Introduction
In 1904, the first strain of endophytic fungus was
discovered. Since that date, more and more endophytic fungi have
been isolated from different natural plants including mangroves
(1–5). Research has demonstrated that the
secondary metabolites from endophytic fungi contain multiple
bioactive compounds with novel structures (1–3). The
advantages of endophytic fungi include their short culture cycle,
mild conditions for growth, little exposure to environmental
pollution and easy industrialization. Thus, these fungi can
facilitate the development of traditional pharmaceutical agents.
Therefore, the identification of novel natural drugs from
endophytic fungi living in different environments and ecosystems
offers numerous opportunities.
Mangroves live in high saline and humid
environments. Thus, numerous bioactive compounds can be generated
under these environmental conditions (4,5). A
large number of bioactive compounds have been isolated from
endophytic fungi of mangroves, such as, flavonoids, alkaloids,
terpenes, quinones, cyclic peptide compounds and fatty acids.
Recently, an increased number of novel bioactive compounds have
been obtained from mangrove-derived endophytic fungi, such as,
novel cyclohexenone, cyclopentenone and xanthone derivatives
(6), polyketides (nectriacids A-C
and 12-epicitreoisocoumarinol) (7),
3-epiarigsugacin E (8),
aspergifuranone and isocoumarin derivatives (9), (R)-3-demethyl purpurester A (9), aromatic butyrolactones (flavipesins A
and B) (10) and sulfide
diketopiperazine derivatives (penicibrocazines A-E) (11). Multiple bioactive compounds from
mangrove-derived endophytic fungi have been demonstrated to exhibit
inhibitory activities against acetylcholinesterase (AchE) (8), α-glucosidase (9), bacteria (10–12)
and viruses (13). Particularly,
accumulating evidence indicates that various bioactive compounds
from mangrove-derived endophytic fungi display antitumor activities
(13–18). Furthermore, studies have reported
the underlying mechanisms of the antitumor effects of
mangrove-derived endophytic fungi (19–24).
An anthraquinone compound G503, isolated from the secondary
metabolites of the mangrove endophytic fungus Nigrospora sp.
(no. 2508), was reported to induce apoptosis in gastric cancer
SGC7901 cells through the mitochondrial pathway (19). Xyloketal B, a marine compound
obtained from the mangrove fungus Xylaria sp. (no. 2508)
from the South China Sea, was demonstrated to suppress the
proliferation and migration of glioblastoma U251 cells by
inhibiting the TRPM7-mediated PI3K/Akt and MEK/ERK signaling
pathways (20). The marine
anthraquinone derivative SZ-685C, isolated from the secondary
metabolites of the mangrove endophytic fungus Halorosellinia
sp. (no. 1403), was found to induce apoptosis in primary human
non-functioning pituitary adenoma (21), breast cancer (22) and human nasopharyngeal carcinoma
(NPC) cells (23), and reverse the
adriamycin-resistance in breast cancer cells (24) by the inhibition of the Akt pathway.
Taken together, these findings indicate that the bioactive
compounds from mangrove-derived endophytic fungi can inhibit cancer
cell growth via the induction of apoptosis and the inhibition of
the Akt signaling pathway. However, the effect of mangrove-derived
endophytic fungi on cancer angiogenesis has not yet been
reported.
Angiogenesis, the development of new microvascular
networks, is required for cancer invasion and metastasis and plays
a key role in controlling the development and progression of a
variety of cancers (25). The
inhibition of cancer angiogenesis can suppress the development and
progression of cancers. Therefore, the screening of angiogenic
inhibitors from mangrove-derived endophytic fungi is extremely
important for identifying new chemotherapeutic drugs for the
prevention and treatment of cancers. There are abundant resources
of mangroves in Zhanjiang. Therefore, the present study was to
isolate, purify and identify endophytic fungi from Zhanjiang
mangroves and explore their effects on the growth and angiogenesis
of lung cancer cells.
In the present study, we isolated, purified and
identified 28 strains of endophytic fungi from three types of
mangrove plants, namely Kandelia candel, Rhizophora
stylosa and Rhizophoraceae, and 10 strains of endophytic
fungi significantly suppressed the growth of lung cancer cell
lines, A549 and NCI-H460. Furthermore, to the best of our
knowledge, we demonstrated for the first time, that three strains
of endophytic fungi markedly inhibited lung cancer angiogenesis
in vitro.
Materials and methods
Reagents
Glucose was purchased from the Tianjin Fuchen
Chemical Reagents Factory (Tianjin, China). Potato dextrose agar
(PDA) was obtained from Hangzhou Microbial Reagent Co., Ltd.
(Hangzhou, China). Tryptone and yeast extract reagents were
purchased from Oxoid Ltd. (Basingstoke, Hampshire, UK).
Transfection reagent (Lipofectamine™ 2000) was obtained from
Invitrogen Corp. (Carlsbad, CA, USA). In vitro angiogenesis
assay kit (ECM625) was obtained from Millipore (Temecula, CA, USA).
Fungus genomic DNA extraction kit was purchased from Bioer
Technology Co., Ltd. (Tokyo, Japan). MTT kit was purchased from
Beyotime Biotechnology (Shanghai, China).
Collection of mangrove plants
The healthy leaves, roots and stems of mangroves
(Kandelia candel, Rhizophora stylosa and
Rhizophoraceae) were collected from Haibin Park and Gaoqiao
National Mangrove Nature Reserve (Zhanjiang, Guangdong, China). The
collected leaves, roots and stems of the mangroves were washed for
2 h under running tap water and were cut into ~0.5 × 0.5 cm pieces
within 72 h after collection. The surface of the fragments was
sterilized by sequential immersion in 75% ethanol
(C2H5OH) for 45 sec and 5% sodium
hypochlorite (NaClO) for different times (leaves for 3 min, roots
for 10 min and stems for 5 min), followed by washing four to five
times with sterile distilled water.
Isolation, purification and culture of
the endophytic fungi
The sterilized fragments of mangroves were dried
under sterile conditions. The dried fragments were cultured in
plates with PDA medium (potato extract 10.0 g/l, glucose 20.0 g/l,
agar 13.0 g/l and chloramphenicol 0.1 g/l) at 28°C, and the growth
of the endophytic fungal colonies from the mangrove fragments was
monitored every day. The fungal colonies which grew out from the
mangrove fragments were isolated and transferred to other plates
with PDA medium for purification. The purified endophytic fungal
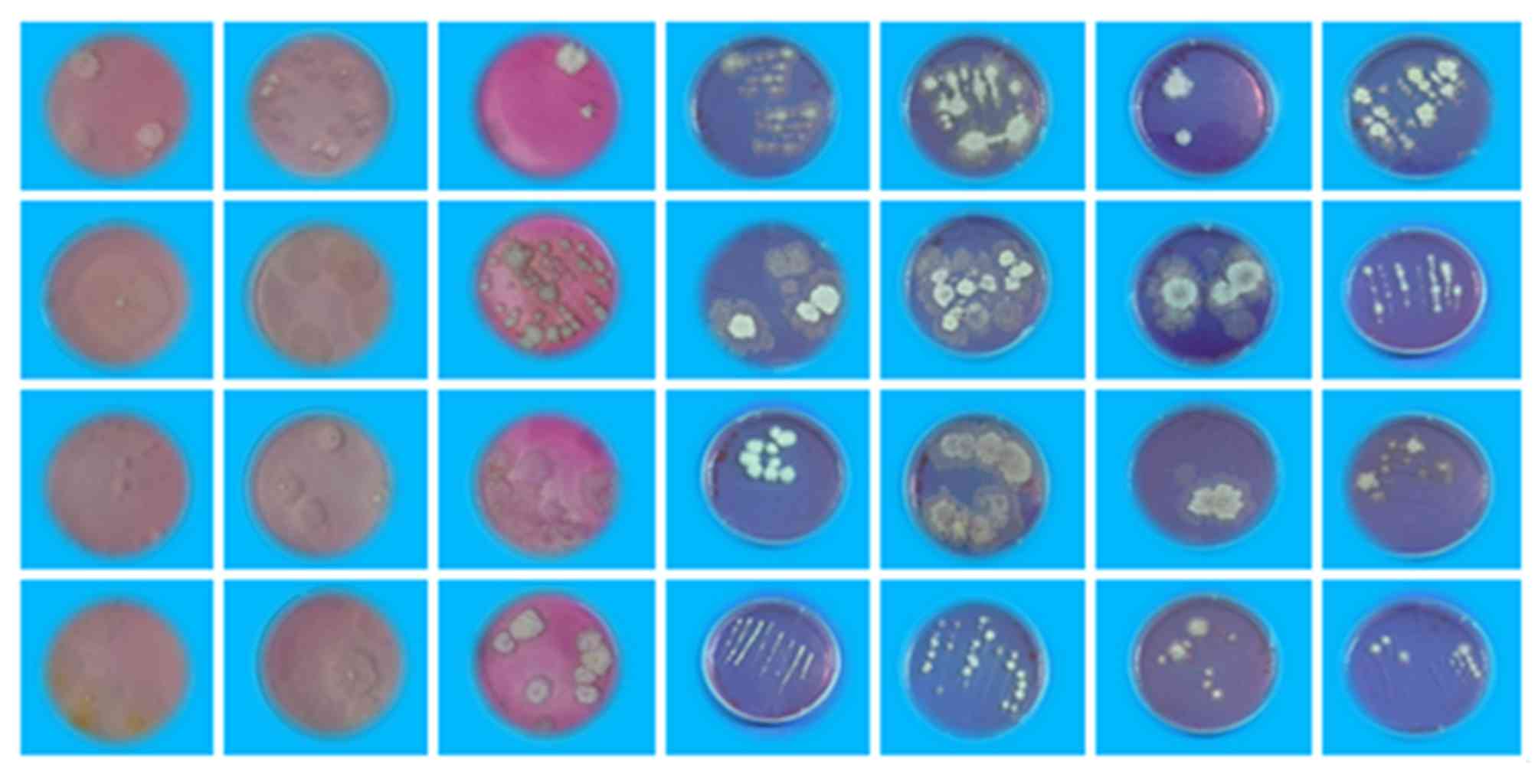
colonies were photographed.
Next, the purified endophytic fungal colonies were
fermented at 28°C in glucose peptone yeast (GPY) extract broth
(tryptone 2.0 g/l, yeast extract 1.0 g/l, glucose 10.0 g/l, and sea
salt 20.0 g/l) in a shaking incubator (160 rpm) at 28°C in the
dark. Seven days later, fungus culture media were filtered using
nylon nets to separate the mycelia and the culture broth. Mycelia
were identified by molecular analysis of the internal transcribed
spacer (ITS) of the genomic DNA. The culture broths were sterilized
by filtration through a 0.22-µm Millipore filter, and the filtrates
were used for MTT and in vitro angiogenesis assays.
Molecular identification of the
endophytic fungi
Genomic DNA was extracted from the separated mycelia
according to the manufacturer's instructions (Bioer Technology Co.,
Ltd.). 18S rDNA fragments were amplified by PCR methods with
universal primers. PCR primers used were: 5′-TCCGTAGGTGAACCTGCGG-3′
(forward) and 5′-TCCTCCGCTTATTGATATGC-3′ (reverse) (GenBank,
NM_006486.2). The primers were synthesized by Sangon Biotech Co.,
Ltd. (Shanghai, China). The thermocycling conditions were as
follows: 94°C for 5 min, followed by 35 cycles at 94°C for 45 sec,
55°C for 45 sec, and 72°C for 60 sec, finally 72°C for 7 min. The
PCR products were detected by 1.5% agarose gel electrophoresis and
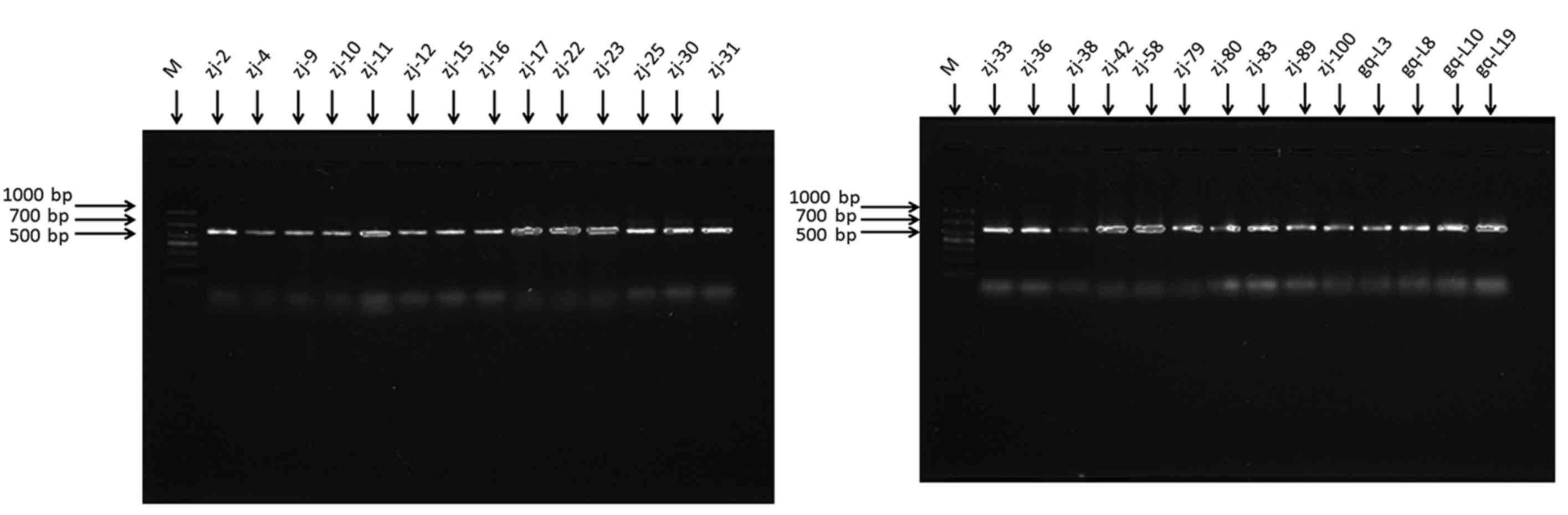
DNA sequencing. The results of agarose gel electrophoresis were
photographed. DNA sequences were analyzed by Sangon Biotech Co.,
Ltd. 18S rDNA fragment sequences of the isolated endophytic fungi
were compared with those in the GenBank database using BLAST at the
National Center for Biotechnology Information (NCBI; Bethesda, MD,
USA), and endophytic fungi were classified by morphologic traits
and molecular identification. The phylogenetic trees were
constructed by Mega 5.0 software.
Cell culture
Human lung adenocarcinoma cell line A549 and human
umbilical vein endothelial cells (HUVECs) were purchased from the
American Type Culture Collection (ATCC; Rockville, MD, USA). Human
lung cancer cell line NCI-H460 was obtained from the Chinese
Academy of Sciences Cell Bank of Type Culture Collection (Shanghai,
China). A549 and NCI-H460 cells were cultured in RPMI-1640 medium
containing 10% fetal bovine serum (FBS). HUVEC cells were grown in
DEME media containing 10% FBS. All cells were maintained in a 5%
CO2 incubator at 37°C.
Transient transfection
Transient transfection was carried out according to
a previously described method (26,27).
The plasmid (p-EGFP-N1-HPV-16 E7), constructed by our laboratory,
was transiently transfected into A549 and NCI-H460 cells using
Lipofectamine™ 2000 according to the manufacturer's instructions,
wherein transfection with the empty vector (p-EGFP-N1) served as
the negative controls. The cells exposed to transfection reagent
alone served as mock transfection controls. The transfection
efficiency was evaluated by observing green fluorescence under a
fluorescence microscope, and the expression of HPV-16 E7
oncoprotein was confirmed in our previous studies (26,27).
MTT assay
The MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
assay was performed to determine the effects of endophytic fungi on
the growth of A549 and H460 cells. A cell suspension was added to
96-well plates at the density of 5×104/ml (100 µl/well),
and cultured in a CO2 incubator overnight at 37°C.
Afterwards, the culture medium was replaced with fresh medium, and
20 µl culture broth of the different endophytic fungi was added
into the wells for culture at 37°C for 72 h. Each culture broth of
the endophytic fungi was repeated four times. Seventy-two hours
later, the supernatant was removed, followed by addition of 20 µl
MTT [5 mg/ml in phosphate-buffered saline (PBS)] into each well,
and the cells were incubated for an additional 4 h. After removing
the supernatant, 150 µl dimethyl sulfoxide (DMSO) was added into
each well. The cells were incubated for 10 min at room temperature
in order to fully dissolve the formed crystals. Absorbance values
at 595 nm were measured using a microplate reader and cell
viability was calculated.
In vitro angiogenesis assay
An in vitro angiogenesis assay kit (ECM625)
was employed to analyze the formation of capillary tube-like
structures according to the manufacturer's instructions. Briefly,
HUVECs were seeded at a density of 5×103 cells/well onto
the surface of 96-well cell culture plates pre-coated with
polymerized ECMatrix™. Subsequently, the conditioned media, derived
from HPV-16 E7-transfected A549 or NCI-H460 cells treated with 20
µl culture broth of the different endophytic fungi, were
respectively added into different wells. Tubule formation was
observed under a phase-contrast microscope, and Scion image
software was used to analyze the total tube length in three random
view fields/well, and the average value was calculated. The
experiment was repeated in triplicate.
Statistical analysis
The experiment was repeated at least three times.
One way ANOVA and LSD were employed for statistical analysis using
SPSS 19.0. P<0.05 was considered to indicate a statistically
significant result.
Results
Results of the isolation, purification
and identification of the endophyte fungi
Sixty-two strains of endophytic fungi were isolated
from three types of mangrove plants (Kandelia candel,
Rhizophora stylosa and Rhizophoraceae) in the
Zhanjiang region. The number of endophytic fungus strains isolated
from Kandelia candel, Rhizophora stylosa and
Rhizophoraceae was 26, 20 and 16, respectively. After
sequencing, 34 strains of endophytic fungi were found to have the
same sequences. After removal of the repeated ones, 28 different
strains of endophytic fungi were successfully isolated in the
present study (Fig. 1). To further
identify the 28 strains of endophytic fungi, the 18S rDNA was
amplified by PCR. The results from agarose gel electrophoresis of
the PCR products are shown in Fig.
2. As shown in Fig. 2, the size
of the PCR products was from 500 to 750 bp, indicating that the 28
strains belonged to fungi. Compared with the sequences in the
GenBank database, 28 different strains of endophytic fungi were
successfully identified (Table I).
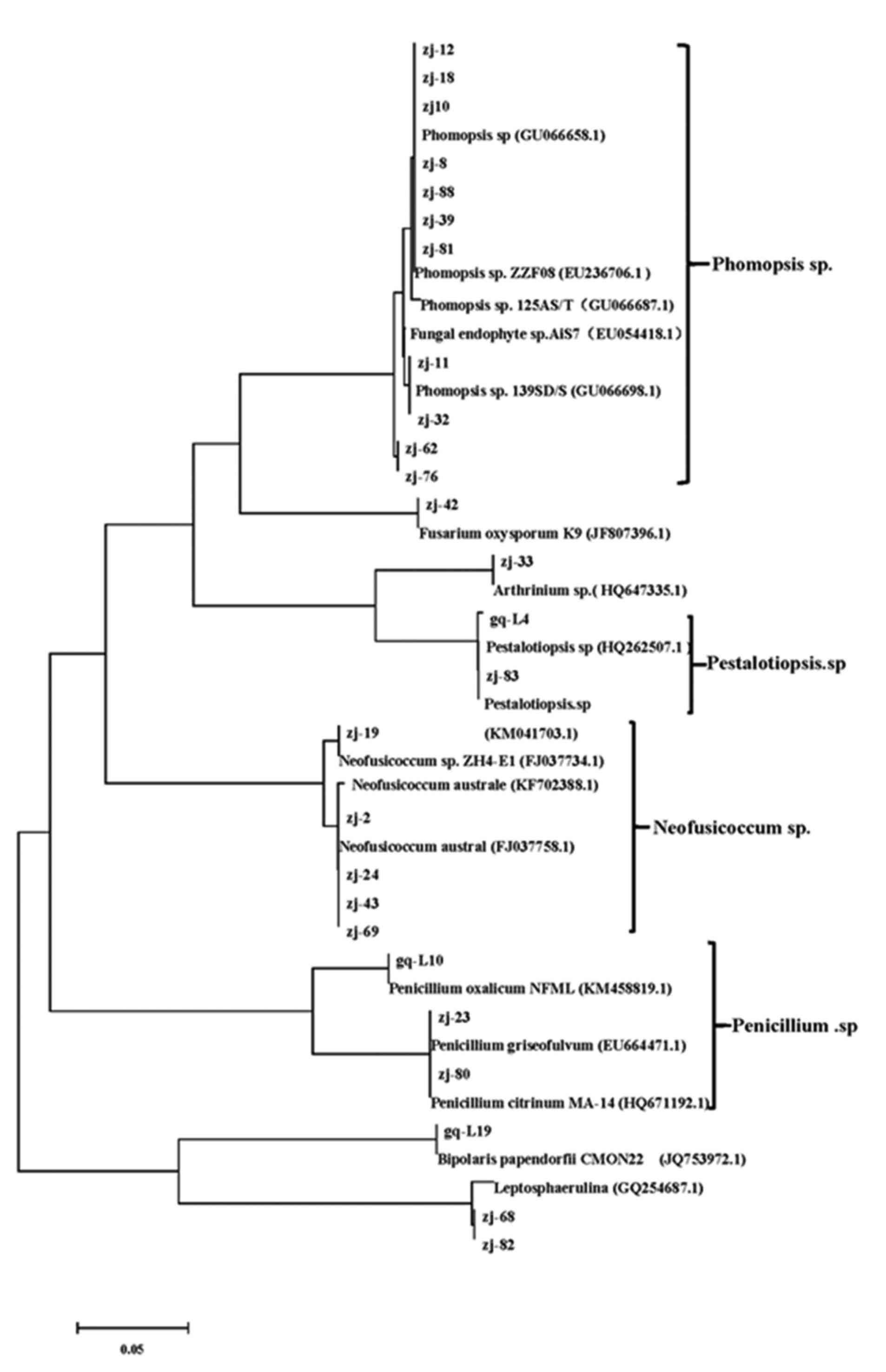
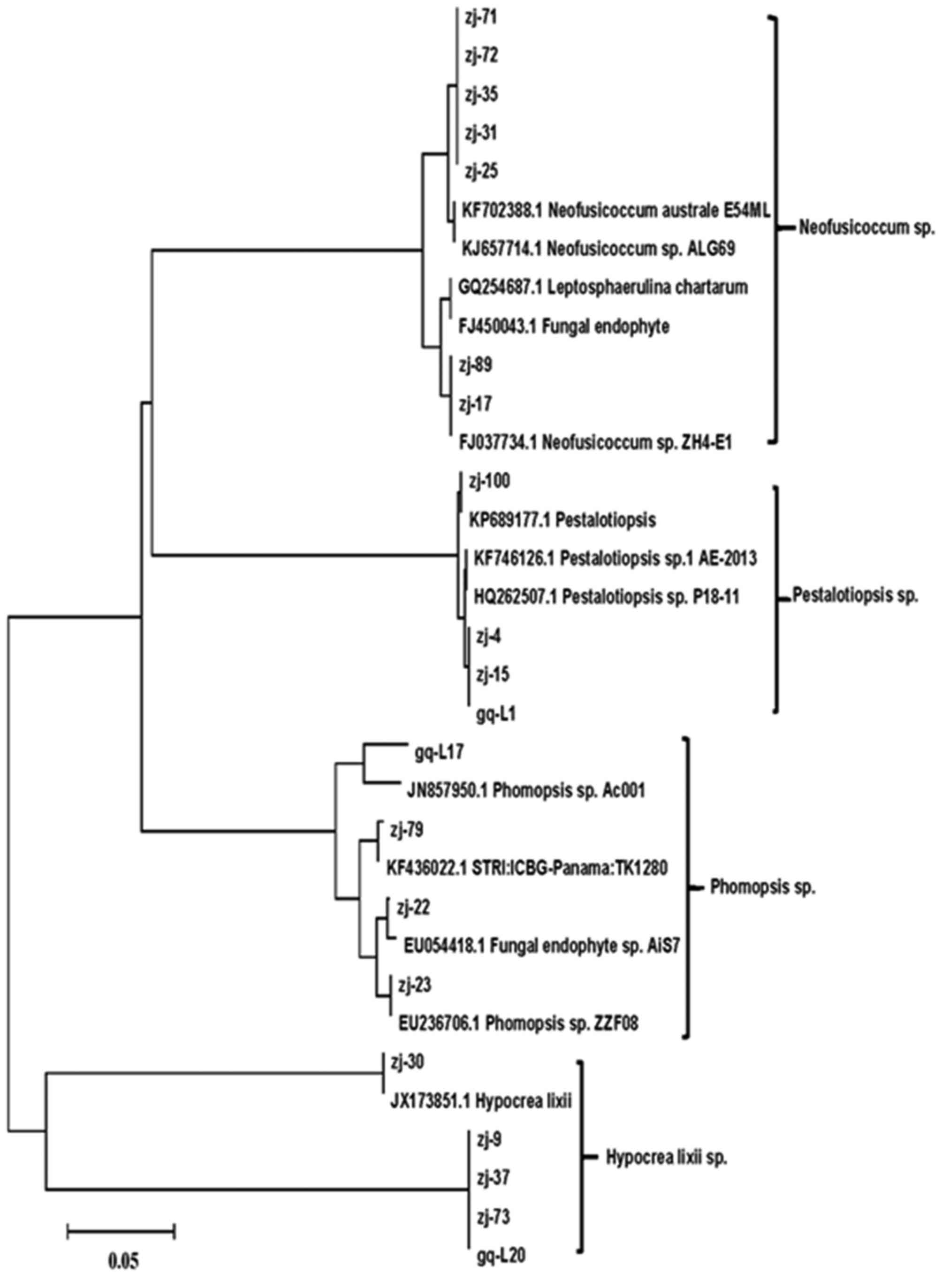
Next, the phylogenetic trees were constructed using Mega 5.0
software. The phylogenetic trees of three types of mangroves,
Kandelia candel, Rhizophora stylosa, and
Rhizophoraceae, are shown in Figs. 3–5,
respectively.
 | Table I.Results of the identification of
isolated endophytic fungi from mangrove plants. |
Table I.
Results of the identification of
isolated endophytic fungi from mangrove plants.
| No. | Strain code | Similar
strains | Sources of similar
strains | Rates of similarity
(%) | Results of
identification | No. | Strain code | Similar
strains | Sources of similar
strains | Rates of similarity
(%) | Results of
identification |
|---|
| 1 | zj-2 | Neofusicoccum
austral | Mangrove | 100 | Neofusicoccum
sp. | 15 | zj-55 | Phomopsis sp.
125AS/T | Annona squamosa
stem | 99 | Phomopsis sp. |
|
| zj-85 | (FJ037758.1) |
|
|
|
| zj-10 | (GU066687.1) |
|
|
|
|
| zj-101 |
|
|
|
|
| zj-56 |
|
|
|
|
|
|
|
|
|
|
|
| zj-88 |
|
|
|
|
|
|
|
|
|
|
|
| zj-70 |
|
|
|
|
| 2 | zj-16 | Neofusicoccum
austral | Myrtus
communis | 99 | Neofusicoccum
sp. | 16 | zj-11 | Phomopsis
sp.139SD/S | Spondias
dulcis seed | 99 | Phomopsis sp. |
|
| zj-20 | E54ML
(KF702388.1) |
|
|
|
| zj-43 | (GU066698.1) |
|
|
|
|
| zj-35 |
|
|
|
|
|
|
|
|
|
|
|
| zj-14 |
|
|
|
|
|
|
|
|
|
| zj-67 |
|
|
|
|
|
|
|
|
|
|
| 3 | zj-17 | Neofusicoccum sp.
ZH4-E1 | Mangrove | 99 | Neofusicoccum
sp. | 17 | gq-L12 | Phomopsis sp.
ZZF08 | Excoecaria
agallocha | 99 | Phomopsis sp. |
|
| zj-19 | (FJ037734.1) |
|
|
|
| zj-23 | (EU236706.10) |
|
|
|
|
| zj-77 |
|
|
|
|
| zj-81 |
|
|
|
|
| 4 | zj-31 | Neofusicoccum
sp. | Unknown | 99 | Neofusicoccum
sp. | 18 | gq-L17 | Phomopsis sp. | Cocos
nucifera | 95 | Phomopsis sp |
|
|
| ALG69
(KJ657714.1) |
|
|
|
| zj-36 | Ac001
(JN857950.1) | flower |
| 5 | zj-89 | Neofusicoccum
parvum | Unknown | 99 | Neofusicoccum
sp. | 19 | zj-58 | Phomopsis sp.
20SO/L |
Saccharum | 99 | Phomopsis sp. |
|
| zj-93 | UY754
(EU080926.1) |
|
|
|
|
| (GQ407098.1) | officinarum
leaf |
| 6 | zj-25 | Penicillium
griseofulvu | Mangrove | 99 | Penicillium
sp. | 20 | zj-9 |
Leptosphaerulina | Unknown | 96 |
Leptosphaerulina |
|
| zj-74 | 091402
(EU664471.1) |
|
|
|
| zj-37 |
chartarum |
|
| zj-86 |
|
|
|
|
| zj-82 | (GQ254687.1) |
|
|
|
|
|
|
|
| zj-29 |
| 7 | gq-L10 | Penicillium
oxalicum | Unknown | 99 | Penicillium
sp. | 21 | zj-22 | Fungal endophyte
sp. | Unknown | 99 | Fungal endophyte
sp. |
|
|
| NFML_CH42_88 |
|
|
|
| zj-32 |
AiS7(EU054418.1) |
|
|
| (KM458819.1) |
| 8 | zj-80 | Penicillium
citrinum MA-14 | Soil | 99 | Penicillium | 22 | zj-38 | Fungal sp.
NIS3 | Tectona
grandis bark | 91 | Fungal sp. |
|
|
| (HQ671192.1) |
|
|
citrinum |
| zj-61 | (KF910769.1) |
| 9 | zj-4 | Pestalotiopsis sp.
1 AE-2013 |
Bradypus | 99 | Pestalotiopsis
sp. | 23 | gq-L8 |
Trichoderma | Ginger | 99 |
Trichoderma |
|
| zj-45 | F4875
(KF746126.1) |
variegatus |
|
|
|
| CHR2FC55 | Rhizosphere
soil |
|
|
|
|
|
|
|
|
| (KJ591703.1) |
| 10 | zj-48 | Pestalotiopsis
FL21 | Huperzia
serrata | 100 | Pestalotiopsis
sp. | 24 | zj-30 | Hypocrea
lixii SZMC | Unknown | 99 | Hypocrea
lixii |
|
| zj-100 | (KP689177.1) |
|
|
|
| zj-98 | 20858
(JX173851.1) |
| 11 | gq-L1 | Pestalotiopsis sp.
P18-11 | Unknown | 99 | Pestalotiopsis
sp. | 25 | zj-33 | Arthrinium
sp. 7 | Bamboo | 99 | Arthrinium
sp. |
|
| zj-15 | (HQ262507.1) |
|
|
|
|
| (HQ647335.1) |
| 12 | gq-L5 | Pestalotiopsis sp.
S149/2013 | Mushroom | 99 | Pestalotiopsis
sp. | 26 | gq-L19 | Bipolaris
papendorfii | Phaseolus
vulgaris | 99 |
Bipolaris |
|
| gq-L3 | (KM041695.1) |
|
|
|
|
| CMON22
(JQ753972.1) |
| 13 | zj-50 | Pestalotiopsis
DBT179/2013c2 | Mushroom | 99 | Pestalotiopsis | 27 | zj-42 | Fusarium
oxysporum | Pigeon
pea | 99 | Fusarium
oxysporum |
|
| zj-83 | (KM041703.1) |
|
|
|
| zj-68 | K9
(JF807396.1) |
| 14 | zj-39 | Phomopsis sp.
89CN/F | Cocos
nucifera | 99 | Phomopsis sp. | 28 | zj-79 |
STRI:ICBG-Panama: |
Saccharum | 99 | Unknown |
|
| zj-87 | (GU066658.1) | flower |
|
|
|
| TK1280
(KF436022.1) |
|
| zj-12 |
|
| zj-64 |
Results of the MTT assay
To assess the antitumor activities of the 28
isolated strains of endophytic fungi, MTT assay was performed to
observe the effects of these fungi on the growth of human lung
cancer cells, A549 and NCI-H460. The results from the MTT assay are
shown in Table II. As shown in
Table II, 10 strains of endophytic
fungi, including 4 Neofusicoccum sp. strains (zj-2, zj14,
zj-17 and zj-67), 4 Phomopsis sp. strains (zj-12, zj-23,
zj-36 and zj-70), 1 Leptosphaerulina sp. strain (zj-9) and 1
Penicillium sp. strain (zj-25), significantly inhibited the
growth of lung cancer A549 and NCI-H460 cells. The average
inhibitory rates of 10 strains in the A549 cells were 64.4, 59.5,
81.9, 43.9, 58.3, 56.2, 48.3, 42.4, 93.0 and 49.7%, respectively.
The average inhibitory rates of 10 strains in the NCI-H460 cells
were 41.2, 49.3, 82.7, 40.7, 53.9, 52.6, 56.8, 64.3%, 91.0 and
45.6%, respectively. The zj-9 and zj-17 strains showed a stronger
inhibitory effects on both the A549 and NCI-H460 lung cancer cell
lines. Particularly, zj-9 exhibited the strongest growth inhibitory
activity in the two lung cancer cell lines.
 | Table II.Results from the MTT assay. |
Table II.
Results from the MTT assay.
| No. | Strain code | Average Average
inhibitory rates in A549 cells (%) | Average Average
inhibitory rates in NCI-H460 cells (%) | No. | Strain code | Average Average
inhibitory rates in A549 cells (%) | Average Average
inhibitory rates in NCI-H460 cells (%) |
|---|
| 1 | zj-2 | 64.4 | 41.2 | 15 | zj-55 | 9.4 | 0.14 |
|
| zj-85 |
|
|
| zj-10 |
|
| zj-101 |
|
|
| zj-56 |
|
|
|
|
|
| zj-88 |
|
|
|
|
|
| zj-70 |
| 2 | zj-16 | 56.9 | 14.4 | 16 | zj-11 | 18.9 | 10.2 |
|
| zj-20 |
|
|
| zj-43 |
|
| zj-35 |
|
| zj-14 |
|
| zj-67 |
| 3 | zj-17 | 81.9 | 82.7 | 17 | gq-L12 | 16.0 | 37.2 |
|
| zj-19 |
|
|
| zj-23 |
|
| zj-77 |
|
|
| zj-81 |
| 4 | zj-31 | 43.6 | 13.7 | 18 | gq-L17 | 48.3 | 56.8 |
|
|
|
|
|
| zj-36 |
| 5 | zj-89 | 20.2 | 39.9 | 19 | zj-58 | 16.0 | 39.4 |
|
| zj-93 |
| 6 | zj-25 | 49.7 | 45.6 | 20 | zj-9 | 93.0 | 91.0 |
|
| zj-74 |
|
|
| zj-37 |
|
| zj-86 |
|
|
| zj-82 |
|
|
|
|
|
| zj-29 |
| 7 | gq-L10 | 42.4 | 64.3 | 21 | zj-22 | 47.7 | 32.1 |
|
|
|
|
|
| zj-32 |
| 8 | zj-80 | 7.6 | 11.5 | 22 | zj-38 | 37.4 | 36.0 |
|
|
|
|
|
| zj-61 |
| 9 | zj-4 | 39.7 | 54.8 | 23 | gq-L8 | 15.1 | 17.9 |
|
| zj-45 |
| 10 | zj-48 | 6.8 | 4.9 | 24 | zj-30 | 64.9 | 7.5 |
|
| zj-100 |
|
|
| zj-98 |
| 11 | gq-L1 | 4.6 | 11.7 | 25 | zj-33 | 1.7 | 4.1 |
|
| zj-15 |
| 12 | gq-L5 | 15.4 | 37.7 | 26 | gq-L19 | 17.0 | 9.7 |
|
| gq-L3 |
| 13 | zj-50 | 17.4 | 24.2 | 27 | zj-42 | 13.4 | 17.3 |
|
| zj-83 |
|
|
| zj-68 |
| 14 | zj-39 | 21.4 | 47.7 | 28 | zj-79 | 11.4 | 16.7 |
|
| zj-87 |
|
| zj-12 |
|
| zj-64 |
Results of the in vitro angiogenesis
assay
To further explore the underlying mechanism of the
antitumor activity of the endophytic fungi, an in vitro
angiogenesis assay was performed to observe the effects of
endophytic fungi on the inhibition of lung cancer angiogenesis. In
our previous studies, we found that human papillomavirus (HPV) type
16 E7 (HPV-16 E7) oncoprotein significantly enhanced lung cancer
cell angiogenesis in vitro (26,27).
Therefore, in the present study, we established an in vitro
angiogenesis model induced by HPV-16 E7 oncoprotein. As shown in
Fig. 6, HPV-16 E7 oncoprotein
markedly stimulated microtubule formation (Fig. 6, image 2) as compared with the empty
vector control (Fig. 6, image 1),
which was in accordance with our previous studies (26,27),
indicating that the model was successfully established. In the
present study, we further found that endophytic fungi zj-14 (image
6), zj-17 (image 7) and zj-36 (image 10) markedly inhibited HPV-16
E7-stimulated microtubule formation (Fig. 6; P<0.01) in both A549 (Fig. 6A) and NCI-H460 (Fig. 6C) cells, which was further confirmed
by total tube length (Fig. 6B and
D; P<0.01). Additionally, endophytic fungus zj-23 (lane 8)
inhibited HPV-16 E7-stimulated microtubule formation in the A549
cells (Fig. 6A and B; P<0.01)
and endophytic fungus zj-12 (lane 5) inhibited HPV-16 E7-stimulated
microtubule formation in the NCI-H460 cells (Fig. 6C and D; P<0.05).
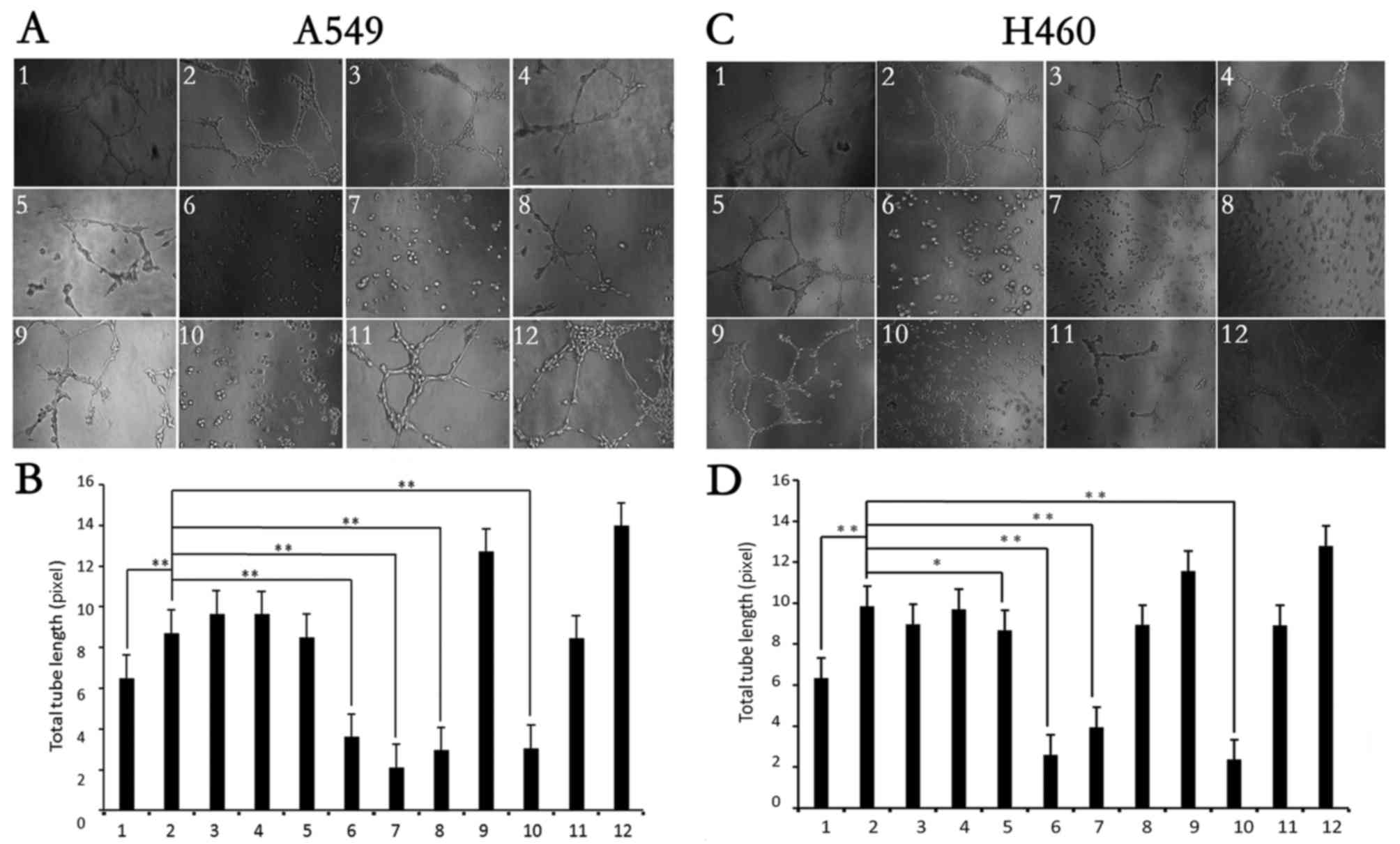 | Figure 6.Effects of endophytic fungal
fermentation on HPV-16 E7-stimulated lung cancer angiogenesis in
vitro. HUVECs were incubated at 37°C for 6–8 h in the
conditioned media derived from HPV-16 E7-transfected A549 or
NCI-H460 cells treated with different culture broths of endophytic
fungi (zj-2, zj-9, zj-12, zj-14, zj-17, zj-23, zj-25, zj-36, zj-67
and zj-70). (A and C) Tube formation was observed under a
phase-contrast microscope (magnification, ×200). (B and D) The
total tube length in three random view-fields/well was determined
using Scion image software, and the average value was calculated.
Lane 1, empty vector transfection control; lane 2, HPV-16 E7
transfection control (abbreviation, E7); lane 3, zj-2 + E7; lane 4,
zj-9 + E7; lane 5, zj-12 + E7; lane 6, zj-14 + E7; lane 7, zj-17 +
E7; lane 8, zj-23 + E7; lane 9, zj-25 + E7; lane 10, zj-36 + E7;
lane 11, zj-67 + E7; lane 12. zj-70 + E7. All data are expressed as
mean ± SD of three independent experiments; *P<0.05,
**P<0.01. |
Discussion
In the present study, we isolated 26, 20 and 16
strains of endophytic fungi from three types of mangrove plants,
Kandelia candel, Rhizophora stylosa and
Rhizophoraceae, respectively. There are a variety of
endophytic fungi that may be isolated from one type of mangrove
plant, but one to three endophytic fungi are dominant. We found
that the dominant endophytic fungi of Kandelia candel,
Rhizophora stylosa and Rhizophoraceae were
Pestalotiopsis sp. (42.3%), Pestalotiopsis sp. (20%)
and Phomopsis sp. (43.8%), respectively. Notably,
Neofusicoccum sp. (31.3%) was also found to be a major
advantage endophytic fungi isolated from Rhizophoraceae in
addition to Phomopsis sp.
A growing body of evidence indicates that multiple
bioactive compounds isolated from mangrove-derived endophytic fungi
inhibit the growth of cancer cells (15–18). A
new sesquiterpene named botryosphaerin F from the mangrove fungus
Aspergillus terreus (no. GX7-3B) was reported to inhibit the
growth of human breast cancer MCF-7 and leukemia HL-60 cells with
IC50 values of 4.49 and 3.43 µM, respectively (15). Five highly oxygenated chromones,
rhytidchromones A-E, were isolated from the culture broth of a
mangrove-derived endophytic fungus, Rhytidhysteron rufulum,
and all compounds, except for rhytidchromone D, displayed
cytotoxicity against Kato-3 cancer cells with IC50
values ranging from 16.0 to 23.3 µM, while rhytidchromones A and C
showed activity against breast cancer MCF-7 cells with
IC50 values of 19.3 and 17.7 µM, respectively (16). Four new lasiodiplodins (1–4) were
isolated from a mangrove endophytic fungus, Lasiodiplodia
sp. 318#., and compound 4 exhibited moderate cytotoxic activities
against human cancer lines THP1, MDA-MB-435, A549, HepG2 and
HCT-116 (17). Two of the three new
resveratrol derivatives, namely resveratrodehydes, isolated from
the mangrove endophytic fungus Alternaria sp. R6, were found
to exhibit cytotoxic activities against human breast cancer cell
line MDA-MB-435 and human colon cancer cell line HCT-116s
(IC50 <10 µM) (18).
Additionally, Tao et al (28) separated 87 compounds from mangrove
endophytic fungus in Southern China, of which 14% of the compounds
had antitumor activity. In the present study, we found that 10
strains, including Neofusicoccum sp. 4 strains (zj-2, zj14,
zj-17 and zj-67), Phomopsis sp. 4 strains (zj-12, zj-23,
zj-36 and zj-70), Leptosphaerulina sp. 1 strain (zj-9) and
Penicillium sp. 1 strain (zj-25), significantly inhibited
the growth of the lung cancer cells, A549 and NCI-H460.
Angiogenesis plays a key role in cancer invasion and
metastasis. Thus, inhibition of angiogenesis is an effective
strategy for cancer prevention (25,29,30).
At present, multiple natural compounds have been reported in in
vitro and in vivo experiments to inhibit angiogenesis,
and most of them from terrestrial plants (29–33).
Recently, the screening of marine medicines from the sea and its
surroundings has also attracted increased attention. A number of
angiogenic inhibitors from marine organisms have been found
(34,35). Moreover, anti-angiogenic drugs can
also be isolated from endophytic fungi (36–38).
Altersolanol, isolated from an Alternaria sp. endophytic
fungus, was reported to show promising anti-angiogenic activity
ex vivo, in vitro and in vivo by the
suppression of proliferation, tube formation and migration
(36). The phenolic compounds,
isolated from an endophytic fungus Coccomyces proteae
collected from a Costa Rican rainforest, were reported to have
anti-angiogenic activity via inhibition of capillary morphogenesis
gene protein 2 (CMG2) (37).
Particularly, toluquinol, isolated from marine fungus secondary
metabolites, was also demonstrated to inhibit angiogenesis both
in vitro and in vivo partly by the suppression of
VEGF and FGF-induced Akt activation (38). In the present study, we established
an HPV-16 E7 oncoprotein-induced lung cancer angiogenic model
according to our previous findings (26,27),
and further observed the effects of endophytic fungi which have
high inhibitory effect on angiogenesis in vitro. We found
that zj-14, zj-17 and zj-36 endophytic fungi significantly
inhibited lung cancer angiogenesis in vitro. Notably, strain
zj-9 was found to have the strongest inhibitory effect on the
growth of lung cancer cells, but it did not exhibit anti-angiogenic
activity, indicating that strain zj-9 may have cell cytotoxicity
but not anti-angiogenic activity, and this issue warrants further
study.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81372511) (to
X.T.), the Guangdong Provincial Department of Science and
Technology (Research and Development of Industrial Technology in
Guangdong Province) (no. 2013B031100002) (to X.T.), and the
Zhanjiang Municipal Governmental Specific Financial Fund Allocated
for Competitive Scientific and Technological Projects (no.
2012C0303-56) (to X.T.).
References
|
1
|
Campos FF, Junior PA Sales, Romanha AJ,
Araújo MS, Siqueira EP, Resende JM, Alves TM, Martins-Filho OA,
Santos VL, Rosa CA, et al: Bioactive endophytic fungi isolated from
Caesalpinia echinata Lam. (Brazilwood) and identification of
beauvericin as a trypanocidal metabolite from Fusarium sp. Mem Inst
Oswaldo Cruz. 110:65–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun ZH, Liang FL, Chen YC, Liu HX, Li HH
and Zhang WM: Two new xyloketals from the endophytic fungus
Endomelanconiopsis endophytica derived from medicinal plant Ficus
hirta. J Asian Nat Prod Res. 18:1036–1041. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun W, Chen X, Tong Q, Zhu H, He Y, Lei L,
Xue Y, Yao G, Luo Z, Wang J, et al: Novel small molecule 11β-HSD1
inhibitor from the endophytic fungus Penicillium commune. Sci Rep.
6:264182016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu DB, Ye WW, Han Y, Deng ZX and Hong K:
Natural products from mangrove actinomycetes. Mar Drugs.
12:2590–2613. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Debbab A, Aly AH, Lin WH and Proksch P:
Bioactive compounds from marine bacteria and fungi. Microb
Biotechnol. 3:544–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Ding W, Wang R, Du Y, Liu H, Kong
X and Li C: Identification and bioactivity of compounds from the
mangrove endophytic fungus Alternaria sp. Mar Drugs. 13:4492–4504.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui H, Liu Y, Nie Y, Liu Z, Chen S, Zhang
Z, Lu Y, He L, Huang X and She Z: Polyketides from the
mangrove-derived endophytic fungus Nectria sp. HN001 and their
α-glucosidase inhibitory activity. Mar Drugs. 14:pii: E862016.
View Article : Google Scholar
|
|
8
|
Ding B, Wang Z, Huang X, Liu Y, Chen W and
She Z: Bioactive α-pyrone meroterpenoids from mangrove endophytic
fungus Penicillium sp. Nat Prod Res. Apr 11;1–8. 2016.(Epub ahead
of print).
|
|
9
|
Liu Y, Chen S, Liu Z, Lu Y, Xia G, Liu H,
He L and She Z: Bioactive metabolites from mangrove endophytic
fungus Aspergillus sp. 16-5B. Mar Drugs. 13:3091–3102. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bai ZQ, Lin X, Wang Y, Wang J, Zhou X,
Yang B, Liu J, Yang X, Wang Y and Liu Y: New phenyl derivatives
from endophytic fungus Aspergillus flavipes AIL8 derived of
mangrove plant Acanthus ilicifolius. Fitoterapia. 95:194–202. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou XM, Zheng CJ, Chen GY, Song XP, Han
CR, Tang XZ, Liu RJ and Ren LL: Two new stemphol sulfates from the
mangrove endophytic fungus Stemphylium sp. 33231. J Antibiot.
68:501–503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng LH, Zhang P, Li XM and Wang BG:
Penicibrocazines A-E, five new sulfide diketopiperazines from the
marine-derived endophytic fungus Penicillium brocae. Mar Drugs.
13:276–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lv F, Daletos G, Lin W and Proksch P: Two
new cyclic depsipeptides from the endophytic fungus Fusarium sp.
Nat Prod Commun. 10:1667–1670. 2015.PubMed/NCBI
|
|
14
|
Wang M, Zhang W, Xu W, Shen Y and Du L:
Optimization of genome shuffling for high-yield production of the
antitumor deacetylmycoepoxydiene in an endophytic fungus of
mangrove plants. Appl Microbiol Biotechnol. 100:7491–7498. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng C, Huang C, Wu Q, Pang J and Lin Y: A
new sesquiterpene from the mangrove endophytic fungus Aspergillus
terreus (No. GX7-3B). Nat Prod Res. 27:1882–1887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chokpaiboon S, Choodej S, Boonyuen N,
Teerawatananond T and Pudhom K: Highly oxygenated chromones from
mangrove-derived endophytic fungus Rhytidhysteron rufulum.
Phytochemistry. 122:172–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Xue Y, Yuan J, Lu Y, Zhu X, Lin Y
and Liu L: Lasiodiplodins from mangrove endophytic fungus
Lasiodiplodia sp. 318. Nat Prod Res. 30:755–760. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Cox DG, Ding W, Huang G, Lin Y and
Li C: Three new resveratrol derivatives from the mangrove
endophytic fungus Alternaria sp. Mar Drugs. 12:2840–2850. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang L, Zhang T, Li S, Duan J, Ye F, Li
H, She Z, Gao G and Yang X: Anthraquinone G503 induces apoptosis in
gastric cancer cells through the mitochondrial pathway. PLoS One.
9:e1082862014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen WL, Turlova E, Sun CL, Kim JS, Huang
S, Zhong X, Guan YY, Wang GL, Rutka JT, Feng ZP, et al: Xyloketal B
suppresses glioblastoma cell proliferation and migration in vitro
through inhibiting TRPM7-regulated PI3K/Akt and MEK/ERK signaling
pathways. Mar Drugs. 13:2505–2525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Tan T, Mao ZG, Lei N, Wang ZM, Hu
B, Chen ZY, She ZG, Zhu YH and Wang HJ: The marine metabolite
SZ-685C induces apoptosis in primary human nonfunctioning pituitary
adenoma cells by inhibition of the Akt pathway in vitro. Mar Drugs.
13:1569–1580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie G, Zhu X, Li Q, Gu M, He Z, Wu J, Li
J, Lin Y, Li M, She Z, et al: SZ-685C, a marine anthraquinone, is a
potent inducer of apoptosis with anticancer activity by suppression
of the Akt/FOXO pathway. Br J Pharmacol. 159:689–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang D, Wang S, Liu Q, Wang M, Wang C and
Yang H: SZ-685C exhibits potent anticancer activity in both
radiosensitive and radioresistant NPC cells through the
miR-205-PTEN-Akt pathway. Oncol Rep. 29:2341–2347. 2013.PubMed/NCBI
|
|
24
|
Zhu X, He Z, Wu J, Yuan J, Wen W, Hu Y,
Jiang Y, Lin C, Zhang Q, Lin M, et al: A marine anthraquinone
SZ-685C overrides adriamycin-resistance in breast cancer cells
through suppressing Akt signaling. Mar Drugs. 10:694–711. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29 Suppl 16:S15–S18. 2002.
View Article : Google Scholar
|
|
26
|
Li G, He L, Zhang E, Shi J, Zhang Q, Le
AD, Zhou K and Tang X: Overexpression of human papillomavirus (HPV)
type 16 oncoproteins promotes angiogenesis via enhancing HIF-1α and
VEGF expression in non-small cell lung cancer cells. Cancer Lett.
311:160–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang E, Feng X, Liu F, Zhang P, Liang J
and Tang X: Roles of PI3K/Akt and c-Jun signaling pathways in human
papillomavirus type 16 oncoprotein-induced HIF-1α, VEGF, and IL-8
expression and in vitro angiogenesis in non-small cell lung cancer
cells. PLoS One. 9:e1034402014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tao YW, Lin YC, She Z-G, Lin MT, Chen PX
and Zhang JY: Anticancer activity and mechanism investigation of
beauvericin isolated from secondary metabolites of the mangrove
endophytic fungi. Anticancer Agents Med Chem. 15:258–266. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi J, Liu F, Zhang W, Liu X, Lin B and
Tang X: Epigallocatechin-3-gallate inhibits nicotine-induced
migration and invasion by the suppression of angiogenesis and
epithelial-mesenchymal transition in non-small cell lung cancer
cells. Oncol Rep. 33:2972–2980. 2015.PubMed/NCBI
|
|
30
|
He L, Zhang E, Shi J, Li X, Zhou K, Zhang
Q, Le AD and Tang X: (−)-Epigallocatechin-3-gallate inhibits human
papillomavirus (HPV)-16 oncoprotein-induced angiogenesis in
non-small cell lung cancer cells by targeting HIF-1α. Cancer
Chemother Pharmacol. 71:713–725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee J, Yi JM, Kim H, Lee YJ, Park JS, Bang
OS and Kim NS: Cytochalasin H, an active anti-angiogenic
constituent of the ethanol extract of Gleditsia sinensis thorns.
Biol Pharm Bull. 37:6–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee SR, Park JY, Yu JS, Lee SO, Ryu JY,
Choi SZ, Kang KS, Yamabe N and Kim KH: Odisolane, a novel oxolane
derivative, and antiangiogenic constituents from the fruits of
mulberry (Morus alba L.). J Agric Food Chem. 64:3804–3809. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park JY, Shin MS, Kim SN, Kim HY, Kim KH,
Shin KS and Kang KS: Polysaccharides from Korean Citrus hallabong
peels inhibit angiogenesis and breast cancer cell migration. Int J
Biol Macromol. 85:522–529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goey AK, Chau CH, Sissung TM, Cook KM,
Venzon DJ, Castro A, Ransom TR, Henrich CJ, McKee TC, McMahon JB,
et al: Screening and biological effects of marine
pyrroloiminoquinone alkaloids: Potential inhibitors of the
HIF-1α/p300 interaction. J Nat Prod. 79:1267–1275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baharara J, Amini E and Mousavi M: The
anti-proliferative and anti-angiogenic effect of the methanol
extract from brittle star. Rep Biochem Mol Biol. 3:68–75.
2015.PubMed/NCBI
|
|
36
|
Pompeng P, Sommit D, Sriubolmas N,
Ngamrojanavanich N, Matsubara K and Pudhom K: Antiangiogenetic
effects of anthranoids from Alternaria sp., an endophytic fungus in
a Thai medicinal plant Erythrina variegata. Phytomedicine.
20:918–922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao S, Cryan L, Habeshian KA, Murillo C,
Tamayo-Castillo G, Rogers MS and Clardy J: Phenolic compounds as
antiangiogenic CMG2 inhibitors from Costa Rican endophytic fungi.
Bioorg Med Chem Lett. 22:5885–5888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
García-Caballero M, Marí-Beffa M, Cañedo
L, Medina MÁ and Quesada AR: Toluquinol, a marine fungus
metabolite, is a new angiosuppresor that interferes with the Akt
pathway. Biochem Pharmacol. 85:1727–1740. 2013. View Article : Google Scholar : PubMed/NCBI
|