Introduction
The increasing worldwide incidence of cancer is a
serious global health problem. Despite significant advances in
cancer treatment and drug development, there is an urgent need for
additional and better tolerated anticancer drugs to treat primary
and recurrent cancers. Unfortunately, the development and discovery
of new anticancer drugs is expensive, time-consuming, and in the
worst case, must be terminated during clinical trials due to the
occurrence of intolerable side effects or poor effectiveness
(1,2).
Testing of already approved therapeutic drugs for
additional purposes, so-called drug repositioning, is a recently
growing approach to discover new applications for already existing
drugs (1,3). When screening tests or preclinical
data indicate a new possible application for a specific drug,
pre-existing knowledge on its pharmacokinetics, bioavailability,
and side effects facilitate the assessment of its possible
application in humans. Recent studies have indicated that HIV drugs
may represent a valuable source of possible new anticancer drugs
(4,5). In particular, the HIV protease
inhibitor nelfinavir was found to be highly active on a variety of
human cancer cells (6–10), and is currently being tested in
several clinical studies on cancer patients (7,11–13).
A screening of anti-retroviral drugs for their
efficacy on human cancer cells revealed a striking sensitivity of
cancer cells to the non-nucleoside reverse transcriptase inhibitor
(NNRTI) efavirenz (14–17). Recently we observed that efavirenz
could influence cell viability of endothelial cells (14). Moreover, this inhibition was
associated with an increase in oxidative stress markers,
endoplasmic reticulum (ER) stress markers, and autophagy (14). A cytotoxic effect has been
demonstrated on several tumor cell lines including colorectal,
pancreatic and the human leukemia cell lines Jurkat (acute T-cell
leukemia cells) (17). Blood cancer
cells are highly sensitive to cytostatic drugs but, depending on
the cancer type, often become resistant after initial therapy,
necessitating second and even third line treatment therapies
(7,11,18).
Thus, there is a need for additional new anticancer drugs that
induce specific cell death pathways in leukemia cells (11,18).
The present study describes the effect of efavirenz on the leukemia
cancer cell lines Jurkat (acute T-cell leukemia), HL60 (acute
promyelocytic leukemia), and IM9 (EBV-transformed
B-lymphoblastoid).
Materials and methods
Cells and cell culture
The human leukemia cell lines IM9 (ATCC CCL-159),
and HL60 (ATCC CCL-240) were purchased from ATCC/LGC Standards
(Wesel, Germany). Jurkat cells were provided by U. Schleicher
(Erlangen, Germany). Cells were cultured in RPMI-1640 medium
supplemented with 10% fetal calf serum and antibiotics at 37°C in a
humidified atmosphere with 5% CO2 and was performed as
previously described (11,14). All cell culture reagents were from
PAA Laboratories (Pasching, Austria).
Reagents
Efavirenz was from Bristol-Myers Squibb (Munich,
Germany) and recovered from 50 mg capsules by means of repeated
ethanol extraction and speed-vac concentration (Eppendorf
Concentrator 5301; Eppendorf, Hamburg, Germany). The efavirenz
extract was finally dissolved in 1 ml of ethanol to obtain a stock
solution of 50 mg/ml in ethanol.
Chemo-sensitivity assays (ATP and MTT
assay)
To test viability of cancer cells, the
bioluminescent ATP assay was performed as previously described
(14). For the ATP assay, 5,000
cells in a total volume of 200 µl were plated in flat bottom
96-well plates (Nunc, Wiesbaden, Germany) and incubated with the
indicated cytostatic drugs for 48 h at 37°C. For cell extraction,
50 µl tumor cell extraction buffer (DCS Innovative Diagnostic
Systems, Hamburg, Germany) was added to each well, mixed thoroughly
and incubated for 20 min at room temperature. Leukemia cells were
collected by centrifugation before extraction in 50 µl cell
extraction buffer. Using a MicroLumat LB 96P bioluminometer
(EG&G Berthold, Bad Wildbad, Germany), Luciferin-Luciferase
agent (DCS Innovative Diagnostic Systems) was added automatically
to each sample and analyzed for bioluminescence.
FACScan analyses
Annexin binding assay
FITC-labeled Annexin V (BioCat, Heidelberg, Germany)
was applied to viable cells as recommended by the supplier in
combination with propidium iodide and analyzed by FACScan with an
FL-1 setting (propidium iodide) of 575 nm and an FL-2 setting
(FITC) of 530 nm. FACScan analysis was performed using a Beckman
Coulter Epics XL-MCL flow cytometer (Beckman Coulter, Munich,
Germany).
Cell cycle analysis
For cell cycle analysis, HL60 cells were treated for
24 h with efavirenz, washed with PBS, fixed with 70% methanol, and
stained with 50 µg/ml propidium iodide in PBS, containing 1 mg/ml
RNAse (Sigma, Munich, Germany) prior to FACScan analysis (620 nm
filter).
Reactive oxygen species (ROS)
Detection of ROS by FACScan analysis used
2,7-dichlorodihydrofluorescein diacetate substrate (DCFH-DA;
Sigma). HL60 cells were treated with efavirenz for 24 h. Cells were
then incubated for 45 min with 1 µg/ml of DCF-DA in cell culture
medium under cell culture conditions, and subjected to FACScan
analysis with a 575 nm filter for detection of green
fluorescence.
Western blot analysis
For immunoblot analysis, cancer cells were cultured
in 10 cm diameter cell culture plates, and cell extracts were
prepared by cell lysis in RIPA-buffer (50 mM Tris, pH 8.0, 150 mM
NaCl, 1% NP-40, 0.5% doxycholine, 0.1% SDS) as previously described
(11,14). Samples containing 20 µg protein each
as determined by the Bio-Rad Bradford assay (Bio-Rad, München,
Germany) were subjected to SDS-polyacrylamide gel electrophoresis,
and proteins were transferred to PVDF membranes in a Bio-Rad Mini
Protean II (Bio-Rad) at 1 mA/cm2 membrane in 10%
methanol, 192 mM glycine, 25 mM Tris, pH 8.2. Membranes were
blocked with 4% non-fat milk powder in PBS with 0.05% Tween-20 for
4 h. Primary antibodies were applied in blocking buffer and
incubated at room temperature overnight. All primary antibodies
were purchased from Cell Signaling Technology (NEB, Frankfurt,
Germany). Secondary, alkaline phosphatase (AP)-coupled antibodies
against the corresponding primary antibodies were from Dianova
(Hamburg, Germany). AP detection was performed by the chromogenic
BCIP/NBT assay (Promega, Mannheim, Germany).
Results
Effect of efavirenz on cell survival and
apoptosis in leukemia-derived cell lines
The three leukemia-derived cell lines HL60 (acute
promyeolocytic leukemia), Jurkat (acute T-cell leukemia), and IM9
(B-lymphoblastoid) proved to be highly sensitive to efavirenz,
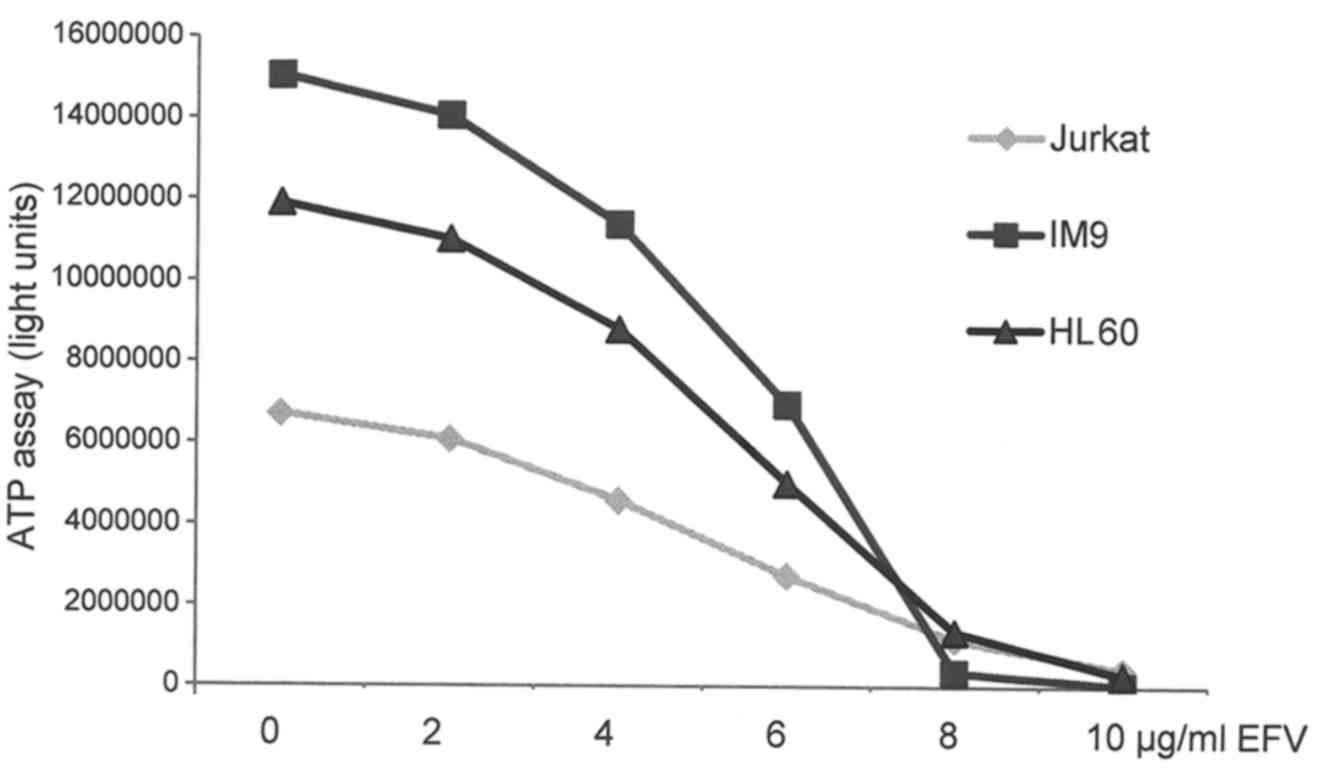
revealing a near complete loss of cell viability at a concentration
of 10 µg/ml of efavirenz (Fig. 1).
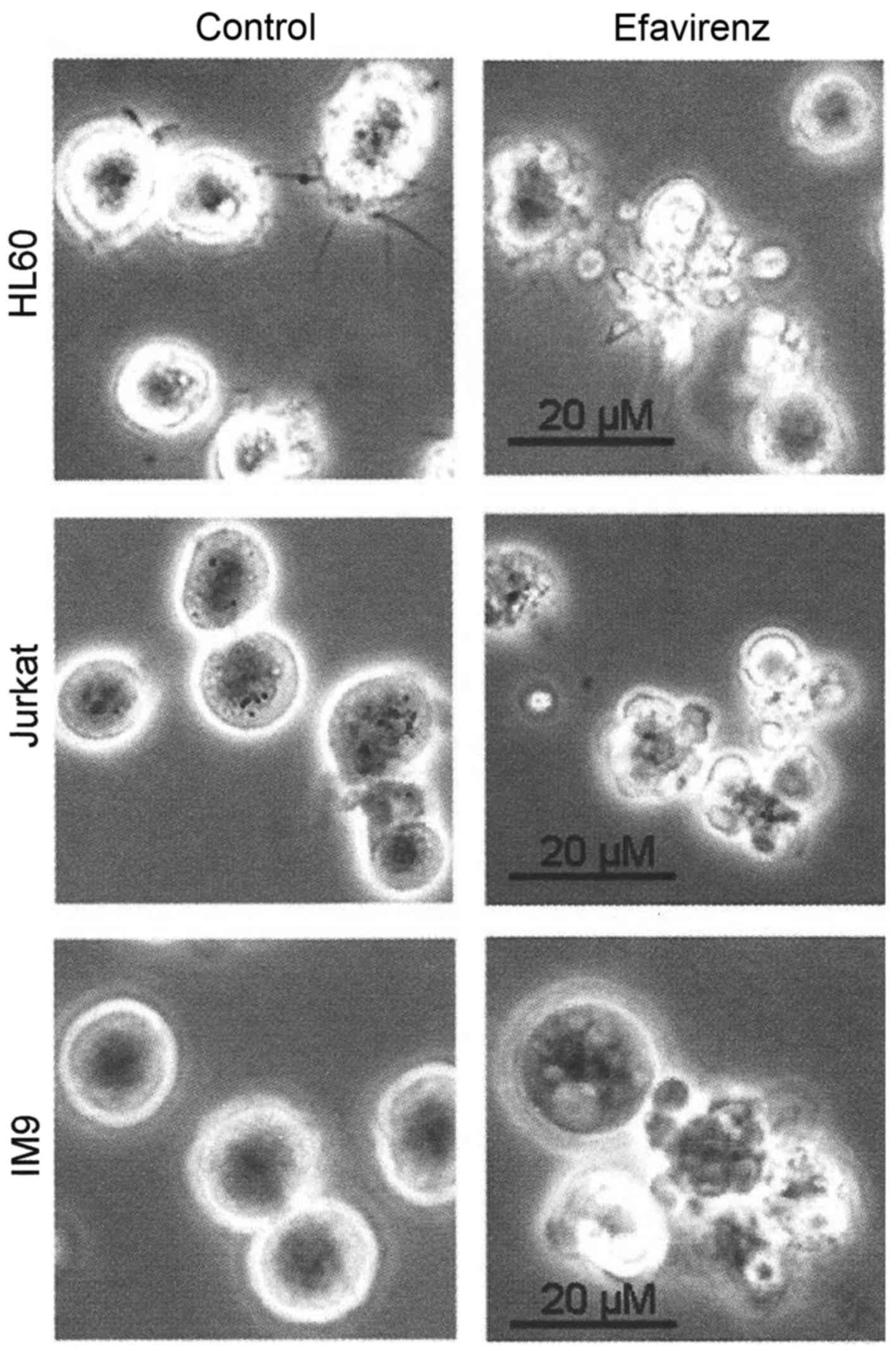
Analysis of efavirenz-treated leukemia cells by microscopy revealed
typical morphological signs of apoptosis, such as cellular
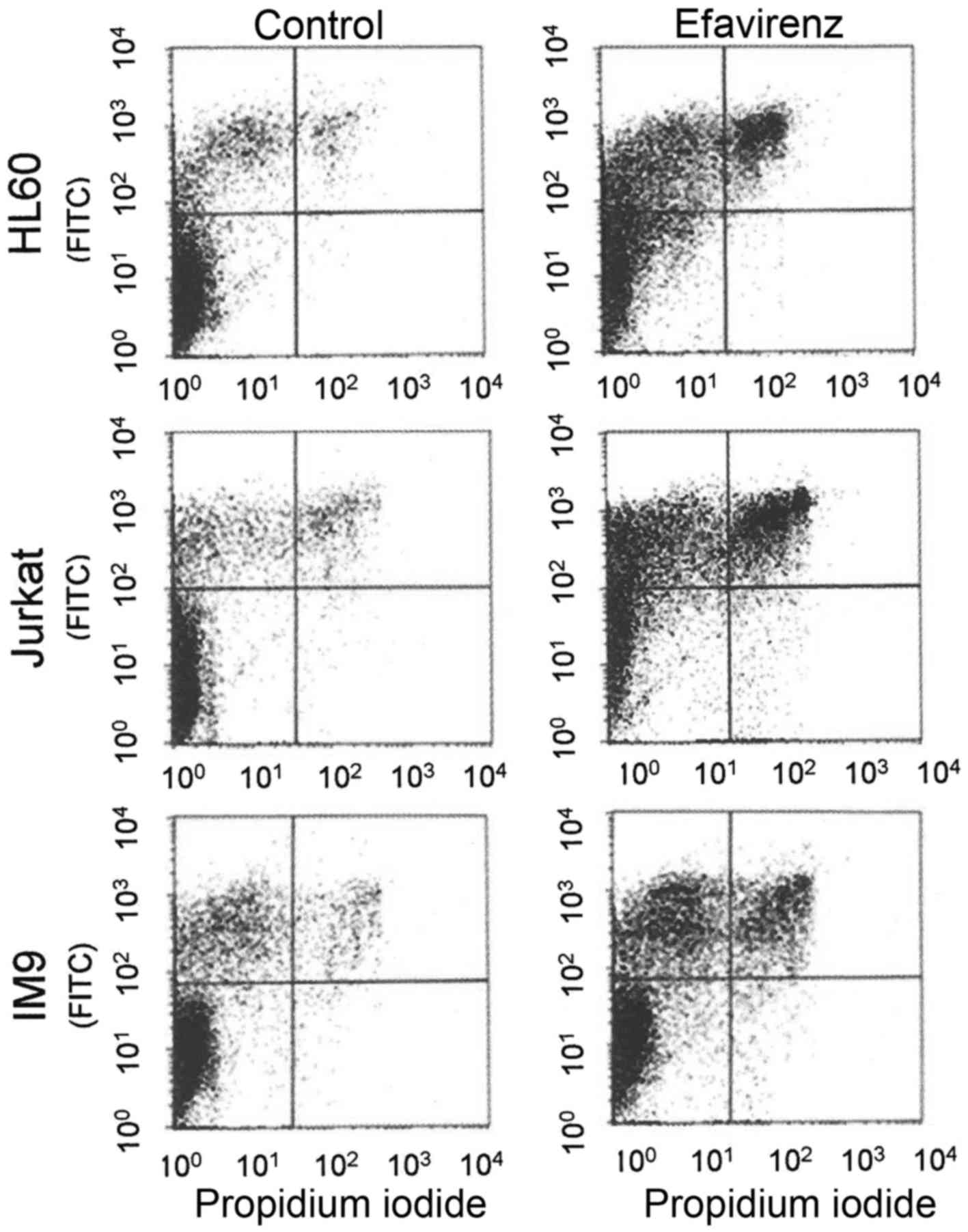
fragmentation and membrane blebbing (Fig. 2). FACScan analysis of
efavirenz-treated cancer cells by propidium iodide/Annexin staining
confirmed the occurrence of apoptosis in addition to necrosis by
biochemical means (Fig. 3).
Efavirenz induces activation of p53 and H2AX
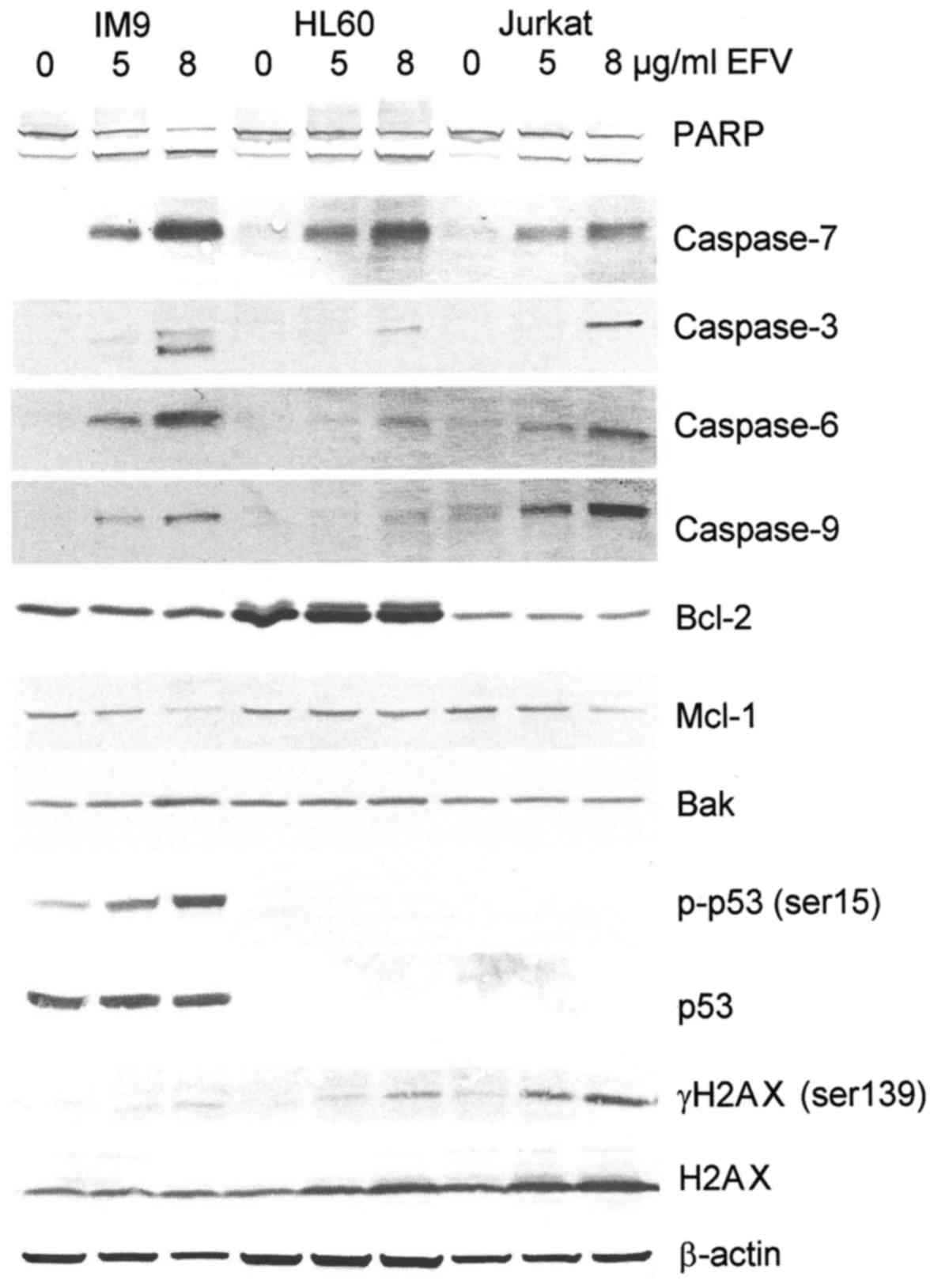
To gain further insight into the molecular and cell
biological effects of efavirenz in human cancer cells, western blot
analysis of efavirenz-treated cancer cells was performed (Fig. 4). Increasing concentrations of
efavirenz resulted in the cleavage of all relevant caspases of the
intrinsic apoptotic pathway (Fig.
4), confirming the induction of the canonical apoptotic
pathway. In IM9 and Jurkat cells, downregulation of the
anti-apoptotic mitochondrial membrane protein mcl-1 was observed
(Fig. 4). Enhanced phosphorylation
of p53 and upregulation of the p53 target gene bak were observed in
the p53 wild-type leukemia cell line IM9 (Fig. 4). As these effects were absent in
the p53 negative cell lines Jurkat and HL60, further
p53-independent pathways that contribute to cell death induced by
efavirenz were investigated. Because phosphorylation of p53 in IM9
cells indicated induction of DNA damage, the expression and
phosphorylation of the DNA damage-associated histone H2AX in
efavirenz-treated leukemia cells was tested. A strong increase in
H2AX phosphorylation (γH2AX) was observed in efavirenz-treated
Jurkat cells (Fig. 4) and, to a
lesser extent, in IM9 and HL60 cells (Fig. 4).
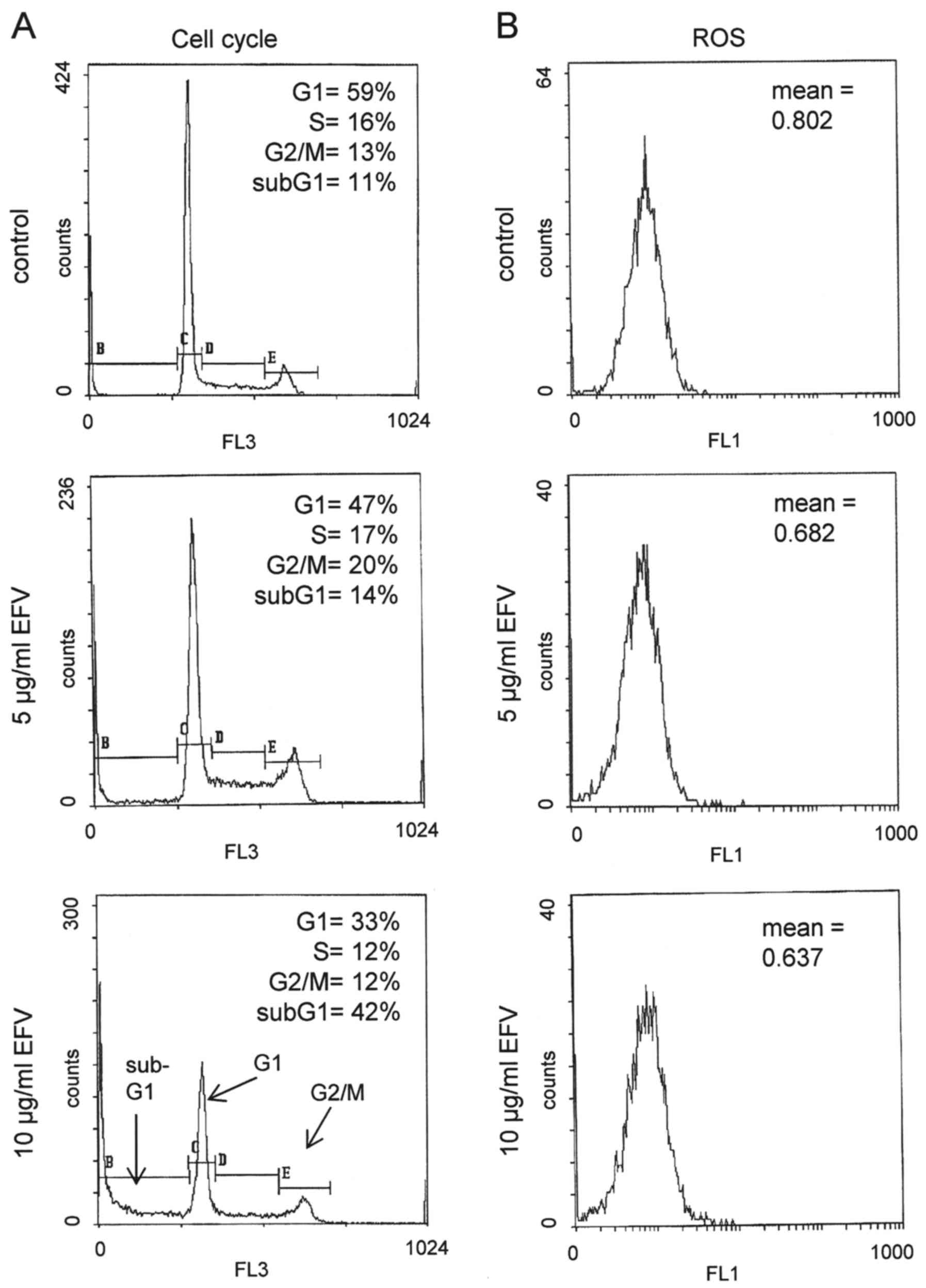
Effect of efavirenz on cell cycle progression and
generation of reactive oxygen species
Induction of DNA damage and activation of p53 often
results in cell cycle arrest, although, in the presence of
efavirenz, cell death by apoptosis appeared to prevail when higher
concentrations of efavirenz were applied. In fact, the application
of 5 µg/ml efavirenz increased the number of G2/M phase cells from
14 to 20%, whereas 10 µg/ml efavirenz predominantly caused cell
death in HL60 cells, as visualized by a high percentage of sub-G1
phase cells, representing apoptotic or necrotic cells (Fig. 5). Since DNA damage may also be a
direct, though unspecific effect of the generation of ROS, we also
analyzed the generation of ROS by efavirenz. Unexpectedly, no
generation of ROS could be detected in HL60 cells even when high
concentrations (10 µg/ml) of efavirenz were applied (Fig. 5).
Discussion
The results of the present study reveal that the HIV
drug efavirenz effectively induces apoptosis in leukemia cells.
This indicates a possible new perspective for the application of
efavirenz in the treatment of human non-solid cancer. Efavirenz
induces the classical apoptotic pathway associated with an enhanced
phosphorylation of H2AX, suggesting efavirenz-induced DNA damage as
a possible trigger for apoptosis. The H2AX histone subtype is not a
DNA repair enzyme itself, but forms a platform and recognition site
for DNA repair enzymes and DNA damage-associated signaling factors,
especially at DNA double strand breaks (19,20).
Recently we demonstrated that efavirenz can induce
cell stress and reduced cell proliferation of endothelial cells
(14). However, concentrations of
up to 10 µg/ml, which efficiently induce cell death in leukemia
cells, left endothelial cells viable and did not destroy
endothelial cell meshwork formations (14). Efavirenz has demonstrated a
significant anti-proliferative effect in pancreatic cancer cells
(15). A cytotoxic effect has been
demonstrated on several tumor cell lines including colorectal,
pancreatic and Jurkat leukemia cells (17). Interestingly, while a change in the
phosphorylation of ERK and AKT was not observed, the tumor
suppressor protein p53 revealed an increased activation of
phosphorylation (17). Moreover, a
synergistic effect with cannabinoid agonists has been observed
(17).
The exact mechanism of efavirenz-induced DNA damage
remains to be elucidated, especially since efavirenz does not
represent a nucleoside analogue. The induction of oxidative stress
has often been suggested as the mechanism mediating DNA damage
caused by xenobiotics (21).
Indeed, Apostolova et al recently described the induction of
oxidative stress in efavirenz-treated human hepatocytes and
hepatoma cells (22), and we have
recently described enhanced oxidative stress by efavirenz in human
endothelial cells (14). However,
we did not observe the induction of oxidative stress in
efavirenz-treated leukemia cancer cells as analyzed by the same
standardized ROS assay. Therefore, the different tissue sensitivity
against efavirenz resulting in oxidative stress still remains to be
elucidated.
In a phase II trial with 53 patients with a
metastatic castration-resistant prostate cancer (mCRPC), the use of
an increased dosage regime of efavirenz may be beneficial for
treatment (16). In the treatment
of leukemia, efavirenz could be tested as a single agent, based on
data showing that the effective drug concentrations (~10 µg/ml) are
not substantially higher than the doses currently used for the
long-term treatment of HIV-infected persons receiving daily
efavirenz treatment. The long-term pharmacological experience with
this drug, its oral bioavailability, tolerable side effects, and
its good compliance (23,24) are further aspects that could
facilitate the design and implementation of clinical trials on
efavirenz with leukemia patients.
Acknowledgements
We would like to thank Mrs. Martina Rahmeh
(Department of Obstetrics and Gynecology, Campus Grosshadern,
Ludwig-Maximilians University of Munich) for excellent technical
assistance.
References
|
1
|
Dueñas-González A, García-López P, Herrera
LA, Medina-Franco JL, González-Fierro A and Candelaria M: The
prince and the pauper. A tale of anticancer targeted agents. Mol
Cancer. 7:822008.
|
|
2
|
Zaenker KS and Entschladen F: Paving roads
for new drugs in oncology. Recent Patents Anticancer Drug Discov.
4:137–145. 2009. View Article : Google Scholar
|
|
3
|
Gupta SC, Sung B, Prasad S, Webb LJ and
Aggarwal BB: Cancer drug discovery by repurposing: Teaching new
tricks to old dogs. Trends Pharmacol Sci. 34:508–517. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bernstein WB and Dennis PA: Repositioning
HIV protease inhibitors as cancer therapeutics. Curr Opin HIV AIDS.
3:666–675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chow WA, Jiang C and Guan M: Anti-HIV
drugs for cancer therapeutics: Back to the future? Lancet Oncol.
10:61–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gills JJ, Lopiccolo J, Tsurutani J,
Shoemaker RH, Best CJ, Abu-Asab MS, Borojerdi J, Warfel NA, Gardner
ER, Danish M, et al: Nelfinavir, A lead HIV protease inhibitor, is
a broad-spectrum, anticancer agent that induces endoplasmic
reticulum stress, autophagy, and apoptosis in vitro and in vivo.
Clin Cancer Res. 13:5183–5194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brüning A, Gingelmaier A, Friese K and
Mylonas I: New prospects for nelfinavir in non-HIV-related
diseases. Curr Mol Pharmacol. 3:91–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiang T, Du L, Pham P, Zhu B and Jiang S:
Nelfinavir, an HIV protease inhibitor, induces apoptosis and cell
cycle arrest in human cervical cancer cells via the ROS-dependent
mitochondrial pathway. Cancer Lett. 364:79–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kushchayeva Y, Jensen K, Recupero A,
Costello J, Patel A, Klubo-Gwiezdzinska J, Boyle L, Burman K and
Vasko V: The HIV protease inhibitor nelfinavir down-regulates RET
signaling and induces apoptosis in medullary thyroid cancer cells.
J Clin Endocrinol Metab. 99:E734–E745. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun L, Niu L, Zhu X, Hao J, Wang P and
Wang H: Antitumour effects of a protease inhibitor, nelfinavir, in
hepatocellular carcinoma cancer cells. J Chemother. 24:161–166.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brüning A, Rahmeh M, Gingelmaier A and
Friese K: The mitochondria-independent cytotoxic effect of
nelfinavir on leukemia cells can be enhanced by sorafenib-mediated
mcl-1 downregulation and mitochondrial membrane destabilization.
Mol Cancer. 9:192010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rengan R, Mick R, Pryma D, Rosen MA, Lin
LL, Maity AM, Evans TL, Stevenson JP, Langer CJ, Kucharczuk J, et
al: A phase I trial of the HIV protease inhibitor nelfinavir with
concurrent chemoradiotherapy for unresectable stage IIIA/IIIB
non-small cell lung cancer: A report of toxicities and clinical
response. J Thorac Oncol. 7:709–715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shim JS, Rao R, Beebe K, Neckers L, Han I,
Nahta R and Liu JO: Selective inhibition of HER2-positive breast
cancer cells by the HIV protease inhibitor nelfinavir. J Natl
Cancer Inst. 104:1576–1590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weiss M, Kost B, Renner-Müller I, Wolf E,
Mylonas I and Brüning A: Efavirenz causes oxidative stress,
endoplasmic reticulum stress, and autophagy in endothelial cells.
Cardiovasc Toxicol. 16:90–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hecht M, Erber S, Harrer T, Klinker H,
Roth T, Parsch H, Fiebig N, Fietkau R and Distel LV: Efavirenz has
the highest anti-proliferative effect of non-nucleoside reverse
transcriptase inhibitors against pancreatic cancer cells. PLoS One.
10:e01302772015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Houédé N, Pulido M, Mourey L, Joly F,
Ferrero JM, Bellera C, Priou F, Lalet C, Laroche-Clary A, Raffin
MC, et al: A phase II trial evaluating the efficacy and safety of
efavirenz in metastatic castration-resistant prostate cancer.
Oncologist. 19:1227–1228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hecht M, Harrer T, Büttner M, Schwegler M,
Erber S, Fietkau R and Distel LV: Cytotoxic effect of efavirenz is
selective against cancer cells and associated with the cannabinoid
system. AIDS. 27:2031–2040. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Woyach JA and Johnson AJ: Targeted
therapies in CLL: Mechanisms of resistance and strategies for
management. Blood. 126:471–477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gochhait S, Dar S, Pal R, Gupta P and
Bamezai RN: Expression of DNA damage response genes indicate
progressive breast tumors. Cancer Lett. 273:305–311. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ivashkevich A, Redon CE, Nakamura AJ,
Martin RF and Martin OA: Use of the γ-H2AX assay to monitor DNA
damage and repair in translational cancer research. Cancer Lett.
327:123–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Monks TJ, Xie R, Tikoo K and Lau SS:
Ros-induced histone modifications and their role in cell survival
and cell death. Drug Metab Rev. 38:755–767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Apostolova N, Gomez-Sucerquia LJ, Moran A,
Alvarez A, Blas-Garcia A and Esplugues JV: Enhanced oxidative
stress and increased mitochondrial mass during efavirenz-induced
apoptosis in human hepatic cells. Br J Pharmacol. 160:2069–2084.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Best BM and Goicoechea M: Efavirenz -
still first-line king? Expert Opin Drug Metab Toxicol. 4:965–972.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maggiolo F: Efavirenz: A decade of
clinical experience in the treatment of HIV. J Antimicrob
Chemother. 64:910–928. 2009. View Article : Google Scholar : PubMed/NCBI
|