Introduction
A class of BTB (bric-à-brack,
tram-track, broad complex) domain-containing
proteins, the KCTD family (potassium channel tetramerization domain
protein family which includes 26 members) shares relatively
conserved N-terminal domains and variable C-termini (1). However, the KCTD family involved in
biological processes including biochemical and structural
properties are rather poorly characterized. Potassium channel
tetramerization domain containing 12 (KCTD12, also known as Pfetin)
belongs to the KCTD family, and was initially identified in human
fetal cochlea. KCTD12 is expressed in a variety of fetal organs,
particularly with highest expression levels in the cochlea and
brain, whereas, it is detected at extremely low levels in adult
organs (2). Recent studies have
demonstrated that KCTD12 not only stabilizes γ-aminobutyric acid
type B (GABAB) receptors, which regulate emotionality
and neuronal excitability, but also upregulates GABAB
receptor signaling by stably interacting with GABAB
receptors (3–5). Using two-dimensional differential gel
electrophoresis and mass spectrometry approaches, Suehara et
al (6) predicted that KCTD12
may be a prognostic biomarker of gastrointestinal stromal tumors
(GISTs). This speculation was confirmed by later investigation.
Commercially obtained KCTD12-specific antibody was used for
assessing the expression level of KCTD12 in surgical specimens of
primary tissues from GIST patients (7). Analyzed data indicate that KCTD12
expression appears more specific for GISTs from neoplastic and
non-neoplastic adult tissues other than brain, suggesting the
potential predictor of KCTD12 in GISTs (8). Most recently, downregulation of KCTD12
was observed in cancer stem cells (CSCs) in colorectal cancer
(CRC). Overexpression of ectopic KCTD12 markedly suppressed CRC
cell stemness by inhibiting the extracellular signal-regulated
kinase (ERK) pathway, suggesting that KCTD12 may serve as a novel
therapeutic target for patients with CRC (9).
So-called uveal melanoma usually refers to melanomas
of the choroid, ciliary body and iris of the eye (10). In fact, choroidal and ocular
melanomas are the alternative terms of this type of cancer. Uveal
melanoma is the most common primary intraocular malignancy in
adults. In the US, the incident rate of these cancers is ~5%, 85%
of which are uveal in origin (11).
Patients with uveal melanoma experience painless loss or distortion
of vision in the early stage, which even leads to retinal
detachment at advanced stage (12).
In addition, uveal melanoma is highly metastatic; patients with
uveal melanoma are at risk for metastatic disease to the liver,
lung and skin (13,14). Therefore, uveal melanoma is
associated with high mortality in up to half of uveal-affected
patients (15,16). Although the tumorigenesis and the
metastasis of uveal melanoma are complex processes (17), genetic mutations such as germline
breast cancer 1 (BRCA1)-associated protein 1 (BAP1) (18) and splicing factor 3B subunit 1
(SF3B1) (19), and signaling
pathways such as MAPK (20) have
been found to be involved in uveal melanoma. However, to date,
there have been no studies on the effects of KCTD12 on uveal
melanomas. In the present study, we generated an OCM-1 cell line
stably expressing Flag-KCTD12 and evaluated the functional
properties of KCTD12 in OCM-1 cells.
Materials and methods
Antibodies
Anti-KCTD12 rabbit polyclonal antibody was purchased
from ProteinTech™ Group (Wuhan, China). Anti-E-cadherin,
anti-β-catenin and anti-β-tubulin polyclonal antibodies were
purchased from Ruiying Biological (Suzhou, China). Anti-vimentin,
anti-Snail and anti-Slug antibodies were obtained from Wanleibio
(Shenyang, China).
Cell culture and maintenance
Human uveal melanoma cell line (OCM-1) purchased
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
was cultured and maintained in RPMI-1640 medium (Sigma, St. Louis,
MO, USA) supplemented with 10% fetal bovine serum (FBS) (Kangyuan
Biology, Tianjin, China) and 1% penicillin-streptomycin mixture
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in the
presence of 5% CO2.
Lentiviral vector construction,
lentivirus infection and scree-ning
The full length human KCTD12 gene (NM_138444.3)
fragment with restriction enzyme cutting site was cloned from 293T
cDNA. After the DNA sequence of KCTD12 in the pGEM-T vector was
confirmed, the gene fragment was ligated into the pLVX-Puro
transfer plasmid (Lenti-X™; Clontech Laboratories, Mountain View,
CA, USA). The transfer and packaging plasmids (pRRE, pRSV-Rev and
pCMV–VSVG) were mixed at the quality ratio of 800:400:140:200. 293T
cells cultured in 6-cm plates were then transfected with 5 µg of
DNA plasmids using Lipofectamine 2000 reagent (Invitrogen,
Carlsbad, CA, USA). The medium was replaced after 24 h, and then
the cell supernatants were collected by centrifugation at 10,000 ×
g for 10 min at room temperature and stored at −80°C. OCM-1 cells
were infected with a double volume of recombinant viruses and the
cells were selected with 1 µg/ml puromycin for 2 weeks according to
the manufacturer's protocol. The antibiotic-resistant colonies were
then expanded for further analysis. Stably expressing Flag-KCTD12
cell line was confirmed by western blotting and immunofluorescence
staining.
Immunofluorescence staining
Stably expressing Flag-KCTD12 cells in 24-well
plates containing a coverslip (8D1007, Nest) on each well were
grown to 80% confluence. Then, the cells were washed with
phosphate-buffered saline (PBS) buffer, fixed with 4%
paraformaldehyde (PFA) for 15 min at room temperature, and
permeabilized in 0.5% Triton X-100 in PBS buffer for 5 min. Cells
were then incubated with the KCTD12 primary antibody (1:500) at
room temperature, and stained with the FITC-conjugated secondary
antibody (rabbit/green 1:300; sc-2012). Cell nuclei were stained
using DAPI containing Vectashield (Vector Laboratories, Inc.,
Burlingame, CA, USA). Fluorescence images were observed with an
Olympus BX40F microscope (Olympus Corporation, Tokyo, Japan).
MTS assays
Cells (~3×103) were plated into 96-well
plates. After 48 h, the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-
carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] (CellTiter
96; Promega, Madison, WI, USA) solution was added into the wells
and incubated at 37°C for 1 h. The absorbance was measured at a
wavelength of 490 nm with a microplate reader (Bio-Rad
Laboratories, Hercules, CA, USA).
Colony formation assays
Cells (~103) were seeded into 24-well
plates. After 10 days of culture, formed colonies were stained with
0.1% crystal violet. Colonies containing >20 cells were scored
as positive. Colonies were photographed by Gel Imaging System
(Liuyi Instrument Plant, Beijing, China).
Soft agar assays
Briefly, 5×103 cells were resuspended in
medium containing 10% FBS with 0.3% agarose, and layered on top of
0.6% agar in medium supplemented with 20% FBS on 35-mm plates.
After 14 days of culture, the cells were stained with 0.05% crystal
violet for 1 h. Colonies were photographed and the number of foci
>100 µm was counted.
Wound healing assay
Cells were cultured in 6-well plates and wounds were
introduced by scraping the confluent cell cultures with the tip of
a 10-µl pipette. Floating cells were removed and medium without
serum was added. The wound healing process was monitored under a
microscope.
Migration and invasion assays
For the migration and invasion assays, 24-well
Transwell chambers (Corning Inc., Corning, NY, USA) were used.
Filters (8-µm pore size) were used for estimating cell migration,
and filters coated with Matrigel (BD Biosciences, Franklin Lakes,
NJ, USA) were used for estimating cell invasion. Cells in 0.1 ml of
serum-free RPMI-1640 medium were placed into the upper chamber
(0.5×105 cells/filter). Complete medium was placed in
the lower chamber as a chemoattractant. Migration and invasion were
scored at 24 h after cells were seeded. Cells were fixed in
methanol for 5 min at room temperature, stained with crystal violet
for 5 min, and counted under microscopy. The mean numbers of
cells/microscopic field over 5 fields/filter were calculated.
In vivo tumorigenicity
experiments
Male BALB/c nude mice (6-week old, 15–17 g) were
randomly divided into 2 groups (n=3/group) for the OCM-1 cell
xenograft growth experiment. For tumor cell implantation, the OCM-1
cells stably expressing Flag-KCTD12 or a vector control
(1×106) suspended in 100 µl PBS were injected into the
armpits of the mice. The weight of mouse and the volume (V) of
tumors were examined (V = tumor length × width2 ×
0.5236). All experiments were performed in accordance with the
Institutional Animal Care and Use Committee of Jilin
University.
Cell cycle analysis
OCM-1 cells stably expressing Flag-KCTD12 or a
vector control were grown to 80–90% confluency. In the cell cycle
arrest experiment, as previously described, cells were synchronized
by treatment with 1 mM hydroxyurea (HU) for 24 h, and were then
harvested by trypsinization 0, 2, 4, 6, 8, 10 and 18 h after
removal of HU (21). Data
collection was performed using EPICS XL™ flow cytometer (Beckman
Coulter, Brea, CA, USA). Acquired data were analyzed using FlowJo
software. The experiment was repeated 3 times under the same
conditions.
Analysis of apoptosis
Apoptosis in OCM-1 cells was determined by an
Annexin V-fluorescein isothiocyanate (FITC) staining kit according
to the manufacturer's protocol (KeyGen Biotec, Nanjing, China).
Propidium iodide (PI) was used to differentiate apoptotic cells
with membrane integrity (Annexin V+/PI−) from
necrotic cells that had lost their membrane integrity (Annexin
V+/PI+). The percentage of apoptotic cells
was calculated using an Accuri Cytometer and the data were analyzed
by CFlow software.
Statistical analysis
All the data were analyzed via the Student's t-test
(Microsoft Excel). The statistical difference of p<0.05 was
considered to indicate a statistically significant result.
Results
Stably expressing Flag-KCTD12 is
established in OCM-1 cells
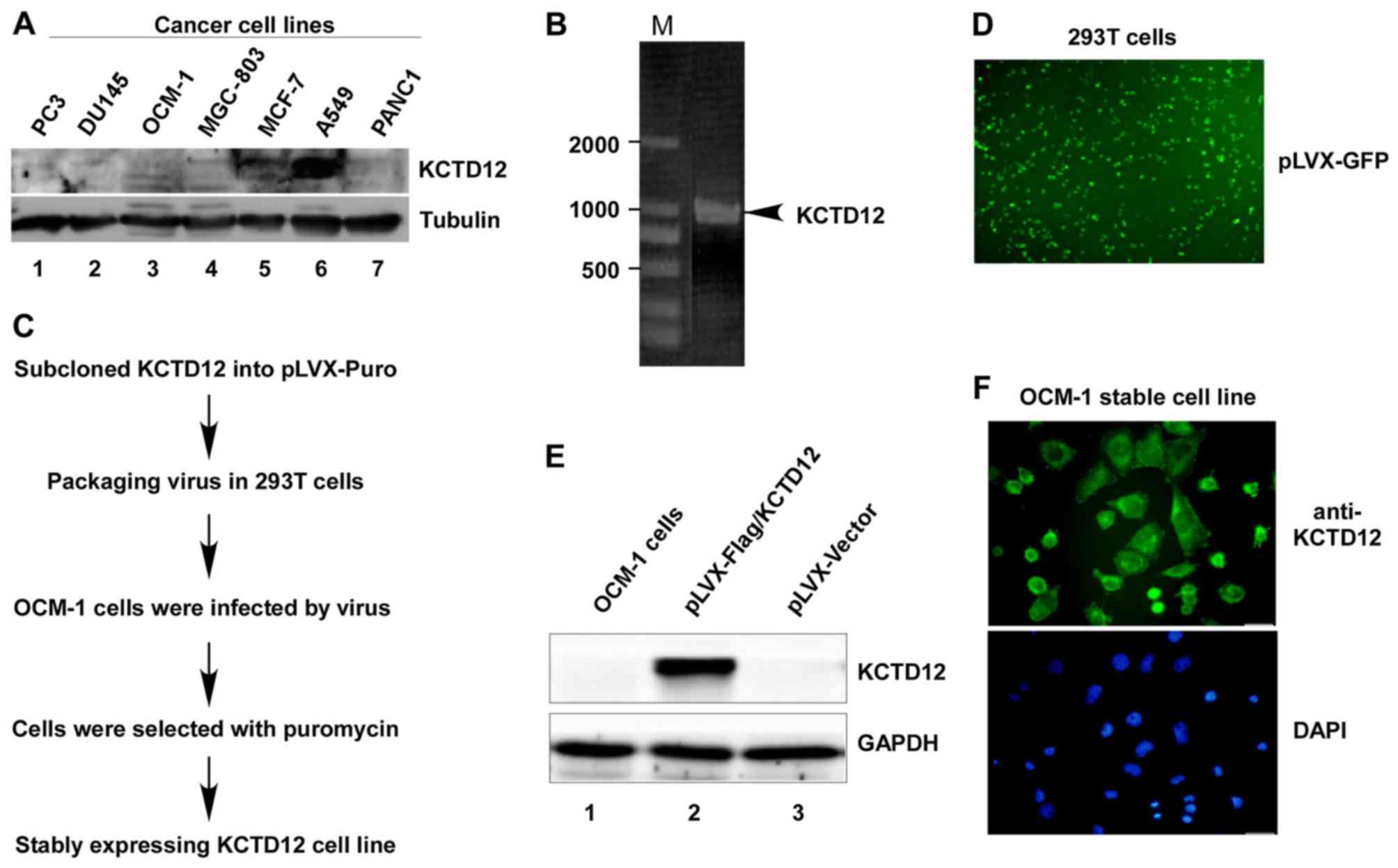
To determine the expression levels of KCTD12 in
cancer cells, we first analyzed the endogenous KCTD12 protein
levels in different cancer cell lines. As shown in Fig. 1A, except for lung carcinoma type II
epithelium-like A549 cells, KCTD12 protein levels in other cancer
cell lines including prostate cancer PC3 and DU145 cells, OCM-1,
gastric cancer MGC-803 cells, human breast adenocarcinoma MCF-7
cells, and pancreatic cancer cell PANC1 cells were extremely low.
In order to further investigate the roles of KCTD12 in cancer
cells, OCM-1 cells were chosen as a test subject. Amplified PCR
products of KCTD12 (Fig. 1B) were
subcloned into the pLVX-Puro vector following the procedure
(Fig. 1C and D) to establish the
stably expressing Flag-KCTD12 OCM-1 cell line. The established
stable cell line was successfully confirmed by western blotting
(Fig. 1E) and immunofluorescence
staining (Fig. 1F).
Overexpression of KCTD12 suppresses
the proliferation of OCM-1 cells
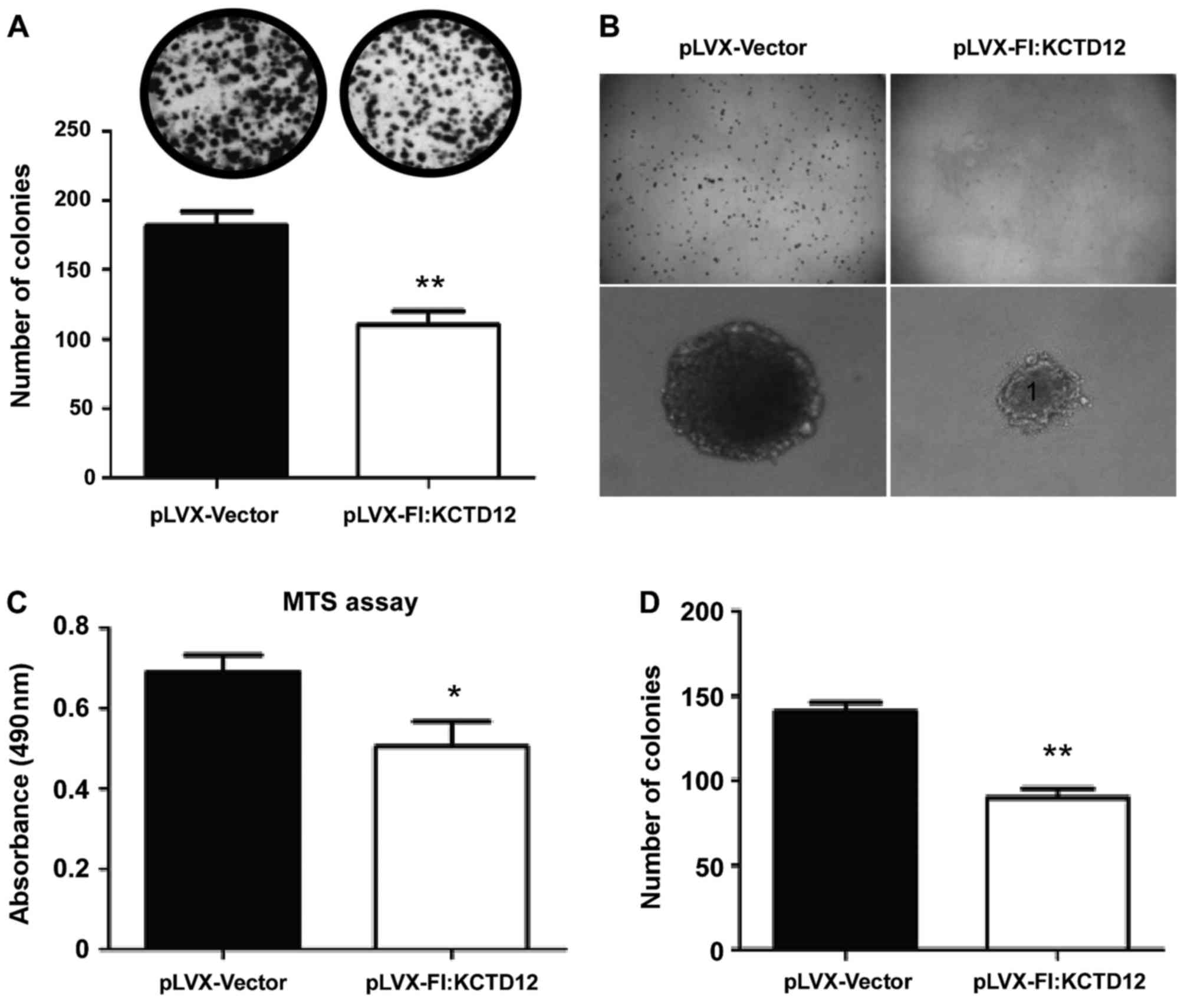
Using the established stable cell line, we first
performed the MTS assays. As shown in Fig. 2C, attenuated viability was observed
in the KCTD12-overexpressing cells (p<0.05). Furthermore, to
explore the functions of KCTD12 in the proliferation and
tumorigenesis of OCM-1 cells, colony formation and soft-agar assays
were performed in vitro. In both assays, significantly
decreased colony formation (Fig.
2A, upper images) and much smaller colony size (Fig. 2B) were observed in the
KCTD12-overexpressing OCM-1 cells. Significant difference in colony
numbers between the KCTD12-overexpressing and vector controls OCM-1
cells was found (p<0.01 in both assays) (Fig. 2A, lower, and D).
Overexpression of KCTD12 inhibits
OCM-1 cell motility
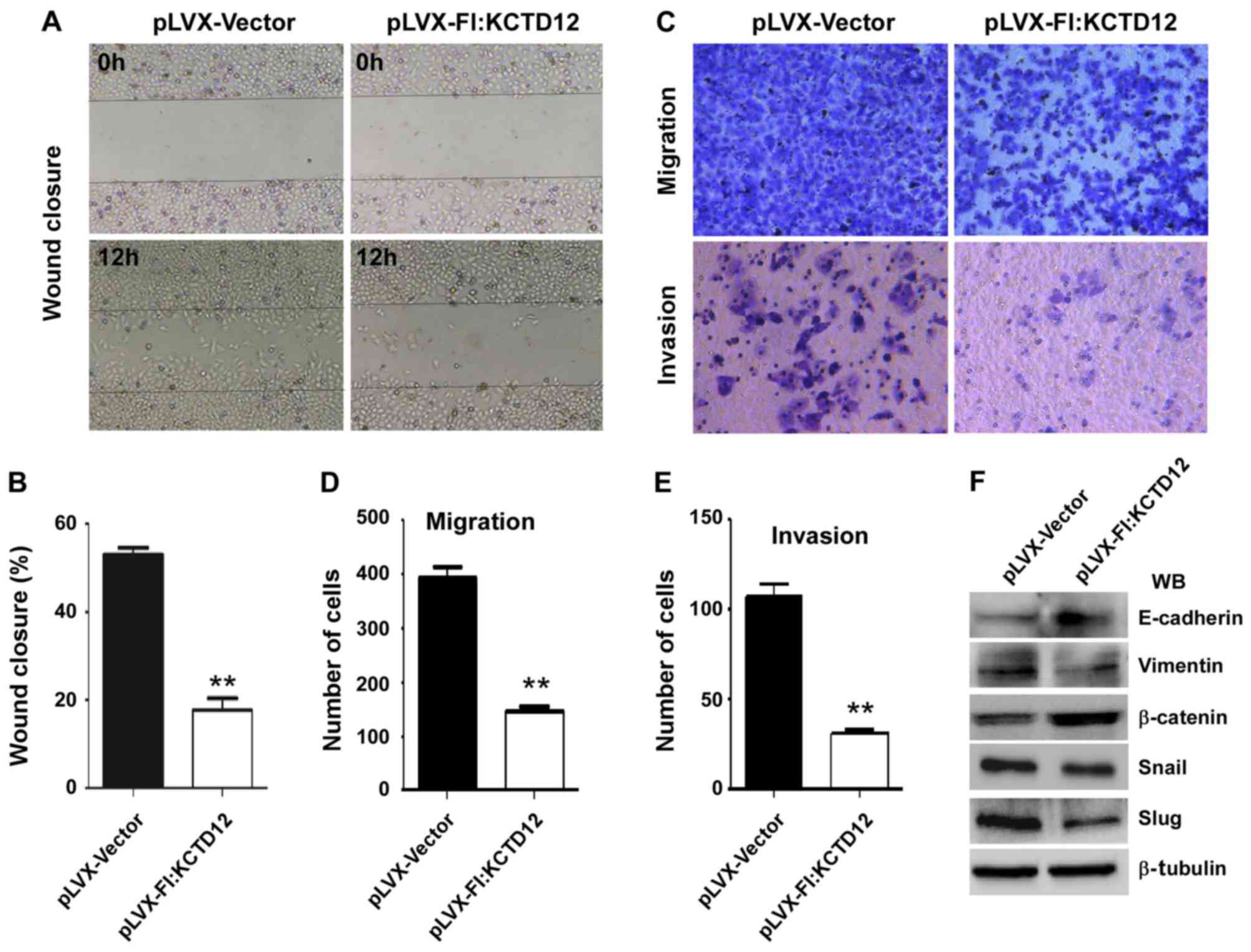
To further observe the effects of KCTD12 on OCM-1
cell motility, we performed wound-healing assays. The images after
12 h of the initial scratch wound are shown in Fig. 3A. The progression of wound closure
in the KCTD12 stably expressing OCM-1 cells was much slower than
that in the control cells. Compared to the control cells, the
percentage of wound closure was significantly decreased (p<0.01)
(Fig. 3B). Then, Transwell
migration (Fig. 3C, upper) and
invasion assays (Fig. 3C, lower)
were carried out to examine the roles of KCTD12 in metastasis.
Quantified number of cells which penetrated the membrane was
largely decreased compared with the control cells (p<0.01)
(Fig. 3D). Similarly,
overexpression of KCTD12 markedly reduced the invasive ability of
the OCM-1 cells (p<0.01) (Fig.
3E). Western blot analysis revealed that the expression levels
of various epithelial-mesenchymal transition (EMT)-related
molecules were altered. As shown in Fig. 3F, elevated E-cadherin and β-catenin
protein levels and decreased mesenchymal (MSC) markers vimentin,
Snail and Slug were noted in the KCTD12-overexpressing OCM-1
cells.
Overexpression of KCTD12 prolongs the
progression of the G2/M phase and induces apoptosis in the OCM-1
cells
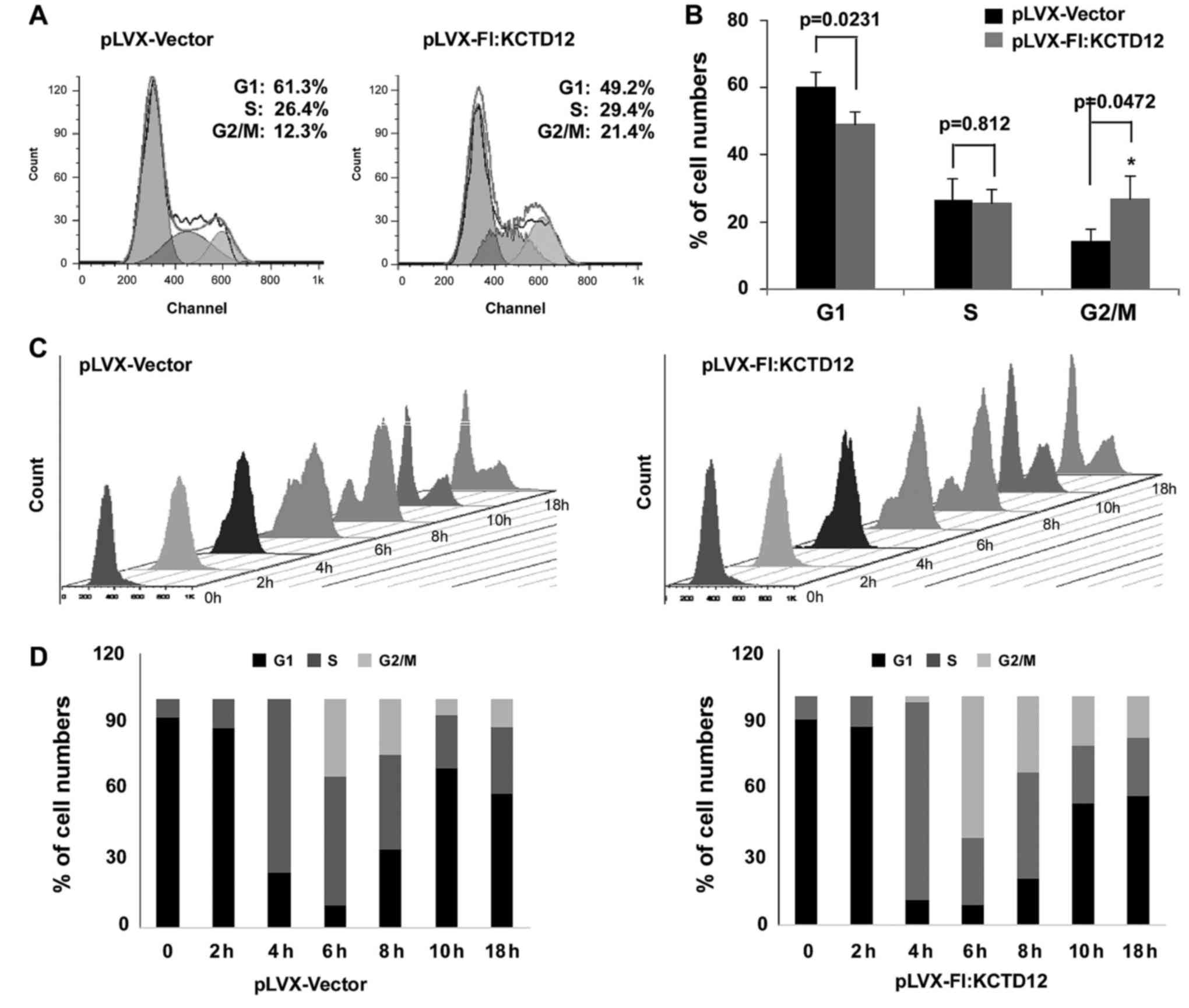
Given that overexpression of KCTD12 significantly
inhibits OCM-1 cell proliferation, we speculated that KCTD12 is
involved in cell cycle progression. Thus, FACS analysis was
performed on KCTD12-overexpressing or control OCM-1 cells. Compared
to the control cells, stably expressing KCTD12 cells had delayed
G2/M phase progression (Fig. 4A).
The percentages of the subpopulation of cells in cell cycle phases
G1, S and G2/M are shown in Fig.
4B. The percentage of KCTD12-expressing cells in cell cycle
phase G2/M was statistically increased compared to the percentage
in the pLVX-Vector cells (p<0.05). To further confirm this
observation, both cells were treated with 1 mM HU to block cells at
the G1/S phase, so that, no new G2/M cells could be generated. Cell
flow cytometry and FACS analysis in both cell lines were performed
at 0, 2, 4, 6, 8, 10 and 18 h after removal of HU (Fig. 4C). The gradual increase in the G2/M
phase population was observed in the KCTD12-overexpressing cells
(Fig. 4D). To further confirm
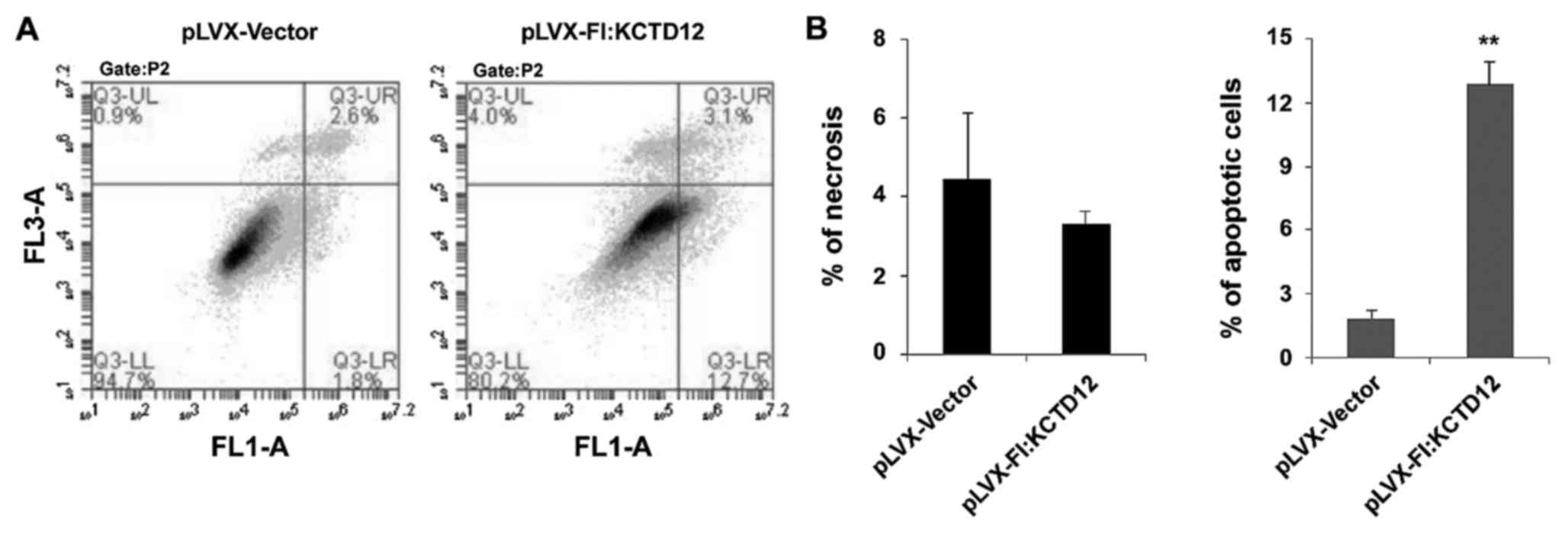
whether overexpression of KCTD12 can affect cell apoptosis, flow
cytometry of Annexin V binding/PI uptake was assessed in the
KCTD12-overexpressing cell line and vector only control (Fig. 5A). Apoptosis is depicted in Fig. 5B. Overexpression of KCTD12 in OCM-1
cells significantly increased apoptosis compared to that in the
vector only control (p<0.01) (Fig.
5B, right). However, there was no significant statistical
difference in necrotic cells between the vector and
KCTD12-overexpressing group (p>0.05) (Fig. 5B, left).
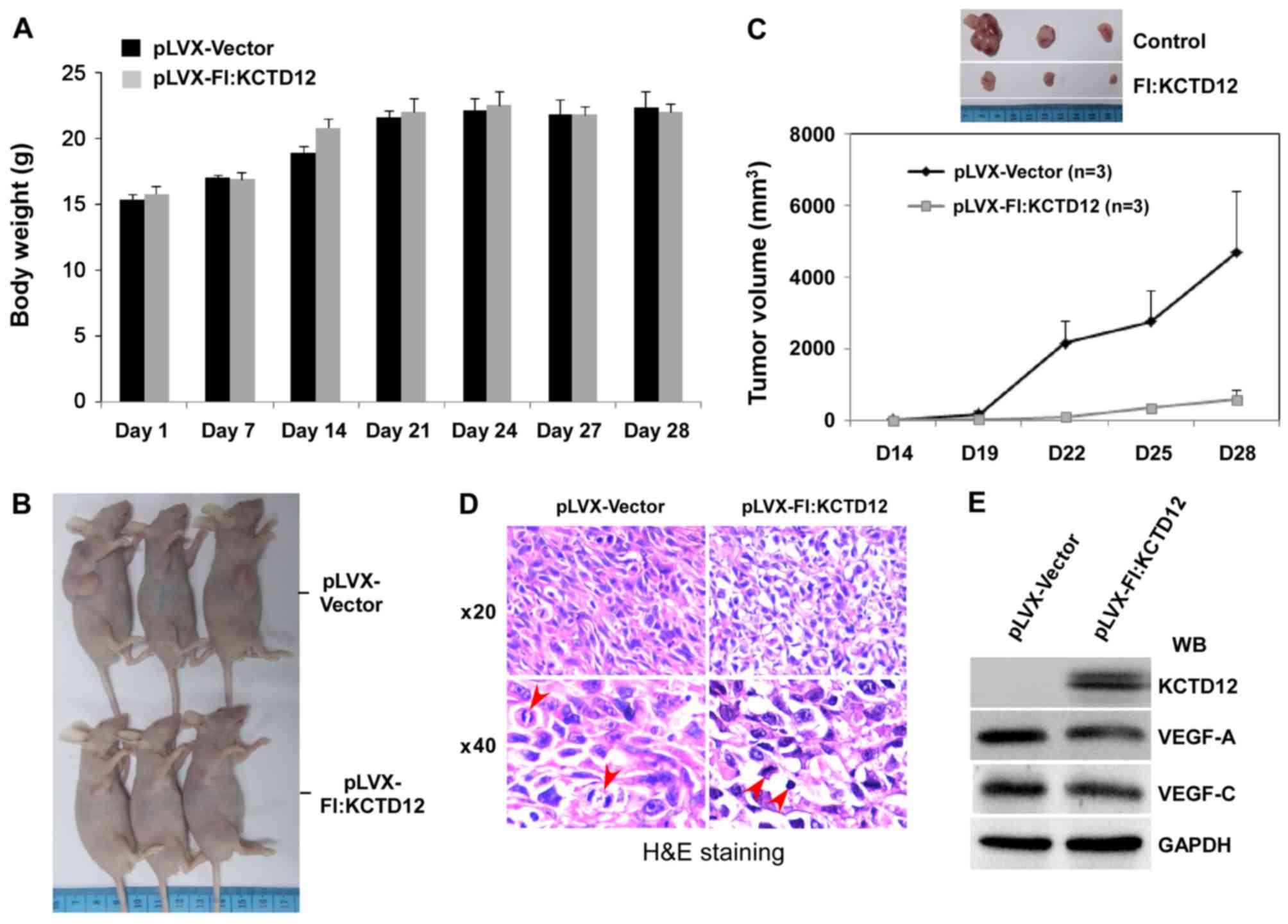
Overexpression of KCTD12 restricts
OCM-1 cell xenograft growth
The above-described series of in vitro
experiments confirmed the effective inhibition of the cell growth
of KCTD12 in OCM-1 cells. To further verify the functions of KCTD12
on the proliferation and tumorigenesis of OCM-1 cells, a xenograft
model consisting of BALB/c nude mice was designed. Stably
expressing KCTD12 OCM-1 cells were injected into the armpits of
6-week mice, and the cells were allowed to grow 28 days. During
this time, the body weight of the mice between the vector and
KCTD12-overexpressing groups had no obvious difference (Fig. 6A). However, overexpression of KCTD12
significantly blocked OCM-1 cell xenograft growth (Fig. 6B). As shown in Fig. 6C, the OCM-1 cell xenograft growth
was slower in the KCTD12-overexpressing group than that noted in
the vector control group (lower). In addition, differences in tumor
size between the two groups are visualized in Fig. 6C (upper images). Cell and nuclear
morphology of the tumor tissues was visualized by hematoxylin and
eosin (H&E) staining. Microscopic images of H&E staining
are shown in Fig. 6D. Morphological
integrity of the cells and a plurality of cells in the mitotic
phase can be seen in the vector control group (left panel). In
contrast, irregularly shaped cells and nuclear condensation were
found in the stably expressing KCTD12 OCM-1 cell group (right
panel). In addition, decreased vascular endothelial growth factor
VEGF-A and VEGF-C were detected by western blot analysis using
prepared whole cell lysate from the tumor tissues (Fig. 6E), suggesting the involvement of
KCTD12 in regulating VEGF.
Discussion
In the present study, we first clarified that
lentiviral-mediated overexpression of KCTD12 inhibits tumor
proliferation in human uveal melanoma OCM-1 cells using both in
vitro and in vivo approaches. A series of in
vitro assays including MTS, colony formation, migration and
invasion assays presented evidence that stably expressing
Flag-tagged KCTD12 significantly suppressed the proliferation and
tumorigenesis of OCM-1 cells. In addition, overexpression of KCTD12
inhibited tumor growth in a xenograft model.
The KCTD proteins with sequence similarity between
its N-terminal region and the tetramerization domain in some
voltage-gated potassium channels are involved in various cellular
biological processes (22). For
example, KCDT proteins as adaptor molecules interact with the Cul3
ubiquitin ligase and its substrate (23,24).
In addition, increasing evidence suggests that the KCTD family is
implicated in proliferation, differentiation, apoptosis and
metabolism of certain cancer cells (25,26).
Based on alignment of the amino acids in the potassium
tetramerization domains, KCTD proteins can be divided into 7 groups
of A to G (22). KCTD12 with KCTD8
and KCTD16 together make up the D-group that directly binds to the
GABAB receptor and an associated G protein (3,4).
KCTD12 as a potential prognostic biomarker of GISTs was screened by
2-D gel combined mass spectrometry approaches. Later studies
confirmed that KCTD12 produced desensitization of the channel to
GABAB receptor signaling by inhibiting the interaction
of Gβγ and Kir3 channels, thereby suppressing proliferation in
GISTs (8). Although the precise
mechanism is unclear, our experimental results verified that
overexpression of KCTD12 markedly suppressed the proliferation of
OCM-1 cells. Stable expression of KCTD12 in OCM-1 cells decreased
the cell viability in MTS assays, and reduced colony numbers in
colony formation and soft agar assays, suggesting the role of
KCTD12 in the proliferation of OCM-1 cells (Fig. 2). Furthermore, overexpression of
KCTD12 in OCM-1 cells not only prolonged the G2/M phase population
(Fig. 4), but also increased
apoptosis (Fig. 5), suggesting that
the ability of KCTD12 to inhibit OCM-1 cell proliferation may be
partly due to the delay in the cell cycle which causes
apoptosis.
A recent study demonstrated that KCTD12 is involved
in the regulation of colorectal cancer cell (CRC) stemness.
Silencing of KCTD12 markedly enhanced CRC cell stemness, while
overexpression of KCTD12 repressed CRC cell stemness and its
markers such as CD44, CD133 and CD29 (9). In the present study, overexpression of
KCTD12 markedly suppressed OCM-1 cell xenograft growth in nude mice
(Fig. 6). Based on H&E staining
of grown tumor tissues, increased numbers of irregularly shaped and
cells with nuclear condensation were found in the
KCTD12-overexpressing OCM-1 cell group. In addition, decreased
VEGF-A and VEGF-C were detected by western blot analysis. Taken
together, KCTD12 is implicated in the tumorigenesis of OCM-1
cells.
It has been found that uveal melanoma is highly
metastatic. Thus, patients with uveal melanoma are at risk of
developing metastatic disease to other organs.
Epithelial-mesenchymal transition (EMT) is a critical process
driving the early phase of cancer metastasis. The process of EMT
can be regulated by many transcription factors. For example, loss
of E-cadherin and β-catenin can trigger the EMT process (27). In contrast, activation of Snail and
Slug often occur in EMT (28). In
the present study, overexpression of KCTD12 significantly
restricted the migration and invasion of OCM-1 cells in in
vitro assays. In addition, an increase in E-cadherin and
β-catenin, and a reduction in Snail and Slug were confirmed by
western blot analysis (Fig. 3),
suggesting the roles of KCTD12 in inhibiting the metastasis of
uveal melanoma by regulating the above genes.
In conclusion, although further research is needed
to clarify the precise mechanisms of uveal melanoma, our data
demonstrated that lentiviral-mediated overexpression of KCTD12
suppressed the proliferation and metastasis of uveal melanoma OCM-1
cells in vitro and in vivo, suggesting that KCTD12
plays an important role in the tumorigenesis of uveal melanoma, and
may serve as a novel therapeutic target for patients with uveal
melanoma.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 31371311 and 31571316),
by the National Laboratory of Biomacromolecules (O5SY02110A and
2012kf04), and by the Project of Jilin Province Science and
Technology Development Program (20130413002GH and
20140414057GH).
References
|
1
|
Ji AX, Chu A, Nielsen TK, Benlekbir S,
Rubinstein JL and Privé GG: Structural insights into KCTD protein
assembly and cullin3 recognition. J Mol Biol. 428:92–107. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Resendes BL, Kuo SF, Robertson NG, Giersch
AB, Honrubia D, Ohara O, Adams JC and Morton CC: Isolation from
cochlea of a novel human intronless gene with predominant fetal
expression. J Assoc Res Otolaryngol. 5:185–202. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwenk J, Metz M, Zolles G, Turecek R,
Fritzius T, Bildl W, Tarusawa E, Kulik A, Unger A, Ivankova K, et
al: Native GABA(B) receptors are heteromultimers with a family of
auxiliary subunits. Nature. 465:231–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Turecek R, Schwenk J, Fritzius T, Ivankova
K, Zolles G, Adelfinger L, Jacquier V, Besseyrias V, Gassmann M,
Schulte U, et al: Auxiliary GABAB receptor subunits
uncouple G protein βγ subunits from effector channels to induce
desensitization. Neuron. 82:1032–1044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cathomas F, Stegen M, Sigrist H, Schmid L,
Seifritz E, Gassmann M, Bettler B and Pryce CR: Altered
emotionality and neuronal excitability in mice lacking KCTD12, an
auxiliary subunit of GABAB receptors associated with
mood disorders. Transl Psychiatry. 5:e5102015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suehara Y, Kondo T, Seki K, Shibata T,
Fujii K, Gotoh M, Hasegawa T, Shimada Y, Sasako M, Shimoda T, et
al: Pfetin as a prognostic biomarker of gastrointestinal stromal
tumors revealed by proteomics. Clin Cancer Res. 14:1707–1717. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kubota D, Mukaihara K, Yoshida A, Suehara
Y, Saito T, Okubo T, Gotoh M, Orita H, Tsuda H, Kaneko K, et al:
The prognostic value of pfetin: A validation study in
gastrointestinal stromal tumors using a commercially available
antibody. Jpn J Clin Oncol. 43:669–675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hasegawa T, Asanuma H, Ogino J, Hirohashi
Y, Shinomura Y, Iwaki H, Kikuchi H and Kondo T: Use of potassium
channel tetramerization domain-containing 12 as a biomarker for
diagnosis and prognosis of gastrointestinal stromal tumor. Hum
Pathol. 44:1271–1277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Duan T, Wang X, Zhang RH, Zhang M,
Wang S, Wang F, Wu Y, Huang H and Kang T: KCTD12 regulates
colorectal cancer cell stemness through the ERK pathway. Sci Rep.
6:204602016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chattopadhyay C, Kim DW, Gombos DS, Oba J,
Qin Y, Williams MD, Esmaeli B, Grimm EA, Wargo JA, Woodman SE, et
al: Uveal melanoma: From diagnosis to treatment and the science in
between. Cancer. 122:2299–2312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang AE, Karnell LH and Menck HR: The
American College of Surgeons Commission on Cancer and the American
Cancer Society: The National Cancer Data Base report on cutaneous
and noncutaneous melanoma: A summary of 84,836 cases from the past
decade. Cancer. 83:1664–1678. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eskelin S and Kivelä T: Mode of
presentation and time to treatment of uveal melanoma in Finland. Br
J Ophthalmol. 86:333–338. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shields CL and Shields JA: Ocular
melanoma: Relatively rare but requiring respect. Clin Dermatol.
27:122–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh AD, Turell ME and Topham AK: Uveal
melanoma: Trends in incidence, treatment, and survival.
Ophthalmology. 118:1881–1885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh AD and Topham A: Survival rates with
uveal melanoma in the United States: 1973–1997. Ophthalmology.
110:956–961. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gill HS and Char DH: Uveal melanoma
prognostication: From lesion size and cell type to molecular class.
Can J Ophthalmol. 47:246–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patel SP: Latest developments in the
biology and management of uveal melanoma. Curr Oncol Rep.
15:509–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harbour JW, Roberson ED, Anbunathan H,
Onken MD, Worley LA and Bowcock AM: Recurrent mutations at codon
625 of the splicing factor SF3B1 in uveal melanoma. Nat Genet.
45:133–135. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Field MG and Harbour JW: Recent
developments in prognostic and predictive testing in uveal
melanoma. Curr Opin Ophthalmol. 25:234–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu FX, Zhang K and Guan KL: YAP as
oncotarget in uveal melanoma. Oncoscience. 1:480–481. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao L, Ding J, Dong L, Zhao J, Su J, Wang
L, Sui Y, Zhao T, Wang F, Jin J, et al: Negative regulation of
p21Waf1/Cip1 by human INO80 chromatin remodeling complex
is implicated in cell cycle phase G2/M arrest and abnormal
chromosome stability. PLoS One. 10:e01374112015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Z, Xiang Y and Sun G: The KCTD family
of proteins: Structure, function, disease relevance. Cell Biosci.
3:452013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Canettieri G, Di Marcotullio L, Greco A,
Coni S, Antonucci L, Infante P, Pietrosanti L, De Smaele E,
Ferretti E, Miele E, et al: Histone deacetylase and
Cullin3-RENKCTD11 ubiquitin ligase interplay regulates
Hedgehog signalling through Gli acetylation. Nat Cell Biol.
12:132–142. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Staropoli JF, Karaa A, Lim ET, Kirby A,
Elbalalesy N, Romansky SG, Leydiker KB, Coppel SH, Barone R, Xin W,
et al: A homozygous mutation in KCTD7 links neuronal ceroid
lipofuscinosis to the ubiquitin-proteasome system. Am J Hum Genet.
91:202–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mancarelli MM, Zazzeroni F, Ciccocioppo L,
Capece D, Po A, Murgo S, Di Camillo R, Rinaldi C, Ferretti E,
Gulino A, et al: The tumor suppressor gene KCTD11REN is
regulated by Sp1 and methylation and its expression is reduced in
tumors. Mol Cancer. 9:1722010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Faryna M, Konermann C, Aulmann S, Bermejo
JL, Brugger M, Diederichs S, Rom J, Weichenhan D, Claus R, Rehli M,
et al: Genome-wide methylation screen in low-grade breast cancer
identifies novel epigenetically altered genes as potential
biomarkers for tumor diagnosis. FASEB J. 26:4937–4950. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu Q, Deng F, Qin Y, Zhao Z, Wu Z, Xing Z,
Ji A and Wang QJ: Long non-coding RNA regulation of
epithelial-mesenchymal transition in cancer metastasis. Cell Death
Dis. 7:e22542016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|