Introduction
Thioridazine
(10-[2-(1-methyl-2-piperidyl)ethyl]-2-methyl-thiophenothiazine,
THIO), a member of the phenothiazine family, was originally used to
treat psychotic disorders such as psychosis and schizophrenia
(1–3). In addition, in advanced cancer
patients, this drug has been used to treat cancer-related sweating
(4,5), as well as depression (6). In recent years, however, some studies
have demonstrated that THIO could inhibit the growth of some cancer
cell lines (7–14). It has also shown selectivity for
cancer stem cells (CSCs), such as leukemic cancer stem cells
(15), glioblastoma stem cells
(3) and breast cancer stem cells
(16). Thus, THIO is currently
regarded as a drug with potential usefulness in anticancer
chemotherapy (9,17).
Recent evidence suggests that cancer development is
mainly driven by a rare population of cells, the CSCs (16,17).
Moreover, some scientists argue that conventional chemotherapeutics
are ineffective against human CSCs (18). Therefore, the development of new
drugs targeting CSCs holds special significance in clinical cancer
research. Sachlos et al (15) demonstrated that THIO could
selectively target neoplastic cells and impair human somatic CSCs
capable of in vivo leukemic disease initiation without any
effect on normal blood stem cells. Their study identified the
potential of THIO to target CSCs directly. However, its exact
effect on CSCs of other types of cancers still requires further
investigation.
Colon cancer is one of the most prevalent cancers,
with incidence rates that have been increasing steadily worldwide
(19,20). In recent years, despite a series of
remarkable advances in colon cancer chemotherapy, the increased
resistance to anticancer drugs has been a serious obstacle to the
efficient treatment of the disease. Overcoming drug resistance and
targeting CSCs are key for the improvement of chemotherapy response
(15,20,21).
Therefore, the development of novel effective drugs for colon
cancer is urgently required.
In this study, we mainly investigated the
anti-proliferation and anti-invasion effects of THIO on CSCs
isolated from a human colon cancer cell line (HCT116) and further
determined the underlying mechanisms. These findings may contribute
to the development of THIO-based chemotherapy for patients with
colon cancer resistant to traditional anticancer drugs.
Materials and methods
Cell lines and culture
The human colon cancer cell line HCT116 and the
human lung fibroblast cell line HELF were obtained from the Cell
Bank of the Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences (Shanghai, China). HCT116 and HELF cells were
maintained in RPMI-1640 medium (Gibco/Thermo Fisher Scientific,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco) and penicillin-streptomycin mixed solution. The CSCs
isolated from HCT116 cells were cultured in DMEM/F12 medium (Gibco)
with 10 µg/ml EGF (Sigma-Aldrich, St. Louis, MO, USA), 10 µg/ml
bFGF (Sigma-Aldrich) and 1% B27 supplement (Gibco). The media were
changed every other day. The cells were incubated in a humidified
incubator with 5% CO2 at 37°C, and were passaged by a
dilution of 1:3 every 4 or 5 days.
EpCAM+ and CD44+
cell selection
The cell selection method followed the protocol
published by Zhang et al (21). Briefly, HCT116 cells were rinsed
with phosphate-buffered saline (PBS) and detached with trypsin at
37°C. After centrifugation, cells were first incubated with
anti-human CD44 monoclonal antibody conjugated with biotin
(eBioscience, Inc., San Diego, CA, USA), and then fractionated
using a CELLection Biotin Binder kit (Invitrogen, Carlsbad, CA,
USA), according to the manufacturers recommendations. The isolated
cells were further incubated with anti-human EpCAM monoclonal
antibody conjugated with biotin (eBioscience) and positive cells
were then isolated with the same kit. Cells were cultured in CSC
medium, and the purity of such CSCs was evaluated by flow cytometry
and immunohistochemistry.
Flow cytometric analysis
The CSCs were dissociated into single cells, further
fixed with fixation buffer (eBioscience) and prepared at a
concentration of 2.0×106/ml of PBS. Anti-human CD44
antibody conjugated with FITC (BD Biosciences, San Jose, CA, USA)
and anti-human EpCAM antibody conjugated with PE (BD Biosciences)
were added and incubated for 30 min at 4°C. After washing twice
with PBS, the cells were acquired and analyzed by FACScalibur (BD
Biosciences).
Immunohistochemistry
The single CSCs were seeded into plates covered in
Matrigel and cultured for 24 h. They were fixed in 4% formaldehyde
for 20 min at 4°C and permeabilized with 0.1% Triton X-100 for 10
min at 25°C. For immunohistochemistry, the primary antibodies used
were anti-human CD44 monoclonal antibodies (1:50; eBioscience) and
anti-human EpCAM monoclonal antibodies (1:50; eBioscience). After
12–14 h of incubation at 4°C, samples were washed three times with
PBS and processed using an ABC kit and DAB solution (both purchased
from Vector Laboratories, Inc., Burlingame, CA, USA). Finally, the
sections were imaged with an Axio Scope A1 and AxioCAM MRc 5
(Carl-Zeiss, Oberkochen, Germany).
Colony formation assay
The CSCs were dissociated into single cells and
seeded into a 96-well plate at a concentration of 0.5 cells/well.
Briefly, the cell suspension was diluted at concentration of 50
cells/ml and 100 µl of cell suspension was added into each well. As
a result, there is 1 cell or 0 cell seeded into each well. We
observed and imaged cell cloning. The plate was incubated in a
humidified incubator with 5% CO2 at 37°C and half of the
medium was changed every 3 days. Colonies gradually formed over the
next 3 weeks.
Inhibitory effects of THIO on cell
proliferation
In order to analyze the anticancer effect of THIO on
CSCs derived from HCT116 cells, a CCK-8 assay was performed. In
order to explore the effect of THIO on fibroblast cells,
representing one of the largest amount of cells in humans, HELF
cells were also selected in CCK8-assay. The cells were seeded in
96-well plates at a concentration of 1.0×105 cells/well,
and were treated with THIO (Sigma-Aldrich) at different
concentrations (0, 10, 20 or 50 µM) for 24 h. The medium without
any cells was used as the blank group, while 100 µM cisplatin (DDP)
was used as the positive control group. The proliferation index of
each group was determined using the Cell Counting kit-8 (CCK-8;
Dojindo Laboratories, Tokyo, Japan) according to the manufacturers
instructions (21). In brief, 10 µl
of CCK-8 solution was added into each well (containing 100 µl of
medium) and cultured for 1–2 h at 37°C. The absorbance at 450 nm,
which was directly proportional to the number of living cells, was
observed for each group (n=4). The inhibition ratio was used to
measure cell proliferation in the present study and it was
described as (absorbance of 0 µM group - absorbance of each
experimental group)/(absorbance of 0 µM group - the absorbance of
the blank group).
Cell invasion assay
To assess cell invasion, a Transwell system was used
(pore size: 8 µm; Corning, Inc., Corning, NY, USA) following the
manufacturers protocol. The CSCs were seeded onto the upper insert
covered with Matrigel at a concentration of 1×105 cells
per insert in serum-free medium. Outer wells were filled with
RPMI-1640 medium containing 10% FBS as a chemoattractant. Then,
cells were incubated for 48 h at 37°C. Non-invading cells were
removed by swabbing the top layer, and cells able to migrate
through the gel and attach to the lower surface of the membrane
were stained with crystal violet. The number of cells in four
randomly selected microscopy fields was counted for each
filter.
Real-time qPCR
Total RNA was extracted using TRIzol reagent
(Invitrogen). For each sample, RNA (2 mg) was reverse-transcribed
using an RT-PCR kit (Takara, Shiga, Japan), and qPCR was performed
with a Thermal Cycler Dice™ Real-Time System and SYBR-Green Premix
EX Taq™ (Takara). In the present study, GAPDH was used for
qPCR normalization, and all measurements were performed in
triplicate. The primer sequences used (5→3) are shown in Table I.
 | Table I.Real-time qPCR primer sequences. |
Table I.
Real-time qPCR primer sequences.
| Gene | Primer sequence
(5–3) |
|---|
| Nanog | F:
ATGCCTGTGATTTGTGGGCC |
|
| R:
GCCAGTTGTTTTTCTGCCAC |
| CLDN-6 | F:
GCCAGATGCAGTGCAAGGTGT |
|
| R:
GATGACAAAGACAATCCCAGAGGTG |
| Bax | F:
TAACCAAGGTGCCGGAACTGA |
|
| R:
GGGAGGAGTCTCACCCAACCA |
| Caspase-3 | F:
CATGGAAGCGAATCAATGGACT |
|
| R:
CTGTACCAGACCGAGATGTCA |
| Bcl-2 | F:
GGGGAGGATTGTGGCCTTCTTT |
|
| R:
TAATGTGCAGGTGCCGGTTCAG |
| GAPDH | F:
ACCACAGTCCATGCCATCAC |
|
| R:
TCCACCACCCTGTTGCTGTA |
Western blotting
Western blotting was carried out to test for cleaved
caspase-3 using the same protocol as the one we reported in a
previous publication (21).
Briefly, cells were lysed with lysis buffer (50 mM Tris pH 7.0, 1
mM EDTA, 150 mM NaCl, 1% NP40, 10 mM NaF, 1 mM
Na3VO4) containing protease inhibitor
cocktail (Roche, Basel, Switzerland), and protein concentrations
were determined using a BCA assay kit (Beyotime Institute of
Biotechnology, Nanjing, China). Protein bands were separated by
electrophoresis in a 12% sodium dodecyl sulfate polyacrylamide gel
(SDS-PAGE) and electroblotted onto polyvinylidene fluoride (PVDF)
membranes (Millipore, Bedford, MA, USA). After blocking them with
4% non-fat dry milk in Tris-buffered saline (TBS), the membranes
were incubated overnight at 4°C with primary antibodies (rabbit
anti-caspase-3; Santa Cruz Biotechnology, Santa Cruz, CA, USA)
diluted 1:1,000 in TBS. They were washed three times with TBS
containing 0.5% Tween-20, and then incubated for 1 h at 25°C with
secondary antibodies conjugated with a 1:5,000 dilution of
horseradish peroxidase (HRP) in TBS. Membranes were then washed
three times in TBS containing 0.5% Tween-20 at 25°C. Finally,
protein bands were visualized on X-ray film using enhanced
chemiluminescence (ECL; GE Healthcare, Bethesda, MD, USA).
Analysis of cell apoptosis
To analyze cell apoptosis, acridine orange/ethidium
bromide (AO/EB) staining and Annexin V-FITC/PI staining were used
according to the manufacturers instructions (BD Biosciences).
Moreover, to determine the effect of THIO on mitochondrial membrane
potential in CSCs, JC-1 apoptosis detection kit (BD Biosciences)
was used according to the manufacturers instructions and assessed
by fluorescence-activated cell sorting (FACS).
Statistical analysis
Statistical analysis was performed with the SPSS
17.0. The results are expressed as mean ± SEM. The differences
between the groups were assessed by one-way ANOVA followed by
t-tests. P<0.05 was considered statistically significant.
Results
Characterization of CSCs from HCT116
cells
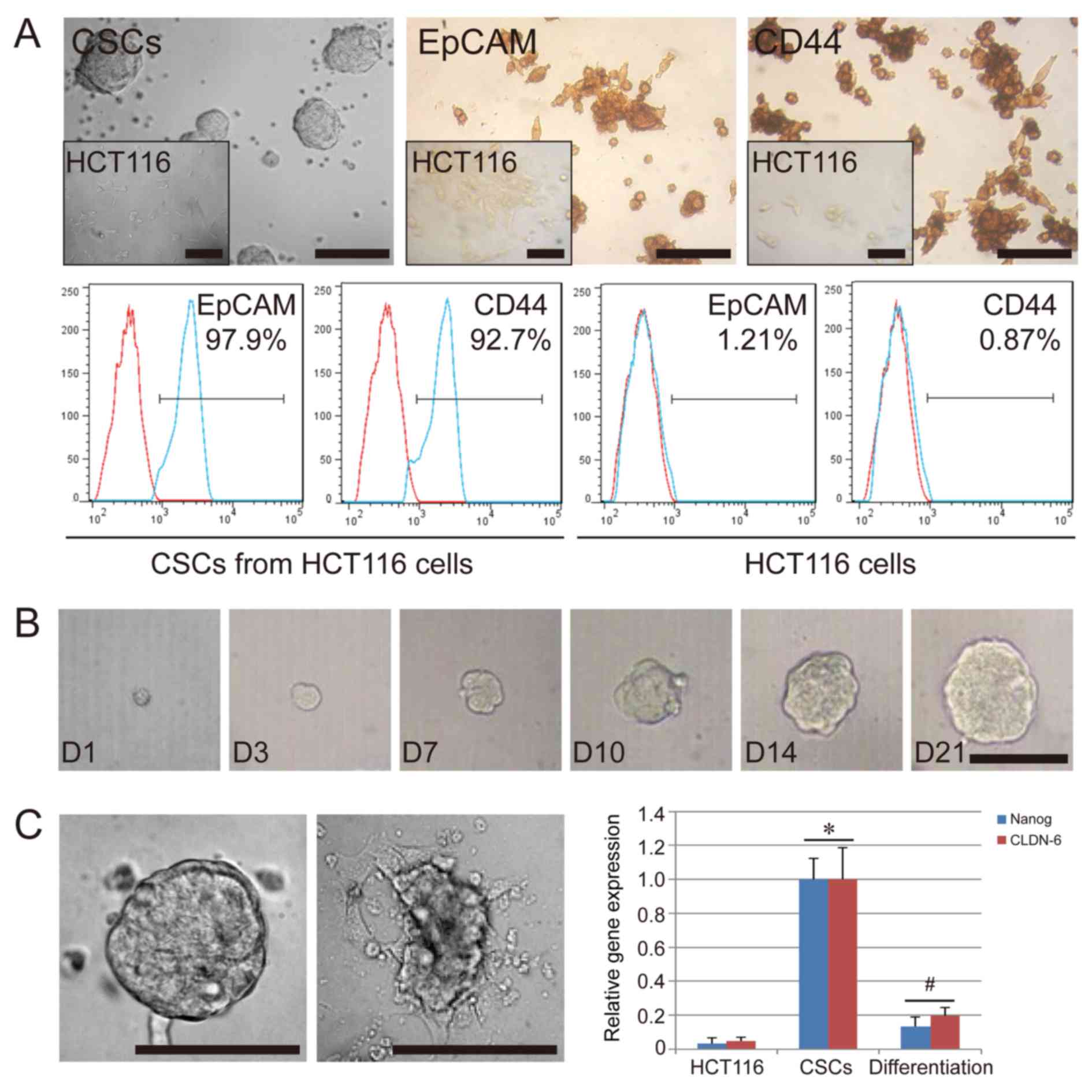
The CSCs isolated from HCT116 formed tumor spheres
in suspension when cultured in vitro. Their appearance was
distinct from the original HCT116 cells, which were spindle-shaped
and grew in adherent state (Fig.
1A). After three to four passages, the cells were further
characterized by immunohistochemistry and flow cytometry. The
results indicated that the CSCs used in the present study were
positive for EpCAM (97.9%) and CD44 (92.7%), while HCT116 cells
used as the negative control group displayed a low expression of
EpCAM (1.21%) and CD44 (0.87%) (Fig.
1A). To further confirm the colony-forming ability of the CSCs,
we separated the CSC spheres into single cells and analyzed their
colony-formation ability in 96-well plates. As expected, a single
CSC could proliferate and grow; in fact, over 40% of the CSCs
formed a tumor sphere after 21 days of single-cell culture
(Fig. 1B). In addition, we
confirmed that the serum-free condition was necessary for the
colony-formation ability and the stem cell-specific gene expression
of CSCs. We compared the in vitro cell culture in basal
conditions plus 10% FBS and in serum-free conditions. Cultures
under FBS conditions could lead to a layer of adherent confluent
cells. Compared with HCT116 cells, the CSCs showed a high
expression of stem cell-specific genes, NANOG, and CLDN-6
(P<0.05), while downregulation of these genes occurred in the
CSCs cultured under FBS conditions (P<0.05) (Fig. 1C). These results suggest that the
tumor sphere-like colonies could be obtained from the HCT116 cell
line, and that these cells had some stem cell characteristics. In
serum-free medium supplemented with EGF and bFGF, the CSCs
differentiated even under conditions of an extra-low cell
concentration, such as single cell conditions, ruling out the
possibility that CSCs may aggregate owing to the high concentration
of cells in cultures.
Effect of THIO on the proliferation
and invasion of CSCs from HCT116 cells
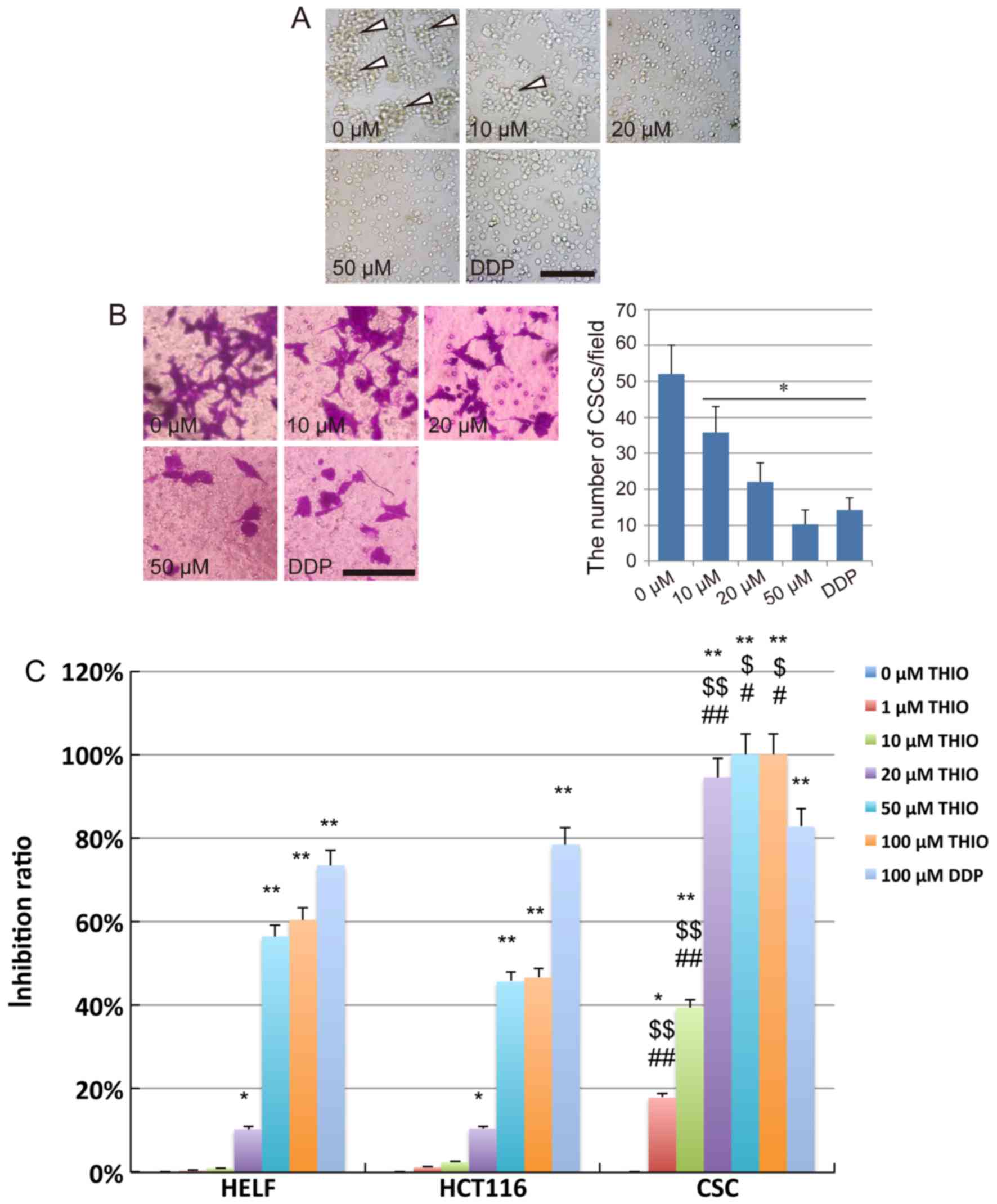
After treatment with THIO for 24 h at different
concentrations, severe morphological alterations were observed in
the majority of CSCs cells. We found that the CSCs could still form
tumor sphere-like colonies when treated with THIO at 10 µM.
However, the number of colonies formed at 10 µM was considerably
lower than at 0 µM. No colony formation could be observed at THIO
concentrations of 20 µM and above and after treatment with DDP
(Fig. 2A).
In vitro invasion assays were
performed using CSCs from HCT116 cells treated with THIO in a
Transwell system
After crystal violet staining, we observed that the
CSC invasion across the membrane from the upper chamber to the
lower surface of the membrane was suppressed by THIO. The number of
cells on the lower surface of the membrane clearly decreased in the
THIO-treated groups at concentrations of 20 and 50 µM, while a
slight inhibition of the cell invasion ability was observed in the
10 µM concentration group (Fig.
2B). After counting the number of cells on the lower surface of
the membrane, we concluded that THIO could significantly suppress
the invasion of CSCs (P<0.05). This effect occurred in a
concentration-dependent manner (52.0±8.0 cells for the 0 µM group,
35.6±7.3 cells for the 10 µM group, 22.0±5.4 cells for the 20 µM
group, 10.3±4.0 cells for the 50 µM group, and 14.3±3.3 cells for
the DDP group) (Fig. 2B).
To further analyze the anticancer effect of THIO on
CSCs derived from HCT116 cells, a CCK-8 assay was performed using
treated cells (normal human lung fibroblast cell line, HELF cells,
HCT116 cells and CSCs). The results indicated that the
proliferation of HELF and HCT116 cells was significantly inhibited
by THIO at 50 and 100 µM, while that of CSCs was significantly
inhibited by THIO at a concentration as low as 1 µM. The inhibition
ratio of THIO at 1 and 10 µM was 19.2 and 39.5%, respectively.
However, THIO concentrations of 20, 50 and 100 µM exhibited
inhibition at very high rates (94.5, 100 and 100%, respectively).
The positive control group (10 µM DDP) showed an inhibition rate of
82.7%. Interestingly, THIO at 20 µM did affect the vitality of HELF
cells, but the inhibition rate was only 10.6%, while that of CSCs
was 94.5% (Fig. 2C). These results
indicate that, at a suitable concentration, THIO may be an optimal
novel agent for colon cancer treatment. However, further studies
are necessary to elucidate the mechanism of action driving this
anticancer activity.
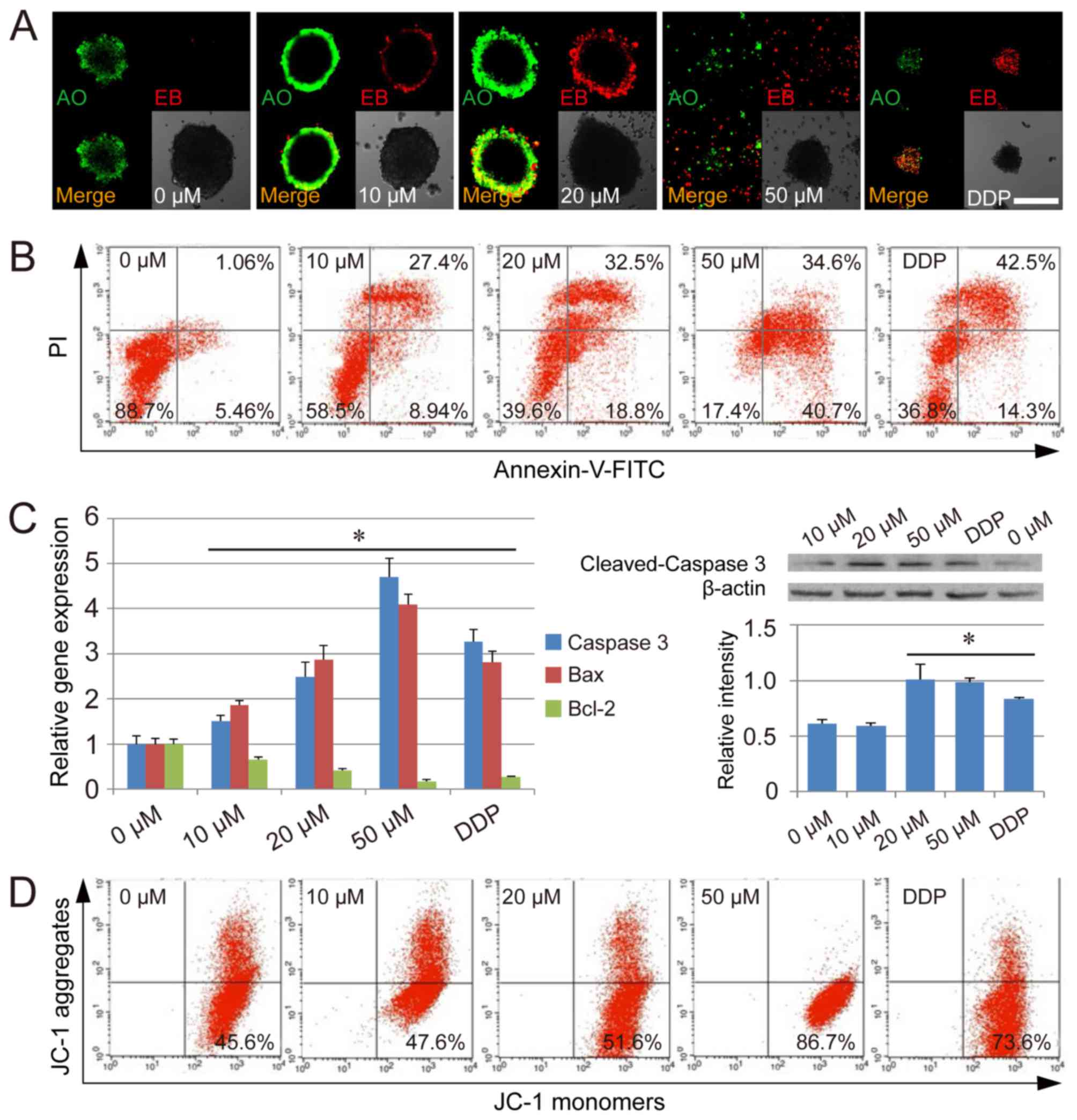
Analysis of THIO-induced apoptosis in
CSCs
THIO has been reported to induce cervical and
endometrial cancer cell apoptosis (7). To detect whether THIO could induce
apoptosis in CSCs derived from HCT116 cells, we carried out AO/EB
staining. The CSC spheres were labeled by AO/EB after treatment
with THIO at different concentrations, and dual staining was
examined using confocal microscopy. No significant apoptosis was
detected in the negative control group. However, in the group
treated with THIO at a concentration of 10 µM, many early-stage
apoptotic cells (marked by yellow-green AO nuclear staining) and
some late-stage apoptotic cells (marked by orange-red nuclear EB
staining) were detected. At higher concentrations (20 and 50 µM),
the number of late-stage apoptotic cells was even higher (Fig. 3A). At 50 µM, CSC spheres were much
smaller than at lower concentrations, and many single cells could
be observed in late-stage apoptosis (Fig. 3A). Further detection by the Annexin
V/PI double staining assay revealed that THIO treatment could lead
to an increase in the proportion of early-stage apoptotic cells
(Annexin V-positive and PI-negative cells), as well as late-stage
apoptotic cells (Annexin V-positive and PI-positive cells) in CSCs
(Fig. 3B). Further analysis
indicated that this induction of apoptosis by THIO occurred in a
concentration-dependent manner. The percentage of early-stage
apoptotic cells was 5.46, 8.94, 18.8, 40.7 and 14.3% at THIO
concentrations of 0, 10, 20 and 50 µM, and DDP treatment,
respectively. The percentage of late-stage apoptotic cells was
1.06, 27.4, 32.5, 34.6 and 42.5% at THIO concentrations of 0, 10,
20 and 50 µM, and DDP treatment, respectively (Fig. 3B).
To elucidate the mechanisms of cell apoptosis
induced by THIO, cell apoptosis genes and mitochondrial membrane
potential were assayed as part of this study. qPCR results
indicated that apoptosis genes such as caspase-3 and Bax were
significantly upregulated in THIO-treated CSCs (P<0.05), while
the anti-apoptosis gene Bcl-2 was significantly downregulated under
the same conditions (P<0.05) (Fig.
3C). Moreover, the expression of the caspase-3 gene was
confirmed by the western blot analysis. To evaluate the
THIO-induced cell apoptosis at the subcellular organelle level,
JC-1 staining was carried out to determine the alterations in
mitochondria. The percentage of cells with loss of mitochondrial
membrane potential increased in a concentration-dependent manner
(45.6% for the 0 µM group, 47.6% for the 10 µM group, 51.6% for the
20 µM group, 86.7% for the 50 µM group, and 73.6% for the DDP
group) (Fig. 3D). The results
suggest that THIO may induce apoptosis in CSCs from HCT116 cells
via the loss of mitochondrial membrane potential.
Discussion
THIO has been used in human clinical studies for
over 50 years, especially for tuberculosis (TB) therapy (22,23),
alleviation of schizophrenia (24)
and reduction of pain in cancer (25). In recent years, THIO has been
reported to suppress cell proliferation and induce cell apoptosis
in several types of cancers (2,7,9,26,27).
However, the cytotoxic effect of THIO on colon cancer has not been
examined, and the effects of THIO on the viability of CSCs, which
are responsible for apoptosis resistance, self-renewal and
differentiation, should be investigated thoroughly. The present
study revealed that THIO could reduce the viability of CSCs from
colon cancer cells (HCT116) and induce apoptosis of CSCs via the
mitochondrial pathway. Previous studies in our group revealed that
THIO also induces apoptosis in CSCs derived from other cancer cell
lines, such as DU145 (human prostate carcinoma cell line) (data not
shown). Thus, THIO-based chemotherapy may prove useful in the
treatment of various types of cancers. This must be evaluated in
future clinical studies.
Although the anticancer effect of THIO has been
demonstrated in vivo using mouse models (26,28,29),
few clinical trials have been carried out in cancer patients
(30) for the complex mechanisms of
the THIO anticancer effect to be clearly understood. In the present
study, JC-1 results showed that the mitochondrial membrane
potential in CSCs was downregulated during apoptosis, which
indicates that THIO-induced apoptosis in CSCs may be related to the
mitochondrial pathway. In addition to the direct cytotoxic effect
on cancer cells, THIO can potentially induce CSC differentiation to
overcome neoplastic self-renewal, and compel CSCs to enter the
normal cellular lifecycle, via antagonism of D2-family DRs
differentially expressed in CSCs (15,31).
However, this theory may only apply to a therapy targeting CSCs,
and the effect of THIO on CSCs has so far been demonstrated only in
human leukemia and breast cancer studies (15,16).
Therefore, the specific effects of THIO on CSCs from other cancers,
as well as the associated mechanisms, still require
exploration.
THIO can prevent the exclusion of some small
molecules from cancer cells (26,32,33).
This may explain the anti-CSC ability of THIO that was discovered
through small-molecule library screening (26,34). A
previous study has indicated that THIO could reverse
chemoresistance of cancer cells and achieve a significant
therapeutic outcome in combination therapy with verapamil (35). A recent study also demonstrated that
the combination of THIO and doxorubicin using polymeric micelles
might provide a promising strategy for breast cancer treatment by
targeting both cancer cells and cancer stem cells (16). Thus, THIO may be useful as a type of
adjuvant in combination with other chemotherapeutic drugs.
In clinical studies of cancer, THIO is mainly used
for managing depression and psychosis (30). Apart from understanding the
therapeutic mechanisms, it is important to consider the optimal
dose in order to avoid serious side-effects, which can include
movement disorders and cardiac and central nervous system toxicity.
Thus, the potential toxic effects of high-dose THIO treatments
should be evaluated carefully.
Conclusion, this research showed that THIO could
suppress proliferation, reduce invasion, and induce apoptosis in
CSCs via the mitochondrial pathway in a concentration-dependent
manner. Even though the anticolon cancer effect of THIO still needs
to be evaluated in vivo, and in well-designed human clinical
trials, there is enough evidence to suggest that THIO may be a
promising novel agent as an adjuvant for the treatment of colon
cancer, and possibly other cancers.
Acknowledgements
The present study was supported by the Jilin
Province Science Foundation (20120960 and 20160204036YY).
References
|
1
|
Zhelev Z, Ohba H, Bakalova R, Hadjimitova
V, Ishikawa M, Shinohara Y and Baba Y: Phenothiazines suppress
proliferation and induce apoptosis in cultured leukemic cells
without any influence on the viability of normal lymphocytes.
Phenothiazines and leukemia. Cancer Chemother Pharmacol.
53:267–275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Min KJ, Seo BR, Bae YC, Yoo YH and Kwon
TK: Antipsychotic agent thioridazine sensitizes renal carcinoma
Caki cells to TRAIL-induced apoptosis through reactive oxygen
species-mediated inhibition of Akt signaling and downregulation of
Mcl-1 and c-FLIP(L). Cell Death Dis. 5:e10632014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng HW, Liang YH, Kuo YL, Chuu CP, Lin
CY, Lee MH, Wu AT, Yeh CT, Chen EI, Whang-Peng J, et al:
Identification of thioridazine, an antipsychotic drug, as an
antiglioblastoma and anticancer stem cell agent using public gene
expression data. Cell Death Dis. 6:e17532015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cowap J and Hardy J: Thioridazine in the
management of cancer-related sweating. J Pain Symptom Manage.
15:2661998.PubMed/NCBI
|
|
5
|
Zhukovsky DS: Fever and sweats in the
patient with advanced cancer. Hematol Oncol Clin North Am.
16579–588. (viii)2002.viii. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ly KL, Chidgey J, Addington-Hall J and
Hotopf M: Depression in palliative care: A systematic review. Part
2. Treatment. Palliat Med. 16:279–284. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang S, Dong SM, Kim BR, Park MS, Trink B,
Byun HJ and Rho SB: Thioridazine induces apoptosis by targeting the
PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells.
Apoptosis. 17:989–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rho SB, Kim BR and Kang S: A gene
signature-based approach identifies thioridazine as an inhibitor of
phosphatidylinositol-3-kinase (PI3K)/AKT pathway in ovarian cancer
cells. Gynecol Oncol. 120:121–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi AR, Kim JH and Yoon S: Thioridazine
specifically sensitizes drug-resistant cancer cells through highly
increase in apoptosis and P-gp inhibition. Tumour Biol.
35:9831–9838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu M, Li J, Luo Z, Zhang S, Xue S, Wang K,
Shi Y, Zhang C, Chen H and Li Z: Roles of dopamine receptors and
their antagonist thioridazine in hepatoma metastasis. Onco Targets
Ther. 8:1543–1552. 2015.PubMed/NCBI
|
|
11
|
Gong L, Wang Y, Tong S, Liu L, Niu L, Yuan
Y and Bao Y: Mechanism of killing effect of thioridazine on human
lung cancer PC9 cells. Zhongguo Fei Ai Za Zhi. 18:727–733. 2015.(In
Chinese). PubMed/NCBI
|
|
12
|
Yin T, He S, Shen G, Ye T, Guo F and Wang
Y: Dopamine receptor antagonist thioridazine inhibits tumor growth
in a murine breast cancer model. Mol Med Rep. 12:4103–4108.
2015.PubMed/NCBI
|
|
13
|
Meng Q, Sun X, Wang J and Wang Y:
Mechanism of thioridazine plus medroxyprogesterone in the treatment
of endometrial cancer. Zhonghua Yi Xue Za Zhi. 95:1540–1543.
2015.(In Chinese). PubMed/NCBI
|
|
14
|
Liu JK, Hao YJ, Huang JW, Li X, Cai HB and
Peng K: Mechanism of thioridazine-induced apoptosis of human
colorectal cancer SW480 cells. Nan Fang Yi Ke Da Xue Xue Bao.
35:511–515. 2015.(In Chinese). PubMed/NCBI
|
|
15
|
Sachlos E, Risueño RM, Laronde S,
Shapovalova Z, Lee JH, Russell J, Malig M, McNicol JD, Fiebig-Comyn
A, Graham M, et al: Identification of drugs including a dopamine
receptor antagonist that selectively target cancer stem cells.
Cell. 149:1284–1297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ke XY, Lin Ng VW, Gao SJ, Tong YW, Hedrick
JL and Yang YY: Co-delivery of thioridazine and doxorubicin using
polymeric micelles for targeting both cancer cells and cancer stem
cells. Biomaterials. 35:1096–1108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dick JE: Looking ahead in cancer stem cell
research. Nat Biotechnol. 27:44–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guan Y, Gerhard B and Hogge DE: Detection,
isolation, and stimulation of quiescent primitive leukemic
progenitor cells from patients with acute myeloid leukemia (AML).
Blood. 101:3142–3149. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim EJ, Kang JI, Kwak JW, Jeon CH, Tung
NH, Kim YH, Choi CH, Hyun JW, Koh YS, Yoo ES, et al: The anticancer
effect of (1S,2S,3E,7E,11E)-3,7,11,
15-cembratetraen-17,2-olide(LS-1) through the activation of TGF-β
signaling in SNU-C5/5-FU, fluorouracil-resistant human colon cancer
cells. Mar Drugs. 13:1340–1359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang C, Tian Y, Song F, Fu C, Han B and
Wang Y: Salinomycin inhibits the growth of colorectal carcinoma by
targeting tumor stem cells. Oncol Rep. 34:2469–2476.
2015.PubMed/NCBI
|
|
22
|
Amaral L, Kristiansen JE, Abebe LS and
Millett W: Inhibition of the respiration of multi-drug resistant
clinical isolates of Mycobacterium tuberculosis by thioridazine:
Potential use for initial therapy of freshly diagnosed
tuberculosis. J Antimicrob Chemother. 38:1049–1053. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boeree MJ: Global clinical trials for the
treatment of TB with thioridazine. Recent Pat Antiinfect Drug
Discov. 6:99–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kleibel F: A method of alleviation of pain
in cancer patients. Clinical trial of thioridazine (Melleril and
Mellerettes) in 251 patients. Munch Med Wochenschr. 103:2341–2343.
1961.(In German). PubMed/NCBI
|
|
25
|
Smith RC, Baumgartner R, Burd A,
Ravichandran GK and Mauldin M: Haloperidol and thioridazine drug
levels and clinical response in schizophrenia: Comparison of
gas-liquid chromatography and radioreceptor drug level assays.
Psychopharmacol Bull. 21:52–58. 1985.PubMed/NCBI
|
|
26
|
Mu J, Xu H, Yang Y, Huang W, Xiao J, Li M,
Tan Z, Ding Q, Zhang L, Lu J, et al: Thioridazine, an antipsychotic
drug, elicits potent antitumor effects in gastric cancer. Oncol
Rep. 31:2107–2114. 2014.PubMed/NCBI
|
|
27
|
Tuynder M, Fiucci G, Prieur S, Lespagnol
A, Géant A, Beaucourt S, Duflaut D, Besse S, Susini L, Cavarelli J,
et al: Translationally controlled tumor protein is a target of
tumor reversion. Proc Natl Acad Sci USA. 101:15364–15369. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Basta-Kaim A, Budziszewska B, Jagła G,
Nowak W, Kubera M and Lasoń W: Inhibitory effect of antipsychotic
drugs on the Con A- and LPS-induced proliferative activity of mouse
splenocytes: A possible mechanism of action. J Physiol Pharmacol.
57:247–264. 2006.PubMed/NCBI
|
|
29
|
Park MS, Dong SM, Kim BR, Seo SH, Kang S,
Lee EJ, Lee SH and Rho SB: Thioridazine inhibits angiogenesis and
tumor growth by targeting the VEGFR-2/PI3K/mTOR pathway in ovarian
cancer xenografts. Oncotarget. 5:4929–4934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hercbergs A: Thioridazine: A radiation
enhancer in advanced cervical cancer? Lancet. 2:7371988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gatto F and Hofland LJ: The role of
somatostatin and dopamine D2 receptors in endocrine tumors. Endocr
Relat Cancer. 18:R233–R251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Akiyama S, Shiraishi N, Kuratomi Y,
Nakagawa M and Kuwano M: Circumvention of multiple-drug resistance
in human cancer cells by thioridazine, trifluoperazine, and
chlorpromazine. J Natl Cancer Inst. 76:839–844. 1986.PubMed/NCBI
|
|
33
|
Efferth T and Volm M: Reversal of
doxorubicin-resistance in sarcoma 180 tumor cells by inhibition of
different resistance mechanisms. Cancer Lett. 70:197–202. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sutton LP and Rushlow WJ: The dopamine D2
receptor regulates Akt and GSK-3 via Dvl-3. Int J
Neuropsychopharmacol. 15:965–979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Castaing M, Loiseau A and Cornish-Bowden
A: Synergy between verapamil and other multidrug -resistance
modulators in model membranes. J Biosci. 32:737–746. 2007.
View Article : Google Scholar : PubMed/NCBI
|