Introduction
The smooth muscle is, as all other human tissues,
subject to tumor transformation. Their diffused localization in the
body allows subdivision of these tumors into groups and subgroups
corresponding to their anatomical regions. In particular, regarding
the female genital tract, the uterine smooth muscle is a site with
frequent onset of three main groups of tumors: leiomyoma (benign),
smooth muscle tumors of uncertain malignant potential (STUMPs) and
leiomyosarcoma (malignant).
Leiomyosarcoma is a rare tumor, accounting for only
about 1.3% of all uterine malignancies and it usually exhibits an
extremely malignant clinical course. The risk of local/metastatic
recurrence is high and the reported 5-year survival rate is only
12–25% (1).
STUMPs are a rare class of uterine neoplasms still
partially unknown and while an anatomopathological classification
has been defined, their risk of relapse and progression into
leiomyosarcoma is largely undefined. Therefore, counseling and
follow-up strategies for patient with this disease remain
undetermined. STUMPs are usually clinically benign but in some
cases, recurrence can occur many years following surgery (both
conservative and aggressive) (2).
In order to improve our knowledge of the prognostic
factors of STUMPs, we evaluated, from a clinical, pathological and
genetic point of view, three patients with recurrences who required
diagnosis and treatment at the Gynecologic Oncology Clinic at San
Gerardo Hospital in Monza.
The first case was a woman who showed a uterine
primitive STUMP and a local recurrence, histologically diagnosed as
undifferentiated sarcoma. The second patient had a primitive STUMP
followed by a second STUMP. The third case showed a primitive STUMP
and a recurrence diagnosed as leiomyosarcoma.
In this study, we report for the first time to the
best of our knowledge a genomic study on primitive STUMP and its
relapsed tumor. Additionally, fluorescence in situ
hybridization and immunohistochemical staining were performed on
these samples and on two other cases of primitive STUMPs and their
corresponding relapsed tumors.
Patients and methods
Case 1
Case 1 was a 46-year-old lady, referred to our
clinic after laparoscopic operative myomectomy for a 7-cm myoma in
February 2007, who underwent a hysterectomy, due to a pathological
diagnosis of STUMP, confirmed by pathological review of the
specimen.
In December 2008 the patient presented femoral vein
thrombosis and hydronephrosis due to a mixed (mainly solid) 9-cm
right pelvic mass involving the iliac vessels; lung metastases were
diagnosed with a CT-scan. A few days later she developed
haemoperitoneum.
In the emergency room a laparotomy was performed,
with a partial tumorectomy and ileocholic resection (according to
Hartmanns procedure). A diagnosis of high malignant leiomyosarcoma
was determined by the pathologist.
The patient received four courses of chemotherapy
with doxorubicin (75 mg/m2) and ifosfamide (10
g/m2) with progressive disease after initial partial
response. A second line of chemotherapy with gemcitabine and
docetaxel was therefore performed, with no results.
The patient succumbed to the disease 12 months after
the diagnosis of recurrence and 34 months from the initial
diagnosis of STUMP (December 2009).
Case 2
Case 2 had a total abdominal hysterectomy and
bilateral salpingo-oophorectomy at 47 years of age (1999) with a
preoperative diagnosis of a 5-cm myoma. The pathological diagnosis
was STUMP, confirmed by a pathological review of the specimen.
Seven years later (June 2006), the patient developed
a retroperitoneal (right external iliac - obturatory) mass of 6×4
cm. Laparotomic tumorectomy was performed. Following a pathological
diagnosis of STUMP with estrogen (ER) and progesterone (PgR)
positivity at 70%, hormonal treatment with megestrol acetate 160 mg
daily was recommended.
In August 2010, the patient developed a new right
pelvic recurrence of STUMP and was treated with surgery and
external beam radiotherapy (45 Gy). The patient is alive and
disease-free.
Case 3
Case 3 was admitted to our hospital in October 2002,
who at 41 years of age underwent multiple myomectomy (5-cm myomas)
with a postoperative diagnosis of STUMP. The follow-up was
uneventful until May 2006 (43 months of DFI), when a large, rapidly
growing myoma of 7.5 cm in diameter was diagnosed. A total
hysterectomy and bilateral salpingo-oophorectomy were performed,
with a diagnosis of high-grade leiomyosarcoma. A PET/CT scan was
negative for distant metastases however four courses of adjuvant
postoperative chemotherapy with doxorubicin and ifosfamide were
administered. In December 2007, a large abdominal recurrence with
liver metastases was diagnosed. Three further courses of
chemotherapy with docetaxel, trabectedin, POMB-ACE and hormonal
treatment did little in controlling the disease. The patient
succumbed 86 months after the initial diagnosis of STUMP and 43
months after recurrence (December 2009). Written informed consent
to the use of biological specimen was obtained from all patients at
the time of surgery.
Array comparative genomic
hybridization analysis (array-CGH)
Genomic DNA extraction from formalin-fixed
paraffin-embedded (FFPE) sections, sample preparation, slide
hybridization and analysis were performed using a SurePrint G3
Human CGH Microarray kit 8×60K (Agilent Technologies, Inc., Santa
Clara, CA, USA) following the manufacturers instructions. The
arrays were scanned at 2-µm resolution using an Agilent microarray
scanner and analyzed using Feature Extraction v10.10 and Agilent
Genomic Workbench v.6.0software (Agilent Technologies). The
aberration detection method-2 (ADM-2) algorithm was used to compute
and assist in the identification of aberrations for a given sample.
Significant chromosomal aberrations were determined using the
algorithm ADM-2 (threshold, 5; absolute minimum average log2 ratio,
0.50; with at least three or more consecutive probe sets).
Fluorescence in situ hybridization
(FISH) analysis
Fluorescence in situ hybridization analysis
of FFPE tissue sections was performed using an IGH/BCL2
translocation, dual fusion probe (Cytocell, Cambridge, UK), an
AneuVysion Multicolor DNA Probe kit (Abbott Molecular) and a
UroVysion bladder cancer kit (Vysis) according to the manufacturers
instructions. All digital images were captured using a Leitz
microscope (Leica DM 5000B) equipped with a charge-coupled device
(CCD) camera and analyzed by means of Chromowin software (Tesi
Imaging, Milano, Italy).
Immunohistochemistry
Immunohistochemical staining was performed on FFPE
(4% formalin) sections of 1-µm thickness. The entire pre-treatment
process of deparaffinization, rehydration and epitope retrieval was
performed using PT LINK (Dako). Then the sections were placed into
the Autostainer Link 48 with the EnVision FLEX visualization
system. The evaluated markers were ER, PgR, p16, p53 and Ki67 for
mitotic activity.
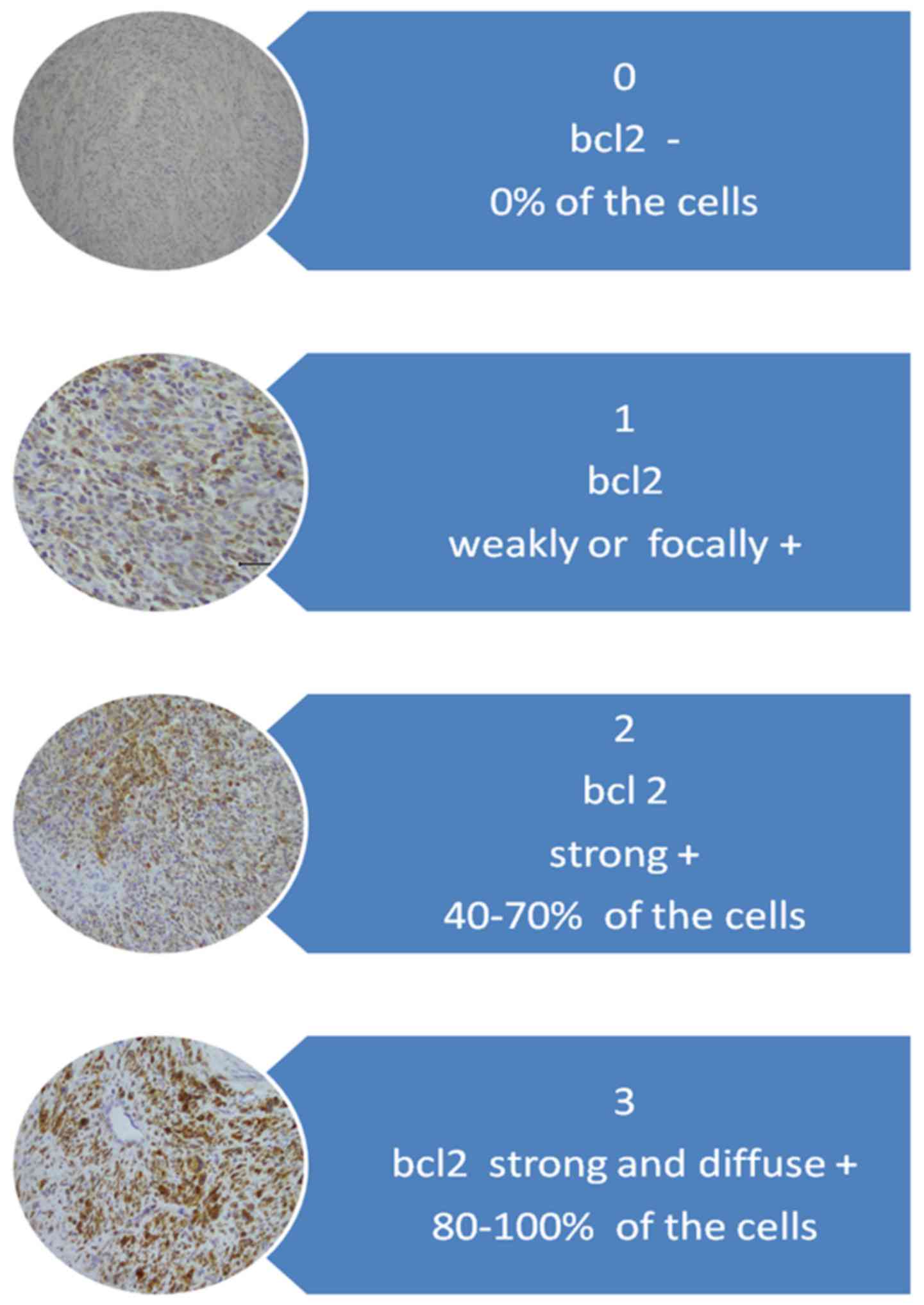
For interpretation of immunohistochemical staining,
the system proposed by Ip et al (3) was adopted. For the Bcl-2 protein
evaluation system, a scale of staining intensity and localization
is described in Fig. 1.
Results
Array-CGH results
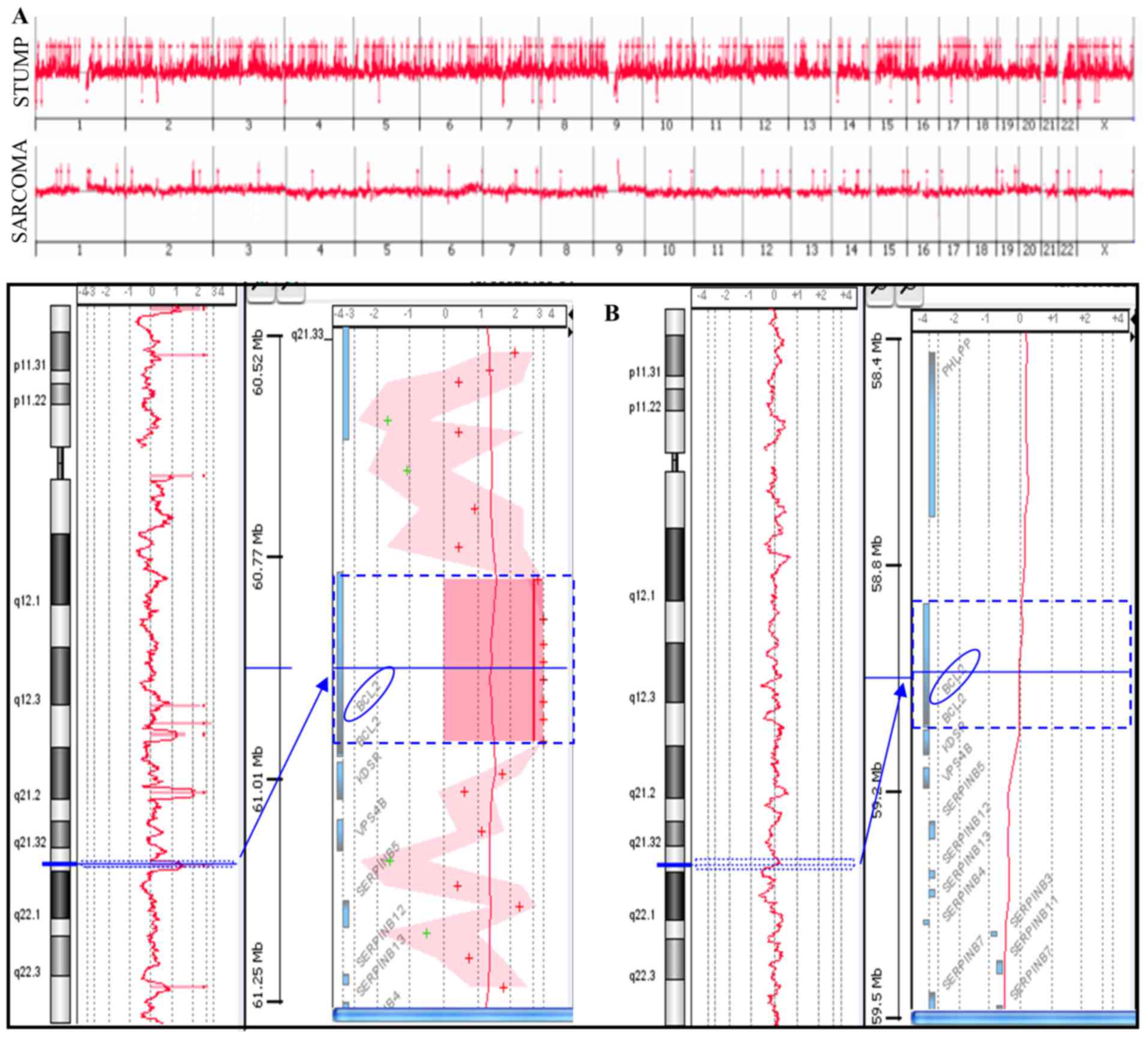
Array-CGH analysis was performed on a primitive
STUMP and on the relapsed undifferentiated sarcoma (Fig. 2A). The STUMP showed a higher number
of copy number alterations (CNAs) compared to the sarcoma (365 vs.
28); gains represent a totality of the sarcoma variations and the
majority of STUMP (342/365). Among the losses, 18 were complete
losses (homozygosity), three were heterozygous losses and two
losses were mosaic and apparently randomly distributed.
The two tumors shared only eight CNAs containing
genes associated with cancer (Table
I). For example, CNA in the 1p22.2-p22.1 included the
transforming growth factor β type III receptor (TGFBR3)
gene, whose expression has been observed in various types of
cancers.
 | Table I.Shared CNAs between STUMP (upper
value) and sarcoma (bottom value). |
Table I.
Shared CNAs between STUMP (upper
value) and sarcoma (bottom value).
| Chr | Cytoband | AvgCGHLR | Gene names |
|---|
| 1 | p22.2-p22.1 | 3.37 | TGFBR3 |
|
|
| 3.39 |
|
| 2 | q32.2 | 3.25 | COL5A2 |
|
|
| 2.95 |
|
| 12 | q24.11 | 2.82 | SART3, ISCU,
TMEM119, |
|
|
| 2 | SELPLG,
CORO1C, SSH1 |
| 13 | q32.1 | 2.51 | UGCGL2 |
|
|
| 1.51 |
|
| 19 | q13.43 | 3.64 | CHMP2A,
UBE2M, |
|
|
| 1.98 | LOC100131691,
MZF1 |
| 21 | q22.3 | 3.94 | C2CD2, ZNF295 |
|
|
| 2.02 |
|
| 22 | q12.1 | 2.26 | PITPNB |
|
|
| 2.21 |
|
| X | q28 | 1.46 | ARHGAP4, ARD1A, |
|
|
| 1.35 | RENBP, HCFC1 |
To identify possible functional groups highly
represented in the genes included in the shared CNAs, a gene
ontology analysis was performed using the GOstat software, but no
GO term was statistically significant, probably due to the small
number of shared genes.
Among the unshared CNAs, a BCL2 gene
amplification (4.7 log2 ratio, 52 copy number) emerged. BCL2
is an anti-apoptotic gene that prevents the normal course of
apoptotic cell death in a variety of cells, playing an important
role in the growth of tumors. Notably, this amplification was not
observed in sarcoma (Fig. 2B).
FISH results
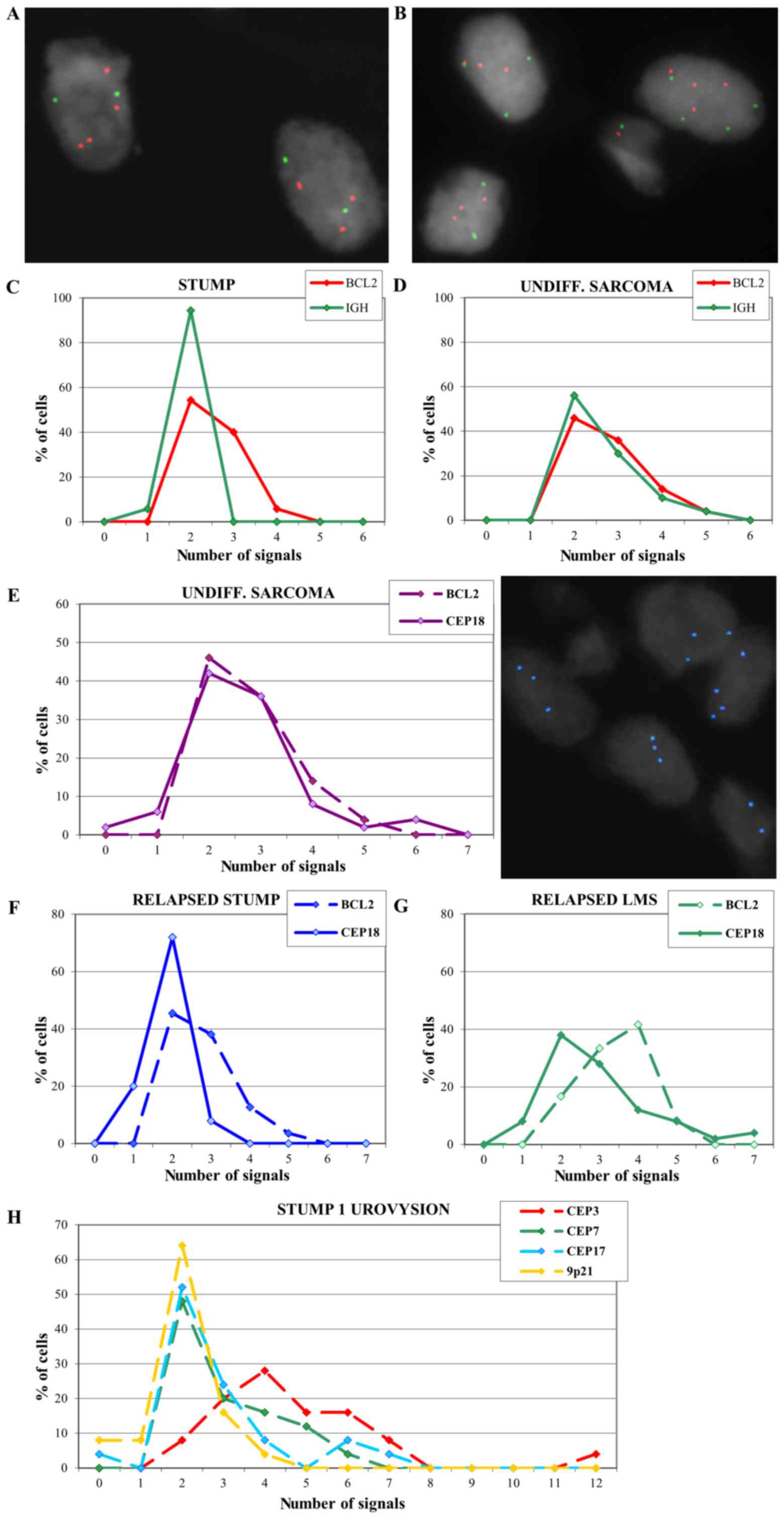
To confirm the array-CGH results, FISH analysis was
performed with an IGH/BCL2 translocation probe. Multiple
copies of the BCL2 gene in the primitive STUMP of case 1
were confirmed and IGH appeared disomic (Fig. 3A and C).
FISH on the undifferentiated sarcoma showed multiple
copies of both BCL2 and IGH signals (Fig. 3B and D) and the analysis was
extended using a probe for centromere 18 (Fig. 3E). Results revealed a signal
distribution for centromere 18 that overlaps with the BCL2
signal distribution, suggesting a polysomy of chromosome 18 that
could explain the absence of amplification in array-CGH. FISH
analysis performed on the relapsed cases 2 and 3 exhibited multiple
copies of BCL2 (Fig. 3F and
G).
A UroVysion test was also performed on the STUMP of
case 1 in order to evaluate a possible polyploidy that could not be
seen by array-CGH technique. Results showed 92% of the cells with
chromosome 3 polysomy (ranging from 3 to 12 copies), 52 and 48% of
the cells with chromosome 7 and 17 polysomy respectively (Fig. 3H), supporting the hypothesis of a
polyploid genome.
Immunohistochemistry results
The histological diagnosis and the
immunohistochemical results are reported in Table II. As expected, the sarcomas
presented a major proliferative trend compared to the STUMP; in
particular the unique case of a STUMP recurrence as
undifferentiated sarcoma showed a marked difference in the levels
of tested protein expression (for instance p53).
 | Table II.Histological diagnosis and
immunocharacterization of the samples. |
Table II.
Histological diagnosis and
immunocharacterization of the samples.
| Case no. | Lesion | Year of
surgery | Mitoses/10 HPF | Ki67 | Markers | Estrogen | Progesterone | p16 | p53 | Βcl2 |
|---|
| 1 | STUMP | 2008 | 4–5 | <1% |
Actin++ | Neg. | Neg. | Neg. | Neg. | 1 |
|
| Undifferent.
Sarcoma | 2009 | 14 | 20% |
Vimentin+, CD10+,
actin+/−; desmin+/−, calponin+/−,
S100−, calretinin−, CD117−,
CD34−, cytokeratin pool− | Neg. | Neg. | Neg. | 3+ | Neg. |
| 2 | STUMP | 1999 | NA | NA | NA | NA | NA | NA | NA | NA |
|
| STUMP | 2006 | 2 | 3% |
Actin+++, desmin+++,
CD10− | 90% | 70% | Neg. | 2+ | 1 |
| 3 | STUMP | 2002 | 3 | <5% | Actin++,
desmin+++; CD10+ focal | 30% | 60% | Neg. | 2+ | 2 |
|
| LMS | 2006 | 15 | 40% | Actin+,
desmin+++, CD10+ | Neg. | 60% | Neg. | Neg. | 2 |
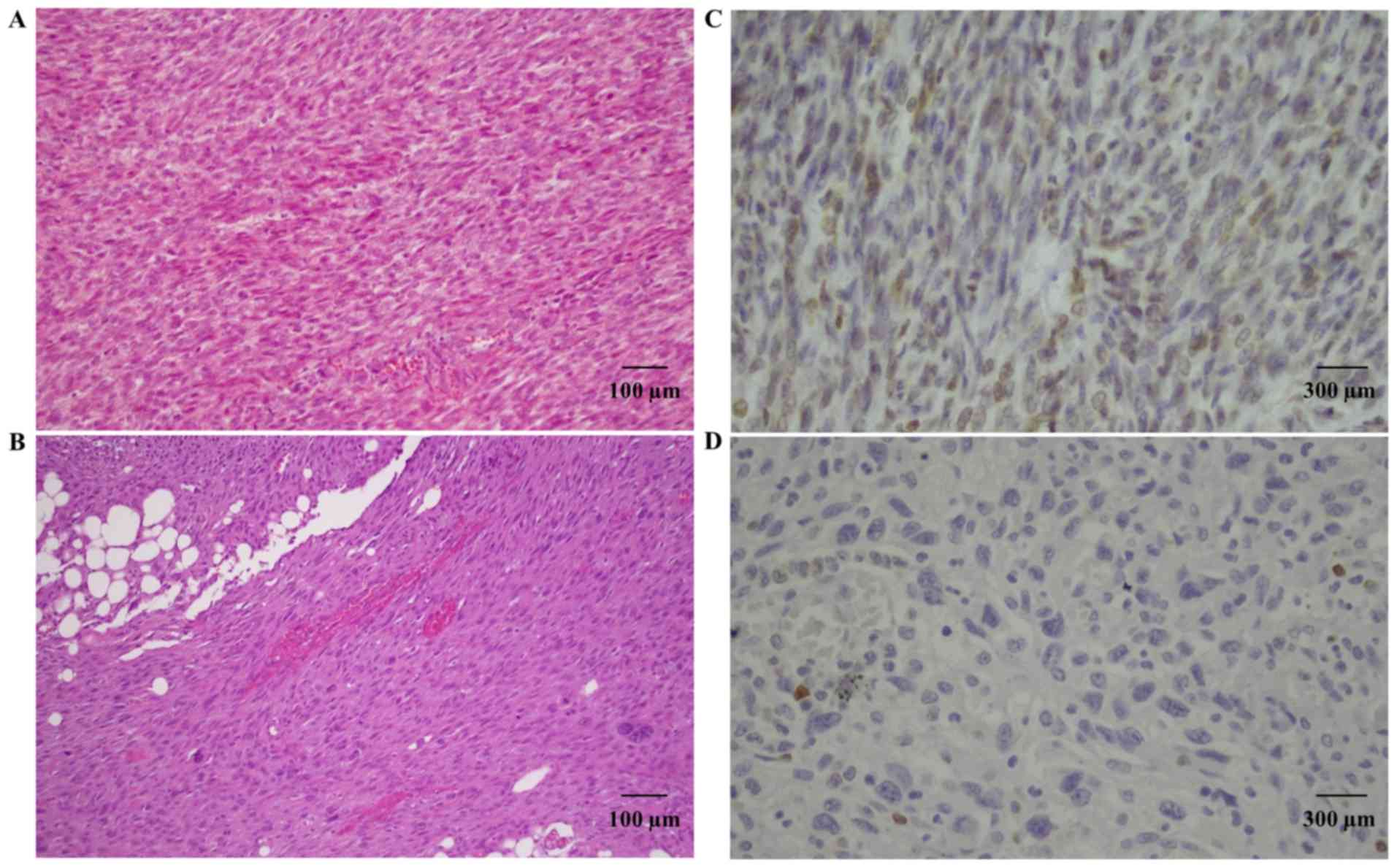
Bcl-2 staining was present in two primitive STUMPs
and in two relapsed tumors (one STUMP and one LMS). In particular,
case 1 showed positive Bcl-2 expression in the primitive tumor,
that was not maintained in the relapsed tumor (Fig. 4). On the contrary, case 3 showed
high expression of Bcl-2 in both tumors. For case 2, the primitive
tumor was not available for study, but the relapsed STUMP showed
Bcl-2 expression.
Discussion
Uterine smooth muscle tumors are the most common
female genital tract neoplasms. They are classified into:
leiomyomas, STUMPs and leiomyosarcomas (4). While leiomyosarcoma is a malignant
tumor and has been studied at length, STUMP remains ambiguous and
has many unresolved issues. For this reason, we performed a study
on three cases of STUMP with different types of relapse.
To our knowledge the present study showed for the
first time an array comparative genomic hybridization analysis of a
primitive STUMP and its relapsed tumor.
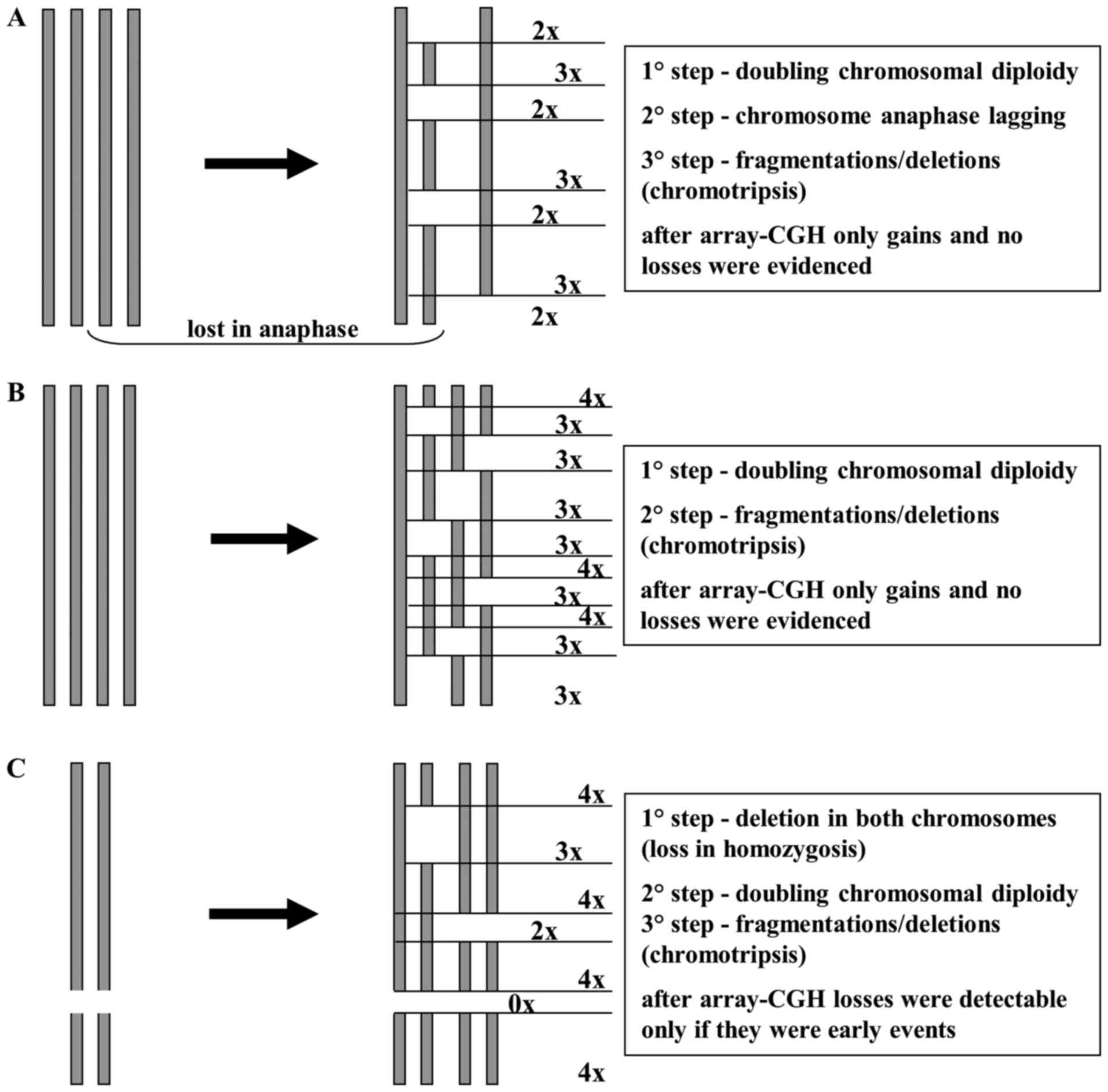
Array-CGH data showed a high number of gains and a
very low number of losses (especially in primitive STUMP). This may
be explained by a chromosome endoreduplication followed by a random
loss in anaphase and/or deletions/chromotripsis of chromosome
pieces (Fig. 5). In this
hypothesis, losses would be ‘masked’ from the initial chromosome
endoreduplication and the few losses may be attibuted to early
events that arose prior to endoreduplication. This hypothesis is
supported by the presence of polysomy in chromosomes 3, 7 17 and 18
detected by FISH, as reported in our results. The two tumors shared
eight CNAs containing many genes, some implicated in other types of
cancer, but never before reported in STUMPs and leiomyosarcomas.
For example, TFGBR3 is considered a tumor-suppressor gene,
commonly lost in various types of cancers (5–7), but
it was recently reported to have a dual role in bladder cancer,
acting as both a tumor suppressor and as a tumor promoter (8). In our cases, TGFBR3 appeared to
play a tumor-promoting role, but this postulation should be further
confirmed using a larger number of samples.
Another shared gained region included CORO1C,
whose amplification has been reported in primary effusion lymphoma
(9) and whose gene expression
silencing seems to inhibit cancer cell proliferation, migration and
invasion in lung squamous cell carcinoma (10).
BCL2 is an anti-apoptotic gene, whose
expression appears to play an important role in the growth of human
carcinomas. The role of BCL2 has been well characterized and
amplification of this gene may result in blockage of apoptosis
(11). For this reason we decided
to evaluate its copy number in our samples. The STUMP presented a
real amplification of the BCL2 gene in the array-CGH
analysis, that was not observed in the sarcoma and which was
detected in FISH as multiple copies of the gene. This disparity
with the array-CGH data may be explained by the idiosyncracies of
the two techniques and the different information provided by them,
as already reported in our previous study (12).
Several studies have shown an inverse correlation
between Bcl-2 expression and p53 expression in different human
malignancies (13–16). Our results suggest that Bcl-2
expression was independent of p53 expression both in the primitive
and in the relapsed tumors, as evident in Table II.
It was also reported that Bcl-2 expression is linked
to a good clinical outcome and a better prognosis in non-small cell
lung carcinoma (15), colorectal
cancer (16) and leiomyosarcomas
(1). Despite the fact that the
number of analyzed samples was limited, our data appear to be
contrary to these other studies, because multiple copies of
BCL2 or Bcl-2 expression were present in the two primitive
STUMPs and two relapsed tumors in a total of five tumors analyzed.
Bcl-2 expression was confirmed in IHC in four of the five tested
samples and moreover two out of the three patients succumbed to the
disease.
The Bcl-2 family of proteins regulates apoptosis by
controlling mitochondrial permeability. Several of these proteins,
both anti-apoptotic and pro-apoptotic, have C-terminal
transmembrane domains that are inserted in the outer membrane of
mitochondria (17). In particular,
the anti-apoptotic proteins Bcl-2 and Bcl-xL inhibit cytochrome
c release and therefore the activation of the apoptotic
pathway.
For this reason copy number gain of BCL2
appears notably also as a therapeutic target. Strategies for
inhibition of the Bcl-2 family of proteins currently in clinical
trials essentially include: ⅰ) the use of antisense-based
mechanisms to knock down Bcl-2 or Bcl-xL expression, or ⅱ) the use
of synthetic BH3 mimetic molecules (e.g., obatoclax, AT-101 and
ABT-737) (18). Furthermore, it has
recently been reported that Bcl-2 inhibition by siRNA might is
useful for therapy in association with chemotherapeutic agents in
MCF-7 breast cancer cells (19).
Despite the limited number of analyzed samples,
BCL2 could be evaluated as a potential marker of malignancy
and recurrence in STUMPs, however, this ambitious hypothesis must
be confirmed using a larger number of patient samples.
References
|
1
|
Zhai YL, Nikaido T, Toki T, Shiozawa A,
Orii A and Fujii S: Prognostic significance of bcl-2 expression in
leiomyosarcoma of the uterus. Br J Cancer. 80:1658–1664. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kotsopoulos IC, Barbetakis N, Asteriou C
and Voutsas MG: Uterine smooth muscle tumor of uncertain malignant
potential: A rare cause of multiple pulmonary nodules. Indian J Med
Paediatr Oncol. 33:176–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ip PP, Cheung AN and Clement PB: Uterine
smooth muscle tumors of uncertain malignant potential (STUMP): A
clinicopathologic analysis of 16 cases. Am J Surg Pathol.
33:992–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hewedi IH, Radwan NA and Shash LS:
Diagnostic value of progesterone receptor and p53 expression in
uterine smooth muscle tumors. Diagn Pathol. 7:12012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Copland JA, Luxon BA, Ajani L, Maity T,
Campagnaro E, Guo H, LeGrand SN, Tamboli P and Wood CG: Genomic
profiling identifies alterations in TGFβ signaling through loss of
TGFβ receptor expression in human renal cell carcinogenesis and
progression. Oncogene. 22:8053–8062. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong M, How T, Kirkbride KC, Gordon KJ,
Lee JD, Hempel N, Kelly P, Moeller BJ, Marks JR and Blobe GC: The
type III TGF-β receptor suppresses breast cancer progression. J
Clin Invest. 117:206–217. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Turley RS, Finger EC, Hempel N, How T,
Fields TA and Blobe GC: The type III transforming growth factor-β
receptor as a novel tumor suppressor gene in prostate cancer.
Cancer Res. 67:1090–1098. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu XL, Xiao K, Xue B, Yang D, Lei Z, Shan
Y and Zhang HT: Dual role of TGFBR3 in bladder cancer. Oncol Rep.
30:1301–1308. 2013.PubMed/NCBI
|
|
9
|
Luan SL, Boulanger E, Ye H, Chanudet E,
Johnson N, Hamoudi RA, Bacon CM, Liu H, Huang Y, Said J, et al:
Primary effusion lymphoma: Genomic profiling revealed amplification
of SELPLG and CORO1C encoding for proteins important for cell
migration. J Pathol. 222:166–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mataki H, Enokida H, Chiyomaru T, Mizuno
K, Matsushita R, Goto Y, Nishikawa R, Higashimoto I, Samukawa T,
Nakagawa M, et al: Downregulation of the microRNA-1/133a cluster
enhances cancer cell migration and invasion in lung-squamous cell
carcinoma via regulation of Coronin1C. J Hum Genet. 60:53–61. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Petrini I, Meltzer PS, Zucali PA, Luo J,
Lee C, Santoro A, Lee HS, Killian KJ, Wang Y, Tsokos M, et al: Copy
number aberrations of BCL2 and CDKN2A/B identified by array-CGH in
thymic epithelial tumors. Cell Death Dis. 3:e3512012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Panzeri E, Conconi D, Antolini L, Redaelli
S, Valsecchi MG, Bovo G, Pallotti F, Viganò P, Strada G, Dalprà L,
et al: Chromosomal aberrations in bladder cancer: Fresh versus
formalin fixed paraffin embedded tissue and targeted FISH versus
wide microarray-based CGH analysis. PLoS One. 6:e242372011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Silvestrini R, Veneroni S, Daidone MG,
Benini E, Boracchi P, Mezzetti M, Di Fronzo G, Rilke F and Veronesi
U: The Bcl-2 protein: A prognostic indicator strongly related to
p53 protein in lymph node-negative breast cancer patients. J Natl
Cancer Inst. 86:499–504. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alderson LM, Castleberg RL, Harsh GR IV,
Louis DN and Henson JW: Human gliomas with wild-type p53 express
bcl-2. Cancer Res. 55:999–1001. 1995.PubMed/NCBI
|
|
15
|
Fontanini G, Vignati S, Bigini D, Mussi A,
Lucchi M, Angeletti CA, Basolo F and Bevilacqua G: Bcl-2 protein: A
prognostic factor inversely correlated to p53 in non-small-cell
lung cancer. Br J Cancer. 71:1003–1007. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaklamanis L, Savage A, Whitehouse R,
Doussis-Anagnostopoulou I, Biddolph S, Tsiotos P, Mortensen N,
Gatter KC and Harris AL: Bcl-2 protein expression: Association with
p53 and prognosis in colorectal cancer. Br J Cancer. 77:1864–1869.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yip KW and Reed JC: Bcl-2 family proteins
and cancer. Oncogene. 27:6398–6406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scarfò L and Ghia P: Reprogramming cell
death: BCL2 family inhibition in hematological malignancies.
Immunol Lett. 155:36–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Talaiezadeh A, Jalali F, Galehdari H and
Khodadadi A: Time depended Bcl-2 inhibition might be useful for a
targeted drug therapy. Cancer Cell Int. 15:1052015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fischer H, Stenling R, Rubio C and
Lindblom A: Colorectal carcinogenesis is associated with stromal
expression of COL11A1 and COL5A2. Carcinogenesis. 22:875–878. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang LH, Xiang J, Yan M, Zhang Y, Zhao Y,
Yue CF, Xu J, Zheng FM, Chen JN, Kang Z, et al: The mitotic kinase
Aurora-A induces mammary cell migration and breast cancer
metastasis by activating the Cofilin-F-actin pathway. Cancer Res.
70:9118–9128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stadler ZK, Esposito D, Shah S, Vijai J,
Yamrom B, Levy D, Lee YH, Kendall J, Leotta A, Ronemus M, et al:
Rare de novo germline copy-number variation in testicular cancer.
Am J Hum Genet. 91:379–383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mudduluru G, Vajkoczy P and Allgayer H:
Myeloid zinc finger 1 induces migration, invasion, and in vivo
metastasis through Axl gene expression in solid cancer. Mol Cancer
Res. 8:159–169. 2010. View Article : Google Scholar : PubMed/NCBI
|