Introduction
Breast cancer is one of the most common female
malignant tumors worldwide. According to the statistics from the
International Agency for Research on Cancer (IARC), ~1.4 million
women suffer from breast cancer, and ~500,000 individuals die of
breast cancer every year (1). The
latest statistics released by Cancer Journal for Clinicians
indicated that ~230,000 women in the US were diagnosed with breast
cancer in 2011, accounting for 30% of all newly diagnosed female
malignant tumors, with an incidence rate ranked as no. 1 among all
female malignant tumors (2).
Although China is a region with a relatively low incidence of
breast cancer, in recent years, the incidence shows an increasing
trend (3). Particularly in Beijing,
Shanghai and other big cities, the incidence rate has risen to
50–60/100,000 individuals, achieving the highest incidence rate of
all female cancers (4). In the past
20 years, with the development and application of chemotherapy,
hormonal and targeted therapies, and other combined modality
therapeutic methods, breast cancer patients have a significantly
prolonged lifespan, and the 5-year survival rate increased from 55%
during the 1970s to the present rate of 80%. In some countries, the
breast cancer mortality rate has shown a downward trend (5).
In recent years, researchers have given more
attention to the relationship between molecular markers and
chemotherapeutic drug resistance, and have achieved some results
(6). Platinum agents are a group
structurally similar to bifunctional alkylating agents, which forms
a complex with bases on nucleophilic groups by covalent bonding,
resulting in DNA replication and transcription by structural
changes and disorders, thereby inhibiting cell division (7). Numerous studies have mainly focused on
the expression of TUBB3 and taxane resistance (8). Research has shown that TUBB3 is not
only a major component of tubulin, with important significance for
the maintenance of cell shape, but its expression in tumor tissues
is also related to the sensitivity of chemotherapeutic agents
(6). Research has shown that TUBB3
expression is closely related to drug resistance of taxanes. TUBB3
can reduce the gathering speed of tubulin and resist against the
tubulin aggregation caused by taxane chemotherapy drugs, leading to
a decrease in the sensitivity to chemotherapy drugs, thus causing
the failure of chemotherapy (9).
In the 1970s, it was found that the main mechanism
of anthracycline is to inhibit TOP2A, leading to the breakage of
double-stranded DNA, thereby promoting tumor cell apoptosis by
interacting with the TOP2A-DNA complex (10). TOP is a key ribozyme necessary for
DNA replication and transcription processes, which can be
classified into TOP1 and TOP2 according to the nature. TOP1 causes
the breakage of single-stranded DNA on DNA sugar-phosphate
backbone, while TOP2 mainly causes the breakage of double-stranded
DNA (11). In eukaryotic organisms,
TOP2 can be classified into two types of isoenzymes, TOP2A and
TOP2B. The TOP2A gene is adjacent to the HER-2 gene, located in the
17q21-q22 region (as the encoding gene of DNATC) P20t. The gene
product is a homodimer protein with a relative molecular mass of
170,000, involved in the processes of DNA recombination, repair,
replication and transcription (12). The TOP2A protein level has a
significant cycle-specificity in proliferative cells, of which the
expression is low in the G1 phase, increased in the S phase, and
reaches a peak after the G2-M phase. The overexpression of TOP2A
may reflect cell proliferation activity and aggressive invasive
behaviors and poor prognosis (10).
Therefore, TOP2A plays an important role in the study of
cytobiology.
As an important tumor-suppressor gene following P53
and PRb, the PTEN gene is closely related to the development of
various human malignant tumors, biological behavior and prognosis.
The PTEN gene is located in the 10q23.3 region that has high
deletion incidence of human chromosomes and heterozygotes, and is
the first tumor-suppressor gene shown to have phosphatase activity
to date (13). Researchers have
found abnormalities in mutation, deletion and protein expression,
thus losing the negative regulatory effect on cell growth, which
may eventually lead to tumorigenesis (14). In the normal human, the PTEN
expression level is high in placenta, heart, brain, lung and kidney
and other tissues, and it is also expressed in peripheral blood
mononuclear cells. Meanwhile PTEN is a self-renewal regulatory gene
for normal hematopoietic stem cells and a leukemia promoter gene,
which is also essential for the maintenance of normal
hematopoiesis. Mutations, deletion and decreased expression of PTEN
are found in leukemia, lymphoma and multiple myeloma and other
blood and lymphoid malignancies, highly suggestive of the important
molecular regulatory function of PTEN in hematopoietic cell
proliferation, apoptosis and malignant transformation (14). In the present study we examined the
PTEN/AKT signaling effects on TUBB3 and TOP2A expression and cell
growth of human breast cancer MCF-7 cells.
Materials and methods
Patients and treatment
A total of 57 breast cancer patients were recruited
for the present study from the Department of Oncology, The First
Affiliated Hospital of Jinan University, between February 2010 and
October 2010. After surgery, all patients were surveyed for 2
months. All participants provided written informed consent and the
present study was approved by the Ethics Committee of The First
Affiliated Hospital of Jinan University.
Immunohistochemical
Breast cancer and para-carcinoma tissues were fixed
with 10% formalin for 24 h and embedded in paraffin. Tissues were
sliced into 4-µm sections and baked for 1 h at 65°C. The sections
were exposed to 10 mM citrate buffer (Beyotime, Shanghai, China)
for 10 min at 37°C and incubated with the mouse monoclonal
anti-human-TUBB3 and anti-human-TOP2A (both from Sigma-Aldrich, St.
Louis, MO, USA). The tissue sections were incubated with polyclonal
goat anti-rabbit IgG biotinylated secondary antibodies at 37°C for
1 h. Then, the sections were incubated with
streptavidin-horseradish peroxidase complex (Sigma-Aldrich) at 37°C
for 10 min. The sections were evaluated independently under a light
microscope (CX21; Olympus Corporation, Tokyo, Japan).
qPCR analysis
Total RNA was isolated from tissue samples using
TRIzol reagent (Takara, Shiga, Japan). Total RNA (1–2 µg) was
reverse transcribed into cDNA using a reverse transcription kit
(Takara). The expression of TUBB3 and TOP2A was measured using
SYBR-Green I Master Mix (Takara) and qPCR (7500 ABI System; Applied
Biosystems, Foster City, CA, USA). The qPCR reaction was performed
as follows: 95°C for 5 min, followed by 40 cycles of denaturation
at 95°C for 20 sec and annealing/extension at 60°C for 30 sec.
Primer sequences used in the study are listed in Table I.
 | Table I.Primer sequences used in the present
study. |
Table I.
Primer sequences used in the present
study.
| Gene name | Primer sequences |
|---|
| TUBB3 | F |
5′-ACTACAACGAGGCCTCTTCTCAC-3′ |
|
| R |
5′-TTGTTGCCGGCCCCACTCTGACC-3′ |
| TOP2A | F |
5′-ATCCTGCCAAAACCAAGAATCG-3′ |
|
| R |
5′-GTACAGATTTTGCCCGAGGAGC-3′ |
| GAPDH | F |
5′-GTGAACCATGAGAAGTATGACAA-3′ |
|
| R |
5′-CATGAGTCCTTCCACGATAC-3′ |
Cell culture
Human breast cancer MCF-7 cells were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and
antibiotic solution (100 µg/ml streptomycin and 100 U/ml penicillin
(all from HyClone, Logan, UT, USA) at 37°C in a 5% CO2
incubator.
Cell viability assay
Cell viability was evaluated using the MTT assay.
MCF-7 cells (2×104/well) in a 96-well plate were treated
with VO-OHpic trihydrate (5 µM), a specific PTEN inhibitor; or
3-methyladenine (3-MA; 10 µM), a PI3K inhibitor; or ATP (5 µM) for
0, 24 and 48 h. Ten milliliters (5 mg/ml) of MTT solution was added
to each well for a 4-h period at 37°C. Dimethyl sulfoxide (DMSO)
was added into each well and dissolved for 30 min. Absorbance
(OD490) was assessed using a FLUOstar OPTIMA microplate reader (BMG
Labtech, Cary, NC, USA) at 490 nm.
Cell apoptosis analysis
MCF-7 cells (1–2×106/well) in a 6-well
plate were treated with VO-OHpic trihydrate (5 µM) or 3-MA (10 µM)
for 48 h. After collection and washing with phosphate-buffered
saline (PBS), the cells were stained with Annexin V and propidium
iodide (PI) using the Annexin V-PI detection kit (Roche, Mannheim,
Germany). Cell apoptosis was measured using flow cytometric
analysis using a FACScan (BD Biosciences, San Jose, CA, USA).
Caspase-3 activity assay
Cells were lysed using RIPA lysis buffer and the
protein content of the cell lysate was quantified by the BCA
protein assay (both from Beyotime). Caspase-3 activity was measured
in 200 µg of cell lysate using Ac-DEVD-pNA (Beyotime) for 2 h at
37°C. Absorbance (OD490) was measured using a FLUOstar Optima
microplate reader (BMG Labtech, Cary, NC, USA) at a wavelength of
405 nm.
Western blot analysis
Cells were washed twice with PBS and were lysed in
RIPA lysis buffer for 30 min at 4°C. The concentration of total
protein was measured uisng a Bradford kit (#500-0205; Bio-Rad,
Hercules, CA, USA). Equal amounts of protein (50 µg) were boiled
and 10–12% SDS-PAGE was carried out. The proteins were transferred
onto nitrocellulose membranes (Bio-Rad). Blots were blocked in 5%
skim milk in Tris-buffered saline Tween-20 (TBST) and incubated
with anti-Bax (14796; 1:1,000), anti-TUBB3 (5568; 1:1,000),
anti-TOP2A (12286; 1:1,000), anti-p-AKT (4060; 1:1,000) (all from
Cell Signaling Technology, Inc., Danvers, MA, USA) and GAPDH
(1:2,000; Beyotime) at 4°C overnight. Blots were washed twice with
TBST and incubated with secondary antibodies (6401–05; Amyjet
Scientific, Inc., Wuhan, China) prior to identification of bands
with chemiluminescence (ECL; Beyotime). The density was quantified
using ImageJ software 3.0 (Bio-Rad).
Statistical analysis
All data are presented as mean values and standard
deviation (mean ± SD). One-way ANOVA with Tukey's post hoc
comparisons at P<0.05 was considered to indicate a statistically
significant result.
Results
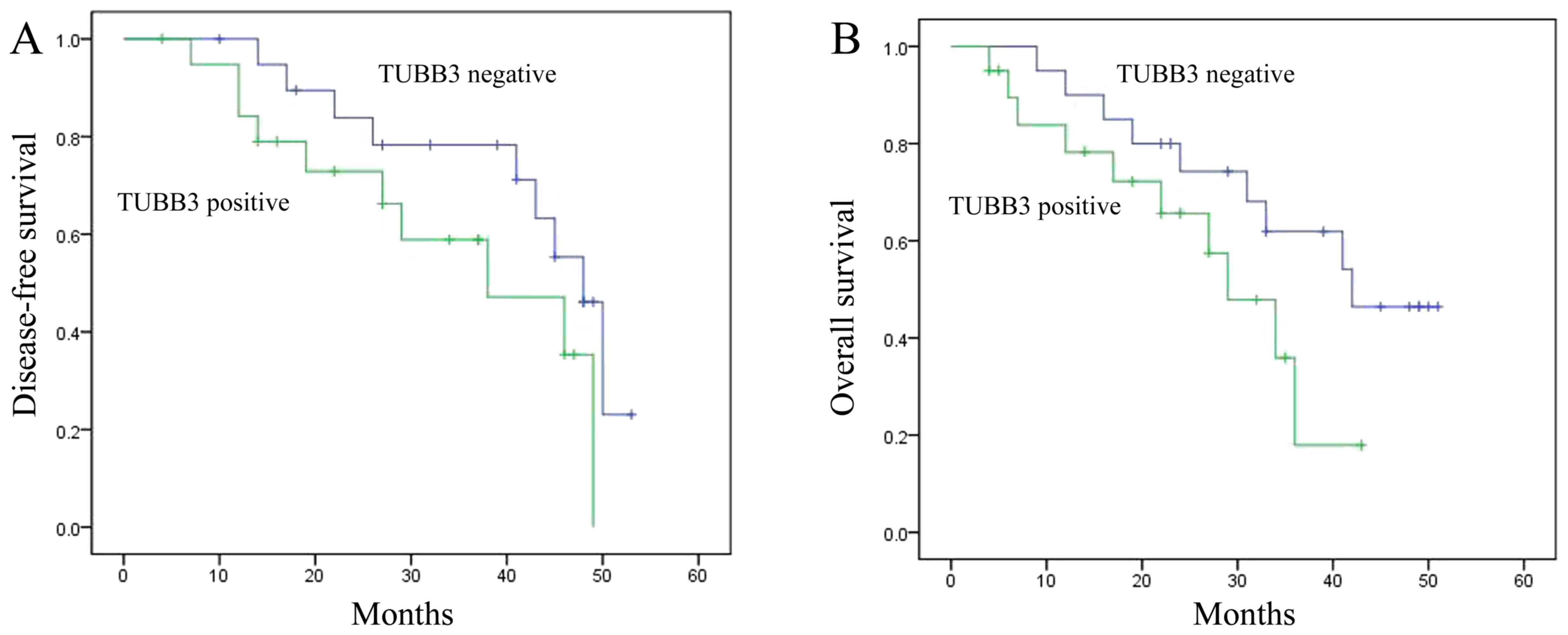
Disease-free survival (DFS) or overall
survival (OS) of the breast cancer patients
Firstly, we determined the DFS and OS of human
breast cancer patients and analyzed the relationships of DFS and OS
with expression of TUBB3 and TOP2A. As shown in Fig. 1A and B, the DFS and OS of the breast
cancer patients with TUBB3-positive tumors were reduced when
compared with the DFS and OS in patients with TUBB3-negative
tumors. Meanwhile, the DFS and OS of breast cancer patients with
TOP2A-positive tumors were also reduced when compared with the DFS
and OS in patients with TOP2A-negative tumors (Fig. 1C and D).
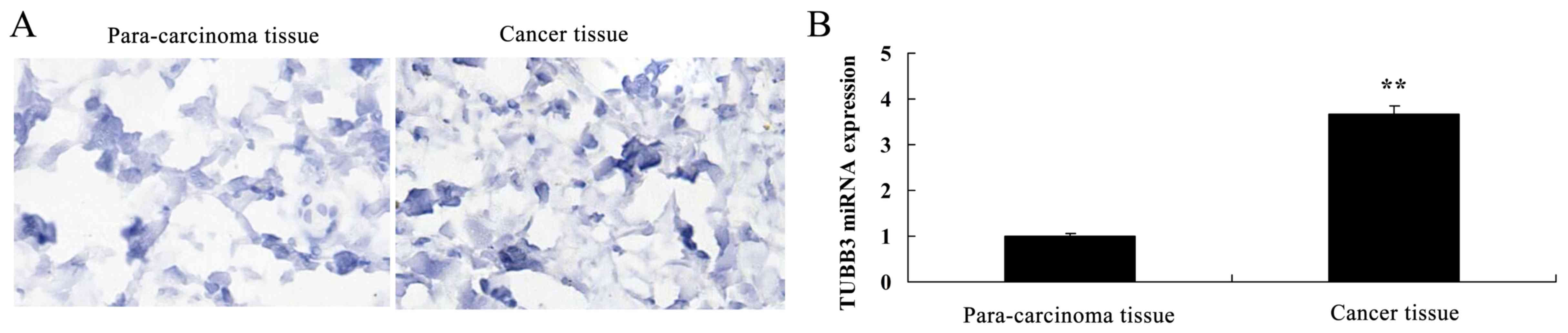
Expression of TUBB3 and TOP2A in the
breast cancer patient tissue
Next, we found that the TUBB3 protein and miRNA
expression in breast cancer tissues were observably higher than
these levels in the para-carcinoma tissue (Fig. 2A and B). TOP2A protein and miRNA
expression in breast cancer tissue were observably higher than
these levels in the para-carcinoma tissues (Fig. 2C and D).
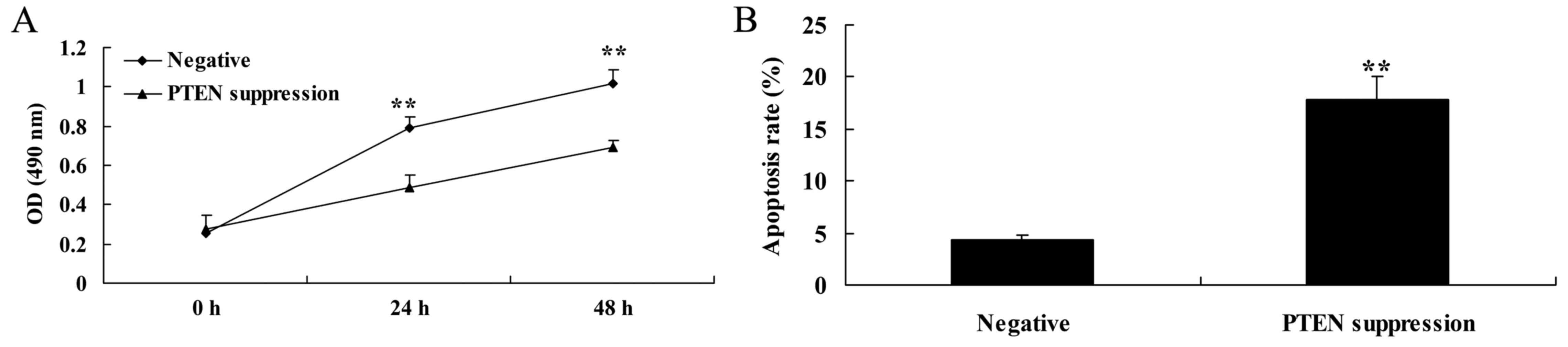
Suppression of PTEN decreases cell
proliferation and induces apoptosis in MCF-7 cells
In order to determine whether PTEN is involved in
the regulation of MCF-7 cell death, a PTEN inhibitor was used to
suppress the expression of PTEN protein. As shown in Fig. 3A, the PTEN inhibitor significantly
decreased cell viability of the MCF-7 cells in a time-dependent
manner, compared with the negative group. As shown in Fig. 3B, PTEN inhibitor also significantly
induced apoptosis in the MCF-7 cells, compared with that noted in
the negative group.
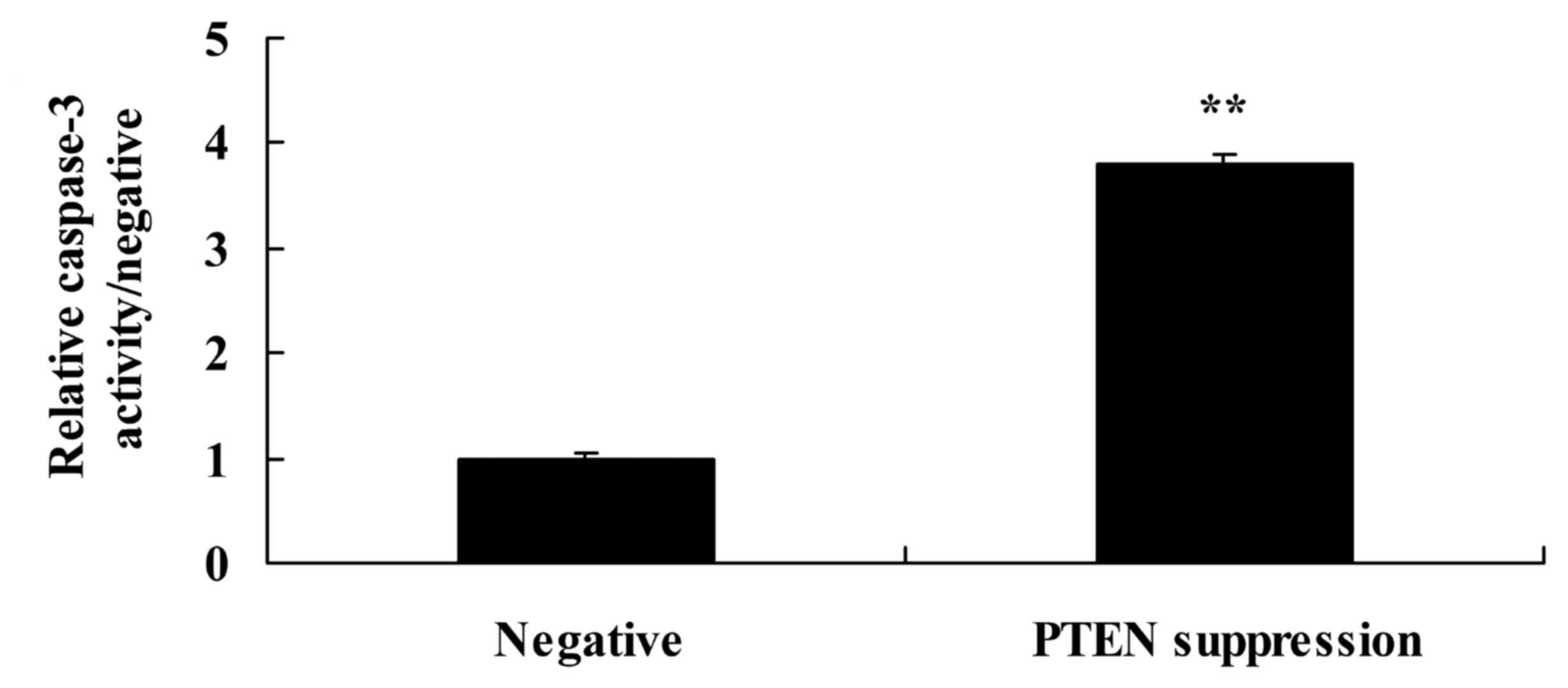
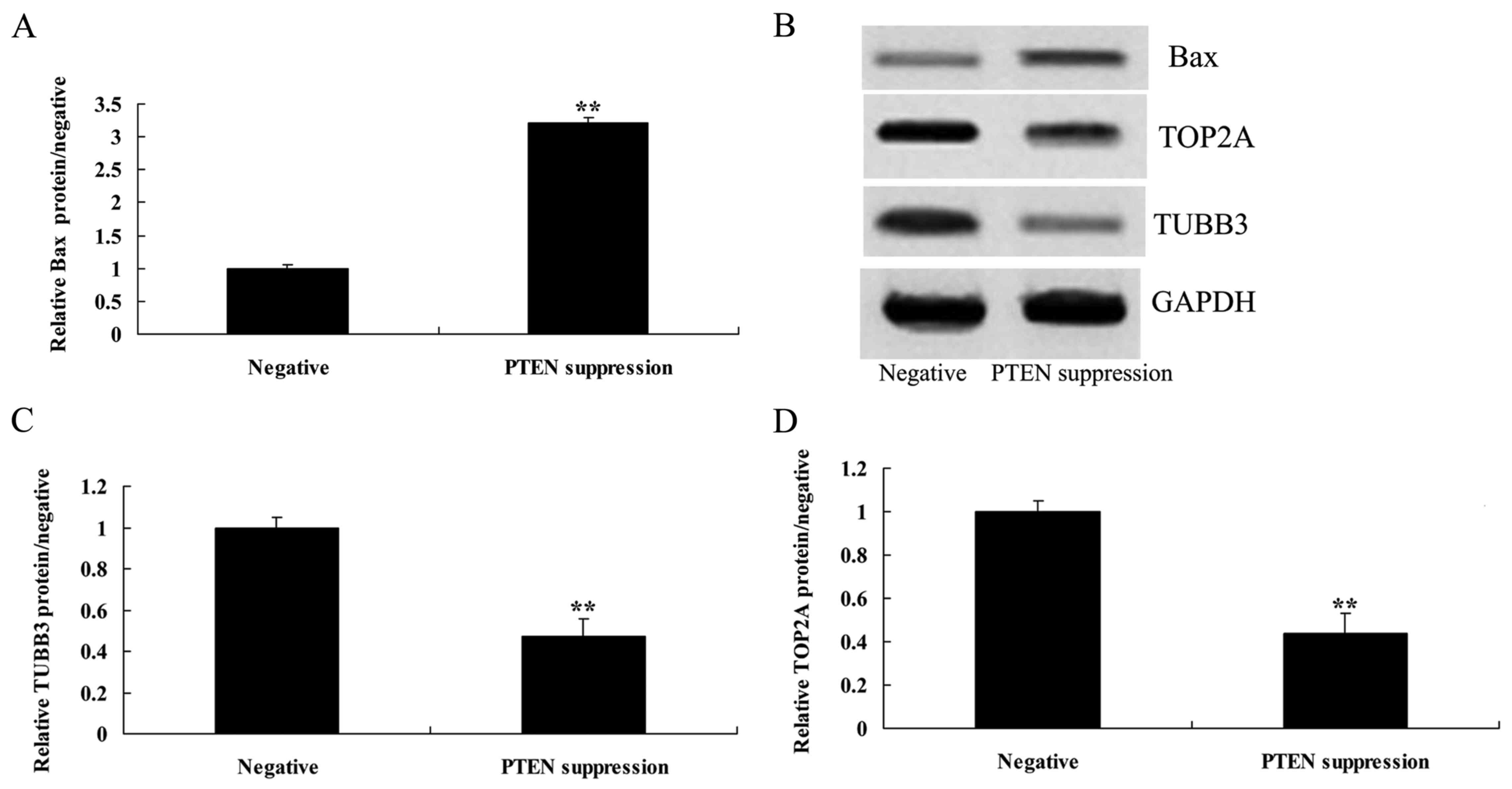
Suppression of PTEN increases
caspase-3 activity and Bax protein expression in MCF-7 cells
We demonstrated that suppression of PTEN increased
caspase-3 activity and Bax protein expression in the MCF-7 cells.
As shown in Figs. 4, and 5A and B, suppression of PTEN significantly
increased caspase-3 activity and Bax protein expression in the
MCF-7 cells.
Suppression of PTEN reduces the TUBB3
protein expression in MCF-7 cells
To understand the mechanism of PTEN underlying MCF-7
cell death, TUBB3 protein expression was measured using western
blot analysis. As shown in Fig. 5B and
C, the suppression of PTEN significantly reduced the protein
expression of TUBB3 in the MCF-7 cells, compared with that noted in
the negative group.
Suppression of PTEN reduces the TOP2A
protein expression in MCF-7 cells
To understand the mechanism of PTEN underlying MCF-7
cell death, TOP2A protein expression was also measured using
western blot analysis. As indicated in Fig. 5B and D, the suppression of PTEN
significantly reduced the protein expression of TOP2A in the MCF-7
cells, compared with that noted in the negative group.
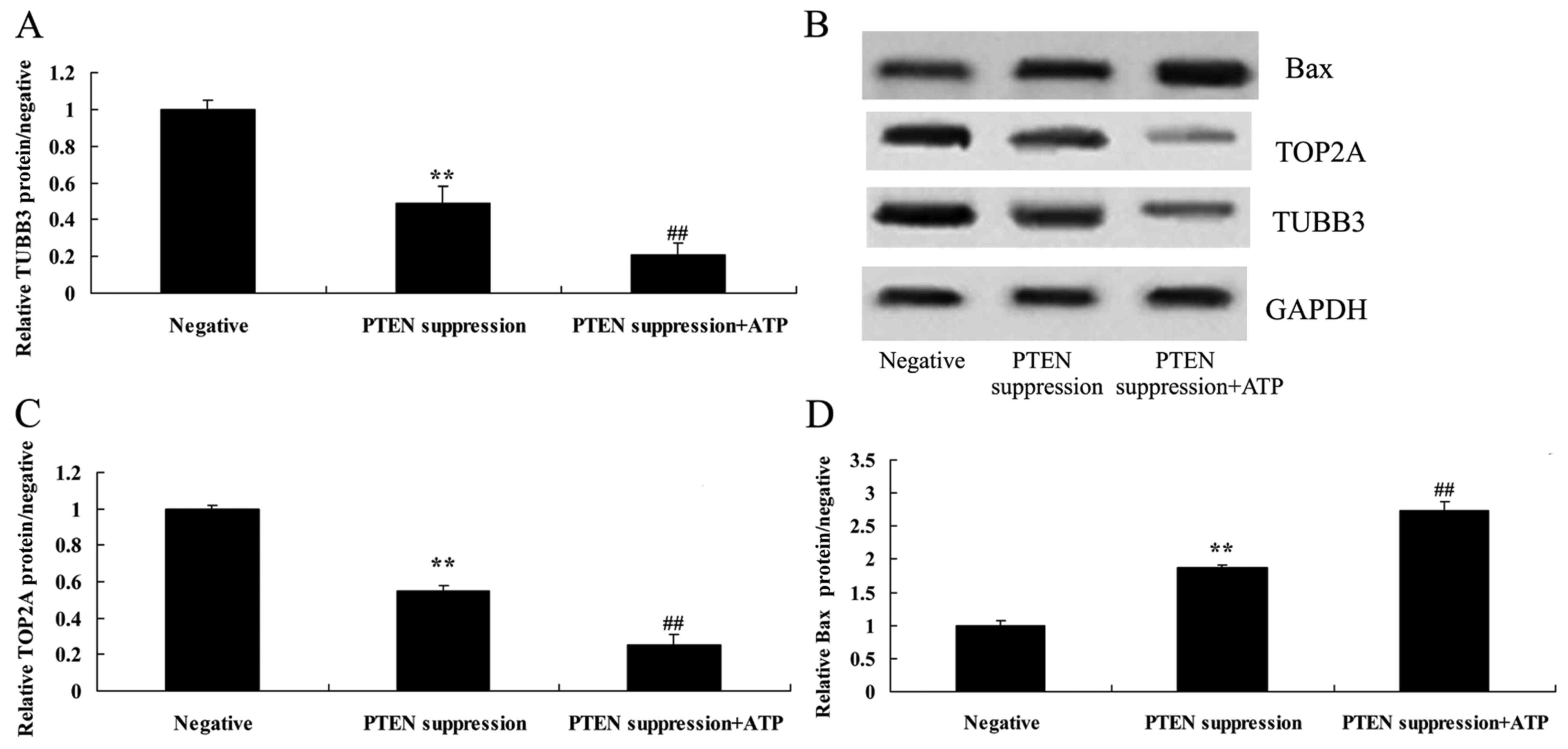
ATP inhibits TUBB3 protein expression
in MCF-7 cells following suppression of PTEN
To ascertain whether ATP regulates the effect of
PTEN on TUBB3 expression in MCF-7 cells, ATP (5 µM) was added to
the MCF-7 cells following suppression of PTEN. Particularly, ATP
inhibited TUBB3 expression in the MCF-7 cells following suppression
of PTEN, compared with MCF-7 cells following suppression only of
PTEN (Fig. 6A and B).
ATP reduces TOP2A protein expression
in MCF-7 cells following suppression of PTEN
To ascertain whether ATP regulates the effect of
PTEN on TOP2A expression in MCF-7 cells, MCF-7 cells were treated
with ATP (5 µM) following suppression of PTEN. Analogously,
increased ATP suppressed TOP2A protein expression in the MCF-7
cells following suppression of PTEN, compared with MCF-7 cells
following suppression only of PTEN (Fig. 6B and C).
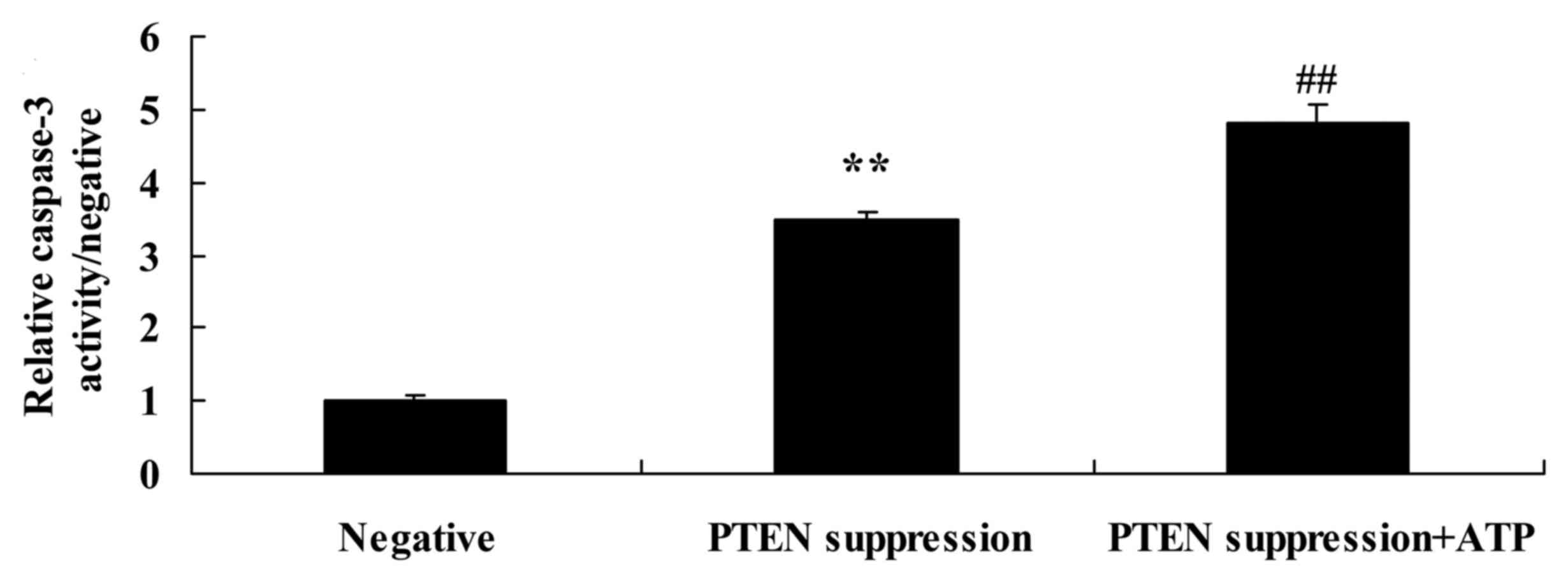
Treatment of ATP further increases
caspase-3 activity and Bax protein expression in MCF-7 cells
following suppression of PTEN
To ascertain whether ATP regulates the effect of
PTEN on MCF-7 cell apoptosis, following suppression of PTEN, ATP
was added to the MCF-7 cells. We found that treatment with ATP
increased caspase-3 activity and Bax protein expression in the
MCF-7 cells following suppression of PTEN, compared with MCF-7
cells following suppression only of PTEN (Figs. 6B and D, and 7).
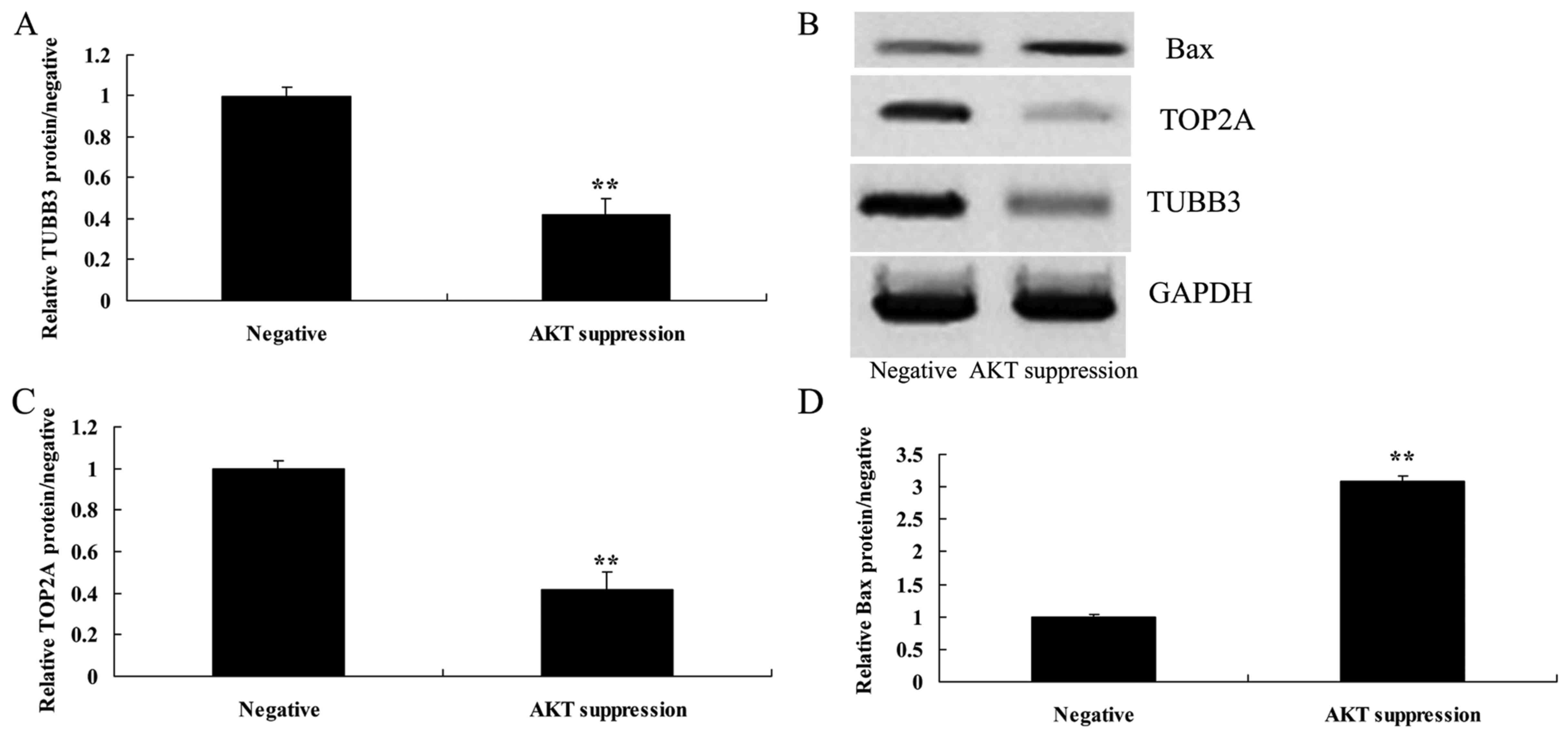
Suppression of p-AKT reduces TUBB3
protein expression in MCF-7 cells
To further understand the mechanism of p-AKT in
MCF-7 cell death, TUBB3 protein expression was selected and
measured using western blot analysis. As shown in Fig. 8A and B, the suppression of p-AKT
significantly suppressed the protein expression of TUBB3 in the
MCF-7 cells, compared with the level in the negative group.
Suppression of p-AKT reduces TOP2A
protein expression in MCF-7 cells
To further understand the mechanism of p-AKT in
MCF-7 cell death, TOP2A protein expression was also selected and
measured using western blot analysis. As indicated in Fig. 8B and C, the protein expression of
TOP2A in MCF-7 cells was significantly reduced following the
suppression of p-AKT, as compared with the level in the negative
group.
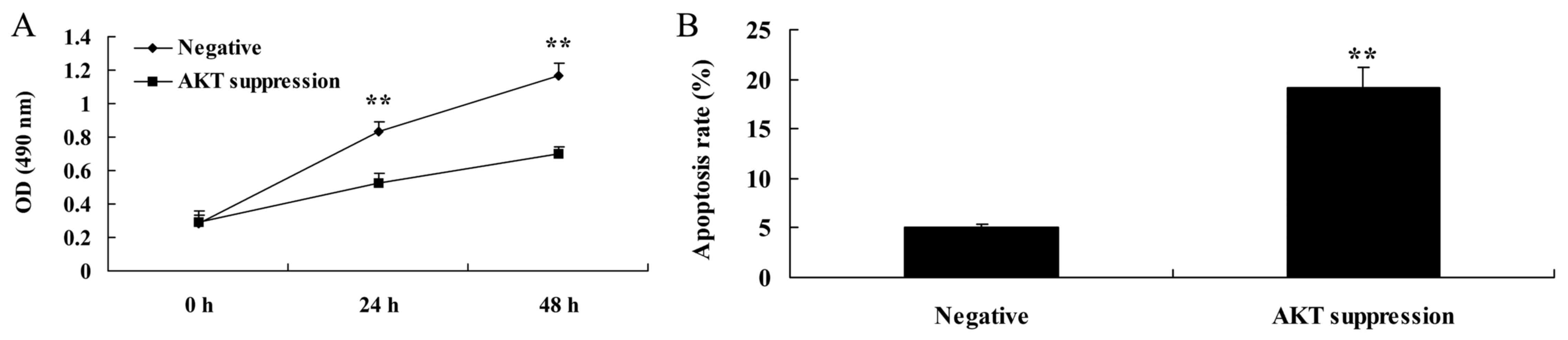
Suppression of p-AKT reduces cell
proliferation and induced apoptosis in MCF-7 cells
In order to further determine whether AKT is
involved in the regulation of MCF-7 cell death, p-AKT inhibitor was
used to suppress the expression of PTEN protein. As shown in
Fig. 9A, the p-AKT inhibitor
significantly decreased the cell viability of the MCF-7 cells in a
time-dependent manner, compared with that noted in the negative
group. As shown in Fig. 9B, MCF-7
cell apoptosis was also induced by the p-AKT inhibitor, compared
with the rate noted in the negative group.
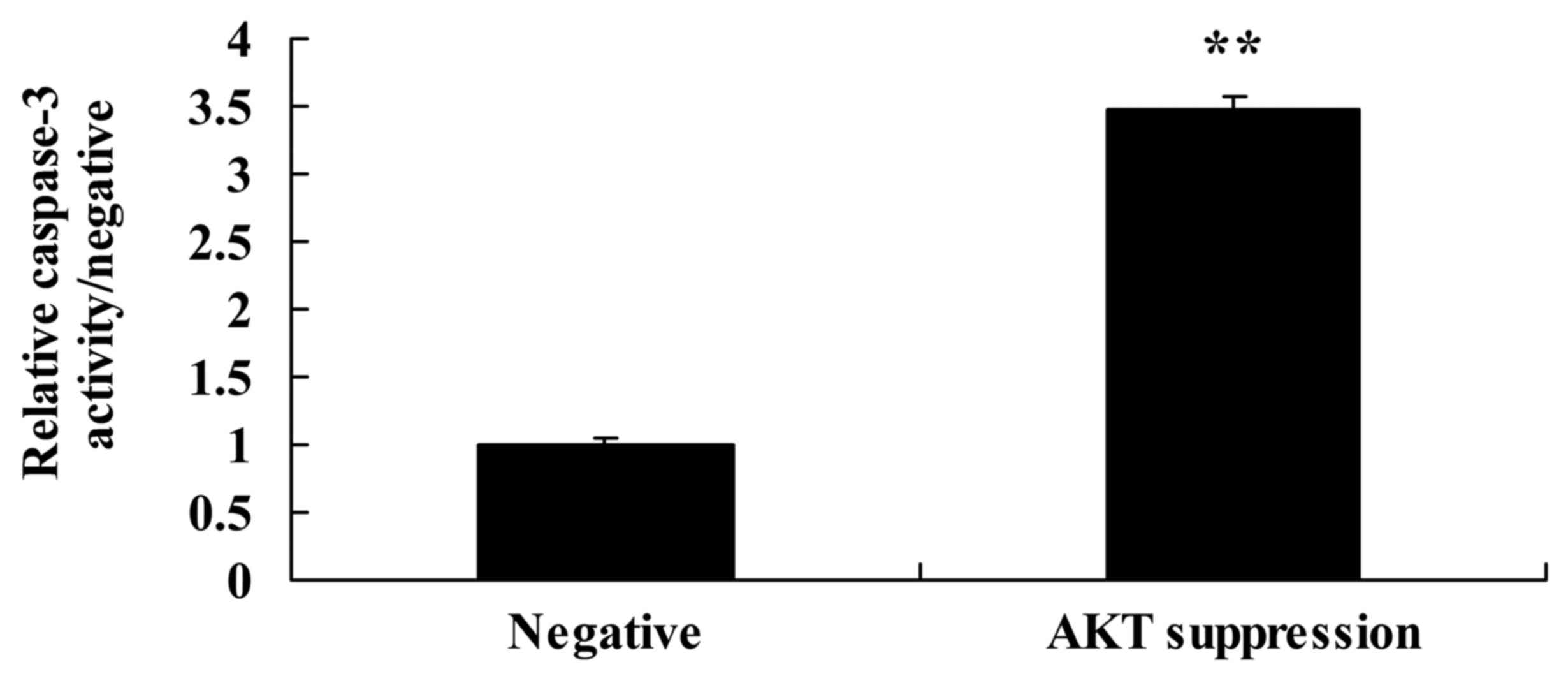
Suppression of p-AKT increases
caspase-3 activity and Bax protein expression in MCF-7 cells
We also demonstrated the effect of the suppression
of p-AKT on caspase-3 activity and Bax protein expression in MCF-7
cells. As shown in Figs. 8B and D,
and 10, the suppression of p-AKT
significantly increased Bax protein expression and caspase-3
activity in the MCF-7 cells, compared with these levels in the
negative group.
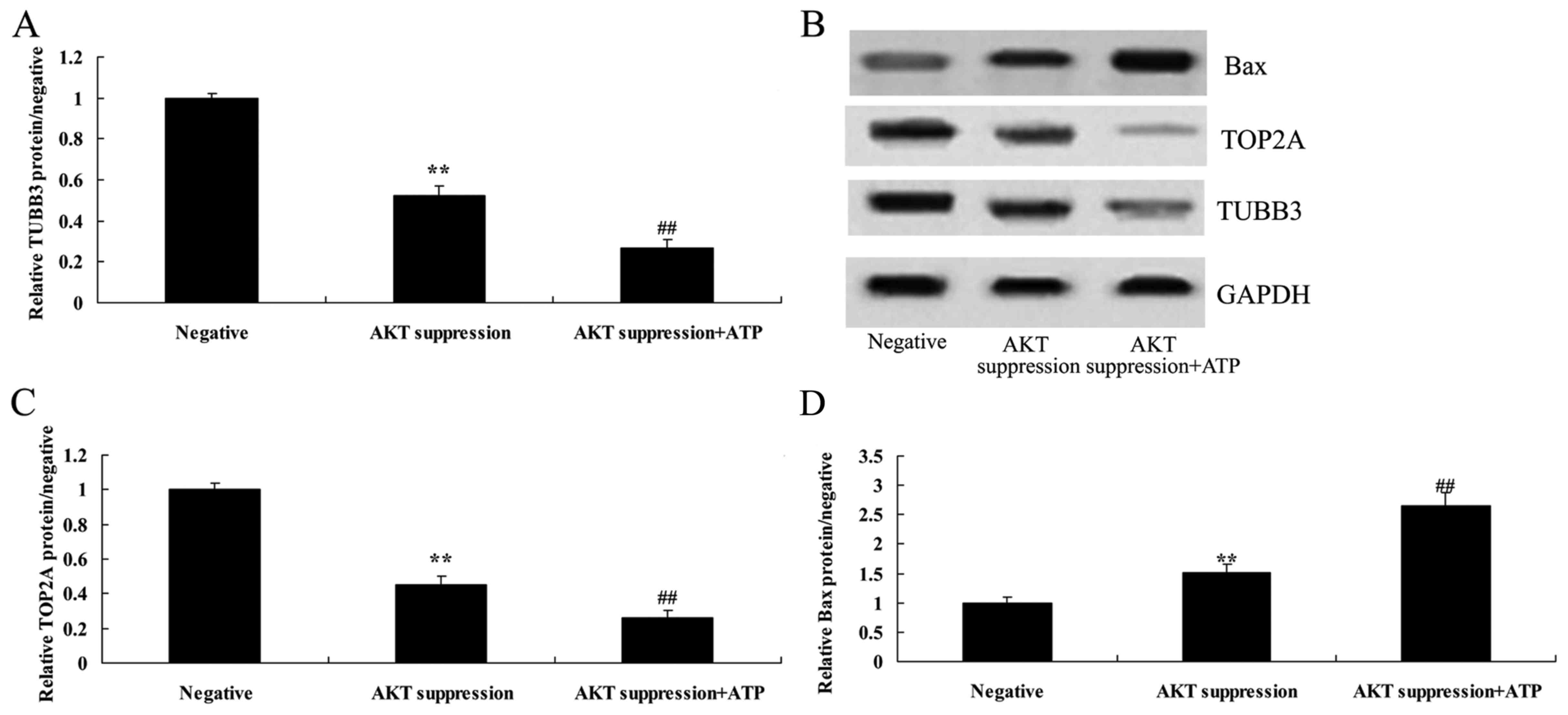
Treatment with ATP inhibits TUBB3
protein expression in MCF-7 cells following suppression of
p-AKT
To further ascertain whether ATP regulates the
effect of p-AKT on TUBB3 expression in MCF-7 cells, ATP (5 µM) was
added to the MCF-7 cells following suppression of p-AKT.
Specifically, ATP significantly inhibited TUBB3 expression in the
MCF-7 cells following suppression of p-AKT, compared with that
noted in the MCF-7 cells following suppression only of p-AKT
(Fig. 11A and B).
Treatment of ATP suppresses TOP2A
protein expression in MCF-7 cells following suppression of
p-AKT
To ascertain whether ATP regulates the effect of
p-AKT on TOP2A expression in MCF-7 cells, ATP (5 µM) was added to
the MCF-7 cells following suppression of p-AKT. Analogously,
treatment with ATP suppressed TOP2A protein expression in the MCF-7
cells following suppression of p-AKT, compared with that noted in
the MCF-7 cells following suppression only of p-AKT (Fig. 11B and C).
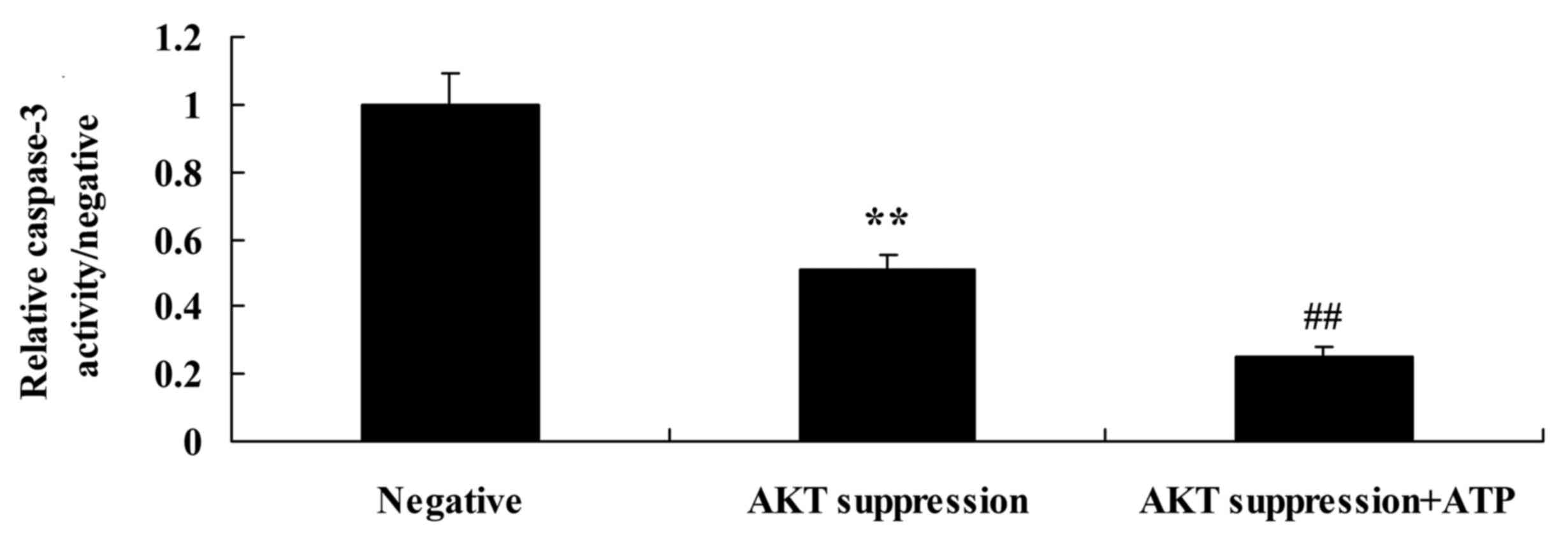
Treatment with ATP decreases caspase-3
activity and increases Bax protein expression in MCF-7 cells
following suppression of p-AKT
To ascertain whether ATP regulates the effect of
PTEN on MCF-7 cell apoptosis following suppression of PTEN, ATP was
added to the MCF-7 cells. We found that treatment with ATP
decreased caspase-3 activity in the MCF-7 cells following
suppression of p-AKT, compared with that in the MCF-7 cells
following suppression only of p-AKT (Fig. 12). Meanwhile, treatment with ATP
decreased Bax protein expression in the MCF-7 cells following
suppression of p-AKT, compared with that noted in the MCF-7 cells
following suppression only of p-AKT (Fig. 11B and D).
Discussion
Breast cancer has become a serious disease that
endangers the health of all women as well as Chinese women,
particularly those in the cities of China (4). During the past 20 years, along with
the development of chemotherapy, hormonal and targeted therapies,
the treatment of breast cancer has significantly improved (15). Cytotoxic chemotherapeutic drugs with
anthracycline as representative have been widely used in breast
cancer; however, in recent years, there are few studies concerning
biomarkers related to the prediction of the outcome of chemotherapy
(16).
Multiple studies have shown that TUBB3 is not only
expressed in normal tissues, but also in cancer tissues (such as
non-small cell lung cancer). However, the amount in adjacent normal
tissues is far less than that in cancerous tissues (17). TUBB3 is mainly expressed in the
nucleus, and immunohistochemistry showing brown granules indicates
positive expression (18). Research
has confirmed the correlation between TUBB3 expression and the
chemosensitivity of taxanes, and TUBB3 has been confirmed as a
prognostic predictor of various solid tumors such as breast,
ovarian, prostate and lung cancer (6). In the present study, the DFS and OS of
human breast cancer patients with TUBB3- or TOP2A-positive tumors
were lower than the DFS and OR of the patients with TUBB3- or
TOP2A-negative tumors.
During the past 10 years, a large number of research
results suggest that HER2-positive breast cancer may be resistant
to CMF and TAM regimens. Compared with the non-anthracycline drugs,
HER2-positive breast cancer is more sensitive to the treatment of
anthracycline-containing drugs. However, recent clinical studies
have shown that, in fact, the predictive value of the efficacy of
anthracycline drugs on HER2-positive breast cancer may be affectd
by the synergistic amplification of the TOP2A gene (19). The TOP2A gene is adjacent to the
HER2 gene, located in the 21 site of the long arm of chromosome 17,
which is expressed as amplification or deletion in breast cancer
with HER2 gene amplification. TOP2A encoded protein-topoisomerase
is a key protease involved in DNA repair, cell cycle regulation and
chromosome division (12).
Topoisomerase 11 is also a target of anthracycline drugs, such as
adriamycin or epirubicin. Therefore, TOP2A is considered to be a
more valuable marker than HER2 for the predication of anthracycline
efficacy. More importantly, we found that the suppression of PTEN
reduced cell proliferation and induced apoptosis and caspase-3
activity, suppressed the protein expression of TUBB3 and TOP2A in
MCF-7 cells through regulation of ATP.
An abnormality in the HER2 signaling pathway is an
important mechanism for the development of breast cancer, in which
the key gene is a predictor for the targeted drug efficacy of
breast cancer (20). Patients with
HER2 overexpression can achieve significant efficacy using
trastuzumab; however, patients with loss of PTEN or PI3K/AKT
mutations show resistance to trastuzumab. The abnormality of the
key genes mentioned above leads to the activation of downstream
signaling of HER2. Although trastuzumab has adequate targets, it
cannot control the abnormal changes in the downstream signal, and
cannot achieve tumor cell proliferation. With the widespread use of
anthracyclines and the gradual increase in patient resistance, it
was found through clinical detection that breast cancer patients
with HER2 overexpression show anthracycline sensitivity, and as
TOP2A and HER2 genes are located on chromosome 17, and very close,
there may be mutual regulation in the coding region (12). Research has demonstrated that HER2
overexpression is also a significant predictor for anthracycline
efficacy (11).
The PTEN/PI3K/Akt signaling pathway exists in a wide
variety of tumor cells, and pathway activation can inhibit
apoptosis to promote cell cycle progression, thereby promoting cell
growth and proliferation. It is also involved in tumor
angiogenesis, which plays an important role in tumor formation, and
in tumor invasion and metastasis (21). PTEN can inhibit tumor formation by
the negative regulation of signaling pathways, and inactivation or
the mutation of the PTEN gene can reduce or lose the inhibitory
effect on the pathway to cause cancer cell growth (22). In the PI3K/AKT signal transduction
process, when PI3K is activated, PIP3 as a second messenger can
activate numerous downstream signaling molecules, thereby resulting
in further signal transduction. The PI3K signaling pathway plays an
important role in many pathophysiological processes such as cell
differentiation, apoptosis, proliferation, migration, vesicular
transport, angiogenesis and cell malignant transformation (23). Our results also suggest that the
suppression of AKT reduced cell proliferation and induced apoptosis
and caspase-3 activity, and suppressed the protein expression of
TUBB3 and TOP2A in MCF-7 cells through regulation of ATP.
In conclusion, the present study also demonstrated
that PTEN/AKT signaling regulates the expression of TUBB3 and TOP2A
and affects the cell growth and apoptosis of human breast cancer
MCF-7 cells through ATP and caspase-3 signaling pathway. Therefore,
this report suggests a potential predictive role of TUBB3 and TOP2A
in therapeutic outcome of human breast cancer to avoid the use of
ineffective therapies.
Acknowledgements
The present study was supported by the Guangdong
Province Science and Technology Plan (2014A020212498).
References
|
1
|
Forbes JF, Sestak I, Howell A, Bonanni B,
Bundred N, Levy C, von Minckwitz G, Eiermann W, Neven P, Stierer M,
et al: IBIS-II investigators: Anastrozole versus tamoxifen for the
prevention of locoregional and contralateral breast cancer in
postmenopausal women with locally excised ductal carcinoma in situ
(IBIS-II DCIS): A double-blind, randomised controlled trial.
Lancet. 387:866–873. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pu Z, Zhang X, Chen Q, Yuan X and Xie H:
Establishment of an expression platform of OATP1B1 388GG and 521CC
genetic polymorphism and the therapeutic effect of tamoxifen in
MCF-7 cells. Oncol Rep. 33:2420–2428. 2015.PubMed/NCBI
|
|
3
|
Aihara T, Yokota I, Hozumi Y, Aogi K,
Iwata H, Tamura M, Fukuuchi A, Makino H, Kim R, Andoh M, et al:
Anastrozole versus tamoxifen as adjuvant therapy for Japanese
postmenopausal patients with hormone-responsive breast cancer:
Efficacy results of long-term follow-up data from the N-SAS BC
03 trial. Breast Cancer Res Treat. 148:337–343. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Xu B, Yuan P, Ma F and Li Q, Zhang
P, Cai R, Fan Y, Luo Y and Li Q: Capecitabine combined with
docetaxel versus vinorelbine followed by capecitabine maintenance
medication for first-line treatment of patients with advanced
breast cancer: phase 3 randomized trial. Cancer. 121:3412–3421.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Witt CM, Außerer O, Baier S, Heidegger H,
Icke K, Mayr O, Mitterer M, Roll S, Spizzo G, Scherer A, et al:
Effectiveness of an additional individualized multi-component
complementary medicine treatment on health-related quality of life
in breast cancer patients: A pragmatic randomized trial. Breast
Cancer Res Treat. 149:449–460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu YC, Zhang FC, Li JJ, Dai JQ, Liu Q,
Tang L, Ma Y, Xu Q, Lin XL, Fan HB, et al: RRM1, TUBB3, TOP2A,
CYP19A1, CYP2D6: Difference between mRNA and protein expression in
predicting prognosis of breast cancer patients. Oncol Rep.
34:1883–1894. 2015.PubMed/NCBI
|
|
7
|
Leng XF, Chen MW, Xian L, Dai L, Ma GY and
Li MH: Combined analysis of mRNA expression of ERCC1, BAG-1, BRCA1,
RRM1 and TUBB3 to predict prognosis in patients with non-small cell
lung cancer who received adjuvant chemotherapy. J Exp Clin Cancer
Res. 31:252012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang ZL, Cao X, Luo RZ, Chen YF, Zhu LC
and Wen Z: Analysis of ERCC1, BRCA1, RRM1 and TUBB3 as predictors
of prognosis in patients with non-small cell lung cancer who
received cisplatin-based adjuvant chemotherapy: A prospective
study. Oncol Lett. 11:299–305. 2016.PubMed/NCBI
|
|
9
|
Narvi E, Jaakkola K, Winsel S,
Oetken-Lindholm C, Halonen P, Kallio L and Kallio MJ: Altered TUBB3
expression contributes to the epothilone response of mitotic cells.
Br J Cancer. 108:82–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huijsmans CJ, van den Brule AJ, Rigter H,
Poodt J, van der Linden JC, Savelkoul PH, Hilbink M and Hermans MH:
Allelic imbalance at the HER2/TOP2A locus in breast cancer. Diagn
Pathol. 10:562015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fasching PA, Weihbrecht S, Haeberle L,
Gasparyan A, Villalobos IE, Ma Y, Ekici AB, Wachter DL, Hartmann A,
Beckmann MW, et al: HER2 and TOP2A amplification in a
hospital-based cohort of breast cancer patients: Associations with
patient and tumor characteristics. Breast Cancer Res Treat.
145:193–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fountzilas G, Valavanis C, Kotoula V,
Eleftheraki AG, Kalogeras KT, Tzaida O, Batistatou A, Kronenwett R,
Wirtz RM, Bobos M, et al: HER2 and TOP2A in high-risk early breast
cancer patients treated with adjuvant epirubicin-based dose-dense
sequential chemotherapy. J Transl Med. 10:102012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li SZ, Qiao SF, Zhang JH and Li K:
Quercetin increase the chemosensitivity of breast cancer cells to
doxorubicin via PTEN/Akt pathway. Anticancer Agents Med Chem.
15:1185–1189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su R, Nan H, Guo H, Ruan Z, Jiang L, Song
Y and Nan K: Associations of components of PTEN/AKT/mTOR pathway
with cancer stem cell markers and prognostic value of these
biomarkers in hepatocellular carcinoma. Hepatol Res. Mar
2–2016.(Epub ahead of print). doi: 10.1111/hepr.12687. View Article : Google Scholar
|
|
15
|
Zheng S, Song QK, Zhao L, Huang R, Sun L,
Li J, Fan JH, Zhang BN, Yang HJ, Xu F, et al: Characteristics of
mammary Paget's disease in China: A national-wide multicenter
retrospective study during 1999–2008. Asian Pac J Cancer Prev.
13:1887–1893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seidman AD, Chan S, Wang J, Zhu C, Xu C
and Xu B: A pooled analysis of gemcitabine plus docetaxel versus
capecitabine plus docetaxel in metastatic breast cancer.
Oncologist. 19:443–452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun S, Shi W, Wu Z, Zhang G, Yang BO and
Jiao S: Prognostic significance of the mRNA expression of ERCC1,
RRM1, TUBB3 and TYMS genes in patients with non-small cell lung
cancer. Exp Ther Med. 10:937–941. 2015.PubMed/NCBI
|
|
18
|
Zou ZQ, Du YY, Sui G and Xu SN: Expression
of TS, RRM1, ERCC1, TUBB3 and STMN1 genes in tissues of non-small
cell lung cancer and its significance in guiding postoperative
adjuvant chemotherapy. Asian Pac J Cancer Prev. 16:3189–3194. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakagawa M, Bando Y, Nagao T, Takai C,
Ohnishi T, Honda J, Moriya T, Izumi K, Takahashi M, Tangoku A, et
al: Among triple-negative breast cancers, HER2(0) breast cancer
shows a strong tendency to be basal-like compared with HER2(1+)
breast cancer: preliminary results. Breast Cancer. 19:54–59. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang F, Lyu S, Dong S, Liu Y, Zhang X and
Wang O: Expression profile analysis of long noncoding RNA in
HER-2-enriched subtype breast cancer by next-generation sequencing
and bioinformatics. Onco Targets Ther. 9:761–772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu X, Tian J, Zhang L, Chen Y and Hao Q:
Involvement of microRNA-93, a new regulator of PTEN/Akt signaling
pathway, in regulation of chemotherapeutic drug cisplatin
chemosensitivity in ovarian cancer cells. FEBS Lett. 586:1279–1286.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shih MC, Chen JY, Wu YC, Jan YH, Yang BM,
Lu PJ, Cheng HC, Huang MS, Yang CJ, Hsiao M, et al: TOPK/PBK
promotes cell migration via modulation of the PI3K/PTEN/AKT pathway
and is associated with poor prognosis in lung cancer. Oncogene.
31:2389–2400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qadir XV, Han C, Lu D, Zhang J and Wu T:
miR-185 inhibits hepatocellular carcinoma growth by targeting the
DNMT1/PTEN/Akt pathway. Am J Pathol. 184:2355–2364. 2014.
View Article : Google Scholar : PubMed/NCBI
|