 7
(statistical table.xlsx). The relative expression of HSP-70/β-actin
in the control group and groups A, B, C, D and E was 5.40±0.18,
5.84±0.13, 7.51±0.19, 5.55±0.15, 5.73±0.13 and 7.95±0.15,
respectively. The relative expression of MMP-9/β-actin was
10.39±0.17, 12.76±0.22, 9.16±0.15, 9.19±0.09, 9.55±0.13 and
8.07±0.08. The relative expression of HIF-1α/β-actin was 9.75±0.12,
10.70±0.13, 11.63±0.17, 9.85±0.12, 10.86±0.12 and 11.55±0.14. The
relative expression of caspase-3/β-actin was 8.40±0.12, 8.28±0.14,
9.81±0.16, 9.70±0.12, 9.08±0.13 and 10.68±0.18. Compared with the
control group, the expression level of HSP-70 and caspase-3 mRNA
remained unchanged, while the expression level of HIF-1α and MMP-9
mRNA increased in group A (P<0.05). However, the expression
level of caspase-3, HSP-70 and HIF-1α mRNA increased, while the
expression level of MMP-9 decreased in groups B and E. The
expression level of caspase-3 mRNA increased while that of MMP-9
mRNA decreased, and no change was observed in the expression level
of HIF-1α and HSP-70 mRNA in group C. The expression level of
HIF-1α and caspase-3 mRNA increased while that of MMP-9 mRNA
decreased, and no change was found in the expression level of
HSP-70 mRNA in group D. The present findings indicated that the
combined treatment of hyperthermic CO2 pneumoperitoneum
and 5-FU was able to promote cell apoptosis by upregulating the
expression of HIF-1α, HSP-70 and caspase-3 and inhibited cell
invasion by downregulating the expression of MMP-9.
In vivo tumor growth and
metastasis
Of the 72 nude mice 55 cecum vaccination nodular
lesions were seen (Fig. 8),
diameter from 1.0 to 9.0 mm, the number from 1 to several. The
tumorigenic success rate was 76.4% (55/72), 30 nude mice had liver,
abdominal wall (Fig. 9), spleen,
kidney, gastrointestinal metastases. Viscera metastasis rate was
41.7% (30/72). The number of tumorigenic success in the control
group and groups A, B, C, D and E was 10, 11, 8, 9, 9 and 8. The
weight of tumor was 0.76±0.05, 0.84±0.06, 0.67±0.06, 0.65±0.05,
0.74±0.05 and 0.45±0.03 g. Compared with the control group, the
tumor inhibition rate of groups A, B, C, D and E was 110.5, 88.2,
85.5, 97.4 and 59.2%. Metastasis rate was 70, 72.7, 50, 44.4, 55.6
and 25%. Compared with the control group, the tumor weight and
metastasis rate decreased in groups B, C, D and E, but not in group
A. The most decrease was seen in group E, indicating that
hyperthermic CO2 pneumoperitoneum and 5-FU chemotherapy
both possess inhibition of tumor growth and metastasis,
hyperthermic CO2 pneumoperitoneum was able to reinforce
the inhibition induced by 5-FU.
Discussion
Laparoscopy has been widely used since its adoption
for the first time in colorectal surgery in 1991, and has been an
important surgical option in colorectal cancer treatment.
CO2 is commonly used to create pneumoperitoneum in
laparoscopic surgeries. A concern exists among some surgeons that
CO2 pneumoperitoneum may be associated with tumor cell
migration and invasion. The potential mechanisms have been
suggested to be the velocity and pressure created by CO2
pneumoperitoneum-induced tumor cell detachment and spread (6), tumor cells seeded at the puncture site
of casing pipes (7), aerosol
dissemination of tumor cells by ultrasonic knife, peritoneal acidic
hypoxic microenvironment created by CO2 pneumoperitoneum
(8), and cellular immunity
alteration (9). However, other
researchers believed that CO2 pneumoperitoneum had no
obvious effect on tumor invasion and metastasis (10). Therefore, it was necessary to avoid
any possibility of tumor invasion and metastasis caused by
CO2 pneumoperitoneum. Hyperthermia is a novel
therapeutic method, which increases temperature systematically or
locally to treatment temperature (42–45°C) to eliminate tumor cells
by altering cell signaling pathway or gene network transduction.
The major mechanisms are to impair the checkpoint of cell cycle and
DNA replication, alter microenvironment, and activate
transcriptional factors for lysosomal enzymes, which eventually
lead to tumor cell necrosis or apoptosis (11–13).
In addition, CO2 gas is a carrier with good heat
conduction and dispersion, which could conduct heat rapidly within
the abdominal cavity. Furthermore, 5-FU is the first-line drug for
colorectal carcinoma. Marked killing effect on tumor cells could be
achieved by using 5-FU as intraperitoneal chemotherapy with high
local concentration and prolonged action time. Intraoperative IHCP
could inhibit tumor cell migration and eliminate the free tumor
cells and micrometastases during operations, thus preventing or
reducing tumor invasion and metastasis (14). However, most of IHCP application and
studies were conducted in open surgeries; hence, information was
lacking on the use of IHCP in laparoscopic operations. Peng et
al discovered that hyperthermic CO2 pneumoperitoneum
could inhibit colon cancer cell proliferation (15). In the present study, a simulated
laparoscopic operation combined with hyperthermic CO2
pneumoperitoneum and 5-FU intraperitoneal chemotherapy was
performed to observe the effect on colon cancer in vitro and
in vivo.
Hyperthermic CO2 pneumoperitoneum
reinforces the inhibitory effect of 5-FU on colon cancer cell
proliferation. The inhibitory effect of hyperthermic CO2
pneumoperitoneum and 5-FU on colon cancer cells was observed under
a microscope and using the CCK-8 test. The combination of
hyperthermic CO2 pneumoperitoneum and 5-FU demonstrated
the strongest inhibition. The apoptosis of colon cancer cells
induced by either hyperthermic CO2 pneumoperitoneum or
5-FU, or combined treatment was observed by FACS analysis; the
combined treatment had the most significant effect. It showed that
hyperthermic CO2 pneumoperitoneum and 5-FU chemotherapy
both inhibited tumor growth, and the combined treatment had the
most significant effect in vivo. The present findings
indicated that hyperthermic CO2 pneumoperitoneum could
reinforce the effect of 5-FU by inhibiting cell proliferation and
inducing apoptosis. HSP-70 is a molecular chaperone involved in
protein synthesis, processing, folding, and transportation, and
related to the occurrence, development, drug resistance, and
prognosis of tumors (16).
Hyperthermic CO2 pneumoperitoneum alone or combined with
5-FU was shown to upregulate the expression of HSP-70 gene and
protein in the present study, which could promote tumor cell
apoptosis and necrosis. Caspase-3 is a key member participating in
the signaling pathways of apoptosis; it is activated in the early
stage of apoptosis, to degrade the substrates in cytoplasm and
nuclei, eventually leading to apoptosis (17). Caspase-3 gene and protein were both
increased after hyperthermic CO2 pneumoperitoneum, or
5-FU, or their combined treatment in this study, which could
initiate apoptosis and promote cell death. The expression of HIF-1α
could be increased under hypoxic conditions, to sustain high energy
metabolism and promote angiogenesis by regulating the expression of
multiple transcriptional factors and coping with hypoxia, hence
promoting tumor invasion, metastasis, and drug resistance (18). In the present study, HIF-1α gene and
protein both increased after hyperthermic CO2
pneumoperitoneum, or 5-FU, or their combined treatment, and it was
speculated that transcriptional factors were modulated by an
increased level of HIF-1α to promote cell apoptosis and inhibit
tumor invasion. The findings indicated that hyperthermic
CO2 pneumoperitoneum and 5-FU combined treatment could
promote cell apoptosis by upregulating the expression of HSP-70,
HIF-1α and caspase-3.
Hyperthermic CO2 pneumoperitoneum
reinforces the inhibitory effect of 5-FU on colon cancer cell
invasion. The inhibitory effect of either hyperthermic
CO2 pneumoperitoneum or 5-FU on colon cancer cell
invasion was observed by Transwell assay, and the reinforced effect
was achieved by the combined treatment. It showed that hyperthermic
CO2 pneumoperitoneum and 5-FU chemotherapy both
inhibited tumor metastasis and the combined treatment had the most
significant effect in vivo. MMP-9 is a kind of zinc
ion-dependent endopeptidase degrading fibrinogen type IV, the major
component of extracellular matrix (ECM), leading to tumor invasion
and metastasis. MMP-9 has been considered as a marker of tumor
invasion and metastasis (19,20).
In the present study, the expression of MMP-9 decreased by
hyperthermic CO2 pneumoperitoneum, or 5-FU, or their
combined treatment, leading to inhibition of ECM degradation and
cancer cell invasion.
In conclusion, the hyperthermic CO2
pneumoperitoneum reinforced the inhibitory effect of 5-FU on colon
cancer cell proliferation and invasion, by upregulating the
expression of HSP-70, HIF-1α and caspase-3 at both mRNA and protein
levels, downregulating the expression of MMP-9.
Acknowledgements
This study was supported by the finding from the
Shanghai Municipal Commission of Health and Family Planning (no.
20134260), (ZHYY-ZXYJHZX-2-02).
References
|
1
|
Bing F and Hong Z: The effect of
CO2 pneumoperitoneum on the growth and metastasis of
malignant tumors of the rectum. J Exp Sur. 22:10212005.
|
|
2
|
Jingli C, Rong C and Rubai X: Influence of
colorectal laparoscopic surgery on dissemination and seeding of
tumor cells. Surg Endosc. 20:1759–1761. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin F, Pan L, Li L, Li D and Mo L: Effects
of a simulated CO2 pneumoperitoneum environment on the
proliferation, apoptosis, and metastasis of cervical cancer cells
in vitro. Med Sci Monit. 20:2497–2503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhenggang Z: Comprehensive treatment and
issues related to gastric cancer recurrence. Chin J Sur.
25:181–183. 2005.
|
|
5
|
Kodach LL, Bos CL, Durán N, Peppelenbosch
MP, Ferreira CV and Hardwick JC: Violacein synergistically
increases 5-fluorouracil cytotoxicity, induces apoptosis and
inhibits Akt-mediated signal transduction in human colorectal
cancer cells. Carcinogenesis. 27:508–516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng M, Lin S and Sun J: Impact of
laparoscopic colorectal surgery on port-site and viscera
metastasis. Chin J Pract Surg. 22:337–339. 2002.
|
|
7
|
Ceccarelli G, Casciola L, Nati S, Bartoli
A, Spaziani A, Stefanoni M, Conti D, Fettucciari V, Di Zitti L,
Valeri R, et al: Neoplastic residues in the trocar tract in
oncologic laparoscopic surgery. Minerva Chir. 59:243–248. 2004.(In
Italian). PubMed/NCBI
|
|
8
|
Jacobi CA, Ordemann J, Zieren HU and
Müller JM: Effect of intra-abdominal pressure in laparoscopy on
intraperitoneal tumor growth and development of trocar metastases.
An animal experiment study in the rat model. Langenbecks Arch Chir
Suppl Kongressbd. 115:(Suppl I). 529–533. 1998.[(In German)].
PubMed/NCBI
|
|
9
|
Wildbrett P, Oh A, Carter JJ, Schuster H,
Bessler M, Jaboci CA and Whelan RL: Increased rates of pulmonary
metastases following sham laparotomy compared to CO2
pneumoperitoneum and the inhibition of metastases utilizing
perioperative immunomodulation and a tumor vaccine. Surg Endosc.
16:1162–1169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buunen M, Veldkamp R, Hop WC, Kuhry E,
Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, et
al: Colon Cancer Laparoscopic or Open Resection Study Group:
Survival after laparoscopic surgery versus open surgery for colon
cancer: Long-term outcome of a randomised clinical trial. Lancet
Oncol. 10:44–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hildebrandt B, Wust P, Ahlers O, Dieing A,
Sreenivasa G, Kerner T, Felix R and Riess H: The cellular and
molecular basis of hyperthermia. Crit Rev Oncol Hematol. 43:33–56.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai W, Dong F, Wang Z, Yang X, Zheng M and
Che X: Heated and humidified CO2 pneumoperitoneum
inhibits tumour cell proliferation, migration and invasion in colon
cancer. Int J Hyperthermia. 30:201–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tabuchi Y, Wada S, Furusawa Y, Ohtsuka K
and Kondo T: Gene networks related to the cell death elicited by
hyperthermia in human oral squamous cell carcinoma HSC-3 cells. Int
J Mol Med. 29:380–386. 2012.PubMed/NCBI
|
|
14
|
Al-Shammaa HA, Li Y and Yonemura Y:
Current status and future strategies of cytoreductive surgery plus
intraperitoneal hyperthermic chemotherapy for peritoneal
carcinomatosis. World J Gastroenterol. 14:1159–1166. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng Y, Zheng M, Feng B, Chen X, Yu B, Lu
A, Wang M, Li J, Ma J and Xu L: Hyperthermic CO2
pneumoperitoneum induces apoptosis in human colon cancer cells
through Bax-associated mitochondrial pathway. Oncol Rep. 19:73–79.
2008.PubMed/NCBI
|
|
16
|
Ito A, Shinkai M, Honda H, Yoshikawa K,
Saga S, Wakabayashi T, Yoshida J and Kobayashi T: Heat shock
protein 70 expression induces antitumor immunity during
intracellular hyperthermia using magnetite nanoparticles. Cancer
Immunol Immunother. 52:80–88. 2003.PubMed/NCBI
|
|
17
|
Depraetere V and Golstein P: Dismantling
in cell death: Molecular mechanisms and relationship to caspase
activation. Scand J Immunol. 47:523–531. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu H, Feng Y, Zhang J, Zhou X, Hao B,
Zhang G and Shi R: Inhibition of hypoxia inducible factor 1α
expression suppresses the progression of esophageal squamous cell
carcinoma. Cancer Biol Ther. 11:981–987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu D, Guo H, Li Y, Xu X, Yang K and Bai
Y: Association between polymorphisms in the promoter regions of
matrix metalloproteinases (MMPs) and risk of cancer metastasis: A
meta-analysis. PLoS One. 7:e312512012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chu D, Zhao Z, Zhou Y, Li Y, Li J, Zheng
J, Zhao Q and Wang W: Matrix metalloproteinase-9 is associated with
relapse and prognosis of patients with colorectal cancer. Ann Surg
Oncol. 19:318–325. 2012. View Article : Google Scholar : PubMed/NCBI
|