Introduction
It is well-known that renal cell carcinoma (RCC) is
a high-risk and high-mortality cancer and is notoriously resistant
to traditional chemotherapies and radiotherapies (1), marked by adverse tumor biology and its
low CSS ranking among urological malignancies (2). RCC patients either receive surgery or
chemoradiotherapy, have poor prognosis and low five-year survival
rate (3,4). Because of the outcomes of
chemotherapy, radiotherapy and hormone therapy are unsatisfactory,
it is necessary to develop an effective adjuvant therapy.
Adriamycin (ADM), a kind of antitumor antibiotic,
inhibits the synthesis of DNA and RNA, has strong cytotoxicity
(5) and induces toxicity through
oxidative stress (6). In clinic,
ADM has been used for sarcomatoid-type tumors, including RCC
(7,8); however, the chronic toxicity and acute
toxicity of ADM limit its clinical application (9,10).
YS-1, a recombinant human p43 protein, not only has been confirmed
to have anti-angiogenesis and antitumor effects, in vitro
and in vivo (11); but also
showed potential antitumor properties for primary and metastatic
solid tumors. In our previous study, YS-1 could directly inhibited
angiogenesis through Dll4-Notch1 signal transduction pathway
(12), and caused renal toxicity
mainly by the activation of ERK1/2 in kidney cells (13). Thus, inhibiting the effects of ADM
on ERK1/2 pathway may be a key mechanism to improve the anticancer
effect of ADM. According to our previous research, YS-1 might
inhibit ERK1/2 activation through affecting the Notch1 pathway.
Taking advantage of combination therapies (i.e., avoiding the risk
of the development of resistance, increasing the effectiveness of
the therapy, and the effectiveness of clinical combination
therapies with ADM), we designed YS-1 combined with ADM, to reduce
the expression of p-ERK1/2 and increase the effectiveness of ADM
therapy for RCCs.
The ERK1/2 cascade regulates a variety of cellular
processes by phosphorylating multiple target proteins (14). The outcome of its activation ranges
from stimulation of cell survival and proliferation to triggering
tumor suppressor responses such as cell differentiation, cell
senescence, and apoptosis (15).
Recent studies have shown that inhibition of ERK1/2 can effectively
reverse the multidrug resistance of prostate cancer, gastric
cancer, and cancer of the blood system (16–18).
The signaling via the ERK cascade is mediated by sequential
phosphorylation and activation of protein kinases in the different
tiers of the cascade, and the main core phosphorylation chain of
the cascade includes Raf kinases, MEK1/2, ERK1/2 (ERKs) and RSKs.
The Ras/Raf/MEK/ERK pathway has been reported to be activated in
over 50% of acute myelogenous leukemia and acute lymphocytic
leukemia and is also frequently activated in other cancer types
(e.g., breast and prostate cancers). Importantly, this increased
expression is associated with a poor prognosis (19). The Ras/Raf/MEK/ERK interacts with
each other to regulate growth and in some cases tumorigenesis, and
it is commonly thought to have anti-apoptotic and drug resistance
effects on cells (20,21).
In this study, we assessed the antitumor activity of
ADM alone and in combination with YS-1 on RCCs. We also identified
the optimal dose of YS-1 which can decrease p-ERK1/2 expression
without causing toxicity. We propose that inhibition of the ERK1/2
activation by YS-1 may be a promising therapeutic target for
enhancing the sensitivity of cancer cells to ADM. These findings
highlighted the possibility of using such natural, safe, relatively
inexpensive compounds as potential adjunct treatment in improving
the overall treatment response of patients with RCC.
Materials and methods
Cell culture and siRNA
Human cancer (95D, SGC-7901, BEL-7402, MDA-MB-435
and 786-O) cell lines were purchased from the Shanghai Institute of
Life Science, Chinese Academy of Sciences. BEL-7402 cells were
maintained in DMEM with 10% fetal bovine serum (FBS, Gibco) and
antibiotics (100 U/ml penicillin, and 100 mg/ml streptomycin). The
other cells were grown in RMPI-1640 with 10% FBS and antibiotics
(100 U/ml penicillin, and 100 mg/ml streptomycin). Cells were
incubated in a humidified atmosphere with 5% CO2 at
37°C. ERK1/2 siRNA was purchased from Shanghai GenePharma Co., Ltd.
(Shanghai, China). Lipofectamine 2000 reagent was obtained from
Invitrogen (Shanghai, China).
Cell growth inhibition assay
Cells were seeded in 96-well plates in 180 µl of
medium and incubated for 12 h. These cells were then cultured in
the presence of YS-1, ADM individually as well in combination for
72 h. Afterwards, 5 mg/ml MTT solution (20 µl/well) was added and
cultured in 5% CO2 incubator at 37°C for 4 h, then the
supernatant was discarded and DMSO was added (150 µl/well). The
suspension was placed on a micro-vibrator for 5 min and the
absorbance was measured at 570 nm using a Universal Microplate
Reader (EL800; Bio-Tek Instruments Inc.). Experiments were
performed in triplicate in a parallel manner for each concentration
and the results are presented as the mean ± SD. The inhibitory
ratio was calculated by the following formula: inhibitory ratio (%)
= (1 - average absorbance of treated group/average absorbance of
control group) ×100.
Colony formation assays
The 786-O cells were seeded in a dish (100 mm × 20
mm) at a density of 300 cells per dish and cultured in the presence
of YS-1, ADM individually as well in combination for two weeks. At
the end of the incubation, the cells were fixed with 4%
paraformaldehyde and stained with Giemsa. Megascopic cell colonies
were counted using Image-Pro Plus 6.0 software (Media Cybernetics,
Bethesda, MD, USA). Each measurement was performed in triplicate
and the experiments were conducted at least three times. The clone
formation rate was calculated by the following formula: clone
formation rate (%) = (number of colonies cells / number of
vaccination cells) ×100.
Cell cycle analysis
The 786-O cells (1×106) were seeded into
dish (100 mm × 20 mm) and incubated overnight, and then cultured in
the presence of YS-1, ADM individually as well in combination for 6
h. Next, the cells were harvested, washed with cold PBS, and then
fixed with 70% cold ethanol at 4°C overnight. After being washed
twice with cold PBS, fixed cells were resuspended with 100 µg/ml
RNase, incubated with 50 µg/ml PI at 37°C for 30 min in the dark.
Data acquisition and analysis were performed in Becton Dickinson
FACS Calibur flow cytometer using Cell Quest software (Franklin
Lakes, NJ, USA).
Wound closure assay
The 786-O cells were cultured to confluence or near
confluence (>90%) in a 6-well dish. A sterile 10 µl pipette tip
was used to scratch a cross-shaped wound through the cells. Cells
were rinsed with PBS, and were cultured in the presence of YS-1,
ADM individually and in combination. Wounds were imaged at 0, and 6
h under a microscope with an attached camera. The TScratch program
(Computational Science and Engineering Laboratory, Zurich,
Switzerland) (22) was used to
measure the open areas and analyze the data.
Migration assay
Cells (5×104) were suspended in 200 µl
serum-free RMPI-1640 medium and seeded into the upper chamber of
each insert of Transwell (Millipore). Then, 700 µl of RMPI-1640
containing 10% FBS was added to a 24-well plate. After incubation
at 37°C for 6 h, the cells that migrated were fixed and stained for
30 min in a 0.1% Crystal Violet solution in PBS. The migrated cells
were quantified by manual counting by using Image-Pro Plus 6.0
software (Media Cybernetics), and five randomly chosen fields were
analyzed for each group.
Annexin V/PI double staining
Cells were incubated for 6 h with YS-1, ADM
separately or in combination. Apoptotic cells were identified by
the Annexin V-FITC Apoptosis Detection kit (Vazyme) in accordance
with the manufacturer's instructions. Flow cytometric analysis was
performed immediately after supravital staining.
Cell transfection
Cells were transfected with ERK1/2 siRNA using
Lipofectamine 2000 (Invitrogen) according to the manufacturer's
protocol. The transfection medium (OptiMEM; Gibco) was replaced
with complete medium 12 h after transfection, and the cells were
incubated for the indicated times.
Western blot analysis
Cells were treated with YS-1 alone, ADM alone or
combination both for 6 h. As previously described (23), then cellular protein extraction and
Western blot analysis were performed. All first antibodies were
purchased from Cell Signaling Technology, Inc. Horseradish
peroxidase (HRP) linked anti-mouse immunoglobulin G (Sigma) and
anti-rabbit immunoglobulins G (CST) were used as the secondary
antibodies. Different protein bands were made visible by enhanced
chemiluminescence reagents (Amersham Pharmacia Biotech).
Quantitative real-time PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen). Complimentary DNA (cDNA) was synthesized with the
Prime-Script RT reagent kit (Takara). The mRNA level was measured
with the SYBR Green master mix (Vazyme). The amount of mRNA for
each gene was standardized with internal control (GAPDH mRNA). Each
treatment group was compared with the control group to show the
relative mRNA level. The gene-specific primer pairs were as
follows: GAPDH (F) 5′-GGTGGTCTCCTCTGACTTCAACA-3′, GAPDH (R)
5′-GTTGCTGTAGCCAAATTCGTTGT-3′; hERK1 (F)
5′-CATGAGAATGTCATCGGCATCC-3′, hERK1 (R)
5′-CCATCAGGTCCTGCACAATGTAG-3′; hERK2 (F)
5′-GACATTATTCGAGCACCAACCATC-3′, hERK2 (R)
5′-GAGGTGTTGTGTCTTCAAGAGCTTG-3′.
In vivo tumorigenicity
Female athymic BALB/c nude mice (5–6 weeks old) with
body weight from 18 to 22 g were supplied by the Shanghai Institute
of Materia Medica, Chinese Academy of Sciences. 786-O cells were
collected in serum-free RMPI-1640 medium and cell suspension
(107 cells/100 µl), then cell suspension was injected
subcutaneously into mice in one flank (n=6). Animal care and
surgery protocols were approved by Animal Care Committees of China
Pharmaceutical University. All animals were treated appropriately
and used in a scientifically valid and ethical manner.
Statistical analysis
All of the results are presented as mean ± SD from
triplicate experiments which were performed in a parallel manner
unless otherwise indicated. Statistically significant differences
(one-way ANOVAs followed by Bonferroni's multiple comparison test)
were determined using GraphPad Prism 6 software. P<0.05 was
considered significant, and P<0.01 and P<0.001 were
considered highly significant.
Results
YS-1 combined with ADM has a
synergistic anticancer effect on cell death in RCCs
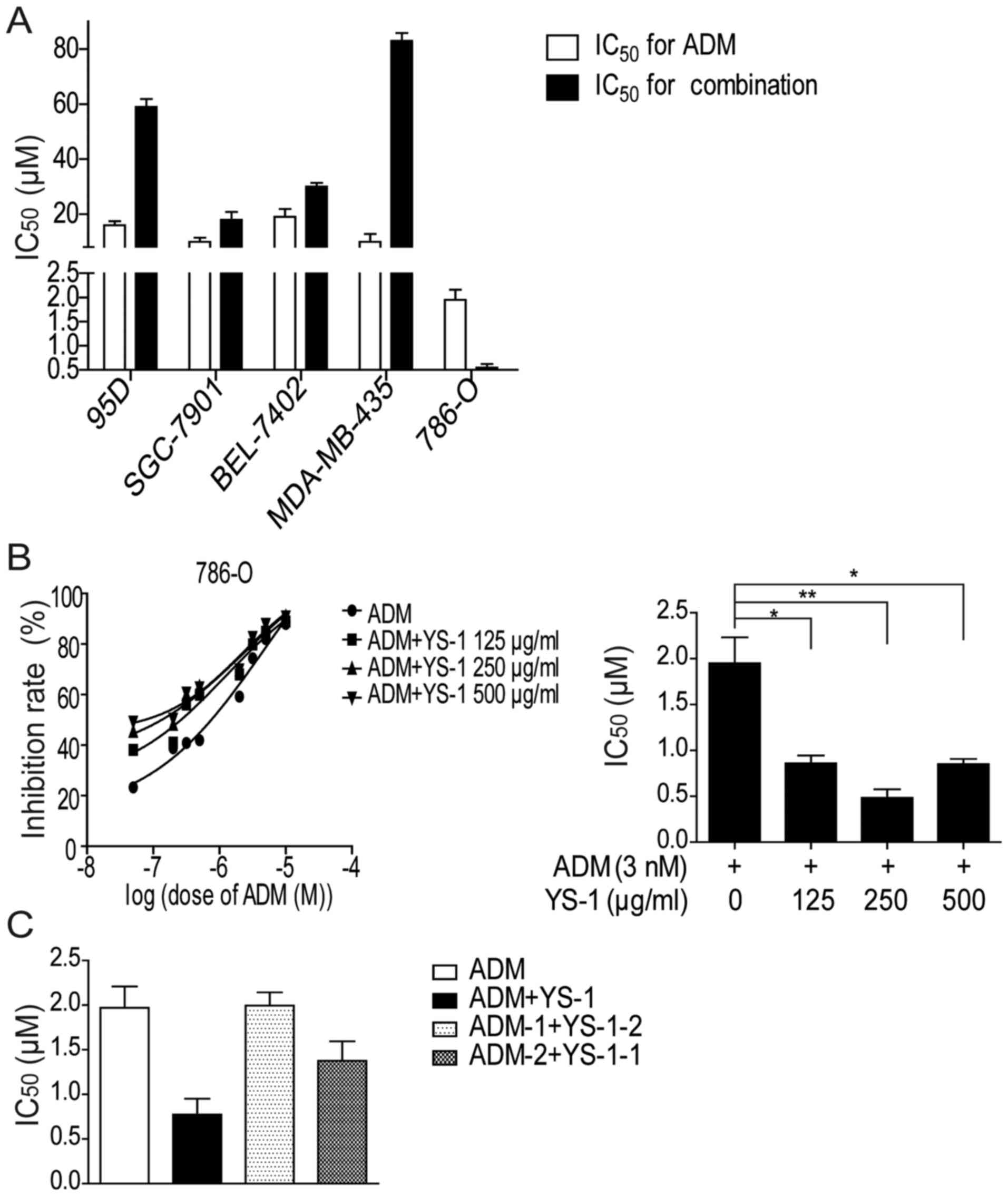
We measured the inhibitory effect of YS-1 combined
with ADM on the survival of different cancer cell lines: 95D,
SGC-7901, BEL-7402, MDA-MB-435, 786-O. After 72 h of treatment, we
found YS-1 combined with ADM only exhibited a synergistic
inhibitory effect on the survival of 786-O cells (Fig. 1A).
In order to determine the optimum concentration of
YS-1 when combined with ADM, 786-O cells were treated with ADM and
YS-1 (125, 250, and 500 µg/ml, respectively) for 72 h, we found
that when combining ADM with 250 µg/ml YS-1, the IC50
value reduced most markedly (Fig.
1B). Next, we investigated the inhibitory effect of the
different drug delivery order of 3 nM ADM with 250 µg/ml YS-1 on
786-O cells, as shown in Fig. 1C,
only when YS-1 and ADM were added simultaneously, the
IC50 value reduced most significantly. These findings
clearly showed that 250 µg/ml YS-1 combined with 3 nM ADM exhibited
a synergistic inhibitory effect on the survival of 786-O cells.
YS-1 combined with ADM promote
anti-proliferative activity in 786-O cells
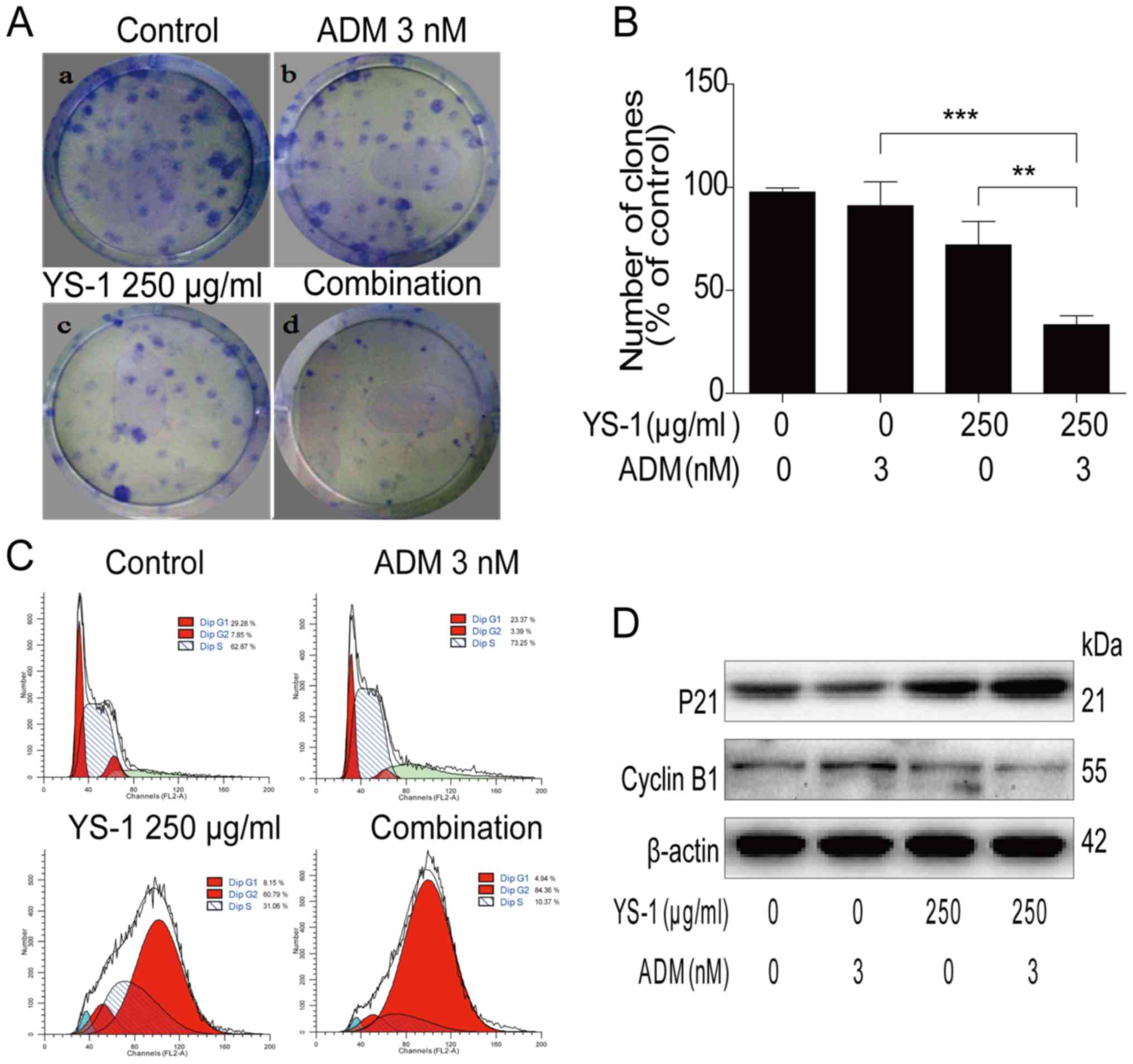
To investigate the mechanism of the combined
treatment on the survival of 786-O cells, we measured the effect of
combination treatment on anti-proliferative activity. As shown in
Fig. 2A and B, the combination
treatment group inhibited the clone formation of 786-O cells
significantly. Moreover, the combination group induced apparent
G2/M cell cycle arrest in 786-O cells as assessed by flow analysis
(Fig. 2C). Then we measured the
levels of proteins involved in the G2/M cell cycle arrest pathway.
The combination treatment increased the expression of P21 and
reduced the expression of cyclin B1 (Fig. 2D). These findings clearly showed
YS-1 combined with ADM promoted the anti-proliferative activity in
786-O cells.
Synergistic anticancer effect of YS-1
combined with ADM did not increase the pro-apoptotic activity in
786-O cells
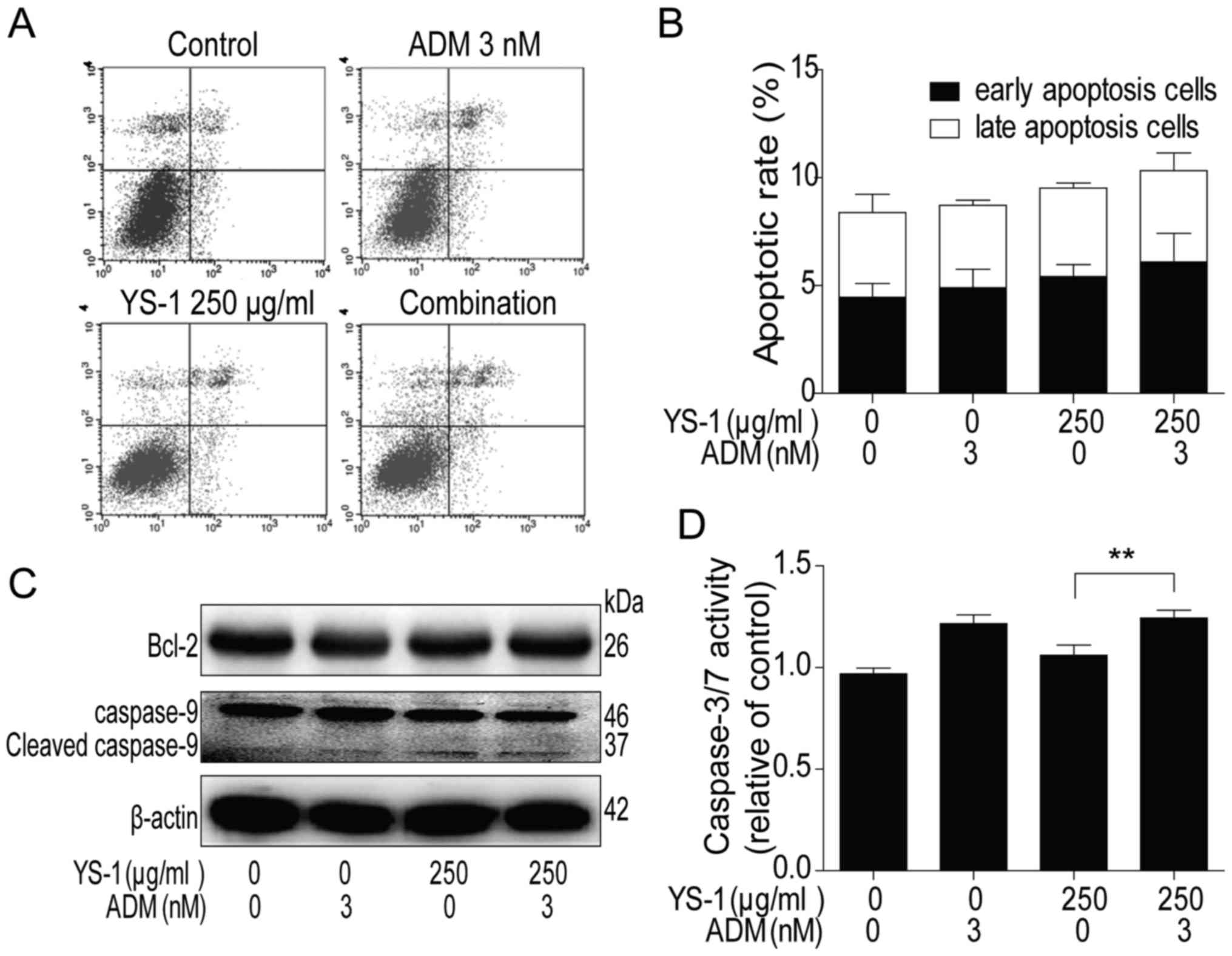
Apoptosis is an important mechanism contributing to
the cell survival reduction (24),
so in our further investigation, we measured the effect of the
combined treatment on the pro-apoptotic activity. As shown in
Fig. 3A and B, after 6 h treatment,
compared with the monotherapy group, the comb-therapy group could
not show any synergistic pro-apoptotic effect, by flow analysis in
786-O cells. After that, we analysed the protein levels of Bcl-2
and the cleaved level of caspase-9 by western blot analysis in
786-O cells, and found that the combination group did not
downregulate Bcl-2 or upregulate cleaved caspase-9 (Fig. 3C) compared with ADM monotherapy
treatment group. Furthermore, the activity of caspase-3/7 was
consistent with the results of flow analysis and western analysis
(Fig. 3D). These finding clearly
showed YS-1 combined with ADM could not induce pro-apoptotic
activity in 786-O cells.
YS-1 combined with ADM regulate
anti-proliferative activity via down-regulating Ras/Raf/MEK/ERK1/2
pathway
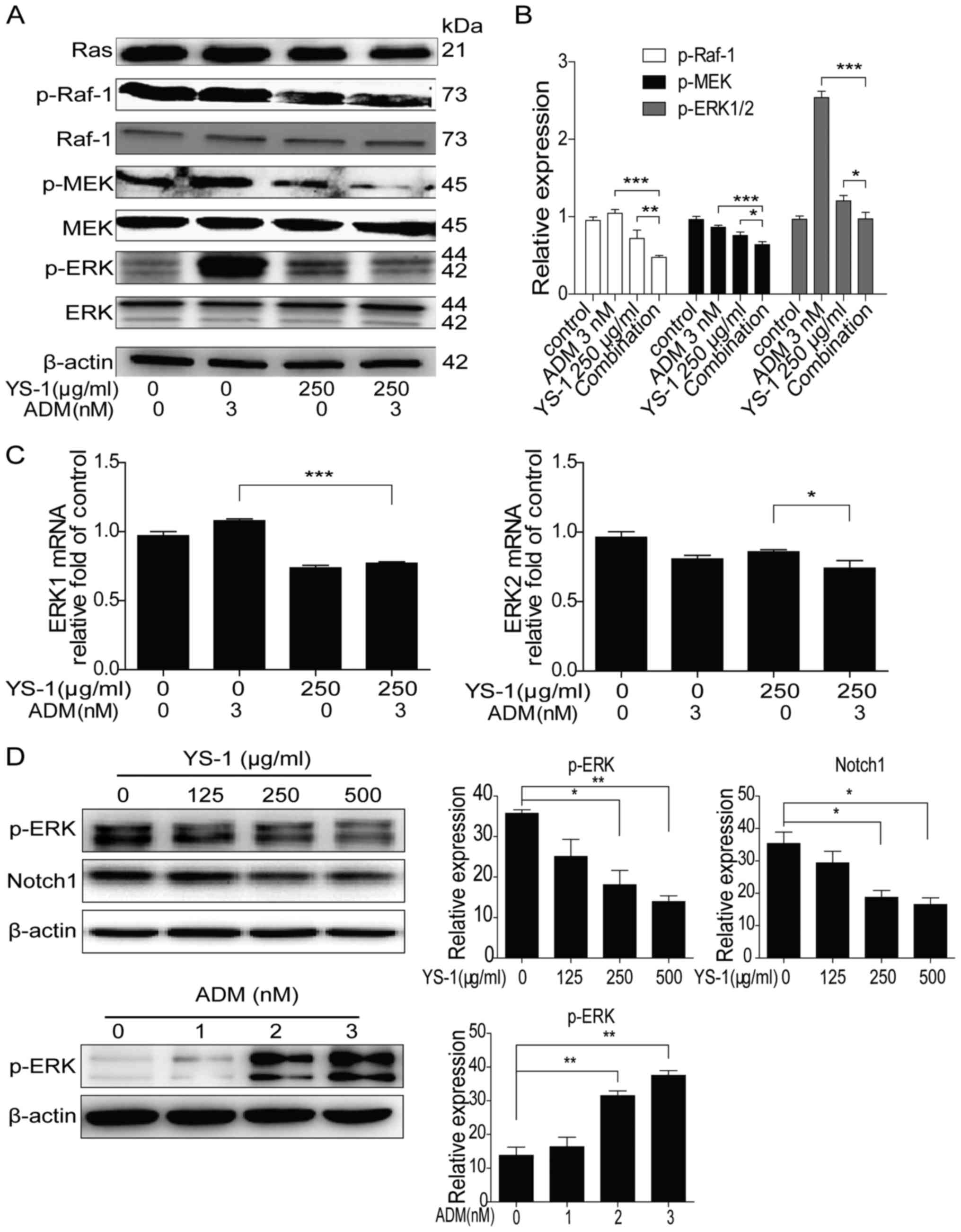
Ras/Raf/MEK/ERK1/2 pathway could influence cell
growth and drug resistance (19).
Recently, research showed that the renal toxicity of ADM was mainly
induced by the activation of ERK1/2 signaling pathway in kidney
cells (13). Therefore, we
investigated the effect of combination therapy on ERK1/2 pathway.
As shown in Fig. 4A and B, the
combination treatment group significantly reduced
Ras/Raf/MEK/ERK1/2 pathway activity in 786-O cells compared with
YS-1 or ADM monotherapy treatment group. Furthermore, the mRNA
levels of ERK1 and ERK2 were slightly decreased when YS-1 was
combined with ADM (Fig. 4C). The
combination treatment reduced phosphorylated ERK1/2. However, it is
not clear to which drug (or both) the function is due. Thus, we
measured the effect of YS-1 and ADM separately on p-ERK1/2 in 786-O
cells. As shown in Fig. 4D, a
decrease in the expression of p-ERK1/2 as well as Notch1 was
observed after 786-O cells were treated with YS-1 for 6 h;
similarly, the expression of p-ERK1/2 was significantly increased
after treatment of 786-O cells with ADM alone for 6 h. These
results indicated that YS-1 promoted ERK1/2 inactivation through
regulating Notch1 pathway, and YS-1 combined with ADM exerts its
anti-proliferative activity via inhibiting the activation of ERK1/2
pathway.
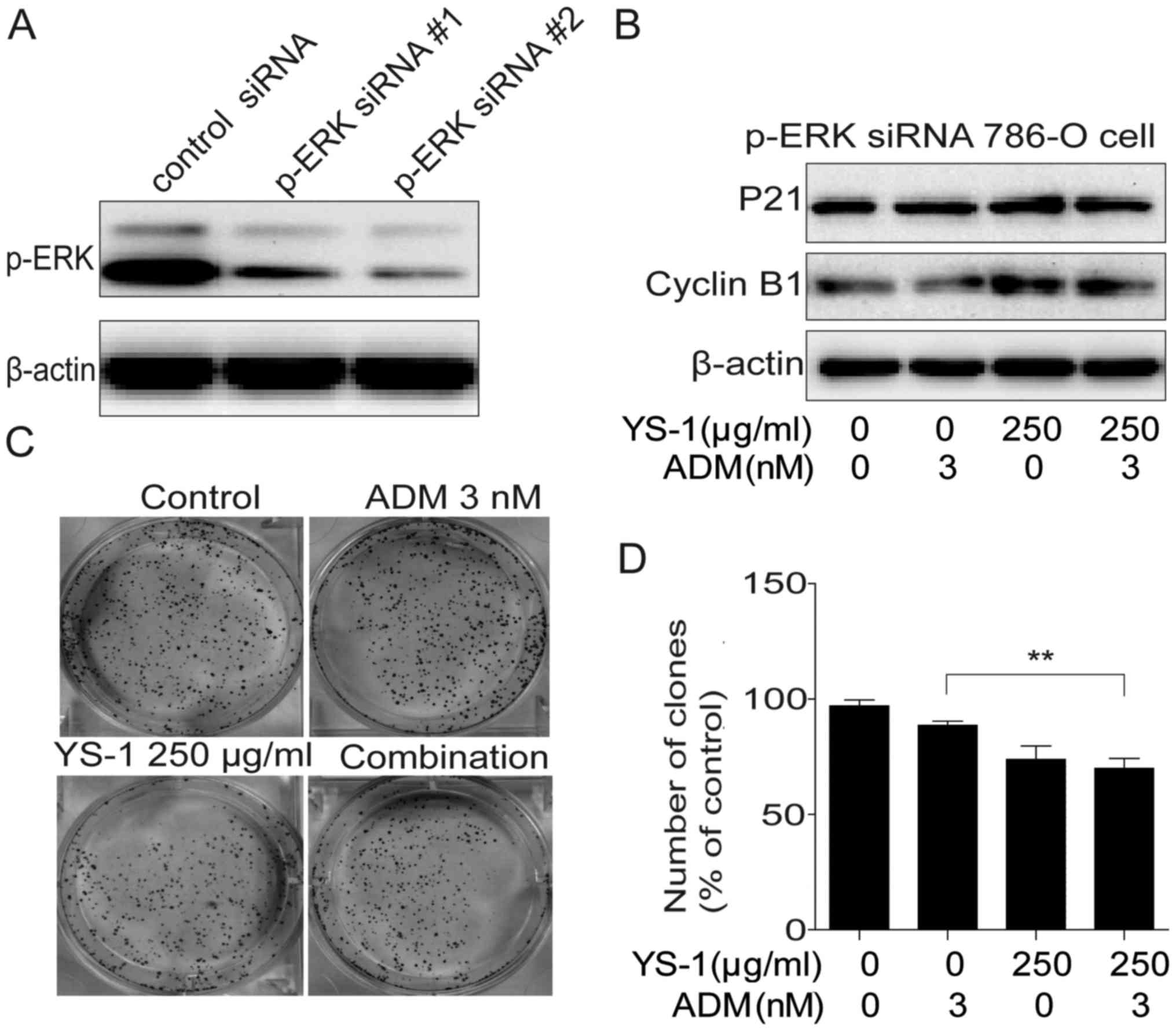
YS-1 combined with ADM exerted
synergistic effects through p-ERK1/2 inhibition
To confirm the involvement of p-ERK1/2 in the
inhibition of proliferation by YS-1 combined with ADM, we knocked
down ERK1/2 by using ERK1/2 siRNA. The ERK1/2 and p-ERK1/2 levels
of 786-O cells were decreased remarkably (Fig. 5A). Then we examined the
anti-proliferative effect of YS-1 combined with ADM on ERK1/2
knockdown 786-O cells, data showed that the combination treatment
could not induce cell cycle arrest (Fig. 5B), and could not inhibit the clone
ability of 786-O cells (Fig. 5C and
D). These data confirmed that YS-1 combined with ADM exerted
synergistic effects through p-ERK1/2 inhibition.
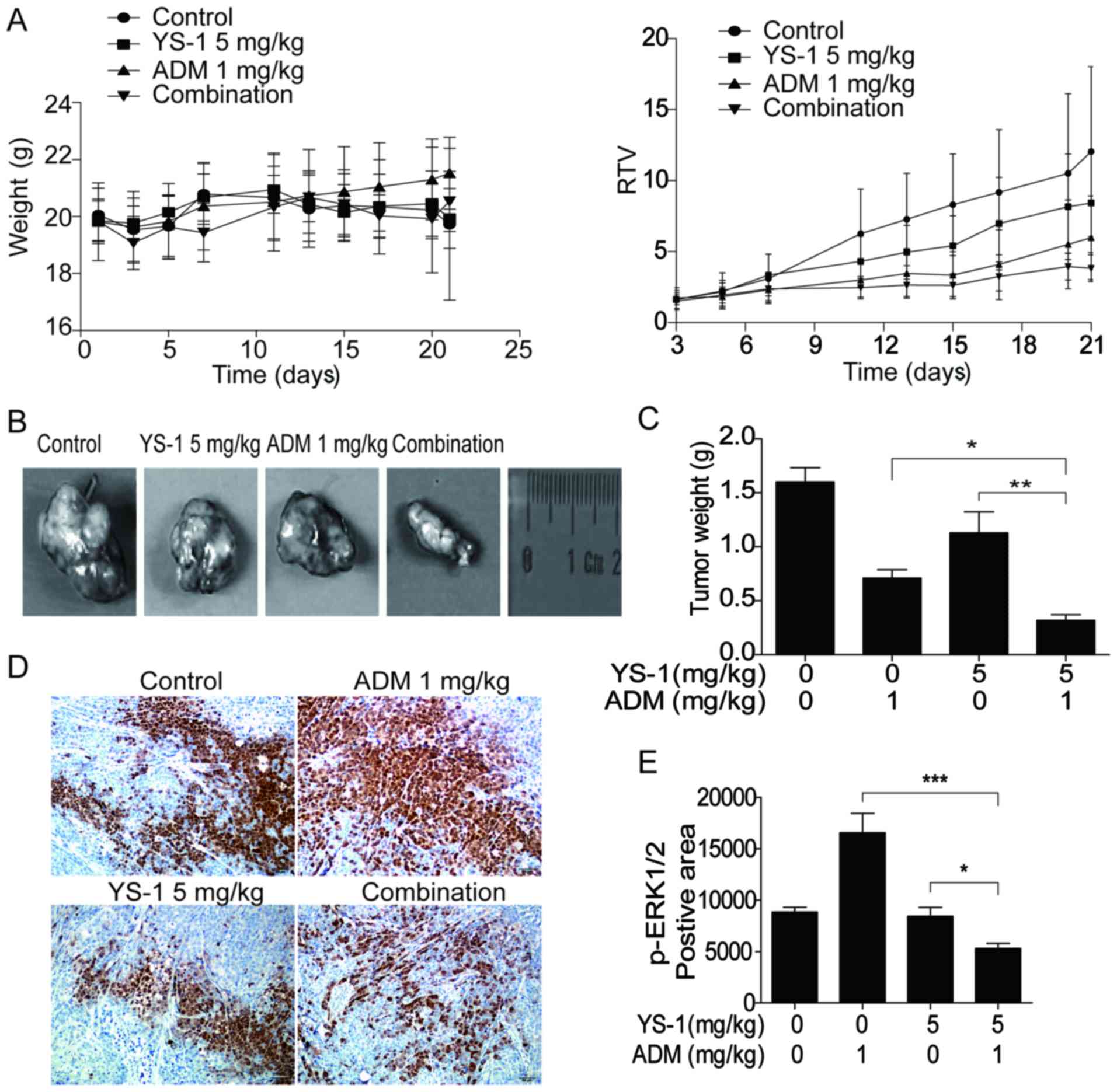
Combination treatment inhibits tumor
growth in 786-O-xenografted nude mice
To assess the efficiency of combination therapy YS-1
and ADM in vivo, we established the 786-O cell xenograft
model and evaluated the RTV and ERK1/2 activation. As shown in
Fig. 6A, the value of RTV was
decreased in the combination group, without showing any obvious
toxic effect. Results showed that 5 mg/kg YS-1 combined with 1
mg/kg ADM exerted a significant synergistic inhibitory effect on
786-O xenografts (Fig. 6B and C).
In tumor tissues from the 786-O-xenografted nude mice treated with
YS-1/ADM combination, the positive areas for p-ERK1/2 were reduced
significantly (Fig. 6D and E).
These results suggested that YS-1 combined with ADM boosted the
anti-proliferative effect in vivo via p-ERK1/2 inhibition,
which was consistent with the in vitro data.
Discussion
In the present investigation, we demonstrated that
p-ERK1/2 was involved in the chemoresistance to ADM in RCC cells;
ADM monotherapy caused renal toxicity by promoting the activation
of ERK1/2 in kidney cells. We found that YS-1, a recombinant human
p43 protein, could increase the sensitivity of 786-O cells to ADM
by inhibiting the p-ERK1/2 level in 786-O cells. YS-1 promoted
ERK1/2 inactivation through regulating the Notch1 pathway. By using
ERK1/2 siRNA, we found the synergistic anti-proliferative effect of
YS-1 combined with ADM was reversed. In 786-O cell xenograft model,
we also found a significantly synergistic inhibitory effect on
786-O xenografts when 5 mg/kg YS-1 was combined with 1 mg/kg ADM.
These findings suggested that YS-1 might synergistically combine
with ADM to inhibit the RCCs through decreasing p-ERK1/2 modulation
of EGFR downstream signaling.
As an important adjuvant treatment, chemotherapy
serves as a necessary component of postoperative therapy for renal
cancer (25). However, most
traditional chemotherapeutic drugs lead to drug resistance and this
has become a major obstacle in chemotherapy (26). Therefore, it is imperative to
identify novel therapeutic targets which are involved in the
acquisition of drug resistance.
Because of the advantages of combination with low
toxicity, high efficiency, there have been many studies on the
combined application of vascular inhibitors and cytotoxic drugs,
such as: Combination of TNP-470 and mitomycin C (MMC), ADM,
cisplatin, gemcitabine and 5-fluorouracil (5-FU) in nude mouse
xenograft model, which significantly increased its antitumor
activity (27–29); Doxorubicin with sorafenib augments
cytotoxicity to RCC through p-ERK1/2 inhibition, this opposite
response of p-eIF2α to sorafenib treatment may determine cell fate
after sorafenib administration because increases of p-ERK1/2 and
p-eIF2α can rescue cell death (30). In addition, some anticancer agents
combined with anti-angiogenic drugs could improve therapeutic index
through their anti-angiogenic effects (31). As shown in the mouse model,
doxorubicin combined with anti-VEGFR2 antibody exerted an excellent
therapeutic effect (32), YS-1 plus
ADM might be another novel method in terms of antiangiogenic
therapeutics.
However, further investigation of the effect of the
YS-1 and ADM combination in clinical trials is necessary to confirm
the promising in vitro and in vivo results reported
here.
Acknowledgements
This work was financially supported by the National
Natural Science Foundation of China (81573456, 81302794) and the
National High Technology Research and Development Program of China
(2014AA022208). We are highly thankful to both organizations for
this sponsorship.
References
|
1
|
Guo H, German P, Bai S, Barnes S, Guo W,
Qi X, Lou H, Liang J, Jonasch E, Mills GB, et al: The PI3K/AKT
pathway and renal cell carcinoma. J Genet Genomics. 42:343–353.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Breda A, Lucarelli G, Rodriguez-Faba O,
Guirado L, Facundo C, Bettocchi C, Gesualdo L, Castellano G,
Grandaliano G, Battaglia M, et al: Clinical and pathological
outcomes of renal cell carcinoma (RCC) in native kidneys of
patients with end-stage renal disease: A long-term comparative
retrospective study with RCC diagnosed in the general population.
World J Urol. 33:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bangalore N and Bhargava P, Hawkins MJ and
Bhargava P: Sustained response of sarcomatoid renal-cell carcinoma
to MAID chemotherapy: Case report and review of the literature. Ann
Oncol. 12:271–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun W, Kalen AL, Smith BJ, Cullen JJ and
Oberley LW: Enhancing the antitumor activity of adriamycin and
ionizing radiation. Cancer Res. 69:4294–4300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Granados-Principal S, Quiles JL,
Ramirez-Tortosa CL, Sanchez-Rovira P and Ramirez-Tortosa MC: New
advances in molecular mechanisms and the prevention of adriamycin
toxicity by antioxidant nutrients. Food Chem Toxicol. 48:1425–1438.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diaz RR, Kwon JK, Lee JY, Nahm JH, Cho KS,
Ham WS, Cho NH and Choi YD: Renal pelvic urothelial carcinoma with
vena caval thrombus mimicking renal cell carcinoma. Korean J Urol.
55:624–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu XX, Zeng Y, Jin XH and Kakehi Y:
Enhanced susceptibility of adriamycin-treated human renal cell
carcinoma cells to lysis by peripheral blood lymphocytes and tumor
infiltrating lymphocytes. Oncol Rep. 18:353–359. 2007.PubMed/NCBI
|
|
9
|
Milner LS, Wei SH and Houser MT:
Amelioration of glomerular injury in doxorubicin hydrochloride
nephrosis by dimethylthiourea. J Lab Clin Med. 118:427–434.
1991.PubMed/NCBI
|
|
10
|
Kojima S, Icho T, Hayashi M, Kajiwara Y,
Kitabatake K and Kubota K: Inhibitory effect of
5,6,7,8-tetrahydroneopterin on adriamycin-induced cardiotoxicity. J
Pharmacol Exp Ther. 266:1699–1704. 1993.PubMed/NCBI
|
|
11
|
Lee YS, Han JM, Kang T, Park YI, Kim HM
and Kim S: Antitumor activity of the novel human cytokine AIMP1 in
an in vivo tumor model. Mol Cells. 21:213–217. 2006.PubMed/NCBI
|
|
12
|
Sun L, Yang Q, Wang P, Liu D, Liang W, Lin
S and Yuan S: The influence of YS-1 on the Dll4-Notch1 signaling
pathway. Acta Biochim Biophys Sin (Shanghai). 46:56–64. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park EJ, Kwon HK, Choi YM, Shin HJ and
Choi S: Doxorubicin induces cytotoxicity through upregulation of
pERK-dependent ATF3. PLoS One. 7:e449902012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deschênes-Simard X, Kottakis F, Meloche S
and Ferbeyre G: ERKs in cancer: Friends or foes? Cancer Res.
74:412–419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Collisson EA, Trejo CL, Silva JM, Gu S,
Korkola JE, Heiser LM, Charles RP, Rabinovich BA, Hann B, Dankort
D, et al: A central role for RAF→MEK→ERK signaling in the genesis
of pancreatic ductal adenocarcinoma. Cancer Discov. 2:685–693.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kisucká J, Barancík M, Bohácová V and
Breier A: Reversal effect of specific inhibitors of
extracellular-signal regulated protein kinase pathway on
P-glycoprotein mediated vincristine resistance of L1210 cells. Gen
Physiol Biophys. 20:439–444. 2001.PubMed/NCBI
|
|
17
|
Lin JC, Chang SY, Hsieh DS, Lee CF and Yu
DS: Modulation of mitogen-activated protein kinase cascades by
differentiation-1 protein: Acquired drug resistance of hormone
independent prostate cancer cells. J Urol. 174:2022–2026. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Steelman LS, Abrams SL, Shelton JG,
Chappell WH, Bäsecke J, Stivala F, Donia M, Nicoletti F, Libra M,
Martelli AM, et al: Dominant roles of the Raf/MEK/ERK pathway in
cell cycle progression, prevention of apoptosis and sensitivity to
chemotherapeutic drugs. Cell Cycle. 9:1629–1638. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Franklin RA, Montalto G, Cervello M, Libra M, Candido S,
Malaponte G, et al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade
inhibitors: How mutations can result in therapy resistance and how
to overcome resistance. Oncotarget. 3:1068–1111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCubrey JA, Steelman LS, Abrams SL, Lee
JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA,
D'Assoro AB, et al: Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT
pathways in malignant transformation and drug resistance. Adv
Enzyme Regul. 46:249–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gebäck T, Schulz MM, Koumoutsakos P and
Detmar M: TScratch: A novel and simple software tool for automated
analysis of monolayer wound healing assays. Biotechniques.
46:265–274. 2009.PubMed/NCBI
|
|
23
|
Zhao R, Sun L, Lin S, Bai X, Yu B, Yuan S
and Zhang L: The saponin monomer of dwarf lilyturf tuber, DT-13,
inhibits angiogenesis under hypoxia and normoxia via
multi-targeting activity. Oncol Rep. 29:1379–1386. 2013.PubMed/NCBI
|
|
24
|
Kalimuthu S and Se-Kwon K: Cell survival
and apoptosis signaling as therapeutic target for cancer: Marine
bioactive compounds. Int J Mol Sci. 14:2334–2354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoon EL, Yeon JE, Lee HJ, Suh SJ, Lee SJ,
Kang SH, Kang K, Yoo YJ, Kim JH, Yim HJ, et al: Systemic cytotoxic
chemotherapy of patients with advanced hepatocellular carcinoma in
the era of sorafenib nonavailability. J Clin Gastroenterol.
48:e22–e29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brown R, Curry E, Magnani L,
Wilhelm-Benartzi CS and Borley J: Poised epigenetic states and
acquired drug resistance in cancer. Nat Rev Cancer. 14:747–753.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kato T, Sato K, Kakinuma H and Matsuda Y:
Enhanced suppression of tumor growth by combination of angiogenesis
inhibitor O-(chloroacetyl-carbamoyl)fumagillol (TNP-470) and
cytotoxic agents in mice. Cancer Res. 54:5143–5147. 1994.PubMed/NCBI
|
|
28
|
Saijo N and Kenmotsu H: Recent development
of molecular-targeted drugs in lung cancer. Intern Med.
49:1923–1934. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jia L, Zhang MH, Yuan SZ and Huang WG:
Antiangiogenic therapy for human pancreatic carcinoma xenografts in
nude mice. World J Gastroenterol. 11:447–450. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shiota M, Eto M, Yokomizo A, Tada Y,
Takeuchi A, Masubuchi D, Inokuchi J, Tatsugami K, Kuroiwa K,
Uchiumi T, et al: Sorafenib with doxorubicin augments cytotoxicity
to renal cell cancer through PERK inhibition. Int J Oncol.
36:1521–1531. 2010.PubMed/NCBI
|
|
31
|
El-Kenawi AE and El-Remessy AB:
Angiogenesis inhibitors in cancer therapy: Mechanistic perspective
on classification and treatment rationales. Br J Pharmacol.
170:712–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang L, Hannay JA, Liu J, Das P, Zhan M,
Nguyen T, Hicklin DJ, Yu D, Pollock RE and Lev D: Vascular
endothelial growth factor overexpression by soft tissue sarcoma
cells: Implications for tumor growth, metastasis, and
chemoresistance. Cancer Res. 66:8770–8778. 2006. View Article : Google Scholar : PubMed/NCBI
|