Introduction
The mitogen extracellular-signal-regulated kinase
kinase 5/extracellular-signal-regulated kinase 5 (MEK5/Erk5) signal
pathway was first characterized in 1995 (1) and categorized as a member of MEK/Erk
family. MEK5 is a 444 amino acid protein with a mass of 50 kDa
(MEK5α) and 40 kDa (MEK5β), respectively (2). MEK5 protein conserves a partial domain
of other MEK family kinases such as MEK1 and MEK2 with an overall
40% homology (1). Physiologically,
MEK5/Erk5 pathway has its functional role during normal muscle and
neuronal development (3–5). It has been reported that MEK5
regulates skeletal and myocardium development through upregulation
of Erk5 and MEF2 (6). Recent
studies showed that targeted deletion of MEK5 led to embryonic
lethality due to blockade of the MEK5/Erk5 signal pathway, which
normal cardiac and embryonic development depends on (7,8). These
studies suggest that MEK5/Erk5 is an essential regulatory signal
pathway for stem cell survival. MEK5 is the only known and
characterized Erk5 kinase direct regulator. MEK5/Erk5 signaling
pathway regulates a number of transcription factors. For example,
MEF2, c-Fos, Fra-1 and NF-κB (6,9–11).
Muscle differentiation and neuronal survival have been implicated
to be associated with Erk5-regulated MEF2 signaling in vitro
studies (7).
In addition, a few reports demonstrated that MEK5
protein was overexpressed and associated with certain types of
cancer (12,13), for example, cancers of breast, lung,
colorectal, prostate and leukemia and neuroblastoma (14–22).
Besides, MEK5/Erk5 signal pathway has been reported to regulate
angiogenesis and tumor cell proliferation (12,23).
Notably, MEK5 has also been observed to play a role in EMT during
breast cancer progression and metastasis by proteomics analysis
(16,24,25).
However, the function and regulation of MEK5 signaling in cancer
metastasis remain to be elucidated in detail.
With respect to the regulation of MEK5 upstream and
downstream signal pathways, some reports demonstrated that MEK5 was
regulated by TNF-α/JNK signal pathway (26,27).
MEKK2 and MEKK3 have been demonstrated as MEK5/Erk5 direct upstream
regulators (28–30). It has been proved that MEK5
signaling function acts through regulating MEF2C, a member of the
MEF2 transcription factor family (6). MEK5 has been reported to promote
prostate cancer metastasis through upregulation of MMP9 (14). Erk5 is MEK5-regulated immediate
downstream kinase that was responsible for further activating
MMP-9, which functions to enhance cancer cell migration and
invasion. Moreover, MEK5 expression was also associated with poor
prognosis in prostate cancer patients. MEK5 is also overexpressed
in breast cancer (16), especially
in cells that Stat3 signaling is persistently activated (31). The overexpression of constitutively
active Stat3 (Stat3C) could upregulate total level of MEK5 protein
expression and activation in breast cancer, suggesting that Stat3
might participate in MEK5 upstream regulation in development of
breast cancer. Stat3 is frequently expressed and activated in
triple-negative breast cancer (TNBC). In addition, MEK5 direct
downstream target, Erk5, has been identified as an important factor
responsible for poor prognosis and low survival rate of TNBC. This
evidence suggests that MEK5/Erk5 inhibitors could have potential to
mitigate malignancy and improve outcomes of TNBC (32–34).
Therefore, therapeutical targets related to kinases, which are
responsible for EMT such as Erk5 and MAPK7, are attractive for
developing new generation of anticancer drugs (34,35).
In order to understand cellular regulatory role of
MEK5 signaling in breast cancer invasion and metastasis, we
compared MEK5 expression and activation in comparison of invasive
and non-invasive breast cancer in the present study. Our data
demonstrated that MEK5 was activated in invasive breast cancer cell
lines that we tested, but not non-invasive breast cancer cell line
and/or immortalized cell line. Ectopic expression of MEK5 could
lead to non-invasive MCF7 breast cancer cells to change morphology
through EMT. The knockdown of MEK5 in highly invasive MDA-MB-231
cells resulted in the loss of its invasive and metastatic ability.
The mechanism study suggested that active Stat3 was closely
correlated with high level of MEK5 expression and activation.
Together, our observations suggested that MEK5 expression was
transcriptionally upregulated by Stat3 activation.
Although active Stat3 was critical to activate MEK5
and further induce cancer cell EMT, Stat3 activation was not
essential to initiate these cellular processes. Instead, we
provided evidence to show that the activation of MEK5 through
serine/threonine phosphorylation was essential for the initiation
of cell invasion and metastasis. Taken together, the present study
unveiled a new insight into the mechanism by which
transcriptionally upregulated MEK5 by active Stat3 was essential to
initiate cell EMT and resulted in cancer cell invasion and
metastasis. The significance of our observations may implicate that
the blockade of Stat3 and MEK5/Erk5 pathways could potentially
benefit the prevention of breast cancer metastasis (13,24,25,36).
Materials and methods
Cell lines and chemicals
MCF-7, MCF10A, MDA-MB-231 and MDA-MB-468 cell lines
were purchased from the American Type Culture Collection (ATCC;
http://www.atcc.org/). Spontaneous immortalized
MCF10A breast cells (37) were
cultured as previously described (31). Breast carcinoma cell lines, MCF-7,
MDA-MB-231 and MDA-MB-468, were cultured in Dulbeccos modified
Eagles medium (DMEM) containing 10% fetal bovine serum (FBS) with
appropriate antibiotics. All chemical reagents were purchased from
Sigma-Aldrich (St. Louis, MO, USA) or Thermo Fisher Scientific
(Pittsburgh, PA, USA) and were of analytically pure grade if not
otherwise noted.
Western blot analysis
For western blot analysis, 100 µg of cell lysate was
resolved on SDS-PAGE and transferred to PVDF membrane. Antibodies
against MEK5 (BD Transduction Laboratories, San Diego, CA, USA),
phos-Stat3 (Tyrosine705), Stat3, Erk5, phospho-Erk5 (Cell Signaling
Technology, Inc., Beverly, MA, USA), phospho-MEK5 (S311/T315),
phospho-MEKK1, MEKK1, MEKK2, MEKK3 (Abcam, Cambridge, MA, USA),
phospho-MEKK2 (Thermo Fisher Scientific), phospho-MEKK3
(Sigma-Aldrich), E-cadherin, N-cadherin, Snail, Slug, vimentin
(Santa Cruz Biotechnology, Santa Cruz, CA, USA) and tubulin
(Sigma-Aldrich) were used to detect corresponding proteins,
respectively.
Cell invasion assay
Cell invasion assay was performed using an invasion
chamber system (BD Transduction Laboratories) following the
manufacturers instructions. In brief, 2.5×104 cancer
cells in 0.5 ml culture medium were seeded into upper compartment
in two layer chamber invasion plates. The cells were cultured for
24 h prior to staining of the Matrigel membrane. Cell penetration
through the membrane was detected by staining the cells on the
porous membrane with a Diff-Quik stain kit (Dade Behring, Inc.,
Newark, DE, USA). Each experimental group was performed at least in
triplicate. The results are denoted as mean ± standard deviation
(SD).
Chromatin immunoprecipitation (ChIP)
assays and real-time PCR
To detect Stat3 binding with MEK5 promoter region, a
ChIP assay kit (Upstate Biotechnology, Lake Placid, NY, USA) was
employed in the present study. The assay was performed according to
the manufacturers instructions and protocols previously described
(31). For measuring MEK5
expression, a real-time PCR was performed and relative expression
level was calculated accordingly.
Infection of breast cancer cells by
viral constructs
The viral plasmid constructs, pWZL-MEK5 and
pMX-Stat3C were used for expression of MEK5 and active Stat3C,
respectively. The construct for expression of constitutively
activated MEK5DD (S311D/T315D) was generated by site-directed
mutagenesis and confirmed by DNA sequencing. MEK5 knockdown was
achieved by using lentiviral shRNA particles (Santa Cruz
Biotechnology) following the manufacturers instructions. Breast
cancer MCF-7 or MD-MB-231 cells were infected for expression of
Stat3 or MEK5 following standard procedures. The infected cells
were selected by neomycin for 3 weeks and pooled for next
experiments or further infection by lentiviral MEK5 shRNA, if
needed. The MDA-MB-231 cells were infected by lentiviral MEK5 shRNA
and selected by puromycin for two weeks and pooled for assays.
Immunohistochemical (IHC)
analysis
Human breast cancer tissue array slides were
obtained from the National Cancer Institute (Philadelphia, PA, USA)
and Chemicon International, Inc. (Temecula, CA, USA).
Immunostaining was conducted by using Chemicon IHC Select™
detection system (Chemicon International). The array slides were
stained and probed by using relevant antibodies according to the
standard procedures and the manufacturers instructions.
Statistical analysis
The data are described as mean ± standard derivation
or odds ratio. Statistical analyses were performed by the Students
t-test, ANOVA and Chi-square analysis. P<0.05 was considered as
statistically significant.
Results
MEK5 is overexpressed in Stat3
activated breast cancer cells, but not in normal breast epithelial
cells or non-invasive cells
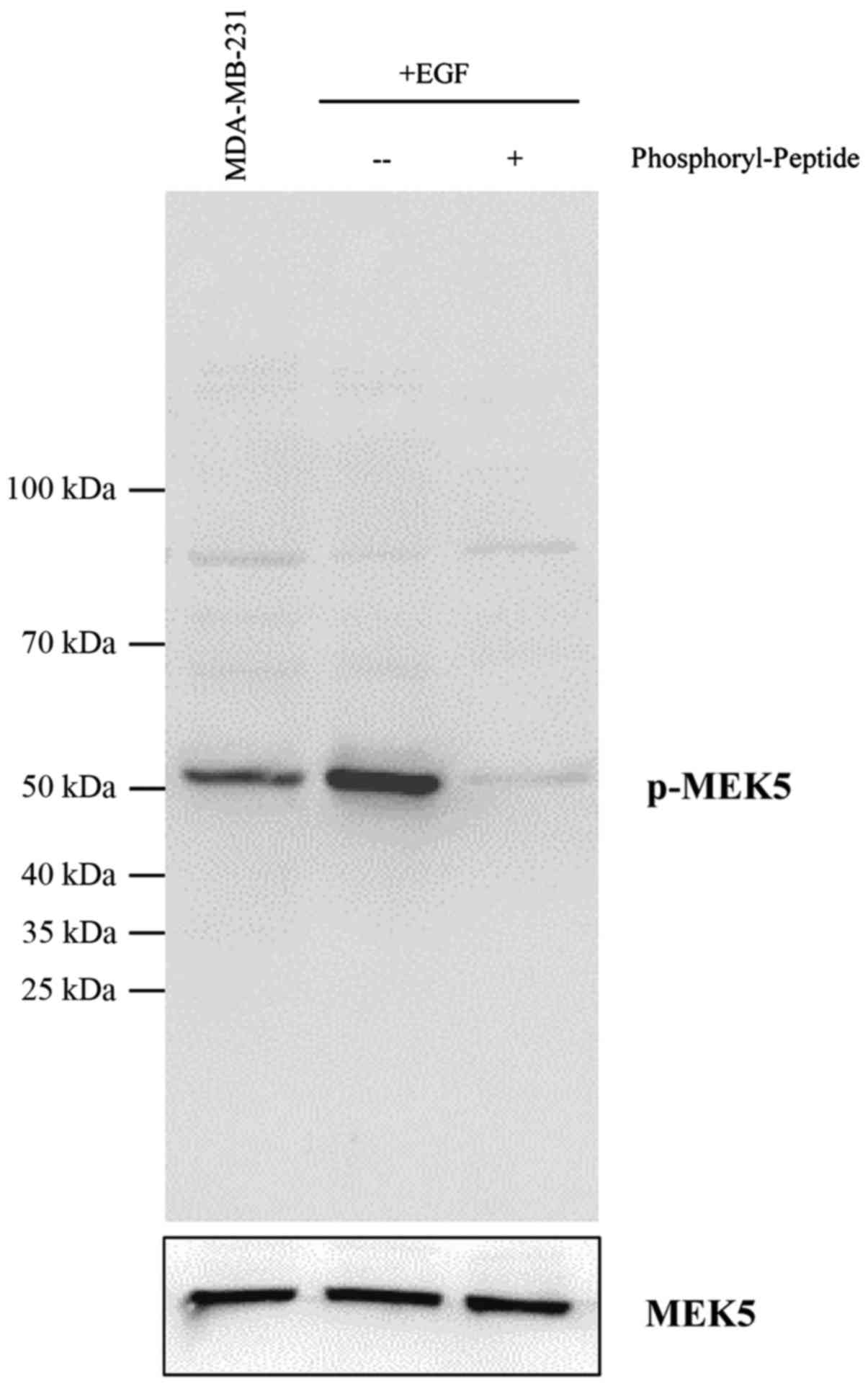
We previously showed that MEK5 was overexpressed in
a majority of breast cancer cells that we tested (31). To prove that this is the case in
breast cancer tissues, we first evaluated an appropriate
anti-phospho-MEK5 antibody that is suitable for tissue
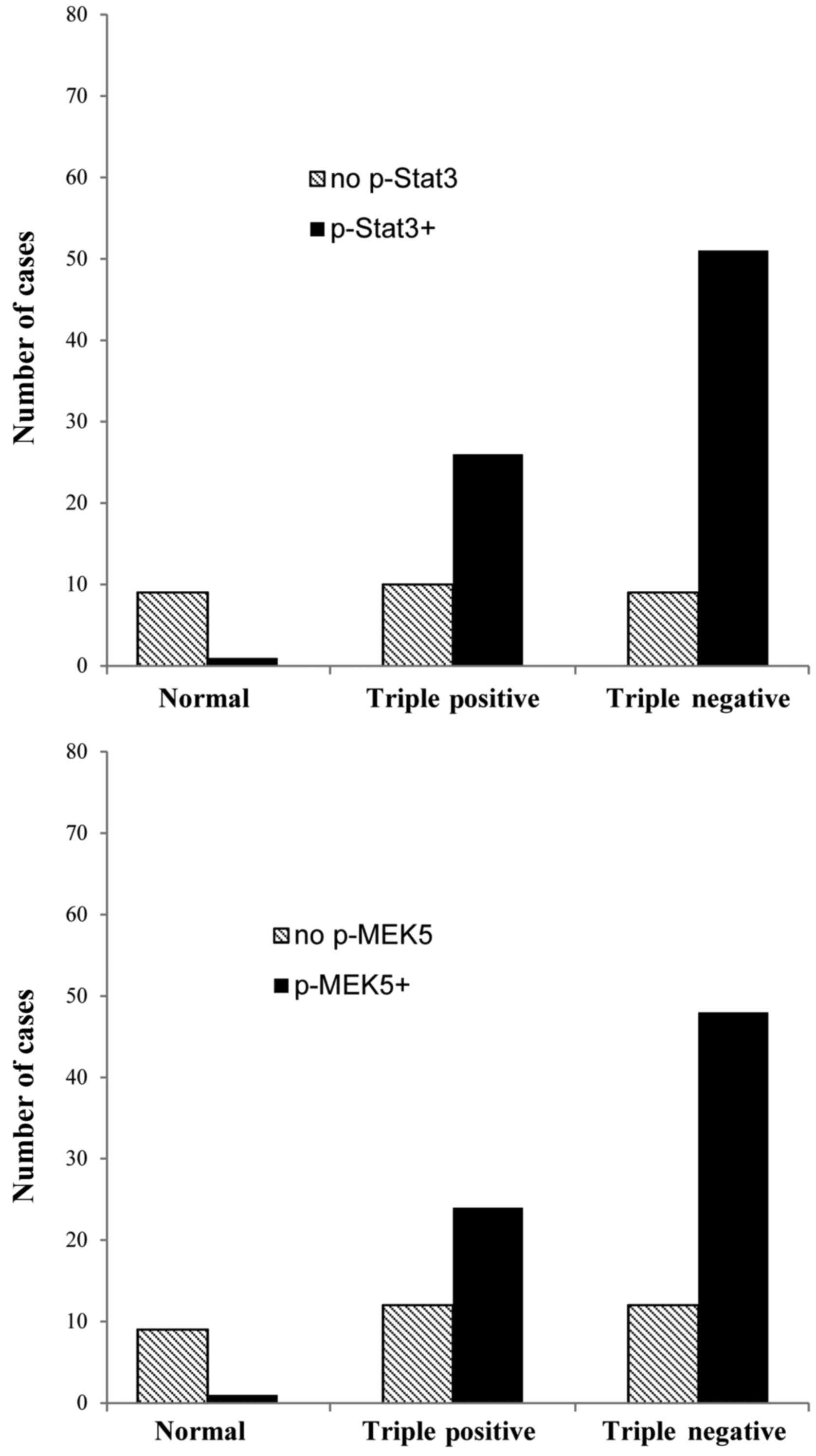
immunostaining (Fig. 1). A group of
samples with 106 cases, including 10 normal breast tissues, 36
triple-positive tissues and 60 TNBCs, were studied in this
investigation. The results showed that odds ratio of Stat3 and MEK5
phosphorylation in breast cancer tissues is 36.47 (P<0.001) and
25.92 (P<0.001) respectively, which was significantly higher
than that in normal tissues (Fig.
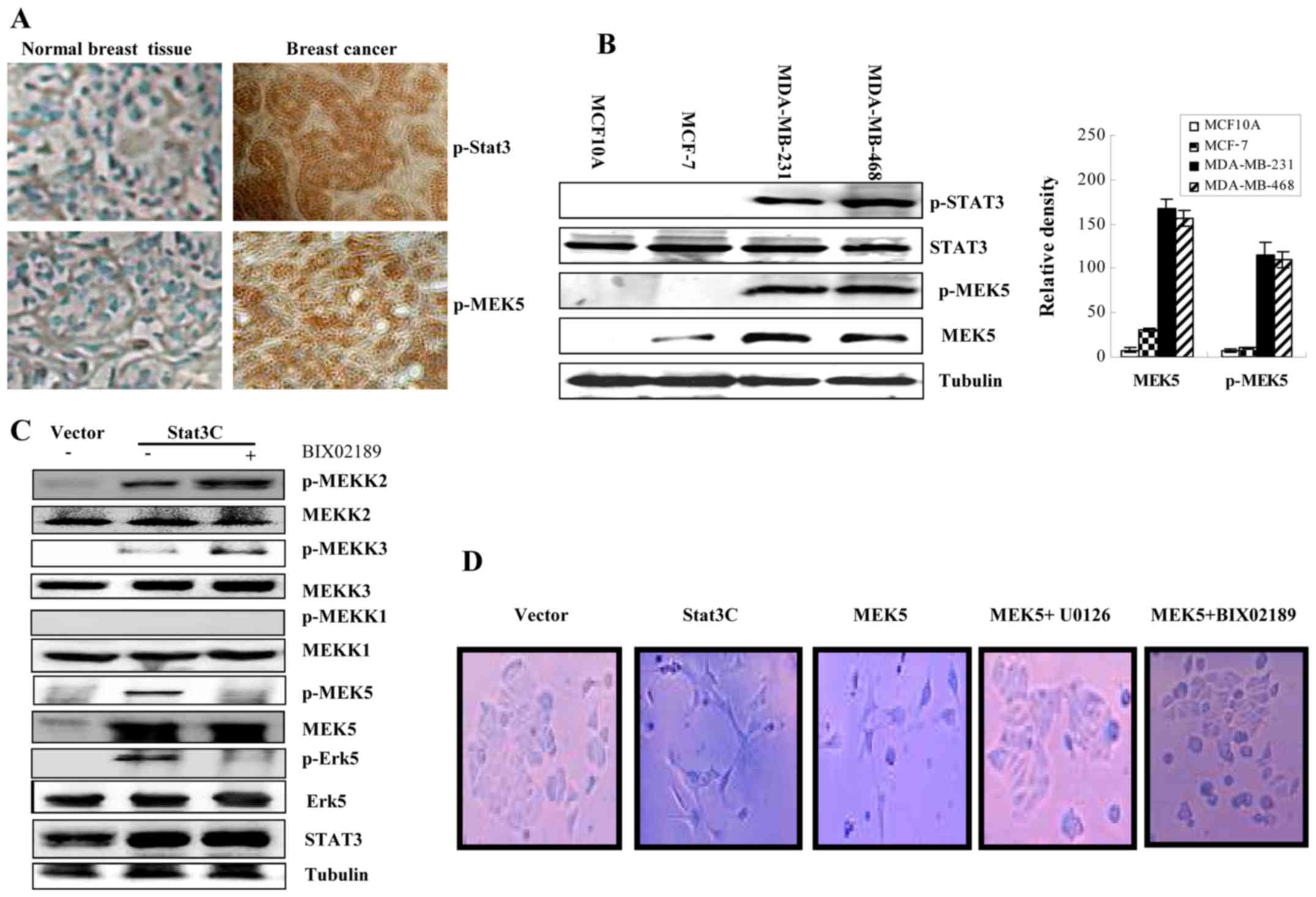
2). As Fig. 3A demonstrates,
breast tissue IHC staining showed that active Stat3 was correlated
with high level of MEK5 expression and activation. MEK5 is
overexpressed and activated in invasive breast cancer cell lines
MDA-MB-231 and MDA-MB-468, but not in immortalized MCF10A cells.
Although MEK5 protein could be detected in non-invasive MC-F7 cells
(Fig. 3B), the phosphorylation of
MEK5 was undetectable, suggesting that MEK5 might be inactive.
Further studies identified that constitutively activated Stat3
could increase phosphorylation of MEKK2/3 but not MEKK1. MEK5/Erk5
specific inhibitor, BIX02189 can reduce Stat3-mediated
phosphorylation of MEK5/Erk5. However, phosphorylation of MEKK2/3
was not affected by such an inhibitor (Fig. 3C). Ectopic expression of either
active Stat3C, which could mimic active phosphorylated Stat3 by
constitutively forming dimers or MEK5 caused the change of cell
morphology. The inhibition of MEK5 by MEK5/Erk5 inhibitor could
block such a change of cell morphology (Fig. 3D). Together, suggesting that both
active Stat3 and MEK5 are able to induce cancer cells to change
morphology and MEK5 may be activated by MEKK2/3 and play an
important role in this cellular process.
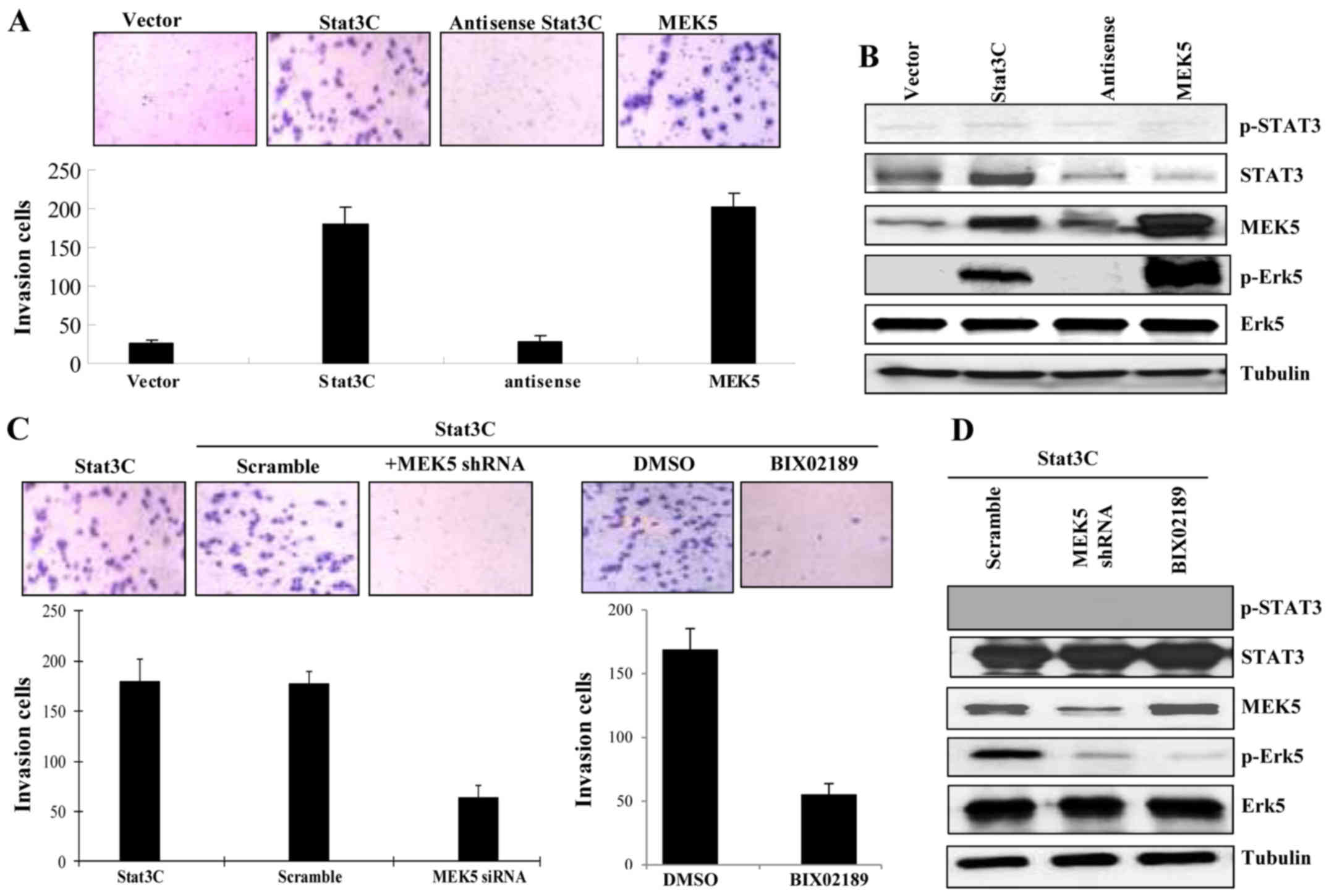
MEK5 promotes non-invasive MCF-7 cells
to become invasive cancer cells
MCF-7 cells are non-invasive breast cells and were
negative for EMT markers such as vimentin (38–40).
However, upon ectopic expression either Stat3C or MEK5, MCF-7 cells
became invasive (Fig. 4A). Western
blot analysis from cell lysates showed that ectopic expression of
both Stat3C and MEK5 could activate the MEK5/Erk5 signal pathway
through serine or threonine phosphorylation (Fig. 4B). Both knockdown of MEK5 by shRNA
and inhibition of MEK5 by a MEK5/Erk5 inhibitor BIX02189 could
block Stat3C-induced invasiveness in the cells that ectopically
expressed active Stat3C (Fig. 4C and
D), suggesting that active Stat3 acted through activation of
MEK5 and MEK5/Erk5 signal pathway played a key role in this
cellular process. Together, these observations indicate that MEK5
may be essential for regulating State3 mediated breast cancer cell
invasion and metastasis.
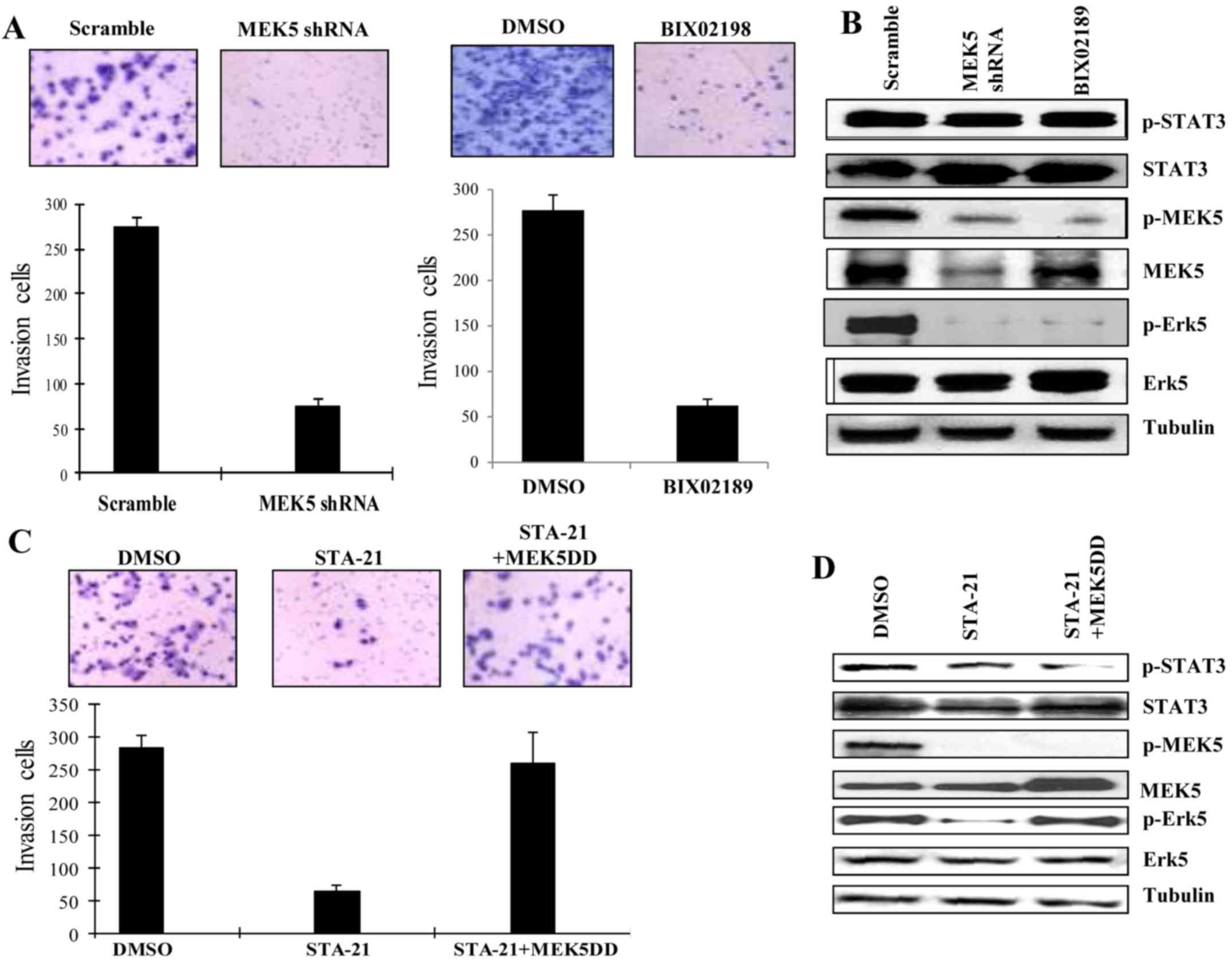
Knockdown of MEK5 inhibits cell
invasion of invasive MDA-MB-231 breast cancer cells
MDA-MB-231 cells express high level of activated
Stat3 and MEK5 and aggressively metastasize to the lung. Knockdown
of MEK5 or treatment by a MEK5 inhibitor could significantly
inhibit its invasive phenotype through blockade of activation of
MEK5/Erk5 pathway (Fig. 5A and B).
The inhibition of Stat3C by a Stat3 inhibitor, STA-21 (41), also reduced its invasive ability
(Fig. 5C and D). However, in the
cells that expressed constitutively active MEK5DD (S311D/T315D),
STA-21 was not able to inhibit cell invasion. This result suggests
that Stat3 functioned through MEK5 activation, which was essential
for EMT and cell invasion. However, the inhibition of MEK5 by both
shRNA and a MEK5/Erk5 inhibitor, BIX02189, significantly reduced
invasion (Figs. 4 and 5). Even though MDA-MB-231 expressed
constitutively activated Stat3C, the blockade of MEK5 was still
able to impede cell invasion (Fig.
4C). These results suggest that MEK5 plays a key role in the
regulation of breast cancer cell invasion. To summarize, this
evidence indicates that Stat3-induced cancer cell invasion and
metastasis may, at least in part, depend on activation status of
MEK5. In other words, MEK5 was essential for enabling breast cancer
invasion and further metastasis.
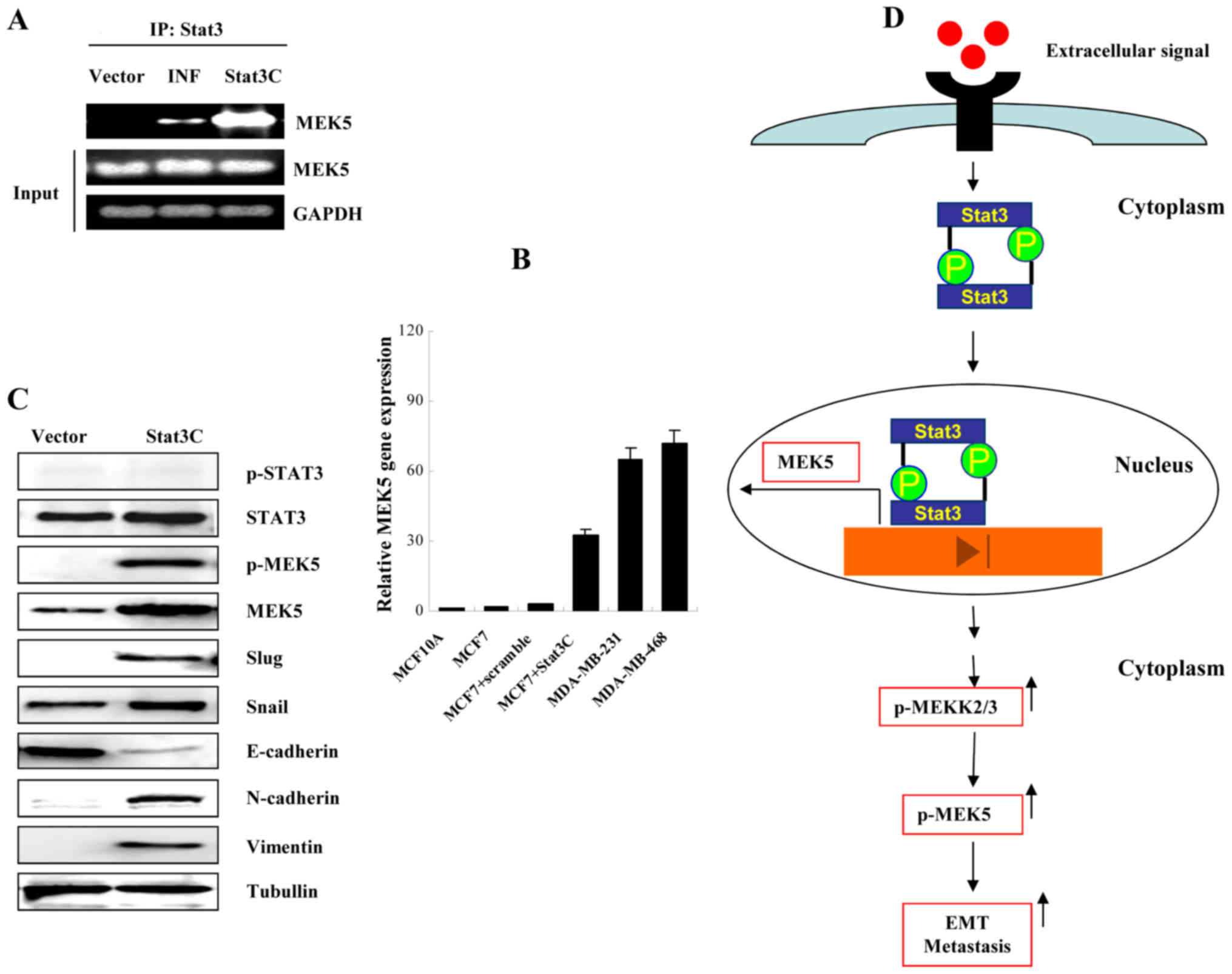
Stat3 transcriptionally upregulates
MEK5 expression
We previously reported that Stat3 directly
upregulated MEK5 expression through microarray analysis. As
reported by us (31), there are
twelve candidate Stat3-binding sites that exist in the 3.2 kb MEK5
promoter region. Ten out of the twelve Stat3-binding sites are
located at the position from −1776 to −1037 upstream of the
transcription initiation site. The sequence TTCTGGAAA between −1770
and −1762 was used for ChIP assay. As showed in Fig. 6A, interferons and active Stat3C were
able to induce MEK5 expression suggesting that Stat3 directly
regulated MEK5 in its promoter region (Fig. 6A). Real-time PCR also demonstrated
that Stat3C enhanced MEK5 mRNA expression (Fig. 6B). Further study showed that active
Stat3C was a causative factor that increased MCF-7 cells to express
EMT markers, including increased expression of MEK5, p-MEK5,
N-cadherin, vimentin, slug, snail and a decrease of E-cadherin
(Fig. 6C). Together, these data
support that active Stat3-mediated upregulation of MEK5 directly
contributed to cancer cell EMT, which subsequently resulted in
cancer invasion and metastasis. To illustrate this concept, we
propose a model that explains a possible mechanism of MEK5-induced
breast cancer cell EMT and metastasis (Fig. 6D). In summary, either
stimulus-activated or constitutively activated Stat3 could
transcriptionally upregulate MEK5 expression and activate MEK5
through increasing phosphorylation of MEKK2/3. Subsequently, an
increased level of active MEK5 causes breast cancer cell EMT and
metastasis. During this process, MEK5/Erk5 plays an essential role
in breast cancer invasion and metastasis.
Discussion
Stat3 plays a critical role in development of normal
embryonic stem cells and tumorigenesis. Constitutively activated
Stat3 was linked to various types of cancer such as cancers of the
breast, prostate, lung, brain, colorectal and leukemia. Moreover,
Stat3 has also been reported to be involved in the initiation and
progression of cancer stem cells, including glioblastoma, hepatoma,
sarcoma, breast, head and neck and skin cancer (42–47).
In addition, Stat3 seems to be a key modulator that regulates
cancer cell EMT and promotes cancer metastasis (16,46,48).
However, our observations suggest that Stat3 has to act through
MEK5 in order to trigger breast cancer invasion and metastasis. It
is of interest that similar observation of MEK5-promoted EMT has
also been reported (16,24). Therefore, this evidence indicates
that Stat3-MEK5/Erk5 pathway has a critical role in the regulation
of EMT, thereafter cancer metastasis.
Although MEK5/Erk5 pathway plays an important role
in various cellular processes (1,2,49,50),
little is known about how MEK5 functions during the development of
tumorigenesis and/or cancer metastasis. MEK5 overexpression has
been reported in invasive breast and prostate cancer (14,32).
Especially in prostate cancer, MEK5 has been reported to play a
pivotal role in prostate cancer progression and metastasis.
However, the underlying molecular mechanism by which MEK5 regulates
these cellular processes remains elusive. The understanding of the
role of MEK5/Erk5 pathway in cancer metastasis may help to develop
an effective cancer therapeutic regimen and prevent cancer from
reoccurrence and/or metastasis.
In the present study, we observed that
constitutively activated Stat3 not only increased the level of MEK5
expression, but also enhanced MEK5 phosphorylation by increasing
phosphorylated MEKK2 and MEKK3. One possibility could be that
Stat3-mediated stress responses are similar to oxidative stress
response. Further study needs to be conducted in order to
understand molecular mechanisms of how activated Stat3 can augment
phosphorylation of MEKK2/3.
MEK5 played an essential role in controlling cancer
cell EMT and subsequent metastasis. The evidence from ectopic
expression and knockdown of MEK5 suggested that MEK5 is a key
player in these processes. Although Stat3 constitutive activation
alone could activate the cancer cell EMT process, blockade of MEK5
by either shRNA or a small molecule inhibitor could impede this
development, suggesting that Stat3 acted through MEKK2/3-activated
MEK5 (Figs. 3C and 6D). Taken together, these data suggest
that MEK5 may play a pivotal role in the initiation of cancer cell
invasion and metastasis. MEK5 participated in regulation of breast
cancer progression and metastasis through increasing cancer cell
EMT. Stat3 along with MEK5 could possibly be effective therapeutic
targets for the treatment of breast cancer, especially TNBCs. The
implications of the present study may suggest that blockade of
Stat3 and MEK5/Erk5 pathways along with other conventional
interventions could potentially benefit breast cancer therapy and
improve therapeutic outcomes by blocking cancer cell
metastasis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China NSFC 31271495 (to H.S.).
References
|
1
|
Zhou G, Bao ZQ and Dixon JE: Components of
a new human protein kinase signal transduction pathway. J Biol
Chem. 270:12665–12669. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
English JM, Vanderbilt CA, Xu S, Marcus S
and Cobb MH: Isolation of MEK5 and differential expression of
alternatively spliced forms. J Biol Chem. 270:28897–28902. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dinev D, Jordan BW, Neufeld B, Lee JD,
Lindemann D, Rapp UR and Ludwig S: Extracellular signal regulated
kinase 5 (ERK5) is required for the differentiation of muscle
cells. EMBO Rep. 2:829–834. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shalizi A, Lehtinen M, Gaudilliere B,
Donovan N, Han J, Konishi Y and Bonni A: Characterization of a
neurotrophin signaling mechanism that mediates neuron survival in a
temporally specific pattern. J Neurosci. 23:7326–7336.
2003.PubMed/NCBI
|
|
5
|
Liu L, Cavanaugh JE, Wang Y, Sakagami H,
Mao Z and Xia Z: ERK5 activation of MEF2-mediated gene expression
plays a critical role in BDNF-promoted survival of developing but
not mature cortical neurons. Proc Natl Acad Sci USA. 100:8532–8537.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kato Y, Kravchenko VV, Tapping RI, Han J,
Ulevitch RJ and Lee JD: BMK1/ERK5 regulates serum-induced early
gene expression through transcription factor MEF2C. EMBO J.
16:7054–7066. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Merritt AJ, Seyfried J, Guo C,
Papadakis ES, Finegan KG, Kayahara M, Dixon J, Boot-Handford RP,
Cartwright EJ, et al: Targeted deletion of mek5 causes early
embryonic death and defects in the extracellular signal-regulated
kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol Cell
Biol. 25:336–345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan L, Carr J, Ashby PR, Murry-Tait V,
Thompson C and Arthur JS: Knockout of ERK5 causes multiple defects
in placental and embryonic development. BMC Dev Biol. 3:11–32.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Terasawa K, Okazaki K and Nishida E:
Regulation of c-Fos and Fra-1 by the MEK5-ERK5 pathway. Genes
Cells. 8:263–273. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pearson G, English JM, White MA and Cobb
MH: ERK5 and ERK2 cooperate to regulate NF-kappaB and cell
transformation. J Biol Chem. 276:7927–7931. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kato Y, Zhao M, Morikawa A, Sugiyama T,
Chakravortty D, Koide N, Yoshida T, Tapping RI, Yang Y, Yokochi T,
et al: Big mitogen-activated kinase regulates multiple members of
the MEF2 protein family. J Biol Chem. 275:18534–18540. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lochhead PA, Gilley R and Cook SJ: ERK5
and its role in tumour development. Biochem Soc Trans. 40:251–256.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Drew BA, Burow ME and Beckman BS:
MEK5/ERK5 pathway: The first fifteen years. Biochim Biophys Acta.
1825:37–48. 2012.PubMed/NCBI
|
|
14
|
Mehta PB, Jenkins BL, McCarthy L, Thilak
L, Robson CN, Neal DE and Leung HY: MEK5 overexpression is
associated with metastatic prostate cancer, and stimulates
proliferation, MMP-9 expression and invasion. Oncogene.
22:1381–1389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramsay AK, McCracken SR, Soofi M, Fleming
J, Yu AX, Ahmad I, Morland R, Machesky L, Nixon C, Edwards DR, et
al: ERK5 signalling in prostate cancer promotes an invasive
phenotype. Br J Cancer. 104:664–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou C, Nitschke AM, Xiong W, Zhang Q,
Tang Y, Bloch M, Elliott S, Zhu Y, Bazzone L, Yu D, et al:
Proteomic analysis of tumor necrosis factor-alpha resistant human
breast cancer cells reveals a MEK5/Erk5-mediated
epithelial-mesenchymal transition phenotype. Breast Cancer Res.
10:R1052008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu F, Yang L, Fang W, Li Y, Yang R, Yang
X, Deng J, Huang B, Xie C, Zhou Y, et al: A functional polymorphism
in the promoter of ERK5 gene interacts with tobacco smoking to
increase the risk of lung cancer in Chinese populations.
Mutagenesis. 28:561–567. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Diao D, Wang L, Wan J, Chen Z, Peng J, Liu
H, Chen X, Wang W and Zou L: MEK5 overexpression is associated with
the occurrence and development of colorectal cancer. BMC Cancer.
16:3022016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Simões AE, Pereira DM, Gomes SE, Brito H,
Carvalho T, French A, Castro RE, Steer CJ, Thibodeau SN, Rodrigues
CM, et al: Aberrant MEK5/ERK5 signalling contributes to human colon
cancer progression via NF-κB activation. Cell Death Dis.
6:e17182015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mansour MA, Hyodo T, Ito S, Kurita K,
Kokuryo T, Uehara K, Nagino M, Takahashi M, Hamaguchi M and Senga
T: SATB2 suppresses the progression of colorectal cancer cells via
inactivation of MEK5/ERK5 signaling. FEBS J. 282:1394–1405. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Pesakhov S, Harrison JS, Danilenko
M and Studzinski GP: ERK5 pathway regulates transcription factors
important for monocytic differentiation of human myeloid leukemia
cells. J Cell Physiol. 229:856–867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Umapathy G, El Wakil A, Witek B, Chesler
L, Danielson L, Deng X, Gray NS, Johansson M, Kvarnbrink S, Ruuth
K, et al: The kinase ALK stimulates the kinase ERK5 to promote the
expression of the oncogene MYCN in neuroblastoma. Sci Signal.
7:ra1022014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giurisato E and Tournier C: Can tumor
cells proliferate without ERK5? Cell Cycle. 15:619–620. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Antoon JW, Martin EC, Lai R, Salvo VA,
Tang Y, Nitzchke AM, Elliott S, Nam SY, Xiong W, Rhodes LV, et al:
MEK5/ERK5 signaling suppresses estrogen receptor expression and
promotes hormone-independent tumorigenesis. PLoS One. 8:e692912013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Dong L, Wei D, Wang X, Zhang S and
Li H: Fatty acid synthase mediates the epithelial-mesenchymal
transition of breast cancer cells. Int J Biol Sci. 10:171–180.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshizumi M, Abe J, Tsuchiya K, Berk BC
and Tamaki T: Stress and vascular responses: atheroprotective
effect of laminar fluid shear stress in endothelial cells: possible
role of mitogen-activated protein kinases. J Pharmacol Sci.
91:172–176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dent P, Yacoub A, Fisher PB, Hagan MP and
Grant S: MAPK pathways in radiation responses. Oncogene.
22:5885–5896. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chayama K, Papst PJ, Garrington TP, Pratt
JC, Ishizuka T, Webb S, Ganiatsas S, Zon LI, Sun W, Johnson GL, et
al: Role of MEKK2-MEK5 in the regulation of TNF-alpha gene
expression and MEKK2-MKK7 in the activation of c-Jun N-terminal
kinase in mast cells. Proc Natl Acad Sci USA. 98:4599–4604. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chao TH, Hayashi M, Tapping RI, Kato Y and
Lee JD: MEKK3 directly regulates MEK5 activity as part of the big
mitogen-activated protein kinase 1 (BMK1) signaling pathway. J Biol
Chem. 274:36035–36038. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun W, Kesavan K, Schaefer BC, Garrington
TP, Ware M, Johnson NL, Gelfand EW and Johnson GL: MEKK2 associates
with the adapter protein Lad/RIBP and regulates the MEK5-BMK1/ERK5
pathway. J Biol Chem. 276:5093–5100. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song H, Jin X and Lin J: Stat3 upregulates
MEK5 expression in human breast cancer cells. Oncogene.
23:8301–8309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miranda M, Rozali E, Khanna KK and Al-Ejeh
F: MEK5-ERK5 pathway associates with poor survival of breast cancer
patients after systemic treatments. Oncoscience. 2:99–101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ortiz-Ruiz MJ, Álvarez-Fernández S,
Parrott T, Zaknoen S, Burrows FJ, Ocaña A, Pandiella A and
Esparís-Ogando A: Therapeutic potential of ERK5 targeting in triple
negative breast cancer. Oncotarget. 5:11308–11318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Al-Ejeh F, Miranda M, Shi W, Simpson PT,
Song S, Vargas AC, Saunus JM, Smart CE, Mariasegaram M, Wiegmans
AP, et al: Kinome profiling reveals breast cancer heterogeneity and
identifies targeted therapeutic opportunities for triple negative
breast cancer. Oncotarget. 5:3145–3158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Javaid S, Zhang J, Smolen GA, Yu M,
Wittner BS, Singh A, Arora KS, Madden MW, Desai R, Zubrowski MJ, et
al: MAPK7 regulates EMT features and modulates the generation of
CTCs. Mol Cancer Res. 13:934–943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li JQ, Xue H, Zhou L, Dong LH, Wei DP and
Li H: Mechanism of fatty acid synthase in drug tolerance related to
epithelial-mesenchymal transition of breast cancer. Asian Pac J
Cancer Prev. 15:7617–7623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song H, Ethier SP, Dziubinski ML and Lin
J: Stat3 modulates heat shock 27kDa protein expression in breast
epithelial cells. Biochem Biophys Res Commun. 314:143–150. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q,
Tang F, Chen ZQ, Liu XP and Xu ZD: Twist1-mediated
adriamycin-induced epithelial-mesenchymal transition relates to
multidrug resistance and invasive potential in breast cancer cells.
Clin Cancer Res. 15:2657–2665. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kumar A, Xu J, Brady S, Gao H, Yu D,
Reuben J and Mehta K: Tissue transglutaminase promotes drug
resistance and invasion by inducing mesenchymal transition in
mammary epithelial cells. PLoS One. 5:e133902010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD
and Wang LH: Twist transcriptionally up-regulates AKT2 in breast
cancer cells leading to increased migration, invasion, and
resistance to paclitaxel. Cancer Res. 67:1979–1987. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song H, Wang R, Wang S and Lin J: A
low-molecular-weight compound discovered through virtual database
screening inhibits Stat3 function in breast cancer cells. Proc Natl
Acad Sci USA. 102:4700–4705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Villalva C, Martin-Lannerée S, Cortes U,
Dkhissi F, Wager M, Le Corf A, Tourani JM, Dusanter-Fourt I, Turhan
AG and Karayan-Tapon L: STAT3 is essential for the maintenance of
neurosphere-initiating tumor cells in patients with glioblastomas:
A potential for targeted therapy? Int J Cancer. 128:826–838. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Denysenko T, Gennero L, Roos MA, Melcarne
A, Juenemann C, Faccani G, Morra I, Cavallo G, Reguzzi S,
Pescarmona G, et al: Glioblastoma cancer stem cells: Heterogeneity,
microenvironment and related therapeutic strategies. Cell Biochem
Funct. 28:343–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee JH, Jung C, Javadian-Elyaderani P,
Schweyer S, Schütte D, Shoukier M, Karimi-Busheri F, Weinfeld M,
Rasouli-Nia A, Hengstler JG, et al: Pathways of proliferation and
antiapoptosis driven in breast cancer stem cells by stem cell
protein piwil2. Cancer Res. 70:4569–4579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Honoki K, Fujii H, Kubo A, Kido A, Mori T,
Tanaka Y and Tsujiuchi T: Possible involvement of stem-like
populations with elevated ALDH1 in sarcomas for chemotherapeutic
drug resistance. Oncol Rep. 24:501–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Masuda M, Wakasaki T, Suzui M, Toh S, Joe
AK and Weinstein IB: Stat3 orchestrates tumor development and
progression: The Achilles heel of head and neck cancers? Curr
Cancer Drug Targets. 10:117–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yao Z and Mishra L: Cancer stem cells and
hepatocellular carcinoma. Cancer Biol Ther. 8:1691–1698. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fang X, Cai Y, Liu J, Wang Z, Wu Q, Zhang
Z, Yang CJ, Yuan L and Ouyang G: Twist2 contributes to breast
cancer progression by promoting an epithelial-mesenchymal
transition and cancer stem-like cell self-renewal. Oncogene.
30:4707–4720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hii CS, Anson DS, Costabile M, Mukaro V,
Dunning K and Ferrante A: Characterization of the MEK5-ERK5 module
in human neutrophils and its relationship to ERK1/ERK2 in the
chemotactic response. J Biol Chem. 279:49825–49834. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sato Y, Harada K, Kizawa K, Sanzen T,
Furubo S, Yasoshima M, Ozaki S, Ishibashi M and Nakanuma Y:
Activation of the MEK5/ERK5 cascade is responsible for biliary
dysgenesis in a rat model of Carolis disease. Am J Pathol.
166:49–60. 2005. View Article : Google Scholar : PubMed/NCBI
|