Introduction
Colorectal cancer (CRC) is the third most frequent
type of cancer diagnosed and the second leading cause of
cancer-related deaths in the United States, with an estimated
132,700 new diagnoses and 49,700 deaths in 2015 (1). Approximately 25% of patients present
with metastatic disease, while another 25–35% of patients develop
metastatic disease during or after treatment, with the liver being
the most common site of metastases (2). Surgical resection has been shown to be
the only potentially curative option for colon cancer metastasis to
the liver (3), resulting in a
25–40% overall survival at 5 years compared to 0–5% in those not
having a liver resection. However, only about 15% of patients are
considered to be resectable at presentation (2). While improvements in chemotherapy
regimens and increasingly aggressive surgical approaches have
resulted in improvements in survival rates for metastatic patients
(4), patients with unresectable,
chemo-refractory colorectal cancer liver metastases (CRCLM) have
limited local treatment options. In addition to being prognostic
for overall survival, the presence of hepatic disease is the major
contributor to the cause of death in approximately half of the
metastatic CRC patients (5),
indicating that local control of the liver is an important aspect
of disease management and outcomes.
For unresectable liver metastases, local therapies
include stereotactic body radiation therapy (SBRT), hepatic
arterial infusion of chemotherapy, arterially directed embolic
therapy, radiofrequency ablation, and liver directed radiation.
Selection of these different modalities often depends on the
extent, size, and location of the disease (6). Yttrium-90 (90Y)
radioembolization exploits the physiological difference in the
blood flow between tumors and the normal liver to preferentially
deliver radiation to the metastases, thereby shrinking the tumors
while sparing the healthy liver (7). Liver directed radiation has shown
promising results in a variety of institutional studies,
demonstrating promising survival outcomes and low toxicities
(8–10). A recent randomized phase III trial,
SIRFLOX, which compared first line chemotherapy vs. the same
chemotherapy with selective internal radiation, demonstrated a
significant delay of disease progression in the liver (11).
CRC is a heterogeneous disease composed of multiple
disease subtypes (12). While about
25% of CRCs are associated with a family history, suggesting a role
for inherited genetic mutations, only a small percentage of family
historylinked cancers are associated with highly penetrant
mutations in major genes. The majority of CRCs occur sporadically,
developing in a multi-step process, involving an accumulation of
mutations in tumor-suppressor genes and oncogenes (13). Mutations in KRAS have been
associated with 33–48% of CRCs. The KRAS protein is a downstream
effector of EGFR receptor tyrosine kinase activity, which activates
intracellular signaling cascades mediated by the RAF/MEK, MAPK, AKT
and PI3K pathways (14). Mutations
in KRAS have been linked to CRC progression, with KRAS mutants
having worse progression-free survival and overall survival
compared to wild-type (WT) patients (13,15–17).
The impact of KRAS mutation on radiation response is less clear,
with one group reporting a greater survival benefit after
90Y radioembolization in WT vs. KRAS mutant patients.
However, as there was no evaluation of local control after therapy,
the differential assessment of radiation response in the two groups
remains unclear (18).
The presence of abnormally high levels of
circulating cell-free DNA (ccfDNA) in the plasma of cancer patients
has been well established and is thought to result from a variety
of different mechanisms including apoptosis, necrosis, and
circulating tumor cell lysis (19,20).
In CRC patients, an elevated plasma DNA concentration has been
shown to be diagnostic for benign vs. malignant disease and to
correlate with worse progression-free survival and overall survival
after chemotherapy (21–23). DNA fragmentation index (FI), which
is measured as the ratio of longer to shorter DNA fragments, has
been shown to be higher in patients with advanced stages of breast
cancer and with post-operative recurrence (24). In addition, recently, the level of
ccfDNA fragmentation has also been shown to correlate with survival
in metastatic colon cancer patients (22). However, changes in ccfDNA
concentration and FI after 90Y radioembolization have
not been assessed.
Here we report on our institutional experience on
the efficacy of resin-based 90Y radioembolization for
the treatment of CRCLM and the changes in ccfDNA levels and FI with
liver directed internal radiation, investigating whether gene
mutations in KRAS could serve as prognostic factors for
90Y treatment of CRCLM.
Materials and methods
Patient selection
This retrospective review and prospective blood
evaluation was approved by the Institutional Review Board (IRB
2008-344) of Georgetown University. From May 2011 to March 2015, a
total of 58 patients underwent 90Y treatment for CRCLM
at our institution. Patients eligible for study inclusion had
histologically-confirmed CRC with liver metastases. Patients were
included irrespective of prior treatment, including prior
chemotherapy, surgery, or radiation therapy. All patients were not
considered candidates for surgical resection and many of the
patients were considered refractory to chemotherapy. Lesions were
considered for treatment in any location within the liver. Patients
generally were expected to have a life expectancy of at least 6
months and adequate hepatic function.
Pre-treatment imaging, planning, and
treatment
All patients underwent pre-procedural imaging with
computed tomography (CT), FDG PET-CT, and/or MRI to determine liver
tumor location, size, and volume. Three dimensional volumetric
software was utilized to evaluate the total tumor volume, total
liver volume, and total liver tumor burden (ratio of TV/LV). A
pre-procedure planning angiogram and embolization of extrahepatic
arteries at risk of nontarget perfusion of 90Y
microspheres was performed. Hepatopulmonary shunt estimation was
performed using Tc-99m-MAA, and this was judged to be adequate if
<20%. Therapy with 90Y microspheres was performed
within 2 weeks of initial planning angiography. 90Y
microsphere infusion followed the manufacturer's guidelines.
Following delivery, a SPECT of the abdomen was performed to assess
the distribution of the microspheres. The patients were discharged
home the same day.
Depending on extent of disease within the liver at
presentation, patients received either bilobar (right and left
lobe) or unilobar treatment. For bilobar treatment, the first lobe
treatment was followed by the second lobe treatment approximately
30 days later. Infusion of 90Y microspheres was
performed by both the interventional radiologist and radiation
oncologist. Patients were treated to a mean dose of 43.87 Gy and
mean activity of 24.28 mCi.
Plasma isolation, ccfDNA extraction,
qPCR, and AFM preparation
Sample handling was in accordance with published NCI
biospecimen best practices. A 10 ml sample of blood was collected
in a K2-EDTA tube. Blood samples were processed within 30–60 min
with an initial spin at 800 × g for 10 min at 4°C. The plasma was
transferred to another tube and centrifuged at 13,000 × g for 10
min to remove the remaining blood cells. The supernatant plasma
samples were then aliquoted into 2 ml/tube and either immediately
processed for DNA extraction or stored at −80°C. ccfDNA was
extracted from 5 ml of plasma using the QIAamp DNA Mini blood kit
(Qiagen, Valencia, CA, USA) according to the ‘Blood and Body Fluid
Protocol’. ccfDNA samples were kept at −20°C until use.
Plasma was prospectively collected from 9 patients
both pre- and post-treatment, and ccfDNA concentrations and FI
values were determined using quantitative PCR as previously
described (25). Briefly, qPCR
amplifications were performed in duplicate in a 20-µl PCR mix,
including the amplification primer and DNA extract. Thermal cycling
consisted of three repeated steps: a 3-min Hot Start polymerase
activation-denaturation step at 95°C, followed by 40 repeated
cycles at 95°C for 10 sec and 60°C for 30 sec. Melting curves were
obtained by increasing the temperature from 55 to 90°C with a plate
reading every 0.2°C. Serial dilutions of genomic DNA were used as a
standard for quantification. The quantity of ccfDNA fragments of
different sizes was assessed using an integrated PCR system that
targeted intronic sequences within the same regions. The degree of
ccfDNA FI was assessed by calculating the ratio of the
concentration determined by using the primer set amplifying a large
target (>250 bp) to the concentration determined by using the
primer set amplifying a short target (<100 bp). These values
were normalized, and the ratio of large fragments (~ 250 bp) to
small fragments (<100 bp) was recorded as the DNA FI. The ratio
of DNA FI values before and after treatment (pre-treatment
FI/post-treatment FI) were stratified by values less than or
greater than 1.
Samples for atomic-force microscopy (AFM) analysis
were prepared as previously described (26). Briefly, a 2 µl ccfDNA sample in a
Tris and EDTA solution was deposited onto APS-mica. The sample was
then rinsed with deionized water and dried with nitrogen gas. It
was mounted on a Nanoscope IIIa AFM for imaging (Veeco/Digital
Instruments, Santa Barbara, CA, USA). Images were acquired in
tapping mode in air using silicon tapping mode probes. Ten images
were acquired for each ccfDNA sample. Acquired AFM images were
analyzed using commercially available software, FemtoScan Online
(Advanced Technologies Center, Moscow, Russia), for measurements of
ccfDNA fragment lengths. Measured DNA fragments were sorted by
size, and the fragment size distribution was obtained by dividing
the number of fragments in each bin by the total number of
fragments. DNA fragment size conversions were made on the
assumption that 1 bp was equivalent to a length of 0.34 nm. AFM was
performed on 6 patients, 4 KRAS WT and 2 KRAS mutants.
Follow-up and statistical
analysis
A retrospective review was conducted for clinical
outcomes, demographic information, and tumor mutational status. In
47 patients, interval imaging, including CT, PET-CT, and/or MRI,
was available for tumor response assessment using RECIST criteria
as a score of complete response, partial response, stable disease,
or progressive disease (27).
Because use of 90Y microspheres is a liver-directed
therapy, tumor response was limited to an assessment of hepatic
lesions in the liver lobe or lobes treated, with whole liver local
control assessed for bilobar treatments and treated liver lobe
local control assessed for unilobar treatments. Tumor mutational
status was available for 49 patients based on the review of
documented pathology reports. Actuarial survival and
progression-free survival were evaluated by the Kaplan-Meier
method. Univariate analysis was performed using Kaplan-Meier, and
multivariate analysis was assessed using Cox regression analysis. A
two-tailed t-test was used to assess changes in fragment size with
AFM in WT and KRAS mutant patients before and after single lobe
90Y treatment.
Results
Patient characteristics
Patient characteristics are noted in Table I. Our patient population consisted
of 58 patients diagnosed with colon cancer at a median age of 56
years (range, 31–85 years). Half of the patients were male, 86% had
a performance status ≤1, and 67% of the patients had metastatic
disease outside the liver. Patients received 90Y
treatment for a median of 20 months (range, 5–52 months) after
diagnosis with hepatic metastases. As summarized in Table II, our patients received a median
of two chemotherapy treatment lines prior to 90Y
treatment, ranging from 1 to 5. Every patient had exposure to
either 5-FU or capecitabine prior to treatment.
 | Table I.Patient characteristics, n=58. |
Table I.
Patient characteristics, n=58.
| Patient
characteristics | n (%) |
|---|
| Age at
90Y treatment (years) |
|
| Median
(range) | 56 (31–85) |
|
≤40 | 3 (5) |
| >40
to ≤50 | 13 (22) |
| >50
to ≤60 | 16 (28) |
| >60
to ≤70 | 16 (28) |
|
>70 | 10 (17) |
| Race |
|
|
White | 36 (62) |
|
Black | 12 (21) |
|
Other | 10 (17) |
| Gender |
|
|
Male | 29 (50) |
|
Female | 29 (50) |
| Performance status
(ECOG) |
|
| 0 | 14 (24) |
| 1 | 36 (62) |
| ≥2 | 7 (12) |
|
Unknown | 1 (2) |
| Location of lesion
treated |
| Right
lobe | 50 (86) |
| Left
lobe | 40 (69) |
| Both
lobes | 32 (55) |
| Presence of
extrahepatic disease |
|
|
Absent | 19 (33) |
|
Present | 39 (67) |
 | Table II.Chemotherapy characteristics,
n=58. |
Table II.
Chemotherapy characteristics,
n=58.
| Chemotherapy
characteristics | n (%) |
|---|
| No. of previous
chemotherapy lines |
|
| 1 | 12 (21) |
| 2 | 29 (50) |
| ≥3 | 17 (29) |
| Exposure to
5-fluoruracil or capecitabine |
|
| No | 0 (0) |
|
Yes | 58 (100) |
| Exposure to
leucovorin |
|
| No | 9 (16) |
|
Yes | 49 (84) |
| Exposure to
oxaliplatin |
|
| No | 5 (9) |
|
Yes | 53 (91) |
| Exposure to
irinotecan |
|
| No | 27 (47) |
|
Yes | 31 (53) |
| Exposure to
bevacizumab |
|
| No | 14 (24) |
|
Yes | 44 (76) |
| Exposure to
cetuximab or panitumumab |
|
| No | 52 (90) |
|
Yes | 6 (10) |
| Exposure to
nilotinib or sunitinib |
|
| No | 54 (93) |
|
Yes | 4 (7) |
| Exposure to other
agentsa |
|
| No | 44 (76) |
|
Yes | 14 (24) |
As summarized in Table
III, tumor mutation information was available for 49 patients,
with 27 (55%) being KRAS mutant, 21 (43%) being WT, and 1 (2%)
being Her2/neu overexpressing. A larger proportion of KRAS WT
patients were treated with anti-EGFR-targeted agents before
90Y radioembolization, but the overall exposure to EGFR
inhibitors was low (10%, Table
II).
 | Table III.Mutation status, n=49. |
Table III.
Mutation status, n=49.
| Mutation
status | n (%) | Median age
(years) |
|---|
| KRAS WT | 21 (43) | 53 |
| KRAS mutated | 27 (55) | 60 |
| Her2/neu | 1 (2) | 38 |
90Y radioembolization
procedure
A total of 90 treatments were delivered to 58
patients (Table I). Eight patients
received treatment to the left lobe alone, 18 received treatment to
the right lobe alone, and 32 received treatment to both liver lobes
spaced approximately a month apart. The delivered activity during
90Y treatment ranged from 8.9 to 59.4 mCi.
Imaging response and liver
progression-free survival
In 47 patients, interval imaging, including CT,
PET/CT and/or MRI, was available for tumor response assessment.
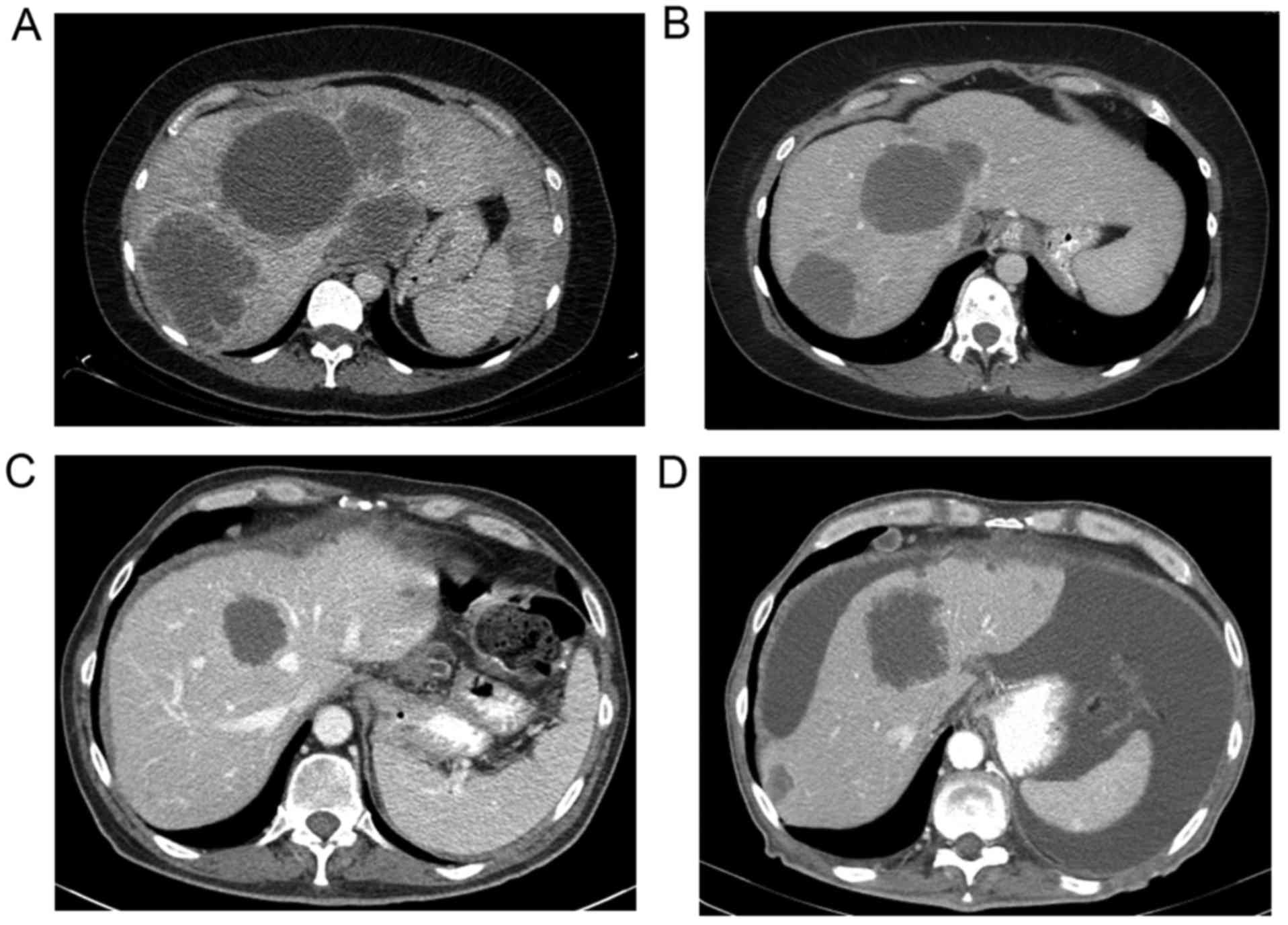
Fig. 1 shows representative CT
images before (Fig. 1A and C) and
after treatment (Fig. 1B and D) in
a WT (Fig. 1A and B) and KRAS
mutant (Fig. 1C and D) patient. The
median time of imaging after the procedure was 2 months following
treatment. RECIST criteria were utilized to measure the response in
each liver lobe treated (28).
Imaging assessment showed a partial response in 9 patients (19%),
stable disease in 20 (43%), and progressive disease in 18
(38%).
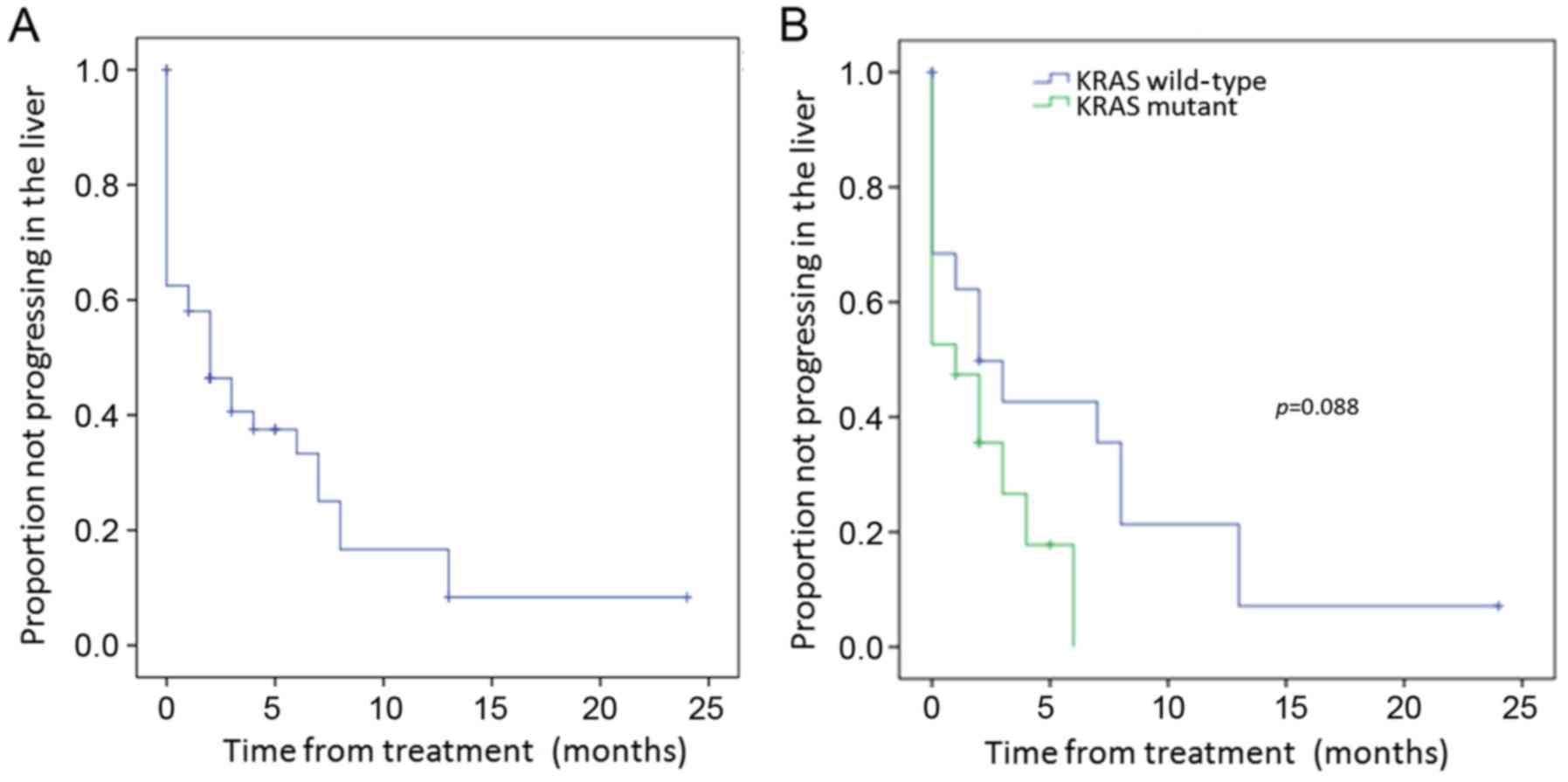
Median liver lobe progression-free survival duration
after 90Y treatment was 2 months [95% confidence
interval (CI), 0.34–3.66 months] for the entire cohort (Fig. 2A). The median local control duration
assessed according to tumor mutation was longer in the WT vs. KRAS
mutant patients of 2 vs. 1 month, respectively (p=0.088) (Fig. 2B). While this was not significant
with univariate analysis, it was significant with multivariate
analysis (p=0.031) (Table IV).
Progression-free survival in the liver was not significantly
different by univariate or multivariate analysis in patients
stratified by prior treatment number of chemotherapy lines
(Table IV). However, patients with
prior exposure to nilotinib or sunitinib had worse progression-free
survival in the liver with univariate analysis (5.6±1.3 vs. 0.5±0.5
months, p=0.034), but this was not significant with multivariate
analysis (Table V).
 | Table IV.Univariate and multivariate analyses
for progression-free survival and overall survival. |
Table IV.
Univariate and multivariate analyses
for progression-free survival and overall survival.
|
|
| Progression-free
survival in liver | Overall survival
treatment | Overall survival
diagnosis |
|---|
|
|
|
|
|
|
|---|
|
|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Variables | Reference | P-value | HR (95% CI) | P-value | P-value | HR (95% CI) | P-value | P-value | HR (95% CI) | P-value |
|---|
| Age (years) | <60 | 0.230 | 2.12
(0.41–10.99) | 0.370 | 0.044 | 0.82
(0.22–3.09) | 0.766 | 0.598 | 1.92
(0.47–7.95) | 0.366 |
| Gender | Female | 0.065 | 1.55
(0.60–3.98) | 0.364 | 0.442 | 0.87
(0.38–1.98) | 0.739 | 0.458 | 0.82
(0.34–1.94) | 0.644 |
| Performance
status | ≤1 vs. ≥2 | 0.395 | 0.63
(0.13–3.08) | 0.568 | 0.001 | 0.24
(0.09–0.61) | 0.003 | 0.017 | 0.21
(0.07–0.62) | 0.005 |
| Smoking | No | 0.742 | 1.24
(0.36–4.27) | 0.731 | 0.444 | 1.38
(0.51–3.76) | 0.524 | 0.013 | 2.03
(0.62–6.68) | 0.245 |
| Presence of
extrahepatic disease | No | 0.445 | 0.83
(0.30–2.30) | 0.723 | 0.775 | 0.90
(0.40–2.01) | 0.801 | 0.722 | 1.36
(0.58–3.18) | 0.475 |
| Mutation
status | KRAS WT | 0.088 | 0.29
(0.09–0.90) | 0.031 | 0.059 | 0.58
(0.26–1.33) | 0.200 | 0.322 | 0.69
(0.28–1.74) | 0.435 |
| FI | <1 | 0.716 | NA |
| 0.170 | NA |
| 0.040 | NA |
| No. of previous
chemotherapy lines | Continuous |
| 3.87
(0.47–32.16) | 0.211 |
| 1.11
(0.17–7.18) | 0.911 |
| 0.2 (0.05-.89) | 0.035 |
|
| 1 vs. ≥2 | 0.387 | 2.96
(0.23–38.73) | 0.409 | 0.652 | 1.60
(0.19–13.59) | 0.667 | 0.278 | 0.21
(0.03–1.47) | 0.115 |
|
| ≤2 vs. ≥3 | 0.703 | 5.07
(0.35–73.60) | 0.235 | 0.845 | 1.00
(0.11–9.28) | 0.998 | 0.801 | 0.24
(0.04–1.50) | 0.128 |
 | Table V.Univariate and multivariate analyses
for progression-free survival and overall survival with
chemotherapy exposure. |
Table V.
Univariate and multivariate analyses
for progression-free survival and overall survival with
chemotherapy exposure.
|
| Progression-free
survival in liver | Overall survival
treatment | Overall survival
diagnosis |
|---|
|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|
|
|---|
| Variables | P-value | HR (95% CI) | P-value | P-value | HR (95% CI) | P-value | P-value | HR (95% CI) | P-value |
|---|
| Oxaliplatin | 0.5 | 0.61
(0.19–2.0) | 0.413 | 0.009 | 0.12
(0.02–0.90) | 0.039 | 0.039 | 0.174
(0.02–1.33) | 0.092 |
| Leucovorin | 0.464 | 1.35
(0.51–3.55) | 0.545 | 0.063 | 1.89
(0.84–4.28) | 0.127 | 0.391 | 0.94
(0.41–2.15) | 0.886 |
| Irinotecan | 0.514 | 0.79
(0.34–1.81) | 0.569 | 0.689 | 0.71
(0.38–1.34) | 0.291 | 0.114 | 1.28
(0.64–2.56) | 0.479 |
| Bevacizumab | 0.073 | 0.39
(0.12–1.32) | 0.132 | 0.426 | 1.36
(0.68–2.75) | 0.385 | 0.858 | 1.0 (0.50–2.0) | 0.988 |
| Cetuximab or
panitumab | 0.799 | 0.56
(0.17–1.84) | 0.338 | 0.708 | 1.34
(0.46–3.87) | 0.59 | 0.332 | 1.65
(0.55–4.93) | 0.372 |
| Nilotinib or
sunitinib | 0.034 | 0.45
(0.15–1.34) | 0.15 | 0.01 | 0.32
(0.11–0.97) | 0.045 | 0.1 | 0.47
(0.16–1.41) | 0.176 |
Survival outcomes
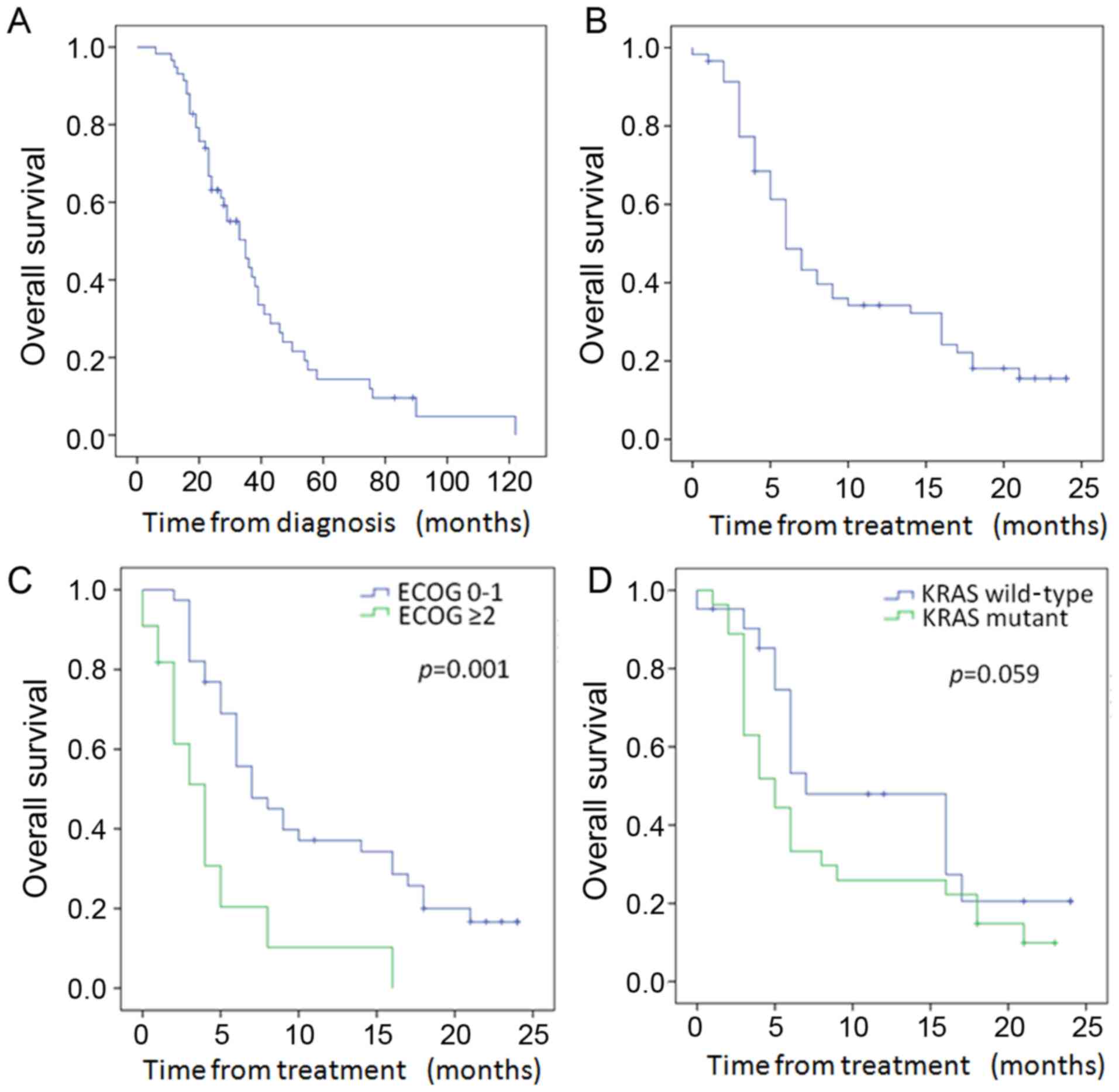
Of the 58 patients, 46 died during follow-up. As
shown in Fig. 3A, the median
survival from diagnosis was 35 months (95% CI, 26.9–43.1 months).
The median survival after 90Y was 6 months (95% CI,
4.55–7.45 months) in the entire cohort (Fig. 3B), with a 12-month survival of 33%.
Patients with ECOG performance status ≤1 had significantly improved
median survival over those with ECOG ≥2 (7 vs. 4 months, p=0.001)
(Fig. 3C and Table IV). In addition, with univariate
analysis, patients younger than 60 also had improved survival after
treatment (p=0.044) (Table IV).
The median survival from treatment stratified by mutational status
(Fig. 3D and Table IV) was longer in the WT as compared
to the KRAS mutant patients at 7 months (95% CI, 1.8–12.2) vs. 5
months (95% CI, 3.0–7.0), but did not achieve statistical
significance (p=0.059). While overall survival from treatment
stratified by the number of prior chemotherapy lines was not
significant with either univariate or multivariate analysis
(Table IV), prior exposure to
oxaliplatin was associated with improved survival from treatment
(22.5±1.1 vs. 9.0±1.0 months, p=0.009) and prior exposure to
nilotinib/sunitinib was associated with a worse survival from
treatment (10.5±1.1 vs. 3.8±0.48 months, p=0.01) (Table V). Futhermore, with multivariate
analysis, survival after treatment was significantly associated
with performance status [p=0.003; HR, 0.24 (CI, 0.09–0.61);
Table IV] and prior exposure to
oxaliplatin and nilotinib/sunitinib (p=0.039 and p=0.045,
respectively; Table V).
Survival from diagnosis was improved in those
patients with ECOG performance status ≤1 (42.3±4.5 vs. 27.3±6.5
months, p=0.17; Table IV). With
multivariate analysis, both the performance status and the number
of previous chemotherapy lines were significant for survival from
cancer diagnosis (Table IV). Prior
exposure to oxaliplatin was statistically significant for overall
survival from diagnosis (70.5±8.8 vs. 37.4±3.8 months, p=0.039)
with univariate analysis, but did not maintain significance with
multivariate analysis (Table
V).
ccfDNA analysis
ccfDNA, which was collected immediately prior to
90Y administration and 2–4 weeks after single lobe
treatment, was detected in all of the 9 analyzed samples by PCR. In
the WT and KRAS mutant patients, DNA FI was decreased from a median
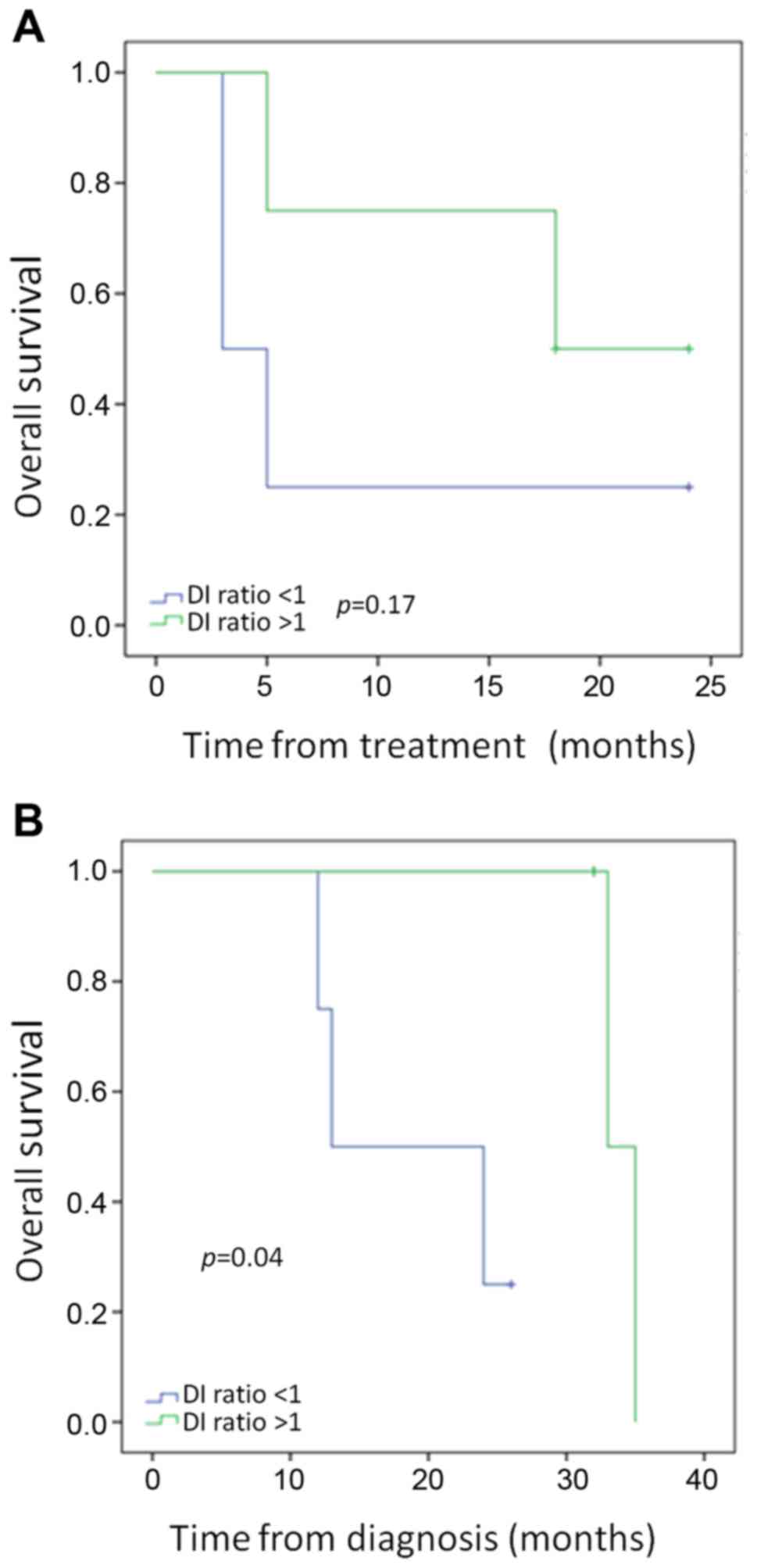
of 0.73–0.65 after treatment. As shown in Fig. 4, a decrease in the ratio of DNA FI
(pre-treatment FI/post-treatment FI), or an increase in the
fraction of small DNA fragments, after single lobe treatment was
associated with improved outcomes. While overall survival from
treatment did not meet statistical significance in patients with a
DNA FI ratio pre/post-treatment of >1 vs. <1 (Fig. 4A, p=0.17), overall survival from
diagnosis was significantly improved in those patients (Fig. 4B, p=0.04).
AFM analysis
Analysis by AFM of paired pre- and post-treatment
samples was performed in 4 WT and 2 KRAS mutant patients. An
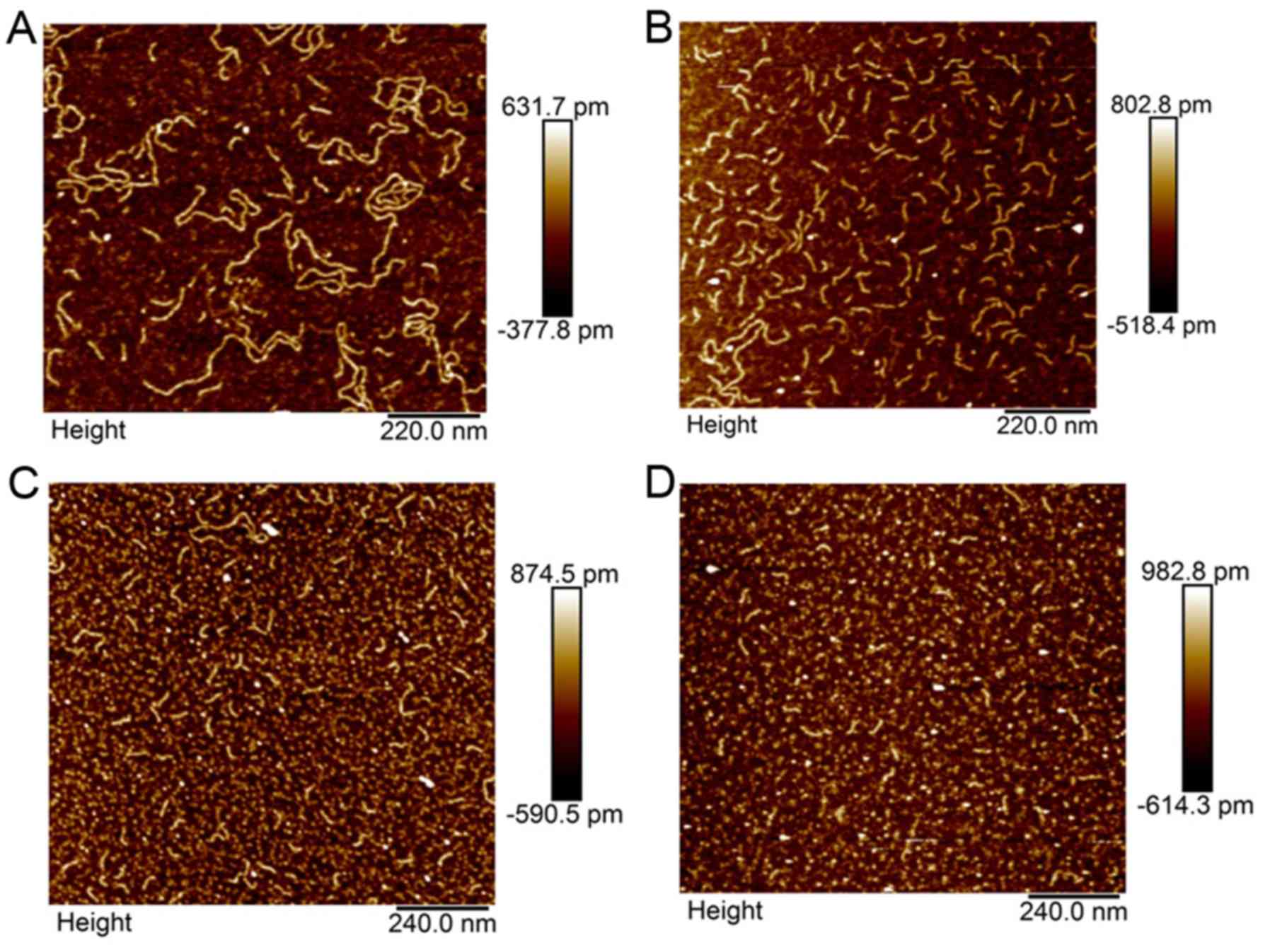
illustrative example is shown in Fig.
5 of sample AFM images of WT (Fig.
5A and B) and KRAS mutant (Fig. 5C
and D) DNA before (Fig. 5A and
C) and after (Fig. 5B and D)
single liver lobe exposure to 90Y. This figure
demonstrates the capability of AFM in measuring individual DNA
fragments and illustrates the treatment related differences in WT
and KRAS mutant patient ccfDNA.
In a pooled analysis of 4 WT and 2 KRAS mutant
patients, the average size of ccfDNA bp change after single lobe
treatment was significantly larger in the WT patients, with the WT
ccfDNA decreasing from 251.86 to 154.65 bp as compared to the KRAS
mutant ccfDNA decreasing from 177.98 to 155.09 bp (p=0.013).
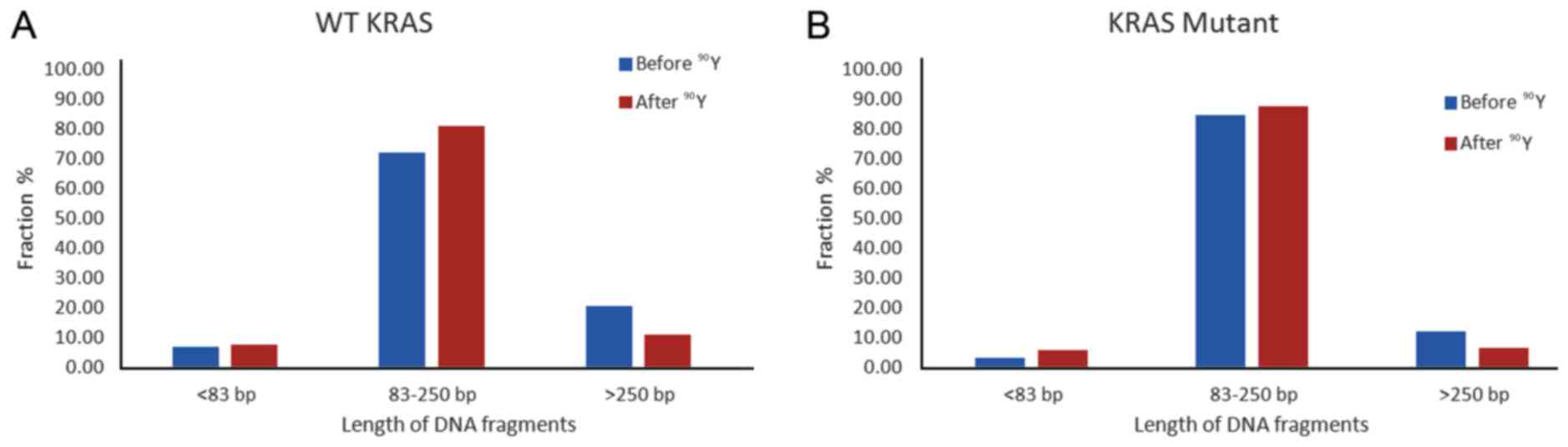
Quantification of changes in different fragment size groups before
and after treatment is shown in Fig.
6. While both WT and KRAS mutant patient ccfDNA had decreased
percentage of >250 bp size range and higher proportion of 85–350
bp ccfDNA after single lobe treatment, the magnitude of change was
larger in the WT patients, with the 85–350 bp size fragments
increasing by 12.3% in the WT (Fig.
6A) and 3.6% in the KRAS mutant (Fig. 6B).
Discussion
In this study we demonstrated that KRAS mutant
patients had less radiation-related changes in ccfDNA after
treatment than WT patients and that radiation treatment changes in
ccfDNA, as measured by DNA FI, were predictive for patient overall
survival. While several studies have previously described the
predictive potential of ccfDNA levels in overall survival after
chemotherapy for metastatic CRC patients (23,29),
this is the first study to demonstrate the treatment FI ratio as a
potential predictive marker for survival.
Molecular biomarker research has revealed CRC to be
a heterogeneous disease with multiple molecular disease subtypes
(12). CRC biomarker research has
led to the development of new chemotherapy regimens and novel
monoclonal antibody therapies targeting critical molecules in cell
signaling pathways, and these treatment advances have resulted in
improvements in overall survival for metastatic CRC (4). There is evidence that tumor response
to chemotherapy is predictive for overall survival. In a study by
Adam et al, response to chemotherapy correlated with
improved overall survival in patients who received neoadjuvant
chemotherapy prior to curative liver metastatic tumor resection
(30). Mechanisms of resistance to
targeted therapies are abundant, with the majority of KRAS WT
tumors not responding to anti-EGFR therapy (31). Anti-EGFR therapy resistance has been
associated with other gene mutations, epigenetic silencing, and
augmented expression of other receptor tyrosine kinases, resulting
in a complex tumor microenvironment that may be difficult to
predict treatment response (12).
Multiple clinical trials have confirmed the lack of
clinical benefit of anti-EGFR therapy against RAS mutant tumors
(32,33). The meta-analysis from Sorich et
al in 2015 demonstrated that tumors without RAS mutations
exhibited an improved clinical response compared to RAS mutant
tumors, regardless of the specific targeted therapies or partner
chemotherapy used (34). In
addition, KRAS mutant tumors have been associated with poor
survival outcomes and treatment responses in the absence of
targeted agents (16,35,36),
which may indicate that these tumors are less responsive to
systemic therapies. The impact of KRAS mutation status on radiation
treatment response is less clear, but is an area of investigation.
Lahti et al reported KRAS mutation status to be predictive
for overall survival after 90Y radioembolization for
unresectable CRC liver metastases (18). While overall survival after
90Y treatment in KRAS mutant vs. WT patients did not
quite meet statistical significance in our study, there was a clear
separation in the curves. Notably, mutation status was significant
on multivariate analysis for liver progression-free survival in our
study. Intriguingly, it is possible that the same differential
chemotherapy responses of WT and KRAS mutant tumors that have been
well described are also true for radiation treatment.
El Messaoudi et al recently demonstrated that
higher ccfDNA concentrations, higher levels of mutant ccfDNA, and
the level of ccfDNA fragmentation correlated with shorter overall
survival in metastatic CRC patients (22). While there was no difference in
overall survival from treatment as a function of DNA integrity
index in our study, analysis of survival from diagnosis was
statistically longer in those patients with a decrease in their
fragment ccfDNA size after treatment. The discrepancy in survival
may be a function of the small number of patients in our study.
However, it may also be a function of the limitations of
90Y in treating metastatic CRC, which have been
previously outlined in the recently published SIRFLOX study
(11), with the overall aim of
90Y treatment being to control liver disease and not to
control systemic burden. Radiation treatment-related DNA FI changes
are likely reflective of tumor response capability. Indeed, DNA FI
may reflect tumoricidal changes and could serve as a biomarker to
assess treatment response.
Our study sample with molecular data is small. While
our findings are hypothesis driving, definitive conclusions are
limited until a more robust analysis can be performed on a larger
number of patients. In addition, while all of our patients received
chemotherapy prior to 90Y treatment and many were
considered chemotherapy refractory, our patient population was
diverse in their overall level of health and in the natural history
of their disease. It is not surprising that overall survival was
improved in patients with better performance statuses. While no
overall survival differences were observed among patients with or
without extrahepatic disease, the overall survival differences seen
based on the number and types of chemotherapy exposures likely
reveals variation in patient performance status and disease course.
Further molecular studies on a larger group of patients are
necessary to validate our observational findings.
In conclusion, 90Y radioembolization is
an effective treatment for CRCLM in extending local control for
liver dominant metastatic disease. However, KRAS mutant tumors may
be more radio-resistant to treatment. In this study, changes in
ccfDNA FI were correlated with overall survival, likely indicating
that these changes are reflective of treatment response.
Measurements of FI may have the potential to be another molecular
biomarker that could predict treatment response to therapy.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Nordlinger B, Adam R, Köhne
CH, Pozzo C, Poston G, Ychou M and Rougier P: European Colorectal
Metastases Treatment Group: Towards a pan-European consensus on the
treatment of patients with colorectal liver metastases. Eur J
Cancer. 42:2212–2221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stangl R, Altendorf-Hofmann A, Charnley RM
and Scheele J: Factors influencing the natural history of
colorectal liver metastases. Lancet. 343:1405–1410. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gallagher DJ and Kemeny N: Metastatic
colorectal cancer: From improved survival to potential cure.
Oncology. 78:237–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Helling TS and Martin M: Cause of death
from liver metastases in colorectal cancer. Ann Surg Oncol.
21:501–506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xing M, Kooby DA, El-Rayes BF, Kokabi N,
Camacho JC and Kim HS: Locoregional therapies for metastatic
colorectal carcinoma to the liver - an evidence-based review. J
Surg Oncol. 110:182–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kennedy A, Coldwell D, Sangro B, Wasan H
and Salem R: Radioembolization for the treatment of liver tumors
general principles. Am J Clin Oncol. 35:91–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sato KT, Lewandowski RJ, Mulcahy MF,
Atassi B, Ryu RK, Gates VL, Nemcek AA Jr, Barakat O, Benson A III,
Mandal R, et al: Unresectable chemorefractory liver metastases:
Radioembolization with 90Y microspheres - safety,
efficacy, and survival. Radiology. 247:507–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hickey R, Lewandowski RJ, Prudhomme T,
Ehrenwald E, Baigorri B, Critchfield J, Kallini J, Gabr A,
Gorodetski B, Geschwind JF, et al: 90Y Radioembolization
of colorectal hepatic metastases using glass microspheres: Safety
and survival outcomes from a 531-patient multicenter study. J Nucl
Med. 57:665–671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maleux G, Deroose C, Laenen A, Verslype C,
Heye S, Haustermans K, De Hertogh G, Sagaert X, Topal B, Aerts R,
et al: Yttrium-90 radioembolization for the treatment of
chemorefractory colorectal liver metastases: Technical results,
clinical outcome and factors potentially influencing survival. Acta
Oncol. 55:486–495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Hazel GA, Heinemann V, Sharma NK,
Findlay MP, Ricke J, Peeters M, Perez D, Robinson BA, Strickland
AH, Ferguson T, et al: SIRFLOX: Randomized phase III trial
comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus
mFOLFOX6 (plus or minus bevacizumab) plus selective internal
radiation therapy in patients with metastatic colorectal cancer. J
Clin Oncol. 34:1723–1731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Linnekamp JF, Wang X, Medema JP and
Vermeulen L: Colorectal cancer heterogeneity and targeted therapy:
A case for molecular disease subtypes. Cancer Res. 75:245–249.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Migliore L, Migheli F, Spisni R and
Coppedè F: Genetics, cytogenetics, and epigenetics of colorectal
cancer. J Biomed Biotechnol. 2011:7923622011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Waring P, Tie J, Maru D and Karapetis CS:
RAS mutations as predictive biomarkers in clinical management of
metastatic colorectal cancer. Clin Colorectal Cancer. 15:95–103.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Osumi H, Shinozaki E, Suenaga M, Matsusaka
S, Konishi T, Akiyoshi T, Fujimoto Y, Nagayama S, Fukunaga Y, Ueno
M, et al: RAS mutation is a prognostic biomarker in colorectal
cancer patients with metastasectomy. Int J Cancer. 139:803–811.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andreyev HJ, Norman AR, Cunningham D,
Oates JR and Clarke PA: Kirsten ras mutations in patients with
colorectal cancer: The multicenter ‘RASCAL’ study. J Natl Cancer
Inst. 90:675–684. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hutchins G, Southward K, Handley K, Magill
L, Beaumont C, Stahlschmidt J, Richman S, Chambers P, Seymour M,
Kerr D, et al: Value of mismatch repair, KRAS, and BRAF mutations
in predicting recurrence and benefits from chemotherapy in
colorectal cancer. J Clin Oncol. 29:1261–1270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lahti SJ, Xing M, Zhang D, Lee JJ,
Magnetta MJ and Kim HS: KRAS status as an independent prognostic
factor for survival after Yttrium-90 radioembolization therapy for
unresectable colorectal cancer liver metastases. J Vasc Interv
Radiol. 26:1102–1111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schwarzenbach H, Hoon DS and Pantel K:
Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev
Cancer. 11:426–437. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bettegowda C, Sausen M, Leary RJ, Kinde I,
Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, et al:
Detection of circulating tumor DNA in early- and late-stage human
malignancies. Sci Transl Med. 6:224ra242014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spindler KL, Appelt AL, Pallisgaard N,
Andersen RF, Brandslund I and Jakobsen A: Cell-free DNA in healthy
individuals, noncancerous disease and strong prognostic value in
colorectal cancer. Int J Cancer. 135:2984–2991. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
El Messaoudi S, Mouliere F, Du Manoir S,
Bascoul-Mollevi C, Gillet B, Nouaille M, Fiess C, Crapez E, Bibeau
F, Theillet C, et al: Circulating DNA as a strong multi-marker
prognostic tool for metastatic colorectal cancer patient management
care. Clin Cancer Res. 22:3067–3077. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spindler KL, Pallisgaard N, Vogelius I and
Jakobsen A: Quantitative cell-free DNA, KRAS, and BRAF mutations in
plasma from patients with metastatic colorectal cancer during
treatment with cetuximab and irinotecan. Clin Cancer Res.
18:1177–1185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Umetani N, Giuliano AE, Hiramatsu SH,
Amersi F, Nakagawa T, Martino S and Hoon DS: Prediction of breast
tumor progression by integrity of free circulating DNA in serum. J
Clin Oncol. 24:4270–4276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mouliere F, El Messaoudi S, Pang D,
Dritschilo A and Thierry AR: Multi-marker analysis of circulating
cell-free DNA toward personalized medicine for colorectal cancer.
Mol Oncol. 8:927–941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pang D, Winters TA, Jung M, Purkayastha S,
Cavalli LR, Chasovkikh S, Haddad BR and Dritschilo A:
Radiation-generated short DNA fragments may perturb non-homologous
end-joining and induce genomic instability. J Radiat Res (Tokyo).
52:309–319. 2011. View Article : Google Scholar
|
|
27
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC, et al: New guidelines to evaluate the
response to treatment in solid tumors. European Organization for
Research and Treatment of Cancer, National Cancer Institute of the
United States, National Cancer Institute of Canada. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Spindler KL, Pallisgaard N, Andersen RF,
Ploen J and Jakobsen A: Gemcitabine and capecitabine for heavily
pre-treated metastatic colorectal cancer patients - a phase II and
translational research study. Anticancer Res. 34:845–850.
2014.PubMed/NCBI
|
|
30
|
Adam R, Pascal G, Castaing D, Azoulay D,
Delvart V, Paule B, Levi F and Bismuth H: Tumor progression while
on chemotherapy: A contraindication to liver resection for multiple
colorectal metastases? Ann Surg. 240:1052–1064. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Roock W, Claes B, Bernasconi D, De
Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V,
Papamichael D, Laurent-Puig P, et al: Effects of KRAS, BRAF, NRAS,
and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy
in chemotherapy-refractory metastatic colorectal cancer: A
retrospective consortium analysis. Lancet Oncol. 11:753–762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sorich MJ, Wiese MD, Rowland A,
Kichenadasse G, McKinnon RA and Karapetis CS: Extended RAS
mutations and anti-EGFR monoclonal antibody survival benefit in
metastatic colorectal cancer: A meta-analysis of randomized,
controlled trials. Ann Oncol. 26:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Andreyev HJ, Norman AR, Cunningham D,
Oates J, Dix BR, Iacopetta BJ, Young J, Walsh T, Ward R, Hawkins N,
et al: Kirsten ras mutations in patients with colorectal cancer:
The ‘RASCAL II’ study. Br J Cancer. 85:692–696. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Richman SD, Seymour MT, Chambers P,
Elliott F, Daly CL, Meade AM, Taylor G, Barrett JH and Quirke P:
KRAS and BRAF mutations in advanced colorectal cancer are
associated with poor prognosis but do not preclude benefit from
oxaliplatin or irinotecan: Results from the MRC FOCUS trial. J Clin
Oncol. 27:5931–5937. 2009. View Article : Google Scholar : PubMed/NCBI
|