Introduction
Lung cancer is the first leading cause of
cancer-related deaths worldwide (1,2). More
than 75% of lung cancers are non-small cell lung cancer (NSCLC)
(3,4), and only a handful of NSCLC patients
are diagnosed at the early stage (5). To date, the main treatment for NSCLC
is surgical resection, while the relapse rate after surgery is very
high and stage II–III patients are treated with adjuvant
chemotherapy, which has been shown to finitely improve patient
survival in several randomized clinical trials (3,6).
Although there has been great advances in traditional treatments,
such as modus operandi and supplementation with adjuvant
chemotherapy, a considerable number of patients with NSCLC are
diagnosed at an advanced stage resulting in poor prognosis
(7,8). Therefore, novel bio-targets for
therapeutic approaches to prevent cancer cell invasion and
metastasis are critical for NSCLC treatment (9,10).
It has been reported that FBXW7 acts as a tumor
suppressor in several types of cancers and targets multiple
transcriptional activators and proto-oncogenes for
ubiquitin-mediated degradation (11), including cyclin E, c-Myc (12,13),
Notch (14), c-Jun (15), p53, mammalian target of rapamycin,
and MCL1 (16,17). Abnormal expression of the FBXW7 gene
is found in ovarian, breast, endometrial, renal, lymphoma and
colorectal cancers (18–24). Therefore, altered expression of
FBXW7 is recognized as one of the major causes of carcinogenesis or
cancer development. Previous studies have clarified that loss of
FBXW7 is significantly associated with a poor prognosis in breast
cancer, gastric cancer, and glioma. Similarly, low expression of
FBXW7 was reported to be correlated with a poor prognosis in NSCLC
(25). However, the specifical
mechanism underlying the above correlation remains unknown.
MicroRNAs (miRNAs) are a large class of endogenous
non-coding RNAs that regulate human gene expression (26). Increasing evidence suggests that
miRNAs can act as oncogenes or tumor suppressors during the
development and progression of cancers through sequence-specific
binding to their mRNA targets (27). These miRNAs play a vital role in a
wide variety of complex biological processes, including cellular
development and differentiation, but investigations have only begun
to verify their significance in carcinogenesis. Given the critical
correlations between FBXW7 and the prognosis of NSCLC (28), we hypothesized that FBXW7 is
regulated by miRNAs at the post-transcriptional level. In our
previous study, we identified miR-367 as a regulator of FBXW7
expression in human NSCLC cells. Our findings demonstrated that
miR-367 promoted the invasion and metastasis and prevented the
apoptosis of NSCLC cells via direct regulation of FBXW7. In
addition, we also focused on the correlation between miR-367 and
prognosis of NSCLC and found that miR-367 may be a potential
biomarker for predicting the survival of NSCLC patients.
Materials and methods
Clinical tissues and cell culture
We analyzed tumor specimens from 113 patients with
lung cancer who underwent surgery for excision of a primary tumor
at the Department of Thoracic Surgery, The First Affiliated
Hospital of Nanjing Medical University (Nanjing, China). Written
informed consent was obtained from the patients, in accordance with
the institutional guidelines, before sample collection, and the
study was approved by the Committees for the Ethical Review of
Research at Nanjing Medical University. Fresh-frozen and/or
formalin-fixed and paraffin-embedded samples were used for miR-367
and FBXW7 expression analysis. The human lung cancer cell lines
H226, H1792, and A549 were cultured in RPMI-1640 medium
supplemented with 10% FBS (both from Invitrogen Life Technologies,
Carlsbad, CA, USA), 100 U/ml penicillin, and 100 µg/ml streptomycin
in a 5% CO2/95% air at 37°C. These cell lines were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA).
Plasmids and transfection
Luciferase constructs were generated by ligating
oligonucleotides containing the wild-type or mutant putative target
site of the FBXW7 3′-UTR into the luciferase reporter plasmid
pEZX-MT01 vector (GeneCopoeia, Inc., Rockville, MD, USA) downstream
of the luciferase gene. FBXW7 CDS sequences were cloned into the
pcDNA3 vector. All sequences were completely sequenced. We
conducted the transfections by using a Lipofectamine 2000 kit
(Invitrogen). Cell lines were transfected with miR-367 mimics or
inhibitors and their controls (GenePharma, Shanghai, China). The
short interfering RNAs targeting FBXW7 (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) were transfected into cells using
Lipofectamine 2000 reagent (Invitrogen) according to the
manufacturer's instructions. The transfection efficiency is >70%
(microRNA) and 60% (plasmid) (data not shown).
RNA isolation and real-time PCR
Total RNA was isolated from tissues and cells using
TRIzol (Invitrogen) and small RNA enrichment was conducted using a
miRVana miRNA isolation kit (Ambion, Inc., Austin, TX, USA),
according to the manufacturer's instructions. Small RNAs were
reverse transcribed to cDNA using the TaqMan MicroRNA reverse
transcription kit (Applied Biosystems, San Diego, CA, USA)
according to the manufacturer's instructions. Then cDNA was used as
a template for the real-time PCR amplification. Real-time PCR was
run on a StepOnePlus™ real-time PCR instruments (Applied
Biosystems). The primers used are available upon request. Real-time
PCR using SYBR-Green (Takara Bio, Inc., Otsu, Japan) was performed
to compare the relative expression levels of FBXW7 mRNA according
to the manufacturer's instructions. For miR-367 real-time PCR, a
commercial Hairpin-it™ miRNAs qPCR Quantification kit with primers
was purchased from GenePharma. The primers for the mRNAs for
real-time PCR are: FBXW7 forward, 5′-GGCCAAAATGATTCCCAGCAA-3′ and
reverse, 5′-ACTGGAGTTCGTGACACTGTTA-3′.
Western blot assay
Total proteins were extracted from the cultured
cells or tissues and quantified using a BCA protein assay kit
(Beyotime, Jiangsu, China) with BSA as a standard. Equal amounts of
protein from different cells were separated by 10% SDS-PAGE and
transferred to a PVDF membrane. The membranes were blocked with 5%
BSA (5% w/v in PBS + 0.1% Tween-20) and incubated with primary
antibodies at room temperature. The antibodies against FBXW7 and
GAPDH were used according to the manufacturer's instructions (Santa
Cruz Biotechnology, Inc.). After using the secondary antibodies
(Santa Cruz Biotechnology, Inc.) at 1:2,000 (v/v) dilutions in PBS
+ 0.1% Tween-20, the signals were detected by the SuperSignal West
Pico Chemiluminescent Substrate kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA) according to the manufacturer's
instructions.
Luciferase assay
H226 and H1792 cells were co-transfected with the
luciferase reporter plasmid pEZX-MT01 (GeneCopoeia, Inc.), and the
miRNA-367 and controls. Twenty-four hours after transfection,
firefly and Renilla luciferase activities were measured
using a Luc-Pair™ miR Luciferase Assay kit (GeneCopoeia, Inc.).
Each transfection was performed in triplicate and repeated three
times.
Cell proliferation and apoptosis
assay
Cell proliferation was performed with Cell Counting
Kit-8 (CCK-8) (Dojindo, Tokyo, Japan). According to the
instructions, CCK-8 reagent was added at 0, 24, 48 and 72 h
respectively after seeding 4×103 cells/well in a 96-well
plate, and incubated at 37°C for 2 h. The optical density (OD) at
450 nm was detected using a microplate reader (Bio-Rad
Laboratories, Richmond, CA, USA). Apoptosis was evaluated using an
Annexin V/PI assay kit (Miltenyi Biotec, Bergisch Gladbach,
Germany) according to the manufacturer's instructions. Flow
cytometry with a FACSCalibur (BD Biosciences, San Jose, CA, USA)
was performed to evaluate the result.
Transwell invasion assay
Cell invasion assays were evaluated using Transwell
cell plates (Corning Costar, Inc., Corning, NY, USA) and 8-µm pore
size Matrigel invasion chambers (BD Biosciences) according to the
manufacturer's instructions (29).
Cells (1.0×104) were seeded in serum-free medium into
the upper chamber and allowed to invade towards 10% FBS in the
lower chamber. After a 24-h incubation at 37°C and 5%
CO2, the cells invaded through the membrane and adhered
to the underside of the membrane. Then the invaded and migrated
cells were fixed and stained with crystal violet. The images were
acquired by using NIS Elements image analysis software (Nikon,
Tokyo, Japan). For the membrane images, we calculated the number of
migrated cells using image analysis software Image-Pro Plus 6.0
(Media Cybernetics, Bethesda, MD, USA).
Statistical analysis
All results are expressed as the mean ± SD. The
Student's t-test was used to analyze significant differences
between samples. The correlation between miR-367 and FBXW7
expression levels was determined by calculating the Spearman's
correlation coefficient. All the histograms were constructed using
performing GraphPad Prism, version 4.0 (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 indicates a statistically significant
result.
Results
Clinicopathological significance of
miR-367 in NSCLC
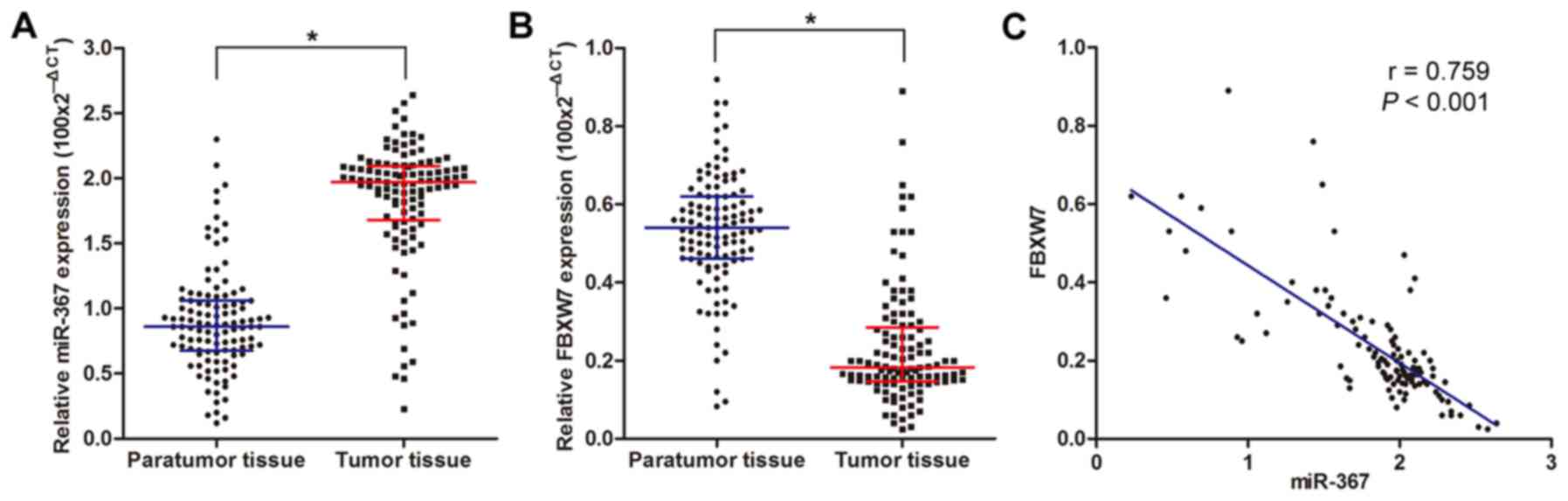
We examined the expression level of miR-367 in 113
NSCLC clinical samples by utilizing real-time PCR, with quantified
values used to calculate miR-367/U6 ratios. Our results
demonstrated that the relative expression level of miR-367 was
significantly higher in the cancer tissues compared with the level
noted in the non-cancer tissues (Fig.
1A). Based on the median value of the miR-367 expression level,
we divided the 113 NSCLC patients into two groups: miR-367
high-expression group (57 cases) and miR-367 low-expression group
(56 cases). Then, we performed Chi-square (χ2) tests to
explore the association between the clinicopathological
characteristics and miR-367 expression. The findings are summarized
in Table I. There were significant
differences in tumor size, tumor stage and metastatic status
between the groups.
 | Table I.Correlation between miR-367
expression and clinicopathological characteristics of the NSCLC
patients (n=113). |
Table I.
Correlation between miR-367
expression and clinicopathological characteristics of the NSCLC
patients (n=113).
|
Characteristics | All patients | miR-367 low
expression (< mediana) | miR-367 high
expression (≥ mediana) | P-value
(χ2 test) |
|---|
| Total cases
(N) | 113 | 56 | 57 |
|
| Age (years) |
|
|
|
|
|
<60 | 46 | 23 | 23 | 0.938 |
|
≥60 | 67 | 33 | 34 |
|
| Gender |
|
|
|
|
|
Male | 59 | 28 | 31 | 0.641 |
|
Female | 54 | 28 | 26 |
|
| Histology |
|
|
|
|
| AC | 69 | 33 | 36 | 0.645 |
|
SCC | 44 | 23 | 21 |
|
| Tumor size |
|
|
|
|
|
T1-T2 | 36 | 23 | 13 | 0.037b |
|
T3-T4 | 77 | 33 | 44 |
|
| Tumor stage |
|
|
|
|
|
I–II | 39 | 25 | 14 | 0.025b |
|
III–IV | 74 | 31 | 43 |
|
| Metastasis |
|
|
|
|
|
Yes | 30 | 20 | 10 | 0.029b |
| No | 83 | 36 | 47 |
|
miR-367 is a negative regulator of
FBXW7 in NSCLC and miR-367 modulates FBXW7 expression by directly
targeting its 3′-UTR
Increasing evidence indicates that FBXW7 is
significantly associated with the prognosis of NSCLC patients.
Therefore, we hypothesized that miR-367 is involved in the
regulation of FBXW7. Then, we assessed the expression level of
FBXW7 in the 113 NSCLC tissues via real-time PCR and found that the
FBXW7 expression level in the cancer tissues was obviously lower
than that in the non-cancer tissues (Fig. 1B). Pearson's correlation analysis
demonstrated that the FBXW7 expression level was negatively
correlated with the expression level of miR-367 in NSCLC (Fig. 1C).
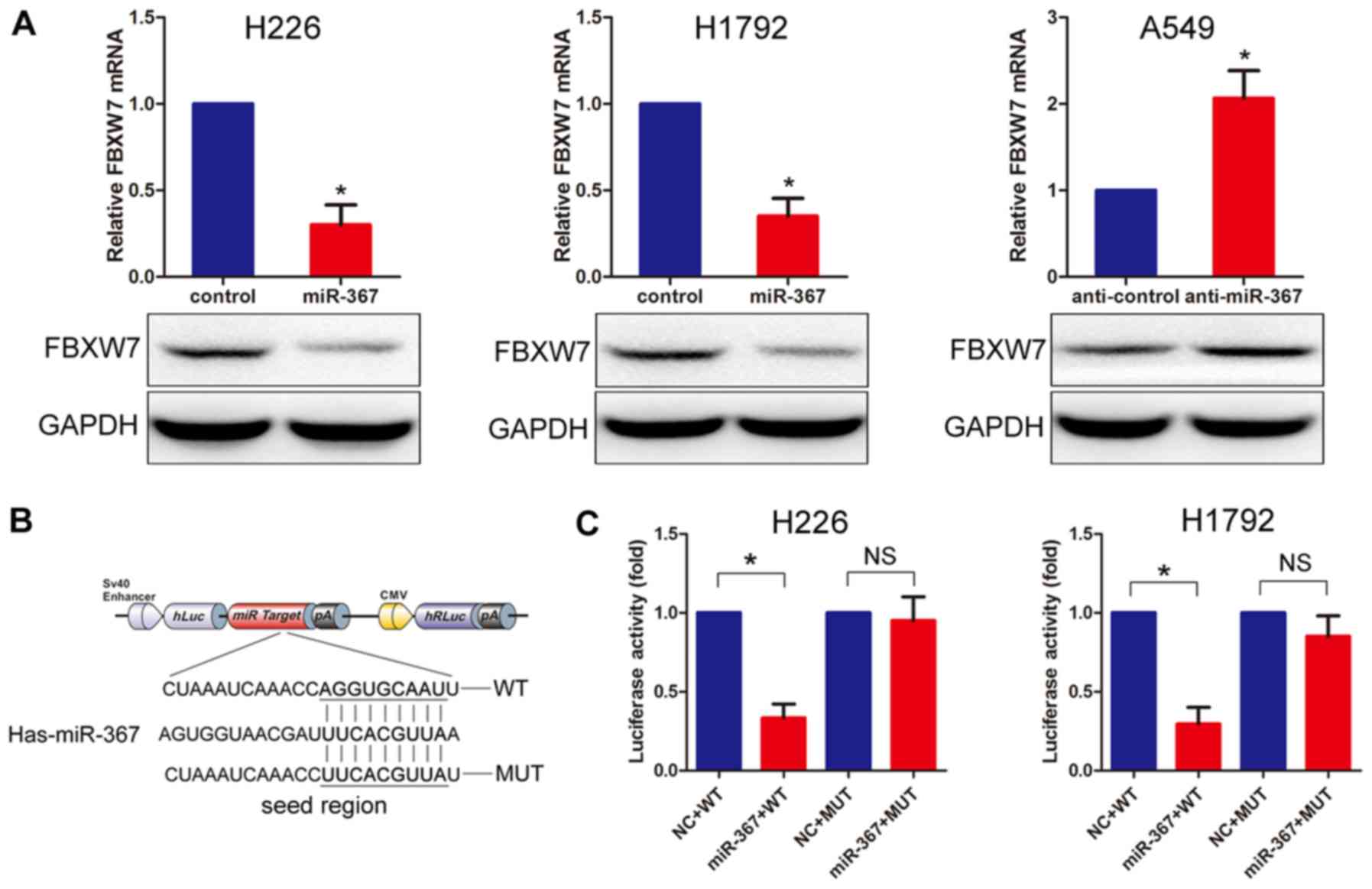
To investigate the association between miR-367 and
NSCLC, we transfected human NSCLC H228 and H192 cells with miR-367
mimics to upregulate the expression of miR-367, and then we
assessed the expression levels of miR-367 and FBXW7 using real-time
PCR. The results showed that miR-367 expression was upregulated
(data not shown) while FBXW7 was significantly downregulated
compared with the negative control cells. Notably, the expression
of FBXW7 in the A49 NSCLC cells with downregulated miR-367 was
obviously upregulated (Fig. 2A).
The miRNA prediction programs, TargetScan (http://www.targetscan.org) and miRanda (http://www.microrna.org), were utilized, which
predicted that a fragment of the FBXW7 3′-untranslated region
(3-UTR) contained a putative miR-367 binding site. To further
confirm the prediction, miR-367 mimics were co-transfected with a
luciferase reporter construct containing wild-type and mutant FBXW7
3′-UTR (Fig. 2B). As shown in
Fig. 2C, we co-transfected the
miR-367 mimics with the luciferase reporter construct containing
wild-type or mutant FBXW7 3′-UTR, and the cells transfected with
the miR-367 mimics had downregulated luciferase activity compared
with transfection with the control. Together, the results indicated
that miR-367 is a potential regulator of FBXW7.
miR-367 promotes proliferation and
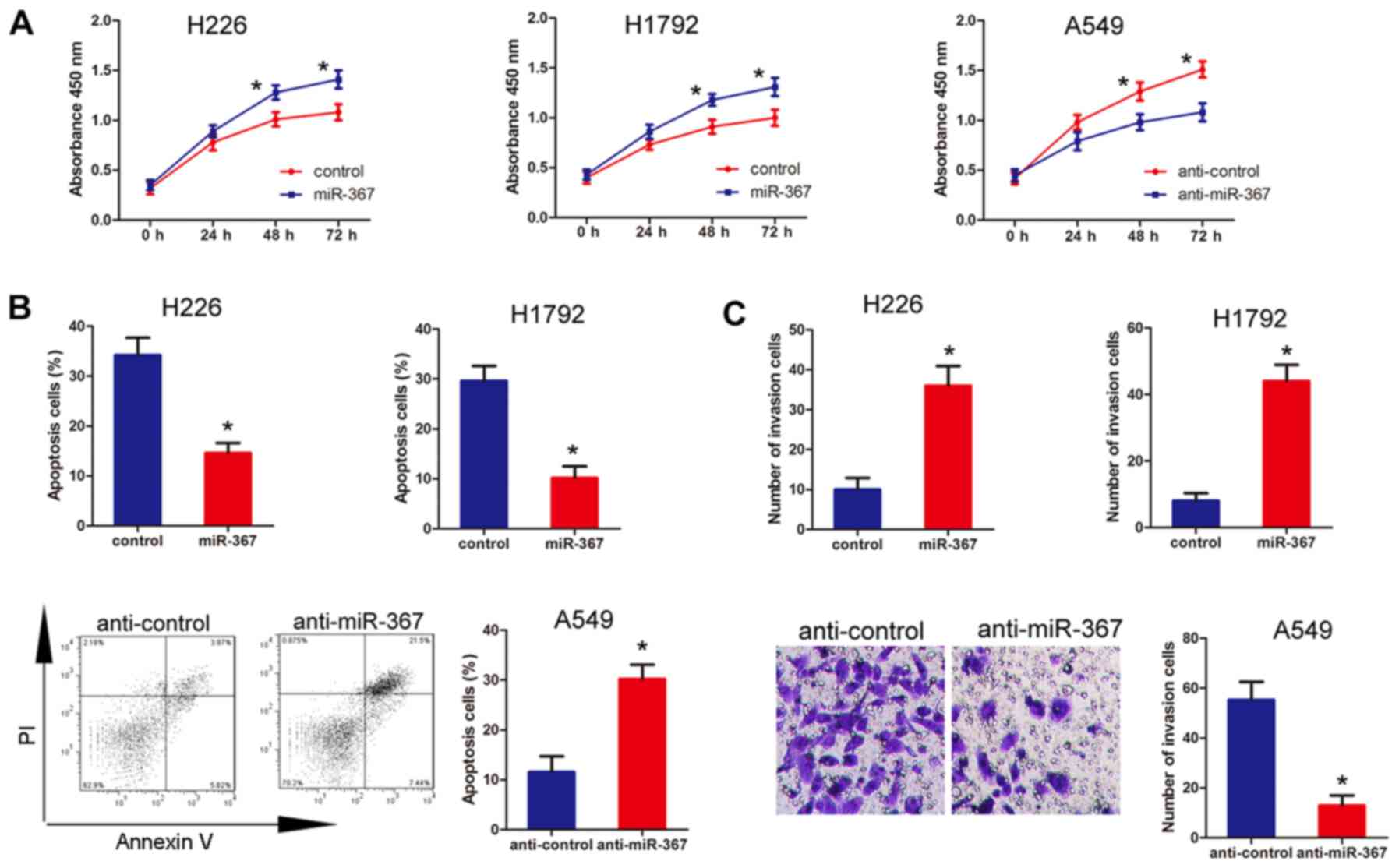
invasion and inhibits apoptosis in NSCLC cells
The significant correlation between miR-367
expression and clinicopathological characteristics of the NSCLC
cases suggested that miR-367 may play a vital role in the
development and progression of NSCLC. Based on the expression
pattern of miR-367, the influence of the overexpression of miR-367
on cell proliferation, apoptosis, and invasion was examined in
human NSCLC cell lines H229 and H1792. The cell lines were
transfected with miR-367 mimics and the expression of miR-367 was
confirmed by real-time PCR (data not shown). The results
demonstrated that upregulation of miR-367 promoted the
proliferation, invasion and prevented the apoptosis ability
compared with the negative control in NSCLC cells (Fig. 3). Moreover, to further investigate
the effects of miR-367 in vitro, we transfected NSCLC A549
cells with miR-367 inhibitors to downregulate miR-367 expression.
Results of the loss-of-function experiments indicated that
downregulation of miR-367 prevented proliferation, invasion and
induced apoptosis compared with the negative control (Fig. 3). Taken together, our findings
verified that miR-367 could promoted proliferation, invasion and
inhibit apoptosis in NSCLC cells.
FBXW7 mediates the function of miR-367
in NSCLC cells
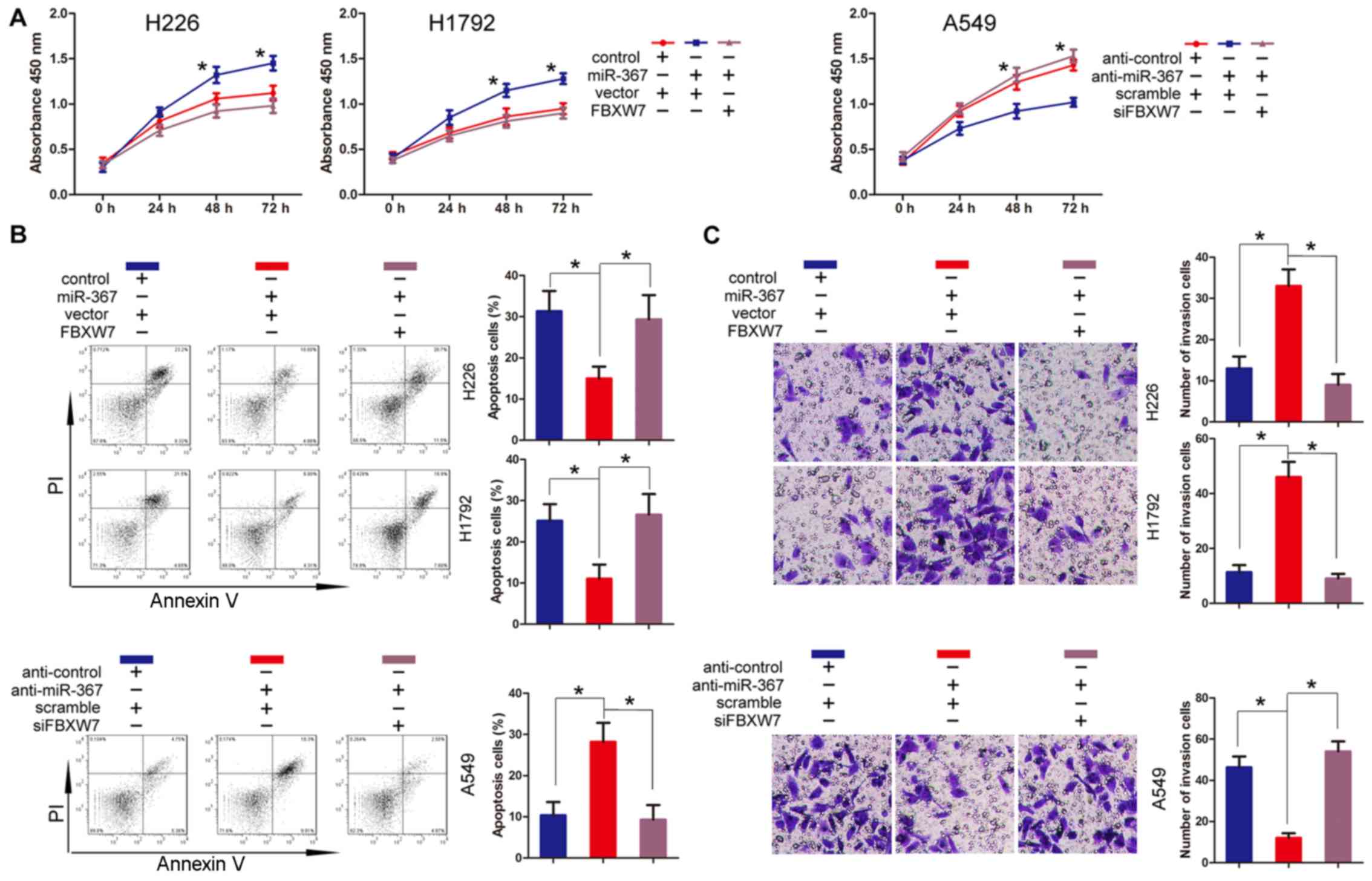
Given the correlation between miR-367 and FBXW7, we
performed a rescue experiment to further examine whether the
functional effects of miR-367 on NSCLC cell lines was exerted via
targeting FBXW7. According to our previous results, FBXW7 was
downregulated in the NSCLC cells which were then transfected with
miR-367 mimics. Therefore, we co-transfected NSCLC cell lines, H226
and H1792, with pcDNA3-FBXW7 and miR-367 mimics. The transfection
cells transfected with the miR-367 mimics and the co-transfected
cells with the vector control and miR-367 mimics were used as the
control groups. The proliferation assay showed that overexpression
of FBXW7 partially abolished the promotion of cell proliferation
induced by miR-367 (Fig. 4A). Then,
we performed an apoptosis assay. The results indicated that
apoptosis partially increased when H226 and H1792 cells were
co-transfected with pcDNA3-FBXW7 and miR-367 mimics (Fig. 4B). Similarly, invasive ability was
reversed to some extent when FBXW7 was overexpressed in the cells
with high-expression of miR-367 compared to the control (Fig. 4C). Moverover, we co-transfected
human NSCLC cell line A549 with siRNA-FBXW7 and miR-367 inhibitors.
Then, we performed the proliferation, apoptosis, and Transwell
invasion assay. Notably, the findings indicated that proliferation,
apoptosis, and invasion were reversed to some extent when we
silenced FBXW7 in cells with low expression of miR-367 compared to
the control (Fig. 4). In
conclusion, these results indicated that the effect of miR-367 on
NSCLC cell lines was partially dependent on FBXW7.
Discussion
Our present study demonstrated that miR-367 was
obviously upregulated in human NSCLC cancer tissues compared with
that in the corresponding non-cancer tissues, and that the NSCLC
patients with a high miR-367 expression had a significantly poorer
prognosis than those with a low expression. Pearson's correlation
analysis also provided evidence that a negative association existed
between the expression of miR-367 and the FBXW7 protein in NSCLC
patients. Furthermore, increasing evidence indicates that FBXW7 is
significantly associated with the prognosis of cancer patients
(25,30–32).
These findings suggest that the overexpression of miR-367
correlates with the poor prognosis of NSCLC patients, possibly due
to the repression of the function of the FBXW7 protein.
Research has found that FBXW7-deficient mice died
in utero of vascular abnormalities at embryonic days
10.5–11.5 (33). Additionally,
although p53-deficient mice do not develop tumors, knockdown of
FBXW7 verifies the tumor profile in p53-deficient mice and that
they develop lung cancer (34).
Moreover, suppression and mutation of FBXW7 have been reported to
be involved in various types of malignancies (35–37). A
number of studies have indicated that the loss or mutation of FBXW7
may exert a vital role in the development and progression of lung
carcinogenesis. FBXW7 functions as a tumor regulator by controlling
the expression of oncoproteins such as cyclin E, MYC, TOP2A, MCL1,
and P53 which are all correlated with tumor development and
progression (12,13). These data, together with the
correlation of FBXW7 between the prognosis of patients with tumors,
suggest that FBXW7 functions as a tumor suppressor in human
tumorigenesis. In the present study, we found that the expression
of FBXW7 was significantly downregulated in the NSCLC clinical
samples compared with the control and the expression levels of
FBXW7 in cancer tissues were negatively associated with miR-367. As
is well known, miRNAs modulate the expression of their target genes
at the post-transcriptional level (38). They prevent their translation by
directly binding to the corresponding complementary sequences of
their target mRNAs, thereby downregulating protein expression. Many
studies indicate that abnormal expression of miRNAs is associated
with various human diseases, including malignancies (39–42).
For instance, Lei et al identified the oncogenic function of
miR-92b in NSCLC through targeting reversion-inducing cysteine-rich
protein with Kazal motifs (RECK). Zhang et al reported that
miR-150 promoted the proliferation of NSCLC cells by targeting p53
(43). In contrast, miR-638 and
miR-625 act as tumor suppressors via targeting sex determining
region Y (SRY)-box 2 (SOX2), respectively (44). However, whether FBXW7 is
post-transcriptionally regulated by miR-367 remains unclear.
Therefore, we aimed to identify whether miR-367 was involved in the
regulation of FBXW7. Notably, via using miRNA prediction programs,
we found that a fragment of the FBXW7 3′-UTR contained the putative
miR-367 binding site. To further confirm the prediction, miR-367
mimics were co-transfected with a luciferase reporter construct
containing wild-type FBXW7 3′-UTR. Our results indicated that
transfection with miR-367 mimics downregulated luciferase activity
compared with transfection with NC. Furthermore, the inverse
experiments further confirmed the above results, suggesting that
miR-367 is a potential regulator of FBXW7. Therefore, we focused on
miR-367 that may have significant effects on the progression and
development of NSCLC. χ2 tests showed a significant
statistical correlation between tumor size, tumor stage, metastasis
and miR-367 expression. Based on the above correlation, we
performed a series of functional experiments to test the effect of
miR-367 on NSCLC cells. Our results demonstrated that
overexpression of miR-367 promoted proliferation, invasion and
inhibited apoptosis in various types of NSCLC cell lines. We first
demonstrated that overexpression of miR-367 was correlated with a
poorer prognosis of NSCLC patients. Because of the function of
FBXW7 in cancers, we developed a series of rescue experiments to
further study whether the functional effect of miR-367 on NSCLC
cell lines was exerted via targeting FBXW7. Our findings confirmed
our hypothesis that proliferation, invasion and apoptosis were
reversed to some extent when we silenced FBXW7 in cells with low
expression of miR-367 compared to the control. Additionally, we
also confirmed that high miR-367 expression and low FBXW7
expression could serve as independent prognostic factors,
respectively.
In conclusion, we identified and characterized the
miR-367/FBXW7 axis, which is involved in proliferation, apoptosis
and invasion of NSCLC cells. Based on our findings, miR-367 and
FBXW7 may act as a therapeutic target for the treatment of human
NSCLC, especially cancers with high invasive potential.
Additionally, miR-367 and FBXW7 expression could respectively serve
as independent prognostic factors.
Acknowledgements
This study was supported in part by the National
Natural Science Foundation of China (81572263), the Jiangsu
Province Natural Science Foundation (BK20151584) and the Jiangsu
Top Expert Program in Six Professions (WSW-028).
References
|
1
|
Villaruz LC, Kalyan A, Zarour H and
Socinski MA: Immunotherapy in lung cancer. Transl Lung Cancer Res.
3:2–14. 2014.PubMed/NCBI
|
|
2
|
Ricciuti B, Mecca C, Crinò L, Baglivo S,
Cenci M and Metro G: Non-coding RNAs in lung cancer. Oncoscience.
1:674–705. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Landi L and Cappuzzo F: Management of
NSCLC: Focus on crizotinib. Expert Opin Pharmacother. 15:2587–2597.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gridelli C, Peters S, Sgambato A, Casaluce
F, Adjei AA and Ciardiello F: ALK inhibitors in the treatment of
advanced NSCLC. Cancer Treat Rev. 40:300–306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uramoto H and Tanaka F: Recurrence after
surgery in patients with NSCLC. Transl Lung Cancer Res. 3:242–249.
2014.PubMed/NCBI
|
|
6
|
Vallières E: Oligometastatic NSCLC: The
changing role of surgery. Transl Lung Cancer Res. 3:192–194.
2014.PubMed/NCBI
|
|
7
|
McElnay P and Lim E: Adjuvant or
neoadjuvant chemotherapy for NSCLC. J Thorac Dis. 6:(Suppl 2).
S224–S227. 2014.PubMed/NCBI
|
|
8
|
Shcherba M, Liang Y, Fernandes D,
Perez-Soler R and Cheng H: Cell cycle inhibitors for the treatment
of NSCLC. Expert Opin Pharmacother. 15:991–1004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gentzler RD, Yentz SE, Johnson ML,
Rademaker AW and Patel JD: The changing landscape of phase II/III
metastatic NSCLC clinical trials and the importance of biomarker
selection criteria. Cancer. 120:3853–3858. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gómez AM, Sarceda JR Jarabo, García-Asenjo
JA, Fernandez C, Hernandez S, Sanz J, Fernandez E, Calatayud J,
Torres A and Hernando F: Relationship of immunohistochemical
biomarker expression and lymph node involvement in patients
undergoing surgical treatment of NSCLC with long-term follow-up.
Tumour Biol. 35:4551–4559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grim JE: Fbxw7 hotspot mutations and human
colon cancer: Mechanistic insights from new mouse models. Gut.
63:707–709. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tu K, Zheng X, Zan X, Han S, Yao Y and Liu
Q: Evaluation of Fbxw7 expression and its correlation with the
expression of c-Myc, cyclin E and p53 in human hepatocellular
carcinoma. Hepatol Res. 42:904–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo Z, Zhou Y, Evers BM and Wang Q: Rictor
regulates FBXW7-dependent c-Myc and cyclin E degradation in
colorectal cancer cells. Biochem Biophys Res Commun. 418:426–432.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Izumi N, Helker C, Ehling M, Behrens A,
Herzog W and Adams RH: Fbxw7 controls angiogenesis by regulating
endothelial Notch activity. PLoS One. 7:e411162012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Babaei-Jadidi R, Li N, Saadeddin A,
Spencer-Dene B, Jandke A, Muhammad B, Ibrahim EE, Muraleedharan R,
Abuzinadah M, Davis H, et al: FBXW7 influences murine intestinal
homeostasis and cancer, targeting Notch, Jun, and DEK for
degradation. J Exp Med. 208:295–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ren H, Koo J, Guan B, Yue P, Deng X, Chen
M, Khuri FR and Sun SY: The E3 ubiquitin ligases β-TrCP and FBXW7
cooperatively mediates GSK3-dependent Mcl-1 degradation induced by
the Akt inhibitor API-1, resulting in apoptosis. Mol Cancer.
12:1462013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren H, Zhao L, Li Y, Yue P, Deng X,
Owonikoko TK, Chen M, Khuri FR and Sun SY: The PI3 kinase inhibitor
NVP-BKM120 induces GSK3/FBXW7-dependent Mcl-1 degradation,
contributing to induction of apoptosis and enhancement of
TRAIL-induced apoptosis. Cancer Lett. 338:229–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li N, Lorenzi F, Kalakouti E, Normatova M,
Babaei-Jadidi R, Tomlinson I and Nateri AS: FBXW7-mutated
colorectal cancer cells exhibit aberrant expression of
phosphorylated-p53 at Serine-15. Oncotarget. 6:9240–9256. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou C, Shen L, Mao L, Wang B, Li Y and Yu
H: miR-92a is upregulated in cervical cancer and promotes cell
proliferation and invasion by targeting FBXW7. Biochem Biophys Res
Commun. 458:63–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Calcagno DQ, Freitas VM, Leal MF, de Souza
CR, Demachki S, Montenegro R, Assumpção PP, Khayat AS, Smith MA,
dos Santos AK, et al: MYC, FBXW7 and TP53 copy number variation and
expression in gastric cancer. BMC Gastroenterol. 13:1412013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ibusuki M, Yamamoto Y, Shinriki S, Ando Y
and Iwase H: Reduced expression of ubiquitin ligase FBXW7 mRNA is
associated with poor prognosis in breast cancer patients. Cancer
Sci. 102:439–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan Y, Sangfelt O and Spruck C: The
Fbxw7/hCdc4 tumor suppressor in human cancer. Cancer Lett.
271:1–12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuiper RP, Vreede L, Venkatachalam R,
Ricketts C, Kamping E, Verwiel E, Govaerts L, Debiec-Rychter M,
Lerut E, van Erp F, et al: The tumor suppressor gene FBXW7 is
disrupted by a constitutional t(3;4)(q21;q31) in a patient with
renal cell cancer. Cancer Genet Cytogenet. 195:105–111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park MJ, Taki T, Oda M, Watanabe T,
Yumura-Yagi K, Kobayashi R, Suzuki N, Hara J, Horibe K and Hayashi
Y: FBXW7 and NOTCH1 mutations in childhood T cell acute
lymphoblastic leukaemia and T cell non-Hodgkin lymphoma. Br J
Haematol. 145:198–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morra F, Luise C, Merolla F, Poser I,
Visconti R, Ilardi G, Paladino S, Inuzuka H, Guggino G, Monaco R,
et al: FBXW7 and USP7 regulate CCDC6 turnover during the cell cycle
and affect cancer drugs susceptibility in NSCLC. Oncotarget.
6:12697–12709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qin C, Huang RY and Wang ZX: Potential
role of miR-100 in cancer diagnosis, prognosis, and therapy. Tumour
Biol. 36:1403–1409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bennett PE, Bemis L, Norris DA and
Shellman YG: miR in melanoma development: miRNAs and acquired
hallmarks of cancer in melanoma. Physiol Genomics. 45:1049–1059.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yokobori T, Yokoyama Y, Mogi A, Endoh H,
Altan B, Kosaka T, Yamaki E, Yajima T, Tomizawa K, Azuma Y, et al:
FBXW7 mediates chemotherapeutic sensitivity and prognosis in
NSCLCs. Mol Cancer Res. 12:32–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Z, Zhang Y, Sun XX, Ma X and Chen
ZN: microRNA-146a inhibits cancer metastasis by downregulating VEGF
through dual pathways in hepatocellular carcinoma. Mol Cancer.
14:52015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Natarajan V, Bandapalli OR, Rajkumar T,
Sagar TG and Karunakaran N: NOTCH1 and FBXW7 mutations favor better
outcome in pediatric South Indian T-cell acute lymphoblastic
leukemia. J Pediatr Hematol Oncol. 37:e23–e30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Naganawa Y, Ishiguro H, Kuwabara Y, Kimura
M, Mitsui A, Katada T, Tanaka T, Shiozaki M, Fujii Y and Takeyama
H: Decreased expression of FBXW7 is correlated with poor prognosis
in patients with esophageal squamous cell carcinoma. Exp Ther Med.
1:841–846. 2010.PubMed/NCBI
|
|
32
|
Milne AN, Leguit R, Corver WE, Morsink FH,
Polak M, de Leng WW, Carvalho R and Offerhaus GJ: Loss of
CDC4/FBXW7 in gastric carcinoma. Cell Oncol. 32:347–359.
2010.PubMed/NCBI
|
|
33
|
Tsunematsu R, Nakayama K, Oike Y,
Nishiyama M, Ishida N, Hatakeyama S, Bessho Y, Kageyama R, Suda T
and Nakayama KI: Mouse Fbw7/Sel-10/Cdc4 is required for notch
degradation during vascular development. J Biol Chem.
279:9417–9423. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mao JH, Perez-Losada J, Wu D, Delrosario
R, Tsunematsu R, Nakayama KI, Brown K, Bryson S and Balmain A:
Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor
gene. Nature. 432:775–779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang H, Lu X, Liu Z, Chen L, Xu Y, Wang Y,
Wei G and Chen Y: FBXW7 suppresses epithelial-mesenchymal
transition, stemness and metastatic potential of cholangiocarcinoma
cells. Oncotarget. 6:6310–6325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sato M, Rodriguez-Barrueco R, Yu J, Do C,
Silva JM and Gautier J: MYC is a critical target of FBXW7.
Oncotarget. 6:3292–3305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Z, Sun Y, Chen X, Squires J,
Nowroozizadeh B, Liang C and Huang J: p53 mutation directs AURKA
overexpression via miR-25 and FBXW7 in prostatic small cell
neuroendocrine carcinoma. Mol Cancer Res. 13:584–591. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pfeffer SR, Yang CH and Pfeffer LM: The
role of miR-21 in Cancer. Drug Dev Res. 76:270–277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Y, Xie YJ, Xu Q, Chen JX, Shan NC and
Zhang Y: Down-regulation of miR-1246 in cervical cancer tissues and
its clinical significance. Gynecol Oncol. 138:683–688. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ye H, Pang L, Wu Q, Zhu Y, Guo C, Deng Y
and Zheng X: A critical role of mir-199a in the cell biological
behaviors of colorectal cancer. Diagn Pathol. 10:652015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Han S, Yang S, Cai Z, Pan D, Li Z, Huang
Z, Zhang P, Zhu H, Lei L and Wang W: Anti-Warburg effect of
rosmarinic acid via miR-155 in gastric cancer cells. Drug Des Devel
Ther. 9:2695–2703. 2015.PubMed/NCBI
|
|
42
|
Liang J, Li X, Li Y, Wei J, Daniels G,
Zhong X, Wang J, Sfanos K, Melamed J, Zhao J, et al: LEF1 targeting
EMT in prostate cancer invasion is mediated by miR-181a. Am J
Cancer Res. 5:1124–1132. 2015.PubMed/NCBI
|
|
43
|
Zhang N, Wei X and Xu L: miR-150 promotes
the proliferation of lung cancer cells by targeting P53. FEBS Lett.
587:2346–2351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Z, Qiao Q, Chen M, Li X, Wang Z, Liu
C and Xie Z: miR-625 down-regulation promotes proliferation and
invasion in esophageal cancer by targeting Sox2. FEBS Lett.
588:915–921. 2014. View Article : Google Scholar : PubMed/NCBI
|