Introduction
Colorectal cancer is the third most common type of
cancer in both females and males in the United States; however, its
incidence and mortality rates have been decreasing in recent years
(1). This is attributed to many
factors, such as more frequent performance of surveillance
colonoscopy, improvements in surgical and radiotherapy techniques,
and development of new chemotherapy and molecular targeted drugs
(2,3). Although chemotherapy and targeted
therapy drugs have progressed over the past few decades, drug
resistance is still a major problem in the treatment of colorectal
cancer. Combination chemotherapy with FOLFOX (folinic acid
(LV)/5-fluorouracil (5-FU)/oxaliplatin) and FORFIRI
(LV/5-FU/irinotecan) provides a higher response rate and has now
become the standard treatment regimen for colorectal cancer, but
resistance to combination chemotherapy eventually occurs, resulting
in tumor recurrence or metastasis.
Annexin A1 (ANXA1), which belongs to the Annexin
superfamily, is a 37 kDa calcium-dependent phospholipid-linked
protein involved in apoptosis, anti-inflammatory effects, and the
regulation of cellular differentiation and proliferation (4,5).
Through these functions, ANXA1 is considered to be associated with
cancer development in various malignant tumors, including
colorectal cancer (6,7). We previously reported a case of
positive ANXA1 expression by immunohistochemical (IHC) staining in
breast cancer that was associated with triple-negative breast
cancer (P=0.007) and venous invasion (P=0.028) (8). In vitro cell experiments
revealed that ANXA1 enhanced breast cancer invasion and metastasis
under hypoxia, indicating that ANXA1 was significantly associated
with worse breast cancer patient outcome. We also reported a case
of positive ANXA1 expression by IHC staining in colon cancer
associated with venous invasion (P=0.023) as well as lymph node
metastasis (P=0.042) (9). These
positive cases were not statistically associated with poor
survival, but appeared to be associated with worse colon cancer
patient outcome.
According to recent studies, there is no doubt that
drug resistance is one of the major reasons of poor patient outcome
following cancer treatment. Those studies identified that ANXA1 is
associated with drug resistance through modulation of the drug
export mechanism of P-glycoprotein and the multidrug resistance
protein (10). One of the key drugs
of the FORFIRI and FOLFOX regimens is 5-FU; thus, we established a
5-FU-resistant cell line to focus on the pivotal role of ANXA1 in
5-FU resistance. The present study also implicates the 5-FU
resistance by modulating ANXA1 expression in colon cancer
cells.
Materials and methods
Cell culture
The colon cancer cell lines used in this study were
originally obtained from the American Type Culture Collection
(Rockville, MD, USA) and were cultured in the recommended media
with 10% fetal bovine serum (FBS). These monolayer cells were
maintained in a 37°C incubator with 5% CO2. Cells were
checked regularly under a light microscope and subcultured when
they reached 80–90% confluence. For hypoxia exposure, each cell
type was cultured for 24 h in a modulator incubator chamber
(Billups-Rothenberg, Del Mar, CA, USA) at 37°C with 1%
O2, 5% CO2, and 94% N2. To mimic
hypoxia, the cells were cultured for 24 h with 100 µM cobalt
chloride (CoCl2) (Sigma-Aldrich, St. Louis, MO,
USA).
IHC staining and evaluation
Colon cancer cell lines were immunostained for ANXA1
(clone 29; BD Biosciences, San Jose, CA, USA) and evaluated for
staining intensity. Briefly, colon cancer cells were fixed in 10%
formalin and embedded in paraffin then cut into thin (4 µm)
sections and stained. The expression of ANXA1 protein was evaluated
by the ratio of the number of positively stained cells to
negatively stained cells. Ten colon cancer cell lines were
dichotomized as positive (≥5% staining) or negative (<5%
staining) for ANXA1.
Establishment of 5-FU resistant SW480
cells
A 5-FU-resistant SW480 cell line (SW480/5-FU) was
established by repeated exposure to stepwise increasing
concentrations of 5-FU up to 1 µM, as previously described
(11). The cells were cultured in
RPMI-1640 medium with 10% FBS in a 37°C incubator with 5%
CO2.
Quantitative reverse transcription
polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cells using TRIzol
reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to
the manufacturer's instructions as previously described (8). Complementary DNA (cDNA) was
synthesized from 5 µg of total RNA with a random hexamer using the
SuperScript III First-Strand synthesis system (Thermo Fisher
Scientific). The cDNAs were used for the measurement of gene
expression with a 7500 Real-time PCR system (Thermo Fisher
Scientific) using TaqMan probes. The experiments were performed in
triplicate with blinded patient information. Expression assays were
purchased from Thermo Fisher Scientific (ANXA1, Hs00167549_m1) and
β-actin was used as an internal control (Hs99999903_m1). Relative
ANXA1 expression was calculated using the 2−ΔΔCT method,
according to the supplier's protocol (Thermo Fisher
Scientific).
Western blotting
Western blot analysis was performed as previously
described (8). Cells were washed
twice with ice-cold PBS and scraped. The cells were then
centrifuged at 15,000 rpm to pellet cellular debris and stored
overnight at −80°C. The protein lysates were collected using
ice-cold RIPA buffer containing Halt Protease Inhibitor single-use
cocktail (Thermo Fisher Scientific). Protein concentration was
determined by the Bradford method (Bio-Rad, Hercules, CA, USA).
SDS-PAGE were prepared by mixing aliquots of the protein with Novex
Tris-Glycine SDS sample buffer (Thermo Fisher Scientific) and
heated at 100°C for 3 min. The protein samples were run on 10%
Bis-Tris gels at 100 V for 90 min with MES SDS running buffer
(Thermo Fisher Scientific). For western blot analysis, gels were
electro-transferred to a nitrocellulose membrane using the iBlot
Dry Blotting system (Thermo Fisher Scientific). Proteins were
blocked using Starting Block (PBS) blocking buffer (Pierce,
Rockford, IL, USA) and detected using anti-ANXA1 (clone 29),
anti-hypoxia-inducible factor-1α (HIF-1α) (BD Novus Bioscience
Biologicals, Littleton, CO, USA), anti-β-actin (Santa Cruz
Biotechnology, Dallas, TX, USA), and a goat anti-mouse secondary
antibody phosphatase (Novagen, Billerica, MA, USA). Western blot
analyses were then incubated with Super Signal West Pico detection
system (Pierce) and detected using LAS-4000 IR MultiColor
(Fujifilm, Tokyo, Japan).
Overexpression of ANXA1 expression
using an expression vector
To generate the plasmid pcDNA3.1-ANXA1, ANXA1 was
amplified by PCR using primers designed as follows: ANXA1-F,
5′-AGCTAGCACACTTTTTCAAAAATGGCAATGG-3′; ANXA1-R,
5′-AGGATCCGGGAATGTTTAGTTTCCTCCACA-3′. These primers contained
extragenic NheI and BamHI recognition sites
(underlined), respectively. Total RNA was extracted by TRIzol
reagent from the SW837 cell line, which highly expressed ANXA1, and
1 µg RNA was reverse-transcribed into cDNA by the SuperScript III
First-Strand Synthesis system. PCR was performed using Pfx50 DNA
Polymerase (Thermo Fisher Scientific) to obtain a high fidelity PCR
product. The reaction mix was initially heated to 94°C for 2 min,
and amplification was performed at 94°C for 15 sec, 55°C for 30
sec, and 68°C for 90 sec, in 35 cycles, with a final 10 min
extension at 72°C. The PCR product was identified by 1% agarose gel
electrophoresis then isolated by QIAquick Gel Extraction kit and
purified by QIAquick PCR Purification kit (both from Qiagen,
Valencia, CA, USA). Finally, the PCR product was digested with the
restriction enzymes NheI and BamHI, and inserted into
the corresponding sites of the pcDNA3.1(+) vector (Thermo Fisher
Scientific) using Ligation high (Toyobo, Tokyo, Japan).
One Shot TOP10 Competent Cells (Thermo Fisher
Scientific) were used for transformation, then Qiagen Plasmid Midi
kit (Qiagen) was used to isolate plasmids. One day before plasmid
transfection, 5×105 RKO cells were plated in 6-cm
plates. The cells were transfected with 1 µg of plasmid DNA using
Lipofectamine 2000 regent (Thermo Fisher Scientific) according to
the manufacturer's instructions. After that, we selected a stable
transfected cell line using the antibiotic 400 µg/ml G418 (Roche,
Indianapolis, IN, USA).
Knockdown of ANXA1 expression using
siRNA technology
The siRNA oligonucleotides for ANXA1 (HSS100502 and
HSS100503) and the control were purchased from Thermo Fisher
Scientific. The ANXA1 siRNA sequences were designed as follows:
siRNA-1, 5-CAACCAUCAUUGACAUUCUAAC UAA-3; siRNA-2,
5-GCCUUGCAUAAGGCCAUAAUGG UUA-3. A scrambled siRNA was used as an
internal control and 5×105 HCT116 cells grown in 6-cm
dishes were transfected with 40 nM of each siRNA using
Lipofectamine RNAiMAX (Thermo Fisher Scientific) according to the
manufacturer's instructions.
Measurement of cell proliferation
The cell proliferation rate was assessed by cell
counting kit-8 (CCK-8) (Dojindo, Tokyo, Japan), according to the
manufacturer's instructions. Briefly, 1×104 cells of
colon cancer cells, including control cells, were plated per well
in 96-well plates and 5-FU was added. After 72 h, 10 µl of CCK-8
reagent was added to each well. After 1 h of incubation at 37°C,
the absorbance was measured at 450 nm using a Benchmark Plus
microplate reader (Bio-Rad).
Statistical data analysis
Statistical analysis was carried out with an
unpaired Student's t-test using GraphPad Prism v5.0 (GraphPad
Software Inc., La Jolla, CA, USA). P<0.05 was considered
significant.
Results
ANXA1 expression in colon cancer cell
lines
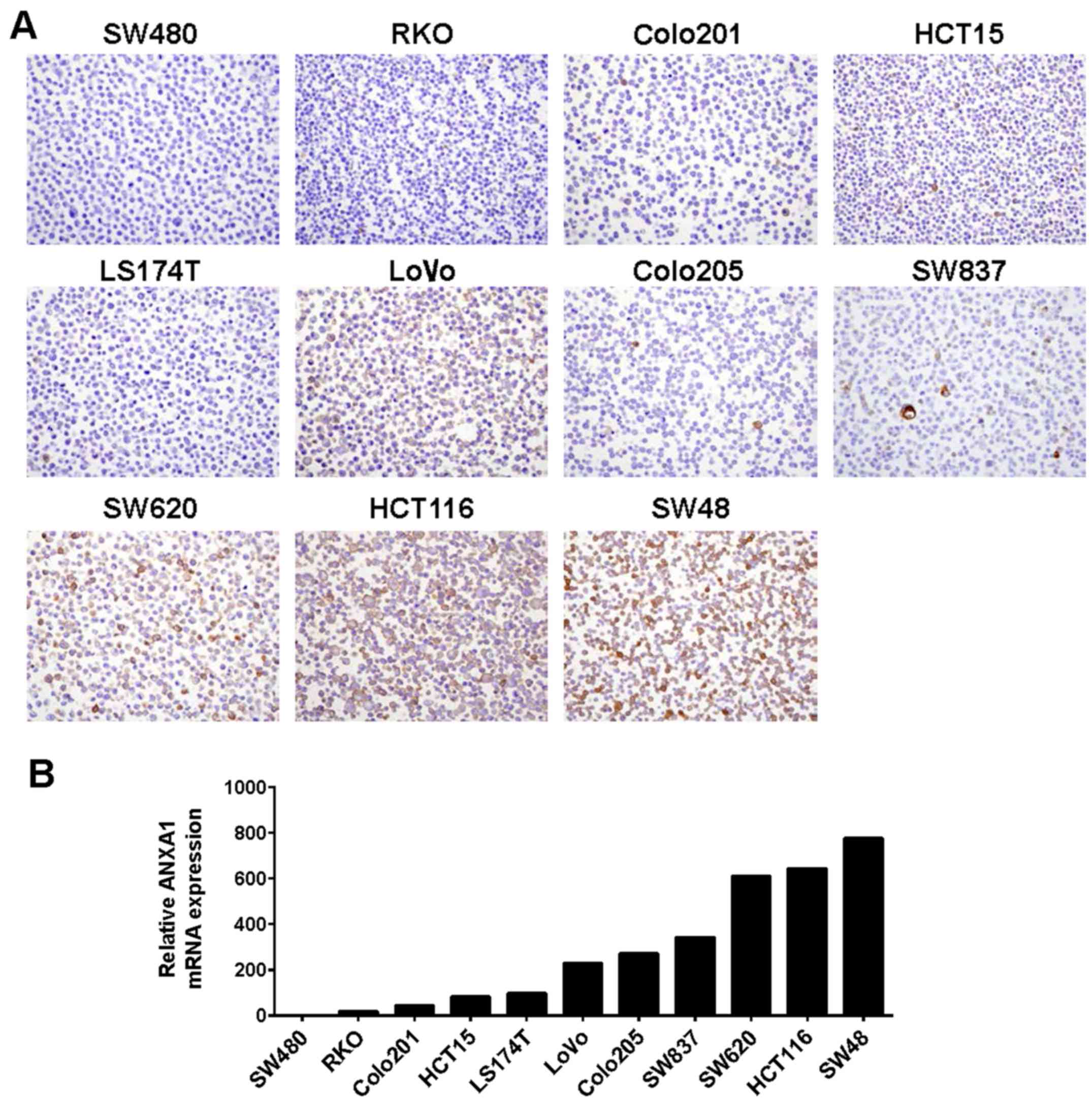
ANXA1 protein expression in ten human colon cancer
cell lines (SW480, RKO, Colo201, HCT15, LS174T, LoVo, Colo205,
SW620, HCT116 and SW48) were examined by IHC staining using an
anti-ANXA1 antibody. Positive staining of ANXA1 was detected in the
nucleus and cytoplasm of the SW620, HCT116 and SW48 cells, while
negative staining of ANXA1 was found in the SW480, RKO, Colo201,
HCT15 and LS174T cells (Fig.
1A).
To confirm ANXA1 expression by IHC evaluation in the
cancer cell lines, we performed qRT-PCR using the same colon cancer
cell lines. Consistent with the IHC results, the ANXA1 mRNA was
highly expressed in the SW620, HCT116 and SW48 cells and
significantly decreased in the SW480 and RKO cells, suggesting that
ANXA1 expression was upregulated at the transcriptional level
(Fig. 1B).
Overexpression of ANXA1 correlates to
5-FU resistance in colon cancer cells
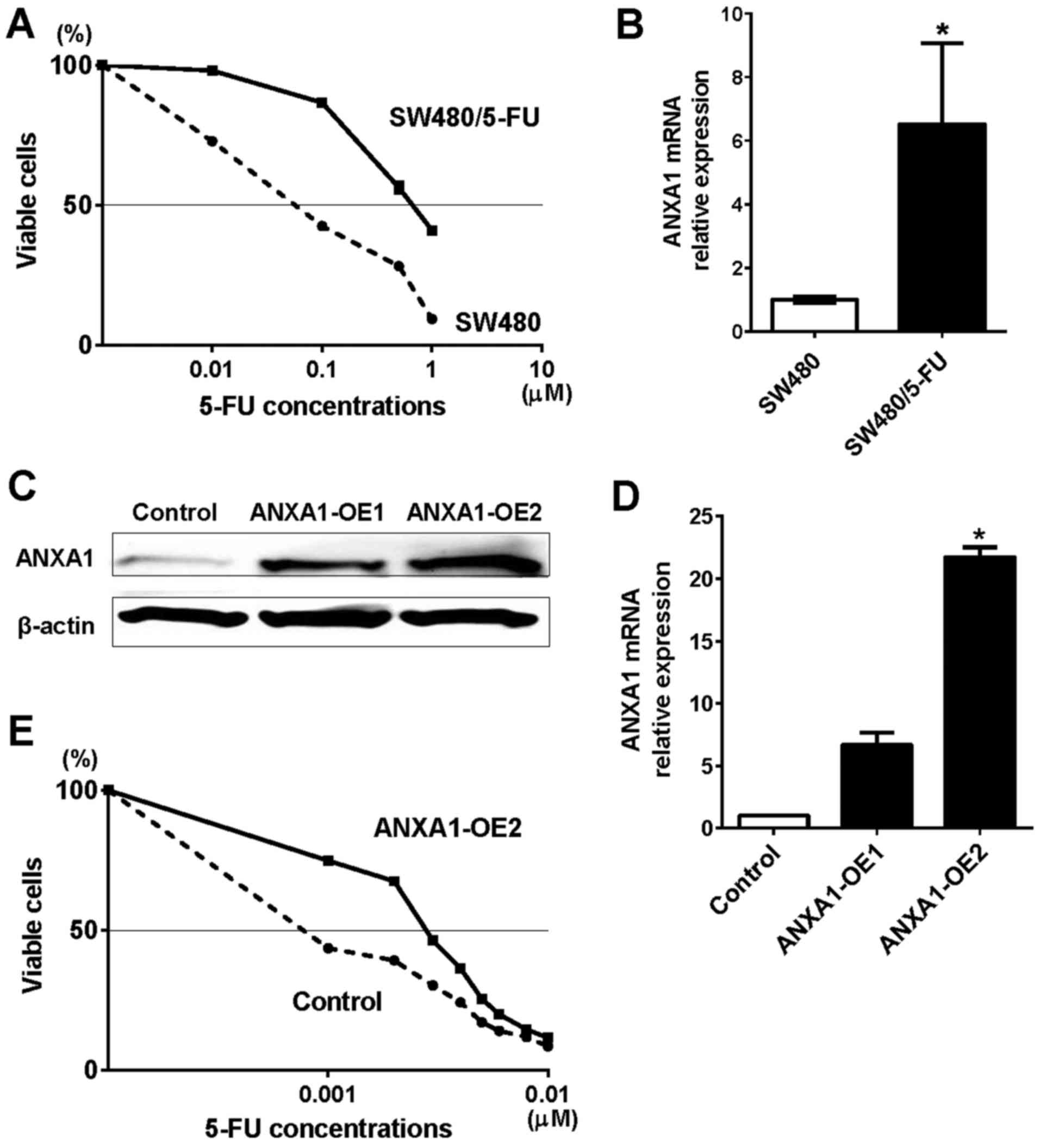
To investigate the role of ANXA1 in 5-FU resistance,
we constructed a 5-FU-resistant SW480 cell line (SW480/5-FU).
First, we confirmed that there were no significant differences in
cell proliferation between SW480 and SW480/5-FU cells (data not
shown). To compare the growth inhibitory effect of 5-FU between the
SW480 and SW480/5-FU cells, we then treated these cells with five
different concentrations of 5-FU (0, 0.01, 0.1, 0.5 and 1 µM)
(Fig. 2A). The inhibitory
concentration 50 (IC50) indicated that the 5-FU
resistance level of the SW480/5-FU cells was 8-fold greater than
that of the control cells. The mRNA expression of ANXA1 in
SW480/5-FU cells was 6.5-fold higher than that of the control cells
(Fig. 2B). These results suggest
that 5-FU resistance induced ANXA1 expression in the colon cancer
cells.
To examine whether ANXA1 overexpression affected
5-FU resistance, we overexpressed ANXA1 in the colon cancer cells
and investigated cell proliferation. Both protein and mRNA
expression of ANXA1 was upregulated by two independent clones
(ANXA1-OE1 and -OE2) in RKO cells that originally expressed
downregulated ANXA1 (Fig. 2C and
D). While no morphological changes were observed in the
ANXA1-OE2 cells, cell viability was attenuated (Fig. 2E). The IC50 indicated
that the 5-FU resistance level of the ANXA1-OE2 cells was 3-fold
greater than that of the control cells. These results further
suggested that high ANXA1 expression is associated with 5-FU
resistance in colon cancer cells.
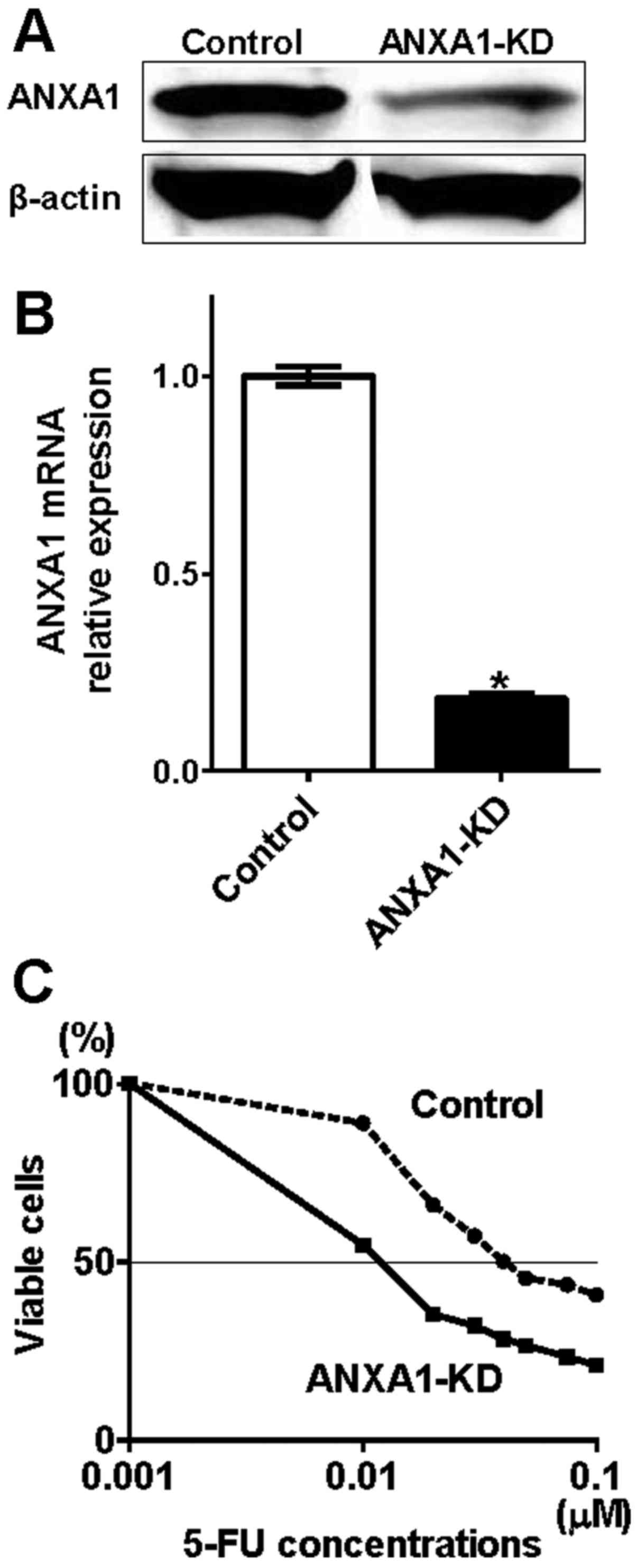
Knockdown of ANXA1 correlates 5-FU
sensitivity in colon cancer cells
According to our present and previous results, which
indicated that ANXA1 expression was correlated with
clinicopathological factors in colon cancer, we hypothesized that
knockdown of ANXA1 improves 5-FU sensitivity. To verify this
hypothesis, we used gene knockdown technology to confirm that both
protein and mRNA ANXA1 expressions were downregulated by siRNA
oligonucleotide in HCT116 cells (ANXA1-KD), which originally
expressed upregulated ANXA1 (Fig. 3A
and B). While no morphological changes were observed in the
ANXA1-KD cells, cell viability was attenuated (Fig. 3C). The IC50 indicated
that the 5-FU resistance level of the ANXA1-KD cells was 4-fold
lower than that of the control cells. These results suggest that
downregulation of ANXA1 significantly improved 5-FU sensitivity,
reconfirming that ANXA1 is associated with 5-FU resistance in colon
cancer cells. Of note, the growth inhibitory effect of 5-FU between
SW480 and HCT116 cells was not significantly different (control
cells in Figs. 2A and 3C). This result suggests that the
spontaneous expression of ANXA1 affects 5-FU resistance only
slightly in colon cells.
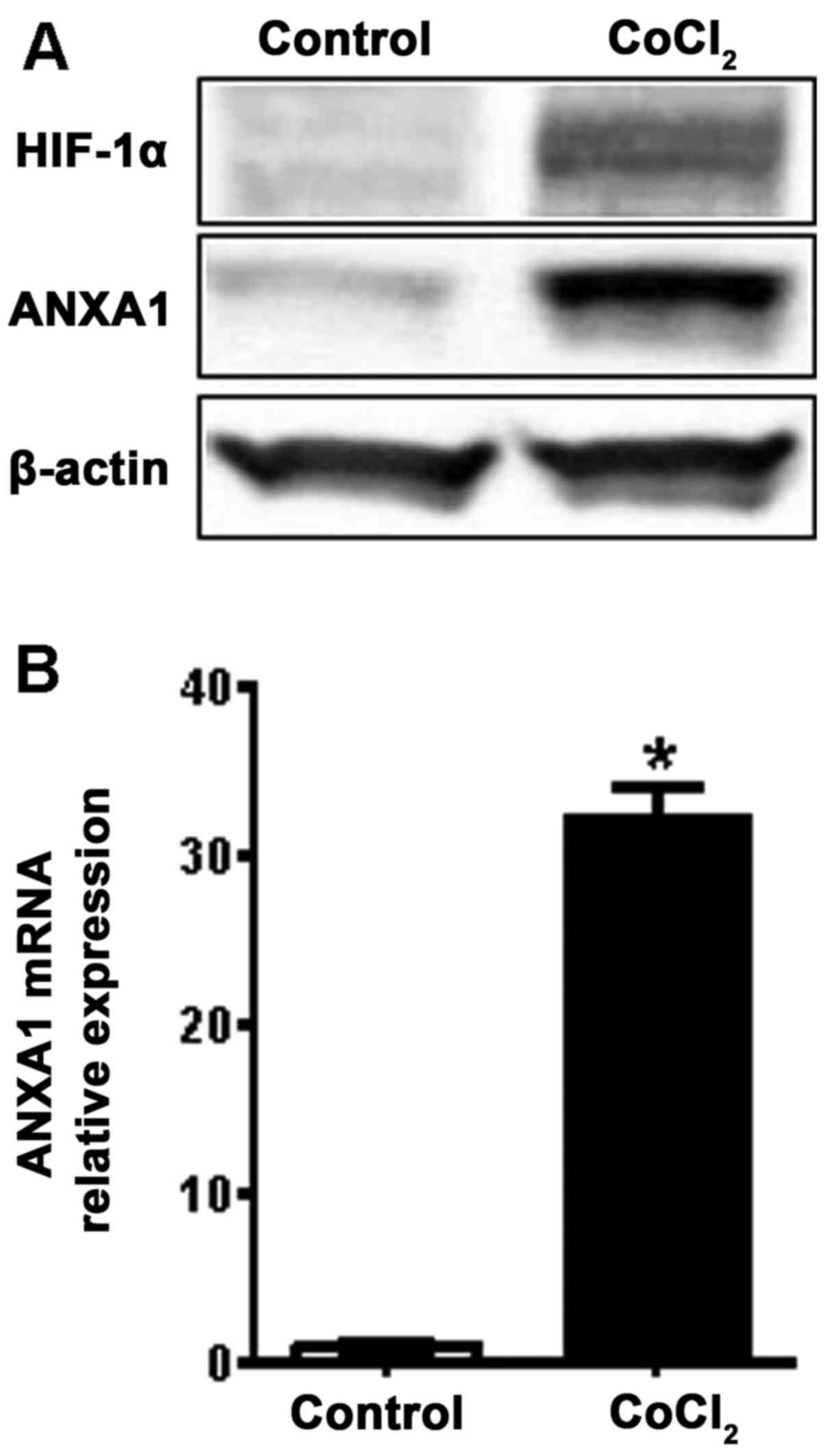
Induction of ANXA1 in hypoxia
To further assess the induction of ANXA1 during
colon cancer progression, we examined ANXA1 expression under
hypoxic conditions, is one of the main characteristic features of
malignant tumors. Following treatment of the SW480 cells with a
hypoxia mimic induced by CoCl2, ANXA1 and HIF-1α
expressions were evaluated. We observed that the HIF-1α and ANXA1
proteins were induced by hypoxia (Fig.
4A). This induction of ANXA1 was also confirmed by examining
mRNA expression (Fig. 4B). These
results suggest that hypoxia may affect 5-FU resistance through
induction of ANXA1 in colon cancer cells.
Discussion
In this study, we demonstrated that ANXA1 is
associated with 5-FU resistance in colon cancer cells. We
previously reported that upregulated ANXA1 was associated with
cancer invasion and lymph node metastasis in colon cancer (9). Based on these findings, we further
investigated the biological significance of ANXA1 by using
overexpression and knockdown methods in colon cancer cells.
Consistent with our findings, a recent study has also revealed that
upregulated ANXA1 was associated with resistance to various
chemotherapeutic agents (10).
ANXA1 activates NF-κB, which is known as the association of
resistance to 5-FU, resulting in progression of metastasis
(12). The investigation of
resistance mechanisms to chemotherapies is strongly required to
develop anticancer therapies with high efficacy and minimum risk of
adverse events. Although the exact mechanisms of ANXA1 in cancer
remain unknown, it is worth investigating the role of ANXA1 in drug
resistance.
This study implicated that overcoming 5-FU
resistance by ANXA1 modulation may be of benefit for patients with
colon cancer as well as other cancers. Because 5-FU is a key drug
in both FOLFOX and FORFIRI regimens, which are the standard base
regimens and used as a first- or second-line therapy for the
treatment of colorectal cancer (13–16).
Although 5-FU is one of the most investigated drugs in terms of
anticancer activity, the molecular mechanism of resistance to 5-FU
has yet to be fully elucidated (17). Both FOLFOX and FORFIRI regimens are
highly effective; however, resistance to these therapies eventually
occurs during treatment, resulting in tumor recurrence and
metastasis. Therefore, the understanding of 5-FU resistance
mechanism leads to the possibility to provide longer treatment
effect and improve patient outcome when either FOLFOX or FORFIRI
are used.
Drug resistance is also associated with hypoxia in
malignant tumors (18). Hypoxia
reduces chemotherapeutic effects by inhibiting tumor cell
proliferation, inducing cell cycle arrest, and affecting various
protein expressions (19). We have
previously demonstrated that hypoxia and the hypoxia mimic induced
by CoCl2 increased ANXA1 and HIF-1α expression in breast
cancer cells (8). HIF-1α is a
transcription factor that contains a basic helix-loop-helix motif
as well as a PAS domain. HIF-1α is induced by hypoxia and promotes
tumor progression by interacting with TP53 (20–23).
This study also revealed that HIF-1α and ANXA1 were induced by
hypoxia, re-suggesting a significant relationship between hypoxia
and drug resistance by modulating HIF-1α and ANXA1 expressions in
colon cancer. However, the interaction between HIF-1α and ANXA1 in
colon cancer is currently unknown. Inhibition of HIF-1α rescues
multidrug resistance in colon cancer cells (24); therefore, inhibition of ANXA1 is
also expected to overcome 5-FU resistance. Of note, one clinical
trial (NCT00984048) to identify biomarkers in metastatic colorectal
cancer patients that have acquired clinical resistance to
first-line chemotherapy (FOLFOX/bevacizumab or FOLFIRI/bevacizumab)
has started. This study allowed us to further understand drug
resistance via a molecular signature.
In conclusion, we report that ANXA1 is upregulated
in 5-FU-resistant colon cancer cells and ANXA1 inhibition can
overcome 5-FU resistance. This suggests that modulating ANXA1
expression may provide an interesting strategy to overcome 5-FU
resistance and provide benefits for colorectal cancer patients.
Acknowledgements
This study was supported by the JSPS KAKENHI, grant
no. 15k10143.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Venook AP, Weiser MR and Tepper JE:
Colorectal cancer: All hands on deck. Am Soc Clin Oncol Educ Book.
34:83–89. 2014. View Article : Google Scholar
|
|
3
|
Mayer RJ, Venook AP and Schilsky RL:
Progress against GI cancer during the American Society of Clinical
Oncology's first 50 years. J Clin Oncol. 32:1521–1530. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rescher U and Gerke V: Annexins - unique
membrane binding proteins with diverse functions. J Cell Sci.
117:2631–2639. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim LH and Pervaiz S: Annexin 1: The new
face of an old molecule. FASEB J. 21:968–975. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ydy LR, do Espírito Santo GF, de Menezes
I, Martins MS, Ignotti E and Damazo AS: Study of the Annexin A1 and
its associations with carcinoembryonic antigen and mismatch repair
proteins in colorectal cancer. J Gastrointest Cancer. 47:61–68.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duncan R, Carpenter B, Main LC, Telfer C
and Murray GI: Characterisation and protein expression profiling of
annexins in colorectal cancer. Br J Cancer. 98:426–433. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okano M, Kumamoto K, Saito M, Onozawa H,
Saito K, Abe N, Ohtake T and Takenoshita S: Upregulated Annexin A1
promotes cellular invasion in triple-negative breast cancer. Oncol
Rep. 33:1064–1070. 2015.PubMed/NCBI
|
|
9
|
Sato Y, Kumamoto K, Saito K, Okayama H,
Hayase S, Kofunato Y, Miyamoto K, Nakamura I, Ohki S, Koyama Y, et
al: Upregulated Annexin A1 expression in gastrointestinal cancer is
associated with cancer invasion and lymph node metastasis. Exp Ther
Med. 2:239–243. 2011.PubMed/NCBI
|
|
10
|
Wang Y, Serfass L, Roy MO, Wong J, Bonneau
AM and Georges E: Annexin-I expression modulates drug resistance in
tumor cells. Biochem Biophys Res Commun. 314:565–570. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi K, Tanaka M, Inagaki A,
Wanibuchi H, Izumi Y, Miura K, Nagayama K, Shiota M and Iwao H:
Establishment of a 5-fluorouracil-resistant triple-negative breast
cancer cell line. Int J Oncol. 43:1985–1991. 2013.PubMed/NCBI
|
|
12
|
Anbalagan D, Yap G, Yuan Y, Pandey VK, Lau
WH, Arora S, Bist P, Wong JS, Sethi G, Nissom PM, et al: Annexin-A1
regulates microRNA-26b* and microRNA-562 to directly target NF-κB
and angiogenesis in breast cancer cells. PLoS One. 9:e1145072014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000.PubMed/NCBI
|
|
14
|
Goldberg RM, Sargent DJ, Morton RF, Fuchs
CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC and Alberts
SR: A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan, and oxaliplatin combinations in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
22:23–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Douillard JY, Cunningham D, Roth AD,
Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J,
Alakl M, et al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: A multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Longley DB, Harkin DP and Johnston PG:
5-Fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gottesman MM: Mechanisms of cancer drug
resistance. Annu Rev Med. 53:615–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gardner LB, Li Q, Park MS, Flanagan WM,
Semenza GL and Dang CV: Hypoxia inhibits G1/S transition through
regulation of p27 expression. J Biol Chem. 276:7919–7926. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, Turley H, Talks K, Pezzella F, Gatter KC and Harris AL: Relation
of hypoxia inducible factor 1 alpha and 2 alpha in operable
non-small cell lung cancer to angiogenic/molecular profile of
tumours and survival. Br J Cancer. 85:881–890. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo
C, Han S, Liu J, Sun S, Han Z, et al: Hypoxia-inducible factor-1
alpha contributes to hypoxia-induced chemoresistance in gastric
cancer. Cancer Sci. 99:121–128. 2008.PubMed/NCBI
|
|
22
|
An WG, Kanekal M, Simon MC, Maltepe E,
Blagosklonny MV and Neckers LM: Stabilization of wild-type p53 by
hypoxia-inducible factor 1alpha. Nature. 392:405–408. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ravi R, Mookerjee B, Bhujwalla ZM, Sutter
CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL and Bedi A:
Regulation of tumor angiogenesis by p53-induced degradation of
hypoxia-inducible factor 1alpha. Genes Dev. 14:34–44.
2000.PubMed/NCBI
|
|
24
|
Chen J, Ding Z, Peng Y, Pan F, Li J, Zou
L, Zhang Y and Liang H: HIF-1α inhibition reverses multidrug
resistance in colon cancer cells via downregulation of
MDR1/P-glycoprotein. PLoS One. 9:e988822014. View Article : Google Scholar : PubMed/NCBI
|